ABSTRACT
The current project was planned for the extraction and characterization of the bioactive moieties from coriander seed (CS), black cumin seed (BCS), and fenugreek seed (FS) with special reference to their synergistic effect. Purposely, the solvent extraction method was applied by using the water and aqueous methanol (70:30 v/v) at constant temperature (40°C) for 7 h. For the estimation of antioxidant profile of resultant extracts, the indices such as total phenolic content (TPC), total flavonoid content (TFC), DPPH (1,1-diphenyl-2-picrylhydrazyl), β-carotene bleaching assay, FRAP (ferric reducing antioxidant power), and ABTS (2,2′-azino-bis,3-ethylbenzothiazoline-6-sulfonic acid) assay were adapted. Moreover, high performance liquid chromatography (HPLC) characterization of the extracts was also carried out for their active ingredients estimation. The results indicated that the combination of spices in some treatments exhibited more antioxidant activity as compared to their single one. The maximum TPC, TFC, DPPH, β-carotene bleaching assay, FRAP, and ABTS assay were exhibited by methanolic extract of T2 (BCS) as 292.5 ± 9.14 (mgGAE/100 g), 188.8 ± 5.69 (mgQE/100 g), 46.3 ± 0.32 (IC50 μg/ml), 76.96 ± 0.81 (%), 5.53 ± 0.08 (mg/TEg), and 67.18 ± 0.82 (μmolTE/g), respectively, followed by T4 (combination of CS and BCS) as 290.5 ± 8.54 (mgGAE/100 g), 184.2 ± 5.88 (mgQE/100 g), 48.2 ± 0.48 (IC50 μg/ml), 75.62 ± 0.87 (%), 5.49 ± 0.04 (mg/TEg), and 64.56 ± 0.46 (μmolTE/g) values for respective parameters, whilst least was observed in T6 (combination of BCS and FS) of respective antioxidant indices as 287.2 ± 9.57 (mgGAE/100 g), 175.9 ± 5.79 (mgQE/100 g), 50.7 ± 0.96 (IC50 μg/ml), 73.24 ± 0.59 (%), 5.40 ± 0.09 (mg/TEg), and 61.96 ± 0.58 (μmolTE/g). Moreover, HPLC characterization conforms to the presence of thymoquinone, diosgenin, and phenolic acids, including (chlorogenic acid, caffeic acid, and kaempferol) in BCS, FS, and CS, respectively. Conclusively, spices extraction was dependent upon the type of solvent and showed promising antioxidant potential not only alone but also in their combinations thus can be utilized for mitigating the oxidative-stress-related maladies.
Introduction
In dietary regimen, phytonutrients-based products/interventions have capacity to provide protection against the plethora of diseases owing to their capability of preventing oxidative stress. There are proven facts that elucidate the inverse association of metabolic syndromes and phytochemical consumption. Among the different tools of dietary intervention, polyphenols have attained paramount potential owing to their therapeutic potential. The outcomes of numerous studies have showed beneficial impact of polyphenol rich diet against different oxidative stress induced maladies owing to their free radical scavenging perspective.[Citation1] Flavonoids and phenolic compounds are widely distributed among the flora population and showed various biological effects, including anti-inflammatory, anti-carcinogenic activities, etc. The association between the antioxidative properties of food and health has recently been extensively investigated. Natural antioxidants are also in high demand for application as nutraceuticals/functional foods and biopharmaceuticals because of consumer preferences and their therapeutic effect.[Citation2] The beneficial role of plant-based commodities against lipid peroxidation has been established through many scientific studies; advocating the importance of a higher consumption of phytochemicals in the promotion of health.[Citation3] An organism may protect itself against free radicals with the help of various antioxidant defense mechanisms in the body, including both exogenous and endogenous sources. The examples for exogenous include β-carotene vitamin C, E, whereas catalase and glutathione peroxidase/reductase represent the endogenous sources.[Citation4] In recent era, the poor dietary habits and sedentary lifestyle trigger to the onset of oxidative stress that demands higher exogenous antioxidant consumption to ameliorate its deteriorative consequences.[Citation5] Several recent epidemiological studies depict significant positive correlation between the consumption of plant-derived foods and reduced incidences of diseases like aging, cancer, coronary heart disease, and Alzheimer’s disease.[Citation6] In this context, spices have gained paramount attention of scientific community as antioxidant-rich commodities owing to their popularity and versatile utilization. The spices lipid peroxidation diminishing perspective has been attributed to their rich phytochemistry dominated by polyphenols. Among the spices, BCS, FS, and CS are widely utilized not only for the culinary purposes but also as folk medicine against different ailments. The bioactive compounds from spices could be extracted and isolated through the solvent extraction method by using organic solvents or water.[Citation7] Several studies have revealed that the extracted phytochemicals have exhibited an extensive range of biological properties owing to their in vitro and in vivo antioxidant potential.[Citation8,Citation9] No doubt, extensive work has already been done on the spices extraction and characterization, but the research on their synergistic role has been lacking. This aspect has great industrial application as their combination is directly linked to their consumer acceptability. Moreover, in Pakistan the scientific research in this aspect on spices has not been widely explored. Therefore, the prime objective of current research is to investigate the effect of different solvents on the extraction efficiency of the spices polyphenols alone and in combination and their extensive antioxidant profiling through different indices. Furthermore, HPLC characterization was also carried out for the estimation of active ingredients.
Materials and methods
The present research project was carried out in the Institute of Home and Food Sciences at Government College University, Faisalabad. In this study, three spices were analyzed to determine their antioxidant profile. Materials to be used and protocols to be followed are described below.
Procurement of raw materials
Seeds of three different spices, i.e., coriander, black cumin, and fenugreek were procured from Vegetable Research Section, Ayub Agriculture Research Institute, Faisalabad. Various analytical and HPLC grade reagents and standards were purchased from Merck (Merck KGaA, Darmstadt, Germany) and Sigma-Aldrich (Sigma-Aldrich Tokyo, Japan).
Raw materials handling
The spices were washed thoroughly under running tap water to remove adhered dirt, dust, and other foreign debris. After washing, the seeds were dried at room temperature for few days. The dried materials were ground further to fine powder by using a small laboratory grinder (Panasonic, Japan, Model MJ-W176P) and passed through a sieve for further refining, and it was packed separately in air-tight plastic jars for further analysis.
Preparation of extracts
The seeds of coriander, black cumin, and fenugreek were ground and divided into separate and blended combinations as T1 (CS, T2 (BCS), T3 (FS), T4 (CS + BCS), T5 (CS + FS), T6 (BCS + FS), and T7 (CS + BCS + FS). Bioactive from the abovementioned spices and their blends were extracted with methanol:water (70:30 v/v) following a 1:10 sample-to-solvent ratio and water. The extracts were separated from solids by filtering through Whatman No. 1 filter paper. Afterward, extracts were concentrated under reduced pressure using rotary evaporator and were stored at 4°C until tested and analyzed.
Antioxidant indices profiling
The resultant extracts were analyzed for their antioxidant potential through various indices like total phenolics, total flavonoids, 1,1-diphenyl-2-picrylhydrazyl (DPPH)-free radical scavenging activities, 2,2′-azino-bis,3-ethylbenzothiazoline-6-sulfonic acid (ABTS) assay, ferric reducing antioxidant power (FRAP), and β-carotene bleaching assay by adapting the following protocols: Total polyphenols were estimated by using the Folin–Ciocalteu procedure following the method of Singleton et al.[Citation10] and absorbance was recorded at 765 nm and estimated as gallic acid equivalent (mg gallic acid/g). Total flavonoids were determined conferring to colorimetric method as described by Zou et al.[Citation11] and was assessed in quercetin equivalents per gram (mg quercetin/g); absorbance was determined at wavelength 510 nm. Similarly, DPPH of extracts was estimated following the method of Muller et al.[Citation12] The absorbance was measured at 520 nm. On the other hand, ABTS assay was measured following the protocol of Böhm et al.[Citation13] The absorbance was determined at 734 nm. However, FRAP was measured conferring to the method of Yuan et al.[Citation14] The absorbance was measured at 700 nm. During the analysis, an increase in the absorbance (A) of the reaction mixture indicated the reducing power. On the other hand antioxidant activity was measured through β-carotene and linoleic acid assay through UV/visible spectrophotometer (IRMECO, U2020) at wavelength of 470 nm.[Citation15] Antioxidant activity was measured with a UV/visible spectrophotometer (IRMECO, U2020).
HPLC quantification of bioactive compounds
Bioactive constituents (thymoquinone and diosgenin) and polyphenols (chlorogenic acid, caffeic acid, and kaempferol) in resultant extracts were estimated through HPLC. Purposely, for polyphenols HPLC (PerkinElmer, Series 200, USA) used in this analysis comprised SCL-10A system control unit, UV–visible detector (SPD-10AUV λmax 360 nm), Rheodyne injector, CTO-10A column oven, and LC-10 AS pumps by adapting the guidelines of Ref. [Citation16]. Thymoquinone quantification was performed on HPLC using chromatographic separations using a symmetry LC-18 stainless steel column (150 mm × 3.9 mm, 5 μm). An isocratic mobile phase consisted of a 20-mM KH2PO4 buffer (pH adjusted to 2.7 ± 0.05 using 50% orthophosphoric acid) and acetonitrile at a ratio of 60:40 eluted at a flow rate of 1 ml/min. Then HPLC quantification of diosgenin was performed using an HPLC instrument applying the standard method using a C18 column at 203 nm UV. Then the quantitative analysis for diosgenin concentration in fenugreek extract was calculated.
Results and discussion
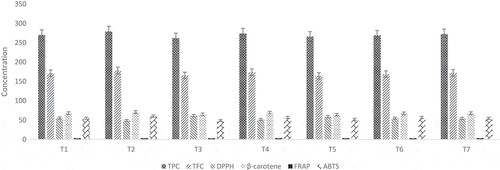
The resultant extraction probed from their bioactive constituent estimation and results expounded significant (p ≥ 0.001) variations among the different treatments for different antioxidant indices like total phenolic content (TPC), total flavonoid content (TFC), DPPH, β-carotene-linoleic acid assay, FRAP, and ABTS. Among the treatments, highest TPC was exhibited in T2 (BCS) 278.65 ± 8.2 mgGAE/100 g followed by T4 (CS + BCS) 273.65 ± 8.2 mgGAE/100 g, T7 (CS + BCS + FS) 271.6 ± 8.5 mgGAE/100 g, T1 (CS) 269.85 ± 8.6 mgGAE/100 g, T6 (BCS + FS) 268.5 ± 8.5 mgGAE/100 g, T5 (CS + FS) 265.5 ± 8.3 mgGAE/100 g, and minimum output in T3 (FS) 261.65 ± 8.9 mgGAE/100 g. However, among the solvents, methanolic extract showed highest TPC as 287.89 ± 8.6 mgGAE/100 g in comparison with water extract as 252 ± 8.8 mgGAE/100 g (). Likewise, a trend was observed for TFC, T2 showed highest contents 178.2 ± 4.5 mgQE/100 g trailed by T4, T7, T1, T6, T3, and T5 as 173.7 ± 5.6, 172.2 ± 4.5, 171.1 ± 3.3, 168.7 ± 5.7, 165.7 ± 6.9, and 164.4 ± 6.5 mgQE/100 g, respectively. A similar trend was noticed regarding solvents; methanol revealed maximum TFC 179.1 ± 5.2 mgQE/100 g followed by water 162 ± 5.3 mgQE/100 g ().
Table 1. Mean values for total phenolic contents (mgGAE/100 g), total flavonoid contents (mgQE/100 g) of water and aqueous methanolic extract of spices
DPPH activity observed in the extracts showed T1 (CS) 55.6 ± 0.5 IC50 μg/ml, T2 (BCS) 49.1 ± .0.2 IC50 μg/ml, T3 (FS) 61.5 ± 0.9 IC50 μg/ml, T4 (CS + BCS) 51.7 ± 0.4 IC50 μg/ml, T5 (CS + FS) 59.05 ± 0.8 IC50 μg/ml, T6 (BCS + FS) 55.05 ± 0.7 IC50 μg/ml, and T7 (CS + BCS + FS) 54.4 ± 0.4 IC50 μg/ml, whereas the recorded DPPH value for solvents was 58.9 ± 0.9 IC50 and 51.4 ± 0.7 IC50 μg/ml in water and methanol, respectively. β-carotene linoleic assay in showed the maximum value in T2 (BCS) 70.8 ± 0.4%, trailed by T4 (CS + BCS) 69.4 ± .0.6%, T1 (CS) 68.05 ± 0.6%, T7 (CS + BCS + FS) 67.9 ± 0.4%, T6 (BCS + FS) 67.7 ± 0.9%, T3 (FS) 65.02 ± 0.8 %, and T5 (CS + FS) 64.09 ± 0.7%, whereas water extract showed 61.2 ± 0.5% and methanol extract as 73.9 ± 0.9 for β-carotene linoleic assay ().
Table 2. Mean values for DPPH (IC50 μg/ml), β-carotene (%) of water and aqueous methanolic extract of spices
Mean FRAP and ABTS values for different spice treatments, i.e., T1 (CS extract), T2 (BCS extract), T3 (FS extract), T4 (CS + BCS extract), T5 (CS + FS extract), T6 (BCS + FS extract), and T7 (CS + BCS + FS extract) were 4.31 ± 0.03 mgTE/g and 54.9 ± 0.9 μmolTE/g, 4.41 ± 0.05 mgTE/g and 60.4 ± 0.7 μmolTE/g, 4.17 ± 0.04 mgTE/g and 49.2 ± 0.7 μmolTE/g, 4.35 ± 0.02 mgTE/g and 56.9 ± 0.9 μmolTE/g, 4.23 ± 0.06 mgTE/g and 52.2 ± 0.9 μmolTE/g, 4.28 ± 0.08 mgTE/g and 56.9 ± 0.5 μmolTE/g, and 4.32 ± 0.09 mgTE/g and 54.5 ± 0.7 μmolTE/g, respectively. Likewise, methanolic extract showed FRAP and ABTS values as 5.41 ± 0.09 mgTE/g and 61.6 ± 0.6 μmolTE/g, and water extract as 3.18 ± 0.06 mgTE/g and 48.4 ± 0.5 μmolTE/g, respectively (). Likewise, in treatments the order of effectiveness for TPC, TFC, FRAP, DPPH, β-carotene bleaching assay, and ABTS was BCS > CS + BCS > CS + BCS + FS > CS > BCS + FC > CS + FS > FS. It is further evident that the combination of some spices elucidated better results as compared to alone thus showing synergism (). The HPLC characterization of resultant extracts for their bioactive constitutes revealed highest (p ≥ 0.001) chlorogenic acid, caffeic acid, and kaempferol in T1 (CS extract) 139.6 ± 7.8, 83.73 ± 2.4, and 228.6 ± 9.7 μg/g, correspondingly. However, maximum thymoquinone was noticed in T2 (BCS) 5.13 ± 0.06 μg/g, and maximum disogenin was observed in T3 (FS) 27.72 ± 0.52 μg/g ().
Table 3. Mean values for FRAP (mg/TEg), ABTS (μmolTE/g) of water and aqueous methanolic extract of spices
Table 4. Mean values for chlorogenic acid, caffeic acid, kaempferol, thymoquinone and disogenin (μg/g) in different spices
Figure 1. Comparison of antioxidant potential of different treatments
The current study was unique in its role as it might be the first time to analyze the synergistic role of BCS, CS, and FS and probing the effect of organic and inorganic solvents for their extraction. The outcomes of the project showed a higher efficiency of methanol for antioxidant indices extraction as compared to water. Likewise, it was observed that all the tested materials showed promising antioxidant indices not in alone but also in their combination. The exceptional antioxidant profile of BCS and suitability of methanol for spices antioxidant extraction in current study are in harmony with the earlier work of Ghosh et al.[Citation17] who explicated TPC in FS, BCS, and CS in methanol extract and observed maximum in BCS (2.1 mgGAE/g) in comparison with FS (0.4 mgGAE/g) and CS (0.31 mgGAE/g). Later, Souri et al.[Citation18] also investigated different spices including FS and BCS for their antioxidant capacities. They found a higher phenolic content (194.63 mgGAE/100 g) in FS than that of BCS (122.67 mgGAE/100 g). The difference in TPC contents in the present investigation with earlier findings may be due to the variations in the variety, nature of the compound, type of solvent, and extraction time.[Citation19,Citation20] In contrary, AL-Mashkor[Citation21] analyzed TPC in FS extracted with 50% methanol, 70% methanol, and observed 22.2 and 18.5 mgGAE/100 g, respectively. The difference in polyphenolic yield from our findings might be due to the difference in the solvent-to-material ratio and the time of extraction.[Citation22] The findings of Deepa and Anuradha[Citation23] showed 12.2 GAE/g TPC in CS aqueous extract. The phenolic metabolism of spices may alter due to the presence of certain factors like the extraction techniques, differences in polarity of solvents as well as other environmental factors such as soil composition, sun exposure, and climate.[Citation24] Moreover, it may be due to the complexity of phenolic compounds composition and their chemical diversity in plant sources.
Likewise, Norziah et al.[Citation25] examined TFC in FS methanol, water, and hot water extracts and found maximum in methanolic samples as compared to water extracts. The effectiveness of methanol as a solvent over water for spices bioactive moieties is well reflected by the earlier work of Bukhari et al.[Citation26] who carried out the total flavonoids estimation from FS through water and methanol and observed higher activity in methanolic extracts. Likewise, Anita et al.[Citation27] investigated the CS methanolic extract for their antioxidant activity and noticed 608.903 μgQE/g TFC. Earlier, Deepa and Anuradha[Citation23] documented 12.6 QE/g TFC in CS aqueous extract. They deduced that difference is due to the polarity of solvents used. These variations in TFC are expected due to the variation in flavonoids solubility and the nature of solvent extraction.[Citation28] Moreover, Rice-Evans et al.[Citation29] and Brand-Williams et al.[Citation30] expressed that the antioxidant activity is largely uttered by their molecular structure. The estimation of the antioxidant activity of spice phenolic extracts is limited to an evaluation of the total antioxidant activity of the system.
The present findings regarding the promising free radical scavenging activity of spices are inline with the earlier work of Harron et al.[Citation31] who elucidated the antioxidant activity of methanolic extracts of five black cumin cultivars through DPPH assay and observed variations in this trait from 1.49 to 7.50 IC50 mg/ml. Likewise, El-Agbar et al.[Citation32] observed 168.8 IC50 μg/ml DPPH activity in black cumin methanolic extract. Afterwards, Gupta[Citation33] assessed DPPH inhibition of the CS and BCS methanolic extracts and observed 0.27% and 6.98%, respectively. The results attained verified the correlation between antioxidant activity and phenolic content, i.e., samples high in phenolic content also display high activity for DPPH and FRAP assays. Likewise, Singh et al.[Citation34] proposed that the antioxidant activity of BCS is equivalent to commercially available antioxidants like butylated hydroxytoluene, butylated hydroxyanisole, and propyl gallate. However, the extraction technique and nature of the solvent affect the DPPH scavenging activity of the spices.[Citation35] The outcomes of the present research are corroborated with the earlier work of Al-Zubaidy et al.[Citation36] who observed higher DPPH scavenging activity as 122 μg/ml IC50 in methanolic extract of FS. In contrary, Seasotiya et al.[Citation37] observed maximum DPPH activity in FS ethyl acetate extract (69.9%) followed by methanol (67.7%), chloroform (57.5%), and hexane (50.9%). It is also observed that extract at high concentration showed significant reduction in the absorbance of DPPH radicals.
Likewise, Harron et al.[Citation31] documented the β-carotene-linoleic acid activity in methanolic extracts of five different black cumin cultivars in the range of 63.99–96.95%. However, the findings of Souri et al.[Citation18] are not inline with our findings as they observed more antioxidant activity in FS 91.66 IC50 μg/ml as compared to BCS 146.86 IC50 μg/ml. Similarly, Sen et al.[Citation38] estimated 94.59% β-carotene activity in the methanol extract of BCS. Likewise, Sriti et al.[Citation39] observed the promising β-carotene bleaching activity of CS methanolic extract. Later, Subhasini et al.[Citation40] observed 0.202 IC50 mg/ml of β-carotene bleaching assay in FS ethanol extract. The β-carotene-linoleic acid assay is more appropriate to determine lipophilic antioxidants as lower inhibition activity was noticed in seed samples in comparison with oil samples. The seed samples contain both lipophilic and the hydrophilic compounds, whereas the oil samples only have lipophilic compounds. Hence, the hydrophilic antioxidants in the seed samples were not measured, thus resulting lower antioxidant activity. Both, the β-carotene-linoleic acid assay and DPPH radical-scavenging assay were explicated to assess the antioxidant activity of the spices, although both the assays were probed on different traits of the antioxidants. The β-carotene-linoleic acid assay concentrated on the inhibition of oxidation by free radicals, while the DPPH assay focused on the scavenging aspect of the antioxidants. So, one offers protection, while the other hunts.[Citation31]
The FRAP assay is based on the ability of antioxidants to reduce Fe3+ to Fe2+ in the presence of 2,4,6-tri(2-pyridyl)-s-triazine (TPTZ), forming an intense blue Fe2+-TPTZ.[Citation41] The recorded FRAP activities in the current research are inline with the earlier findings of Tubesha et al.[Citation42] who observed the FRAP value as 24 mgGAE/100 g in BCS. Likewise, Gupta et al.[Citation33] investigated CS and BCS methanolic extracts for their FRAP activity and observed 0.96 mMFeSO4 and 2.361 mMFeSO4, respectively. However, Toma et al.[Citation43] observed more FRAP value in the ethanol extract of BCS as compared to methanol. Moreover, Șen et al.[Citation38] observed dose-dependent FRAP reducing activity for different spices. Likewise, Deepa et al.[Citation44] investigated FRAP assay in CS and reported the maximum in methanol extract 7.53 mg/g than in the aqueous extract 6.9 mg/g. They deduced that FRAP activity is correlated to high phenolic and flavonoid compounds, namely, kaempferol, quercetin, and quercitrin. So, the spice extract could act as electron donors and may react with free radicals and convert them to stable products, hence terminating the radical chain reaction.[Citation45]
Similarly, ABTS•+ scavenging assay is an excellent method to determine the antioxidant activity of hydrogen donating and chain breaking antioxidants. The outcomes of recent investigation regarding the ABTS activity of spices are in accordance with the earlier study of Toma et al.[Citation43] who examined 77.7 IC50 μg/ml ABTS in ethanolic extract of BCS. Similarly, Goga et al.[Citation46] also observed promising ABTS activity exhibited by methanolic and ethanolic extracts of BCS. The better antioxidant perspective of BCS over other spices is well reflected through the findings of Ghosh et al.[Citation17] who compared the antioxidant activity of BCS, FS, and CS and noticed higher antioxidant potential of BCS as compared to other.
However, the outcomes of present investigation regarding ABTS are not inline with the findings of some earlier studies conducted by Gallo et al.[Citation47] and Kaviarasan et al.[Citation48] who observed a higher ABTS value in FS instead of BCS. The considerate factors for the variability may be extraction time and technique. They carried out ultrasound extraction instead of conventional solvent extraction.
The HPLC quantification showed that spices extract under the study hold promising bioactive molecules. The phenolic acids like hydroxycinnamic acids, caffeic acid, and chlorogenic acid are widely present in spices that are well documented in the findings of Zekovi et al.[Citation49] who recorded the appreciable amount of phenolic acids in BCS, FS, and cumin seed; however, the highest amount was observed in FS. Similarly, Benayad[Citation50] observed 0.45% and 11.22% of caffeic acid and of kaempferol, correspondingly in FS extracts. Moreover, Khole et al.[Citation51] also observed a higher amount of phenolic acid in FS extracts. One of the peers, El-Dakak[Citation52] carried out HPLC characterization of FS and noticed appreciable amount of chlorogenic acid and kaempferol. Thymoquinone is considered as the active constituent of BCS. The results regarding the presence of thymoquinone in BCS are in corroboration with the earlier work of Iqbal et al. and Alam et al.[Citation53,Citation54] who observed a higher amount of thymoquinone in methanolic extract of BCS as compared to water. Later, Fahmi[Citation55] reported thymoquinone in the range of 0.176–0.929 mg/ml in BCS. Diosgenin is a spirostanol saponin comprising a hydrophobic steroid aglycone linked to a hydrophilic sugar moiety and is analogous to cholesterol and other steroids. Subsequently after disogenin discovery, a major sapogenins present in FS and the single key precursor were used in the production of synthetic steroids in the pharmaceutical industry.[Citation56] Diosgenin in FS were estimated through HPLC and found 29.65 μg/ml and 5.087 mg/g, respectively.[Citation57,Citation58]
Conclusion
Decisively, all the spices under study depicted promising antioxidant potential not only alone but also in combination. Among the solvents, methanol showed a better performance as compared to water, whereas, amongst the treatments, BCS-based samples exhibited a better antioxidant profile in comparison with CS and FS. However, it is recommended that in future effect of supercritical fluid extraction and ultra sound extraction techniques should be evaluated.
Acknowledgments
The contribution of each author for this paper was as follows: SH and AI designed the experimental study for exploring the effect of spices using the animal model study. SH and AI also carried out the trials and collected data, and SH, MUA, MN, MSA, and FS also drafted the manuscript. It is evident that all the authors read and approved the final manuscript.
Additional information
Funding
References
- Singh, B. N.; Singh, B. R.; Singh, R. L.; Prakesh, D.; Sarma, B. K.; Singh, H. B. Antioxidant Antiquorum Sensing Activities of Green Pod of Acacia Nilotica L. Food. Chem. Toxicol. 2009, 47, 778–786. DOI: 10.1016/j.fct.2009.01.009.
- Aggarwal, K. B.; Ranjan, J. K.; Rathore, S. S.; Saxena, S. N.; Mishra, B. K. Changes in Physical and Biochemical Properties of Fenugreek (Trigonella Sp. L.) Leaf during Different Growth Stages. Int. J. Seed Spices. 2013, 3(1), 31–35.
- Enayde, D. A.; Lima, V.; Maciel, M. Polyphenol, Ascorbic Acid and Total Carotenoids Content in Common Fruit and Vegetables. Brazilian J. Of Food Tech. 2006, 9(2), 89–96.
- Singh, R. P.; Sharad, S.; Kapur, S. Free Radicals and Oxidative Stress in Neurodegenerative Diseases: Relevance of Dietary Antioxidants. J. Ind. Acad. Clinic. Medi. 2004, 5(3), 218–225.
- Singh, P.; Singh, U.; Shukla, M.; Singh, R. L. Antioxidant Activity Imparting Bio-Molecules in Cassia Fistula. J. Adv. Life Sci. 2008, 2(3–4), 23–28.
- Tangkanakul, P.; Trakoontivakorn, G.; Auttaviboonkul, P.; Niyomvit, B.; Wongkrajang, K. Antioxidant Activity of Northern and North-Eastern Thai Foods Containing Indigenous Vegetables. Kasetsart J. Nat. Sci. 2006, 40, 47–58.
- Srinivasan, K.;. Antioxidant Potential of Spices and Their Active Constituents. Crit. Rev. Food Sci. Nutr. 2014, 54, 352–372. DOI: 10.1080/10408398.2011.585525.
- Khoobchandani, M.; Ojeswi, B. K.; Ganesh, N.; Srivastava, M. M.; Gabbanini, S.; Matera, R.; Iori, R.; Valgimigli, L. Antimicrobial Properties and Analytical Profile of Traditional Eruca Sativa Seed Oil: Comparison with Various Aerial and Root Plant Extracts. Food Chem. 2010, 120, 217–224. DOI: 10.1016/j.foodchem.2009.10.011.
- Goncalves, S.; Gomes, D.; Costa, P.; Romano, A. The Phenolic Content and Antioxidant Activity of Infusions from Mediterranean Medicinal Plants. Ind. Crops. Prod. 2013, 43, 465–471. DOI: 10.1016/j.indcrop.2012.07.066.
- Singleton, V. L.; Orthofer, R.; Lamuela-Raventos, R. M. Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin- Ciocalteu Reagent. Meth. Enzy. 1999, 299, 152–178.
- Zou, Y.; Lu, Y.; Wei, D. Antioxidant Activity of Flavonoid-Rich Extract of Hypericum Perforatum L in Vitro. J. Agri. Food Chem. 2004, 52, 5032–5039. DOI: 10.1021/jf049571r.
- Muller, L.; Frohlich, K.; Bohm, V. Comparative Antioxidant Activities of Carotenoids Measured by Ferric Reducing Antioxidant Power (FRAP), ABTS Bleaching Assay (αTEAC), DPPH Assay and Peroxyl Radical Scavenging Assay. Food Chem. 2011, 129, 139–148. DOI: 10.1016/j.foodchem.2011.04.045.
- Böhm, V.; Puspitasari-Nienaber, N. L.; Ferruzzi, M. G.; Schwartz, S. J. Trolox Equivalent Antioxidant Capacity of Different Geometrical Isomers of A-Carotene, Bcarotene, Lycopene and Zeaxanthin. J. Agri. Food Chem. 2002, 50, 221–226. DOI: 10.1021/jf010888q.
- Yuan, Y.; Velev, O. D.; Lenhoff, A. M. Mobility of Adsorbed Proteins Studied by Fluorescence Recovery after Photobleaching. Langmuir. 2003, 19(9), 3705–3711. DOI: 10.1021/la026368m.
- Taga, M. S.; Miller, E. E.; Pratt, D. E. China Seeds as Source of Natural Lipid Antioxidation. J. Am. Oil Chem. Soc. 1984, 61, 928–931. DOI: 10.1007/BF02542169.
- Ok-Kim, D.; Lee, C. Y. HPLC Separation of Polyphenolics. Current Protoc. Food Anal. Chem. 2002, 3, 1.
- Ghosh, M.; Das, K.; Dhar, T. M. A Comparative Study of the Antioxidative Properties of the Different Seed Spices Available in India. J. Adv. Pharm. Edu. Res. 2015, 5, 1.
- Souri, E.; Amin, G.; Farsam, H.; Tehrani, B. M. Screening of Antioxidant Activity and Phenolic Content of 24 Medicinal Plant Extracts. DARU. 2008, 16(2), 83–87.
- Wijekoon, M. M. J. O.; Bhat, R.; Karim, A. A. Effect of Extraction Solvents on the Phenolic Compounds and Antioxidant Activities of Bunga Kantan (Etlingera Elatior Jack) Inflorescence. J. Food Comp. Anal. 2011, 24(4), 615–619. DOI: 10.1016/j.jfca.2010.09.018.
- Kowalczyk, D.; Swieca, M.; Cichocka, J.; Gawlik- Dziki, U. The Phenolic Content and Antioxidant Activity of the Aqueous and Hydroalcoholic Extracts of Hops and Their Pellets. J. Inst. Brewing. 2013, 119(3), 103–110.
- AL-Mashkor, I. M. A.;. Phenolic Content and Antioxidant Activity of Fenugreek Seeds Extract. Int. J. Pharma. Phytochem. Res. 2014, 6(4), 841–844.
- Rostagno, M. A.; Palma, M.; Barroso, C. G. Ultrasound-Assisted Extraction of Soy Isoflavones. J. Chromato. A. 2003, 1012(2), 119–128. DOI: 10.1016/S0021-9673(03)01184-1.
- Deepa, B.; Anuradha, C. V. Antioxidant Potential of Coriandrum Sativum L. Seed Extract. Ind. J. Experi. Bio. 2011, 49, 30–38.
- Djeridane, A.; Yousfi, M.; Nadjemi, B.; Boutassouna, D.; Stocker, P.; Vidal, N. Antioxidant Activity of Some Algerian Medicinal Plants Extracts Containing Phenolic Compounds. Food Chem. 2006, 97(4), 654–660. DOI: 10.1016/j.foodchem.2005.04.028.
- Norziah, M. H.; Fezea, F. A.; Bhat, R.; Ahmad, M. Effect of Extraction Solvents on Antioxidant and Antimicrobial Properties of Fenugreek Seeds (Trigonella Foenum-Graecum L.). Int. Food Res. J. 2015, 22(3), 1261–1271.
- Bukhari, S. B.; Bhanger, M. I.; Memon, S. Antioxidative Activity of Extracts from Fenugreek Seeds (Trigonella Foenum-Graecum). Pak. J. Anal. Environ. Chem. 2008, 9(2), 78–83.
- Anita, D.; Sharad, A.; Amanjot, K.; Ritu, M. Antioxidant Profile of Coriandrium Sativum Methanolic Extract. Int. Res J. Pharm. 2014, 5, 3.
- Chebil, L.; Humeau, C.; Anthoni, J.; Dehez, F.; Engasser, J. M.; Ghoul, M. Solubility of Flavonoids in Organic Solvents. J. Chem. Engin. Data. 2007, 52, 1552–1556. DOI: 10.1021/je7001094.
- Rice-Evans, C.; Miller, N.; Paganga, G. Structure-Antioxidant Activity Relationships of Flavonoids and Phenolic Acids. Free Radical Bio. Med. 1996, 20, 933–956. DOI: 10.1016/0891-5849(95)02227-9.
- Brand-Williams, W.; Cuvelier, M. E.; Berset, C. Use of Free Radical Method to Evaluate Antioxidant Activity. Lebensmittel Wissenschaft und Technologie. 1995, 28, 25–30. DOI: 10.1016/S0023-6438(95)80008-5.
- Harron, H.; Grace-Lynn, C.; Shahar, S. Comparison of Physicochemical Analysis and Antioxidant Activities of Nigella Sativa Seeds and Oils from Yemen, Iran and Malaysia. Sains Malays. 2014, 43(4), 535–542.
- El-Agbar, Z. A.; Shakya, A. K.; Khalaf, N. A.; Al-Haroon, M. Comparative Antioxidant Activity of Some Edible Plants. Turk. J. Biol. 2008, 32, 193–196.
- Gupta, V.;. Hypolipidemia Effect of Fenugreek and Garlic on Experimentally Induced Hyperlipidemia in Rabbits: A Randamozed Control Trial. Int. J. Basic App. Physio. IJBAP. 2013, 2, 193.
- Singh, G.; Marimuthu, P.; Heluani, D. C. S.; Catalan, C. Chemical Constituents and Antimicrobial and Antioxidant Potentials of Essential Oils and Acetone Extract of Nigella Sativa Seeds. J. Sci Food. Agri. 2005, 85(13), 2297–2306. DOI: 10.1002/jsfa.2255.
- Anjum, F.; Shahid, M.; Bukhari, S. A.; Anwar, S.; Latif, S. Study of Quality Characteristics and Efficacy of Extraction Solvent/Technique on the Antioxidant Activity of Bitter Gourd Seed. J. Food Process. Technol. 2013, 4(2), 1–8.
- Al-Zubaidy, A. A.; Sahib, H. B.; Sadiq, M. H. Anti-Angiogenig Activity of Fenugreek Extracts: In Vivo and Ex Vivo Study. Int. J. Pharm. Sci. Rev. Res. 2016, 36(2), 184–189.
- Seasotiya, L.; Siwach, P.; Bai, S.; Malik, A.; Dalal, S. Free Radical Scavenging Activity, Phenolic Contents and Phytochemical Analysis of Seeds of Trigonella Foenum Graecum. Asian Pac. J. Health Sci. 2014, 1(3), 219–226.
- Șen, N.; Kar, Y.; Tekeli, I. Antioxidant Activities of Black Cumin (Nigella Sativa L.) Seeds Cultivating in Different Regions of Turkey. J. Food Biochem. 2010, 34, 105–119. DOI: 10.1111/j.1745-4514.2009.00309.x.
- Sriti, J.; Wannes, W. A.; Talou, T.; Jemia, M. B.; Kchouk, M. E.; Marzouk, B. Antioxidant Properties and Polyphenol Contents of Different Parts of Coriander (Coriandrum Sativum L.) Fruit. Rivista Italiana Delle Sostanze Grasse. 2012, LXXXIX, 253–261.
- Subhasini, N.; Thangathirupathi, A.; Lavanya, N. Antioxidant Activity of Trigonella Foenum Graecum Using Various in Vitro and Ex Vivo Models. Int. J. Pharm. Pharm. Sci. 2011, 3(2), 96–102.
- Benzie, I. F. F.; Strain, J. J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Analytic. Biochem. 1996, 239, 70–76. DOI: 10.1006/abio.1996.0292.
- Tubesha, Z.; Iqbal, S.; Ismail, M. Effects of Hydrolysis Conditions on Recovery of Antioxidants from Methanolic Extracts of Nigella Sativa Seeds. J. Medi. Plants Res. 2011, 5(22), 5393–5399.
- Toma, C.; Olah, N.; Vlase, L.; Mogoșan, C.; Mocan, A. Comparative Studies on Polyphenolic Composition, Antioxidant and Diuretic Effects of Nigella Sativa L. (Black Cumin) and Nigella Damascena L. (Lady-In-A-Mist) Seeds. Molecules. 2015, 20, 9560–9574. DOI: 10.3390/molecules20069560.
- Deepa, G.; Ayesha, S.; Nishtha, K.; Thankamani, M. Comparative Evaluation of Various Total Antioxidant Capacity Assays Applied to Phytochemical Compounds of Indian Culinary Spices. Food Res. J. 2013, 20(4), 1711–1716.
- Oktay, M.; Culcin, I.; Kufrevioglu, O. I. Determination of in Vitro Antioxidant Activity of Fennel (Foenniculum Vulgare) Seed Extracts. Lebensm. Wiss. Technol. 2003, 36, 263–271.
- Goga, A.; Hasić, S.; Bećirović, Š.; Ćavar, S. S. Phenolic Compounds and Antioxidant Activity of Extracts of Nigella Sativa L. Bullet. Chem. Technol. Bosnia Herzegovina. 2012, 39, 15–20.
- Gallo, M.; Ferracane, R.; Graziani, G.; Ritieni, A.; Fogliano, V. Microwave Assisted Extraction of Phenolic Compounds from Four Different Spices. Molecules. 2010, 15, 6365–6374. DOI: 10.3390/molecules15096365.
- Kaviarasan, S.; Naik, G. H.; Gangabhagirathi, R.; Anuradha, C. V.; Priyadarsini, K. I. In Vitro Studies on Antiradical and Antioxidant Activities of Fenugreek (Trigonella Foenum Graecum) Seeds. Food Chem. 2007, 103(1), 31–37. DOI: 10.1016/j.foodchem.2006.05.064.
- Zekovi´C, Z.; Buˇsi´C, A.; Komes, D.; Vladi´C, J.; Adamovi´C, D.; Pavli´C, B. Coriander Seeds Processing: Sequential Extraction of Non-Polar and Polar Fractions Using Supercritical Carbon Dioxide Extraction and Ultrasound-Assisted Extraction. Food Bio. Prod. Process. 2015, 95, 218–227. DOI: 10.1016/j.fbp.2015.05.012.
- Benayad, Z.; Go´mez-Cordove´s, C.; Es-Safi, N. E. Identification and Quantification of Flavonoid Glycosides from Fenugreek (Trigonella Foenum-Graecum) Germinated Seeds by LC_DAD_ ESI/MS Analysis. J. Food Compos. Anal. 2014, 35(1), 21–29. DOI: 10.1016/j.jfca.2014.04.002.
- Khole, S.; Chatterjee, S.; Variyar, P.; Sharma, A.; Devasagayam, T. P. A. Bioactive Constituents of Germinated Fenugreek Seeds with Strong Antioxidant Potential. J. Function. Foods. 2014, 6, 270–279. DOI: 10.1016/j.jff.2013.10.016.
- El-Dakak, A. M. N. H.; El-Rahman, H. S. M. A.; El-Nahal, D. M. M. Comparison Studies between Aqueous Lepidium Sativum L., Lupinus Albus and Trigonella Foenum- Gracum Seeds, and Their Mixture Extracts on Streptozotocin-Induced Diabetic Rats. J. Appl. Sci. Res. 2013, 9(4), 2965–2982.
- Iqbal, M.; Alam, P.; Anwer, M. T. High Performance Liquid Chromatographic Method with Fluorescence Detection for the Estimation of Thymoquinone in Nigella Sativa Extracts and Marketed Formulations. Open Access Sci. Rep. 2013, 2, 2.
- Alam, P.; Yusufoglu, H.; Alam, A. HPTLC Densitometric Method for Analysis of Thymoquinone in Nigella Sativa Extracts and Marketed Formulations. Asian Pac. J. Trop. Dis. 2013, 3(6), 467–471. DOI: 10.1016/S2222-1808(13)60102-4.
- Fahmi, A.; Ismail, H.; Doolaanea, A. A.; Mohamed, F.; Mansor, N. I.; Shafri, M. A. M.; Yusof, F. A.; Development, M. Validation Using UV Spectrophotometry for Nigella Sativa Oil Microparticles Quantification. J. App. Pharma. Sci. 2015, 5(09), 082–088.
- Raju, J.; Rao, C. V. Diosgenin, a Steroid Saponin Constituent of Yams and Fenugreek: Emerging Evidence for Applications in Medicine. In Bioactive Compounds in Phytomedicine, IntechOpen. 2012; pp 125–142.
- Kaid, A. N. A.; Norbaiyah, M. B.; Imad, M. A.; Norazian, M. H. Quantification of Anti-Fertility compound-Diosgenin Concentration in the Fenugreek Seeds Aqueous Extract (FSA). The International Medical Journal Malaysia. 2016, 15(1), 75–80.
- Laila, O.; Murtaza, I.; Abdin, M. Z.; Ahmad, S.; Ganai, N. A.; Jehangir, M. Development and Validation of HPTLC Method for Simultaneous Estimation of Diosgenin and Quercetin in Fenugreek Seeds (Trigonella Foenum-Graceum). ISRN Chromatogr. 2014, 583047, 1–8. DOI: 10.1155/2014/583047.
