?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Kelulut honey was dehydrated at 40, 55, and 70°C up to 84 h in a dehydrator. The changes of its properties and qualities in terms of moisture content, water activity, hygroscopicity, moisture adsorption isotherm, colour intensity, total phenolic content (TPC), viscosity, glass transition temperature (Tg), surface stickiness, hydroxymethylfurfural (HMF) content, and diastase activity were evaluated. The dehydration process for 18 h between temperatures of 55 and 70°C can safely produce Kelulut honey product with less than 8% moisture content and water activity below 0.6. Similar quality of Kelulut honey dehydrated at lower temperature between 40 and 55°C requires up to 36 h of dehydration. These recommended dehydration conditions were able to increase TPC of honey from 7.86% from its original value for the shorter duration of 18 h and lower dehydration temperature of 40°C and up to 70.9% for the longer duration of 36 h and higher temperature of 70°C. Dehydrated honey was darker, more viscous, and stickier. The increase of HMF content in dehydrated honey at 40 and 55°C up to 36 h was not significant which are at 0 and 5.81 mg/kg honey, respectively, and at 70°C, it was about 80 mg/kg honey. The honey was found to have very low diastase activity ranging from 0 to 0.75 DN, thereby causing its changes to be insignificant during dehydration.
Introduction
Raw stingless bee (Heterotrigona Itama) honey, the Kelulut, has a unique sweet and sour flavour. It has been reported to have higher antioxidant activity, flavonoids, and polyphenol content[Citation1,Citation2] and brings significant benefits to health.[Citation3] Despite being an excellent product, Kelulut honey has severe storage problems as it undergoes rapid alcoholic fermentation once harvested due to its high water content of above 30%[Citation4] and the presence of osmophilic yeast.[Citation5] Alcoholic fermentation produces undesirable substances such as ethanol and carbon dioxide which lead to off-flavour of honey,[Citation6] giving it a more acidic taste and undesirable appearance.[Citation7] As the presence of osmophilic yeast and fungi in honey is unavoidable during bees’ nectar collection for honey production, the Kelulut honey needs processing to prevent it from fermentation during storage.
Conventionally, honey processing prior to storage is by subjecting honey to thermal treatment. Honey undergoes preheating at 40°C, straining, filtering, indirect heating at 60–65°C for 25–30 min and followed by rapid cooling.[Citation8] Heating of honey is also done at different temperatures and durations from mild temperatures of 50 to 90°C for 15 to 120 min,[Citation9] 50 to 80°C for 15 to 60 min,[Citation10] 60 to 100°C for 2–20 min[Citation11] and 75 to 100°C for 15 to 90 min[Citation12] to high temperatures above 100°C such as those by Tosi et al.[Citation13] at 160°C for 0 to 90 s and at 100 to 140°C for 10 to 30 s by Tosi et al.[Citation14] These heat treatments help to kill microorganisms in honey that are responsible for spoilage and at the same time reduces its moisture content to a safe level. It has been reported that reduction of moisture content of honey to below 17% retards the growth of yeast and inhibits the fermentation process.[Citation15] However, the application of heat degrades quality of honey. Heating of honey causes the loss of aromatic substances, decreases its diastase activity[Citation11,Citation16,Citation17] and increases its hydroxymethylfulfural (HMF) content.[Citation8,Citation9,Citation12,Citation13,Citation18,Citation19] Tosi et al.[Citation11] showed that the diastase activity decreased and HMF content of honey increased more significantly at higher heating temperature and longer heating duration. The adverse effect of heating on honey is proportional to the temperature and duration of heat applied.
In order to minimize the deterioration of honey quality during processing, other approaches used include the spray-drying,[Citation20] vacuum-drying,[Citation21] freeze-drying,[Citation22] vacuum evaporation,[Citation23] and microwave-vacuum drying.[Citation24] Drying of honey refers to process of removing moisture from liquid honey by introducing heat with intention to produce solid honey usually in powder form. The most commonly tested honey drying method is the spray-drying process at 150–180°C.[Citation9,Citation20,Citation25–Citation27] Besides the deterioration of honey quality due to heat, the sticky nature of honey is also a problem in drying of honey.[Citation28] Due to very low glass transition temperature of natural honey, drying of honey without any additives is impossible. Honey tends to stick to the equipment causing high losses during the drying process. Some form of flour, starch or anticaking agent is added to honey and the slurry then is dried.[Citation20,Citation22,Citation26] This limits the content of honey in the powder form. The microwave and infrared heat processing also affect the quality of honey despite providing rapid drying of honey.[Citation18]
From the literature reviews, in search for an energy efficient honey processing method which can produce a promising quality of dehydrated honey, the modern food dehydrator, an appliance specially designed to dehydrate food seems to fit the description of Bhandari et al.[Citation29] who suggested various manoeuvres including modification of dryer design, use of mild drying temperature conditions and drying aids or carriers in overcoming drying problems of sugar-rich food including honey. The food dehydrator is a chamber with stackable trays and it dries food by using mild heated air circulating inside the chamber. There are few scientific and quantitive evaluation on the benefits of using a dehydrator despite many informal reviews about its convenience, efficiency and economic savings when used for drying foods. Neil[Citation30] presented that the food dehydrators provide a fine control over the dehydrating temperature and are more energy efficient than oven drying while Kendall et al.[Citation31] presented that drying foods with dehydrators usually take two to three times shorter time than oven drying. The dehydrators are commonly used to dry fruits and vegetables and are claimed best in preserving nutrients and enzymes of the foods as it dries food at a relatively low temperature, usually below 70°C.[Citation30] It is able to reduce moisture in food up to 75% and the dried food created with a dehydrator can be considered as ‘raw food’ for those prepared below 46°C.[Citation32]
This research aimed to investigate the quality changes of an extremely high moisture tropical honey dried with a commercial food dehydrator at mild dehydration conditions. Dehydration of honey to different moisture level can help to diversify applications and usage of honey because dried products at intermediate moisture contents such as honey powder, honey flakes, honey spread, and honey candy can be possible.[Citation28]
Materials and method
Honey samples and dehydration
Raw Kelulut (Heterotrigona Itama) honey was harvested from a forest reserve in Teluk Intan, Perak, Malaysia and stored in bottles at ambient temperature. A food dehydrator (Himmel V3, Nature Himmel Marketing Sdn. Bhd., Selangor, Malaysia) was used to dehydrate honey at temperatures of 40, 55, and 70°C for a duration of 12, 24, 36, 48, 60, 72, and 84 h. Liquid honey samples were spread uniformly up to thickness of 3–4 mm onto silicone trays with dimensions of 25.0 cm × 13.5 cm. Two silicon trays were placed on each dehydration tray which was stacked up through a 6 cm × 6 cm air vent which emitted hot air. A diagram on the samples arrangement during dehydration is shown in . One dehydration tray of honey was removed at every 12-h interval for honey properties and qualities analysis whilst the remaining trays of the honey in the dehydrator continued the dehydration process. The dehydration rate for every 12 h was calculated from the moisture content measurements using EquationEquation (1)(1)
(1) . The dehydration process yield was calculation based on EquationEquation (2)
(2)
(2) . A digital kitchen scale (XJ-2K820S, Pastry Pro, Malaysia) of 0.1 g increment was used as follows
where M is the moisture content (dry basis) at dehydration time, t1 and its subsequent dehydration time, t2, and A is tray area of 0.0675 m2:
Honey properties and quality analyses
Physico-chemical properties of moisture content, water activity, colour intensity, viscosity, glass transition temperature and stickiness were measured at every 12 h up to 84 h. Hygroscopicity and water adsorption isotherm of the dehydrated honey were measured at the end of 84 h. Quality of honey was evaluated by its HMF content, total phenolic content (TPC), and diastase activity. The analyses were performed in triplicates and results were expressed as mean ± standard error. Error bars in figures represent the standard error of mean.
Moisture content
Moisture content was determined by using a moisture analyser (MX-50, A&D Co. Ltd., Tokyo) and it was expressed in percent moisture (dry basis).
Water activity
Water activity of honey samples was determined using a water activity meter (Aqualab Pre, Washington, USA). The equipment was calibrated with saturated salt solutions in the water activity range of interest.
Hygroscopicity
Hygroscopicity was determined by placing 1 g of each honey sample at room temperature in airtight plastic containers containing saturated solution of sodium chloride (75.29% RH). After one week, the samples were weighed and hygroscopicity was expressed as grams of absorbed moisture per 100 g dry solids.[Citation33]
Moisture adsorption isotherm
The moisture adsorption isotherm data at 25°C were obtained from gravimetric water content measurements of steady-state water contents at various water activities. Samples of 1 g of fresh and dehydrated honey was weighed in plastic cups and stored in air-tight containers over various saturated salt solutions. The solutions were magnesium nitrate, potassium iodide, sodium chloride, potassium chloride, and potassium sulphate with theoretical relative humidity of 52.9%, 68.9%, 75.3%, 84.3%, and 97.3% at 25°C, respectively. The samples were weighed using an analytical balance (XP 204, Mettler-Toledo, Switzerland) at 7 days interval for 28 days. Samples were equilibrated until constant weight values (±0.001 g) were achieved.
Colour intensity
The colour intensity of the honey was measured following method of Kek et al.[Citation1] A 50% (w/v) of honey solution was prepared by dissolving 5 g of honey in warm distilled water. The solution was filtered to remove any coarse particles. Using distilled water as blank, the absorbance at 450 nm and 720 nm was recorded. Colour intensity was calculated as follows:
where A450 and A720 are the absorbances at 450 nm and 720 nm, respectively.
Total phenolic content
TPC was determined using the Folin-Ciocalteu spectrophotometric method.[Citation34] Minor modification was made by diluting honey to 0.05 g/mL with distilled water, and then 1 ml of the honey solution was mixed with 5 ml of 0.2N Folin-Ciocalteu reagent. After 5 min of incubation, 4 mL of 7.5% (w/v) aqueous sodium carbonate solution was added. The mixture was well-mixed and incubated for 2 h in dark at room temperature. The absorbance of the mixture was determined at 765 nm using distilled water as blank. A standard calibration curve was plotted using gallic acid with concentration range from 10 to 100 µg/mL. TPC was expressed in mg of gallic acid equivalent (GAE) per kg of honey.
Viscosity
A rheometer (AR-G2, TA Instruments, New Castle, USA) with a 20-mm diameter flat plate (1000 µm truncation gap) was used to determine the dynamic viscosity of honey. Dehydrated sample was slowly loaded to avoid any possible bubbles. Before starting rheological tests, all samples were allowed to remain at rest for 1 min to allow stress relaxation induced during sample loading. Steady-state measurements were performed at 20°C, with a shear rate range of 10–100 s−1 in ascending ramp. The temperature was controlled by a circulating water system. The results were analysed using its accompanying software, Rheology Advantage Data Analysis Version 5.7.
Glass transition temperature
Honey samples of 5–10 mg were added in a standard 40 µL aluminium pan. It is hermetically sealed and analysed with a differential scanning calorimetry (DSC-7, Perkin Elmer, USA). Samples were scanned as follow: (1) cooled from 30 to −60°C, (2) held for 1 min, (3) heated from −60 to 20°C, (4) hold for 1 min, and (5) heat from −60 to 20°C. For drier samples, the temperature range for step 3 and 5 were changed to a maximum temperature of 60°C as the glass transition temperature increased. A heating rate of 20°C/min was used. The second scanning of sample was used to reduce enthalpy relaxation of the amorphous substance which appeared in the first scan, thereby enhancing the accuracy of Tg measurement. The transfer of samples from container to DSC pan was done quickly in an air-conditioned room to avoid moisture absorption by the sample. The results were expressed as half-width of the glass transition region which were obtained from the accompanying software, Pyris™.
Surface stickiness
The surface stickiness of the samples was measured using a texture analyser (TA-XTplus, Stable Micro Systems, Surrey, UK). Adhesive test was performed using a 20 mm diameter aluminium cylindrical probe. The pre-test speed of 1.5 mm/s, test speed of 1.0 mm/s, post-test speed of 10 mm/s, and a trigger force of 2.5 g were set. The honey in the glass container was placed centrally under the probe. The probe was set to penetrate 3 mm into the honey. The probe then was withdrawn from the sample at a speed of 10 mm/s and stopped at a distance of 200 mm above the sample surface. The maximum force needed to separate the probe was generated from the texture analyser software macro (TEE 32 Stable Macro System, UK). The values obtained were then converted to maximum tensile pressure by the following formula. The maximum tensile pressure required to separate the probe from the surface of honey was regarded as the surface stickiness:
Hydroxymethylfurfural
HMF content was determined using White’s spectrophotometric method.[Citation35] Distilled water was added to dissolve 5 g of honey samples. The honey solution was made up to 50 mL with distilled water after addition of 0.5 mL of Carrez I solution (consisting 15 g of potassium hexacyanoferrate (II), K4Fe(CN)6.3H2O in 100 mL water), 0.5 mL of Carrez solution II (consisting 30 g of zinc acetate, Zn(CH3.COO)2.2H2O in 100 mL of water), and few drops of ethanol. The solution was then filtered and the first 10 mL of the filtrate was rejected. Aliquots of 5 mL each were transferred to two test tubes. Distilled water of 5 mL was added to the first tube as sample and another 5 mL of 0.2% (w/v) sodium bisulphite solution was added to the second test tube as reference. Absorbance of sample solution was determined against reference solution at 284 nm and 336 nm in quartz cuvettes. HMF was determined as follows[Citation35]:
where A284 and A336 is the absorbance at 284 nm and 336 nm, respectively. The factor 149.7 is a theoretical value linked to the molar extinction coefficient of HMF at 284 nm, D is the dilution factor, when dilution is necessary, and W is the weight of honey sample in g.
Diastase activity
Honey solution was prepared by dissolving 1 g of honey in a 100 ml volumetric flask with 0.1 M, pH 5.2 acetate buffer solution. The procedure was completed within an hour. Test tube was prepared and 5 mL of the solution was transferred into the test tube. Solution was then incubated in 40°C water bath for 5 min. A blank was prepared by placing a 5 mL aliquot of the acetate buffer and treated exactly as the sample solution. To both solutions, a Phadebas tablet (Honey Diastase Test, Magle AB, Lund, Sweden) was added with a tweezer and the timer started. The solutions were mixed using a vortex mixer and returned to the water bath. The reaction was terminated exactly after 15 min by adding 1 mL sodium hydroxide solution. The mixture was mixed again for 5 s. The solutions were immediately filtered through filter papers and its absorbance was measured at 620 nm using water as reference. One diastase number (DN) corresponded to the enzyme activity of 1 g of honey, which can hydrolyse 0.01 g of starch in 1 h at 40°C. For low diastase values of between 0 and 6, the diastase number was calculated from absorbance at 620 nm (A620) using the following equation:
where 35.2 and −0.46 are the slope and the intercept, respectively, of the best fitted line obtained from the linear regression of A620 and DN.
Statistical analysis
The statistical analysis of data was performed using Minitab statistical software (Version 18, Minitab Inc., USA). One-way ANOVA was performed. Tukey’s test was used to examine for any significant differences among the mean values at confidence level of 0.05. Mean values were averaged from triplicate measurements.
Results and discussion
Dehydration curve, rate and yield
shows the dehydration curves of honey at three temperatures based on moisture content (dry basis) measurements and shows its dehydration rate curve. The moisture content of honey dropped very steeply and showed no lag periods in the first 12 h or 15% of the overall dehydration period of 84 h. It is assumed that this short initial dehydration period forms a constant dehydration rate before moving into the two phases of falling rate periods. During the constant rate of drying, the initial moisture removal was easy as the surface of honey is kept “sufficiently wet” due to its very high moisture content.[Citation36] The rate of dehydration was also the highest. The constant dehydration process for honey reached its critical moisture content of 18% (dry basis) after 12 h at which the dehydration rate started to reduce. The dehydration rate reduction phase is known as the falling rate period[Citation36] because the remaining water bound are stronger and thus the moisture loss is harder.[Citation37] The dehydration of Kelulut honey displayed two falling rate periods () similar to most biological products.[Citation38] The first falling period is mainly due to the effect of dehydrating temperature where higher temperature results in a higher dehydration rate. The higher dehydrating temperature accelerates the water migration rate and causes the dehydration rate to be higher. The second falling rate begins when the free water at the honey surface was fully removed and honey is in fully hygroscopic state.[Citation38,Citation39] The dehydration rate decreased slowly and reached almost zero when the equilibrium moisture between the honey and surrounding drying conditions was achieved.[Citation38] The dehydration rate of honey during the second falling rate period does not differ significantly for all the dehydration temperatures. However, the dehydration rates for 40, 55, and 70°C were significantly different (P < 0.05) during the constant rate drying period. Dehydrating at higher temperatures resulted in a higher water diffusivity and thereby increased the rate of moisture removal.[Citation36] Similar result was also shown in many drying studies of agricultural products.[Citation36,Citation40–42]
Figure 2. Effects of dehydration temperature and duration on (a) moisture content, (b) dehydration rate, and (c) yield of honey
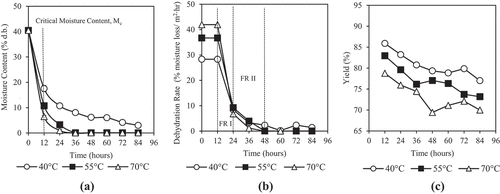
At the point of critical moisture content where the dehydration process changes from constant rate to falling rate, the moisture content of honey was below 20% (dry basis) and met the recommended moisture content of processed Kelulut honey to be less than 22% (dry basis) by the Department of Standards Malaysia.[Citation43] Prolonged dehydration to 84 h produced honey with extremely low moisture content of almost zero, with exception of 40°C giving a residual moisture content of 3.03% (dry basis). Honey dehydrated at 40°C had significantly higher (P < 0.05) moisture content than honey dehydrated at 55 and 70°C () implying that dehydration of honey at 40°C had a significantly lower moisture removal rate. Dehydration of the honey at 55 and 70°C for more than 36 h did not show significant difference in moisture content (P < 0.05). The dried honey had a candy-like structure and is said to have reached its minimum moisture level. The moisture content profiles of this study show that dehydration at 55 and 70°C was able to reduce moisture content of honey from as high as 40% (dry basis) to literally 0% within 36 h, while dehydration at 40°C can produced honey with less than 10% moisture content.
shows the mass and moisture loss of honey during the dehydration process while shows the calculated dehydrated honey yield. As expected, moisture loss in honey was greater with higher temperature and longer duration of dehydration as amount of moisture loss from honey accumulates with time and escalates with temperature. The higher moisture loss from honey results in a lower yield of dehydrated honey.
Table 1. Weight and moisture loss in grams of Kelulut honey during dehydration at three temperatures
Water activity, hygroscopicity, and water adsorption isotherm
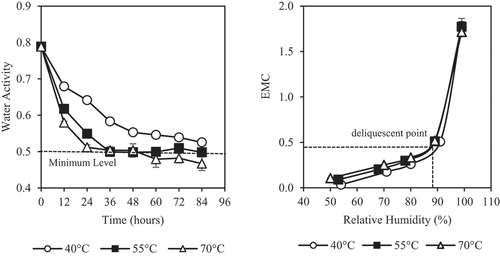
The initial water activity of the honey was very high at 0.788 ± 0.002 causing Kelulut honey to be highly susceptible to natural fermentation. Water activity has often been used to describe the moisture migration and microbial stability of food[Citation44,Citation45] and the minimal water activity for osmophilic yeasts to grow is only 0.6.[Citation45] shows that the dehydration process reduces the water activity of honey in the same trend as the moisture content (. Studies have reported that water activity of honey is linearly correlated with moisture content of honey.[Citation46,Citation47] The Kelulut honey dehydrated at 40°C has significantly higher (P < 0.05) water activity than the other two temperatures. The water activity decreased gradually during the dehydration process and reached a minimum level of about 0.5 after 36 h at 55 and 70°C. For 40°C, 36 h of dehydration managed to reduce water activity of Kelulut honey to be below 0.6. These results show that dehydration process is able to reduce water activity of honey to a level safe in terms of microbial growth. However, at low water activity, honey may be more hygroscopic.[Citation48] Hygroscopicity is the measure of the moisture absorption ability of the honey and is highly dependent on its moisture content.[Citation49,Citation50]
Figure 3. Effects of dehydration temperature and duration on (a) water activity and (b) moisture sorption isotherm of honey
The hygroscopicity of Kelulut honey dehydrated at 40, 55, and 70°C for 84 h were 20.15 ± 0.41, 27.23 ± 0.42, and 29.70 ± 0.29 g of absorbed water per 100 g dry solids, respectively. Kelulut honey dehydrated at higher temperature was more susceptible to moisture absorption due to higher moisture difference between the sample and the surrounding as moisture migration is proportional to the moisture differences.[Citation48] The results obtained for this dehydrated Kelulut honey ranging from 20.15 to 29.7 g of absorbed water per 100 g dry solids are comparable to the spray-dried tamarind pulp powder which ranged from 23.23 to 34.04 g of absorbed water per 100 g dry solids.[Citation33] Dehydrated honey can be classified as amorphous sugar particle and it has been shown that the amorphous sugar particles are very hygroscopic.[Citation51] The study of Martin[Citation5] also showed that honey kept in controlled-humidity chambers absorbed moisture from the surrounding and eventually leads to the occurrence of honey fermentation. Industrial dehydrated honey producers usually control the hygroscopicity of honey by adding in some drying aids like maltodextrins and lactose[Citation52] or using the co-crystallization technique by encapsulating honey in a primary ingredient such as sucrose.[Citation28]
The moisture sorption isotherm present the relationship of equilibrium moisture content (EMC) of food and the relative humidity of the surrounding in graphical form.[Citation53,Citation54] It provides information on the sorption mechanism and honey–water interactions. It tells how the moisture absorption occurs in a food and is often used to estimate the amount of moisture intake if it is exposed to a surrounding at a certain relative humidity.[Citation55] shows that the dehydrated Kelulut honey follows the type III isotherm according to Brunauer et al.[Citation56] classifications. Food which is rich in sugars is usually represented by this isotherm shape due to the solubility of sugars in water.[Citation54] Although the EMC decreased slightly with decreasing dehydration temperature, these changes were not significantly different (P > 0.05) and it did not change the shape of the isotherm curves. The deliquescent point of dehydrated honey was found to be around relative humidity of 87%. It is a point at which the samples began to absorb large amount of moisture from surrounding.[Citation57,Citation58] The moisture uptake was very slow before this point, and above this, moisture content increases rapidly and water is able to dissolve the samples. Similar trend has also been reported in literature for agricultural food materials including dried raisins, prunes, apricot, figs,[Citation59] tamarind pulp powder,[Citation55] honey powder,[Citation21] apples, grapes, and potatoes.[Citation60]
Colour intensity and total phenolic content
shows that the colour intensity of Kelulut honey increased more noticeably at 70°C than those at 40 and 55°C. The darkening of honey is highly dependent on the dehydration temperature. Various studies have reported that darker honey will be produced after subjected to heating treatments.[Citation24,Citation61,Citation62] The darkening of honey was reported to be due to degradation of volatile substances, caramelization of sugar, and the production of brown melanoidins.[Citation63] The colour intensity of honey dehydrated at 40, 55, and 70°C increased by 23, 61, and 215%, respectively. Study of Cui et al.[Citation24] reported that the changes of colour of honey is insignificant at temperature range of 30 to 50°C while Turkmen et al.[Citation61] reported that colour of honey increases significantly at higher temperature range of 50 to 70°C. The darkening of colour of honey due to thermal treatment is because of the formation of polymeric brown pigments through Maillard reaction.[Citation61] Turkmen et al.[Citation61] studied the effects of heating temperatures on the formation of brown pigments and showed that it follows the zero-order kinetics at which the rate of formation of brown pigments is constant along the process. Studies have been reported that colour of honey is strongly correlated with its TPC.[Citation1,Citation63,Citation64]
Figure 4. Effects of dehydration temperature and duration on (a) colour intensity and (b) total phenolic content of honey
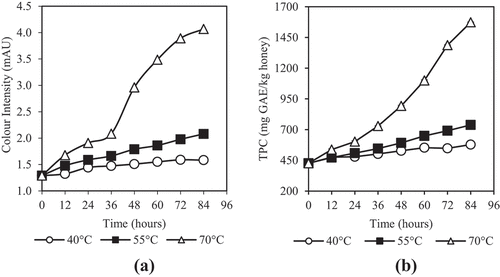
Phenolic content as one of the main source of antioxidant in honey is an important properties of honey. The initial TPC of the Kelulut honey was 419.99 ± 16.36 mg GAE/kg. It falls within the range of 228 to 1058 mg GAE/kg.[Citation1,Citation2] shows that the changes of TPC of Kelulut honey during dehydration process follows the trend of colour intensity changes. At the end of 84 h, the original TPC of 419.99 ± 16.36 mg GAE/kg has increased to 578.30 ± 1.04, 737.67 ± 2.76, and 1573.18 ± 2.20 mg GAE/kg, corresponding to 37.7%, 75.6%, and 274.6% for honey dehydrated at 40, 55, and 70°C, respectively. Average TPCs from the three dehydration temperatures after 18 and 36 h, respectively, was 512.76 ± 49.88 and 592.24 ± 120.47 mg GAE/kg. Similarly, honey that were heated at 50 and 70°C showed an increase in total phenols with increasing thermal processing time in other studies.[Citation65,Citation66] The increase in TPC was reported to be due to the thermal extraction of phenolic compounds during processing.[Citation66,Citation67] In a study using Canadian honeys, there was a strong and significant correlation of 0.950, between Maillard reaction products (MRPs) and total phenolics content.[Citation68] Thus, the increase in TPC was also possibly due to the MRPs which led to the formation of brown melanoidins. Study of Turkmen[Citation61] showed that the formation of MRPs increases the antioxidant activity of honey which is beneficial to human health. These studies show that the dehydration process is not only beneficial in retaining phenolic content in raw honey but also in increasing its value up to 274.6% significantly (p < 0.05).
Viscosity, glass transition temperature and surface stickiness
Viscosity is an important parameter of honey especially during storage, handling, and processing of honey. shows that the viscosity of honey increased more pronouncedly at higher dehydration temperature. The viscosity of honey reached 8.11, 56.21, and 279.88 Pa.s after dehydrated for 18 h at 40, 55, and 70°C, respectively. The increase of honey viscosity is also due to its moisture removal. It is commonly known that viscosity of honey is highly dependent on its moisture content. Previous studies reported that lower moisture honey will have higher viscosity.[Citation69–Citation71] Dehydrated honey, which can be classified as amorphous foods, is highly water-plasticizable.[Citation70] The removal of moisture which act as plasticizer in honey will eventually decrease the molecular mobility and thereby cause honey to become more viscous.[Citation72] The higher dehydration temperature and longer duration which result in higher moisture loss, will give a more viscous honey. Honey will eventually change to a very viscous glassy state due to the continued decrease of molecular mobility and free volume as dehydration persist.[Citation72] Incomplete data points for 55 and 70°C in were due to impossible analysis of honey samples which were too thick and the rheometer reached its maximum torque limit.
Figure 5. Effects of dehydration temperature and duration on (a) viscosity, (b) glass transition temperature, and (c) surface stickiness of honey
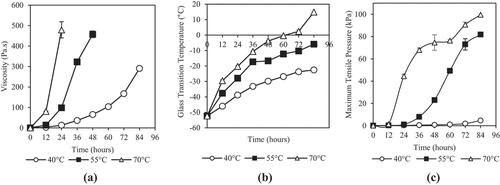
Glass transition temperature, Tg is an important property to be considered during the dehydration process of sugar rich products, including honey as it is the temperature at which an amorphous system changes from glassy to rubbery state.[Citation51] shows that Kelulut honey has very low glass transition temperature before dehydration, which is at −52.35 ± 0.38°C. The Tg for pure unadulterated honey was previously studied and reported to be between −42 and −51°C.[Citation73] Dehydration caused the Tg to increase significantly but remained low and below room temperature. The Tg of honey increased from −52.35 ± 0.38°C to −22.57 ± 0.14, −5.9 ± 0.85, and 14.86 ± 0.85°C after being dehydrated for 84 h at 40, 55, and 70°C, respectively. According to Umesh Hebbar et al.[Citation28] the Tg of honey is highly dependent on its composition where the lower molecular weight material will have a low Tg. Umesh Hebbar et al.[Citation28] reviewed and compared the Tg of various sugars and found that fructose and glucose, both with the lowest molecular weight of 180 had the lowest Tg of 5 and 31°C, respectively. Honey, which mainly constitutes fructose and glucose, have a low Tg. As glass transition temperature, Tg of honey also depends on its moisture content, dehydration process which removes moisture from the honey also causes the increase in Tg.[Citation73] The reported Tg of water is −135oC[Citation74] and it acts as a potent plasticizer and decreases the Tg of amorphous and partially amorphous solids.[Citation75] The plasticizing effect of water weakens in dehydrated honey thus Tg of honey increase.[Citation73] The reduction of Tg with increasing moisture content agreed well with the results for polysaccharides,[Citation76] honeys,[Citation73] and β-casein.[Citation77] Study of Kántor et al.[Citation73] reported that the low glass transition temperature of honey contributes to the sticky nature of honey.
The surface stickiness was measured with probe tack test which mimics the touches of human finger on sticky surfaces.[Citation78,Citation79] The surface stickiness was quantified as the maximum tensile pressure required to separate the probe from the surface of honey. The initial surface stickiness value of the honey was 0.16 ± 0.01 kPa which is comparable to the value obtained by Adhikari et al.[Citation79] of 0.115 kPa. The results in shows that a higher dehydrating temperature and longer duration have resulted in a significantly stickier honey. The honey surface stickiness change was most prominent at higher dehydration temperature. The surface stickiness increased from 0.16 ± 0.01 kPa to 4.69 ± 0.05, 81.86 ± 5.07, and 99.62 ± 2.22 kPa after being dehydrated for 84 h at 40, 55, and 70°C, respectively. The value of stickiness of honey dehydrated at 70°C is about 20 times higher than those dehydrated at 40°C. The increase in stickiness is mainly due to the removal of moisture from honey which originally act as a dispersing medium in honey.[Citation28] Honey becomes a highly viscous glassy state and forms a sticky cohesive mass as its moisture content decreased.[Citation77,Citation79] Stickiness has always been a drawback in processing and drying of sugar rich products like honey.[Citation28] Stickiness can be related to the adhesion and cohesion forces of the honey due to intermolecular and electrostatic forces, liquid bridges, solid bridges, or mechanical interlocking of particles.[Citation52,Citation80] It measures the strength of honey sticking together and to the other surfaces. The higher stickiness indicates that the honey has higher tendency to stick and larger amount force is needed to separate the honey and subsequently causing trouble for the processing. Thus, conventional drying of honey often involve the addition of high molecular weight compounds such as maltodextrin as drying aid to alter its glass transition temperature and decrease its stickiness.[Citation52]
Hydroxymethylfurfural and diastase activity
HMF is a chemical product of the furan group. It is formed through Maillard reaction by dehydration of sugars under acidic conditions during thermal treatments.[Citation81] HMF is often used as a quality evaluation property for honey as it is strongly correlated to ageing and overheating of honey.[Citation16,Citation19,Citation82] Previous studies have reported that HMF increases more significantly at higher heating temperature and time.[Citation12,Citation13,Citation17,Citation19,Citation81] shows dehydration at 40°C did not give any change to HMF and at 55°C, there was a steady increase. At 70°C, HMF content increased to 189.15 ± 5.82 mg/kg and peaked at 60 h and subsequently decreased to 24.69 ± 3.41 mg/kg at 84 h. In another study involving heating of acacia honey from 20 to 240°C for 30 min, it was also observed that the HMF content peaked at 190°C and decreased at 230°C. This may be due to the significant decrease of fructose and glucose due to overheating.[Citation81] HMF is classified as a potential carcinogenic chemical to humans or it might be metabolized by humans to potentially carcinogenic compounds.[Citation83] The study of Capuano & Fogliano[Citation83] reviewed some studies done related to toxicity of HMF and showed that HMF at high concentrations is cytotoxic, irritating to eyes, upper respiratory tract, skin and mucous membranes. Hence, the concentration of HMF should be strictly controlled. The maximum HMF content allowed in honey is 80 mg/kg for honey originating from tropical countries.[Citation84] The results showed that the HMF content in honey heated at 40 and 55°C remained within the limits. For dehydration at 70°C for 36 h, it produced HMF of 83.19 ± 2.05 mg/kg which is slightly higher than 80 mg/kg.
Figure 6. Effects of dehydration temperature and duration on (a) HMF and (b) diastase activity of honey
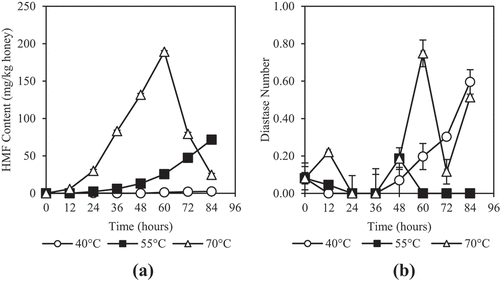
The diastase activity is the measure of the combined activities of both α- and β-amylases and is often used as a quality evaluation parameter for honey.[Citation16,Citation85] The diastase activity is greatly reduced by heating and thus could be used as an indication for overheating of honey.[Citation11,Citation85] The results found that the Diastase Number (DN) of dehydrated honey was very low, ranging from 0 to 0.75 DN, which was far below the minimum value of 3 DN following the standard set by Codex Alimentarius Commission[Citation84] for honey with low enzyme content. The low diastase activity of the honey lead to no significant difference between the samples. Kek et al.[Citation86] reported that the diastase value of Malaysian honey from Apis honey bees and Trigona stingless bee ranged from 0.04 to 1.48 DN while Chua & Adnan[Citation85] reported diastase value ranged from 2.12 to 2.32 DN for Malaysian honey from Apis honey bees. Both studies on Malaysia’s honey showed that the diastase activity were below the minimum value of 3 DN. The low diastase activity indicates that the honey have a low natural enzyme content. The low diastase activity could be due to the tropical climate in Malaysia which can easily go above 30°C. The reduction of enzymatic activities occur even at a relatively low temperature of 30 to 50°C.[Citation16,Citation19]
Conclusion
Dehydration of Kelulut honey at lower temperatures of 40°C for 36 h and higher temperatures of 55 and 70°C for 18 h can produce honey with water activity below 0.6 and moisture content below 8% (dry basis). The loss of water results contributes to the increase of its TPC while experiencing an increase in HMF, colour intensity, viscosity, hygroscopicity, glass transition temperature, and surface stickiness which are all still within acceptable ranges following the Codex Alimentarius Commission.
Nomenclature
| D | = | Dilution factor |
| d.b. | = | Dry basis |
| FRI | = | First falling rate |
| FRII | = | Second falling rate |
| h | = | Hour |
| HMF | = | Hydroxymethylfurfural |
| Mc | = | Critical moisture content |
| Mwt | = | Moisture content at time t |
| RH | = | Relative humidity |
| t | = | Dehydration time |
| Tg | = | Glass transition temperature |
| TPC | = | Total phenolic content |
| W | = | Weight of honey |
Additional information
Funding
References
- Kek, S. P.; Chin, N. L.; Yusof, Y. A.; Tan, S. W.; Chua, L. S. Total Phenolic Contents and Colour Intensity of Malaysian Honeys from the Apis Spp. And Trigona Spp. Bees. Agric. Agric. Sci. Procedia 2014, 2, 150–155.
- Ranneh, Y.; Ali, F.; Zarei, M.; Akim, A. M.; Hamid, H. A.; Khazaai, H. Malaysian Stingless Bee and Tualang Honeys: A Comparative Characterization of Total Antioxidant Capacity and Phenolic Profile Using Liquid Chromatography-Mass Spectrometry. LWT - Food Sci. Technol. 2018, 89, 1–9. DOI: 10.1016/j.lwt.2017.10.020.
- Bogdanov, S.; Jurendic, T.; Sieber, R.; Gallmann, P. Honey for Nutrition and Health: A Review. J. Am. Coll. Nutr. 2008, 27(6), 677–689. DOI: 10.1080/07315724.2008.10719745.
- Souza, B.; Roubik, D.; Barth, O. M.; Heard, T.; Enríquez, E.; Carvalho, C.; Villas-Bôas, J.; Marchini, L.; Locatelli, J.; Persano-Oddo, L., et al. Composition of Stingless Bee Honey: Setting Quality Standards. Interciencia 2006, 31(12), 867–875.
- Martin, E. C. Some Aspects of Hygroscopic Properties and Fermentation of Honey. Bee World 1958, 39(7), 165–178. DOI: 10.1080/0005772X.1958.11095058.
- Mendes-Ferreira, A.; Cosme, F.; Barbosa, C.; Falco, V.; Inês, A.; Mendes-Faia, A. Optimization of Honey-Must Preparation and Alcoholic Fermentation by Saccharomyces Cerevisiae for Mead Production. Int. J. Food Microbiol. 2010, 144(1), 193–198. DOI: 10.1016/j.ijfoodmicro.2010.09.016.
- Sanz, S.; Gradillas, G.; Jimeno, F.; Perez, C.; Juan, T. Fermentation Problem in Spanish North-Coast Honey. J. Food Prot. 1995, 58(5), 515–518. DOI: 10.4315/0362-028X-58.5.515.
- Subramanian, R.; Hebbar, H. U.; Rastogi, N. K. Processing of Honey: A Review. Int. J. Food Prop. 2007, 10(1), 127–143. DOI: 10.1080/10942910600981708.
- Samborska, K.; Czelejewska, M. The Influence of Thermal Treatment and Spray Drying on the Physicochemical Properties of Polish Honeys. J. Food Process. Preserv. 2014, 38(1), 413–419. DOI: 10.1111/j.1745-4549.2012.00789.x.
- Bath, P. K.; Singh, N. Chemical Changes in Helianthus Annuus and Eucalyptus Lanceolatus Honey during Storage. J. Food Qual. 2000, 23(4), 443–451. DOI: 10.1111/j.1745-4557.2000.tb00570.x.
- Tosi, E.; Martinet, R.; Ortega, M.; Lucero, H.; Ré, E. Honey Diastase Activity Modified by Heating. Food Chem. 2008, 106(3), 883–887. DOI: 10.1016/j.foodchem.2007.04.025.
- Turhan, I.; Tetik, N.; Karhan, M.; Gurel, F.; Reyhan Tavukcuoglu, H. Quality of Honeys Influenced by Thermal Treatment. LWT - Food Sci. Technol. 2008, 41(8), 1396–1399. DOI: 10.1016/j.lwt.2007.09.008.
- Tosi, E.; Ciappini, M.; Ré, E.; Lucero, H. Honey Thermal Treatment Effects on Hydroxymethylfurfural Content. Food Chem. 2002, 77(1), 71–74. DOI: 10.1016/S0308-8146(01)00325-9.
- Tosi, E. A.; Ré, E.; Lucero, H.; Bulacio, L. Effect of Honey High-Temperature Short-Time Heating on Parameters Related to Quality, Crystallisation Phenomena and Fungal Inhibition. LWT - Food Sci. Technol. 2004, 37(6), 669–678. DOI: 10.1016/j.lwt.2004.02.005.
- Stephen, W. A. The Relationship of Moisture Content and Yeast Count in Honey Fermentation. Sci. Agric. 1946, 26(6), 258–264.
- White, J. W. The Role of Hmf and Diastase Assays in Honey Quality Evaluation. Bee World 1994, 75(3), 104–117. DOI: 10.1080/0005772X.1994.11099213.
- Sahinler, N.; Aziz, G. Effect of Heating and Storage of Honey Hydroxy Methylfurfural and Diastase Activity. J. Food Technol. 2005, 3, 152–157.
- Hebbar, H. U.; Nandini, K. E.; Lakshmi, M. C. Microwave and Infrared Heat Processing of Honey and Its Quality. Food Sci. Technol. Res. 2003, 9(1), 49–53. DOI: 10.3136/fstr.9.49.
- Karabournioti, S.; Zervalaki, P. The Effect of Heating on Honey HMF and Invertase. Apiacta 2001, 36(4), 177–181.
- Cuevas-Glory, L. F.; Pino, J. A.; Sosa-Moguel, O.; Sauri-Duch, E.; Bringas-Lantigua, M. Optimization of the Spray-Drying Process for Developing Stingless Bee Honey Powder. Int. J. Food Eng. 2016, 13(1). DOI: 10.1515/IJFE-2016-0217.
- Nurhadi, B.; Roos, Y. H. Water Sorption and Water Plasticization Behavior of Vacuum Dried Honey. Int. J. Food Prop. 2016, 19(6), 1370–1380. DOI: 10.1080/10942912.2015.1081607.
- Jedlińska, A.; Samborska, K.; Witrowa, D. Properties of Powders Received by Spray Drying and Freeze Drying of Solutions of Honey (In Polish). Acta Agrophysica. 2012, 19, 3, 563–574.
- Kanayama, T. Process for Producing Solid Honey. U.S. Patent 5,356,650 A, Oct 1994.
- Cui, Z. W.; Sun, L. J.; Chen, W.; Sun, D. W. Preparation of Dry Honey by Microwave-Vacuum Drying. J. Food Eng. 2008, 84(4), 582–590. DOI: 10.1016/j.jfoodeng.2007.06.027.
- Nurhadi, B.; Andoyo, R.; Mahani, R.; Indiarto, R. Study the Properties of Honey Powder Produced from Spray Drying and Vacuum Drying Method. Int. Food Res. J. 2012, 19(3), 907–912.
- Samborska, K.; Gajek, P.; Kamińska-Dwórznicka, A. Spray Drying of Honey: The Effect of Drying Agents on Powder Properties. Polish J. Food Nutr. Sci. 2015, 65(2), 109–118. DOI: 10.2478/pjfns-2013-0012.
- Shi, Q.; Fang, Z.; Bhandari, B. Effect of Addition of Whey Protein Isolate on Spray-Drying Behavior of Honey with Maltodextrin as a Carrier Material. Dry. Technol. 2013, 31(13–14), 1681–1692. DOI: 10.1080/07373937.2013.783593.
- Umesh Hebbar, H.; Rastogi, N. K.; Subramanian, R. Properties of Dried and Intermediate Moisture Honey Products: A Review. Int. J. Food Prop. 2008, 11(4), 804–819. DOI: 10.1080/10942910701624736.
- Bhandari, B. R.; Datta, N.; Howes, T. Problems Associated With Spray Drying Of Sugar-Rich Foods. Dry. Technol. 1997, 15(2), 671–684. DOI: 10.1080/07373939708917253.
- Neil. Drying & Dehydrating: Dehydrator Vs. Oven Drying. https://preserveandpickle.com/dehydrator-vs-oven-drying/(accessed Mar 1, 2018).
- Kendall, P.; DiPersio, P.; Sofos, J. Drying Vegetables. [cited 2018 Mar 1]. https://extension.colostate.edu/topic-areas/nutrition-food-safety-health/drying-vegetables-9-308/
- Cunningham, E. What Is a Raw Foods Diet and are There Any Risks or Benefits Associated with It? J. Acad. Nutr. Diet. 2004, 104(10), 1623.
- Muzaffar, K.; Kumar, P. Parameter Optimization for Spray Drying of Tamarind Pulp Using Response Surface Methodology. Powder Technol. 2015, 279, 179–184. DOI: 10.1016/j.powtec.2015.04.010.
- Singleton, V. L.; Orthofer, R.; Lamuela-Raventos, R. M. Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. Methods Enzymol. 1999, 299C, 152–178. DOI: 10.1016/S0076-6879(99)99017-1.
- White, J. W. Spectrophotometric Method for Hydroxymethylfurfural in Honey. J. Assoc. Off. Anal. Chem. 1979, 62(3), 509–514.
- Botelho, F.; Corrêa, C. P.; Goneli, A.; Martins, M.; Magalhães, F. E. A.; Campos Botelho, S. Periods of Constant and Falling-Rate for Infrared Drying of Carrot Slices, Revista Brasileira de Engenharia Agricola e Ambiental. 2011;15(8), 845-852. DOI: 10.1590/S1415-43662011000800012
- Earle, R. L. Unit Operations in Food Processing, second ed. Pergamon Press, London. 1983.
- Bellagha, S.; Amami, E.; Farhat, A.; Kechaou, N. Drying Kinetics and Characteristic Drying Curve of Lightly Salted Sardine (Sardinella Aurita). Dry. Technol. 2002, 20(7), 1527–1538. DOI: 10.1081/DRT-120005866.
- Karathanos, V. T.; Belessiotis, V. G. Sun and Artificial Air Drying Kinetics of Some Agricultural Products. J. Food Eng. 1997, 31(1), 35–46. DOI: 10.1016/S0260-8774(96)00050-7.
- Gill, R. S.; Hans, V. S.; Singh, S.; Pal Singh, P.; Dhaliwal, S. S. A Small Scale Honey Dehydrator. J. Food Sci. Technol. 2015, 52(10), 6695–6702. DOI: 10.1007/s13197-015-1760-0.
- Krokida, M. K.; Karathanos, V. T.; Maroulis, Z. B.; Marinos-Kouris, D. Drying Kinetics of Some Vegetables. J. Food Eng. 2003, 59(4), 391–403. DOI: 10.1016/S0260-8774(02)00498-3.
- Gunhan, T.; Demir, V.; Hancioglu, E.; Hepbasli, A. Mathematical Modelling of Drying of Bay Leaves. Energy Convers. Manag. 2005, 46(11–12), 1667–1679. DOI: 10.1016/j.enconman.2004.10.001.
- Malaysian Standard. Kelulut (Stingless Bee) Honey- Specification: MS 2683:2017, Department of Standard Malaysia. 2017.
- Roos, Y. H. Water Activity and Physical State Effects on Amorphous Food Stability. J. Food Process Preserv. 1992, 16(1993), 433–447. DOI: 10.1111/j.1745-4549.1993.tb00221.x.
- Gleiter, R. A.; Horn, H.; Isengard, H. D. Influence of Type and State of Crystallisation on the Water Activity of Honey. Food Chem. 2006, 96(3), 441–445. DOI: 10.1016/j.foodchem.2005.03.051.
- Zamora, M. C.; Chirife, J.; Roldán, D. On the Nature of the Relationship between Water Activity and % Moisture in Honey. Food Control 2006, 17(8), 642–647. DOI: 10.1016/j.foodcont.2005.04.002.
- Chirife, J.; Zamora, M. C.; Motto, A. The Correlation between Water Activity and % Moisture in Honey: Fundamental Aspects and Application to Argentine Honeys. J. Food Eng. 2006, 72(3), 287–292. DOI: 10.1016/j.jfoodeng.2004.12.009.
- Ergun, R.; Lietha, R.; Hartel, R. W. Moisture and Shelf Life in Sugar Confections. Crit. Rev. Food Sci. Nutr. 2010, 50(2), 162–192. DOI: 10.1080/10408390802248833.
- Umprayn, K.; Mendes, R. W. Hygroscopicity and Moisture Adsorption Kinetics of Pharmaceutical Solids: A Review. Drug Dev. Ind. Pharm. 1987, 13(4–5), 653–693. DOI: 10.3109/03639048709105213.
- Martin, E. C. The Hygroscopic Properties of Honey, 1939; 32(5), pp 660–663. DOI: 10.1093/jee/32.5.660.
- Abbas, K. A.; Lasekan, O.; Khalil, K. S. The Significance of Glass Transition Temperature in Processing of Selected Fried Food Products: A Review. Mod. Appl. Sci. 2010, 4(5), 3–21. DOI: 10.5539/mas.v4n5p3.
- Ashemi, N. H.; Ilani, E. M.; Ortezavi, S. A. M.; Azdi, F. T. Y. Sticky Point Temperature as a Suitable Method in Evaluation of Shelf Life of Food Powders. Bulletin de la Société Royale des Sciences de Liège. 2017, 86, 7–12.
- Wani, S. A.; Kumar, P. Moisture Sorption Isotherms and Evaluation of Quality Changes in Extruded Snacks during Storage. LWT - Food Sci. Technol. 2016, 74, 448–455. DOI: 10.1016/j.lwt.2016.08.005.
- Al-Muhtaseb, A. H.; McMinn, W. A. M.; Magee, T. R. A. Moisture Sorption Isotherm Characteristics of Food Products: A Review. Food Bioprod. Process. 2002, 80(2), 118–128. DOI: 10.1205/09603080252938753.
- Muzaffar, K.; Kumar, P. Moisture Sorption Isotherms and Storage Study of Spray Dried Tamarind Pulp Powder. Powder Technol. 2016, 291, 322–327. DOI: 10.1016/j.powtec.2015.12.046.
- Brunauer, S.; Deming, L. S.; Deming, W. E.; Teller, E. On a Theory of the Van Der Waals Adsorption of Gases. J. Am. Chem. Soc. 1940, 62(7), 1723–1732. DOI: 10.1021/ja01864a025.
- Fontana, A. J., Jr; Schmidt, S. J.; Labuza, T. P. Water Activity in Foods: Fundamentals and Applications; Blackwell Publishing Ltd, Oxford., 2008; DOI: 10.1002/9780470376454.
- Yao, W.; Yu, X.; Lee, J. W.; Yuan, X.; Schmidt, S. J. Measuring the Deliquescence Point of Crystalline Sucrose as a Function of Temperature Using a New Automatic Isotherm Generator. Int. J. Food Prop. 2011, 14(4), 882–893. DOI: 10.1080/10942910903474393.
- Maroulis, Z. B.; Tsami, E.; Marinos-Kouris, D.; Saravacos, G. D. Application of the GAB Model to the Moisture Sorption Isotherms for Dried Fruits. J. Food Eng. 1988, 7(1), 63–78. DOI: 10.1016/0260-8774(88)90069-6.
- Kaymak-Ertekin, F.; Gedik, A. Sorption Isotherms and Isosteric Heat of Sorption for Grapes, Apricots, Apples and Potatoes. LWT - Food Sci. Technol. 2004, 37(4), 429–438. DOI: 10.1016/j.lwt.2003.10.012.
- Turkmen, N.; Sari, F.; Poyrazoglu, E. S.; Velioglu, Y. S. Effects of Prolonged Heating on Antioxidant Activity and Colour of Honey. Food Chem. 2006, 95(4), 653–657. DOI: 10.1016/j.foodchem.2005.02.004.
- Kędzierska-Matysek, M.; Florek, M.; Wolanciuk, A.; Skałecki, P.; Litwińczuk, A. Characterisation of Viscosity, Colour, 5-Hydroxymethylfurfural Content and Diastase Activity in Raw Rape Honey (Brassica Napus) at Different Temperatures. J. Food Sci. Technol. 2016, 53(4), 2092–2098. DOI: 10.1007/s13197-016-2194-z.
- Sant’Ana, L. D. O.; Buarque Ferreira, A. B.; Lorenzon, M. C. A.; Berbara, R. L. L.; Castro, R. N. Correlation of Total Phenolic and Flavonoid Contents of Brazilian Honeys with Colour and Antioxidant Capacity. Int. J. Food Prop. 2014, 17(1), 65–76. DOI: 10.1080/10942912.2011.614368.
- Piljac-Žegarac, J. Antioxidant Properties and Phenolic Content of Different Floral Origin Honeys. J. Api Prod. ApiMed. Sci. 2009, 1(2), 43–50. DOI: 10.3896/IBRA.4.01.2.04.
- Chaikham, P.; Prangthip, P. Alteration of Antioxidative Properties of Longan Flower-Honey after High Pressure, Ultra-Sonic and Thermal Processing. Food Biosci. 2015. DOI: 10.1016/j.fbio.2015.01.002.
- Akhmazillah, M. F. N.; Farid, M. M.; Silva, F. V. M. High Pressure Processing (HPP) of Honey for the Improvement of Nutritional Value. Innov. Food Sci. Emerg. Technol. 2013, 20, 59–63. DOI: 10.1016/j.ifset.2013.06.012.
- Cao, X.; Bi, X.; Huang, W.; Wu, J.; Hu, X.; Liao, X. Changes of Quality of High Hydrostatic Pressure Processed Cloudy and Clear Strawberry Juices during Storage. Innov. Food Sci. Emerg. Technol. 2012, 16, 181–190. DOI: 10.1016/j.ifset.2012.05.008.
- Brudzynski, K.; Miotto, D. The Relationship between the Content of Maillard Reaction-Like Products and Bioactivity of Canadian Honeys. Food Chem. 2011, 124(3), 869–874. DOI: 10.1016/j.foodchem.2010.07.009.
- Yanniotis, S.; Skaltsi, S.; Karaburnioti, S. Effect of Moisture Content on the Viscosity of Honey at Different Temperatures. J. Food Eng. 2006, 72(4), 372–377. DOI: 10.1016/j.jfoodeng.2004.12.017.
- Zaitoun, S.; Ghzawi, A. A. M.; Al-Malah, K. I. M.; Abu-Jdayil, B. Rheological Properties of Selected Light Colored Jordanian Honey. Int. J. Food Prop. 2001, 4(1), 139–148. DOI: 10.1081/JFP-100002192.
- Oroian, M. Physicochemicalrr and Rheological Properties of Romanian Honeys. Food Biophys. 2012, 7(4), 296–307. DOI: 10.1007/s11483-012-9268-x.
- Roos, Y.; Karel, M. Plasticizing Effect of Water on Thermal-Behavior and Crystallization of Amorphous Food Models. J. Food Sci. 1991, 56(1), 38–43. DOI: 10.1111/j.1365-2621.1991.tb07970.x.
- Kántor, Z.; Pitsi, G.; Thoen, J. Glass Transition Temperature of Honey as a Function of Water Content as Determined by Differential Scanning Calorimetry. J. Agric. Food Chem. 1999, 47, 2327–2330. DOI: 10.1021/jf981070g.
- Roos, Y.; Karel, M. Water and Molecular Weight Effects on Glass Transitions in Amorphous Carbohydrates and Carbohydrate Solutions. J. Food Sci. 1991, 56(6), 1676–1681. DOI: 10.1111/j.1365-2621.1991.tb08669.x.
- Hancock, B. C.; Zografi, G. The Relationship between the Glass Transition Temperature and the Water Content of Amorphous Pharmaceutical Solids. Pharm. Res. 1994, 471–477. doi:10.1023/A:1018941810744.
- Roos, Y.; Karel, M. Phase Transitions of Mixtures of Amorphous Polysaccharides and Sugars. Biotechnol. Prog. 1991, 7(1), 49–53. DOI: 10.1021/bp00007a008.
- Mauer, L. J.; Smith, D. E.; Labuza, T. P. Effect of Water Content, Temperature and Storage on the Glass Transition, Moisture Sorption Characteristics and Stickiness of β-Casein. Int. J. Food Prop. 2000, 3(2), 233–248. DOI: 10.1080/10942910009524630.
- Adhikari, B.; Howes, T.; Lecomte, D.; Bhandari, B. R. A Glass Transition Temperature Approach for the Prediction of the Surface Stickiness of A Drying Droplet during Spray Drying. Powder Technol. 2005, 149(2–3), 168–179. DOI: 10.1016/j.powtec.2004.11.007.
- Adhikari, B.; Howes, T.; Bhandari, B. R.; Truong, V. Characterization of the Surface Stickiness of Fructose-Maltodextrin Solutions during Drying. Dry. Technol. 2003, 21(1), 17–34. DOI: 10.1081/DRT-120017281.
- Papadakis, S. E.; Bahu, R. E. The Sticky Issues of Drying. Dry. Technol. 1992, 10(4), 817–837. DOI: 10.1080/07373939208916484.
- Zhang, Y.; Song, Y.; Zhou, T.; Liao, X.; Hu, X.; Li, Q. Kinetics of 5-Hydroxymethylfurfural Formation in Chinese Acacia Honey during Heat Treatment. Food Sci. Biotechnol. 2012, 21(6), 1627–1632. DOI: 10.1007/s10068-012-0216-9.
- Chuttong, B.; Chanbang, Y.; Sringarm, K.; Burgett, M. Effects of Long Term Storage on Stingless Bee (Hymenoptera: Apidae: Meliponini) Honey. J. Apic. Res. 2016, 54(5), 441–451. DOI: 10.1080/00218839.2016.1186404.
- Capuano, E.; Fogliano, V. Acrylamide and 5-Hydroxymethylfurfural (HMF): A Review on Metabolism, Toxicity, Occurrence in Food and Mitigation Strategies. LWT - Food Sci. Technol. 2011, 44(4), 793–810. DOI: 10.1016/j.lwt.2010.11.002.
- Codex Alimentarius Commission. Revised Codex Standard For Honey Codex Stan 12-1981, Codex Standard, 2001; 12, 1-7.
- Chua, L. S.; Adnan, N. A. Biochemical and Nutritional Components of Selected Honey Samples. Acta Sci. Pol. Technol. Aliment. 2014, 13(2), 169–179. DOI: 10.17306/J.AFS.
- Kek, S. P.; Chin, N. L.; Yusof, Y. A.; Tan, S. W.; Chua, L. S. Classification of Entomological Origin of Honey Based on Its Physicochemical and Antioxidant Properties. Int. J. Food Prop. 2017, 20(sup3), S2723–S2738. DOI: 10.1080/10942912.2017.1359185.