ABSTRACT
Isoeugenol (2-methoxy-4-(prop-1-en-1-yl)phenol), a component of from clove (Eugenia caryophylata) oil, is a starting material for both the biotechnological and synthetic production of vanillin and vanillic acid. In this article, the isoeugenol showed excellent inhibitory effects against some metabolic enzymes such as acetylcholinesterase (AChE) enzymes, α-glycosidase, and α-amylase. Isoeugenol has the IC50 values of 411.5 for α-amylase, 19.25 nM for α-glycosidase and 77.00 nM for AChE. Ki values of isoeugenol were found as 21 ± 9 nM and 16 ± 3 nM against α-glycosidase and AChE, respectively. On the other hand, tacrine as standard AChE inhibitor exhibited IC50 value of 20.38 nM. α-Glycosidase inhibitors, commonly referred to as starch blockers, are anti-diabetic drugs that help reduce edible blood glucose levels. AChE inhibitors are used for the treatment of some neurologic degenerational diseases, which involve Alzheimer’s disease. Also, there is a growing interest and the need to search for newer effective and safe AChE, α-amylase, and α-glycosidase inhibitors for the treatment of some metabolic disorder.
Introduction
Clove (Eugenia caryophylata) is widely used in traditional medicine for treatment of many diseases such as digestive systems, toothaches, bacterial, and fungal infections.[1] Clove (Eugenia caryophylata) oil is very rich in terms of natural phenolic compounds including eugenol, eugenyl acetate, and isoeugenol.[Citation2,Citation3] Indeed, isoeugenol (2-methoxy-4-propenyl-phenol) is present in a variety of spices. Also, it exhibits a large spectrum of biological activity. Isoeugenol is used as a sweetener, natural antioxidant, and storage agent in food and pharmaceutical processes.[Citation4] Isoeugenol had various bioactivities including the antibacterial activity against some common food-borne pathogens.[Citation5] It is well known that phenols including isoeugenol as biological active compounds[Citation6] and important compounds food and pharmaceutical chemistry. In our daily life, phenolic compounds and derivatives have a great importance.[Citation7] They are particularly characterized for their antioxidant effectiveness.[Citation8,Citation9] For example, α-tocopherol is used as the most efficient phenolic compound.[Citation10,Citation11] Functionalization of aromatic rings of phenols has been of great importance in areas such as food, cosmetics, pharmaceuticals, and paint industries. Eugenol and isoeugenol were used as sweeteners, and additives in food products.[Citation12,Citation13] However, differences in biological activities between both phenolic compounds have been rarely studied.
Acetylcholinesterase (AChE; E.C.3.1.1.7) hydrolyzes acetylcholine (ACh), a neurotransmitter in cholinergic synapses. AChE has properties that are not found in other enzymes in terms of active site and catalytic mechanism.[Citation14] Active site of AChE is at the bottom of the narrow gutter structure and consists of two subunits. The first is the negatively charged or the anionic region; the second is the esterified region containing the catalytic moiety, or the catalytic triad (Ser203, Glu334, and His447).[Citation15] The catalytic triad is temporarily attached to the acyl region of the substrate. The hydrophobic subunit and the acyl pocket contain the alcohol group in the case of tetrahedral transition.[Citation16] Oxygenation hole stabilizes the transition state by providing negative carbonyl oxygen. In addition to the catalytic triad, all known AChE contains a second substrate-binding domain termed the peripheral anionic domain. Cholinesterase inhibition is also used in the production of chemical gas to produce lethal acetylcholinergic stimulation as used for therapeutic purposes to increase acetylcholine (ACh) in the synaptic range in diseases such as Alzheimer’s disease (AD), which is one of the most common neurodegenerative disorders. Myasthenia gravis, which persists with ACh insufficiency.[Citation17] The human brain contains mainly two types of cholinesterase including AChE and butyrylcholinesterase (BChE; E.C.3.1.1.8). The human brain contains indicated both cholinesterases.[Citation18] The reaction catalyzed by AChE occurs enzymatically in two steps. Firstly, the enzyme acts as a strong nucleophile; then, it acts as a perfect disintegrating group through a nucleophilic hydroxyl group of a specific serine residue.[Citation19] The main function of AChE is the termination of cholinergic neurotransmission. BChE’s physiological function has not been known; however, it hydrolyzes ACh and other choline esters. AD arises as a result of the reduction of neurotransmitters in the brain. The decrease in the brain in ACh levels is the biggest change in the biochemical sense of AD. There is no definitive treatment of AD. The treatments currently used are intended to eliminate the symptoms of the disease.[Citation20]
Diabetes is one of the most serious health problems of the century. It continues to increase rapidly all over the world and affects more and more people every day.[Citation21,Citation22] Growing pandemic increases the consumption of health resources in every country around the world and causes many people in working age to become inoperable and die prematurely.[Citation23] Diabetes authorities and non-governmental organizations report that if something is not done, diabetes will affect more people in the world. Diabetes is classified into four groups including gestational diabetes, other specific types, Type 1 diabetes (T1DM), and Type 2 diabetes mellitus (T2DM). The first one is more acute in children and adolescents with insulin deficiency due to pancreas β cell destruction. T2DM is a prominent insulin resistance and insulin secretion disorders. Approximately 90–95% of all people with diabetes are on T2DM. While gestational diabetes describes diabetes that occurs during pregnancy, other specific types define the high blood glucose level that occurs for many reasons affecting the pancreas.[Citation24,Citation25]
In the literature, there are negligible studies on the effects of isoeugenol on these metabolic enzymes. Therefore, the study has novelty and completely original. The aim of this paper was to determine the inhibition effects of isoeugenol against some metabolic enzyme including AChE, α-glycosidase, and α-amylase. Another goal of this study is to compare the inhibition effects of isoeugenol with standard enzymes inhibitors including acarbose (for α-amylase and α-glycosidase enzymes) and tacrine (for AChE).
Materials and methods
Chemicals
p-Nitrophenyl α-D-glycopyranoside (p-NPG), achethylycholinesteras from electric eel (Electrophorus electricus), α-amylase from porcine pancreas, α-glycosidase from Saccharomyces cerevisiae, isoeugenol (CAS Number: 97–54-1) and starch were bought from Sigma-Aldrich (G5003; St.Louis, MO).
α-glycosidase activity
For this enzyme, p-NPG was used as the substrate. The samples were prepared by dissolving ethanol (1 mg/mL). Firstly, 100 μL of NaH2PO4 with pH 7.4 and 10 μL of enzyme solution were mixed (). And then, 50–100 μL of the samples were added into the current solution and these were mixed.[Citation26] After, it was preincubated at 35°C for 10 min by adding the p-NPG to initiate the reaction. In addition, 50 μL of p-NPG at pH 7.4 of NaH2PO4 (5 mM) after preincubation was added and the incubation was again carried out at 35°C. IC50 and Ki values were evaluated with the obtained data. Acarbose was used as a positive control. Absorbance values were spectrophotometrically measured at 405 nm.[Citation27]
Table 1. Cuvette content of the study with α-glycosidase method
α-amylase activity
α-Amylase activity and inhibition study of this enzyme were spectrophotometrically measured at 580 nm according to the procedure of Xiao et al.[Citation28] Starch was used as substrate conforming to previous studies.[Citation29] In this work, for preparing starch solution, 0.5 g of starch was dissolved in 20 mL NaOH solution (0.4 M) and heated for 30 min at 80°C, then cooled in iced water, with HCl (2.0 M). The solution pH was adjusted to 6.9 and using water was added to complete 25 mL. Sample solutions were prepared by dissolving 5 mg in 0.5 mL (EtOH: water). Multiple solutions in phosphate buffer were prepared in case of getting entire enzyme inhibition. 35 µL of substrate, 35 µL of phosphate buffer (pH 6.9), and 5 µL of sample solutions were mixed and preincubated at 35°C for 30 min. Then, 20 µL of a 50 µg/mL enzyme was added and re-incubated for 30 min (). The reaction was finished by addition of 50 µL of HCl (0.1 M). The absorbance was spectrophotometrically measured at 580 nm.
Table 2. Cuvette content of the study with α-amylase method
Acetylcholinesterase inhibitory study
The inhibitory effect of isoeugenol on AChE activity was performed according to Ellman’s procedure[Citation30] as described in the previous study.[Citation31] Acetylthiocholine iodide (AChI) was used as a substrate for the cholinergic reaction. In brief, an aliquot (100 µL) of Tris/HCl buffer (pH 8.0, 1.0 M) and different concentration of sample solutions (10–30 µg/mL) were added to 50 µL of AChE solution (5.32 × 10−3 EU). The mixture was incubated at 20°C for 10 min. An aliquot (50 µL, 0.5 mM) of DTNB (5,5ʹ-dithio-bis(2-nitro-benzoic)acid) and AChI were added to incubated mixture and enzymatic reactions were initiated (). AChE activities were spectrophotometrically determined at 412 nm.[Citation31,Citation32]
Table 3. Cuvette content of the study with acetylcholinesterase method
Results and discussion
Isoeugenol as a natural phenol derivative was used medical and cosmetics industries for its antioxidant, anticancer, antigenotoxic and anti-inflammatory properties. It is widely used as preservatives and flavors, sweeteners, antioxidants, and fragrances.[1,Citation33-Citation35] Essential oils, which can be named as essence oil, essential oil or aromatic oil, take place an important role in plant phytochemistry.[Citation34] Mostly, they have a strong odor, colorless or light yellow color, liquid form or freezing properties at room temperature. Essential oils are mostly used as antibacterial compounds in plant extracts. Isoeugenol is component an essential oil naturally found in plants.[Citation35,Citation36]
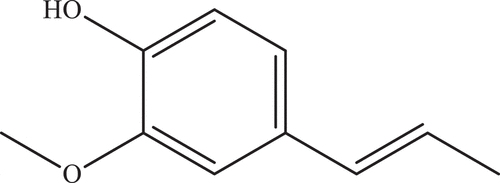
Evaluation of the effects of the isoeugenol on AChE, α-amylase, and α-glycosidase was an important aim of this article and inhibition findings are represented in . The chemical structure of isoeugenol is shown in . Half maximal inhibition concentration (IC50) is a measure of the potency of a substance in inhibiting a specific biological or biochemical function.[Citation39,Citation40] Also, the IC50 value of isoeugenol against some metabolic enzymes was investigated.
Table 4. The enzyme inhibition results of isoeugenol against α-glycosidase, AChE, and α-Amylase enzymes
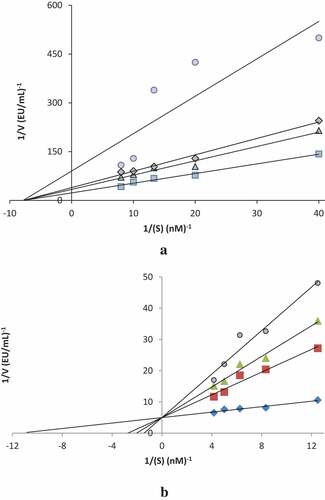
AD is the most common cause of dementia in aged people and is one of the ten main causes of death in the world loss is clinically defined by irreversible cognitive and rising language impairment and extreme behavioral abnormalities and disorders. AD occurs as a result of malfunctions of different biochemical pathways and is usually seen in older people.[Citation41,Citation42] T2DM is an intense endocrinal functionless that causes postprandial hyperglycemia, leading to challenging long-term disorders such as cardiovascular disease, diabetic neuropathy, diabetic retinopathy, and diabetic nephropathy.[Citation42] α-Amylase and α-glycosidase for hydrolyzing of polysaccharides are responsible for the final stage of the digestion of glycogen and starch. Inhibitors of both digestive enzymes are drug candidates and potential functional food factors to postpone postprandial absorption of glucose.[Citation43] α-Amylase, α-glycosidase, and AChE were investigated in the enzyme inhibition stage. The inhibitory effects of the isoeugenol compound against AChE, α-amylase, and α-glycosidase are summarized in . The following results can be clearly observed from the inhibition data. AD causes a decrease in neurotransmitters in the brain.[Citation44] The neurotransmitter with the highest decrease in this disease is ACh. Also, there is a growing interest and the need to search for newer effective and safe AChE, α-amylase and α-glycosidase inhibitors for the treatment of some metabolic disorder. In this research, IC50 and Ki factors of the isoeugenol compound were studied and the results are shown in and . In this paper, we investigated the AChE inhibitory potential of the isoeugenol compound. Starch is a polysaccharide. Consequently, it is not possible to adjust the concentration as the substrate since it is not monomeric. Therefore, Ki values could not be calculated for α-amylase enzyme. For this enzyme, the isoeugenol compound had an IC50 value of 77.00 nM and Ki value of 16 ± 3 nM (). IC50 values of isoeugenol and standard (tacrine) exhibited the following order: Tacrine (20.38 nM) < isoeugenol (77.00 µM). It was reported that plants secondary metabolites with different structural features including sterols, flavonoids, terpenoids, and phenolic compounds have been recognized as AChE inhibitors and promising lead compounds for AD.[Citation45]
Figure 2. Determination of Lineweaver–Burk graphs for isoeugenol inhibitor of α-glycosidase (a) and achethylcholinesterase (AChE) (b) enzymes for determination of Ki values
Enzymes as macromolecule had a large spectrum of biological catalysis and chemical reactions.[Citation46–Citation52] By providing ACh hydrolysis, a neurotransmitter is carried out after the delivery of this substance is achieved. ACh is an ester of great biological importance. While AChE breaks down the ACh neurotransmitter as acetate and choline, other neurotransmitters do not break down serotonin, dopamine, and norepinephrine.[Citation53] Neurotransmitters like norepinephrine, dopamine, and serotonin are absorbed by the synaptic space and take an essential role in central nerve conduction. AChE is mainly found in brain, muscle, nerve cells and erythrocytes. Pseudocholinesterase has the same mechanism, but is present in the plasma and circulates the destruction of ACh.[Citation54] Reactive oxygen species and oxidants produced from activated neutrophils and macrophages get a crucial role in neurodegenerative diseases like AD and painful pathogenic diseases.[Citation10]
Reduction of AChE for treatment or prevention of the disease should be reduced and this is possible by the inhibition of AChE, which breaks down acetylcholine. The AChE active site consists of five major binding sites. Firstly, the oxyanion pit is in equilibrium with tetrahedral transition.[Citation55] The second active region of AChE is the esteratic region. This region consists of serine residues and attacks the esteratic carbonyl group (C = O). The third active site is the binding region of anionic substrates, whereas in this region the negative charge is small, while the aromatic residues are numerous. As known, ACh has a quarternary ammonium tip.[Citation56] The Z electrons of the aromatic groups in this active site are bound by the preferential interaction of ACh to the quaternary nitrogen atom. The fourth active region is the selective aromatic binding region. In this region, the esteratic and anionic regions are close.[Citation57] Therefore, this active site is important for aryl substrates and active site ligands. Finally, the fifth active site is the peripheral anion-binding site. The peripheral anionic binding site may be attached to the hydrophobic moiety of the group, which is separated from the active moiety.[Citation58] Drugs used and designed for AD are used to adjust the level of acetylcholine and are designed accordingly. Acetylcholine is involved in signal transduction in synapses. After signal transduction to the synapses, it is hydrolyzed with AChE to break down into choline and acetate compound. Phenolic compounds remove free radicals and prevent diseases caused by them. One of these is AD. In this study, the AChE inhibition effects of isoeugenol were investigated. The inhibition effect on the AChE enzyme was investigated and the inhibition type, Ki and IC50 value were calculated. Tacrine molecule was used as standard. When all these results are evaluated, it can be a guide in pharmacological studies, the results are thought to be a triggering factor in the investigation of AD and treatment of this disease.[Citation59,Citation60]
α-Glycosidase enzyme (E.C.3.2.1.20) release from intestine cells and hydrolyzes oligosaccharides and polysaccharide to monosaccharide units, such as glucose and fructose in the small intestine.[Citation34,Citation41] In human, α-glycosidase inhibitors (α-GIs) had a great importance for controlling of T2DM and hyperglycemia.[Citation61] Recently, two main chemical classes of N-comprising α-GIs contain sugar-based inhibitors.[Citation37] α-GIs can reduce the uptake of dietary carbohydrates and repress postprandial hyperglycemia and T2DM. Thus, these α-GIs are endowed with sugar molecule such as compete and moieties with the oligosaccharides for binding to the active site of the enzyme, hence effectively reducing the postprandial glucose amounts in T2DM.[Citation38,Citation62,Citation63] On the other hand, for this study, the α-glycosidase assay which performed according to the method of Tao et al.[Citation64], is presented on cells lining the intestine, hydrolyzing monosaccharides to be absorbed through the intestine, isoeugenol exhibited IC50 value of 19.25 nM and Ki value of 21 ± 9 nM (). The results obtained from α-glycosidase assay showed that isoeugenol had effective α-glycosidase inhibition profile than that of acarbose (IC50: 22.8 μM) as α-GIs.[Citation65,Citation66] Also, highly effective IC50 value was obtained against α-amylase with IC50 value of 411.5 nM. The inhibition of digestive enzymes had great importance due to treating and preventing T2DM, postprandial glucose amounts and hyperglycemia.[67]
Conclusion
Isoeugenol was found to be powerful anticholinergic and antidiabetic effects when compared to standard compounds. Acetylcholine is one of the key neurotransmitters for peripheral nervous system. The inhibition of AChE has been proposed as a drug for neurotoxicity. On the other hand, α-amylase and α-glycosidase release from intestine cells and hydrolyzes oligosaccharides and polysaccharide to monosaccharide units. Therefore, isoeugenol had effective inhibition profiles against both digestion enzymes. Hence, isoeugenol can be used for the treatment of mild-to-moderate AD, various other memory diseases and T2DM.
References
- Gulcin, I.; Şat, İ. G.; Beydemir, Ş.; Elmastaş, M.; Küfrevioğlu, Ö. İ. Comparison of Antioxidant Activity of Clove (Eugenia caryophylata Thunb) Buds and Lavender (Lavandula Stoechas L.). Food. Chem. 2004, 87, 393–400. DOI: 10.1016/j.foodchem.2003.12.008.
- Lee, K. G.; Shibamoto, T. Antioxidant Property of Aroma Extract Isolated from Clove Buds [Syzygium aromaticum (L.) Merr. Et Perry]. Food. Chem. 2002, 74, 443–448. DOI: 10.1016/S0308-8146(01)00161-3.
- Gulcin, I.; Elmastaş, M.; Aboul-Enein, H. Y. Antioxidant Activity of Clove Oil: A Powerful Antioxidant Source. Arab. J. Chem. 2012, 5(4), 489–499. DOI: 10.1016/j.arabjc.2010.09.016.
- Liang-Liang, Z.; Li-Fang, Z.; Jian-Guo, X.; Qing-Ping, H. Comparison Study on Antioxidant, DNA Damage Protective and Antibacterial Activities of Eugenol and Isoeugenol against Several Foodborne Pathogens. Food Nutr. Res. 2017, 61(1), 1353356. DOI: 10.1080/16546628.2017.1339555.
- Atsumi, T.; Fujisawa, S.; Tonosaki, K. A Comparative Study of the Antioxidant/Prooxidant Activities of Eugenol and Isoeugenol with Various Concentrations and Oxidation Conditions. Toxicol. In. Vitro. 2005, 19, 1025–1033. DOI: 10.1016/j.tiv.2005.04.012.
- Gulcin, I. Antioxidant Activity of Eugenol: A Structure and Activity Relationship Study. J. Med. Food. 2011, 14(9), 975–985. DOI: 10.1089/jmf.2010.0197.
- Koksal, E.; Bursal, E.; Gulcin, I.; Korkmaz, M.; Çağlayan, C.; Goren, A. C.; Alwasel, S. H. Antioxidant Activity and Polyphenol Content of Turkish Thyme (Thymus vulgaris) Monitored by LC-MS/MS. Int. J. Food Propert. 2017, 20(3), 514–525. DOI: 10.1080/10942912.2016.1168438.
- Gulcin, I.; Beydemir, Ş.; Alici, H. A.; Elmastaş, M.; Büyükokuroğlu, M. E. In vitro Antioxidant Properties of Morphine. Pharmacol. Res. 2004, 49, 59–66.
- Gülçin, İ.; Şat, İ. G.; Beydemir, Ş.; Küfrevioğlu, Ö. İ. Evaluation of the In Vitro Antioxidant Properties of Extracts of Broccoli (Brassica oleracea L.). Ital. J. Food Sci. 2004, 16, 17–30.
- Oztaskin, N.; ÇEtinkaya, Y.; Taslimi, P.; Göksu, S.; Gulcin, I. Antioxidant and Acetylcholinesterase Inhibition Properties of Novel Bromophenol Derivatives. Bioorg. Chem. 2015, 60, 49–57. DOI: 10.1016/j.bioorg.2015.04.006.
- Gulcin, I.; Elias, R.; Gepdiremen, A.; Taoubi, K.; Köksal, E. Antioxidant Secoiridoids from Fringe Tree (Chionanthus Virginicus L.). Wood Sci. Technol. 2009, 43(3–4), 195–212. DOI: 10.1007/s00226-008-0234-1.
- Gulcin, I.; Küfrevioğlu, Ö. İ.; Oktay, M.; Büyükokuroğlu, M. E. Antioxidant, Antimicrobial, Antiulcer and Analgesic Activities of Nettle (Urtica dioica L.). J. Ethnopharmacol. 2004, 90, 205–215. DOI: 10.1016/j.jep.2003.09.028.
- Bulut, N.; Koçyiğit, U. M.; Gecibesler, I. H.; Dastan, T.; Karci, H.; Taslimi, P.; Durna Dastan, S.; Gulcin, I. Cetin, A. Synthesis of Some Novel Pyridine Compounds Containing Bis-1,2,4-Triazole Moiety and Investigation of Their Antioxidant Properties, Carbonic Anhydrase and Acetylcholinesterase Enzymes Inhibition Profiles. J. Biochem. Mol. Toxicol. 2018, 32(1), e22006. DOI: 10.1002/jbt.22006.
- Turan, B.; Sendil, K.; Sengul, E.; Gultekin, M. S.; Taslimi, P.; Gulcin, I.; Supuran, C. T. The Synthesis of Some β-lactams and Investigation of Their Metal Chelating Activity, Carbonic Anhydrase and Achetylcholinesterase Inhibition Profiles. J. Enzyme Inhib. Med. Chem. 2016, 31(S1), 79–88. DOI: 10.3109/14756366.2016.1170014.
- Özbey, F.; Taslimi, P.; Gulcin, I.; Maraş, A.; Goksu, S.; Supuran, C. T. Synthesis, Acetylcholinesterase, Butyrilcholinesterase, Carbonic Anhydrase Inhibitory and Metal Chelating Properties of Some Novel Diaryl Ether. J. Enzyme Inhib. Med. Chem. 2016, 31(S2), 79–85. DOI: 10.1080/14756366.2016.1189422.
- Garibov, E.; Taslimi, P.; Sujayev, A.; Bingöl, Z.; Çetinkaya, S.; Gulcin, I.; Beydemir, S.; Farzaliyev, V.; Alwasel, S. H.; Supuran, C. T. Synthesis of 4,5-Disubstituted-2-Thioxo-1,2,3,4-Tetrahydropyrimidines and Investigation of Their Acetylcholinesterase, Butyrylcholinesterase, Carbonicanhydrase I/II Inhibitory and Antioxidant Activities. J. Enzyme Inhib. Med. Chem. 2016, 31(S3), 1–9. DOI: 10.1080/14756366.2016.1198901.
- Aksu, K.; ÖZgeriş, B.; Taslimi, P.; Naderi, A.; Gulcin, I.; Göksu, S. Antioxidant Activity, Acetylcholinesterase and Carbonic Anhydrase Inhibitory Properties of Novel Ureas Derived from Phenethylamines. Arch. Pharm. 2016, 349(12), 944–954. DOI: 10.1002/ardp.201600183.
- Taslimi, P.; Sujayev, A.; Mamedova, S.; Kalın, P.; Gulcin, I.; Sadeghian, N.; Beydemir, S.; Küfrevioglu, Ö. İ.; Alwasel, S. H.; Farzaliyev, V.; et al. Synthesis and Bioactivity of Several New Hetaryl Sulfonamides. J. Enzyme Inhib. Med. Chem. 2017, 32(1), 137–145. DOI: 10.1080/14756366.2016.1238367.
- Bayrak, Ç.; Taslimi, P.; Gulcin, I.; Menzek, A. The First Synthesis of 4-Phenylbutenone Derivative Bromophenols Including Natural Products and Their Inhibition Profiles for Carbonic Anhydrase, Acetylcholinesterase and Butyrylcholinesterase Enzymes. Bioorg. Chem. 2017, 72, 359–366. DOI: 10.1016/j.bioorg.2017.03.001.
- Aktaş, A.; Taslimi, P.; Gulcin, I.; GöK, Y. Novel NHC Precursors: Synthesis, Characterization and Carbonic Anhydrase and Acetylcholinesterase Inhibitory Properties. Arch. Pharm. 2017, 350(6), e1700045. DOI: 10.1002/ardp.201700045.
- Zengin, M.; Genc, H.; Taslimi, P.; Kestane, A.; Guclu, E.; Ogutlu, A.; Karabay, O.; Gulcin, I. Novel Thymol Bearing Oxypropanolamine Derivatives as Potent Some Metabolic Enzyme inhibitors-Their Antidiabetic, Anticholinergic and Antibacterial Potentials. Bioorg. Chem. 2018, 81, 119–126. DOI: 10.1016/j.bioorg.2018.08.003.
- Huseynova, M.; Taslimi, P.; Medjidov, A.; Farzaliyev, V.; Aliyeva, M.; Gondolova, G.; Şahin, O.; Yalçın, B.; Sujayev, A.; Orman, E. B.; et al. Synthesis, Characterization, Crystal Structure, Electrochemical Studies and Biological Evaluation of Metal Complexes with Thiosemicarbazone of Glyoxylic Acid. Polyhedron. 2018, 155, 25–33. DOI: 10.1016/j.poly.2018.08.026.
- Turkan, F.; Cetin, A.; Taslimi, P.; Gulcin, I.; Some Pyrazoles Derivatives: Potent Carbonic Anhydrase, α-glycosidase and Cholinesterase Enzymes Inhibitors. Arch. Pharm. 2018, 351(10), e1800200. DOI: 10.1002/ardp.201800200.
- Biçer, A.; Taslimi, P.; Yakali, G.; Gulcin, I.; Gültekin, M. S.; Turgut Cin, G. Synthesis, Characterization, Crystal Structure of Novel Bis-Thiomethylcyclohexanone Derivatives and Their Inhibitory Properties against Some Metabolic Enzymes. Bioorg. Chem. 2019, 82, 393–404. DOI: 10.1016/j.bioorg.2018.11.001.
- Huseynova, M.; Medjidov, A.; Taslimi, P.; Aliyeva, M. Synthesis, Characterization, Crystal Structure of the Coordination Polymer Zn(II) with Thiosemicarbazone of Glyoxalic Acid and Their Inhibitory Properties against Some Metabolic Enzymes. Bioorg. Chem. 2019, 83, 55–62. DOI: 10.1016/j.bioorg.2018.10.012.
- Türker, F.; Barut Celepci, D.; Aktaş, A.; Taslimi, P.; Gök, Y.; Aygün, M.; Gulcin, I.; Meta-Cyanobenzyl Substituted Benzimidazole: Synthesis, Characterization, Crystal Structure and Carbonic Anhydrase, α-glycosidase, Butyrylcholinesterase, Acetylcholinesterase Inhibitory Properties. Arch. Pharm. 2018, 351(7), e201800029. DOI: 10.1002/ardp.201800029.
- Taslimi, P.; Çağlayan, C.; Farzaliyev, F.; Nabiyev, O.; Sujayev, A.; Türkan, F.; Kaya, R.; Gulcin, I.; Synthesis and Discovery of Potent Carbonic Anhydrase, Acetylcholinesterase, Butyrylcholinesterase and α- Glycosidase Enzymes Inhibitors: The Novel N,N′-bis-cyanomethylamine and Alkoxymethylamine Derivatives. J. Biochem. Mol. Toxicol. 2018, 32(4), e22042. DOI: 10.1002/jbt.22042.
- Xiao, Z.; Storms, R.; Tsang, A. A Quantitative Starch-Iodine Method for Measuring Alpha-Amylase and Glucoamylase Activities. Anal. Biochem. 2006, 351, 146–148. DOI: 10.1016/j.ab.2006.01.036.
- Taslimi, P.; Gulcin, I. Antidiabetic Potential: In Vitro Inhibition Effects of Some Natural Phenolic Compounds on α-glycosidase and α-amylase Enzymes. J. Biochem. Mol. Toxicol. 2017, 31(10), e21956. DOI: 10.1002/jbt.2017.31.issue-10.
- Ellman, G. L.; Courtney, K. D.; Andres, V.; Featherston, R. M. A New and Rapid Colorimetric Determination of Acetylcholinesterase Activity. Biochem. Pharmacol. 1961, 7, 88–95.
- Taslimi, P.; Sujayev, A.; Garibov, E.; Nazarov, N.; Huyut, Z.; Alwasel, S. H.; Gulcin, I. The Synthesis of New Cyclic Thioureas and Evaluation of Their Metal-Chelating Activity, Acetylcholinesterase, Butyrylcholinesterase and Carbonic Anhydrase Inhibition Profiles. J. Biochem. Mol. Toxicol. 2017, 31(7), e21897. DOI: 10.1002/jbt.21897.
- Öztaşkın, N.; Taslimi, P.; Maras, A.; Göksu, S.; Gulcin, I. Novel Antioxidant Bromophenols with Acetylcholinesterase, Butyrylcholinesterase and Carbonic Anhydrase Inhibitory Actions. Bioorg. Chem. 2017, 74, 104–114. DOI: 10.1016/j.bioorg.2017.07.010.
- Gulcin, I.; Mshvildadze, V.; Gepdiremen, A.; Elias, R. Antioxidant Activity of Saponins Isolated from Ivy: α-Hederin, hederasaponin-C, hederacolchiside-E and hederacolchiside-F. Planta. Med. 2004, 70, 561–563.
- Gulçin, İ.; Taslimi, P.; Aygün, A.; Sadeghian, N.; Bastem, E.; Kufrevioglu, O. I.; Turkan, F.; Şen, F. Antidiabetic and Antiparasitic Potentials: Inhibition Effects of Some Natural Antioxidant Compounds on α-glycosidase, α-amylase and Human Glutathione S-Transferase Enzymes. Int. J. Biol. Macromol. 2018, 119, 741–746. DOI: 10.1016/j.ijbiomac.2018.08.001.
- Gülçin, İ.; Beydemir, S.; Sat, İ. G.; Küfrevioğlu, Ö. İ. Evaluation of Antioxidant Activity of Cornelian Cherry (Cornus mas L.). Acta. Aliment. 2005, 34(2), 193–202. DOI: 10.1556/AAlim.34.2005.2.13.
- Elmastaş, M.; Gulcin, I.; Öztürk, L.; Gökçe, İ. Investigation of Antioxidant Properties of Spearmint (Mentha spicata L.). Asian J. Chem. 2005, 17(1), 137–148.
- Daryadel, S.; Atmaca, U.; Taslimi, P.; Gulcin, I.; Çelik, M. Novel Sulfamate Derivatives of Menthol: Synthesis, Characterization and Cholinesterases and Carbonic Anhydrase Enzymes Inhibition Properties. Arch. Pharm. 2018, 351(11), e1800209. DOI: 10.1002/ardp.201800209.
- Demir, Y.; Taslimi, P.; Ozaslan, M. S.; Oztaskın, N.; Çetinkaya, Y.; Gulcin, I.;Beydemir, Ş.; Göksu, S. Antidiabetic Potential: In Vitro Inhibition Effects of Bromophenol and Diarylmethanones Derivatives on Metabolic Enzymes. Arch. Pharm. 2018, 351(12), e1800263. DOI: 10.1002/ardp.201800263.
- Boztaş, M.; Çetinkaya, Y.; Topal, M.; Gulcin, I.; Menzek, A.; Şahin, E.; Tanc, M.; Supuran, C. T. Synthesis and Carbonic Anhydrase Isoenzymes I, II, IX, and XII Inhibitory Effects of Dimethoxy-Bromophenol Derivatives Incorporating Cyclopropane Moieties. J. Med. Chem. 2015, 58(2), 640–650. DOI: 10.1021/jm501573b.
- Göçer, H.; Akıncıoğlu, A.; Göksu, S.; Gulcin, I.; Supuran, C. T. Carbonic Anhydrase and Acetylcholine Esterase Inhibitory Effects of Carbamates and Sulfamoylcarbamates. J. Enzyme Inhib. Med. Chem. 2015, 30(2), 316–320. DOI: 10.3109/14756366.2014.928704.
- Gulcin, I.; Alici, H. A.; Cesur, M. Determination of in Vitro Antioxidant and Radical Scavenging Activities of Propofol. Chem. Pharm. Bull. 2005, 53(3), 281–285.
- Topal, M.; Gocer, H.; Topal, F.; Kalin, P.; Polat Köse, P.; Gülçin, İ.; Çakmak, K. C.; Küçük, M.; Durmaz, L.; Gören, A. C.; et al. Antioxidant, Antiradical and Anticholinergic Properties of Cynarin Purified from the Illyrian Thistle (Onopordum illyricum L.). J. Enzyme Inhib. Med. Chem. 2016, 31(2), 266–275. DOI: 10.3109/14756366.2015.1018244.
- Taslimi, P.; Aslan, H. E.; Demir, Y.; Oztaskin, N.; Maraş, A.; Gulçin, İ.; Beydemir, S.; Goksu, S. Diarilmethanon, Bromophenols and Diarilmetan Compounds: Discovery of Potent Aldose Reductase, α-Amylase and α-Glycosidase Inhibitors as New Therapeutic Approach in Diabetes and Functional Hyperglycemia. Int. J. Biol. Macromol. 2018, 119, 857–863. DOI: 10.1016/j.ijbiomac.2018.08.004.
- Akıncıoğlu, A.; Topal, M.; Gülçin, İ.; Göksu, S. Novel Sulfamides and Sulfonamides Incorporating Tetralin Scaffold as Carbonic Anhydrase and Acetylcholine Esterase Inhibitors. Arch. Pharm. 2014, 347(1), 68–76. DOI: 10.1002/ardp.201300273.
- Murray, A. P.; Faraoni, M. B.; Castro, M. J.; Alza, N. P.; Cavallaro, V. Natural AChE Inhibitors from Plants and Their Contribution to Alzheimer’s Disease Therapy. Curr. Neuropharmacol. 2013, 11(4), 388–413. DOI: 10.2174/1570159X11311040004.
- Şişecioğlu, M.; Gülçin, İ.; Çankaya, M.; Özdemir, H. The Inhibitory Effects of L-Adrenaline on Lactoperoxidase Enzyme (LPO) Purified from Buffalo Milk. Int. J. Food Propert. 2012, 15(6), 1190–1199. DOI: 10.1080/10942912.2010.511924.
- Aydin, B.; Gülcin, I.; Alwasel, S. H. Purification and Characterization of Polyphenol Oxidase from Hemşin Apple (Malus communis L.). Int. J. Food Propert. 2015, 18(12), 2735–2745. DOI: 10.1080/10942912.2015.1012725.
- Altay, A.; Koktepe, T.; Durmaz, L.; Topal, F.; Gülçin, İ.; Koksal, E. Purification and Selected Biochemical Properties of Peroxidase from Cress (Lepidium sativum Sub Sp. Sativum). Int. J. Food Propert. 2018, 21(1), 2610–2621. DOI: 10.1080/10942912.2018.1540989.
- Altın, S.; Tohma, H.; Gülçin, İ.; Köksal, E. Purification, Characterization and Inhibition Sensitivity of Peroxidase from Wheat (Triticum aestivum Ssp. Vulgare). Int. J. Food Propert. 2017, 20(09), 1949–1959. DOI: 10.1080/10942912.2016.1225308.
- Köktepe, T.; Altın, S.; Tohma, H.; Gulcin, I.; Koksal, E. Purification, Characterization and Some Inhibition Properties of Peroxidase from Haricot Bean (Phaseolus vulgaris L.). Int. J. Food. Propert. 2017, 20(S2), S1944–S1953.
- Köksal, Z.; Kalın, R.; Gulcin, I.; Özdemir, H. Inhibitory Effects of Selected Pesticides on Peroxidases Purified by Affinity Chromatography. Int. J. Food Propert. 2018, 21(1), 385–394. DOI: 10.1080/10942912.2018.1424197.
- Koksal, Z.; Kalın, R.; Gülçin, İ.; Özdemir, H.; Atasever, A. The Impact of Some Avermectins on Lactoperoxidase from Bovine Milk. Int. J. Food. Propert. 2016, 19(6), 1207–1216. DOI: 10.1080/10942912.2015.1076457.
- Gocer, H.; Topal, F.; Topal, M.; Küçük, M.; Teke, D.; Gülçin, İ.; Alwasel, S. H.; Supuran, C. T. Acetylcholinesterase and Carbonic Anhydrase Isoenzymes I and II Inhibition Profiles of Taxifolin. J. Enzyme Inhib. Med. Chem. 2016, 31(3), 441–447. DOI: 10.3109/14756366.2015.1036051.
- Taslimi, P.; Osmanova, S.; Gulcin, I.; Sardarova, S.; Farzaliyev, V.; Sujayev, A.; Kaya, R.; Koc, F.; Beydemir, S.; Alwasel, S. H.; et al. Discovery of Potent Carbonic Anhydrase, Acetylcholinesterase and Butyrylcholinesterase Enzymes Inhibitors: The New Amides and Thiazolidineones Synthesized on an Acetophenone Base. J. Biochem. Mol. Toxicol. 2017, 31(9), e21931. DOI: 10.1002/jbt.21931.
- Taslimi, P.; Akıncıoğlu, H.; Gulcin, I.; Synephrine and phenylephrine act as α-amylase, α-glycosidase, acetylcholinesterase, butyrylcholinesterase and carbonic anhydrase enzymes inhibitors. J. Biochem. Mol. Toxicol. 2017, 31(11), e21973. DOI: 10.1002/jbt.21973.
- Taslimi, P.; Caglayan, C.; Gulcin, I. The Impact of Some Natural Phenolic Compounds on Carbonic Anhydrase, Acetylcholinesterase, Butyrylcholinesterase, and α-Glycosidase Enzymes: An Antidiabetic, Anticholinergic, and Antiepileptic Study. J. Biochem. Mol. Toxicol. 2017, 31(12), e21995. DOI: 10.1002/jbt.21995.
- Burmaoglu, S.; Yilmaz, A. O.; Taslimi, P.; Algul, O.; Kılıç, D.; Gulcin, I.; Synthesis and Biological Evaluation of Phloroglucinol Derivatives Possessing α-Glycosidase, Acetylcholinesterase, Butyrylcholinesterase, Carbonic Anhydrase Inhibitory Activity. Arch. Pharm. 2018, 351(2), e1700314. DOI: 10.1002/ardp.201700314.
- Yamali, C.; Gül, H. İ.; Ece, A.; Taslimi, P.; Gulcin, I.;Synthesis, Molecular Modeling and Biological Evaluation of 4-(5-Aryl-3-(Thiophen-2-Yl)-4,5-Dihydro-1h-Pyrazol-1-Yl)Benzenesulfonamides Towards Acetylcholinesterase, Carbonic Anhydrase I and II Enzymes. Chem. Biol. Drug Des. 2018, 91(4), 854–866. DOI: 10.1111/cbdd.13149.
- Taslimi, P.; GulçIn, İ. Antioxidant and Anticholinergic Properties of Olivetol. J. Food Biochem. 2018, 42(3), e12516. DOI: 10.1111/jfbc.2018.42.issue-3.
- Gulcin, I.; Taslimi, P. Sulfonamide Inhibitors: A Patent Review 2013-Present. Exp. Opin. Ther. Pat. 2018, 28(7), 541–549. DOI: 10.1080/13543776.2018.1487400.
- Gondolova, G.; Taslimi, P.; Medjidov, A.; Farzaliyev, F.; Sujayev, A.; Huseuinova, M.; Şahin, O.; Yalçın, B.; Turkan, F.; Gulcin, I. Synthesis, Crystal Structure and Biological Evaluation of Spectroscopic Characterization of Ni(II) and Co(II) Complexes with N-salicyloil-N’-maleoil-hydrazine as Anticholinergic and Antidiabetic Agents. J. Biochem. Mol. Toxicol. 2018, 32(9), e22197. 250. DOI: 10.1002/jbt.22197.
- Maharramova, G.; Taslimi, P.; Sujayev, A.; Farzaliyev, F.; Durmaz, L.; Gulcin, I. Synthesis, Characterization, Antioxidant, Antidiabetic, Anticholinergic, and Antiepileptic Properties of Novel N-Substituted Tetrahydropyrimidines Based on Phenylthiourea. J. Biochem. Mol. Toxicol. 2018, 32(12), e22221. DOI: 10.1002/jbt.22221.
- Scozzafava, A.; Passaponti, M.; Supuran, C. T.; Gülçin, İ. Carbonic Anhydrase Inhibitors: Guaiacol and Catechol Derivatives Effectively Inhibit Certain Human Carbonic Anhydrase Isoenzymes (Hca I, II, IX, and XII). J. Enzyme Inhib. Med. Chem. 2015, 30(4), 586–591. DOI: 10.3109/14756366.2014.956310.
- Tao, Y.; Zhang, Y.; Cheng, Y.; Wang, Y. Rapid Screening and Identification of α‐glucosidase Inhibitors from Mulberry Leaves Using Enzyme‐Immobilized Magnetic Beads Coupled with HPLC/MS and NMR. Biomed. Chrom. 2013, 27, 148–155. DOI: 10.1002/bmc.2761.
- Torres-Naranjo, M.; Suarez, A.; Gilardoni, G.; Cartuche, L.; Flores, P.; Morocho, V. Chemical Constituents of Muehlenbeckia tamnifolia (Kunth) Meisn (Polygonaceae) and Its in Vitro α-Amilase and α-Glucosidase Inhibitory Activities. Molecules. 2016, 21, 1461–1465. DOI: 10.3390/molecules21111461.
- Teng, H.; Chen, L.; Fang, T.; Yuan, B.; Lin, Q. Rb2 Inhibits α-glucosidase and Regulates Glucose Metabolism by Activating AMPK Pathways in HepG2 Cells. J. Funct. Foods. 2017, 28, 306–313. 256. DOI: 10.1016/j.jff.2016.10.033.
- Yiğit, B.; Kaya, R.; Taslimi, P.; Işık, Y.; Karaman, M.; Yiğit, M.; Özdemir, İ.; Gulcin, I. Imidazolinium Chloride Salts Bearing Wing Tip Groups: Synthesis, Molecular Docking and Metabolic Enzymes Inhibition. J. Mol. Struct. 2019, 1179, 709–718. DOI: 10.1016/j.molstruc.2018.11.038.