ABSTRACT
Hydrodistilled essential oils (HD) of dried aerial parts of Achillea fragrantissima cultivated in Egypt and Madinah Monawara, Saudi Arabia, and their volatiles extracted by solid phase microextraction (SPME) were analyzed using Gas Chromatography – Mass Spectrometry. Thirty – four constituents of the essential oil of Egyptian A. fragrantissima were identified, representing 90.15% of the total oil constituents, while SPME revealed 15 components constituting 94.72% of the volatile material. Santolina alcohol, artemisia ketone, α-thujone, 4(10)-thujen-3-ol, β-thujone, yomogi alcohol and trans-sabinyl acetate were the predominant components in both extracts, with quantities varying with extraction method. Many terpenes e.g. β-pinene, sabinene, α-terpinene, p-cymene, linalool, p-menth-2-en-1-ol, 4(10)-thujen-3-ol, borneol, carvone, p-menth-1-en-3-one, bornyl acetate and germacrene D, were identified for the first time. α-Thujone, 4-terpineol, trans-pinocarveol, and spathulenol were the major components among 42 identified components accounting for 93.65% of the total identified volatiles of Madinah hydrodistillate. Monoterpenes concentration was higher in Madinah SPME volatile extract than in HD essential oil. A. fragrantissima essential oil of Madinah exhibited higher antioxidant activity (IC50 1.09 mg/ml) than did Egyptian oil (IC50 1.72 mg/ml), consistent with the differences in phenolic content and volatile constituents identified in both oils.
Introduction
Food suppliers have applied synthetic antioxidants to their food products for many decades in order to avoid quality deterioration due to autoxidation of food components especially lipid. However, nutrition specialists and the general public are increasingly concerned about their potential health and antiaging benefits. Interests in using natural antioxidants rich in phenolic compounds such as herbs and spices in foods, nutraceuticals and cosmetics is driven by customer trends especially interest in substitutes for synthetic antioxidants such as butylated hydroxyl toluene (BHT) and butylated hydroxyl anisole (BHA), which are associated with adverse effects on health.[1]
Achillea fragrantissima (known in Arabic as Qaysūm, family Asteraceae) is a well – known small perennial aromatic herb, distributed regionally in North Africa, eastern Mediterranean coast, and Middle East.[Citation2] However, it is now cultivated worldwide due to its various medicinal uses. Traditionally, A. fragrantissima is prepared as oil or infusion and used in folk medicine as a stomachic, anthelmintic, diuretic, antichloristic, antispasmodic and antiseptic agent.[Citation3] Many researchers have investigated the antimicrobial, anti-inflammatory, antiviral, anticancer, antifungal and antibacterial effects of the essential oil of A. fragrantissima, but not the antioxidant activity.[Citation4–Citation9]
In order to determine the bioactive components responsible for such activities, studies have investigated the chemical composition of A. fragrantissima hydrodistilled essential oil using Gas Chromatography – Mass Spectrometry (GC-MS), with the detected chemical constituents varying with environmental, climatic, and geographic conditions.[Citation10] For example, El-Shazly et al.[Citation4] identified cis-thujone, santolina alcohol and artemesia ketone as the major constituents of the essential oil of A. fragrantissima collected from Sinai desert, while Choucry[Citation9] reported caryphyllene oxide, 1-terpinene-4-ol and veridiflorol as the abundant components in the oil extracted from the herb cultivated in North coast, Alexandria. Selection of a suitable extraction technique is important to obtain reliable results. Hydrodistillation (HD) is the most common technique applied in many studies. However, it is associated with limitations such as chemical changes in monoterpenes, isomerization, saponification and polymerization due to thermal treatment.[Citation11] Recently, solid – phase microextraction(SPME) has been successfully used as alternative technique with no negative effects for the analyses of aroma, flavors, essential oils and contaminants[Citation10] but has not been applied to A. fragrantissima.
Meanwhile, despite the fact that the flora of Saudi Arabia represents some of the most diverse flora in the Arabian Peninsula and a very important resource for medicinal plants such as A. fragrantissima,[Citation12] neither the chemical constituents nor the antioxidant activity of A. fragrantissima cultivated in different regions of Saudi Arabia (e.g., the western province which is known as Hejaz) have been studied. Hejaz is separated from Egypt on the west by the Red Sea, on the north by Jordan, on the east by the Najd, and on the south by ’Asir Region. It is the most populated province in Saudi Arabia, and Madinah Monawara is the largest region in the province, covering 7.7% of the total area of Saudi Arabia.[Citation13]
Therefore, the aim of the present study was to compare the volatile constituents of A. fragrantissima cultivated in Madinah Monawara, Saudi Arabia, and Nile Delta, Egypt using two different techniques, i.e., HD and SPME. Further, the antioxidant activity of the essential oils extracted using HD was assayed in order to be applied and used as a food additive.
Material and methods
Plants and chemicals
Qaysūm herb (A. fragrantissima) was collected from a Wilderness Area (Khils Region in Madinah, Saudi Arabia), while the Egyptian herb was obtained from Bilbis, Sharkia, Egypt in August 2015. Both Madinah and Egyptian herbs were identified by a taxonomist at the Department of Botany, Faculty of Science, Zagazig University and deposited in Madinah Monawara Municipality Lab for Food, Water Analysis and Environmental Research with voucher specimens number AF-36003932–2015 and AF-36003933–2015. Diethyl ether and methanol were purchased from Fisher Chemicals (Pittsburgh, USA). The mixture of n-alkanes C6–C26, authentic compounds, sodium bicarbonates, linoleic acid (≥99%), Tween 40, β-carotene (≥97%), Folin – Ciocalteu reagent for total phenolics, 2,2ʹ-diphenyl-1-picrylhydrazyl (DPPH), and gallic acid were obtained from Sigma Aldrich Chemical Co. (St. Louis, MO, USA).
Essential oil extraction by HD
Air dried aerial parts of A. Fragrantissima (100 g) in three replicates that were cut into small pieces and subjected to HD for 3 h, using a Clevenger-type apparatus, according to the method of El-massry et al.[Citation14] The extracted essential oils were dried using anhydrous sodium sulfate and stored in airtight glass vials covered with aluminum foil at – 20°C until analysis.
Headspace solid – phase microextraction (HS-SPME)
The air dried herb of A. fragrantissima was cut into 1–2-cm-long pieces before being subjected to SPME. Two grams of leaves were placed in a 20mL SPME vial. The SPME device (fused-silica fiber) coated with a 100-μm layer of polydimethylsiloxane (Supelco, Bellefonte, PA, USA) was used for extraction of the plant volatiles, and the vial was sealed with a silicone septum. The samples were exposed to 60°C for 30 min and immediately introduced into the gas chromatography injector. This method has been used and optimized by many authors.[Citation15–Citation18]
Gas chromatography–mass spectrometry (GC–MS)
The components of the essential oil obtained using HD and the volatiles trapped by the SPME fiber were analyzed using a GC–MS apparatus. Separation was performed on a Trace GC Ultra Chromatography system (Thermo Scientific, USA) equipped with an ISQ-mass spectrometer (Thermo Scientific, USA) with a 60 m × 0.25 mm × 0.25 μm-thick TG-5MS capillary column (Thermo Scientific, USA). The column separation was programmed from 50°C with a holding time of 3 min, and then the temperature was increased at a rate of 4°C per min to 140°C with a holding time of 5 min. Thereafter, the temperature was increased at a rate of 6°C per minute to 260°C for a 5-minisothermal holding time. The injector temperature was 180°C, the ion source temperature was 200°C, and the transition line temperature was 250°C. The carrier gas was helium with a constant flow rate of 1.0 Ml min−1. The mass spectrometer had a scan range from m/z 40–450, and the ionization energy was set at 70 Ev. The identification of compounds was based on matching with the MS computer library (NIST library, 2005 version) and comparison with those of authentic compounds and published data.[Citation19] The relative percentage of the oil constituents was calculated from the GC peak areas. Kovat’s index was calculated for each compound, using the retention times of a homologous series of C6–C26 n-alkanes and by matching with literature.[Citation4,Citation10,Citation14,Citation19,Citation20]
Antioxidant activity measurements
DPPH radical scavenging assay: Potential antioxidant activity of A. fragrantissima oils was assessed according to the methods reported by Hatano et al.[Citation21] in comparison to a synthetic antioxidant used in food industry, tert-butylhydroquinone(TBHQ). The absorbance was measured at 517 nm using a spectrophotometer(Evolution 300 Thermo UV-VIS); all tests were run in three replicates and the results were averaged.
β-Carotene bleaching assay
The antioxidant activity of A. fragrantissima oils was determined using a β-carotene/linoleic acid system, as described by Taga et al.[Citation22] in comparison to TBHQ. The absorbance was measured at 470nm over a 60-min period.
Total phenolic conten
Total phenolic content of the essential oils was determined using the Folin–Ciocalteu reagent according to a method modified from that of Singleton et al.[Citation23] using gallic acid as the standard. The reaction mixtures were incubated in a thermostat at 45°C for 45 min before the absorbance at 765 nm was measured.
Statistical analysis
Statistical analyses were performed using SPSS software version 16. The data were expressed as mean± SD and analyzed using Student’s t – test and analysis of variance.
Results and discussion
Hydrodistillation of dried aerial parts of A. fragrantissima produced pale yellow oil, with an aromatic fragrant odor with 0.53 ± 0.06% in Madinah essential oil and 0.42 ± 0.08% in the Egyptian one. For the essential oil of dried Egyptian A. fragrantissima, a total of 34 components were identified, representing 90.15% of the total oil content (). The major compounds in the HD oil were santolina alcohol (27.21%), artemisia ketone (14.5%), α-thujone (11.77%), 4(10)-thujen-3-ol (8.33%), β-thujone (7.19%), Yomogi alcohol (4.83%) and trans-sabinyl acetate (4.65%). These findings are consistent with the previous reports of Aboutabl et al.,[Citation24] Fleisher and Fleisher,[Citation25] and El-Shazly et al.[Citation4] despite the differences in the nature of the hydrodistilled parts of the herb; dried parts were used in the this study, while the previous studies mentioned above investigated the fresh aerial parts of the plant cultivated in Sinai peninsula, Egypt. Another study by Choucry[Citation9] revealed that the essential oil of A. fragrantissima cultivated in North coast of Egypt had an entirely different profile, in which caryphyllene oxide, 1-terpinene-4-ol and veridiflorol were the major components. This is likely due to differences in climatic and geographic conditions.
Table 1. Volatile constituents identified from the essential oils of A. fragrantissima cultivated in Egypt and Madinah using GC-MS with HD extraction and SPME
Meanwhile, many terpenes were identified in the Egyptian A. fragrantissima HD essential oil for the first time; β-pinene (0.17%), sabinene (0.11%), α-terpinene (0.25%), p-cymene (0.37%), linalool (0.37%), p-menth-2-en-1-ol (0.22%), 4(10)-thujen-3-ol (8.33%), borneol (0.3%), carvone (0.52%), p-menth-1-en-3-one (0.23%), bornyl acetate (0.11%) and germacrene D (0.22%) (, )). Solvent extraction has the advantage of recovering higher molecular weight natural components, which could be interesting from the point of view of bioactivity but also recovers non – volatile compounds.[Citation26] El-Shazly et al.[Citation4] and Choucry[Citation9] used solvent extraction (SE) and hexane to detect additional volatiles and non – volatiles (e.g. sabinene hydrates, methyl eugenol, BHT, bergamal, cis-chrysanthenol, iso-3-thujanol, and phytol) and many fatty acids and sterols. Surprisingly, many of these compounds could be identified in the present study, which depends on HD essential oils such as cis-sabinene hydrate (1.66%), eugenol (0.11%), 4(10)-thujen-3-ol (8.33%) in addition to many other monoterpenes. Well – known negatives of HD and solvent extraction techniques may responsible for the non detection of such compounds previously in the literature.
Figure 1. Volatile extracts chromatograms for Egyptian A. fragrantissima isolated by (a) hydrodistillation and (b) SPME
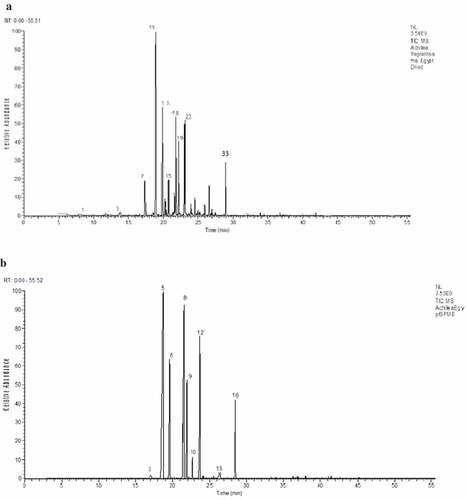
There was a necessity toward find out an alternative optimized extraction technique without known drawback, in order to determine the volatile constituents of the herb with an efficient and reliable recovery. SPME analysis is a simple, rapid, and economic procedure, currently used on a wide scale for studying volatiles of aromatic plants. Fifteen compounds were identified as the adsorbed volatiles of Egyptian A. fragrantissima on SPME fiber, accounting for 94.72% of the total identified components (, )). Santolina alcohol (30.77%), α-thujone (18.92%), artemisyl acetate (12.49%), artemisia ketone (11.83%), β-thujone (8.64%) and trans-sabinyl acetate (8.31%) were the major identified components of the dried Egyptian plant. These compounds are in line with those identified in the HD essential oil () but with significant differences in the quantities; many monoterpenes e.g., thujone isomers, lavandulol and sabinyl acetate were identified among the volatiles adsorbed by SPME. Sesquiterpenes such as germacrene D, spathulenol and eudesmol were identified at a lower amounts using SPME or not detected at all (). This is obviously due to the key differences in these extraction techniques. The use of the HD technique for the extraction of essential oil volatiles is unreliable, because of the possible chemical changes, losses, and degradation of aroma compounds by heat, steam, pH, and thermal or hydrolytic effects.[Citation27] In addition, in line with a report by Zheljazkov et al.,[Citation28] the duration of the HD process may have also affected the essential oil composition. Shorter distillation time yields higher concentrations of monoterpenes, e.g., α-pinene, myrcene, and phellandrenes, whereas the affinity of such compounds toward SPME fiber reduces negative artifacts.[Citation29]
Due to differences in ecological parameters, growing location, agronomical practices, as well as environmental conditions, significant variations are expected between both A. fragrantissima essential oils of Egypt and Madinah ().[Citation10,Citation20] Forty – two compounds could be identified in the HD essential oil of dried Madinah A. fragrantissima (, )). In agreement with the results of Hazem et al.[Citation30] and Alsohaili and Al-Fawwaz[Citation7], who studied the essential oil composition of A. fragrantissima in Jordan, α-thujone (14.3%), 4-terpineol (12.89%), trans-pinocarveol (6.54%), and spathulenol (4.99%) were the predominant compounds among the identified volatiles (). The levels of santolina triene (1.12%), linalool (2.8%) and trans-pinocarveol (6.54%) of Madinah essential oil were considerably higher than those in the Egyptian essential oil, while those of yomogo alcohol (2.08%), santolina alcohol (3.63%), artemisia ketone (1.27%)and β-thujone (2.59%) of the same oil were considerably lower (). 4-Terpineol, iso-3-thujanol, thymol, carvacrol, β-farnesene and germacrene – D 4-ol were dominant among the volatiles of Madinah A. fragrantissima essential oil but were not identified in the Egyptian oil.
Table 2. Ecological, climatic and geographical characteristics of growing areas in Egypt and Madinah Monawara, KSA
Figure 2. Volatile extracts chromatograms for Madinah, Saudi Arabia A. fragrantissima isolated by (a) hydrodistillation and (b) SPME
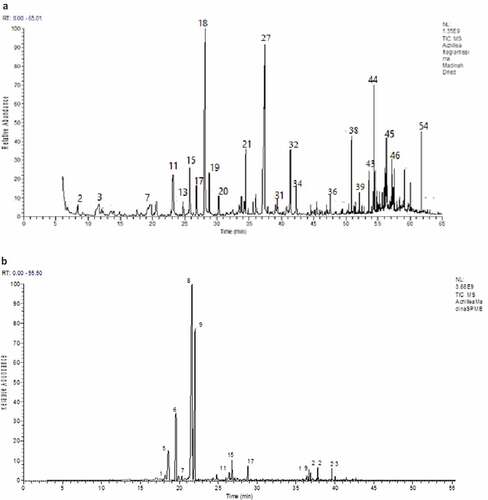
Figure 3. Effect of extraction technique on the area percentage of the main volatiles of A. fragrantissima cultivated in Egypt and Madinah
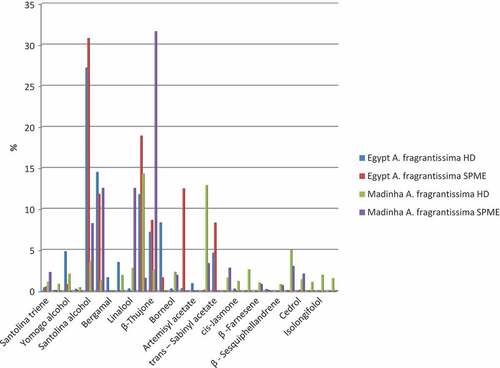
Dramatically higher levels of the monoterpenes of dried Madinah A. fragrantissima adsorbed on SPME than those in the HD oil were also observed, where the levels of α- and β-thujone, artemisia ketone and santolina alcohol were higher by 31.61, 24.25, 12.55 and 8.24% respectively (, ), and ). Sesquiterpenes such as β-farnesene and spathulenol were identified among SPME volatiles but at lower concentrations relative to those in Madinah HD oil. Other compounds such caryophyllene oxide, β-santalole and β-eudesmol were not detected at all ().
Antioxidant activity of both HD essential oils under investigation was tested using DPPH radical scavenging and β-carotene – linoleate bleaching assays. Results are presented in . Madinah essential oil of A. fragrantissima had a lower IC50 (1.088 mg/ml) than Egyptian oil (IC50 1.72 mg/ml) which indicates a higher activity. This is consistent with the total phenolic content and differences in volatile constituents identified in both oils. In agreement with the higher total phenolic content detected in Madinah essential oil (), many phenolic compounds were identified in this oil but not found in Egyptian essential oil e.g., borneol (2.32%), thymol (2.98%), carvacrol (1.6%), eugenol (0.75%) and trans-pinocarveol (6.54%). Linalool was identified in both oils, but at a lower concentration in the Egyptian oil (0.37%) in comparison to Madinah oil (2.8%) (). Phenolic compounds are well – known as antioxidants with very higher activity,[Citation31] in comparison to that of synthetic antioxidants. To date, there is no report concerning the antioxidant activity of A. fragrantissima essential oil, but many researches showed such activity for the extracts. The most relevant research by Shahat et al.[Citation32] showed that, of the many extracts of medicinal plants cultivated in Riyadh KSA, the methanolic extract of A. fragrantissima was the most efficient ion chelator and showed 100% inhibition of the peroxidation of linoleic acid, however the responsible bioactive compounds were not identified. The essential oil of A. fragrantissima was reported as safe, with no effect on the nature and color of embryo fluid as well as experimental animals,[Citation4,Citation6,Citation33] therefore, according to the results introduced in this research, the essential oil of A. fragrantissima can be used safely as antioxidant in foods in replacement to the concerned synthetic antioxidant.
Table 3. Antioxidant activity of essential oils for A. fragrantissima cultivated in Egypt and Madinah in comparison to the synthetic antioxidant TBHQ
Conclusion
In the present study, volatiles of dried A. fragrantissima cultivated in the Nile Delta, Egypt and Madinah Monawara, KSA were extracted using HD and HS-SPME followed by separation and analysis using GC–MS. Due to differences in the cultivation location, agronomical practices, and environmental conditions, significant differences in the quantity and quality of the extracts of both regions were observed. In addition, differences in the extraction techniques applied resulted in differences in the extracted essential oil, such as considerably higher quantity of the monoterpenes adsorbed on SPME than in the HD oil. Madinah HD essential oil showed a higher scavenging ability and greater inhibiting effect toward the oxidation of linoleic acid than that of the Egyptian oil due to the presence of many volatiles with phenolic nature and, therefore, antioxidant activity as well as higher total phenolic content.
Acknowledgments
The authors extend their appreciation to the Deanship of Scientific Research at King Saud University for funding the work through the research group Project no. RGP-VPP-032.
References
- Zheng, W.; Wang, S. Antioxidant Activity and Phenolic Composition in Selected Herbs. J. Agric. Food Chem. 2000, 149, 5165–5170.
- Batanouny, K. H.; Aboutabl, E.; Shabana, M.; Soliman, F. Wild Medicinal Plants in Egypt. Academy of Scientific Research and Technology, Egypt; The World Conservation Union (IUCN): Switzerland, 1999; pp 102–104.
- Boulos, L. Medicinal Plants of North Africa; Reference Publications, Inc.: Algonac, Michigan, USA, 1983; pp 52.
- El-Shazly, A. M.; Hafez, S. S.; Wink, M. Comparative Study of the Essential Oils and Extracts of Achillea Fragrantissima (Forssk.) Sch. Bip. And Achillea Santolina L. (Asteraceae) from Egypt. Pharmazie. 2004, 59, 226–230.
- Aqel, H.; Al-Charchafchi, F.; Ghazzawi, D. Biochemical, Antibacterial and Antifungal Activity of Extracts from Achillea Fragrantissima and Evaluation of Volatile Oil Composition. Der Pharmacia Sinica. 2012, 3, 349–356.
- Zeedan, G. S. G.; Abdalhamed, A. M.; Ottai, M. E.; Abdelshafy, S.; Abdeen, E. Antimicrobial, Antiviral Activity and GC-MS Analysis of Essential Oil Extracted from Achillea Fragrantissima Plant Growing in Sinai Peninsula, Egypt. J. Microbial Biochem. Technol. 2014, S8, 006. DOI: 10.4172/1948-5948.S8-006.
- Alsohaili, S.; Al-Fawwaz, A. Composition and Antimicrobial Activity of Achillea Fragrantissima Essential Using Food Model Media. Eu. Sci. J. 2014, 10, 156–165.
- Elsharkawy, E. Anti-Inflammatory Activity and Chemical Compositions of Essential Oil Achillea Fragrantissima. Natl. J. Physiol. Pharm. Pharmacol. 2016, 6, 258–262. DOI: 10.5455/njppp.2016.6.23022016130.
- Choucry, M. A. Chemical Composition and Anticancer Activity of Achillea Fragrantissima (Forssk.). Sch. Bip. (Asteraceae) Essential Oil from Egypt. J. Pharmacog. Phytother. 2017, 9, 1–5.
- Farouk, A.; Ali, A.; Al-Khalifa, A.; Mohsen, M.; Fikry, R. Aroma Volatile Compounds of Parsley Cultivated in the Kingdom of Saudi Arabia and Egypt Extracted by Hydrodistillation and Headspace Solid – Phase Microextraction. Int. J. Food Proper. 2017, 20, 2868–2877. DOI: 10.1080/10942912.2017.1381707.
- Chialva, F.; Gabri, G.; Liddle, P.; Ulian, F. Qualitative Evaluation of Aromatic Herbs by Direct Head Space(GC)2 Analysis. Applications of the Method and Comparison with the Traditional Analysis of Essential Oils. In Aromatic Plants–Basic and Applied Aspect; Margaris, N., Koedam, A., Vokou, D., Eds.; Martinus Nijhoff Publishers: Netherlands, 1982; pp 183–195.
- Alatar, A. A. Effect of Temperature and Salinity on Germination of Achillea Fragrantissimaand Moringa Peregrine from Saudi Arabia. Afr. J. Biotchnol. 2011, 10, 3393–3398. DOI: 10.5897/AJB10.1882.
- Saudi Geological Survey. Kingdom of Saudi Arabia: Facts and Numbers; SGS Publications: Jeddah, KSA. 2012.
- El-Massry, K. F.; Farouk, A.; Abou-Zeid, M. Free Radical Scavenging Activity and Lipoxygenase Inhibition of Rosmarinus Officinalis L. Volatile Oil. J. Essent. Oil – Bearing Plants. 2008, 11, 536–543. DOI: 10.1080/0972060X.2008.10643663.
- Cătunescu, G. M.; Socaci, S. A.; Rotar, I.; Vidican, R.; Bunghez, F.; Tofană, M.; Pleșa, A.; Muntean, M. Volatile Profile of Minimally Processed Herbs during Cold Storage. Rom. Biotechnol. Lett. 2016, 21, 11923–11931.
- Yeon, B.; Sowndhararajan, K.; Jung, J.; Jhoo, J.; Kim, S. Comparison of Volatile Composition of Supercritical Carbon Dioxide Extract from Rhizomes of Korean Medicinal Plant ‘Chun-Kung’ (Cnidium Officinale Makino) by Direct-And SPME-GC/MS. Int. J. Pharm. Pharm. Sci. 2014, 6, 355–358.
- Moradi, M.; Kaykhaii, M.; Ghiasvand, A. R.; Shadabi, S. Comparison of Solid Headspace Microextraction, Headspace Single Drop Microextraction and Hydrodistillation for Chemical Screening of Volatiles in Myrtus Communis L. Phytochem. Anal. 2012, 23, 379–386. DOI: 10.1002/pca.1368.
- Gazim, Z.; Rezende, C.; Fraga, S.; Filho, B.; Celso-Vataru, N.; Cortez, D. Analysis of the Essential Oils from Calendula Officinalis Growing in Brazil Using Three Different Extraction Procedures. Braz. J. Pharmacol. Sci. 2008, 44, 391–395.
- Adams, R. Identification of Essential Oil Components by Gas Chromatography/Mass Spectroscopy; Allured Publishing Corp.: Carol Stream, IL, 2007.
- Farouk, A.; Fikry, R.; Mohsen, S.; Metwaly, A. Chemical Composition, Antioxidant and Antimicrobial Activity of Cymbopogon Citrates Essential Oil Cultivated in Madinah Monawara, Saudi Arabia and Its Comparison to the Egyptian Chemotype. Int. J. Food Nut. Sci. 2015, 4, 29–33.
- Hatano, T.; Kagawa, H.; Yasuhara, T.; Okuda, T. Two New Flavonoids and Other Constituents in Licorice Root: Their Relative Astringency and Radical Scavenging Effects. Chem. Pharm. Bull. 1988, 36, 2090–2097. DOI: 10.1248/cpb.36.2090.
- Taga, M.; Miller, E.; Pratt, D. Chia Seeds as Source of Natural Lipid Antioxidants. J. Am. Oil Chemists’ Soc. 1984, 61, 928–931. DOI: 10.1007/BF02542169.
- Singleton, V.; Orthofer, R.; Lamuela-Raventos, R. Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. Meth. Enzymol. 1999, 299, 152–178.
- Aboutabl, E. A.; Soliman, F. M.; El-Zalabani, S. M.; Brunke, E. J.; El-Kersh, T. A. Essential Oil of Achillea Fragrantissima (Forssk.). Sch. Bip. Egypt. J. Pharm. Sci. 1986, 27, 215–219.
- Fleisher, Z.; Fleisher, A. Volatiles of Achillea Fragrantissima (Forssk.). Sch. Bip. Aromatic Plants of the Holy Land and the Sinai. Part XI. J. Essent. Oil Res. 1993, 5, 211–214.
- Burbott, A.; Loomis, W. D. Effects of Light and Temperature on the Monoterpenes of Peppermint. Plant Physiol. 1967, 42, 20–28. DOI: 10.1104/pp.42.1.20.
- Khajeh, M.; Yamini, Y.; Sefidkon, F.; Bahramifar, N. Comparison of Essential Oil Composition of Carumcopticum Obtained by Supercritical Carbon Dioxide Extraction and Hydrodistillation Methods. Food Chem. 2004, 86, 587–591. DOI: 10.1016/j.foodchem.2003.09.041.
- Zheljazkov, V.; Astatkie, T.; Schlegel, V. Hydrodistillation Extraction Time Effect on Essential Oil Yield, Composition, and Bioactivity of Coriander Oil. J. Oleo Sci. 2014, 63, 857–865.
- Benyelles, B.; Allali, H.; Dib, M.; Djabou, N.; Tabti, B.; Costa, J. Essential Oil from Rhaponticumacaule L. Roots: Comparative Study Using HS-SPME/GC/GC-MS and Hydrodistillation Techniques. J. Saudi Chem. Soc. 2014, 18, 972–976. DOI: 10.1016/j.jscs.2011.12.001.
- Hazem, A.; Al-Charchafchi, F.; Ghazzawi, D. Biochemical, Antibacterial and Antifungal Activity of Extracts from Achillea Fragrantissima and Evaluation of Volatile Oil Composition. Der Pharmacia Sinica. 2012, 3, 349–356.
- Pripdeevech, P.; Chumpolsri, W.; Suttiarrorn, P.; Wongpornchai, S. The Chemical Composition and Antioxidant Activities of Basil from Thailand Using Retention Indices and Comprehensive Two-Dimensional Gas Chromatography. J. Serbian Chem. Soc. 2010, 75, 1503–1513. DOI: 10.2298/JSC100203125P.
- Shahat, A. A.; Ibrahim, A. Y.; Elsaid, M. S. Polyphenolic Content and Antioxidant Activity of Some Wild Saudi Arabian Asteraceae Plants. Asian Pacific J. Tropical Med. 2014, 545–551. DOI:10.1016/S1995-7645(14)60091-2.
- Shahwar, D.; Rehman, S. U.; Raza, M. A. Acetyl Cholinesterase Inhibition Potential and Antioxidant Activities of Ferulic Acid Isolated from Impatiens Bicolor Linn. J. Med. Plant Res. 2010, 4, 260–266.
