ABSTRACT
The objective of this study was to evaluate the effect of harvest at different times on the chemical composition of phenolic compounds of Artemisia herba-alba. Plant material has been harvested at three times (July, November, and May). Methanolic plant extracts were obtained by means of ultrasound-assisted extraction and then analyzed by HPLC–electrospray ionization–Q-TOF–MS for their phenolic profile. The HPLC allowed the identification of 26 phenolic compounds, 12 of them were identified for the first time for this material. The phenolic compounds detected were 8 phenolic acids, 12 flavones, 4 isoflavone, 1 flavonol, and 1 flavanone. Results showed that during the all harvesting date, phenolic acids were predominant. In November, at the flowering period, it revealed the highest accumulation of total phenolic contents (515 ± 142 mg GAE/g dry matter weight [DMW]) in the methanolic extracts. November at the flowering period was characterized by the highest levels of flavonoids 8.3 mg/g DMW and the lowest contents of phenolic acids 3 mg/g DMW.
Introduction
There has been an increasing interest in the research of flavonoids from dietary sources, due to the growing evidence of the versatile health benefits of flavonoids through epidemiological studies. As occurrence of flavonoids is directly associated with human daily dietary intake of antioxidants, it is important to evaluate flavonoid sources in food.[1] Flavonoids are an integral part of human and animal diet. Being phytochemicals, flavonoids cannot be synthesized by humans and animals.[Citation2]
Flavonoids and phenolic acids make up one of the most pervasive groups of plant phenolics. Due to their importance in plants and human health, it would be useful to have a better understanding of flavonoid concentration and biological activities that could indicate their potentials as therapeutic agents, and also for predicting and controlling the quality of medicinal herbs.[Citation2]
The genus Artemisia belongs to one of the largest and most widely distributed genera of the family Asteraceae (Compositae). It is a diverse and economically important genus and it has more than 500 species. Most plants within this genus have a great importance as medication, foodstuff, ornamentals, or soil stabilizers.[Citation3–Citation6] Phytochemical investigations have proven that this genus is rich in terpenoids, flavonoids, coumarins, acetylenes, caffeoylquinic acids, and sterols and it has been shown that Artemisia has multiple beneficial bioactivities such as antimalarial, antiviral, antitumor, antipyretic, antihemorrhagic, anticoagulant, antianginal, antioxidant, anti-hepatitis, anti-ulcerogenic, antispasmodic, and anticomplementary activities.[Citation7]
Eleven species of Artemisia can be found in Algerian flora.[Citation8] Artemisia herba-alba grows commonly on the Algerian steppe. This plant is widely used in the traditional medicine to treat diabetes, bronchitis, diarrhea, hypertension, and neuralgias.[Citation9,Citation10] Various secondary metabolites have been isolated from A. herba-alba, perhaps the most important being the sesquiterpene lactones. Several researches have been conducted on the chemical composition of the essential oil[Citation11] but very few studies have studied the chemical composition of polyphenols of this plant.
Several research studies have shown that the polyphenol content of a medicinal plant is very variable. In particular, environmental characteristics strongly influence the ability to synthesize secondary metabolites in medicinal plants.[Citation12] The chemical composition of a secondary metabolite is very complex and subject to many variables. Knowing the exact constituents of these metabolites is fundamental, both to verify its quality, to explain its properties, and to predict its potential toxicity.
The aim of this study was to determine the effect of the harvest period on the phenolic compounds of A. herba-alba and evaluate the antioxidant potential of the plant extracts. This is the first study regarding the detailed chemical characterization of its phenol constituents, and the variation in the phenol profile of this plant was collected during its different ontogenic growth in order to determine the most favorable harvest time characterized by the highest content of bioactive compounds.
Experimental methods
Plant material
The aerial parts of A. herba-alba utilized in this study were collected in summer at a late vegetative stage (July, 2013), in winter at full-flowering stage (December 2013), and in spring at early vegetative stage (May, 2014). A. herba-alba samples were collected from Ain Bel (Moudjebara locality). The collection site Moudjebara (34° 30′ 15.45″N, 3° 28′ 18.74″E, 1040 m above the sea) was characterized by a lower semiarid climate with an annual average temperature of 14.7°C and rainfall of 310 mm. The identification of the species was realized by Professor Dahia Mustapha, a botanist at the University of Ziane Achour, Faculty of Sciences of Djelfa, Algeria. A voucher specimen was deposited at the Herbarium of the Laboratory of Biotechnology Plants at the Saad Dahlab University of Blida. Samples were air-dried during 15 days in the laboratory in a room temperature till the weight stayed stable.
Humidity and rainfall for the months of the study were collected from nearby meteorological station belonging to the Algerian Meteorological Service to estimate the monthly mean air humidity and the monthly mean rainfall of sampling site ().
Table 1. Estimated mean of humidity and rainfall for the months of the study
Preparation of Artemisia extracts
Dry plant materials was grounded to a fine powder in a mechanic grinder and extracted by ultrasound-assisted solvent extraction which were carried out as reported by Shu et al.[Citation13] with some modifications. Briefly, plant material (0.5 g) was extracted with 20 mL of methanol in an ultrasonic bath for 30 min and centrifuged at 3500 rpm for 15 min. The extraction was repeated twice. The supernatants were collected, evaporated, and reconstituted in 1 mL of methanol. The final extracts were filtered though Whatman no. 4 filter paper and kept at −4°C before the analysis.
Total phenols (Folin–Ciocalteu)
The extracts of total phenol content were determined using the Folin–Ciocalteu reagent and tannic acid as standard as described by Adedapo et al.[Citation14] The extract sample (0.5 mL) and 7.5 mL of sodium carbonate (20%) were added to 2.5 mL of Folin–Ciocalteu reagent. After 16–18 h of reaction at room temperature, the absorbance was measured at 765 nm in a Secomam UVI Lumière XT5 spectrophotometer. The analyses were performed in triplicate. The amount of total phenolic compounds was calculated as mg of tannic acid equivalents and expressed as mg tannic acid/g dry weight (DW) of the plant material. The calibration equation for tannic acid was y = 0.0039x − 0.0008 (R2 = 0.9962) where y is the absorbance and x is the concentration of tannic acid in mg/mL.
Total flavonoids
Estimation of the total flavonoids in the plant extracts was carried out using the method of Ordon et al.[Citation15] Briefly, a diluted solution (2 mL) of each extract was mixed with an equal volume of aluminum trichloride (AlCl3) in methanol (2%). The absorbance was read at 420 nm after 1 h against a blank sample consisting of a methanol (2 mL) and extract (2 mL) without AlCl3. Quercetin was used as reference compound to produce the standard curve, and results were expressed as g of quercetin equivalents (QEs)/g of dry mass using the following equation based on the calibration curve: y = 0.0322x − 0.0146, R2 = 0.9969, where x was the absorbance and y was the QE (mg/g).
HPLC–MS analysis
Polyphenol analyses were performed using an Agilent 1200 RR HPLC coupled to a diode array detector and mass spectrometer (QSTAR, Elite mode) (Agilent Technologies, Palo Alto, CA, USA). A C18 analytical column of 2.5 mm × 50 mm and 2.6 µm particle size (Kinetex XB-C18) was used. The inlet flow rate was maintained at 0.4 mL/min. The column oven temperature was set at 40°C.
The mobile phase consisted of 1% (v/v) formic acid in water (eluent A) and of 0.1% Acetonitrile and formic acid (10/90, v/v; eluent B). The flow rate was 0.4 mL/min. The gradient program was as follows: 0 min, 6% B; 14 min, 16.5% B; 16 min, 17% B; 18 min, 17.5% B; 20 min, 17.5% B; 22 min, 18.5% B; 24 min, 18.5% B; 27 min, 20% B; 46 min, 100% B; 48 min, 100% B;48.1 min, 6% B; and 54 min, 6% B. The injection volume was 0.5 μL. Phenolic compounds were monitored separately at 365 nm.
Mass spectrometry conditions
Mass spectrometry was performed using a QSTAR Elite LC–MS–MS system coupled with an electrospray ionization (ESI) interface and was operated in negative ion mode. In this study, the parameters were optimized as follows: ESI voltage, −4200 V; nebulizer gas, 60; auxiliary gas, 50; curtain gas, 32; turbo gas temperature, 400°C; declustering potential, −60 V; focusing potential, −190 V; and declustering potential, −15 V. The samples were analyzed with an information-dependent acquisition method, which can automatically select candidate ions for the MS–MS study. The TOF mass range was set from m/z 70 to 800. The collision energy was set to 30 eV to observe the pseudomolecular [M − H]− ion. The accurate-mass capability of the TOF analyzer allowed reliable confirmation of the identity of the detected metabolites, normally with mass errors below 5 ppm in routine analysis, which was sufficient to verify the chemical constituents in A. herba-alba. The mass analyzer was calibrated using Taurocholic acid (2 ng/µL) by direct injection at a flow rate of 5 µL/min. The data were acquired and processed using Analyst QS 2.0 software.
Quantification
A commercial patron called “Ginkgo biloba flavonoids mix” of sigma was acquired – Aldrich containing quercetin, kaempferol, and isorhamnetin. The stock solutions of reference standards were prepared by dissolving them in methanol (100 μg/mL). Calibration curves were established by diluting the stock solutions with methanol in appropriate quantities. The quantities of compounds were calculated from an external standard calibration curves established on four concentrations in the following range: 8.5–100 mg/mL for quercetin, 4–80 mg/mL for kaempferol, and 4–73 mg/mL for isorhamnetin. The data relevant for obtaining the calibration curves are shown in .
Table 2. Regression equation, correlation coefficients, linearity ranges, LOD, and LOQ for three analytes
Results and discussion
Effect of harvest time on yield and components of polyphenols
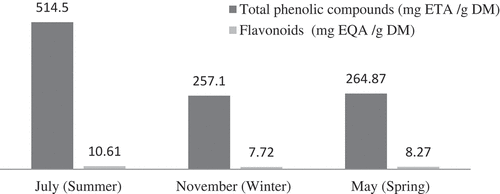
Results of quantitative estimation of phenols in the aerial parts of A. herba-alba during the different harvest date are given in . The content of phenols varied significantly with harvest time. The lowest level of phenols was detected in November (265 ± 48 mg/g DMW), whereas the highest content was enregistered during July late vegetative stage (515 ± 142 mg/g DMW). A consistent decrease in the amounts of phenols was observed during May with 257 ± 87 mg/g DMW. Sellami et al.[Citation16] reported the highest accumulation of total phenolics at the late vegetative stage of Origanum majorana. These results suggest that the late vegetative stage could be characterized by the maximum growth period of this plant. In fact, it could be postulated that during the late vegetative stage, the plant accumulates phenolics to prepare itself to the lignification process in order to slow down its growth. The summer season is known by the simultaneous presence of many types of abiotic stress (high temperatures, lack of water, solar radiation). The accumulation of phenolic compounds for this season may be due to the fact that during this critical period of the year, the protection of the plant is mainly ensured by the synthesis of phenolic compounds strongly accumulated during this season.[Citation16] Indeed, it is well known that flavonoids and phenolic acids play important role in the mechanisms of adaptations of plants to several stresses.[Citation17] In another studying, seasonal change in contents of phenolic compounds in several species, such as Boerhavia diffusa, Sida cordifolia,[Citation18] Hypericum pruinatum,[Citation19] Thymbra spicata, and Satureja thymbra,[Citation20] investigators have found a maximal production of phenolics at flowering. Regarding these variations in the accumulation of secondary metabolites in A. herba-alba plants, it could be concluded that the physiological stage of the plant affects the choice of best harvesting time.
Identification of phenolic compounds by HPLC–DAD–ESI–TOF–MS
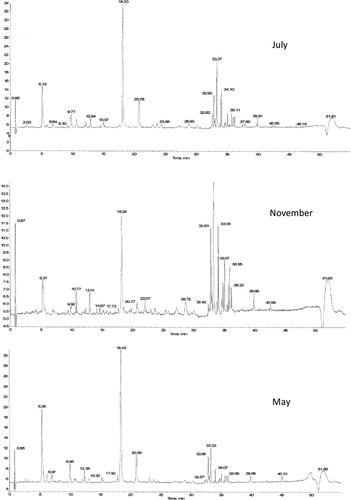
To determine the phenolic profile in A. herba-alba, the extracts were analyzed by HPLC coupled to DAD and ESI–TOF–MS. Peak identification was performed on the basis of their retention times, their UV–vis and mass spectra together with the information previously were reported in the literature. The results are shown in and .
Table 3. Phenolic compounds identified by HPLC/ESI–MS in Artemisia herba-alba extracts
In the present work, a total of 26 phenolic compounds were identified in the methanolic extracts of A. herba-alba, 12 of them (Vitexin, Tomentin, 5,3′-dihydroxy-7,4′-dimethoxyflavanone, Chrysoeriol, Tectorigenin, Iristectorigenin A, Iristectorigenin B, Irigenin, Skullcapflavon I, Skullcapflavon II, Cirsiliol, Chrysoeriol-methyl-ether) were identified for the first time for this material. The substances were 8 phenolic acids, 12 flavones, 4 isoflavone, 1 flavonol, and 1 flavanone. In a previous study, vicenin-2, isoschaftoside, cirsilineol, cirsimaritin, acacetin were identified in extracts of A. herba-alba collected from Sinai.[Citation31,Citation32] Also, ferulic acid was previously identified and described in A. herba-alba and in other Artemisia species.[Citation33] 3,4,5-Tricaffeoylquinique acid, 3,5-dicaffeoylquinique acid, and 3-caffeoylquinic acid have been detected previously in study of Dahmani–Hamzaoui et al.[Citation34] Caffeoylquinic acids were identified in several Artemisia species.[Citation34] Chrysoeriol was reported for the first time in this species. Thus, this compound was detected in Artemisia frigida.[Citation32] Cirsiliol was well described in Artemisia annua, in Artemisia campestris[Citation35] and as far we know, it was tentatively determined in this species for the first time.
Variation of the amounts of different phenolic acids and flavonoids compounds during the three harvest time is given in . Results showed that during the all harvesting date, dicaffeoylquinic acid and its derivatives were predominant. These results are in agreement with those of Carvalho et al.[Citation36] who reported that caffeic acid and ferulic acid conjugates were the most dominant hydroxycinnamic acids in Artemisia leaves of A. annua, A. arborescens, A. ludoviciana, A. oleandica, A. princeps, and A. stelleriana. Dicaffeoylquinic acid showed an important decrease from 12.6 mg/g DW in July to 3 mg/g DW in November but then increased to achieve the amount of 13.1 mg/g DW in May. The levels of caffeic and ferulic acid conjugates in the A. herba-alba during the three harvest date were much higher than those reported by Carvalho et al.[Citation36] in six species of Artemisia. However, Sellami et al.[Citation16] reported high amounts of caffeic acid dimer at the late vegetative stage of O. majorana. November (the flowring stage) was characterized by the highest levels of flavonoids (8.3 mg/g of DMW) and the lowest contents of phenolic acids (3 mg/g of DMW) whereas May (the early vegetative stage) was characterized by the lowest contents of flavonoids (4.9 mg/g of DMW) and the highest levels of phenolic acids (14.2 mg/g DMW). These results are in agreement with those of Papageorgiou et al. (2008) and Sellami et al.[Citation16] who reported that flavonoids were predominant during the flowering stage of O. majorana. Also, Skrzypczak–Pietraszek et al.[Citation37] reported the contents of flavonoids and phenolic acids are lowest at the beginning of vegetative period and increase during summer to achieve the highest amounts at the end of phenological cycle. Our results on flavonoids in A. herba-alba herb suit to that pattern. On the other hand, Tan et al.[Citation38] obtained for Asiatic species – Artemisia scoparia – quite opposite results: lowest contents of phenolic acids and highest levels of flavonoids at the end of vegetative period.
Table 4. Phenolic profiling of A. herba-alba methanolic extracts in the course of three harvest time
Phenolic composition of A. herba-alba varied significantly with growth stage. In fact, in May (spring) and in July (summer), phenolic acids were the major compound (14.2 mg/g of DMW, 13.4 mg/g of DMW, respectively) followed by flavone (2.7 mg/g of DMW, 3.3 mg/g of DMW, respectively), isoflavone (1.9 mg/g of DMW, 3 mg/g of DMW, respectively), and flavonol (0.3 mg/g of DMW, 00 µg/g of DMW, respectively). In November, flavone became the major compound (6.4 mg/g of DMW) followed by phenolic acids (3 mg/g of DMW), isoflavone (1.4 mg/g of DMW), and flavanone (0.5 mg/g of DMW). These are in agreement with the reported results of Sartor et al.[Citation39] who proved that the levels of caffeic acid of Baccharis dentata were, in general, higher in spring, summer. Also the increased concentrations of caffeic acid and chlorogenic acid were found in Nicotiana tabacum and Mahonia repens cultivated under low temperatures.[Citation29]
The UV/vis spectrophotometric determination is one of the most widely used methods for quantification of total flavonoids in raw plant materials due to its simplicity, low cost of implementation, and wide availability in laboratories for quality control. On the other hand, the HPLC analysis is an analytical procedure more sensitive and selective in the area of natural products to quantify isolated substances and is widely used for all classes of flavonoids.[Citation30]
When comparing simple spectrophotometric and HPLC quantification methods to one another, the responses of spectrophotometric methods are far superior to the results observed for HPLC. The reason for this difference in values is that during the HPLC analysis, we were unable to identify and quantify all the flavonoid compounds in our samples where there are five flavonoid compounds remaining unknown.
Despite the wide applicability of the both techniques mentioned above, some questions about the specific city and comparability of their results have been done in view of the broad structural variability presented by the flavonoid compounds, as well as limitations inherent in each methodology. Accordingly, several scientific studies conducted with both procedures have shown conflicting results. Moreover, the choice to quantify a set of compounds or isolated compounds in biological matrices such as herbal material is a very controversial point in the analysis and quality control.[Citation30]
Conclusion
A. herba-alba is a rich source of polyphenol compounds, the levels of phenolic compounds, including flavonoids, varied in quantity and quality depending on the harvest time. Twelve new compounds were successfully characterized for the first time from A. herba-alba sample. The new phenolic compounds detected were 1 C-glycosyl flavone (Vitexin), 1 flavonol aglycone (Tomentin), 2 flavanone aglycones (5,3′-dihydroxy-7,4′-dimethoxyflavanone, Chrysoeriol), and 4 isoflavone aglycones (Tectorigenin, Iristectorigenin A, Iristectorigenin B, Irigenin), 4 flavone aglycones (Cirsiliol, Skullcapflavon I, cirsilineol, Skullcapflavon II). In A. herba-aba samples, phenolic acids predominate in both vegetative stages, while flavonoids are the most important at the flowering stage. The results show is not possible recommended the best time for collecting of A. herba-alba herb because the chemical profile of phenolic compound were greatly affected by growth developmental stages. Finally, the richness of A. herba-alba in phenolic active compounds known for their antioxidant, antimicrobial, and insecticidal activities could support the utilization of this plant in a large field of application including cosmetic, pharmaceutical, agro alimentary, and biological defense.
References
- Yao, L.-H.; Jiang, Y.-M.; Shi, J.; Toma´ S-Barbera´ N, F. A.; Datta, N.; Singanusong, R.; Chen, -S.-S. Flavonoids in Food and Their Health Benefits. Plant. Food. Human Nutr. 2004, 59, 113–122. DOI: 10.1007/s11130-004-0049-7.
- Shashank, K.; Abhay, K. P. Chemistry and Biological Activities of Flavonoids. Hindawi Publishing Corporation. Scie. World. J. 2013. Article ID 162750, 16 pages.
- Hayat, M.-Q.; Ashraf, M.; Khan, M.-A.; Jabeen, S. Ethnobotany of the Genus Artemisia L. (Asteraceae) in Pakistan. Ethnobot. Res. Appl. 2009, 7, 147–162. DOI: 10.17348/era.7.0.147-162.
- Hayat, M.-Q.; Ashraf, M.; Khan, M.-A.; Yasmin, G.; Shaheen, N.; Jabeen, S. Palynological Study of the Genus Artemisia (Asteraceae) and Its Systematic Implications. Pak. J. Bot. 2010, 42(2), 751–763.
- Pareto, G. A.;. Ricerca Ed applicazione. Q. Agric. Suppl. 1985, 2, 1–261.
- Tan, R.-X.; Zheng, W.-F.; Tang, H.-Q. Biologically Active Substances from the Genus Artemisia. Planta. Med. 1998, 64, 295–302. DOI: 10.1055/s-2006-957438.
- Tilaoui, M.; Mouse, H.-A.; Jaafari, A.; Aboufatima, R.; Chait, A.; Zyad, A. Chemical Composition and Antiproliferative Activity of Essential Oil from Aerial Parts of a Medicinal Herb Artemisia Herba-Alba. Rev. Bras. Farmacogn. 2011, 21(4), 781–785. DOI: 10.1590/S0102-695X2011005000114.
- Quezel, P.; Santa, S. Nouvelle Flore de l’Algérie et des régions désertiques méridionales (Tome II); CNRS: Paris, 1963.
- Said, O.; Khalil, K.; Fulder, S.; Azaizeh, H. Ethnopharmacological Survey of Medicinal Herbs in Israel, the Golan Heights and the West Bank Region. J. Ethnopharmacol. 2002, 83(3), 251–265.
- Tahraoui, A.; El-Hilalya, J.; Israili, Z. H.; Lyoussi, B. Ethnopharmacological Survey of Plants Used in the Traditional Treatment of Hypertension and Diabetes in South-Eastern Morocco (Errachidia Province). J. Ethnopharmacol. 2007, 17(1), 105–117.
- Tepe, B.; Daferera, D.; Tepe, A.; Polissiou, M.; Sokmen, A. Antioxidant Activity of the Essential Oil and Various Extracts of Nepta Flavida Hud. -Mor. From Turkey. Food Chem. 2007, 103, 1358–1364. DOI: 10.1016/j.foodchem.2006.10.049.
- Pacifico, S.; Galasso, S.; Piccolella, S.; Kretschmer, N.; Pan, S.; Marciano, S.; Bauer, R.; Monaco, P. Seasonal Variation in Phenolic Composition and Antioxidant and Anti-Inflammatory Activities of Calamintha Nepeta (L.) Savi. Food Res. Int. 2015, 69, 121–132. DOI: 10.1016/j.foodres.2014.12.019.
- Shu, P.; Hong, J.-L.; Wu, G.; Yu, B.-Y.; Qin, M.-J. Analysis of Flavonoids and Phenolic Acids in Iris Tectorum by HPLC-DAD-ESI-MS. Chinese. J. Nat. Med. 2010, 8(3), 0202−0207. DOI: 10.3724/SP.J.1009.2010.00202.
- Adedapo, -A.-A.; Jimoh, F.-O.; Afolayan, A.-J.; Masika, P.-J. Antioxidant Properties of the Methanol Extracts of the Leaves and Stems of Celtis Africana. Rec. Nat. Prod. 2009, 3(1), 23–31.
- Ordon, E.-A.-A. L.; Gomez, J.-D.; Vattuone, M.-A.; Isla, M.-I. Antioxidant Activities of Sechium Edule (Jacq.) Swart Extracts. Food. Chem. 2006, 97, 452–458. DOI: 10.1016/j.foodchem.2005.05.024.
- Sellami, I.-H.; Maamouri, E.; Chahed, T.; Wannes, A.-W.; Kchouk, E.-M.; Marzouk, B. Effect of Growth Stage on the Content and Composition of the Essentialoil and Phenolic Fraction of Sweet Marjoram (Origanum Majorana L.). Ind. Crops. Prod. 2009, 30, 395–402. DOI: 10.1016/j.indcrop.2009.07.010.
- Dixon, R.-A.; Paiva, N.-L. Stress-Induced Phenyl Propanoid Metabolism. Plant Cell. 1995, 7, 1085–1097. DOI: 10.1105/tpc.7.7.1085.
- Verma, V.; Kasera, P.-K. Variations in Secondary Metabolites in Some Arid Zone Medicinal Plants in Relation to Season and Plant Growth. Indian. J. Plant. Phy. 2007, 12, 203–206.
- Ayan, A. K.; Yanar, P.; Cirak, C.; Bilgener, M. Morphogenetic and Diurnal Variation of Total Phenols in Some Hypericum Species from Turkey during Their Phenological Cycles. Bangladesh J. Botany. 2007, 36, 39–46. DOI: 10.3329/bjb.v36i1.1547.
- Müller-Riebau, F.-J.; Berger, B.-M.; Yegen, O.; Cakir, C. Seasonal Variations in the Chemical Compositions of Essential Oils of Selected Aromatic Plants Growing Wild in Turkey. J. Agr. Food. Chem. 1997, 45, 4821–4825. DOI: 10.1021/jf970110y.
- Parejo, I.; Jauregui, O.; Sanchez-Rabaneda, F.; Viladomat, F.; Bastida, J.; Codina, C. Separation and Characterization of Phenolic Compounds in Fennel (Foeniculum Vulgare) Using Liquid Chromatography–Negative Electrospray Ionization Tandem Spectrometry. J. Agric. Food. Chem. 2004, 52, 3679–3687. DOI: 10.1021/jf030813h.
- Jian, H.; Min, Y.; Xue, Q.; Man, X.; Baorong, W.; Dean, G. Characterization of Phenolic Compounds in the Chinese Herbal Drug Artemisia Annua by Liquid Chromatography Coupled to Electrospray Ionization Mass Spectrometry. J. Pharmaceut. Biomed. Anal. 2008, 47(3), 516–525. DOI: 10.1016/j.jpba.2008.02.013.
- Sumei, Y.Weixia, Q. 5, 30-Dihydroxy-7,40-dimethoxyflavanone from Artemisia sphaerocephala Kraschen. Acta Crystallographica Section E. 2008, Structure Reports Online ISSN 1600-5368
- Sánchez-Rabaneda, F.; Jáuregui, O.; Lamuela-Raventós, R.M.; Viladomat, F.; Bastida, J.Codina, C. Qualitative Analysis Of Phenolic Compounds in Apple Pomace Using Liquid Chromatography Coupled to Mass Spectrometry in Tandem Mode. Rapid Communications In Mass Spectrometry. 2004, 18, 553–563 doi:10.1002/(ISSN)1097-0231
- Ferreira, J.-F. S.; Devanand, -L.-L.; Tomikazu, S.-A. H. Flavonoids from Artemisia Annua L. As Antioxidants and Their Potential Synergism with Artemisinin against Malaria and Cancer. Molecules. 2010, 15(5), 3135–3170. DOI: 10.3390/molecules15053135.
- Jian, H.; Min, Y.; Man, X.; Jianghao, S.; Baorong, W.; Dean, G. Characterization Of Flavonoids In The Traditional Chinese Herbal medicine-Huangqin By Liquid Chromatography Coupled With Electrospray Ionization Mass Spectrometry. J. Chromatogr B. 2007, 848(2), 355–362. DOI: 10.1016/j.jchromb.2006.10.061.
- Gobbo-Neto, L.; Lopes, N.-P. Medicinal Plants: Factors of Influence on the Content of Secondary Metabolites. Quim. Nova. 2007, 30, 374–381. DOI: 10.1590/S0100-40422007000200026.
- Graziella, S. M.; Waleska, F. L.; Magaly, A. M. L.; Monize, S. P.; Rebeka, P. M. M.; Larissa, A. R.; Haroudo, S. X.; Pedro, J. R. N.; Luiz, A. L. S. Comparative Evaluation of UV/VIS and HPLC Analytical Methodologies Applied for Quantification of Flavonoids from Leaves of Bauhinia Forficata, Brazil. J. Pharmacog. 2013, 23(1), 51–57.
- Justesen, U.;. Collision-Induced Fragmentation of Deprotonated Methoxylated Flavonoids, Obtained by Electrospray Ionization Mass Spectrometry. J.Mass.Spectrometry. 2001, 36(2), 169–178. DOI: 10.1002/jms.118.
- Falcão, S.-I.; Vale, N.; Gomes, P.; Domingues, M.-R.; Freire, C.; Cardoso, S.-M.; Vilas-Boas, M. Phenolic Profiling of Portuguese Propolis by LC-MS Spectrometry: Un Common Propolis Rich in Flavonoid Glycosides. Phytochem. Anal. 2013, 24(4), 309–318. DOI: 10.1002/pca.2412.
- Nabiel, A.-M. S.; Sabry, I.-E. N.; Mohamed, F.-A.; Mamdouh, M.-A.; Dellamonica, G.; Chopin, J. Flavonoid Glycosides of Artemisia Monosperma and A. Herba Alba. Phytochemistry. 1985, 24(1), 201–203. DOI: 10.1016/S0031-9422(00)80845-6.
- Nabiel, A.-M. S.; Sabry, I.-E. N.; Mamdchjh, M.-A. Z. Flavonoids of Artemisia Judaica, A. Monosperma and A. Herba-Alba. Phytochem. 1987, 26(1), 3059–3064. DOI: 10.1016/S0031-9422(00)84593-8.
- Djeridane, A.; Yousfi, M.; Nadjemi, B.; Vidal, N.; Lesgards, J.-F.; Stocker, P. Screening of Some Algerian Medicinal Plants for the Phenolic Compounds and Their Antioxidant Activity. Eur. Food Res. Technol. 2007, 224, 801–809. DOI: 10.1007/s00217-006-0361-6.
- Dahmani-Hamzani, N.; Baaliouamer, A. Chemical Composition of the Algerian Essential Oil of Artemisia Herba-Alba Native to Dejelfa. Riv. Ital. EPPOS. 2005, 40, 7–13.
- Megdiche-Ksouria, W.; Trabelsia, N.; Mkadminia, K.; Bourgoua, S.; Noumib, A.; Snoussic, M.; Barbriad, R.; Tebourbid, O.; Ksouriaa, R. Artemisia Campestris Phenolic Compounds Have Antioxidant Andantimicrobial Activity. Ind. Crops. Prod. 2015, 63, 104–113. DOI: 10.1016/j.indcrop.2014.10.029.
- Carvalho, I.-S.; Cavaco, T.; Brodelius, M. Phenolic Composition and Antioxidant Capacity of Six Artemisia Species. Ind. Crops. Prod. 2011, 33, 382–388. DOI: 10.1016/j.indcrop.2010.11.005.
- Skrzypczak-Pietraszeka, E.; Pietraszekb, J. Chemical Profile and Seasonal Variation of Phenolic Acid Content in Bastard Balm (Melittis Melissophyllum L., Lamiaceae). J. Pharm. Biomed. Anal. 2012, 66, 154–161. DOI: 10.1016/j.jpba.2012.02.019.
- Tan, X.; Li, Q.; Chen, X.; Wang, Z.; Shi, Z.; Bi, K.; Jia, Y. Simultaneous Determination of 13 Bioactive Compounds in Herba Artemisiae Scopariae (Yin Chen) from Different Harvest Seasons by HPLC-DAD. J. Pharm. Biomed. Anal. 2008, 47, 847–853. DOI: 10.1016/j.jpba.2008.04.010.
- Sartora, T.; Xavierb, V.-B.; Falcãob, M.-A.; Mondinc, C.-A.; Santosd, M.-A.; Casselb, E.; Astaritaa, L.-V.; Santaréma, E. R. Seasonal Changes in Phenolic Compounds and in the Biological Activities of Baccharis Dentata (Vell.). Ind. Crops. 2013, 51, 355–359. DOI: 10.1016/j.indcrop.2013.09.018.