ABSTRACT
The chemical (proximate, amino acid, fatty acid) and mineral composition of raw goat milk from British Alpine, Jamnapari, Saanen, Shami, and Toggenburg breed (n= 30) were investigated. The results showed a significant difference (p< .05) on the chemical and mineral composition between the breeds. Principal component analysis (PCA) grouped the breeds into three groups; Jamnapari, Saanen and Shami, British Alpine and Toggenburg. This study showed that chemical and mineral composition of goat milk can be used as the criteria to classify goat breeds. These findings were important for goat breeder and manufacturer of goat milk to improve their products using breed selection.
Introduction
Goat milk has been shown to play an essential role in human nutrition due to a pivotal relationship between nutritional values in goat milk and human health. Goat milk has a similar composition with other types of milk. It contains protein, lipid, carbohydrate, vitamin, and mineral. However, the unique features of the composition of goat milk made it becomes the choice for manufacturing to cater gourmet foods market and medical needs.
Many studies have investigated lipid and fatty acids composition of goat milk as its main nutritional advantage over cow milk. The fat globules in goat milk were abundant in sizes less than 3.5 μm compared to cow which was 4.55 μm.[Citation1] Some studies reported that the number of fat globules smaller than 5 μm in goat milk is ∼80% compared to ∼60% in cow milk.[Citation2] This characteristic contributes to the softer texture of goat milk products and enhances lipid metabolism, thus making them more digestible.[Citation3] The fat in goat milk contains a higher proportion of medium-chain fatty acids and conjugated linoleic acid, which are associated with the characteristics of cheese flavor and “goaty” odor of goat milk as well as the anti-carcinogenic and anti-atherogenic effect.[Citation1] Other than fat, protein in goat milk has greater buffering capacity and distinct alkalinity, which would be beneficial for the treatment of stomach ulcer. This benefit is due to higher levels of major buffering components, such as proteins, non-protein nitrogen, and phosphate.[Citation3] Another unique characteristic of goat milk is casein micelles. They are less solvated, less heat stable, and lose β-casein more readily than in cow milk. They also played an important role in cheese-making process especially during renneting.[Citation4]
The quality of milk is reliant on milk composition that varies with breed, age, body size and weight, udder size, diet, stage of lactation, season, length of dry period, and environmental temperature.[Citation3] In this study, the effect of breed on milk composition was investigated. Indigenous breed will have a low average milk yield but have high total solid and other milk components.[Citation5] In comparison, selected or improved breed will have high milk yield but with a lower total solid.[Citation6] According to Malaysian Livestock Breeding Policy 2013[Citation7], imported dairy goat breeds available in Malaysia are British Alpine, Saanen, and Toggenburg. Meanwhile, Jamnapari and Shami (Damascus) are among the dual-purpose breeds. British Alpine, Saanen, and Toggenburg are among the major dairy goat breeds in the United States, and they are famous for their high milk productivity. The Saanen is best known as the Holstein of the goat world, producing high milk yields with low-fat levels. The Alpine and Toggenburg breeds produced milk yields and composition between the levels of the Saanen and Nubians in general but with some individual exceptions.[Citation8]
In normal circumstances, goat breeder will choose the animal breed according to yield and productivity, while food manufacturers will favor milk with high functional properties. This study aims to explore the chemical composition of goat milk, which are protein, fat, moisture, ash, carbohydrate, amino acids, fatty acids, and minerals profiles from these five different breeds. The chemical and mineral composition of the milk samples were expected to be significantly different as breed is one of the important factors, which determines the milk composition. Current findings are important for goat breeder and manufacturer of goat milk products to improve breed selection and cross-breeding program, as well as to design nutritionally enhanced functional food products.
The limitation that needs to be highlighted in this study is that no single farm in the country could provide all five breeds; thus, the samples from Shami and Toggenburg were derived from different farms. Samples from these two breeds could be influenced by other factors, such as feeds, environmental factors, and daily care.
Materials and methods
Goat milk samples
Fresh goat milk samples were obtained from five different breeds, i.e. British Alpine, Jamnapari, Saanen, Shami, and Toggenburg. Each breed was represented by six animals, with a total of 30 samples. The animals were in 7–8 months of lactation stage. British Alpine, Jamnapari, and Saanen milk were obtained from a farm in Sungai Siput, Perak. The animals were fed with dairy goat pellet, Guinea grass, and Napier grass. Shami milk was obtained from a farm in Muadzam Shah, Pahang. The animals were fed with dairy pellet and Napier grass. Toggenburg was obtained from a farm in Panglima Garang, Selangor and the animals were fed with dairy goat pellet, Napier grass, and corn. All samples were milked manually and collection was done in the early morning. Samples were kept immediately in sterile glass bottles under aseptic technique. The samples were transferred to the laboratory in an icebox with the temperature maintained at 2–4°C. Fresh goat milk samples were preserved at −20°C until further use.
Chemicals and standards
Sodium chloride was purchased from R&M Chemicals (Essex, UK). Ammonium acetate and Supelco 37 FAME Mix were purchased from Sigma-Aldrich (St. Louis, Missouri, USA). Other chemicals were purchased from Merck (Darmstadt, Germany). The water was purified using a ELGA Pure Lab Classic system (ELGA, Woodridge, IL, USA). All chemicals used were of analytical except for HPLC analysis.
Analytical procedures
Proximate analysis
Samples were analyzed for proximate composition according to standard procedures. Fat content of the samples was measured by Gerber method (AOAC 2000.18)[Citation9], moisture content using oven-drying method (AOAC 934.01)[Citation9], while ash content was determined by furnace-drying method (AOAC 942.05).[Citation9] Carbohydrate content was calculated based on differentiation.
Amino acid analysis
The analysis was carried out according to Rafiq et al. (2016) and Zakaria et al. (2007) with modifications.[Citation10,Citation11] Thawed milk samples were thoroughly mixed and hydrolyzed using 6 N HCl in sealed glass ampules for 24 h at 110°C. The hydrolysate was filtered using 0.2 μm cellulose nitrate membrane filter and subjected to pre-column derivatization with phenylisothiocyanate. The analysis of amino acid was achieved by reversed-phase HPLC using C18 column (Purospher STAR RP18 encapped column, 5 μm, 250 × 4.6 mm). Ammonium acetate (0.1 M, pH 6.5) and 0.1 M ammonium acetate in HPLC grade acetonitrile: methanol: water (46: 1 0: 44) were used. The flow rate was set at 1.0 mL/min. The UV detector response was monitored at 254 nm.
Fatty acid analysis
The analysis was carried out according to Feng et al. (2004) with some modifications.[Citation12] Approximately, 20 mL of thawed milk samples in 50 mL Falcon tube were centrifuged at 14,000 xg for 40 min at 4°C. The aqueous layer was removed and the samples were left to room temperature for 20 min. Then, the samples were subjected to another centrifugation at 10,200 xg for 40 min at 30°C. The separated fat were stored in amber vials, and frozen at −20°C until further analysis. Exactly, 100 mg of fat was weighed into vial and dissolved with 5 mL hexane. The solution was added with 250 μL of sodium methoxide and mixed using vortex for 1 min with paused every 10 s. Next, 5 mL of saturated sodium chloride was added, the vial was closed tightly and shaked vigorously for 15 s. The vial was left at room temperature for 10 min. The hexane layer was removed and transferred to another vial containing 2 mg of sodium sulfate. The hexane layer (FAME solution) was allowed to contact with sodium sulfate for at least 15 min prior to analysis.
Exactly 1 μL of the FAME solution was injected into an Agilent 6890 Gas Chromatograph (Agilent Technologies, California, USA) equipped with a flame ionization detector. Analysis was performed with a DB23 column (60 m × 0.25 mm i.d.) with a film thickness of 0.15 μm (Chrompack, Middelburg, The Netherlands). The gas chromatography condition was as follows: initial temperature of 50°C was maintained for 1 min, raised to 175°C at a rate of 25°C/min, and increased to 230°C at a rate of 4°C/min for 18 min. The split ratio was 1:40, and helium was used as the carrier gas. The injector and detector temperatures were 250°C. The identification of fatty acids was done by comparing retention times of the methyl esters of the samples using reference standard (Supelco 37 FAME Mix, Sigma-Aldrich, Missouri, USA). Quantification of fatty acids was done according to AOAC 2012.13[Citation9] using Pentadecane as the internal standard.
Mineral analysis
Sample preparation was done based on precipitation of casein by trichloroacetic acid (TCA) according to Brooks et al. (1970) [Citation13] and Hankinson (1975).[Citation14] In a 100-mL volumetric flask, 5 mL of milk was mixed with 50 mL of 24% (w/v) TCA and adjusted to volume with deionized water. The sample was shaked at 5 min interval for 30 min and centrifuged for 5 min at 986 x g at room temperature. Exactly, 5 mL of filtrate was filtered using 0.45 μm nylon syringe filter into 50 mL volumetric flask and added volume with deionized water. Major and minor elements were determined using Atomic Absorption Spectrometer 3300 (Perkin-Elmer, Massachusetts, USA).
Statistical analysis
Data (average and standard deviation) were expressed as a mean of three independent experiments. Using Minitab 17 software, one-way ANOVA was conducted for chemical composition analysis, while two-way ANOVA was conducted for amino acids, fatty acids, and mineral analysis. The interaction effect ‘breed x variables’ for each determination was evaluated based on the differences between means at the 95% confidence level using the Tukey Independent test. Principal component analysis (PCA) was performed in order to distinguish groups belonging to different breeds using 23 selected variables from and five cases goat breeds were analyzed.
Table 1. Chemical and mineral composition of goat milk from five different breeds in Malaysia
Results and discussion
As goat breed is one of the major influences on the chemical composition of goat milk, several significant differences were reported between the five different breeds, i.e. British Alpine, Jamnapari, Saanen, Shami, and Toggenburg that are available in Malaysia ().
Chemical composition
Moisture and ash content showed minimum variations among the breeds and ranged from 85.32% to 89.01% and 0.66% to 1.10%, respectively (). Moisture is a critical factor for manufacturing of milk products as it affects the quality, cost, and microbial stability of the products. Water serves as a medium for solution and colloidal suspension for the other components present in milk.[Citation15] The findings of this study on are in agreement with Sung et al. (1999), which reported moisture content in goat milk from Saanen, Toggenburg, and British Alpine were 88.94%, 88.39%, and 88.45%, respectively.[Citation16] In this study, Jamnapari showed lower moisture content than Shami, Saanen, and Toggenburg with 85.32%. For ash content, British Alpine had the highest value with 1.10% compared to other four breeds. Previous study reported that Jamnapari breed had 81.28% of moisture and 0.96% of ash content.[Citation17] Even though similar environmental setting and breed were applied in both studies, other factors, such as age of goat, body size and weight, udder size, diet, and lactation stage, can cause variation in milk constituents[Citation18] .
For fat content, Jamnapari (4.20%) showed significantly higher fat content in comparison to British Alpine (3.70%), Shami (3.12%), and Toggenburg (3.08%). According to Food Regulation 1985[Citation19], milk, raw milk, or fresh milk shall contain not less than 3.25% of milk, fat; and 8.5% of non-fat milk solids. This standard is also applied to goat milk. The fat contents of Shami and Toggenburg in this study failed to meet the prescribed minimum limit of this standard. In the previous study, the fat content for Shami milk was reported to be 4.4–4.55%.[Citation20] As mentioned earlier, these two breeds were derived from different farms hence, the difference contributed to the reported results. In addition, the low reported fat content could also be due to the limited number of animals used for each breed.
Protein content in all four breeds was shown to be significantly higher (p < .05) than British Alpine (2.75%). The protein and fat in this study were in agreement with several other studies.[Citation16,Citation17,Citation21–Citation23] However, since most of the previous studies focused on Saanen, minor differences were noticed in the fat and protein content of this breed.[Citation16,Citation17,Citation21–Citation23] The variation was contributed by differences in the diet, breed, individual, season, feeding, management, environmental condition, locality, and stage of lactation.[Citation18]
As for sugar content, the samples were subjected to lactose analysis according to Sharma et al. (2009). [Citation24] However, the data were not reported due to low content of lactose (ca. 50% less) and non-comparable with other studies.[Citation15,Citation25] This might be due to lactose hydrolysis during sample preparation. Hence, this method may not be suitable for lactose analysis in goat milk.
Amino acid analysis
summarizes the amino acid composition of goat milk from five different breeds. In the previous study, goat milk was characterized by high content of threonine (Thr) and low content of methionine (Met).[Citation26] In this study, Thr was found to be the highest essential amino acid in Jamnapari, British Alpine, and Shami while cysteine (Cys) was the lowest amount found in all breeds except for Shami. Essential amino acids are important as they are part of complex pathways and biological systems, such as protein metabolism, nervous system, and more. Milk protein is a good source of branched chain amino acids (Leu, Ile, and Val) compared to soy protein. The branched amino acids are important in weight control via glucose homeostasis and lipid metabolism.[Citation10] Jamnapari showed the highest content of Leu, Ile, and Val as compared to other breeds and could be a promising source of branched chain amino acids in human nutrition.
Among non-essential amino acids, there was a noticeably higher level of glutamine (Glu) and proline (Pro). Rafiq et al. (2016) [Citation10] reported similar trends that maximum Glu was found in whey protein while casein was represented by good amount of Pro. In this study, Pro was found highest in Jamnapari, while high amount of Glu was reported in Shami and Jamnapari. Glu and Pro are important amino acids involved in healthy brain functions. In addition, Cys and Met are sulfur-containing amino acids that could be utilized in a specific dietary manipulation to enhance immune system by serving as antioxidant.[Citation27]
Fatty acid analysis
Fatty acid composition of goat milk from five different breeds is summarized in . The highest amount of fatty acid found in milk were total oleic acid (C18:1), palmitic acid (C16:0), myristic acid (C14:0), decanoic acid (C10:0) and stearic acid (C18:0). Similar findings were reported by Sumarmono et al. (2015).[Citation28] These five fatty acids (total C18:1, C18:0, C16:0, C14:0 and C10:0) are more than 75% of total fatty acids in goat milk.[Citation13] These fatty acids together with lauric acid (C12:0) were found to account for 83.5% of total fatty acids in Kondyli et al. (2012).[Citation29]
In this study, significant differences between the goat breeds corresponded to the content of long and medium-chain fatty acids. The results indicated that goat milk contains a very high number of minor BCFA (branched-chain fatty acids), mostly monomethylates, that have potential implications for the unique aroma and flavor of the goat milk and its products.[Citation28] With a focus on Jamnapari, this study found lower amount of fatty acids than reported by Sumarmono et al. (2012).[Citation28] While the study determined fatty acid profiles of goat milk from Peranakan Etawah or Jamnapari in Banyumas, Central Java Province, the variation could be contributed by differences in the diet, season, feeding, and stage of lactation. However, these factors were not clarified in the study.
Goat milk has a unique characteristic in the C12:C10 ratio, which has been used to check the authenticity of goat milk. According to Alonso et al. (1999) the ratios were 0.46 and 0.50, which were significantly lower than cow milk, at 1.16 and 1.14, respectively.[Citation30] The ratio becomes proportionally larger with increased substitution of cow’s milk in goat milk. In this study, the ratio of C12:C10 was 0.46, which confirmed the authenticity of the samples were derived from goat. The detection of the extent of adulteration has been used in the dairy industry such as in the production of goat cheese.[Citation31]
Mineral analysis
Mineral composition in goat milk is presented by the ash content. The major minerals in goat milk determined in this study were Ca, P, Mg, K, and Na, while minor minerals were Mn, Zn, Fe, and Cu. According to , K was the highest mineral compared to other major minerals. Jenness (1980)[Citation32] indicated that goat milk provided a great amount of Ca and P and the normal Ca⁄P ratio in milk has been considered as 1.20.[Citation32] However, P was not being included in this study due to the unavailability of standard. The findings of this study on Ca and Mg were in line with Mayer and Fiechter (2012)[Citation33], which determined the major minerals for Saanen and Toggenburg goats in Austria.[Citation33] However, minor differences were noticed for K and Na, where the findings in this study were slightly lower. The Na/K ratio for Saanen in this study was 0.33, slightly higher than the previous studies.[Citation18,Citation33] This supports the fact that milk composition within similar breed may differ between geographical region (environmental temperature, season), lactation stage, nutritional status of animals, and composition of the feeds.[Citation33]
Multivariate analysis
Principal component analysis (PCA)
PCA was used to determine the correlation between the chemical composition of the milk and goat breed. Main chemical compositions were selected from . Two principal components with eigenvalue of more than 1 were extracted to determine the best parameter to classify goat breeds. As shown in , the PC1 and PC2 accounted for 51.1% and 23.4% of the total variation in the data, respectively. The cumulative variance for both principal components was 74.5%.
Figure 1. (a). Score plot for PC2 versus PC1 from PCA for breed classification. (b). Loading plot for PC1 versus PC2
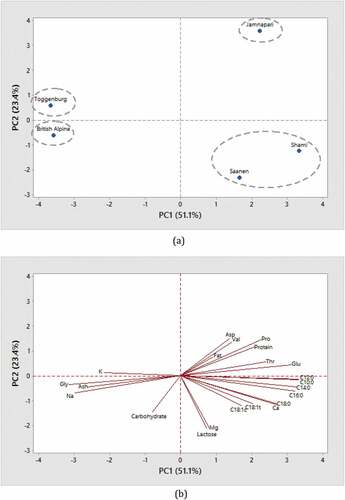
shows the relationship between the chemical composition and goat breed. Based on PC1, Jamnapari was discriminated and well separated from other goat breeds at positive score of PC1, which means Jamnapari had a significantly different chemical composition than other breeds. This suggested that the chemical composition of milk can be used as the potential criteria for classification of goat breeds. According to PC2, Toggenburg and British Alpine were separated in PC2 positive and negative position. This close separation was contributed by significant differences in Na, K, Gly, and ash contents. Saanen and Shami were grouped together according to PCA and HCA results. Both of them had strong association with lactose, Mg, Ca, and fatty acids (C10:0, C12:0, C14:0, C16:0, C18:0, C18:1t, and C18:1c). As mentioned in the previous section, milk from Saanen, British Alpine, and Jamnapari were derived from similar farm. Most of the external factors such as age of animals, lactation stage, type of feeding, environment, and daily care were kept constant among them. However, all these breeds were significantly different based on PCA results. Therefore, this finding has proven that chemical composition of goat milk could be highly influenced by different goat breeds.
Conclusion
The present study provides preliminary data on the chemical composition, amino acid, fatty acid, and mineral profile of goat milk from five different breeds in Malaysia. It is well established and had been shown by different authors that there might be considerable differences in chemical composition and physical properties of milk from different goat breeds. PCA showed that goat breeds from similar farm could be divided into three groups, with Jamnapari in one group. The present findings would be useful for the dairy processing industries since protein and fat are the key ingredients of milk that determine its quality. Apart from the manufacturer, this information may benefit the goat farmer in choosing the highest quality of breed for their milk production and to consider cross-breeding program to reduce their cost. Further studies should be conducted to investigate the nutritional value, rheology, color, and other properties of goat milk from different breeds, which was not analyzed in this study. Comparison between indigenous and selected breed from the farm and lactation stage which received the same types of feed also can be done to show the influence of each factor to the variation in chemical composition of goat milk.
Conflict of interest
All the authors claimed no conflict of interest.
Acknowledgments
This work was supported by the Ministry of Higher Education, Malaysia under Grant no. [UPM/700-2/1/TRGS/5535704] and Universiti Putra Malaysia under Insentif Putra Siswazah [Grant number: GP-IPS/2016/9507900]. We would like to thank the owners of goat farms in Sungai Siput, Muadzam Shah and Panglima Garang for the sample collection.
Additional information
Funding
References
- Haenlein, G. F. W.;. Goat Milk in Human Nutrition. Small Rumin. Res. 2004, 51(2), 155–163. DOI: 10.1016/j.smallrumres.2003.08.010.
- Silanikove, N.; Leitner, G.; Merin, U.; Prosser, C. G. Recent Advances in Exploiting Goat Milk: Quality, Safety and Production Aspects. Small Rumin. Res. 2010, 89((2–3):), 110–124. DOI: 10.1016/j.smallrumres.2009.12.033.
- Park, Y. W.; Haenlein, G. F. W. Milk Production. In Solaiman SG Solaiman (Ed) Goat Science and Production; Wiley-Blackwell Publishers, Oxford, UK, 2010; pp 275–292.
- Meena Goswami, B. S. K.; Tewari, A.; Sharma, H.; Karunakara, K. N.; Khanam, T. Implication of Functional Ingredients of Goat Milk to Develop Functional Foods. J. Anim. Feed Sci. Tech.. 2017, 5, 65–72. DOI: 10.21088/jafst.2321.1628.5217.5.
- Mestawet, T. A.; Girma, A.; Ådnøy, T.; Devold, T. G.; Narvhus, J. A.; Vegarud, G. E. Milk Production, Composition and Variation at Different Lactation Stages of Four Goat Breeds in Ethiopia. Small Rumin. Res.. 2012, 105(1–3), 176–181. DOI: 10.1016/j.smallrumres.2011.11.014.
- Abbas, H. M.; Hassan, F. A.; El-Gawad, M. A. M. A.; Enab, A. K. Physicochemical Characteristics of Goat Milk. Life Sci J. 2014, 11(1), 307–317.
- DVS. Malaysian Livestock Breeding Policy. 2013, pp. 1–42. DOI: 10.1017/CBO9781107415324.004.
- Park, Y. W.; Haenlein, G. F. W.; Wendorff, W. L.(Eds.);. Handbook of Milk of Non-Bovine Mammals (2nd ed.); John Wiley & Sons, Hoboken, NJ, 2017. DOI:10.1002/9781119110316
- AOAC. Official Methods of Analysis of AOAC International; AOAC: Gaithersburg, MD, 2005. 17th Vol. II, 33: 934.01, 942.05, 991.20, 2000.18
- Rafiq, S.; Huma, N.; Pasha, I. Chemical Composition, Nitrogen Fractions and Amino Acids Profile of Milk from Different Animal Species. Asian-Australas. J. Anim. Sci. 2016, 29(7), 1022–1028. DOI: 10.5713/ajas.15.0452.
- Zakaria, Z. A.; Mat Jais, A. M.; Goh, Y. M.; Sulaiman, M. R.; Somchit, M. N. Amino Acid and Fatty Acid Composition of an Aqueous Extract of Channa Striatus (Haruan) that Exhibits Antinociceptive Activity. Clin. Exp. Pharmacol. Physiol.. 2007, 34(3), 198–204. DOI: 10.1111/j.1440-1681.2007.04572.x.
- Feng, S.; Lock, A.; Technical Note:, G. P. A Rapid Lipid Separation Method for Determining Fatty Acid Composition of Milk. J. Dairy Sci. 2004, 87(11), 3785–3788. DOI: 10.3168/jds.S0022-0302(04)73517-1.
- Brooks, I. B.; Luster, G. A.; Easterly, D. G. A Procedure for the Rapid Determination of the Major Cations in Milk by Atomic Absorption Spectrophotometry. Atom. Absorption Newsl. 1970, 9(4), 93–94.
- Hankinson, D. J.;. Potential Sources of Copper Contamination of Farm Milk Supplies Measured by Atomic Absorption Spectrophotometry1. J. Dairy Sci. 1975, 58(3), 326–336. DOI: 10.3168/jds.S0022-0302(75)84569-3.
- Imran, M.; Khan, H.; Hassan, S. S.; Khan, R. Physicochemical Characteristics of Various Milk Samples Available in Pakistan. J. Zhejiang Univ. Sci. B. 2008, 9(7), 546–551. DOI: 10.1631/jzus.B0820052.
- Sung, Y. Y.; Wu, T. I.; Wang, P. H. Evaluation of Milk Quality of Alpine, Nubian, Saanen and Toggenburg Breeds in Taiwan. Small Rumin. Res. 1999, 33(1), 17–23. DOI: 10.1016/S0921-4488(98)00201-6.
- Alyaqoubi, S.; Abdullah, A.; Samudi, M.; Abdullah, N.; Addai, Z. R.; Al-Ghazali, M. Physicochemical Properties and Antioxidant Activity of Milk Samples Collected from Five Goat Breeds in Malaysia. Adv. J. Food. Sci. Technol. 2015, 7(4), 235–241. DOI: 10.19026/ajfst.7.1301.
- Park, Y. W.;. Rheological Characteristics of Goat and Sheep Milk. Small Rumin. Res. 2007, 68(1–2), 73–87. DOI: 10.1016/j.smallrumres.2006.09.015.
- Food Act. Laws of Malaysia: Food Act and Regulations 1983. Section 34, Act 281.
- Gül, S.; Keskin, M.; Güler, Z.; Dursun, A.; Gündüz, Z.; Önel, S. E.; Tüney Bebek, D. Effects of Pre-Milking Resting on Some Lactation Characteristics in Damascus (Shami) and Kilis Goats. J. Anim. Prod. (Hayvansal Üretim Dergisi). 2018, 59(1), 17–24. DOI: 10.29185/hayuretim.372188.
- Trancoso, M.; Trancoso, M. A.; Martins, A. P. L.; Roseiro, L. B. Chemical Composition and Mineral Content of Goat Milk from Four Indigenous Portuguese Breeds in Relation to One Foreign Breed. Int. J. Dairy Technol. 2010, 63(4), 516–522. DOI: 10.1111/j.1471-0307.2010.00625.x.
- Da Costa, W. K. A.; de Souza, E. L.; Beltrao-Filho, E. M.; Vasconcelos, G. K. V.; Santi-Gadelha, T.; de Almeida Gadelha, C. A.; Franco, O. L.; Do Egypto, R. D. C. R.; Magnani, M. Comparative Protein Composition Analysis of Goat Milk Produced by the Alpine and Saanen Breeds in Northeastern Brazil and Related Antibacterial Activities. PLoS One. 2014, 9(3), e93361. DOI: 10.1371/journal.pone.0093361.
- Syd Jaafar, S. H.; Hashim, R.; Hassan, Z.; Ariffin, N. A Comparative Study on Physicochemical Characteristics of Raw Goat Milk Collected from Different Farms in Malaysia. Trop Life Sci. Res. 2018, 29(1), 195–212. DOI: 10.21315/tlsr2018.29.1.13.
- Sharma, R.; Rajput, Y. S.; Dogra, G.; Tomar, S. K. Estimation of Sugars in Milk by HPLC and Its Application in Detection of Adulteration of Milk with Soymilk. Int. J Dairy Technol. 2009, 62(4), 514–519. DOI: 10.1111/j.1471-0307.2009.00532.x.
- Mahmood, A.; Usman, S. A Comparative Study on Physicochemical Parameters of Milk Sampels Collected from Buffalo, Cow, Goat and Sheep of Gujrat, Pakistan. Pakistan J. Nutr. 2010, 9(12), 1192–1197. DOI: 10.3923/pjn.2010.1192.1997.
- Sabahelkheir, M.; Fat, M.; Hassan, A. Amino Acid Composition of Human and Animal’s Milk (Camel, Cow, Sheep and Goat). ARPN Ji Sci. Technol. 2012, 2, 32–34.
- Hall, W. L.; Millward, D. J.; Long, S. J.; Morgan, L. M. Casein and Whey Exert Different Effects on Plasma Amino Acid Profiles, Gastrointestinal Hormone Secretion and Appetite. Br. J. Nutr. 2003, 89(2), 239. DOI: 10.1079/BJN2002760.
- Sumarmono, J.; Sulistyowati, M. Fatty Acids Profiles of Fresh Milk, Yogurt and Concentrated Yogurt from Peranakan Etawah Goat Milk. Procedia Food Sci. 2015, 3, 216–222. DOI: 10.1016/j.profoo.2015.01.024.
- Kondyli, E.; Svarnas, C.; Samelis, J.; Katsiari, M. C. Chemical Composition and Microbiological Quality of Ewe and Goat Milk of Native Greek Breeds. Small Rum. Res. 2012, 103, 194–199. DOI: 10.1016/j.smallrumres.2011.09.043.
- Alonso, L.; Fontecha, J.; Lozada, L.; Fraga, M. J.; Juárez, M. Fatty Acid Composition of Caprine Milk: Major, Branched-Chain, and Trans Fatty Acids. J. Dairy Sci. 1999, 82(5), 878–884. DOI: 10.3168/jds.S0022-0302(99)75306-3.
- Wolf, R. L.;. Contribution of Trans-18:1 Acids from Dairy Fat to European Diets. Jaocs. 1994, 71, 277–283. DOI: 10.1007/BF02638053.
- Jenness, R.;. Composition and Characteristics of Goat Milk: Review 1968–1978. J. Dairy Sci. 1980, 63, 1605–1630. doi: 10.3168/jds.S0022-0302(80)83125-0.
- Mayer, H. K.; Fiechter, G. Physicochemical Characteristics of Goat Milk in Austria-Seasonal Variations and Differences between Six Breeds. Dairy Sci. Technol. 2012, 92(2), 167–177. DOI: 10.1007/s13594-011-0047-0.
