?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Inonotus hispidus (Bull.) P. Karst. has been used as traditional medicine for the treatment of dyspepsia, cancer, and diabetes. Numerous studies have confirmed the antimicrobial, antiviral, antioxidant, anti-inflammatory, immunomodulatory, antiproliferative and cytotoxic biological activities of extracts from this species. The purpose of this study was a comparative analysis of the antioxidant and the antimicrobial activities of methanol extracts from fruit and liquid-cultured mycelia. Four compounds (N-butylbenzenesulfonamide, lauramidopropyl betaine, 3,5-di-tert-butyl-4-hydroxybenzaldehyde, and uplandicine), determined by hybrid HRMS, were found only in mycelia culture extracts. Free radical scavenging, measured by DPPH assay on methanol extracts, showed an activity of about 17.2% and 22.1% of Trolox in fruiting bodies and mycelia, respectively. The I. hispidus methanol extracts from fruit and mycelia culture were found to have varying degrees of antibacterial and antifungal effects against the pathogenic microorganisms tested (minimum inhibitory concentration from 0.17 to 2.56 μg mL−1).
Introduction
Fungi belong to one of the most diverse and important groups of living organisms on earth, not only because of their vital roles in ecosystem functions but also for their nutritional and therapeutic values.[Citation1–Citation5] In fact, fungi produce a variety of secondary metabolites, low-molecular-weight compounds associated with many medical useful biologic activities.[Citation6,Citation7]
Inonotus hispidus (Bull.) P. Karst. (Hymenochaetaceae Donk), commonly known as shaggy bracket, is a necrotrophic species usually occurring on living trees. In the first stage of degradation, I. hispidus behaves as a typical white rot agent by attacking the middle lamella, whereas in the later stage it displays typical soft rot patterns of degradation.[Citation8,Citation9] This species lives preferentially on a variety of deciduous trees such as Quercus cerris L., Q. ilex L., Fraxinus excelsior Thunb., Aesculus hippocastanum L., Malus spp., Morus spp., Pyrus spp., Sophora spp., Prunus spp., Platanus spp.[Citation10] I. hispidus has been used in folk medicine for treating gastric ulcers and it is known for its antiviral activity.[Citation11,Citation12] These functional characteristics are derived from the mushroom chemical composition. Previous studies of this species have reported the presence of hispolon and hispidin, inonotusin A and B, (E)-4-(3,4-dihydroxyphenyl)but-3-en-2-one, hispidin, 3,4-dihydroxybenzaldehyde, hispindic acids A and B, melanin and polysaccharides with a high content of glucose, mannose, and galactose.[Citation13–Citation17]
Extracts from this fungus have exhibited biological activities, including antimicrobial,[Citation18] antiviral,[Citation12] antioxidant,[Citation19] anti-inflammatory,[Citation14] immunomodulatory,[Citation20] antiproliferative,[Citation13] and cytotoxic activities.[Citation21] According to Smolskaite et al.,[Citation18] positive results in antimicrobial activity were obtained when I. hispidus was analyzed versus gram-positive bacteria (Bacillus cereus), gram-negative bacteria (Pseudomonas aeruginosa) and fungi (Candida albicans) by means of agar diffusion method. In the same work, the antioxidant capacity based on methanol extracts was compared in I. hispidus and other fungal species. Interestingly, I. hispidus reported considerably higher antioxidant activities.[Citation18] The inhibitory effect of phenolic compounds and alkaloids of I. hispidus on Candida rugosa lipase were investigated by Benarous et al.[Citation22] The same authors also reported stronger antiradical activity by the phenolic extracts than the alkaloids extracts. Consistently, strong antioxidant activity was demonstrated for the main phenolic metabolites (hispolon, hispidin and its derivatives named inonotusine A and B) from fruiting bodies of I. hispidus.[Citation11,Citation14]
Inonotusin A also showed moderate cytotoxicity against a human breast carcinoma cell line (MCF-7) with IC50 values of 19.6 mM.[Citation14] In screening for immunomodulatory and antiviral agents from some Basidiomycota, inhibitory activity was detected in the ethanolic extracts of fruiting bodies of I. hispidus.[Citation20] Ethanolic extracts of fruiting bodies and mycelial cultures from I. hispidus showed interesting antiviral activity against influenza virus type A and B.[Citation12] Another documentated effect of a compound isolated from the fruiting bodies, hispidinic acid A, is the greater capability to increase melanogenesis and tyrosinase activity to B16 melanoma cells than those of positive control, 8-methoxypsoralen.[Citation15]
Despite the mentioned evidence of antibacterial and antifungal activity, very scarce literature has to date specifically focused on the antimicrobial potential of I. hispidus extracts. Analogously, the chemical composition of this species is mostly unexplored both in the fruiting bodies and mycelia extracts, except for a few phenolic and terpenoid compounds.[Citation15] Use of mycelia cultures of macrofungi for experimental and medical purpose is a potential biotechnological method to obtain an efficient production of secondary metabolites with biological activities. The main advantage of mycelia cultures is their independence from environmental conditions and their ability to continuously produce high-quality material.[Citation23,Citation24] Accordingly, the main purposes of this study were to evaluate and to compare the in vitro antimicrobial and antioxidant potential of fruiting bodies and mycelia extracts of I. hispidus.
Materials and methods
Chemical
1,1-Diphenyl-2-picryl-hydrazyl-hydrate (DPPH), Folin-Ciocalteu reagent, Gallic acid, ß-Carotene, Linoleic acid, Trolox, 2,3,5-Triphenyl-Tetrazolium Chloride (TTC), Butylated Hydroxytoluene (BHT), Mueller-Hinton broth (MHB), Rose Bengal Chloramphenicol Agar (RBCA), Malt Extract Agar (MEA), Sabouraud Dextrose Agar (SDA), RPMI (Roswell Park Memorial Institute) 1640 medium, Morpholinepropanesulphonic acid (MOPS), purity grade organic solvents (Methanol, Chloroform, Dimethyl Sulfoxide), Ciprofloxacin and Fluconazole were purchased from Sigma (Sigma-Aldrich GmbH, Germany).
Mushroom material
The fruiting bodies of the strain I. hispidus PeruMyc_2361 were collected in Ohrid (Former Yugoslavian Republic of Macedonia) in October 2017. After a few days, it was transported to the laboratory of Mycology of the Department of Chemistry, Biology and Biotechnology (DCBB) of Perugia University for identification and further characterization of its main biological activities. In order to isolate mycelium in pure culture, small pieces of context (about 10 mm3) were aseptically drawn from the fruiting bodies and inoculated into Rose Bengal Chloramphenicol agar.[Citation25] Incubation was performed into the dark at 25°C for 14 days.[Citation25] Subsequently, it was regularly sub-cultured on Malt Extract Agar and voucher cultures are kept in the PeruMyc collection of the DCBB.
Morphological identification
Collected specimens were primordia, thus they had not yet developed all the specific macro and micro-morphological features which are usually reported for mature fruiting bodies. Nevertheless, morphological identification was performed by referring to Bernicchia[Citation10] and Ryvarden et Melo[Citation26] dichotomous keys. Zeiss-Axioplan was exploited for microscopy.
Molecular identification
Fifteen days old mycelium grown in ME 2% was used for DNA extraction. Drawn mycelium was washed with sterile, de-ionized water and lyophilized by freeze drier. Total genomic DNA was extracted from freeze-dried samples using a Nucleospin Plant II DNA extraction kit (Macherey Nagel). Genomic DNA was visualized in 0.8% agarose gel in 1x TAE buffer (40 mM Tris, 20 mM acetic acid, 1 mM EDTA, pH 8) with SYBR Safe DNA gel stain (Thermo Fisher) and visualized with Molecular Imager® Gel DocTM XR+ – BIO-RAD. Nanodrop 2000 UV (Thermo Fisher, USA) was used to determine concentration and purity of total extracted DNA; DNA samples were consequently diluted up to 10 µg/µL by nuclease-free water before PCR amplification. PCR amplification was carried out by using ITS1 and ITS4 primers to amplify the ITS1, 5.8S, and ITS2 rDNA regions.[Citation27,Citation28] The mixture for PCR amplification contained 1x Dream Taq Green PCR Master Mix (ThermoScientific), 5 µM ITS1 and 5 µM ITS4 primers and H2O nuclease-free water (Ambion) to volume. Two microliters of diluted DNA were added per sample. Thermal Cycler T100 – BIO-RAD was programmed as follows: one cycle of denaturation at 95°C for 2 min; 34 cycles of denaturation at 95°C for 30 s, annealing at 55°C for 1 min and extension at 72°C for 1 min; one final extension cycle at 72°C for 5 min. Electrophoresis of PCR amplicons was carried out on 1.2% agarose gel as above described.
PCR products were purified by means of Clean Sweep PCR Purification Reagent (Applied Biosystems) and then sequenced by Macrogen (Spain). SEQUENCHER 5.0 was used to adjust the sequences. Matching with sequences in repositories was carried out by MycoBank (CBS) pairwise alignment tool by comparing several reference databases at once (www.mycobank.org). Final results for identification were critically discussed.
Sequence alignment and phylogenetic analysis
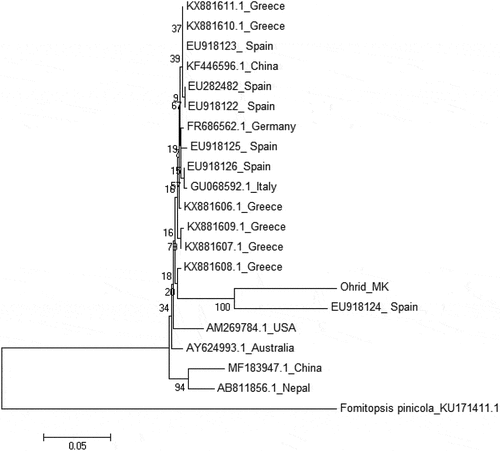
Besides the strain under examination in the present study (PeruMyc_2361), alignment included 20 ITS sequences retrieved from GenBank (https://www.ncbi.nlm.nih.gov/nucleotide/) and cross-checked on Mycobank (http://www.mycobank.org). Only the sequences recognized as belonging to I. hispidus by Mycobank molecular ID were adopted. The geographic origin of the samples was inferred based on the information provided by the respective authors on GenBank. Fomitopsis pinicola (Sw.) P. Karst. KU171411.1 was used as outgroup. CLUSTAL W was used for pairwise and multiple sequence alignment.[Citation29] The aligned sequences were treated on MEGA7 by Neighbor-Joining (NJ) analysis Tamura-Nei Parameter Distance model, where gaps were treated as missing data. Bootstrapping was performed with NJ analysis using 1000 replicates.[Citation30]
Preparation of fungal methanol extracts
Two methanol extracts were obtained from the fungal isolate I. hispidus. The first one was achieved by direct extraction of a portion (100 g) of the fungal fruiting bodies with a single aliquot (500 mL) of methanol at 25°C for two weeks [the extraction was considered completed when the dry weight (daily withdrawal) remained constant]. The fruiting bodies have been previously shade-dried for four weeks and then ground with mortar and pestle prior to undergoing solvent extraction. The second extract was prepared from I. hispidus mycelium. Free mycelium was obtained by culturing the isolated strain in 250 mL Erlenmeyer flasks containing 50 mL of 2% Malt Extract Broth (Oxoid, UK) supplemented with 1% glucose (pH 5.5, from here onward referred to as MEG medium). Four agar plugs (9 mm diameter) were drawn from 7 days old cultures of I. hispidus on MEA and inoculated into each flask. Incubated was performed statically at 25°C for 10 days. The fungal biomass developed in MEG medium was collected aseptically and repeatedly washed with sterile distilled water (5 × 250 mL). The resulting material was then freeze-dried and ground with mortar and pestle. Methanol extraction was performed in 1 L Erlenmeyer flask containing 100 g of mycelium in a 1:5 (w/v) ratio with the organic solvent, kept in static and natural light conditions and for 14 days at 25°C.
Both bodies and mycelia extracts were then filtered through Whatman® n° two filters (Sigma, Germany), prior to completely evaporating the solvent under reduced pressure (40°C, 218 mbar) in a rotary vacuum evaporator (Rotavapor R-100, Büchi, Switzerland). The residue was hence resuspended in a 1:1 mixture of DMSO and H2O and filter-sterilized by using 0.2 μm polytetrafluoroethylene (PTFE) membrane disks (Corning Inc., USA) and stored at −20°C until further use. Fungal fruiting bodies and mycelia extracts were used for estimation of the total phenolic compounds, antioxidant activity, and MIC determinations.
Liquid chromatography (LC) – mass spectrometry (MS) analysis of the fungal extracts
The methanol extract was analyzed by hybrid high-resolution mass spectrometry (HRMS), as described below. HRMS analysis was performed on an Agilent 6540 LC/MS quantitative time-of-flight (Q-TOF) system. The LC was composed of a binary pump, autosampler, column compartment and diode array detector, all Agilent 1290 series. The LC was interfaced to the mass spectrometer by an Agilent Dual JetStream ESI source that operated both positive and negative ionisation with N2 as desolvation gas (Gas temperature and Sheath Gas temperature both at 250°C, Gas flow and Sheath Gas Flow both at 9 L/min, Nebulizer 35 psi, Capillary 4000 V, Fragmentor 110 V). The Q-TOF operated in AutoMS/MS mode in a range of 50–1700 m/z with a collisional energy of 35 V.
The chromatographic separation of the components of the extract was obtained by injecting 1 µL of the diluted sample in a Phenomenex C18 Luna Polar column (2.1×100 mm, 1.6 µm) maintained at 35°C during a 30-min run. Water (A) and Acetonitrile (B) were used as delivery solvent at a constant flow of 0.35 mL/min with the following linear gradient: from 0% to 95.5% of B in 0–25 min, and isocratic condition of 99.5% of B from 25 to 30 min. The results were evaluated using MassHunter software using Metlin PCDL.
Estimation of the total phenolic compounds
A modified method of Folin-Ciocalteu, according to Singleton and Rossi[Citation31,Citation32] was used for determination of the total amount of phenolics. Briefly, 3 mL of distilled water, 0.5 mL of Folin-Ciocalteu reagent and 0.5 mL of methanol extract were mixed. After 5 min, 2 mL of 20% Na2CO3 were added and incubated in darkness at room temperature for 90 min. The absorbance was measured at 685 nm. A calibration curve with gallic acid was made under the same operating condition, and the results were expressed in mg of gallic acid/g of methanol extract dry weight. All measures were run in triplicate and the results were averaged.
DPPH assay
The antiradical activity was determined by the 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical-scavenging method. Briefly, 100 µL of 100 mM Tris-HCl buffer, pH 7.4 was added to variable amounts of methanol extract; the volume was brought to 1 mL with methanol and then added with 1 mL of 0.05 mM DPPH in methanol. The control sample was prepared using methanol. Trolox was employed as a positive standard. Absorbances of the mixtures were measured at 517 nm. The DPPH radical scavenging activity calculated as a percentage of the DPPH pink discoloration using the following equation:
where As is the absorbance of the solution containing the sample, and ADPPH is the absorbance of the DPPH solution. The activity was calculated as IC50. All measures were repeated three times and the results were averaged.[Citation33]
ß-carotene/linoleic acid assay
In this assay, antioxidant capacity was determined by measuring the inhibition of the volatile organic compounds and the conjugated diene hydroperoxides arising from linoleic acid oxidation. A stock solution of ß-carotene/linoleic acid mixture was prepared as follows: 0.5 mg ß-carotene (0.9 mM) was dissolved in 1 mL of chloroform, then 25 µL linoleic acid and 200 mg Tween 40 were added. Then, 100 mL distilled water saturated with oxygen (30 min, 100 mL/min) was added with vigorous shaking; 2.5 mL of this reaction mixture were dispensed into test tubes and 100 µL portions of the methanol extracts were added; the emulsion system was incubated for up to 24 h at room temperature under agitation.
The same procedure was repeated with the synthetic antioxidant butylated hydroxytoluene (BHT) as a positive control, and a blank. After this incubation period, absorbances of the mixtures were measured at 490 nm. The activity was calculated as IC50 value or as % Antioxidant Activity (AA) using the following equation:
where As0 is the absorbance of the sample at 0 min, Ast is the absorbance of the sample at 24 h, Ac0 is the absorbance of control sample at 0 min, and Act is the absorbance of control sample at 24 h. All tests were run in triplicate and averaged.[Citation34]
Antimicrobial susceptibility testing
In vitro antimicrobial activity of methanol extracts from I. hispidus was assessed against six bacterial strains (CLSI M07-A9),[Citation35] namely Pseudomonas aeruginosa (ATCC 15442), Escherichia coli (ATCC 10536), Salmonella typhi (clinical isolate), Staphylococcus aureus (ATCC 6538), Bacillus cereus (ATCC 12826) and Enterococcus faecalis (clinical isolate) and four yeasts and filamentous fungi, namely Candida albicans (YEPGA 6183), C. tropicalis (YEPGA 6184), Aspergillus tubingensis (PeruMicA 21), and A. minutus (PeruMicA 22).
Two yeast strains, Candida parapsilosis (ATCC 22019) and C. krusei (ATCC 6258), were used as quality controls for antifungal assays.[Citation36–Citation39] Voucher microbial cultures are maintained in the PeruMycA culture collection of the Department of Chemistry, Biology and Biotechnology (DCBB) (University of Perugia, Italy) and are available upon request. The two I. hispidus-derived methanol extracts from mycelia and fruiting bodies were used for MIC determination in the range 0.20–3.22 and 0.14–2.16 mg (d.w.) mL−1, respectively. Ciprofloxacin and fluconazole were used in the range 0.12–125 μg mL−1 and 0.063–16 μg mL−1, respectively, as a control for antibacterial and antifungal agents.
Antibacterial activity assay
Determination of Minimum Inhibitory Concentration (MIC) was performed according to the Clinical and Laboratory Standard Institute broth dilution method M07-A9.[Citation35] Working bacterial suspensions (inocula) were prepared as follows: a few colonies from 24-h old cultures on Mueller–Hinton Agar (MHA) plates were transferred to Mueller-Hinton broth (MHB) and incubated statically overnight at 37°C. The cell density of each inoculum was hence adjusted to that of the opacimetric standard Mac Farland 0.5 (1.5 × 108 CFU/mL) at a wavelength of 600 nm. 20 μL of bacterial suspensions were inoculated in 1 mL of MHB medium containing serial dilutions of fungal methanol extracts.
To further assess the viability of bacterial cells at MIC end-points, a colorimetric tetrazolium salt assay was used as optimized by Sabaeifard and collaborators.[Citation40] Following 20 h incubation, 180 μl of bacterial cultures for MIC determination were collected and transferred to 96-wells plates. Hence, 20 μl of a 2,3,5-triphenyl-tetrazolium chloride (TTC) solution in distilled water was added to each well in order to reach a final concentration of 0.4% (w/v). Viability controls consisted of MHB-grown bacterial cultures and uninoculated MHB supplemented with fungus methanol extracts were used as incubation controls. 96-wells plates were incubated aerobically for 6 h at 37°C prior to measuring absorbance measuring at 405 nm in a Tecan Infinite 200 PRO spectrophotometer (Tecan Trading AG, Switzerland). MIC end-points for ciprofloxacin were defined as the lowest concentration that inhibited 50% of the growth when compared with the growth control (MIC50).
Antifungal activity assay
Minimum Inhibitory Concentration (MIC) determination was performed by serial dilution using 99-well microtitre plates (Sarstedt, Milan, Italy), according to the Clinical and Laboratory Standard Institute M27-A3,[Citation36] M38-A2[Citation37] and supplement M61 protocols,[Citation39] respectively. RPMI (Roswell Park Memorial Institute) 1640 medium with L-glutamine and without sodium bicarbonate, supplemented with 2% glucose (w⁄v), buffered with 0.165 mol l−1 morpholinepropanesulphonic acid (MOPS), pH 7.0, was used throughout the study.
The inoculum suspensions were prepared from 7 days old cultures grown on Sabouraud Dextrose Agar (SDA) at 25°C and adjusted spectrophotometrically to optical densities (at a wavelength of 600 nm) that ranged from 0.09 to 0.11 (Mac Farland standard). The non-germinated conidial and yeasts inoculum suspensions were diluted to a ratio of 1:50 in RPMI 1640 to obtain twice an inoculum size ranging from 0.2 to 0.4 × 104–5 CFU mL−1. This was further confirmed by plating serial dilutions of the inoculum suspensions on SDA. Furthermore, each yeast and filamentous fungal strain included in the study was tested for its sensitivity to fluconazole following the M38-A2,[Citation37] M27-S4,[Citation38] and supplement M61,[Citation39] protocols.
MIC end-points (µg mL−1) were determined after 24 h (for Candida albicans and C. tropicalis) and 48 h (for A. tubingensis and A. minutus) of incubation in ambient air at 30°C.[Citation38,Citation39] For the fungal extracts, the MIC end-points were defined as the lowest concentration that showed 100% growth inhibition.[Citation41] The MIC end-points for fluconazole were defined as the lowest concentration that inhibited 50% of the growth when compared with the growth control (MIC50).[Citation38,Citation39] Geometric means and MIC ranges were determined from the three biological replicates to allow comparisons among the activities of plant extracts.
Results and discussion
Fungal identification
The monophyletic ingroup within the phylogenetic tree comprises five main clades, whose distinction appears consistent on a geographic base. Interestingly, sequence PeruMyc_2361 is located in a 100% bootstrap supported subclade within the European clade. Nevertheless, the genetic distance of this subclade is particularly high in comparison to the others. ITS region has recently provided new insights on the global diversity internal to species I. hispidus even when distinctly considered with respect to other regions.[Citation42]
HPLC analysis of the fungal extracts
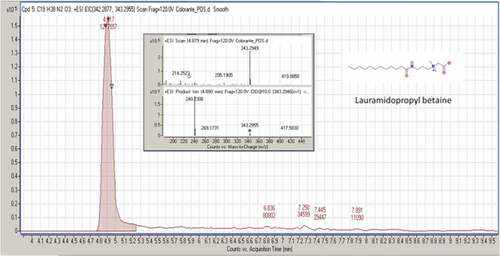
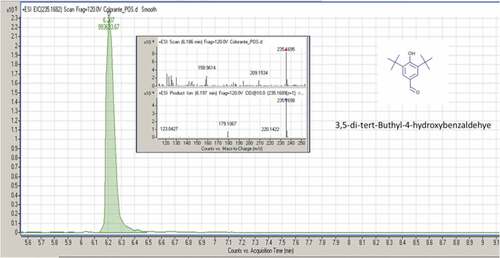
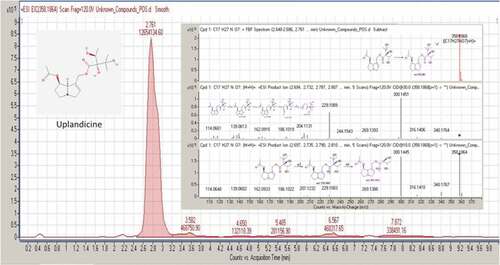
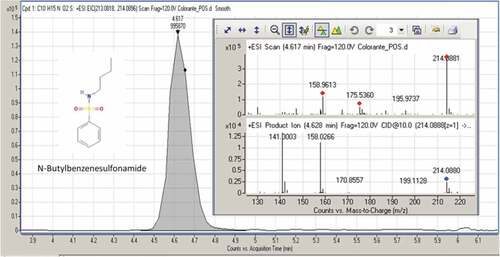
The metabolomic trace HPLC/MS/MS of the methanol extracts of fruiting bodies and mycelia is very different. In the latter case compounds from fruiting bodies methanolic extracts reported in literature were not found, but on the contrary, have been identified for I. hispidus not yet reported in literature. We concentrated on the identification of compounds of mycelia extracts as they were much less studied than the methanol extracts of fruiting bodies. For the analysis of the untargeted compounds, Full Scan/AIF experiment was used in order to obtain both full scan information (exact mass of precursor ions – chemical formula of compounds) and MS/MS delucidation (structural information). The obtained results were elaborated by “Find Compounds by formula” present inside MassHunter software and using Metlin PCDL as compounds database. The obtained results were evaluated to keep only the compounds with a score greater than 80% and subsequently filtered based on compliance between the theoretical and experimental MS/MS spectra. Subsequently a targeted MS/MS was performed in order to obtain a cleanest MS/MS spectrum and evaluate accurately the fragmentation pattern. The identified compounds are N-butylbenzenesulfonamide; lauramidopropyl betaine; 3,5-di-tert-butyl-4-hydroxybenzaldehyde; uplandicine. The Full Scan peak, Full Scan spectrum, and MS/MS spectrum are shown in –.
For all four compounds the carbon isotopic pattern linked with an accurate mass determination (mass error of precursor ions and fragment ions <5 ppm) confirms the chemical formulae of the compounds. Furthermore, the MS/MS fragments confirm the qualitative assessment of compounds due to the presence of characteristic fragments for each compound as shown in . These compounds are already known in literature but have never been reported in I. hispidus. N-butylbenzenesulfonamide (NBBS; CAS registry number 3622–84-2), is one of the compounds naturally found in the bark extract of Prunusa fricana.[Citation43–Citation45] It is a sulfur-containing compound that is widely used as a plasticizer in polyacetals and polyamides. Moreover, it shows high antiandrogenic and antifungal activity.[Citation43,Citation46]
Table 1. Data from the MS/MS spectrum of identified compounds present in the culture mycelia
Lauramidopropyl Betaine (LAPB, CAS registry number 4292–10-8) is a high-foaming and mild surfactant. It is known for the excellent foam and viscosity building with anionic systems. Moreover, it is a thickener with antistatic properties for hair shampoos, hand soaps, shower gels, and pet shampoos. It also has antimicrobial effects.[Citation47] 3,5-Di-tert-butyl-4-hydroxybenzaldehyde (BHT-CHO, CAS registry number 1620–98-0) is a metabolite of the butylated hydroxytoluene (BHT), a food additive. It has less prominent radical-scavenging activity than butylated hydroxytoluene. Like the anthropogenic pollutant,[Citation48] it was detected in human breast adipose tissues.[Citation49] 3,5-di-tert-butyl- 4-hydroxybenzaldehyde was found in the essential oil of Sphaeranthus indicus and in the capsules of Asclepias physocarpa.[Citation50] It is used as a reagent in the synthesis of a series of quinolinone-chalcones, which have anti-parasitic activity. Another use of BHT-CHO is in the synthesis of thiazolo[3,2-b][Citation1,Citation2,Citation4]triazole-6(5H)-one derivatives, a potent analgesic, with radical-scavenging and anti-inflammatory agents.[Citation51,Citation52]
Uplandicine (CAS registry number 74202–10-1) is a simple pyrrolizidine alkaloid, isolated from some plants belonging to Borraginaceae family: Symphytum uplandicum,[Citation53] Echium rauwolfii,[Citation54] Onosma arenaria,[Citation55] Onosma bracteatum.[Citation56] It exhibits antibacterial effects against Escherichia coli, with a MIC of 1.7 mg mL−1.[Citation56]
Total polyphenols content
shows the concentration of the total polyphenols (TPC) contained in the methanol extracts of the fruiting bodies and the mycelia placed in the culture of I. hispidus. The mean values of the ratio GAE/extract are expressed as µmol g−1. In order to compare these values with literature data, one can consider dividing the range from 0 to 5878.2 (µmol equivalent to 1 g of gallic acid) into deciles. The values found for TPC of fruiting bodies and mycelia both fall in the second decile (1045 and 919, respectively).
Table 2. Content of total phenolics (TCP) and DPPH assay of methanol extracts of I. ispidus.
In literature, there are few fungal species with values comparable to those of I. hispidus and among them, one can report Fomitopsis pinicola fruiting bodies with 1982.3 + 56.0 (fourth decile) and Polyporus lipsiensis fruiting bodies with 945.2 + 88.8 (second quartile).[Citation57] Many works report flavonoid concentrations in fungi with a wide range of concentrations. The free flavonoids in ethanol extract of raw Lentinula edodes (Berk.) Pegler, expressed as mg of (+)-catechin equivalents per 100 g sample, were 0.8 mg/100 g mushroom.[Citation58] However, in many other species, they were presented in mg g−1 reaching levels of 349.6 mg g−1 flavonoids as in Cryptoporus volvatus (Peck) Shear (349.6 mg g−1), followed by Cordyceps militaris (L.) Fr. (312.6 mg g−1).[Citation59]
Although the total flavonoid test was positive for the methanol extracts (data not shown) the HPLC/MS/MS metabolomics analysis showed no trace of flavonoid-like compounds. Some authors state that edible mushrooms cannot synthesize flavonoids because they do not have the main enzymes involved in their metabolic pathway, and no significant absorption was noticed from fruiting bodies cultivated in flavonoid-enriched substrates or from mycelia grown of flavonoid-supplemented lab media.[Citation60] Thus, spectrophotometric methods for ‘total flavonoids’ determination should not be applied to fungal samples.[Citation60] The flavonoids identified in several mushroom species using advanced identification devices such as DAD and MS might be due to sample contaminations or other pitfalls within the utilized protocols.[Citation60]
Radical scavenging activity
Extracts are typically complex mixtures of many compounds differing in functional groups, polarity, and chemical behavior. As a consequence, their possible antioxidant activity can give unpredictable results.[Citation61] The 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical-scavenging assay is the more useful assay to predict a general radical scavenging. As determined by scavenging DPPH radical (IC50), the antioxidant activities of the methanol extracts of fruiting bodies and cultivated mycelia were 0.082 ± 0.011 and 0.064 ± 0.007 mg mL−1 respectively. Compared with the activity of Trolox, the positive standard, the methanol extracts showed an activity of about 17.2% and 22.1%, respectively. If the results are expressed as % RSA, then the values of fruiting bodies and mycelia are 53.07 ± 7.62 and 50.56 ± 5.22, respectively.
The antioxidant activity showed highly significant differences among the different mushrooms in DPPH assays. The DPPH radical scavenging activity of the edible mushrooms Amanita caesarea, Boletus edulis, Cantharellus cibarius, Lactarius indigo, and Ramaria sp. were 6.15 ± 1.76, 48.06 ± 6.60, 30.48 ± 3.94, 31.28 ± 2.42 and 20.88 ± 3.91, respectively.[Citation62]
ß-carotene/linoleic acid assay
The antioxidant activity of the methanol extracts of fruiting bodies and cultivated mycelia, as determined by ß-Carotene/linoleic acid assay (IC50) were 0.066 ± 0.009 and 0.132 ± 0.015 mg mL−1 respectively. Compared with the activity of BHT (the positive standard) the methanol extracts showed an activity of 15.44 ± 0.24% and 11.44 ± 0.48%, respectively. If the antioxidant activity is expressed as % AA, the values are 69.96 ± 1.16 and 77.78 ± 3.59, respectively. The protection capacity performed by the double bonds of fatty acids in the methanol solutions of I. hispidus are rather low compared to those of BHT, but it must be considered that BHT is highly specific for this type of antioxidant action.[Citation63]
Antimicrobial activity of fungal extracts
Microbial resistance towards conventional antibiotic drugs is an ever-expanding healthcare threat of worldwide proportions. In order to overcome such serious challenge, seeking for a novel or alternative antimicrobials has now become an urgent priority for international healthcare policies.[Citation64] Several conventional antibiotics (e.g., penicillin, cephalosporins, and griseofulvin) were early isolated from asexual forms currently attributed to Ascomycota, whereas the importance of “higher fungi” belonging to the phylum Basidiomycota has been long overlooked.[Citation65,Citation66] Accordingly, very few commercial antimicrobial agents are derived from basidiomycetous fungi. Among these, derivatives of pleuromutilin produced by Clitopilus passeckerianus (Pilát) Singer and C. scyphoides f. mutilus (Fr.) Singer (formerly known as Pleurotus passeckerianus Pilát and P. mutilus (Fr.) Gillet) are now successfully used both in human and veterinary medicine.[Citation67] In recent years, however, renewed interest has regarded Basidiomycota as a source of new antimicrobials and other bioactive compounds.[Citation67]
The results of bacterial and fungal susceptibility tested towards I. hispidus methanol extract are reported in – and in Supplementary –. Regardless of the bacterial strain used, the antibacterial activity of fruiting bodies extract was consistently higher than that of the mycelia culture. This may be due to a higher concentration of bioactive compounds in fruiting bodies than in mycelia stage. In fact, according to Sulkowska-Ziaja et al.,[Citation68] the amounts of bioactive compounds such as polysaccharides, terpenoids, steroids, and phenolic compounds are much lower under the controlled, reproducible conditions of mycelia cultures. Many authors observed that mushroom-derived extracts are particularly effective towards Gram-positive bacteria.[Citation69] Accordingly, both methanol extracts of I. hispidus were particularly effective in inhibiting the growth of S. aureus, B. cereus, and E. faecalis.
Table 3. Minimum inhibitory concentration (MIC) of Inonotus hispidus methanol extracts and ciprofloxacin towards selected Gram-positive and Gram-negative bacteria
Table 4. Minimum inhibitory concentration (MIC) of I. hispidus methanol extracts and fluconazole towards selected yeast and filamentous fungal strains
Figure 1. Phylogenetic dendrogram obtained by Neighbor-Joining (NJ) from the ITS rDNA alignment of a selected Inonotus hispidus dataset. Values from 1000 bootstrap replicates are reported on branches. Only accessions reporting the respective geographic origins were entered in the dataset
Comparing results of antimicrobial activities is not easy, as the methodology used to produce fungal extracts may vary widely and susceptibility is not only species-specific, but even strain-specific.[Citation69] Nevertheless, MIC values reported in the present study are in the same range as those reported for other Basidiomycota,[Citation69] this being true also for the Gram-negative strains tested. Moreover, fruiting bodies methanol extract showed MIC values against S. typhi and P. aeruginosa in the same range as those of some conventional antibiotics (e.g., chloramphenicol and erythromycin, data not shown).
As for the antifungal activity of I. hispidus extracts, the five tested fungal strains showed an increased susceptibility towards the mushroom fruiting bodies extract (). This result is consistent with those reported above for bacteria (). Regardless of the extract typology, Candida albicans strain YEPGA 6183 and Aspergillus tubingensis strain PeruMycA 21 showed a somewhat lower susceptibility towards I. hispidus methanol extracts. Fluconazole showed good activity in vitro against C. albicans (MIC: 2 µg mL−1) and C. tropicalis (MIC: 4 µg mL−1) while, A. tubingensis and A. minutus strains were resistant to this azole-antifungal agent, as reported by the new CLSI clinical breakpoints (MICs ≥8).[Citation39,Citation70] A. tubingensis and A. minutus strains are also reported as amphotericin- and itraconazole-resistant species.[Citation41] The MIC values of fluconazole for the C. parapsilosis (ATCC 22019) and C. krusei (ATCC 6258) strains were within the established ranges.[Citation38]
Conclusion
Based on the methanol extraction both from mycelia and fruiting bodies, the present study provides a multi-focus characterization of a single strain (PeruMyc_2361). Unlike the majority of literature, this thus allows to reliably compare the performances in different tests, as the fungal material is the same. Four novel compounds never reported for this species were found in the methanol extracts from mycelia culture only, whereas they were not present in the fruiting bodies. This further confirms the importance of a comparative and distinct analysis for metabolites from mycelia and fruiting bodies, respectively. Consistently, antibacterial activity was found significantly higher in the extract from fruiting bodies than from mycelia. Total polyphenols resulted among the highest reported in literature, although the apparently remarkable values of flavonoids are to be considered ambiguous. Therefore, the present study further confirms that common protocols for spectrophotometric estimation of total flavonoids are improper for application to fungal samples. The antioxidant activities (greater for the extract of the mycelia) and antimicrobial (greater for the extract of the fruiting bodies) require further studies to identify the responsible compounds and cannot be justified on the basis of the compounds found so far. Finally, one can observe that values emerged for antioxidant and anti-scavenging activity are quite similar to values reported in literature for other species; nevertheless, most comparison species are mycorrhizal, whereas I. hispidus is easily cultivable.
Additional information
Funding
References
- Pecoraro, L.; Angelini, P.; Arcangeli, A.; Bistocchi, G.; Gargano, M. L.; La Rosa, A.; Lunghini, D.; Polemis, E.; Rubini, A.; Saitta, S.; et al. Macrofungi in Mediterranean Maquis along Seashore and Altitudinal Transects. Plant Biosyst. 2014, 148(2), 367–376. DOI: 10.1080/11263504.2013.877535.
- Vidović, S.; Zeković, Z.; Jokić, S. Clavaria Mushrooms and Extracts: Investigation on Valuable Components and Antioxidant Properties. International. J. Food Prop. 2014, 17, 2072–2081. DOI: 10.1080/10942912.2012.745129.
- Angelini, P.; Tirillini, B.; Properzi, A.; Rol, C.; Venanzoni, R. Identification and Bioactivity of the Growth Inhibitors in Tuber Spp. Methanolic Extracts. Plant Biosyst. 2015, 149(6), 1000–1009. DOI: 10.1080/11263504.2014.983575.
- Angelini, P.; Bistocchi, G.; Arcangeli, A.; Rubini, A.; Venanzoni, R. Inventory, Diversity and Communities of Macrofungi in the Collestrada Forest (Umbria, Central Italy). Plant Biosyst. 2016, 150(5), 1096–1105. DOI: 10.1080/11263504.2015.1108939.
- Angelini, P.; Arcangeli, A.; Bistocchi, G.; Rubini, A.; Venanzoni, R.; Perini, C. Current Knowledge of Umbrian Macrofungi (Central Italy). Plant Biosyst. 2017, 151(5), 915–923. DOI: 10.1080/11263504.2016.1265609.
- Wasser, S. P. Medicinal Mushrooms in Human Clinical Studies. Part I. Anticancer, Oncoimmunological, and Immunomodulatory Activities: A Review. Int. J. Med. Mushrooms. 2017, 19(4), 279–317. DOI: 10.1615/IntJMedMushrooms.v19.i4.10.
- Angelini, P.; Tirillini, B.; Bistocchi, G.; Arcangeli, A.; Rubini, A.; Pellegino, R. M.; Fabiani, R.; Cruciani, G.; Venanzoni, R.; Rosignoli, P. Overview of the Biological Activities of a Methanol Extract from Wild Red Belt Conk, Fomitopsis Pinicola (Agaricomycetes), Fruiting Bodies from Central Italy. Int. J. Med. Mushrooms. 2018, 20(11), 1047–1063. DOI: 10.1615/IntJMedMushrooms.2018028595.
- Koyani, R. D.; Pramod, S.; Bhatt, I. M.; Rao, K. S.; Rajput, K. S. The Delignification Pattern of Ailanthus Excelsa Wood by Inonotus Hispidus (Bull.: Fr.) P. Karst. J. Sustainable For. 2015, 34(5), 502–515. DOI: 10.1080/10549811.2015.1033554.
- Fischer, M.; González García, V. An Annotated Checklist of European Basidiomycetes Related to White Rot of Grapevine (Vitis Vinifera). Phytopathol. Mediterr. 2015, 54(2), 281−298.
- Bernicchia, A. Polyporaceae s.l., Fungi Europaei, 10. Candusso ed 2005. ISBN: 9788890105753
- Yousfi, M.; Djeridane, A.; Bombarda, I.; Hamia, C.; Duhem, B.; Gaydou, E. M. Isolation and Characterization of a New Hispolone Derivative from Antioxidant Extracts of Pistacia Atlantica. Phytother. Res. 2009, 23(9), 1237–1242. DOI: 10.1002/ptr.2543.
- Ali, N. A. A.; Mothana, R. A. A.; Lesnau, A.; Pilgrim, H.; Lindequist, U. Antiviral Activity of Inonotus Hispidus. Fitoterapia. 2003, 74, 483–485. DOI: 10.1016/S0367-326X(03)00119-9.
- Ali, N. A. A.; Lüdtke, J.; Pilgrim, H.; Lindequist, U. Inhibition Of Chemiluminescence Response Of Human Mononuclear Cells And Suppression Of Mitogen-Induced Proliferation Of Spleen Lymphocytes Of Mice By Hispolon And Hispidin. Pharmazie. 1996, 51(9), 667–670.
- Zan, L. F.; Qin, J. C.; Zhang, Y. M.; Yao, Y. H.; Bao, H. Y.; Li, X. Antioxidant Hispidin Derivatives from Medicinal Mushroom Inonotus Hispidus. Chem. Pharm. Bull. 2011, 59(6), 770–772.
- Ren, Q.; Lu, X. Y.; Han, J. X.; Aisa, H. A.; Yuan, T. Triterpenoids and Phenolics from the Fruiting Bodies of Inonotus Hispidus and Their Activations of Melanogenesis and Tyrosinase. Chin. Chem. Lett. 2017, 28(5), 1052–1056. DOI: 10.1016/j.cclet.2016.12.010.
- Hou, R.; Liu, X.; Xiang, K.; Chen, L.; Wu, X.; Lin, W.; Zheng, M.; Fu, J. Characterization of the Physicochemical Properties and Extraction Optimization of Natural Melanin from Inonotus Hispidus Mushroom. Food Chem. 2019, 277, 533–542. DOI: 10.1016/j.foodchem.2018.11.002.
- Liu, X.; Hou, R.; Xu, K.; Chen, L.; Wu, X.; Lin, W.; Zheng, M.; Fu, J. Extraction, Characterization And Antioxidant Activity Analysis Of The Polysaccharide From The Solid-State Fermentation Substrate Of Inonotus Hispidus. Int. J. Biol. Macromol. 2019, 123, 468–476. DOI: 10.1016/j.ijbiomac.2018.11.069.
- Smolskaite, L.; Talou, T.; Venskutonis, P. R. Comprehensive Evaluation of Antioxidant and Antimicrobial Properties of Different Mushroom Species. LWT - Food Sci. Technol. 2015, 60(1), 462–471. DOI: 10.1016/j.lwt.2014.08.007.
- Lee, I. K.; Yun, B. S. Highly Oxygenated and Unsaturated Metabolites Providing a Diversity of Hispidin Class Antioxidants in the Medicinal Mushrooms Inonotus and Phellinus. Bioorg. Med. Chem. 2007, 15(10), 3309–3314. DOI: 10.1016/j.bmc.2007.03.039.
- GrüNdemann, C.; Arnhold, M.; Meier, S.; Bäcker, C.; Garcia-Käufer, M.; Grunewald, F.; Steinborn, C.; Klemd, A. M.; Wille, R.; Huber, R.; et al. Effects of Inonotus Hispidus Extracts and Compounds on Human Immunocompetent Cells. Planta Med. 2016, 82(15), 1359–1367. DOI: 10.1055/s-0042-111693.
- Gonindard, C.; Bergonzi, C.; Denier, C.; Sergheraert, C.; Klaebe, A.; Chavant, L. Synthetic Hispidin, a PKC Inhibitor, Is More Cytotoxic toward Cancer Cells than Normal Cells in Vitro. Cell Biol. Toxicol. 1977, 13(3), 141–153. DOI: 10.1023/A:1007321227010.
- Benarous, K.; Bombarda, I.; Iriepa, I.; Moraleda, I.; Gaetan, H.; Linani, A.; Tahria, D.; Sebaa, M.; Yousfi, M. Harmaline and Hispidin from Peganum Harmala and Inonotus Hispidus with Binding Affinity to Candida Rugosa Lipase: In Silico and in Vitro Studies. Bioorg. Chem. 2015, 62, 1–7. DOI: 10.1016/j.bioorg.2015.06.005.
- Tura, D.; Zmitrovich, I. V.; Wasser, S. P.; Nevo, E. Medicinal Species From Genera Inonotus And Phellinus (Aphyllophoromycetideae): Cultural-Morphological Peculiarities, Growth Characteristics, And Qualitative Enzymatic Activity Tests. Int. J. Med. Mushrooms. 2009, 11(3), 309–328. DOI: 10.1615/IntJMedMushr.v11.i3.
- Elisashvili, V. Submerged Cultivation of Medicinal Mushrooms: Bioprocesses and Products (Review). Int. J. Med. Mushrooms. 2012, 14(3), 211–239. DOI: 10.1615/IntJMedMushr.v14.i3.
- Gams, W.; Hoekstra, E. S.; Aptroot, A. eds. CBS Course of Mycology, 4th ed.; Centraalbureau voor Schimmelcultures Publisher, Baarn, Netherlands, 1998. 165.
- Ryvarden, L.; Melo, I. Poroid Fungi of Europe. Synop. Fungorum. 2014, 31, 1–455.
- Nilsson, R. H.; Kristiansson, E.; Ryberg, M.; Hallenberg, N.; Larsson, K. H. Intraspecific ITS Variability in the Kingdom Fungi as Expressed in the International Sequence Databases and ITS Implications for Molecular Species Identification. Evol. Bioinf. 2008, 4, 193–201. DOI: 10.4137/EBO.S653.
- Schoch, C. L.; Seifert, K. A.; Huhndorf, S.; Robert, V.; Spouge, J. L.; Levesque, C. A.; Chen, W. Nuclear Ribosomal Internal Transcribed Spacer (ITS) Region as a Universal DNA Barcode Marker for Fungi. Proc. National Academy Sci. 2012, 109(16), 6241–6246. DOI: 10.1073/pnas.1117018109.
- Thompson, J. D.; Higgins, D. G.; Gibson, T. J. CLUSTAL W: Improving the Sensitivity of Progressive Multiple Sequence Alignment through Sequence Weighting, Position-Specific Gap Penalties and Weight Matrix Choice. Nucleic Acids Res. 1994, 22(22), 4673–4680.
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 For Bigger Datasets. Mol. Biol. Evol. 2016, 33(7), 1870–1874. DOI: 10.1093/molbev/msw054.
- Singleton, V. L.; Rossi, J. A. J. Colorimetry Of Total Phenolics With Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–153.
- Lamaison, J. L.; Carnat, A. Content of Main Flavonoids in Flowers and Leaves of Crataegus Monogyna Jacq. And Crataegus Laevigata (Poiret) DC. In Relation with Development Stages. Pharm. Acta Helv. 1991, 65, 315–320.
- Peterson, D. M.; Hahn, M. J.; Emmons, C. L. Oat Avenanthramides Exhibit Antioxidant Activities in Vitro. Food Chem. 2002, 79(4), 473–478. DOI: 10.1016/S0308-8146(02)00219-4.
- Caprioli, G.; Alunno, A.; Beghelli, D.; Bianco, A.; Bramucci, M.; Frezza, C.; Iannarelli, R.; Papa, F.; Quassinti, L.; Sagratini, G.; et al. Polar Constituents And Biological Activity Of The Berry-Like Fruits From Hypericum Androsaemum L. Front. Plant Sci. 2016, 7, 232. DOI: 10.3389/fpls.2016.00232.
- CLSI. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically, Approved Standard, 9th Ed., CLSI Document M07-A9, 950 West Valley Road, Suite 2500, Wayne, Pennsylvania 19087; Clinical and Laboratory Standards Institute: USA, 2012.
- CLSI. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts; Approved Standard-Third Edition. CLSI Document M27-A3; Clinical and Laboratory Standards Institute: Wayne, PA, 2008.
- CLSI. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi; Approved Standard-Second Edition. CLSI Document M38-A2; Clinical and Laboratory Standards Institute: Wayne, PA, 2008.
- CLSI. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts; 4th Informational Supplement. CLSI Document M27-S4; Clinical and Laboratory Standards Institute: Wayne, PA, 2012.
- CLSI. Performance Standards for Antifungal Susceptibility Testing of Filamentous Fungi. 1st. Ed. CLSI Supplement M61; Clinical and Laboratory Standards Institute: Wayne, PA, 2017.
- Sabaeifard, P.; Abdi-Ali, A.; Soudi, M. R.; Dinarvand, R. Optimization of Tetrazolium Salt Assay for Pseudomonas Aeruginosa Biofilm Using Microtiter Plate Method. J. Microbiol. Methods. 2014, 105, 134–140. DOI: 10.1016/j.mimet.2014.07.024.
- Pagiotti, R.; Angelini, P.; Rubini, A.; Tirillini, B.; Granetti, B.; Venanzoni, R. Identification and Characterisation of Human Pathogenic Filamentous Fungi and Susceptibility to Thymus Schimperi Essential Oil. Mycoses. 2011, 54, e364–376. DOI: 10.1111/j.1439-0507.2010.01926.x.
- Zhou, L. W.; Vlasák, J.; Decock, C.; Assefa, A.; Stenlid, J.; Abate, D.; Wu, S. H.; Dai, Y. C. Global Diversity and Taxonomy of the Inonotus Linteus Complex (Hymenochaetales, Basidiomycota): Sanghuangporus Gen. Nov., Tropicoporus Excentrodendri and T. Guanacastensis Gen. Et Spp. Nov., And 17 New Combinations. Fungal Diversity. 2016, 77(1), 335–347. DOI: 10.1007/s13225-015-0335-8.
- Papaioannou, M.; Schleich, S.; Roell, D.; Schubert, U.; Tanner, T.; Claessens, F.; Matusch, R.; Baniahmad, A. NBBS Isolated from Pygeum Africanum Bark Exhibits Androgen Antagonistic Activity, Inhibits AR Nuclear Translocation and Prostate Cancer Cell Growth. Invest. New Drugs. 2010, 28(6), 729–743. DOI: 10.1007/s10637-009-9304-y.
- Roell, D.; Baniahmad, A. The Natural Compounds Atraric Acid And N-Butylbenzene-Sulfonamide As Antagonists Of The Human Androgen Receptor And Inhibitors Of Prostate Cancer Cell Growth. Mol. Cell. Endocrinol. 2011, 332(1–2), 1–8. DOI: 10.1016/j.mce.2010.09.013.
- Rider, C. V.; Janardhan, K. S.; Rao, D.; Morrison, J. P.; McPherson, C. A.; Harry, G. J. Evaluation Of N-Butylbenzenesulfonamide (Nbbs) Neurotoxicity In Sprague-Dawley Male Rats Following 27-Day Oral Exposure. NeuroToxicology. 2012, 33(6), 1528–1535. DOI: 10.1016/j.neuro.2012.07.002.
- Kim, K. K.; Kang, J. G.; Moon, S. S.; Kang, K. Y. Isolation And Identification Of Antifungal N-Butylbenzenesulphonamide Produced By Pseudomonas Sp. Ab2. J. Antibiot. (Tokyo). 2000, 53(2), 131–136. DOI: 10.7164/antibiotics.53.131.
- Lindstedt, M.; Allenmark, S.; Thompson, R. A.; Edebo, L. Antimicrobial Activity of Betaine Esters, Quaternary Ammonium Amphiphiles Which Spontaneously Hydrolyze into Nontoxic Components. Antimicrob. Agents Chemother. 1990, 34(10), 1949–1954.
- Fries, E.; PüTtmann, W. Monitoring of the Antioxidant BHT and Its Metabolite BHT-CHO in German River Water and Ground Water. Sci. Total Environ. 2004, 319(1–3), 269–282. DOI: 10.1016/S0048-9697(03)00447-9.
- Hernández, F.; Portolés, T.; Pitarch, E.; López, F. J. Searching For Anthropogenic Contaminants In Human Breast Adipose Tissues Using Gas Chromatography-Time-of-Flight Mass Spectrometry. J. Mass Spectrom. 2009, 44(1), 1–11. DOI: 10.1002/jms.1538.
- Ma, B. J.; Peng, H.; Liu, J. K. Monitoring Of BHT-Quinone And Bht-Cho In The Gas Of Capsules Of Asclepias Physocarpa. J. Biosci. 2006, 61(5–6), 458–460.
- Mullican, M. D.; Wilson, M. W.; Conner, D. T.; Kostlan, C. K.; Schrier, D. J.; Dyer, R. D. Design of 5-(3,5-Di-Tert-Butyl-4-Hydroxyphenyl)-1,3,4-Thiadiazoles, −1,3,4-Oxadiazoles, and −1,2,4-Triazoles as Orally Active, Nonulcerogenic Antiinflammatory Agents. J. Med. Chem. 1993, 36(8), 1090–1099.
- Fujisawa, S.; Kadoma, Y.; Yokoe, I. Radical-Scavenging Activity Of Butylated Hydroxytoluene (Bht) And Its Metabolites. Chem. Phys. Lipids. 2004, 130(2), 189–195. DOI: 10.1016/j.chemphyslip.2004.03.005.
- Culvenor, C. C. J.; Edgar, J. A.; Frahn, J. L.; Smith, L. W. The Alkaloids of Symphytum Uplandicum Russian Comfrey. Aust. J. Chem. 1980, 33(5), 1105–1114. DOI: 10.1071/CH9801105.
- El-Shazly, A.; Abdel-All, M.; Tei, A.; Wink, M. Pyrrolizidine Alkaloids from Echium Rauwolfii and Echium Horridum (Boraginaceae). J. Biosci. 1999, 54(5–6), 295–300.
- El-Shazly, A.; Abdel-Ghani, A.; Wink, M. Pyrrolizidine Alkaloids from Onosma Arenaria (Boraginaceae). Biochem. Syst. Ecol. 2003, 31(5), 477–485. DOI: 10.1016/S0305-1978(02)00177-1.
- Navneet, S. S. G.; Kumar, S. Appraisal of Antimicrobial Properties of Onosma Bracteatum Wall. Fruit Extracts against Respiratory Tract Pathogens. J. Med. Herbs Ethnomed. 2015, 1, 108–112.
- Doskocil, I.; Havlik, J.; Verlotta, R.; Tauchen, J.; Vesela, L.; Macakova, K.; Opletal, L.; Kokoska, L.; Rada, V. In Vitro Immunomodulatory Activity, Cytotoxicity and Chemistry of Some Central European Polypores. Pharm. Biol. 2016, 54(11), 2369–2376. DOI: 10.3109/13880209.2016.1156708.
- Choi, Y.; Lee, S. M.; Chun, J.; Lee, H. B.; Lee, J. Influence of Heat Treatment on the Antioxidant Activities and Polyphenolic Compounds of Shiitake (Lentinus Edodes) Mushroom. Food Chem. 2006, 99(2), 381–387. DOI: 10.1016/j.foodchem.2005.08.004.
- Jeong, S. C.; Koyyalamudi, S. R.; Hughes, J. M.; Khoo, C.; Bailey, T.; Marripudi, K.; Park, J. P.; Kim, J. H.; Song, C. H. Antioxidant And Immunomodulating Activities Of Exo-And Endopolysaccharide Fractions From Submerged Mycelia Cultures Of Culinary-Medicinal Mushrooms. Int. J. Med. Mushrooms. 2013, 15(3), 251–266.
- Gil-Ramírez, A.; Pavo-Caballero, C.; Baeza, E.; Baenas, N.; Garcia-Viguera, C.; Marín, F. R.; Soler-Rivas, C. Mushrooms Do Not Contain Flavonoids. J. Funct. Foods. 2016, 25, 1–13. DOI: 10.1016/j.jff.2016.05.005.
- Sanchez, C. Reactive Oxygen Species and Antioxidant Properties from Mushrooms. Synth. Syst. Biotechnol. 2017, 2(1), 13–22. DOI: 10.1016/j.synbio.2016.12.001.
- López-Vázqueza, E.; Prieto-García, F.; Gayosso-Canales, M.; Otazo Sáncheza, E. M.; Villagómez Ibarra, J. R. Phenolic Acids, Flavonoids, Ascorbic Acid, β-glucans And Antioxidant Activity In Mexican Wild Edible Mushrooms. Ital J. Food Saf. 2017, 29(4), 766–774.
- Adegoke, G. O.; Vijay Kumar, M.; Gopala Krishna, A. G.; Varadaraj, M. C.; Sambaiah, K.; Lokesh, B. R. Antioxidants and Lipid Oxidation in Foods - A Critical Appraisal. J. Food Sci. Technol. 1998, 35(4), 283–298.
- WHO. World Health Organization – Antibiotic resistance. http://www.who.int/news-room/fact-sheets/detail/antibiotic-resistance. ( Accessed Nov 16, 2018).
- Lindequist, U.; Niedermeyer, T. H. J.; Jülich, W. D. The Pharmacological Potential of Mushrooms. Evid. Based Complement. Altern. Med. 2005, 2(3), 285–299. DOI: 10.1093/ecam/neh107.
- Yamaç, M.; Bilgili, F. Antimicrobial Activities of Fruit Bodies And/Or Mycelial Cultures of Some Mushroom Isolates. Pharm. Biol. 2006, 44(9), 660–667. DOI: 10.1080/13880200601006897.
- de Mattos-Shipley, K. M. J.; Ford, K. L.; Alberti, F.; Banks, A. M.; Bailey, A. M.; Foster, G. D.; Good, T. The Bad and the Tasty: The Many Roles of Mushrooms. Stud. Mycol. 2016, 85, 125–157. DOI: 10.1016/j.simyco.2016.11.002.
- Sułkowska-Ziaja, K.; Szewczyk, A.; Galanty, A.; Gdula-Argasińska, J.; Muszyńska, B. Chemical Composition and Biological Activity of Extracts from Fruiting Bodies and Mycelial Cultures of Fomitopsis Betulina. Mol. Biol. Rep. 2018, 45(6), 2535–2544. DOI: 10.1007/s11033-018-4420-4.
- Alves, M. J.; Ferreira, I. C.; Dias, J.; Teixeira, V.; Martins, A.; Pintado, M. A Review on Antimicrobial Activity of Mushroom (Basidiomycetes) Extracts and Isolated Compounds. Planta Med. 2012, 78(16), 1707–1718. DOI: 10.1055/s-0032-1315370.
- Espinel-Ingroff, A.; Barlett, M.; Chaturvedi, V.; Ghannoum, M.; Hazen, K. C.; Pfaller, M. A.; Rinaldi, M.; Walsh, T. J. Optimal Susceptibility Testing Conditions for Detection of Azole Resistance in Aspergillus Spp.: NCCLS Collaborative Evaluation. National Committee for Clinical Laboratory Standards. Antimicrob. Agents Chemother. 2001, 45(6), 1828–1835. DOI: 10.1128/AAC.45.6.1828-1835.2001.