?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Shellac resin ammonium salts (SRAS) were prepared from the natural product of shellac resin and ammonium hydroxide by amidation. Resveratrol (RES) was embedded with SRAS to improve its thermostability, antioxidant activity, and bioavailability. The absorption of RES was promoted in the intestinal tract. Meanwhile, an enteric-coated microcapsule-loaded RES was successfully established by spray drying technology. The RES microcapsule exhibited a spherical shape and perfect stability as well as outstanding sustainable release character in simulated intestinal release experiment. Moreover, SRAS/RES presented a dose-dependent manner with a much higher radical scavenging activity of DPPH· and ABTS+ compared to the pure RES. Therefore, a kind of new pH-sensitive RES microcapsule established may act as a favorable carrier for an insoluble antioxidant ingredient in the potentially new food delivery system on functional food supplements.
Introduction
With the continuous improvement of living standard, people tend to purchase green, healthy, and nutritious food. Antioxidants’ development also gradually changed from synthetic into natural which as an important part of food process. Among them, plant antioxidants such as polyphenols, tannins, vitamin, and others, due to the raw material sources, natural green, non-toxic, health, and other advantages, which has a broad application prospect in food and health-care products and other fields.[Citation1–Citation3] Precisely, resveratrol (RES) is a kind of natural antioxidant with polyphenols structure and widely existed in the plant of grape, peanut, mulberry, and Polygonum cuspidatum. It can remove the destructive free radical, prevent lipid peroxide, inhibit DNA damage, and so on.[Citation4–Citation6] Studies on the application of natural RES to protect the intestinal mucosal injury, regulate intestinal function, and prevent tumors such as colon cancer have been reported.[Citation7–Citation10] However, a large proportion of RES is broken down and lose activity in the process of human liver and intestinal metabolism, lead to poor absorption, and low bioavailability, which seriously limits the application of RES.[Citation11]
Delivery system is a preparation that transports the active component exclusively to the site where it is required and has little or no interaction with other tissues. It is widely used in medicine and food delivery. In the past decades, various types of polymer materials used in the delivery system, including synthesis polymer and macromolecular compounds extracted from natural products such as peptides, polysaccharide, protein, etc.[Citation12–Citation14] Shellac resin (SR) is a natural resinous material, with easy to degradation, non-toxic, and other excellent properties, which are obtained from secretions of scale insects. It was a mixture containing terpenic resin (70–80%), wax (6–7%), coloring matter (4–8%), and other impurities.[Citation15,Citation16] The good mechanical and film-forming properties of SR make it widely used in food, medicine, cosmetics, and coatings industry.[Citation17–Citation19] Moreover, because of the acid insoluble of SR, the coating material which is used as the enteric soluble functional component has a certain pH-response in the aspect of food and medicine.[Citation20] Nevertheless, congenital defects such as poor water solubility and heat resistance limit the application of SR. SR containing a large amount of active groups such as hydroxyl and carboxyl group, so the disadvantages can effectively be improved by using chemical modification,[Citation21] then applied to the coating material of enteric drugs, the goal of controlled release of functional components will be attained.
The aim of our work was to enhance the stability, bioavailability, and antioxidant of RES by using natural SR. To this purpose, the ammonium hydroxide was used to SR modification, and then SRAS was applied to the microcapsules of RES. In particular, we investigated the thermostability, pH-sensitive performance in simulated physiological conditions and antioxidant activity of the microcapsules.
Material and methods
Chemicals and reagents
Chemicals, including Ammonium hydroxide (NH3·H2O), Tween80, sodium dihydrogen phosphate (NaH2PO4), disodium hydrogen phosphate (Na2HPO4), and ethyl acetate were of analytical grade and purchased from Sinopharm Chemical Reagent Co., Ltd. SR was native to Yunnan province of China. DPPH· and ABTS+· agents were brought from Shanghai Yuanye Bio-Technology Co., Ltd. RES (98%) were provided by Huayuan Hengyuan plant biochemical Co., Ltd. All other chemicals used were analytical grade, and water was double-distilled just before use.
Preparation of SRAS
After crushing and screening, SR (20.00 g) was added to 0.1 mol/L NH3·H2O aqueous solution to prepare the SRAS at the temperature of 40°C with continuous stirring. Standing and then cooling after reaction over, take the supernatant liquor to freeze drying. Then, SRAS was obtained.
Preparation of SRAS/RES
SRAS was first dissolved in water at the temperature of 50°C. Then, RES is added to the solution, and a small amount of Tween80 is added dropwise. The prepared solution is stirred for 1 h using a magnetic stirrer. The RES concentration of 0%, 5%, and 10% solutions were prepared, respectively. Then, microcapsules were prepared according to the methodology of Carvalho et al. with modifications.[Citation22] Spray drying was performed in a B-290 laboratory scale spray dryer (BUCHI, Switzerland) with a nozzle atomization system with a 0.7 mm diameter nozzle. The emulsion was pumped into the primary chamber through a peristaltic pump at 25°C using a magnetic stirrer. Feed flow rate was 6 mL/min, and the drying airflow rate was 50 m3/h. Experimental tests were carried out with an inlet air drying temperature of 140°C and outlet air temperature of 65°C. The production by spray drying was performed in duplicate.
Determination of moisture, encapsulation efficiency, and loading capacity
The moisture of fresh microcapsules was determined by free-drying and weighed. Encapsulation efficiency and loading capacity were measured according to the method reported by Wang et al. with a slight modification.[Citation23] In brief, 20 mg sample was washed using ethyl acetate for three times and filtered. The retention liquor was added to the sample. Based on the equation for the RES standard curve, the retention liquor was collected to determine the free RES amount by measuring the absorbance at 305 nm. The standard curve of RES was determined by measuring the absorbance (305 nm) of a known concentration of RES using a TU-19 UV/VIS spectrophotometer (Beijing Purkinje General Instrument Co, Ltd.). Moisture, EE, and LC were calculated by using the following equations:
where W0 and W1 were the weight of microcapsule before and after free-drying, respectively. M0 and M1 were the mass of initial addition RES and un-embedding RES, M was the mass of microcapsule.
Morphology of SRAS/RES
Morphologies of the SRAS/RES microcapsule were observed using a field-emission scanning electron microscopy (SU8010, Hitachi, Japan). All of the samples were sputter-coated with gold just before observation and observed under a working voltage of 10 kV.
Solid-state study
The sample was tested by using Fourier-transform infrared spectroscopy (FT-IR) and Differential scanning calorimetry-thermogravimetric (DSC) analyses. FT-IR of the samples were recorded using an Alpha (Bruker Ltd., USA) at a resolution of 2 cm-1 with 24 scans over the wave-number range of 400~4000 cm−1. All FT-IR experiments were performed in triplicate. DSC analysis (Mettler-Toledo, Switzerland) was performed under a nitrogen atmosphere at a flow rate of 50 mL/min. Samples were accurately weighed and sealed in 40 μL aluminum pans, and heated at temperature from 30°C to 400°C under the purging of nitrogen gas with heating rate of 10°C/min.
Drug release of SRAS/RES
To evaluate the release profiles of RES, a fully calibrated dissolution apparatus using the paddle method were performed in 600 mL phosphate buffers with the pH of 1.0, 6.8, and 7.4, respectively.[Citation24] Briefly, samples contain 100 mg RES were placed to dialysis tube (MD3500, Viskase, USA), clamped and sank at the bottom of the vessel at the beginning of the experiment. The temperature was maintained at 37°C ± 0.5°C, and vessel speed was kept at 100 rpm. 1 mL of supernatant was collected and replenished with 1 mL of fresh PBS, and the accumulated amount of released factor in solution was quantified by a TU-19 UV/VIS spectrophotometer. Accumulated release percentage of the drug was determined as follows:
where Mt was the weight of growth factor released at time t, and M0 was the total amount mass of the RES in the microcapsule theoretically. All experiments were tested in triplicate.
Determination of antioxidant activity on SRAS/RES
DPPH radical scavenging assay and ABTS+ scavenging assay were used to evaluate the antioxidant activity of the SRAS/RES. Precisely, radical scavenging activity of the SRAS/RES samples against the DPPH· was done using a UV/VIS spectrophotometer in accordance with the method reported by Musa et al. with minor modification.[Citation25] Briefly, 0.5 mL of each tested solution (RES concentration were 200, 400, 600, 800, 1000 μg/mL, respectively) was blended with 5.0 mL of 79 mg/L alcoholic solution of DPPH radical. The mixture was shaken energetically and kept in the dark for 1 h at 310.15 K. The absorbance of supernatant was recorded at 517 nm. The percentage of DPPH radical scavenging activity was calculated as follows:
where Ac, Ab, and As were the absorbance of control, blank, and blended solutions at 517 nm, respectively. Radical cation scavenging ability of the SRAS/RES samples against the ABTS+· was assayed in accordance with Intarasirisawat et al. method with slight modification.[Citation26] ABTS radical cation was obtained by the mixture of 3.84 g/L ABTS+· aqueous solution with 1.34 g/L potassium persulfate aqueous solution at a ratio of 1:1 (v/v). The mixed solution was retained for 12 h in the dark at room temperature. Prior to using, necessary absorption 0.7 ± 0.02 at 734 nm was obtained by diluting the mixed solution with PBS solution. The samples (0.5 mL) with different concentrations of RES (40, 60, 80, 100, and 120 μg/mL, respectively) were blended with 10 mL of ABTS radical cation and then all mixtures were left for 1 h at 310.15 K in dark. The absorbance was tested at 734 nm.
where Ac, Ab, and As were the absorbance of control, blank, and blended solutions at 734 nm, respectively.
Statistical analysis
Each experiment was performed three times, and the results are given as the mean ± standard deviation (SD). All data were collected and converted to graphs using Origin 8.0 software.
Results and discussion
Physical parameters
shows that the encapsulation efficiency of RES was preferable by using SRAS. The encapsulation efficiency was significantly reduced after increase in the proportion of RES. The main reason is the limited embedding ability of the wall material on the core material. When the amount of the core material increases significantly, the concentration of the wall material decreases, and the encapsulation efficiency of the core material (RES) decreases significantly.[Citation27,Citation28]
Table 1. Physical parameters of microcapsules
Morphology of SRAS/RES delivery system
In order to confirm the morphological characteristic of the RES-loaded microcapsules, SEM was used as shown in . The results showed the morphology of pure RES (a) demonstrated a slender crystal structure. The 0% SRAS/RES microcapsule (b) had no core material of RES showed adhesion together, and with the increase of the amount of RES (c and d), microcapsule sphere structure gradually forming, surface seemed more smoothly with smaller particle size and uniform distribution, indicating a preferable embedding effect.
Solid-state study
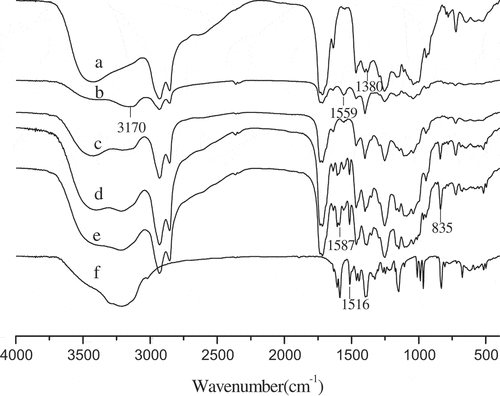
FT-IR was conducted to investigate the change of the functional group and the compatibility between the active ingredients and the wall material in the microcapsule. The FT-IR spectra of SR, SRAS, the microcapsules, and RES were showed in . Except for RES, all spectra displayed a broadband at 3000~3600 cm−1 indicating the overlap of O–H and N–H stretches in the same region. Compared to SR, the FT-IR spectra of 3150 cm−1 has a strong enhancement in this region, and a new spectra of 1559 cm−1 was appeared which represented N–H stretches and R–COO−, respectively. The changes of the spectra of 1380 cm−1 due to the formation of amido bond through ammonolysis by lactone of SR. All the results showed that the amino-group was successfully bonded with the carboxyl group of SR to form amido bond. After embedding RES, the FT-IR spectra of microcapsule showed obvious characteristic absorption peak of RES, especially around the spectra of 1600~1450 cm−1 and 900~500 cm−1 indicating that RES was part of the formation of the microcapsules.
Figure 2. FT-IR spectra of the shellac resin (a), SRAS (b), 0% SRAS/RES (c), 5% SRAS/RES (d), 10% SRAS/RES microcapsules (e), and RES (f)
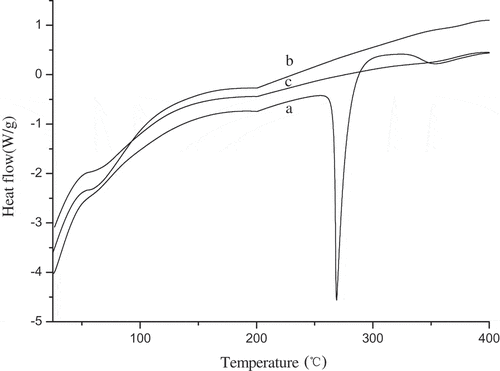
DSC analysis was performed to investigate the thermal stability and physical state of RES in the microcapsules. As shows, the thermograms of all substances showed a slight endothermic peak around 50~100°C which were associated with the loss of water. The pure RES showed an endothermic sharp peak at 266.98°C with a specific enthalpy of transition (DH) 241.2 J/g. This corresponds to the melting of RES, which is in accordance with Kobierski et al.[Citation29] But, it was not found for the microcapsules. Therefore, it was proved that the formation of microcapsules could be achieved by the application of the natural polymers for the encapsulation of RES.
In vitro release study
The studies of the in vitro release of RES loaded microcapsules were performed under simulated gastrointestinal tract on food products. Thus, it was studied the cumulative release of RES from obtained microcapsules in phosphate buffers at a pH of 1.0, 6.8, and 7.4, respectively. In microspheres/microcapsules, the Peppas equation (EquationEquation 7(7)
(7) ) was usually used to explain the type of RES release mechanisms.[Citation30]
where Q is the accumulative release percentage of RES released at time t; k is a rate constant related to structural and geometric characteristics of the material; n is the release exponent that indicates the mechanism of the RES release. (n ≤ 0.43 indicates the dominant release mechanism is the Fickian diffusion; 0.43 ≤ n < 0.85 indicates non Fickian or anomalous transport; n ≥ 0.85 corresponds to zero order release kinetics)
As shows, the release profiles of RES almost the same with different pH conditions. The release rate of RES was rapid in front of 10 h and no any pH-sensitive behavior. However, the microcapsules exhibited a controlled and sustained release profile of RES. More precisely, during the first 2 h at pH 1.0, only a small percentage of RES was released into the PBS solution. There can be considered as the RES dissolution on the surface of microcapsules, and the RES of inner was difficult to transport into solution through the wall. But in simulated intestinal juice, especially at the pH of 6.8, RES can quickly be released from the microcapsules, so as to achieve the aim of colon-targeting release.
Figure 4. Cumulative release profiles of RES, 5% and 10% SRAS/RES at 37℃: (a) pH = 1; (b) pH = 6.8; (c) pH = 7.4
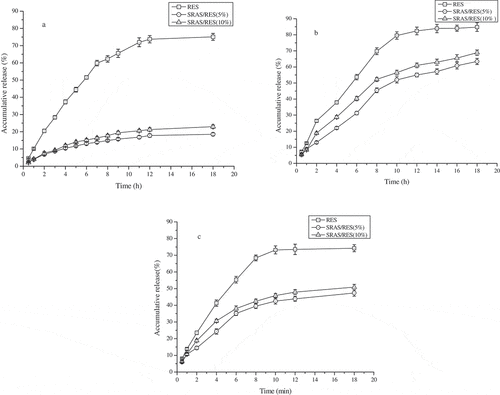
The fitting results by Peppas equation of the release rate showed all of these depend simultaneously by the swelling and the diffusion processes specific to a non Fickian mechanism (). The release kinetics of RES from microcapsules at pH of 6.8 were best described by the Peppas equation (0.990 and 0.982, respectively).
Table 2. Kinetic release parameters of RES, 5% and 10% SRAS/RES microcapsule
Antioxidant activity
There are several methods to evaluate radical scavenging activity, among which, the DPPH (1,1-diphenyl-2-picrylhydrazyl) and ABTS+ [2,2‘-Azinobis-(3-ethylbenzthiazoline-6-sulphonate)] are widely used in measuring the antioxidant capability with stable and efficiency.
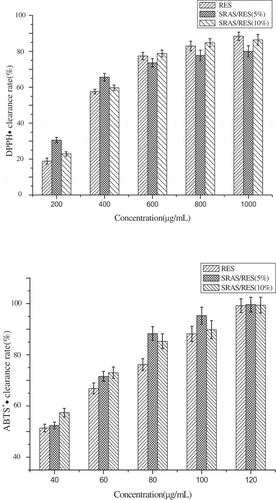
As shows, the reduced percentage of DPPH and ABTS+ radical were all related to the concentration of the different formulations. A dose-dependent manner was discovered for all formulations. However, it was interesting that the microcapsules have a higher ability of reducing free radicals under a low concentration compared with the same concentration of RES. The EC50 value (concentration required to obtain a 50% antioxidant effect) was calculated of each sample against free radicals as shown in .[Citation31] It can be seen that the EC50 value of DPPH and ABTS free radicals were decreased after embedding resveratrol in accord with Pan et al.[Citation32] This may be due to the large surface area of the RES on the microcapsule at the reaction medium. In conclusion, after encapsulated by SR ammonium salt, the antioxidant activities of RES were remained, which has good scavenging ability for DPPH and ABTS+ free radicals.
Table 3. EC50 value of antioxidant formulations on the DPPH· and ABTS+·
Therefore, the new resveratrol carrier manifested the hopeful properties for the new effective intestinal formulation, which could be used into the functional delivery system and regulate the balance of intestinal ecological with taking the lower amount of antioxidants and the high radical scavenging activity. The natural polymers of SR used in coating material have been reported before. In this study, the amino group grafted into SR to regard as wall material, and resveratrol microcapsule were successfully prepared to improve the stability and bioavailability of resveratrol, at the same time improve the antioxidant activity. This new delivery system can act as a favorable carrier for insoluble antioxidants in the potential use of new food delivery system on functional food supplements.
Conclusion
The microcapsule (SRAS/RES) was successfully prepared to enhance the stability, bioavailability, and the radical scavenging activity of RES and make the best use of food delivery system on functional food supplements. The ammonium salts of SR were successfully synthesized and used to fabricate the microcapsule. The microcapsule showed perfect stability and outstanding sustained release character in vitro intestinal release test. Moreover, compared to the pure RES, the microcapsule showed a dose-dependent manner with a higher radical scavenging activity of DPPH· and ABTS+, which can be used widely to the further food or nutrient applied research.
Additional information
Funding
References
- Kong, D. X.; Li, Y. Q.; Bai, M.; Deng, Y. L.; Liang, G. X.; Wu, H. A Comparative Study of the Dynamic Accumulation of Polyphenol Components and the Changes in Their Antioxidant Activities in Diploid and Tetraploid Lonicera Japonica. Plant Physiol. Biochem. 2017, 112, 87–96. DOI: 10.1016/j.plaphy.2016.12.027.
- Ali, M.; Khan, T.; Fatima, K.; Ali, Q. U. A.; Ovais, M.; Khalil, A. T.; Ullah, I.; Raza, A.; Shinwari, Z. K.; Idrees, M. Selected Hepatoprotective Herbal Medicines: Evidence from Ethnomedicinal Applications, Animal Models, and Possible Mechanism of Actions. Phytotherapy Res. 2018, 32, 199–215. DOI: 10.1002/ptr.5957.
- Abbasi, S.; Gharaghani, S.; Benvidi, A.; Latif, A. M. Identifying the Novel Natural Antioxidants by Coupling Different Feature Selection Methods with Nonlinear Regressions and Gas Chromatography-Mass Spectroscopy. Microchem. J. 2018, 139, 372–379. DOI: 10.1016/j.microc.2018.03.012.
- Hung, L. M.; Chen, J. K.; Huang, S. S.; Lee, R. S.; Su, M. J. Cardioprotective Effect of Resveratrol, A Natural Antioxidant Derived from Grapes. Cardiovasc. Res. 2000, 47, 549–555. DOI: 10.1016/S0008-6363(00)00102-4.
- Eybl, V.; Kotyzova, D.; Koutensky, J. Comparative Study Of Natural Antioxidants-Curcumin, Resveratrol And Melatonin-In Cadmium-Induced Oxidative Damage In Mice. Toxicology. 2006, 225, 150–156. DOI: 10.1016/j.tox.2006.05.011.
- Duan, X.; Li, M.; Ma, H. J.; Xu, X.-M.; Jin, Z. Y.; Liu, X. B. Physicochemical Properties and Antioxidant Potential of Phosvitin-Resveratrol Complexes in Emulsion System. Food Chem. 2016, 206, 102–109. DOI: 10.1016/j.foodchem.2016.03.055.
- Borges, S. C.; Ferreira, P. E. B.; Da Silva, L. M.; De PaulaWerner, M. F.; Irache, J. M.; Cavalcanti, O. A.; Buttow, N. C. Evaluation of the Treatment with Resveratrol-Loaded Nanoparticles in Intestinal Injury Model Caused by Ischemia and Reperfusion. Toxicology. 2018, 396-397, 13–22. DOI: 10.1016/j.tox.2018.02.002.
- Martín, A. R.; Villegas, I.; La Casa, C.; De La Lastra, C. A. Resveratrol, A Polyphenol Found in Grapes, Suppresses Oxidative Damage and Stimulates Apoptosis during Early Colonic Inflammation in Rats. Biochem. Pharmacol. 2004, 67, 1399–1410. DOI: 10.1016/j.bcp.2003.12.024.
- Baer-Dubowska, W.; Zielinska-Przyjemska, M.; Ignatowicz, E.; Rimando, A. Effects of Polyphenols: Resveratrol and Its Natural Analogues and Tannic Acid on DNA Oxidative Damage and Apoptosis in Human Neutrophils. Planta Med. 2010, 76, 489–508. DOI: 10.1055/s-0030-1265853.
- Schneider, Y.; Duranton, B.; Gossé, F.; Schleiffer, R.; Seiler, N.; Raul, F. Resveratrol Inhibits Intestinal Tumorigenesis and Modulates Host-Defense-Related Gene Expression in an Animal Model of Human Familial Adenomatous Polyposis. Nutr. Cancer. 2001, 39, 102–107. DOI: 10.1207/S15327914nc391_14.
- Brill, S. S.; Furimsky, A. M.; Ho, M.-N.; Furniss, M. J.; Li, Y.; Green, A. G.; Bradford, W. W.; Green, C. E.; Kapetanovic, I. M.; Iyer, L. V. Glucuronidation of Trans-Resveratrol by Human Liver and Intestinal Microsomes and UGT Isoforms. J. Pharm. Pharmacol. 2006, 58, 469–479. DOI: 10.1211/jpp.58.4.0006.
- Deng, L. L.; Zhang, X.; Li, Y.; Que, F.; Kang, X. F.; Liu, Y. Y.; Feng, F. Q.; Zhang, H. Characterization of Gelatin/Zein Nanofibers by Hybrid Electrospinning. Food Hydrocolloids. 2018, 75, 31271–31277. DOI: 10.1016/j.foodhyd.2017.09.011.
- Hemmati, K.; Ghaemy, M. Synthesis Of New Thermo/pH Sensitive Drug Delivery Systems Based On Tragacanth Gum Polysaccharide. Int. J. Biol. Macromol. 2016, 87, 415–425. DOI: 10.1016/j.ijbiomac.2016.03.005.
- Liu, R.; Liu, D. Y.; Liu, Y.; Song, Y. S.; Wu, T.; Zhang, M. Using Soy Protein SiOx Nanocomposite Film Coating To Extend The Shelf Life Of Apple Fruit. Int. J. Food Sci. Technol. 2017, 52, 2018–2030. DOI: 10.1111/ijfs.13478.
- Alzahrani, H.; Bedir, Y.; Al-Hayani, A. Efficacy of Shellac, A Natural Product, for the Prevention of Wet Gangrene. J. Int. Med. Res. 2013, 41, 795–803. DOI: 10.1177/0300060513483391.
- Licchelli, M.; Malagodi, M.; Somaini, M.; Weththimuni, M.; Zanchi, C. Surface Treatments of Wood by Chemically Modified Shellac. Surf. Eng. 2012, 29, 121–127. DOI: 10.1179/1743294412Y.0000000069.
- Patel, A. R.; Rajarethinem, P. S.; Gredowska, A.; Turhan, O.; Lesaffer, A.; De Vos, W. H.; Van De Walle, D.; Dewettinvk, K. Edible Applications of Shellac Oleogels: Spreads, Chocolate Paste and Cakes. Food Funct. 2014, 5, 645–652. DOI: 10.1039/c4fo00034j.
- Patel, A.; Heussen, P.; Hazekamp, J.; Velikov, K. P. Stabilisation And Controlled Release Of Silibinin From pH Responsive Shellac Colloidal Particles. Soft Matter. 2011, 7, 8549–8555. DOI: 10.1039/C1SM05853C.
- Soradech, S.; Limatvapirat, S.; Luangtana-Anan, M. Stability Enhancement of Shellac by Formation of Composite Film: Effect of Gelatin and Plasticizers. J. Food Eng. 2013, 116, 572–580. DOI: 10.1016/j.jfoodeng.2012.12.035.
- Cui, L.; Liu, Z. P.; Yu, D. G.; Zhang, S. P.; Annie Bligh, S. W.; Zhao, N. Electrosprayed Core-Shell Nanoparticles of PVP and Shellac for Furnishing Biphasic Controlled Release of Ferulic Acid. Colloid Polym. Sci. 2014, 292, 2089–2096. DOI: 10.1007/s00396-014-3226-8.
- Limmatvapirat, S.; Panchapornpon, D.; Limmatvapirat, C.; Nunthanid, J.; Luangtana-Anan, M.; Puttipipatkhachorn, S. Formation of Shellac Succinate Having Improved Enteric Film Properties through Dry Media Reaction. Eur. J. Pharm. Biopharm. 2008, 70, 335–344. DOI: 10.1016/j.ejpb.2008.03.002.
- Carvalho, A. G. S.; Silva, V. M.; Hubinger, M. D. Microencapsulation by Spray Drying of Emulsified Green Coffee Oil with Two-Layered Membranes. Food Res. Int. 2014, 61, 236–245. DOI: 10.1016/j.foodres.2013.08.012.
- Wang, K.; Wang, Y.; Zhao, X.; Li, Y.; Yang, T.; Zhang, X.; Wu, X. Sustained Release of Simvastatin from Hollow Carbonated Hydroxyapatite Microspheres Prepared by Aspartic Acid and Sodium Dodecyl Sulphate. Mater. Sci. Eng. C. 2017, 75, 565–571. DOI: 10.1016/j.msec.2017.02.066.
- Guo, C. J.; Yin, J. G.; Chen, D. Q. Co-Encapsulation of Curcumin and Resveratrol into Novel Nutraceutical Hyalurosomes Nano-Food Delivery System Based on Oligo-Hyaluronic Acid-Curcumin Polymer. Carbohydr. Polym. 2018, 181, 1033–1037. DOI: 10.1016/j.carbpol.2017.11.046.
- Musa, K. H.; Abdullah, A.; Alhaiqi, A. Determination of DPPH Free Radical Scavenging Activity: Application of Artificial Neural Networks. Food Chem. 2016, 194, 705–711. DOI: 10.1016/j.foodchem.2015.08.038.
- Intarasirisawat, R.; Benjakul, S.; Visessanguan, W.; Wu, J. Effects of Skipjack Roe Protein Hydrolysate on Properties and Oxidative Stability of Fish Emulsion Sausage. LWT Food Sci. Technol. 2014, 58, 280–286. DOI: 10.1016/j.lwt.2014.02.036.
- Martins, I. M.; Barreiro, M. F.; Coelho, M.; Rodrigues, A. E. Microencapsulation of Essential Oils with Biodegradable Polymeric Carriers for Cosmetic Applications. Chem. Eng. J. 2014, 245, 191–200. DOI: 10.1016/j.cej.2014.02.024.
- Jyothi, N. V. N.; Prasanna, P. M.; Sakarkar, S. N.; Prabha, K. S.; Ramaiah, P. S.; Srawan, G. Y. Microencapsulation Techniques, Factors Influencing Encapsulation Efficiency. J. Microencapsulation. 2010, 27, 187–197. DOI: 10.3109/02652040903131301.
- Kobierski, S.; Oforikwakye, K.; Müller, R.-H.; Keck, C. M. Resveratrol Nanosuspensions for Dermal Application-Production, Characterization, and Physical Stability. Pharmazie. 2009, 64, 741–747. DOI: 10.1691/ph.2009.9097.
- Dima, C.; Pǎtraşcu, L.; Cantaragiu, A.; Alexe, P.; Dima, Ş. The Kinetics of the Swelling Process and the Release Mechanisms of Coriandrum Sativum L. Essential Oil from Chitosan/Alginate/Inulin Microcapsules. Food Chem. 2015, 195, 39–48. DOI: 10.1016/j.foodchem.2015.05.044.
- Chen, Z.; Bertin, R.; Froldi, G. EC50 Estimation of Antioxidant Activity in DPPH· Assay Using Several Statistical Programs. Food Chem. 2013, 138, 414–420. DOI: 10.1016/j.foodchem.2012.11.001.
- Pan, K.; Zhong, Q.; Baek, S. J. Enhanced Dispersibility and Bioactivity of Curcumin by Encapsulation in Casein Nanocapsules. J. Agric. Food Chem. 2013, 61, 6036–6043. DOI: 10.1021/jf400752a.