 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Different antioxidant mechanisms of lotus root and leaf were exhibited against lipid oxidation. The effect of lotus root (LRE) and leaf (LLE) extracted with 50% ethanol exhibited high antioxidant activities. It also improved quality and/or oxidative stability of pork patties when extracts were applied individually (1%) or in combination (each 0.5%). The pork patties treated with either LLE or that with LRE exhibited significantly lower peroxide and 2-thiobarbituric acid reactive substances (TBARS) value than the control and patties with LRE. However, the lowest TBARS values were observed in patties with LLE alone from day 7 among the treatments. Patties treated with both LRE and LLE had the highest score in off flavor (P< 0.05). Hence, the combined effect of LRE and LLE was uncertain and the addition of 1% LLE in patties showed superior antioxidant activity during storage days with an adverse effect on quality properties, except for color.
Introduction
Lipid oxidation in meat and meat products is one of the major reasons for quality loss including off-flavor, discoloration, and other undesirable changes in sensorial value.[Citation1] This reaction mainly occurs during meat processing such as grinding, heating, and storage, resulting in deterioration of color, flavor, texture, and sensory quality, and reduction in the shelf life of products.[Citation2] Antioxidant compounds possessing free radicals scavenging activity and inhibition activity on auto-oxidation have been used in processed meat products to minimize and retard lipid oxidation.[Citation3] Generally, synthetic antioxidants including butylated hydroxytoluene (BHT), butylated hydroxyanisole, and tertiary-butylhydroquinon are being used. However, their use has been limited due to its health risks, e.g., toxicity and carcinogenic effect.[Citation4] Accordingly, synthetic antioxidants are being replaced with natural materials.[Citation5] Therefore, many studies on natural antioxidant sources such as rosemary[Citation6], green tea[Citation7], and garlic[Citation8] were conducted. In addition, studies on the combined effect of different natural antioxidants were partially reported in meat products.[Citation4,Citation9] This revealed a combined effect from these antioxidants, which could reduce the cost of expensive additive-free products.
Lotus (Nelumbo nucifera), a perennial submerged vegetation of southern Asia and northern Australia origin[Citation10], contains abundant levels of polyphenolic compounds. Lotus roots, a popular vegetable since ancient times, possess antifungal, anti-inflammatory, and anti-anxiety activities.[Citation11] Furthermore, lotus leaves have been used to alleviate body heat and enhance body energy according to Chinese folk medicine.[Citation12] Previous studies have been confirmed the antioxidant activity of lotus roots.[Citation13,Citation14] In addition, lotus leaf contains a variety of antioxidant compounds such as ascorbic acid, phenolic compounds, carotenoids, flavonoids, phenolic acids, and tocopherols.[Citation15] Despite several authors[Citation15–Citation17] have been studied on functional activity of lotus, the effects of combining were scarcely discussed. Furthermore, a standard extraction procedure which can influence the antioxidant potency of the extracts has yet to be established. The pork patties are suitable test subjects for the antioxidant candidates because pork patties are susceptible to oxidation owing to the quite large content of lipid (5–30%) and processing procedures including grinding and heating as aforementioned.[Citation18] In this study, the antioxidant activities of ethanol extracts from lotus root and leaf at different ethanol concentrations were investigated. Extract from lotus root and leaf possessing superior antioxidant activity or/and extraction yield was selected. Subsequently, physicochemical and sensorial properties, and lipid oxidation stability of pork patties with the extract individually or in combination were determined during 10 days of storage at 4 ± 1°C.
Materials and methods
Preparation of lotus root and leaf extracts
Lotus root powder and dried lotus leaf were obtained from Joeunyeon Co. (Inchoen, Korea). The dried lotus leaves were powdered with a blender (HMF-985, Hanil electric, Seoul, Korea). The lotus root and leaf powders were soaked in different levels of ethanol at 1:10 ratio (W:V) for 12 h in a shaking incubator at 20 ± 1°C. They were then filtered through a filter paper (Whatman, φ-110 mm, No. 2). After removing the solvent using a rotary evaporator (EYELA N-1000, Rikakkai Co., Ltd., Tokyo, Japan), extracts were diluted to 10% using ethanol and stored at 4 ± 1°C. The concentrations of the extract solvent (50% and 75%) were selected based on a preliminary study using 0 (distilled water), 50%, 75%, and 95% ethanol as the solvents. Lotus root and leaf extracts with 50% and 75% ethanol (R50, R75, L50, and L75) were prepared for trial. As a positive control, BHT which diluted as the same concentration of the extracts was used.
Effect extract solvent at different levels on antioxidant activity of lotus root and leaf
Extraction yield
The extraction yield for each solvent was calculated by subtracting the weight of residues after evaporation from the weight of the original plant powders using the method of Choe et al.[Citation19]
Diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity
The DPPH is a stable violet radical substance and commonly used to evaluate antioxidant activity. The DPPH radical scavenging activity can be measured from many samples at low concentrations for a short period using color change by hydrogen donation.[Citation19] The assay was achieved based on of Choe et al.[Citation20] An extract of 1 mL was mixed with 3 mL of 100 µmol DPPH in ethanol. The mixture was stored at 20 ± 1°C for 30 min without light. Subsequently, the decrease in absorbance of the resulting solution was observed at 517 nm using a spectrophotometer (Optizen 2120 UV plus, Mecasys Co. Ltd., Seoul, Korea). The radical scavenging activity was expressed in percentages:
Chelating activity
Chelating activity was determined using the method by Decker and Welch.[Citation21] Chelation contributes to the antioxidant effect. An o-quinol group at the B-ring of phenolics in food can chelate metal ions which present pro-oxidant effect.[Citation22] Accordingly, 0.05 mL of FeCl2 (2 mmoL) and 0.2 mL of ferrozine (5 mmoL) were added to 2.5 mL of each extract. The mixtures were then left to react at 20 ± 1°C for 10 min without light. The absorbance of the mixture was then measured at 562 nm using spectrophotometer (Mecasys Co. Ltd.). The chelating activity was expressed in percentages by:
Reducing power
The activities of reductones in extracts were determined via reducing power assays by reacting the samples with active oxygen and free radicals to make them stable.[Citation23] The assay was performed following the method by Choe et al.[Citation20] Correspondingly, 0.5 mL of each extract was mixed with 5 mL of phosphate buffer (0.2 M, pH 6.6) and 2.5 mL of potassium ferricyanide (1%). The mixture was then incubated at 50°C for 20 min and centrifuged at 650 g and 4°C for 10 min. Thereafter, 2.5 mL of the supernatant was mixed with 2.5 mL of distilled water and 0.5 mL of 0.1% ferric chloride. The absorbance of the mixture was measured at 700 nm using a spectrophotometer (Mecasys Co. Ltd., Daejeon, Korea).
Effect of lotus root and leaf extracts on quality properties and lipid oxidation stability of pork patties
Preparation of pork patties: Fresh pork hind leg was purchased from a slaughterhouse 48 h postmortem. All subcutaneous inter-muscular fat and visible connective tissues were removed from the muscles. Lean meat and pork back fat were ground through a 3-mm plate using a meat grinder (PM-98, Fujee Tech., Hwasung, Korea), and then divided into five portions. The pork patties were manufactured to the following formulation: 70% pork hind leg, 20% pork fat, 10% ice water, and 1.5% salt based on the total weight. The LRE and/or LLE were added to the patties at 1% (LRE and LLE, respectively) or together at 0.5% (LRE+LLE). Control patties were made without any extract and pork patties containing 0.02% BHT were used as positive control. In addition, patties with varying levels of the extracts (0.1%, 0.5%, and 1%) were made to compare their effect on lipid oxidation. All ingredients and additives were mixed in a steel bowl manually for 7 min. Subsequently, 90 g of these were thrusted in patty molds (small ground press, Spikomat Ltd., Nottinghamshire, UK). The patties were then steam-cooked in a chamber (1600EL, Kerres GmbH, Backnang, Germany) for 30 min until their core temperature reached 75 ± 1°C. The products were individually vacuum packed in PE/Nylon film bags and stored at 4 ± 1°C for 10 days. In total, three batches of the patties were manufactured at different days for analysis.
The color and pH values of each extract used in this study were as follows: lotus root extract (pH, 5.64 ± 0.01; CIE L*-value, 67.2 ± 3.05; CIE a*-value, 2.2 ± 0.98; CIE b*-value, 27.0 ± 3.52) and lotus leaf extract (pH, 4.54 ± 0.04; CIE L*-value, 18.9 ± 0.18; CIE a*-value, 2.9 ± 1.22; CIE b*-value, 2.1 ± 0.24).
Cooking yield
After the cooking of the patties in a chamber for 30 min, they were cooled at room temperature for an hour. The surfaces of patties were carefully drained using a paper towel and weighed. Their cooking yields were determined by the weight of patties before and after cooking.
Proximate composition
The proximate compositions of meat samples at day 0 were determined using official methods of AOAC.[Citation24] Moisture content was measured based on the weight loss of the sample after drying at 105°C for 12 h in a drying oven (SW-90D, Sang Woo Scientific Co., Bucheon, Korea). Fat content was determined by Soxhlet method using a solvent extraction system (Soxtec® Avanti 2050 Auto System, Foss Tecator AB, Höganas, Sweden). Protein content was obtained by Kjeldahl method using an automatic Kjeldahl nitrogen analyzer (Kjeltec® 2300 Analyzer Unit, Foss Analytical AB, Höganäs, Sweden). Ash content was evaluated according to AOAC.[Citation24]
pH values
Meat samples of mass 5 g and 20 mL of distilled water were homogenized using an Ultra-Turrax SK15 (Janke & Kunkel, Staufen, Germany). The pH value of the homogenate was measured using a pH meter (Model 340, Mettler-Toledo GmbH, Schwerzenbach, Switzerland).
Color measurement
The color of the outer side of each cooked patty was determined using a colorimeter (Chroma meter CR-210, Minolta, Japan; illuminate C, calibrated with a white plate, CIE L* = +97.83, CIE a* = – 0.43, CIE b* = +1.98). CIE L*-value (lightness), CIE a*-value (redness), and CIE b*-value (yellowness) were recorded.
Lipid oxidation
Peroxide value (POV): To compare the lipid oxidation of pork patties, lipids of meat samples were extracted following the method by Folch et al.[Citation25] using a chloroform: methanol solvent system with 2:1 ratio. The extracts were concentrated by completely removing the solvent using a rotary evaporator (EYELA N-1000, Rikakikai. Co. Ltd., Tokyo, Japan). The lipids were then subjected to POV measurements according to the official method of AOAC[Citation25] and expressed as mili-equivalent peroxide/kg sample.
2-Thiobarbituric acid reactive substances (TBARS) value: TBARS value was evaluated for the effect of single usage of each LRE and LLE with varying levels (0%, 0.1%, 0.5%, and 1% respectively; ), as well as the combined effect of the extracts (each 0.5%; ). Lipid oxidation was determined following a slightly modified method by Vyncke et al.[Citation26] with minor modification. Accordingly, 10 g of meat sample was homogenized in 30 mL of 7.5% trichloroacetic acid solution and 0.2 mL of 0.3% BHT using a blender (Ultra-Turrax SK15, Janke & Kunkel, Germany). After filtering through a filter paper (Whatman No. 1), 5 mL of the filtrate was transferred in a stoppered glass tube, mixed with equal volume of 0.02 mol TBA solution, and immersed in a boiling water bath for 40 min for reaction. The tubes were water-cooled for 10 min. Absorbance was measured at 538 nm using a spectrophotometer (Mecasys Co. Ltd.). The calibration curve for the TBARS calculations was prepared using 1,1,3,3-tetraethoxy propane. The TBARS value was expressed in mg malondialdehyde (MDA)/kg meat sample.
Table 1. Antioxidant properties of lotus root and leaf extracts
Table 2. Quality properties of pork patties with lotus root and leaf extracts
Table 3. Thiobarbituric acid reactive substances (TBARS) values of pork patties depending on the addition level of lotus root and leaf extracts
Sensory evaluation
Patty samples were cooked following the previous procedure and were kept at 20 ± 1°C before sensory evaluation. The sensory panel consisted of 10 individuals aged between 20 and 35 years old. According to Choe et al.[Citation27], the panel was semi-trained and instructed to cleanse their palates using water between samples. They evaluated samples for color, flavor, tenderness, juiciness, off-flavor, and overall acceptability using a 9-point descriptive scale on each trait (1 = extremely undesirable, 9 = extremely desirable).
Statistical analysis
The entire experiments were replicated three times at different days and all the samples analyzed in three replicates. All data were expressed as means ± standard deviation (SD). One-way analysis of variance (ANOVA) was used to analyze the data using a general linear model (GLM) procedure of SPSS Statistics18.0 software (SPSS Inc., Chicago, IL, USA). Duncan’s multiple range test was performed to compare mean values and measure significant differences (P < 0.05).
Results and discussion
Effect of different levels of extract solvent on antioxidant activity of lotus root and leaf
Extraction yield
The level of ethanol significantly influenced the extraction yield of lotus root, but not of lotus leaf (). The R50 showed the highest (P < 0.05) extraction yield, while both R75 and L75 exhibited the lowest (P < 0.05). This result may be due to the difference in solubility of active substances dissolved in water and ethanol.[Citation28] The extraction yield was affected by properties and mixing proportions of extract solvent because compounds from plants have different polarities and solubilities, which make their phytochemicals difficult to be extracted with a single solvent.[Citation28] Previous studies reported that a mixed solvent of water and ethanol induced higher extraction yield in onion peels[Citation29] and brown soybeans.[Citation30] In the present study, 0 (distilled water) and 95% ethanol were not used as extract solvent, owing to their low extraction yield on lotus root and leaf. Extracts with 50% ethanol exhibited relatively higher extraction yield and antioxidant activity compared to those of extract with 50% ethanol (, ). Therefore, it is considered as an appropriate extracting concentration for lotus root and leaf.
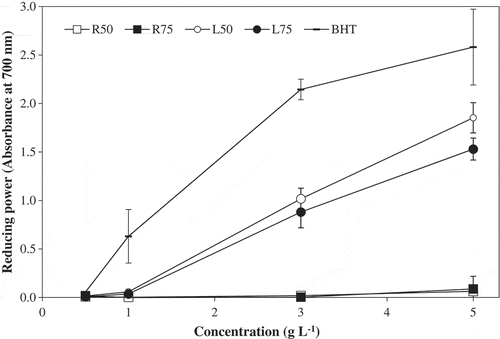
Figure 1. Reducing power of lotus root and leaf extracts. (□) R50, Lotus root extract extracted with 50% ethanol; (■) R75, Lotus root extract extracted with 75% ethanol; (○) L50, Lotus leaf extract extracted with 50% ethanol; (●) L75, Lotus leaf extract extracted with 75% ethanol; (−) BHT, 0.02% BHT (butylated hydroxytoluene). Error bars show the SD
DPPH radical scavenging activity
Comparing the antioxidant effect of extracts, the lowest concentration of samples required to scavenge DPPH radicals by 50% (IC50 value) was exhibited in . BHT indicated an IC50 value L75 (P > 0.05). The leaf extract displayed higher antioxidant activities compared with root extract (P < 0.05). It was in accordance with the report of Choi et al.[Citation31] who compared the antioxidant effect of various parts composing the lotus. In the root extract, 50% ethanol for extraction showed higher (P < 0.05) scavenging activity on DPPH radical compared to 75% ethanol. This difference made by the concentration of solvent ethanol might be caused by the difference in the extracted antioxidant compounds, which have different polarities and solubilities.
Chelating activity
Chelation contributes to the antioxidant effect. An o-quinol group at the B-ring of phenolics in food can chelate metal ions which present pro-oxidant effect.[Citation21] Because of the sufficient amount of hemeproteins and other porphyrins in meat and meat products that accelerate lipid oxidation[Citation32], chelating activity is important in the overall antioxidant effect. The root extracts (R50 and R75) exhibited significantly higher chelating activities with lower EC50 compared to leaf extracts (L50 and L75; ). This results from the superiority of the chelating ability of tannins in lotus roots. Phenolic compounds are multifunctional antioxidants that provide oxidation chain-breaking and chelating activities in the same molecule.[Citation33] However, phenolic compounds with only a single OH group cannot bind with Fe2+ and Cu2+, whereas tannins that are one of the complexed polyphenols can chelate metal ions more effectively as they possess either galloyl (trihydroxyphenyl) or catechol group (o-dihydroxyphenyl), which are essential for the formation of complex metal ions, especially Fe2+ ions that preferentially bind to those groups.[Citation34] Huang et al.[Citation16] reported that tannins in lotus roots were prevalent, more than twice of total tannin contents in its leaf. Thus, the higher chelating effect of the root extracts than that of the leaf extracts, despitelower antioxidant effects, may be attributed to the abundance of tannins in root extracts. This phenomenon can be further applied for the combination of lotus root and leaf extracts due to their complementary mechanism for antioxidant activity. The levels of ethanol did not influence chelating activity in both lotus root and leaf extracts.
Reducing power
The reducing powers were increased with the concentration of extracts (). This is consistent with the result of Choe et al.[Citation20], which demonstrated the concentration-dependent increase in reducing power of persimmon peel extracts using different levels of ethanol. LRE resulted in very low reducing power that was below absorbance of 0.1. However, a report by Jeong et al.[Citation35] presented reducing power of lotus root extracts above absorbance of 1.0 on the fraction of CHCl2 and butanol. Therefore, the result herein may be made by the effect of the used solvent. The difference in reducing power between ethanol concentrations (50% or 75%) of both lotus root and leaf extracts (P > 0.05) were negligible. The BHT exhibited the highest (P < 0.05) reducing power among the treatments.
Antioxidant activity of lotus root and leaf extracts at different ethanol levels was exhibited depending on assay (DPPH radical scavenging or chelating activity). The leaf extract presented antioxidant activities comparable to BHT in DPPH radical scavenging assay. Although they were lower than that of LLE in DPPH radical scavenging and reducing power assay, the root extract provided higher (P < 0.05) chelating activities that promote antioxidant effect against the initiation of lipid oxidation in meat products. Meanwhile, extracts made by 50% solvent ethanol exhibited generally higher extraction yield than 75% ethanol though the difference was hardly found on antioxidant effect on both lotus root and leaf extracts (). Consequently, R50 and L50 were applied to pork patties.
Effect of lotus leaf and root extracts on quality properties and lipid oxidation stability of pork patties
Physicochemical properties of pork patties
Fat, protein, and ash contents, and cooking yield were not affected by the addition of LRE or LLE (; P > 0.05). The moisture contents of pork patties containing BHT was the highest and those treated with LRE or LLE had significantly lower compared to other treatments. The pH values of pork patties did not vary upon treatment of LRE and LLE (, P > 0.05) despite the difference in pH values of LLE (pH 4.54) and LRE (pH 5.64). Generally, pure LRE treatment induced higher L* and lower b* values of pork patties compared with pure LLE or that with LRE (; P < 0.05). This might be due to the inherent colors of LRE and LLE. Similar results have been reported by Hwang et al.[Citation4], revealing that L* and a* values of raw and deep-fried chicken nuggets decreased with the addition of ethanol extract of ganghwayakssuk (Artemisia princeps Pamp.) that had an inherent dark green color. Consequently, the addition of LRE and LLE in pork patties had no significant effect on quality properties.
TBARS value of pork patties containing lotus root and leaf extracts individually
The TBARS values of pork patties with LRE or LLE at different levels (0.1%, 0.5%, and 1%, respectively) were decreased with increasing addition levels of LRE and LLE (). The addition of more than 0.5% LLE induced lower (P < 0.05) TBARS values of pork patties compared to 0.02% BHT. Between extracts, LLE could more effectively inhibit lipid oxidation of pork patties than LRE at above 0.5% was used (P < 0.05). Pork patties added with more than 0.5% of LRE or LLE maintained its TBARS value under the sensory threshold of 1 mg/kg though the others do not.[Citation36]
Lipid oxidation stability of pork patties containing a combination of lotus root and leaf extracts
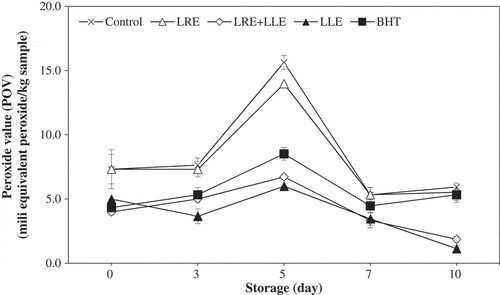
POV measurements were determined the concentration of hydroperoxide, the primary lipid oxidation product, based on the level of oxidation.[Citation4] The POVs of all patties increased until 5 days of storage and decreased thereafter, indicating the decomposition of peroxides after 5 days[Citation37] (). During the entire storage period, pork patties treated with combination LLE and LRE had significantly lower POV than the control and those with LRE. Furthermore, those with BHT also exhibited relatively lower (P < 0.05) POV than the control and sample with LRE alone. However, after 7 days of storage, there was no significant difference among them. Therefore, LLE and LRE could prevent peroxide production, especially when both are administered together in pork patties.
Figure 2. Changes in peroxide value (POV) of pork patties containing lotus loot and leaf extracts during refrigerated storage. (×) Control, no ingredient added; (△) patties with 1% lotus root extract; (◇) patties with 0.5% lotus root extract and 0.5% lotus leaf extract; (▲) patties with 1% lotus leaf extract; (■) patties with 0.02% butylated hydroxytoluene (BHT). Error bars show the SD
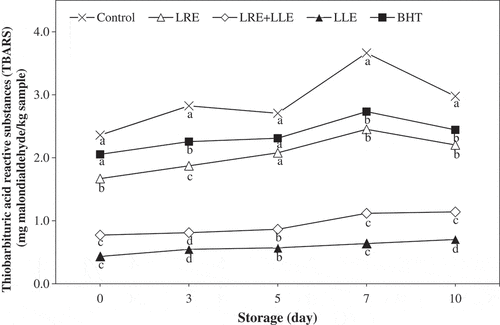
The effect of combined extracts (each 0.5%) was compared with single extracts (1%, respectively) on pork patties during refrigerated storage (). During storage of 10 days, control samples and those treated with LRE and BHT showed maximum TBARS values at day 7 then decreased at day 10. Maqsood and Benjakul[Citation38] reported the decrease in TBARS values caused by the interaction of TBA-reactive substances with proteins. Kim et al.[Citation39] also reported that both microbial growth level and strain during storage influence TBARS values in raw ground pork. During the entire storage days, patties samples treated with 1% LLE exhibited the lowest TBARS values (P < 0.05). The treatment maintained its TBARS values under the sensory threshold level (1.0 mg/kg) of pork.[Citation37] Even though LRE had a less antioxidant effect than LLE, patties treated with combined 0.5% LRE and 0.5% LLE maintained similar (P > 0.05) TBARS value with those treated with 1% LLE until day 7. This is supported by the TBARS value of the single extracts. As shown in , the addition of LLE at 0.5% level alone could not reduce the TBARS value to the extent of 1% addition (P < 0.05). While the combination of LLE and LRE at 0.5% level of each resulted similar (P > 0.05) antioxidant effect to 1% LLE on TBARS and POV value ( and ). In this study, the combined effect of LRE and LLE was uncertain because patties samples with 0.5% LRE or 0.5% LLE individually cannot be compared POV and TBARS values to combined treatment ( and ). However, based on antioxidant activities of extracts () and patties samples with 0.5% samples LRE or LLE (), the differences in antioxidizing mechanisms between LRE and LLE were observed. In the evaluation of antioxidant effects of LRE and LLE suggests that LRE display a significantly higher chelating activity, while LLE provides significantly higher reducing power and DPPH radical scavenging activity (, ). Hence, the antioxidant effect of LLE originates from its ability to reduce free electrons and radicals, and that of LRE is because of its ability to chelate catalyzing transition metals such as Fe2+ and Cu+. Similarly, Bandarra et al.[Citation40] reported that the synergistic antioxidant effect from phosphatidylethanolamine and tocopherol result from their difference in antioxidizing mechanisms.
Figure 3. Changes in thiobarbituric acid reactive substances (TBARS) of pork patties containing lotus root and leaf extracts during refrigerated storage. (×) Control, no ingredient added; (△) patties with 1% lotus root extract; (◇) patties with 0.5% lotus root extract and 0.5% lotus leaf extract; (▲) patties with 1% lotus leaf extract; (■) patties with 0.02% butylated hydroxytoluene (BHT). a-d means that column with different letters are significantly different (P < 0.05)
Sensory evaluation
The sensory evaluations of pork patties treated with LRE and LLE () revealed that samples applied with combined LRE and LLE attained the highest overall acceptance score, followed by patties treated with LLE and those containing BHT. Panels estimated that the patties containing pure or combined extracts had undesirable color compared to the control (P < 0.05). This might be explained by the greenish colors of applied extracts that need to be resolved further. Significant differences among the patties were also observed by instrumental color analysis. Similarly, because of its indigenous taste, the flavor of pork patties treated with LRE received the lowest score in that category (P < 0.05). Moreover, patties with the combined extract treatment scored better as the taste of each extract diminished. The patties treated with LRE received the lowest score in terms of juiciness. This corresponded with the low moisture content of LRE-treated patties (P < 0.05, ). The panels informed that the negative control pork patty had undesirable off-flavor compared with those that were treated (). This review agrees with the POV and TBARS value of the patties, indicating the strong antioxidant effects from the extracts, especially when used in combination.
Table 4. Sensory evaluation of pork patties with lotus root and leaf extracts
Conclusion
The results of this study revealed that the application of 50% ethanol extracts of lotus root and leaf in pork patties effectively retarded lipid oxidation, possessing different antioxidizing mechanisms. However, the synergistic effect of lotus root and leaf was uncertain in pork patties. Hence, lotus root and lotus leaf alone or together could be used as antioxidant depending on the oxidation condition of processed meat products.
Acknowledgment
This work was carried out with the support of the Cooperative Research Program for Agriculture Science & Technology Development (Project No.011617), Rural Development Administration, Korea.
Additional information
Funding
References
- Roldan, M.; Antequera, T.; Armenteros, M.; Ruiz, J. Effect of Different Temperature–Time Combinations on Lipid and Protein Oxidation of Sous-Vide Cooked Lamb Loins. Food Chem. 2014, 149, 129–136. DOI: 10.1016/j.foodchem.2013.10.079.
- Reihani., S.; Tan, T.; Huda, N.; Easa, A. M. Frozen Storage Stability of Beef Patties Incorporated with Extracts from Ulam Raja Leaves (Cosmos Caudatus). Food Chem. 2014, 155, 17–23. DOI: 10.1016/j.foodchem.2014.01.027.
- Rodríguez-Rojo, S.; Visentin, A.; Maestri, D.; Cocero, M. Assisted Extraction of Rosemary Antioxidants with Green Solvents. J. Food Eng. 2012, 109, 98–103. DOI: 10.1016/j.jfoodeng.2011.09.029.
- Hwang, K. E.; Choi, Y. S.; Choi, S. M.; Kim, H. W.; Choi, J. H.; Lee, M. A.; Kim, C. J. Antioxidant Action of Ganghwayakssuk (Artemisia Princeps Pamp.) In Combination with Ascorbic Acid to Increase the Shelf Life in Raw and Deep Fried Chicken Nuggets. Meat Sci. 2013, 95, 593–602. DOI: 10.1016/j.meatsci.2013.05.035.
- Lee, J.; Kunz, B. The Antioxidant Properties of Baechu-Kimchi and Freeze-Dried Kimchi-Powder in Fermented Sausages. Meat Sci. 2005, 69, 741–747. DOI: 10.1016/j.meatsci.2004.11.006.
- Neil, J. H.; Dimick, P. S.; Mast., M. G. Use of Chemical Compounds and a Rosemary Spice Extract in Quality Maintenance of Deboned Poultry Meat. J. Food Sci. 1973, 38, 1080–1081. DOI: 10.1111/j.1365-2621.1973.tb02155.x.
- Tang, S. Z.; Kerry, J. P.; Sheehan, D.; Buckley, D. J. Antioxidative Mechanisms of Tea Catechins in Chicken Meat Systems. Food Chem. 2002, 76, 45–51. DOI: 10.1016/S0308-8146(01)00248-5.
- Cao, Y.; Gu, W.; Zhang, J.; Chu, Y.; Ye, X.; Hu, Y.; Chen, J. Effects of Chitosan, Aqueous Extract of Ginger, Onion and Garlic on Quality and Shelf Life of Stewed-Pork during Refrigerated Storage. Food Chem. 2013, 141, 1655–1660. DOI: 10.1016/j.foodchem.2013.04.084.
- Babuskin, S.; Babu, P. A. S.; Sasikala, M.; Sabina, K.; Archana, G.; Sivarajan, M.; Sukumar, M. Antimicrobial and Antioxidant Effects of Spice Extracts on the Shelf Life Extension of Raw Chicken Meat. Int. J. Food Microbiol. 2014, 171, 32–40. DOI: 10.1016/j.ijfoodmicro.2013.11.011.
- Kim, H.; Lee, C.; Oh, J.; Lee, J.; Lee, S. Quality Characteristics of Sponge Cake with Added Lotus Leaf and Lotus Root Powders. J. Korean Soc. Food Sci. Nutr. 2011, 40, 1285–1291. DOI: 10.3746/jkfn.2011.40.9.1285.
- Du, H.; Zhao, X.; You, J.; Park, J.; Kim, S.; Chang, K. Antioxidant and Hepatic Protective Effects of Lotus Root Hot Water Extract with Taurine Supplementation in Rats Fed a High Fat Diet. J. Biomed. Sci. 2010, 17, 1. DOI: 10.1186/1423-0127-17-S1-S39.
- Wu, M.; Wang, L.; Weng, C.; Yen, J. E. Am. J. Chinese. Med. 2003, 31, 687–698. DOI: 10.1142/S0192415X03001429.
- Jung, I. C.; Park, H. S.; Choi, Y. J.; Park, S. S.; Kim, M. J.; Park, K. S. The Effect of Adding Lotus Root and Leaf Powder on the Quality Characteristics of Cooked Pork Patties. Korean J. Food Cookery Sci. 2011, 27, 783–791. DOI: 10.9724/kfcs.2011.27.6.783.
- Ham, Y. K.; Hwang, K. E.; Song, D. H.; Kim, Y. J.; Shin, D. J.; Kim, K. I.; Lee, H. J.; Kim, N. R.; Kim, C. J. Lotus (Nelumbo Nucifera) Rhizome as an Antioxidant Dietary Fiber in Cooked Sausage: Effects on Physicochemical and Sensory Characteristics. Korean J. Food Sci. An. 2017, 37, 219–227. DOI: 10.5851/kosfa.2017.37.2.219.
- Choe, J. H.; Jang, A.; Lee, E. S.; Choi, J. H.; Choi, Y. S.; Han, D. J.; Kim, H. Y.; Lee, M. A.; Shim, S. Y.; Kim, C. J. Oxidative and Color Stability of Cooked Ground Pork Containing Lotus Leaf (Nelumbo Nucifera) and Barley Leaf (Hordeum Vulgare) Powder during Refrigerated Storage. Meat Sci. 2011, 87, 12–18. DOI: 10.1016/j.meatsci.2010.08.011.
- Huang, B.; He, J.; Ban, X.; Zeng, H.; Yao, X.; Wang, Y. Antioxidant Activity of Bovine and Porcine Meat Treated with Extracts from Edible Lotus (Nelumbo Nucifera) Rhizome Knot and Leaf. Meat Sci.. 2011, 87, 46–53. DOI: 10.1016/j.meatsci.2010.09.001.
- Park, K. S.; Park, H. S.; Choi, Y. J.; Moon, Y. H.; Lee, K. S.; Kim, M. J.; Jung, I. C. Quality Change of Pork Patty Containing Lotus (Nelumbo Nucifera) Leaf and Root Powder during Refrigerated Storage. J. Life Sci. 2011, 21, 1732–1739. DOI: 10.5352/JLS.2011.21.12.1732.
- Barbut, S.; The Science of Poultry and Meat Processing; Principles of meat processing. University of Guelph: Ontario, 2015
- Choe, J.; Jang, A.; Choi, J. H.; Choi, Y. S.; Han, D. J.; Kim, H. Y.; Lee, M. A.; Kim, H. W.; Kim, C. J. Antioxidant Activities of Lotus Leaves (Nelumbo Nucifera) and Barley Leaves (Hordeum Vulgare) Extracts. Food Sci. Biotechnol. 2010, 19, 831–836. DOI: 10.1007/s10068-010-0117-8.
- Choe, J.; Kim, H.; Kim, Y.; Yeo, E.; Kim, C. Antioxidant Activity and Phenolic Content of Persimmon Peel Extracted with Different Levels of Ethanol. Int. J. Food. Prop. 2014, 17, 1779–1790. DOI: 10.1080/10942912.2012.731460.
- Decker, E. A.; Welch, B. Role of Ferritin as a Lipid Oxidation Catalyst in Muscle Food. J. Agric. Food Chem. 1990, 38, 674–677. DOI: 10.1021/jf00093a019.
- Shahidi, F.;. Handbook of Antioxidants for Food Preservation; Woodhead Publishing: Cambridge, 2015.
- Kanatt, S. R.; Chander, R.; Sharma, A. Antioxidant Potential of Mint (Mentha Spicata L.) In Radiation-Processed Lamb Meat. Food Chem. 2007, 100, 451–458. DOI: 10.1016/j.foodchem.2005.09.066.
- Oyaizu, M.;. Antioxidative Activities of Browning Reaction Prepared from Glucosamine. Jpn. J. Nutr. 1986, 44, 307–315. DOI: 10.5264/eiyogakuzashi.44.307.
- Folch, J.; Lees, M.; Sloane-Stanley, G. Extraction of Fatty Acid. J. Biol. Chem. 1957, 226, 497–509.
- Vyncke, W.;. Direct Determination of the Thiobarbituric Acid Value in Trichloracetic Acid Extracts of Fish as a Measure of Oxidative Rancidity. Fett. Wiss. Technol. 1970, 72, 1084–1087. DOI: 10.1002/lipi.19700721218.
- Choe, J.; Kim, Y. H. B.; Kim, H. Y.; Kim, C. J. Evaluation of Physicochemical and Anti-Oxidant Properties of Powdered Leaves from Lotus, Shepherd’s Purse and Goldenrod in Restructured Duck/Pork Patties. J. Food Sci. Technol. 2017, 54, 2494–2502. DOI: 10.1007/s13197-017-2693-6.
- Sun, T.; Ho, C. Antioxidant Activities of Buckwheat Extracts. Food Chem. 2005, 90, 743–749. DOI: 10.1016/j.foodchem.2004.04.035.
- Shim, S. Y.; Choi., Y. S.; Kim., H. Y.; Kim, H. W.; Hwang, K. E.; Song, D. H.; Lee, M. A.; Lee, J. W.; Kim, C. J. Antioxidative Properties of Onion Peel Extracts against Lipid Oxidation in Raw Ground Pork. Food Sci. Biotechnol. 2012, 21, 565–572. DOI: 10.1007/s10068-012-0072-7.
- Lee, C. H.; Hwang, K. E.; Kim, H. W.; Song, D. H.; Kim, Y. J.; Ham, Y. K.; Choi, Y. S.; Jang, S. J.; Jeong, T. J.; Kim, C. J. Antioxidant Activity of Brown Soybean Ethanolic Extracts and Application to Cooked Pork Patties. Korean J. Food Sci. An. 2016, 36, 359–368. DOI: 10.5851/kosfa.2016.36.3.359.
- Choi, H. Y.; Jung, K.; Shin, H. S. Antioxidant Activity of the Various Extracts from Different Parts of Lotus (Nelumbo Nucifera Gaertner). Food Sci. Biotechnol. 2009, 18, 1051–1054.
- Love, J. D.; Pearson, A. Lipid Oxidation in Meat and Meat products—A Review. J. Am. Oil. Chem. Soc. 1971, 48, 547–549. DOI: 10.1007/BF02544559.
- Khokhar, S.; Apenten, R. K. O. Iron Binding Characteristics of Phenolic Compounds: Some Tentative Structure–Activity Relations. Food Chem. 2003, 81, 133–140. DOI: 10.1016/S0308-8146(02)00394-1.
- Karamać, M.;. Chelation of Cu (II), Zn (II), and Fe (II) by Tannin Constituents of Selected Edible Nuts. Int. J. Mol. Sci. 2009, 10, 5485–5497. DOI: 10.3390/ijms10125485.
- Jeong, C. H.; Son, K. B.; Kim, J. H.; Kang, S. K.; Park, E. Y.; Seo, K. I.; Shim, K. H. Antioxidant and Anticancer Activities of Lotus (Nelumbo Nucifera) Leaf and Root. Korean J. Food Preserv. 2010, 17, 131–138.
- Tarladgis, B. G.; Watts, B. M.; Younathan, M. T.; Dugan, L., Jr. A Distillation Method for the Quantitative Determination of Malonaldehyde in Rancid Foods. J. Am. Oil Chem. Soc. 1960, 37, 44–48. DOI: 10.3746/jfn.2004.9.4.312.
- Lee, M. A.; Choi, J. H.; Choi, Y. S.; Kim, H. Y.; Kim, H. W.; Hwang, K. E.; Chung, H. K.; Kim, C. J. Effects of Kimchi Ethanolic Extracts on Oxidative Stability of Refrigerated Cooked Pork. Meat Sci. 2011, 89, 405–411. DOI: 10.1016/j.meatsci.2011.05.004.
- Maqsood, S.; Benjakul, S. Preventive Effect of Tannic Acid in Combination with Modified Atmospheric Packaging on the Quality Losses of the Refrigerated Ground Beef. Food Control. 2010, 21, 1282–1290. DOI: 10.1016/j.foodcont.2010.02.018.
- Kim, J. K.; Jo, C. R.; Kim, H. J.; Lee, K. H.; Kim, Y. J.; Byun, M. W. Relationship of Specific Microbial Growth and TBARS Value in Radiation-Sterilized Raw Ground Pork. J. Food Sci. Nutr. 2004, 9, 312–316. DOI: 10.3746/jfn.2004.9.4.312.
- Bandarra, N. M.; Campos, R. M.; Batista, I.; Nunes, M. L.; Empis, J. M. Antioxidant Synergy of α-tocopherol and Phospholipids. J. Am. Oil Chem. Soc. 1999, 76, 905–913. DOI: 10.1007/s11746-999-0105-4.
