ABSTRACT
1,3-Dioleoyl-2-palmitoylglycerol (OPO) is a kind of structured triglyceride which could instead human milk fat (HMF) for the fat of infant formula, this kind of structured triglyceride is good at absorbing easily. The aim of this paper was to produce OPO though three different processing technologies. The raw material for the first method (Method 1) and second method (Method 2) was palm oil, and their catalyst is Novozym 435 and Lipozyme TL IM. The difference was the Method 1, which obtained tripalmitin (PPP) by acidolysis palm oil and palmitic acid (PA), then obtained OPO by acidolysis PPP and oleic acid (OA), Method 2 was acidolysis palm oil and mixed fatty acids directly without crystallization. The third method (Method 3) produced palm stearin (PS) enriched in PA by catalyzing palm stearin with sodium methoxide firstly, and then palm stearin enriched in PA and mixed vegetable oils catalyzed by Lipozyme TL IM. The content of OPO and sn-2 position PA of three methods was higher than 40.34% and 39.34%. The relative content of PA located at the sn-2 position and OA located at the sn-1, 3 position was higher than 59.97% and 66.01%. Among the three methods, the methods related to enzyme appeared safer, and Method 3 was easier for manufacturing OPO and saved time, so it should be more suitable for industrial production.
Introduction
Human milk is the ideal source of nutrients for newborn infants, which could supply energy and essential nutrients to infants.[Citation1] Furthermore, human milk fat (HMF) represents the primary source of energy for the breastfed baby and it provides 50–60% dietary energy of the infants’ required dietary.[Citation2] Structured triglycerides not only provide main energy for infants but also benefit to digestion and absorption. Therefore, we have to add structured triglycerides in infant formula as energy supplement.
It is about 3–5% HMF in human milk and more than 98% HMF in it is triacylglycerols (TAGs).[Citation3,Citation4] The major saturated fatty acid in human milk fat is PA, which accounts about 20–25% of the total fatty acids,[Citation5] and approximately 70% of PA is located at the sn-2 position of TAGs. Other fatty acids such as OA and linoleic acid, which are unsaturated fatty acids, are mainly located at the sn-1, 3 positions.[Citation6] Therefore, a great number of TAGs in HMF are the form of ABA (A is unsaturated fatty acid and B is saturated fatty acid). The distribution of unique fatty acids in these TAGs has a great influence on the improvement of infants’ growth.
Typically, most TAGs are hydrolyzed by pancreatic lipase (a sn-1, 3-specific lipase) in the small intestine.[Citation7] The hydrolyzates are sn-2 monoacylglycerols (sn-2 MAGs) and free fatty acids of sn-1, 3. The unsaturated fatty acids, short-chain free fatty acids, and sn-2 MAGs are absorbed easily in the small intestine, and sn-2 MAGs are synthesized into TAGs and transferred to different organs through the lymph to provide energy.[Citation7] But there is some different distribution of location in TAGs in HMF and these structured triglycerides which are added to infant formula. When the more PA is located at the sn-1, 3 positions of triglycerides, the TAGs are hydrolyzed by pancreatic lipase and produce free PAs, which have a high melting point and can combine with calcium to form a poorly absorbed calcium soap, then result in loss of calcium and energy, constipation even stone.[Citation8–Citation10] Relatively, the more PA is located at the sn-2 position of TAGs like HMF, the easier it is for small intestinal epithelial cells to absorb chylomicron synthesized by bile salts.[Citation11] Therefore, PA located at the sn-2 position improves the absorption and utilization of fatty acids and calcium in the small intestine of infants, and supply energy for the growth of infants.
OPO is the ideal structured triglyceride in infant formula, it has a similar structure to human milk fat, and it has a variety of beneficial effects on infants, such as remitting constipation, optimizing calcium and fatty acids absorption and enhancing bone development. Therefore, it becomes one of the research hotspots in infant formula. In the past few years, the route for the synthesized OPO is based on a common method – interesterification. There are two types of interesterification methods for obtaining OPO – chemical and enzymatic. Relatively, enzymatic interesterification has gained more attention. Lipases from microorganism are usually used in the enzymatic interesterification, such as Novozym 435, Lipozyme TL IM and Lipozyme RM IM.[Citation12–Citation14] The advantage of enzymatic interesterification is that it can perform under a mild condition, which effectively prevents the migration of fatty acids and production of trans fatty acids, decreases the consumption of energy. Moreover, it makes lipase separate easily from products of specific composition and functionality.[Citation15,Citation16] What is more, the disadvantages of enzymatic interesterification are the high cost of lipase, the long reaction time and the complex and expensive equipment. Chemical interesterification usually utilizes sodium methoxide (CH3ONa) and Sodium alkylate as a catalyst. It is reported that the advantages of sodium methoxide, as a basic catalyst, is cheap, efficient and with high activity that only requires a small amount for catalysis. However, it is susceptible to moisture, the structure of sodium methoxide can be changed by water, completely inactivate the catalyst.[Citation17–Citation19] In the reaction, the higher catalytic temperature is required, which results in a lot of by-products in the end-product and increases the difficulty of purification.
Palm stearin, the by-product of palm oil, which is the solid fraction of palm oil after crystallization and fractionation at a controlled temperature.[Citation20] Palm stearin has a wide melting point and iodine value.[Citation21] However, the characteristics of palm stearin can be modified. Palm stearin contains 47–74% PA, 16–37% oleic acid, 3–10% linoleic acid, 4–6% stearic acid and 1–2% myristic acid.[Citation22] The synthesis of OPO was by a two-step method or three-step method, tripalmitin (PPP) is a main raw material or intermediate compound in both of two. Nevertheless, the latest research has reported that there is a lot of PPP which belongs to TAGs in palm stearin. Palm stearin is a particularly desirable raw material and is widely used in margarine and shortenings.[Citation23] Moreover, it is also a cheaper fractionation product from palm oil. The palm oil, a kind of vegetable oil, which is the largest amount of production, consumption and international trade of world, and is much cheaper than soybean oil, rapeseed oil, and sunflower oil. Different melting point of palm oil has different fatty acid composition. However, all of palm oils contain a high content of PA and OA.[Citation24]
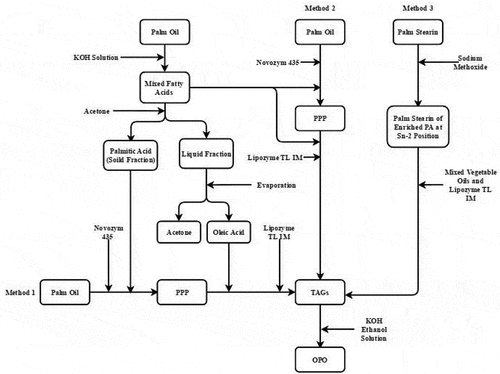
The objectives of this study were to synthesize OPO in three methods as followed: (1) PA and OA were prepared by saponification and separating of palm oil, then the OPO was synthesized by acidolysis fatty acids and palm oil. (2) PPP was obtained by acidolysis of palm oil and mixed fatty acids (contain OA and PA), and then OPO was synthesized by acidolysis of PPP with mixed fatty acids using Lipozyme TL IM. (3) PA-rich in palm stearin at the sn-2 position was obtained by random transesterification of palm stearin under sodium methoxide catalysis. OPO was then produced by transesterification with palm stearin and mixed vegetable oils. The reaction factors (substrate ratio, amount of lipase, temperature and time) were optimized to produce more OPO.
Materials and methods
Materials
Lipozyme TL IM and Novozym 435 were purchased from Beijing Clisent Technology Co., Ltd. (Beijing, China). The standard of 1,3-dioleoyl-2-palmitoyl-glycerol (OPO) was purchased from Sigma-Aldrich (Shanghai, China). Sodium methoxide was obtained from Guangzhou Sunuo Chemical Co., Ltd. (Guangzhou, China). Palm oil with melting point of 33°C was supplied from Guangzhou UBT Feed Technology Co., Lid. (Guangzhou, China). Coconut oil, rapeseed oil, corn oil, and high oleic sunflower oil were purchased from Heilongjiang Biyoute Supermarket (Harbin, China). The standards of fatty acid methyl ester (FAME) were purchased from Sigma-Aldrich (Shanghai, China). Acetone and acetonitrile are chromatographic grade; other solvents and chemicals are analytical reagent purity. All solvents and reagents used in experiment were obtained from normal suppliers.
Preparation of free fatty acids from palm oil
Preparation of mixed fatty acids, PA and OA is shown in , it also exhibits three methods for preparing OPO-rich TAGs. palm oil (25 g) were saponified for 1 h at 60°C thermostat water bath by using a mixture of KOH (5.75 g), distilled water (11 mL), and 95% aqueous ethanol (66 mL). Distilled water (50 mL) was added to the saponified mixture and hexane (2 × 100 mL) was then added for extracting unsaponifiable matter. The hexane layer was discarded after shaking the mixture. The aqueous layer containing the saponification matter was acidified to a pH of 1.0 with 6 mol/L HCl. Hexane (50 mL) was then added to the mixture for extracting free mixed fatty acids. The hexane phase was taken and the mixed fatty acids (consist PA and OA) was recovered by removing the solvent at 40°C rotary evaporation.[Citation25]
Concentration of PA and OA from mixed fatty acids
A typical mixture consisted of mixed fatty acids and acetone (1:40, w/v) were dissolved in Erlenmeyer flask at 40°C thermostat water bath. The mixture was cooled to room temperature, and then stored in the refrigerator at −24°C for 24 h. The PA was filtered with a Buchner funnel under suction (15–20 μm),[Citation26] and the liquid fraction was evaporated at 45°C rotary evaporation for recovering the OA. Then, the PA and OA were stored at −20°C.
Synthesis of OPO with PA and OA (Method 1)
The interesterification mixture consisted of palm oil and PA at molar ratio 1:3, 8% (w/w) Novozym 435 was then added to the reaction mixture, then the reaction mixture was oscillated in an air bath shaker with 200 rpm at 50°C for 24 h. The reaction was stopped by filtration of the lipase with mesh sieve (100 mesh).[Citation27] PPP was stored at −20°C. Novozym 435 was washed with acetone to remove surface excess lipids. The lipase was then dried and placed at 4°C. Acetone was recovered by rotary evaporation at 45°C.
The PPP was mixed with OA (1:2, 1:4, 1:6, 1:8 and 1:10 molar ratio) in an Erlenmeyer flask with a silicone plug, and then added Lipozyme TL IM (4–12%, w/w) to the reaction, then the reaction mixture was placed in an air bath shaker at 30-70°C with 200 rpm for 2–12 h.[Citation28] The collection of triglycerides and the recovery of lipase and acetone were the same as the steps of synthesizing PPP.
Synthesis of OPO with mixed fatty acids directly (Method 2)
PPP was obtained by acidolysis of palm oil and mixed fatty acids at molar ratio 1:3, the reaction was started by adding Novozym 435 (8%, w/w), and the reaction mixture was incubated in an air bath shaker with 200 rpm at 50°C for 24 h. OPO was then synthesized by acidolysis of PPP and mixed fatty acids with Lipozyme TL IM, and the reaction was performed as follows: a mixture of PPP/mixed fatty acids (1:2, 1:4, 1:6, 1:8 and 1:10 molar ratio) and Lipozyme TL IM (4–12%, w/w) was placed in an Erlenmeyer flask with a silicone plug in an air bath with 200 rpm at 30-70°C for 2–12 h. After the reaction reached equilibrium, the reaction was stopped by filtration of lipase. PPP and OPO were stored at −20°C. The lipase was washed with acetone and dried under vacuum, then it was stored at −4°C. Acetone was recovered by rotary evaporation at 45°C.
Synthesis of OPO by sodium methoxide and palm stearin (Method 3)
Palm stearin enriched in PA at the sn-2 position was prepared as follows: palm stearin was put into a round-bottomed flask, heated to 100°C with rotary evaporation under vacuum (0.1 MPa) for removing moisture, and then the sodium methoxide (0.1–0.5%, w/w) was added to round-bottomed flask, heated to 80-110°C and kept 10–60 min. After reaction complete, the 12% citric acid solution was added to the reaction mixture for removing sodium methoxide, oil layer was separated, and then, the citric acid solution was added twice to removing residual sodium methoxide.[Citation29] Palm stearin enriched in PA at the sn-2 position was stored at −20°C.
Palm stearin enriched in PA at the sn-2 position was mixed with mixed vegetable oils (coconut oil: rapeseed oil: corn oil: high oleic sunflower oil = 1:1:1:2) at different molar ratios with 1:2, 1:3, 1:4, 1:5 and 1:6 in Erlenmeyer flask, and then the Lipozyme TL IM (4–12%, w/w) was added to reaction mixture, the reaction was performed in an air bath shaker at 200 rpm, and the temperature was set at 55-70°C for 2–12 h. The collection of triglycerides and the recovery of lipase and acetone were the same as the steps of Method 2.
Purification of the OPO
KOH ethanol solution (0.5 mol/L) was prepared by mixing ethanol and water (3:7, v/v). The OPO stored in −20°C was melted in a water bath at 60°C, and approximately 1.5 times the number of equivalents of KOH ethanol solution was then added to neutralizing the free fatty acids (volume of KOH ethanol solution = 30 × V × concentration of free fatty acids/256, this equivalent number was calculated considering PA (the major fatty acid) as representative of all fatty acids), stirred in a water bath with magnetic agitation at 60°C for 10 min,[Citation30] then allow to stand for 5 min to separate into two layers. The upper layer of light-yellow organic phase is OPO.
Determination of content of OPO product
A C18 chromatography column (250 × 4.6, 25 μm, Elite, Dalian, China) was used for high-performance liquid chromatography (HPLC) with acetone and acetonitrile (80:20, v/v) as mobile phase at a flow rate of 1 mL/min. The HPLC system consisted of a separations module (2695, Waters Corp., Milford, USA) and an evaporative light scattering (ELS) detector (2420, Waters Corp., Milford, USA). The operation temperature of chromatography column was 30°C. The OPO (1 mg) was diluted with hexane (10 mL) and the injection volume was 10 μL for each analysis.
Analysis of fatty acids composition and SN-2 fatty acids
Process for the preparation of 2-MAGs was as follows: 0.1 g OPO in a centrifuge tube was mixed with 2 mL Tris–HCl buffer (pH 7.6), 0.2 mL CaCl2 (220 g/L), 0.2 mL sodium cholate solution (1 g/L) and 20 mg pancreatic lipase, and the centrifuge tube was incubated in a water bath at 40°C for 2 min under shaking condition,[Citation31] vortexed for 1 min and then 1 mL HCl solution (6 mol/L) and 1 ml diethyl ether were added and centrifuged at 6000 rpm for 10 min. The upper diethyl ether layer was transferred to a test tube and was evaporated to 200 µL under nitrogen gas. The 200 µL solution was then spotted on a silica gel G254 thin-layer chromatography (TLC) plate with a developing solvent which was hexane/diethyl ether/formic acid (70:30:1; v/v/v). The bands were sprayed with 2,7-dichlorofluorescein (0.2%, w/v) and visualized under 254 nm ultraviolet (UV) light,[Citation32] and the band corresponding to 2-monoacylglycerols (2-MAGs) was scraped off and as described below for methylation.
Preparation of fatty acids methyl esters (FAMEs) was as follows: the OPO (0.1 g) was hydrolyzed with 8 mL of NaOH-methanol solution (2%, w/v) at 80°C for 20 min in a round-bottomed flask with a condenser. After hydrolyzed, the mixture was esterified with 7 mL of H2SO4-methanol solution (0.5 mol/L) and then heated in a water bath for 15 min.[Citation33] Hexane (10 mL) was then added to the mixture and heated for 1 min. The mixture was cooled to room temperature rapidly and then shook for 2 min. Saturated NaCl solution was added to the mixture, allow to stand for separating into two layers. The upper layer was transferred to a test tube and anhydrous sodium sulfate (5 g) was added to the test tube. The upper solution (2 mL) was transferred to a vial for GC analysis.
Sample analysis was performed by gas chromatography (Agilent 7890A, Agilent Inc., DE, USA) equipped with a capillary column sp-2560 (100 m × 0.25 mm × 0.20 μm, Supelco Inc., Bellefonte, USA), FID (flame ionization detector) and autosampler injector with a split ratio of 100:1. 1 μL sample was injected into the system. The temperature of the injector and detector were 250°C and 260°C. The column oven was initially held at 140°C for 5 min and was then increased to 240°C in 10 min at a rate of 4°C/min and followed by increasing to 260°C at the rate of 4°C/min, maintained at 260°C for 5 min.[Citation22] Nitrogen was used as the carrier gas at a pressure of 0.5 MPa and the flow was 1.1 mL/min.
Statistical analyses
The figures were drawn with Origin 2018. All values are expressed as the average of three experiments and are analyzed by one-way analysis of variance (ANOVA) and Tukey’s-b using SPSS 22.0 software. P values lower than 0.05 were considered as significant difference.
Results
Separation and purification of palmitic and oleic acids from palm oil
These compositions of fatty acids before and after separation and purification from palm oil are displayed in . The results illustrated that the value of PA and OA in palm oil were 30.25% and 44.2% before separation, respectively. After separation, the content of PA and OA in fatty acid mixtures was 29.8% and 43%, respectively. Therefore, it showed that almost all of the fatty acids in palm oil were extracted. The results of crystallization of fatty acid mixtures are also shown in . There were two different components after crystallization, a solid fraction of mainly was saturated fatty acid which was mainly comprised of PA, accounted for 70.8%; another fraction was a liquid fraction which was mainly comprised of unsaturated fatty acid, contained about 72.33% oleic acid. Stearic acid accounted for 21.4% which was found in the solid fraction, linoleic acid accounted for 10.61% which was found in the liquid fraction.
Table 1. Fatty acid compositions of palm oil and result of crystallization (%)
Although stearic acid accounted for 21.4% in solid fraction, the enrichment of PA by removing stearic acid with crystallization is difficult, because it is similar with PA in structure, polarity, and solubility. Although the content of oleic acid can be enhanced by crystallization in acetonitrile.[Citation26] However, the time consumed and the amount of solvent used in this step are not suitable for industrial production, and the fatty acid content in the liquid portion is not low after the fatty acid separation.
Synthesis of OPO with PA and OA
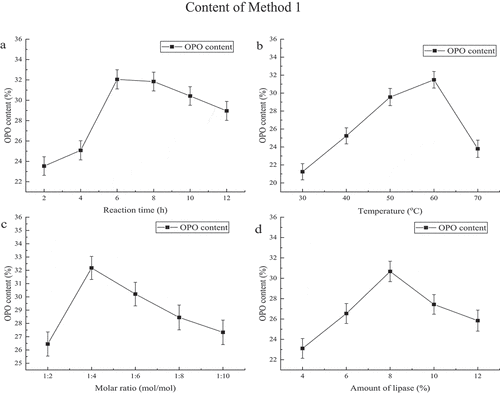
In this subsection, we tested the content of PA at the sn-2 position in PPP, and our experiment also paid more attention to the influence of reaction time, temperature, molar ratio and amount of lipase on the content of OPO. Under the optimal conditions, the content of sn-2 PA in PPP was 68.46%. shows the relationship between OPO content and reaction time. When the reaction time is in the range from 2 h to 12 h, it was detected every 2 h, the contents of OPO were 33.86%, 39.92%, 45.06%, 44.51%, 42.76%, and 41.48%, respectively. It showed the more content of OPO was obtained (45.06%) after 6 h of reaction. shows the relationship between OPO content and the reaction temperature. The contents of OPO were 35.06%, 38.41%, 43.96%, 46.82% and 37.01% at temperatures of 30°C, 40°C, 50°C, 60°C and 70°C, respectively. It indicated the optimal temperature was 60°C. shows the relationship between OPO content and molar ratio. The content of OPO was 48.49% at a molar ratio of 1:4, higher than at molar ratios of 1:2 1:6, 1:8, and 1:10 with the values of 39.67%, 44.68%, 42%, and 41.84%, respectively. shows when the amount of lipase was fixed at 4%, 6%, 8%, 10% and 12% (w/w), the contents of OPO were 35.92%, 40.27%, 45.92%, 42.63% and 40.84%, respectively. So, it was the best choice with the addition of 8% (w/w) of lipase.
Synthesis of OPO with mixed fatty acids directly
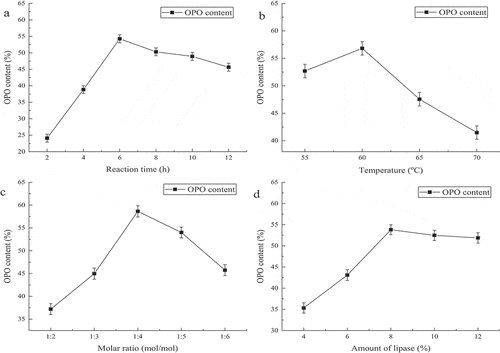
In this subsection, we studied the synthesis of OPO with mixed fatty acids directly without crystallization, and it saved 24 h, all the same indicators as in the previous subsection should be researched. Then, we got 56.39% PA at the sn-2 position in PPP. shows when the reaction time is in the range from 2 h to 12 h, it was also detected every 2 h, the content of OPO was 23.54%, 25.08%, 32.05%, 31.84%, 30.42%, and 28.96%, respectively. The maximum content of OPO was 32.05% at 6 h. shows when temperatures were 30°C, 40°C, 50°C, 60°C and 70°C, the content of OPO was 21.24%, 25.24%, 29.56%, 31.48%, and 23.8%, respectively. It showed when the temperature was 60°C, the OPO content was maximum. shows the relationship between OPO content and molar ratio. The content of OPO was 26.45%, 32.18%, 30.21%, 28.45% and 27.33% at the molar ratios of 1:2, 1:4, 1:6, 1:8 and 1:10, respectively. It indicated the optimal molar ratio was 1:4. shows the relationship between OPO content and amount of lipase. The contents of OPO were 23.11%, 26.54%, 30.67%, 27.43% and 25.84% when the amounts of lipase were 4%, 6%, 8%, 10% and 12% (w/w), respectively. Therefore, it was the best choice with the addition of 8% (w/w) of lipase.
Synthesis of OPO by sodium methoxide and palm stearin
shows the compositions of fatty acids in the palm stearin. However, it had a similar structure with most plant oil that mostly saturated fatty acid was located at the sn-1,3 position of triglyceride and only small content was at the sn-2 position. Then, the transesterification was catalyzed by sodium methoxide to increase the content of PA at the sn-2 position.
Table 2. Fatty acid compositions of palm stearin (%)
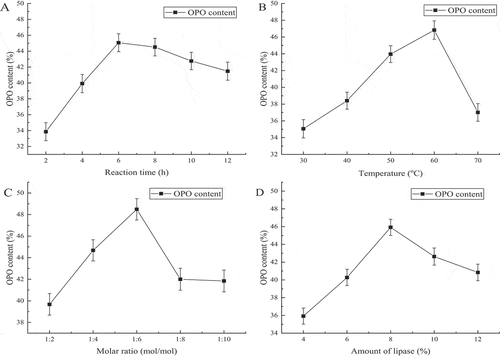
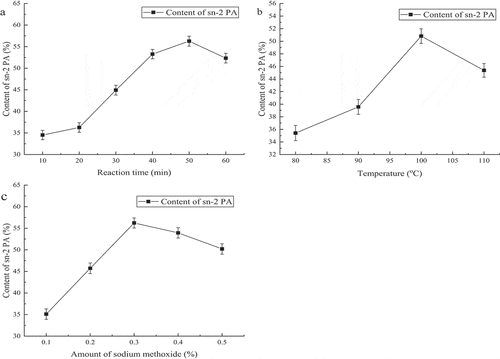
In this subsection, the main experiments included enriching PA at the sn-2 position and obtaining more OPO with palm stearin, the effects of different reaction time, reaction temperatures and amount of catalysis were discussed carefully. And we also discussed the influence of molar ratio on OPO. shows the content of PA at the sn-2 position in palm stearin of enriching PA at sn-2 position would vary with the reaction time, when the time was 10 min, 20 min,30 min,40 min,50 min, and 60 min, the PA content was 34.52%, 36.27%, 44.92%, 53.26%, 56.27% and 52.33%, respectively. shows the influence of the reaction temperature on the content of PA at the sn-2 position in palm stearin. When the temperature increased from 80°C to 110°C every 10°C, the PA content was 35.41%, 39.57%, 50.82%, and 45.37%, respectively. shows the change of PA content at the sn-2 position with the amount of sodium methoxide (0.1% to 0.5%, w/w), it was detected every 0.1%, the PA content was 35.11%, 45.72%, 56.23%, 53.94%, and 50.21%, respectively. Therefore, based on the above studies, the optimal conditions for sodium methoxide to catalyze the palm stearin were as followed: temperature, 100°C; time, 50 min; added an amount of sodium methoxide being 0.3% (w/w). Under the optimal conditions, the content of PA at the sn-2 position in palm stearin was 57.37%.
Figure 4. Influence of Reaction Time, Temperature and Amount of Sodium Methoxide on Sn-2 PA Content in the First Step of Method 3
shows the reaction time ranging from 2 h to 12 h every 2 h, the content of OPO was 24.11%, 38.84%, 54.27%, 50.31%, 48.92%, and 45.65%, respectively. The highest of which has been noted at the reaction time of 6 h. shows the variation of OPO content with the temperature at 55°C, 60°C, 65°C and 70°C, the contents of OPO were 52.69%, 56.82%, 47.56%, and 41.48%, respectively. The maximum content of PA was 56.82% at 60°C. shows the content of OPO is 37.2%, 44.98%, 58.64%, 54% and 45.74% at the molar ratio of 1:2, 1:3, 1:4, 1:5 and 1:6 when the acyl donor is mixed vegetable oils, respectively. It indicated the optimal molar ratio is 1:4. shows when the acyl donor was mixed vegetable oils, the contents of OPO were 35.34%, 43.1%, 53.8%, 52.48% and 51.88% at 4%, 6%, 8%, 10% and 12% (w/w) of lipase dosages, respectively. So, adding an amount of 8% (w/w) lipase is the best choice.
Optimal conditions for synthesis of OPO by three methods
The optimal conditions for three methods to obtain OPO were as followed, respectively: 1) Lipozyme TL IM, 8% (w/w); substrate molar ratio, 1:4; reaction time, 6 h; and reaction temperature, 60°C for acidolysis of PPP and OA. 2) Lipozyme TL IM, 8% (w/w); substrate molar ratio, 1:4; reaction time, 6 h; and reaction temperature, 60°C for acidolysis of PPP and fatty acids. 3) Lipozyme TL IM, 8% (w/w); substrate molar ratio, 1:4; reaction time, 6 h; and reaction temperature, 60°C for acidolysis of palm stearin and mixed vegetable oils. Then, the OPO was synthesized under optimal conditions and purified using KOH ethanol solution. The OPO content of the three methods in the final product after purification was 71.22%, 46.86%, and 79.50% (P< .05), respectively (). shows the yield of OPO after purified, the yields of OPO for three methods were 68.12%, 64.70%, 73.79%, respectively. shows the compositions and distribution of fatty acids in OPO after purified, the contents of PA in the three methods were 34.30%, 30.20%, and 22.42%, respectively. The contents of PA at the sn-2 position of three methods were 65.30%, 54.33% and 52.31% (P < .05), respectively. There are significant differences between them (). The contents of PA at the sn-2 position accounts for total PA content were 63.46%, 59.97%, and 77.77%. The OA contents of three methods were 49.14%, 53.93%, and 57.55%, respectively. The contents of OA at the sn-1,3 positions accounted for total OA content were 66.01%, 68.22% and 73.65%, respectively.
Table 3. The OPO content in final product after purification of the three methods (%, P < .05)
Table 4. The raw material dosage of OPO and the purified yield
Table 5. Fatty acid composition and distribution of the three-final product after purification step under the optimized conditions (%)
Table 6. The PA content located at sn-2 position in OPO (%, P < .05)
Discussion
Palm oil and palm stearin were used as raw material in this study, owing to the source of vegetable oils is widely and the price is cheap. Palm oil does not contain cholesterol. The digestion and absorption rate of body to palm oil is over 97%. As shown in and , the contents of PA in palm oil and palm stearin were 30.25% and 58.56%, respectively. The PA content of palm stearin was approximately twice as much as palm oil, the high PA content in the raw material provides a guarantee for the PA content at the sn-2 position in the OPO, it was a distinct advantage of palm stearin. Moreover, palm stearin is cheaper than palm oil, the price of palm oil is 7500 RMB per ton and the price of palm stearin is 5500 RMB per ton. As shown in and , the contents of OA in palm oil and palm stearin were 44.20% and 28.67%, respectively. They instead of acyl donor provided OA for OPO. Therefore, palm oil and palm stearin not only contained a lot of PA and OA, but also were good for human health, and it was suitable for the raw materials of synthesis OPO, and from the above two points, palm stearin was better than palm oil.
The influence of reaction time, temperature, and amount of sodium methoxide on the intermediate (palm stearin enriched with PA at the sn-2 position) were discussed in order to obtain more PA at the sn-2 position. shows the content of PA at the sn-2 position increased with reaction time prolonged, when it reached 50 min, the transesterification reached an equilibrium state, and the content of PA did not raise as time increased. Moreover, the content of PA descended slightly with the time prolonged. There were two reasons for explaining the answer, the first one was an acyl transfer phenomenon during the reaction, which resulted in the PA content at the sn-2 position decrease,[Citation34] the second reason may be the sodium methoxide activity decline and saponification reaction happened due to long reaction time.[Citation29] Except reaction time, the temperature also led to PA content decrease, as shown in , firstly, the content of PA growing quickly with the temperature increased from 80°C to 100°C, the reaction reaches equilibrium at 100°C and then the content of PA did not change much when the reaction temperature continued to rise. The color of the palm stearin gradually deepened with the reaction temperature rose over 100°C, darkening of color is one of indicators of the palm stearin quality decline, so when the temperature of more than 100°C was not beneficial to palm stearin. As shown in , when the catalyst amount changed from 0.1% to 0.3% (w/w) the content of PA of sn-2 position increased rapidly and subsequently reached equilibrium. As the amount of catalysis continues to increase, the content of PA did not substantially increase. This was because when sodium methoxide was added in a large amount, it will react with oil to produce a large amount of soap, which affected the final yield of the reaction. At the same time, the formed soap greatly increases the viscosity of the system and forms a gel, hinder the further progress of the reaction. Moreover, while the amount of the sodium methoxide was too high, there may be Claisen condensation occurs.[Citation35] Then, the PA content at the sn-2 position in intermediates (PPP and palm stearin enriched with PA at the sn-2 position) of three methods was discussed. The sn-2 PA contents of PPP and PA-rich palm stearin were 68.46%, 56.39% and 57.37% (, P< .05), respectively. Although the content of PA at the sn-2 position in PPP which synthesis by palm oil and PA was higher than other methods; however, it needed much time for processing, so it’s not suitable to commercial production. Compared to most chemical catalysts, lipase-catalyzed had a higher reaction rate, mild reaction conditions and select specificity, produces no by-product during the reaction. However, the sodium methoxide as one of the chemical catalysts, its advantages were obvious. Owing to the high activity, cheap and short reaction time, sodium methoxide is one of the extensively used as a catalyst. Moreover, in China, it was allowed to use as a catalyst in food processing.[Citation36] The PPP synthesized by Novozym 435 needed 24 h, the contents of PA at the sn-2 position were 68.46% in Method 1. The palm stearin of enrich PA at the sn-2 position synthesized by sodium methoxide only needed 50 min and the sn-2 PA content was 57.37%. Novozym 435 spent 28 folds more time than sodium methoxide, the PA content only increased by 11.09%. So, in terms of the cost of time and production, sodium methoxide is more suitable for industrial production. Moreover, sodium methoxide is unstable; thus, it is very easily removed from product.
Table 7. The sn-2 PA content of PPP and palm stearin enriched in PA (%, P < .05)
The reaction time, temperature, substrate molar ratio, and enzyme dosage in the three methods were discussed in order to obtain more OPO. As shown in , , and , with the increase of time, OPO content showed a trend of increasing before 6 h to three producing methods, it may be due to the fact that the transesterification between the substrates occurs continuously during the reaction. And there was another trend of decreasing after 6 h to all methods, and the probable reason was as below, the PA which was displaced rejoined to the transesterification reaction as an acyl donor. Xu et al.[Citation34] found that acyl migration increases with reaction time rises. Lee et al.[Citation37] also observed that time had the great important influence on acyl migration which is the reason for explaining the OPO content decreases with reaction time. As shown in , and , when temperature increased from 30°C to 60°C of Method 1 and Method 2 and temperature rose from 55°C to 60°C of method 3, the content of OPO was increase, the reason for this phenomenon was high temperature which could reduce the viscosity of the reaction mixture and expand the effectual crash of substances, both of them enhanced the reaction speed,[Citation38] increased the yield of the product rapidly. However, when the temperature exceeded 60°C, the content of OPO was decreased. High temperature can also raise the speed of acyl migration, which led to the content of product decreased. Moreover, high temperature destroyed the structure of enzyme, caused it to irreversible inactivation. As shown in , and , the content of OPO was the highest at molar ratio 1:4 in three methods. The transesterification was completed when triglyceride and OA were at a molar ratio 1:2 in theoretically, while it was a reversible reaction which catalyzed by lipase,[Citation39] therefore when increased the ratio of acyl donor, the reaction equilibrium can be shifted to the right, which was advantage to speed up the reaction, and enhanced the content of OPO. However, owing to the existence of acyl migration, the sn-2 fatty acid (PA) could migrate to sn-1 or sn-3 position of triglyceride with the increase of molar ratio and reaction time, which led to decrease in the PA content at the sn-2 position and content of OPO. As shown in , and , the enzyme dosage was an important factor for the reaction rate which was influenced by enzyme activity site in the reaction system, too low enzyme dosage, it had little activity site which would decrease substrates’ reaction rate. High enzyme dosage could reduce the reaction time, which could decrease acyl migration rate, especially, diacylglycerols produced by lipase-catalyzed could also accelerate acyl migration,[Citation40] so it was vital for choosing the optimal enzyme dosage. Above all, it showed that we have to optimize temperature, reaction time, substrate molar ratio and enzyme dosage for making more OPO.
As shown in , the total PA content and PA content at the sn-2 position of Method 1 were all the highest. The contents of total PA and sn-2 PA in OPO synthesized by Method 1 were higher than products processed by Kotani et al.[Citation41] and Nagachinta and Akoh.[Citation42] The PA located at the sn-2 position accounts for total PA of Method 1 was 63.46%. However, the value was not higher than Method 3. Moreover, the relative content of OA in the sn-1, 3 position was also the highest in Method 3. Combined with the digestive pattern of triglycerides in the intestine, it gave us a vital information about PA which located at the sn-2 position would appear a good performance at effective utilization, we also could infer that OPO synthesized by Method 3 is the best digested and absorbed in the intestine.
After purification, the lowest OPO content in three methods was Method 2, which was 46.84%. It was higher than Chinese standard, which requests that the OPO content more than 40%.[Citation43] The OPO content synthesized by the others was more than 70%, which was 71.22% in Method 1 and 79.50% in Method 3. The different OPO content showed, for the two-step synthesis OPO, the content of PA and OA as acyl donor had an essential influence on OPO in the final product. In general, high content of PA and OA as acyl donor increases the OPO content of the final product.[Citation44] However, in the second step, the OA content of the acyl donor of Method 3 was lower than that of Method 1, the OA content was 52.40% and 72.33% in Method 3 and Method 1, respectively. But the OPO content in Method 3 was higher than that in Method 1, there was a richer fatty acid species in acyl donor of Method 3 which contained lauric acid, α-linolenic acid, and arachidonic acid, these three fatty acids were not found in acyl donor of Method 1. Therefore, we supposed that a reasonable proportion of fatty acids in the acyl donor has a positive effect on the OPO content.
Pande et al.[Citation45] reported that the more abundant fatty acids in human milk were PA, OA, and linoleic acid, and these contents were 15.43–24.46%, 28.30–43.83%, and 10.61–25.30%, respectively. The contents of the three fatty acids in the sn-2 position were 52.30%, 13.97%, and 10.95%, respectively. Compared with the content of fatty acids in human milk, the OPO synthesized by Method 3 in this study had a similar content in these three fatty acids, also similar with the content at the sn-2 position, these fatty acids have an important effect on infants’ growth. Moreover, because OPO produced by Method 3 had a similar fatty acids composition and distribution to human milk fatty acids, it was more easily absorbed in the small intestine, rather than being converted to insoluble soap calcium excreted with feces.
Conclusion
This work compared three methods of synthesizing OPO and obtained the optimal process. In terms of product quality, the PA content at the sn-2 position and OPO content of the three methods were all higher than specified in national standards of China. Among three methods, the OPO synthesized by palm stearin and mixed vegetable oils had a higher OPO content and relative content of PA at the sn-2 position than other methods. Therefore, the OPO synthesized by palm stearin and mixed vegetable oils is more suitable for digestion and absorption. In addition, acyl donor is important precursor for the synthesis of OPO, the PA and OA content has an important influence on the OPO synthesis, but in our research, it does not consistent with the previous reports, the OA content in liquid fraction of mixed fatty acids after crystallization was higher than mixed vegetable oils, but the OPO content synthesized by PPP and OA was lower than PA-rich palm stearin and mixed vegetable oils. The OA content in the acyl donor and the OPO content in the product are not positive correlation. Therefore, we supposed that a reasonable proportion of fatty acids in the acyl donor has a positive effect on the OPO content. In addition, by comparing the time cost of three methods, the OPO synthesized by palm stearin and mixed vegetable oils saved much time than other methods, so it is more suitable for industrial production.
Additional information
Funding
References
- Jensen, R. G.;. Lipids in Human Milk. Lipids. 1999, 34(12), 1243–1271. DOI: 10.1007/s11745-999-0477-2.
- Jensen, G. L.; Jensen, R. G. Specialty Lipids for Infant Nutrition. II. Concerns, New Developments, and Future Applications. J. Pediatr. Gastroenterol. Nutr. 1992, 15(4), 382–394. DOI: 10.1097/00005176-199211000-00004.
- Jensen, R. G.;. Human Milk Lipids as a Model for Infant Formula. Lipid Technol. 1998, 10, 34–38.
- Lópezlópez, A.; López-Sabater, M. C.; Campoy-Folgoso, C.; Rivero-Urgell, M.; Castellote-Bargalló, A. I. Fatty Acid and Sn-2 Fatty Acid Composition in Human Milk from Granada (Spain) and in Infant Formulas. Eur. J. Clin. Nutr. 2002, 56(12), 1242–1254. DOI: 10.1038/sj.ejcn.1601470.
- Nelson, C.; Innis, S. Plasma Lipoprotein Fatty Acids are Altered by the Positional Distribution of Fatty Acids in Infant Formula Triacylglycerols and Human Milk. Am. J. Clin. Nutr. 1999, 70(1), 62–69. DOI: 10.1017/S002966519900097X.
- Nielsen, N. S.; Yang, T.; Xu, X.; Jacobsen, C. Production and Oxidative Stability of a Human Milk Fat Substitute Produced from Lard by Enzyme Technology in a Pilot Packed-Bed Reactor. Food Chem. 2006, 94(1), 53–60. DOI: 10.1016/j.foodchem.2004.10.049.
- Zou, X. Q.; Jin, Q. Z.; Guo, Z.; Huang, J. H.; Xu, X. B.; Wang, X. G. Preparation of 1, 3-Dioleoyl-2-Palmitoylglycerol-Rich Structured Lipids from Basa Catfish Oil: Combination of Fractionation and Enzymatic Acidolysis. Eur. J. Lipid Sci. Technol. 2016, 118(5), 708–715. DOI: 10.1002/ejlt.201500226.
- Lien, E. L.;. The Role of Fatty Acid Composition and Positional Distribution in Fat Absorption in Infants. J. Pediatr. 1994, 125(5), 62–68. DOI: 10.1016/S0022-3476(06)80738-9.
- Lien, E. L.; Boyle, F. G.; Yuhas, R.; Tomarelli, R. M.; Quinlan, P. The Effect of Triglyceride Positional Distribution on Fatty Acid Absorption in Rats. J. Pediatr. Gastroenterol. Nutr. 1997, 25, 167–174. DOI: 10.1097/00005176-199708000-00007.
- Lucas, A.; Quinlan, P.; Abrams, S.; Ryan, S.; Meah, S.; Lucas, P. J. Randomised Controlled Trial of a Synthetic Triglyceride Milk Formula for Preterm Infants. Arch. Dis. Child. Fetal Neonatal Ed. 1997, 77(3), 178–184. DOI: 10.1136/fn.77.3.F178.
- Small, D. M.;. The Effects of Glyceride Structure on Absorption and Metabolism. Annu. Rev. Nutr. 1991, 11(11), 413. DOI: 10.1146/annurev.nu.11.070191.002213.
- Turan, D.; Yeşilçubuk, N. Ş.; Akoh, C. C. Enrichment of Sn-2 Position of Hazelnut Oil with Palmitic Acid: Optimization by Response Surface Methodology. LWT Food Sci. Technol. 2013, 50(2), 766–772. DOI: 10.1016/j.lwt.2012.07.009.
- Wei, W.; Feng, Y. F.; Zhang, X.; Cao, X.; Feng, F. Q. Synthesis of Structured Lipid 1,3-Dioleoyl-2-Palmitoylglycerol in Both Solvent and Solvent-Free System. LWT Food Sci. Technol. 2015, 60(2), 1187–1194. DOI: 10.1016/j.lwt.2014.09.013.
- Liu, S. L.; Dong, X. Y.; Wei, F.; Wang, X.; Lv, X.; Zhong, J. Ultrasonic Pretreatment in Lipase-Catalyzed Synthesis of Structured Lipids with High 1,3-Dioleoyl-2-Palmitoylglycerol Content. Ultrason. Sonochem. 2015, 23(23), 100–108. DOI: 10.1016/j.ultsonch.2014.10.015.
- Kim, B. H.; Akoh, C. C. Recent Research Trends on the Enzymatic Synthesis of Structured Lipids. J. Food Sci. 2015, 80(8), C1713–C1724. DOI: 10.1111/1750-3841.12953.
- Srivastava, A.; Akoh, C. C.; Chang, S. W.; Lee, G. C.; Shaw, J. F. Candida Rugosa Lipase Lip1-Catalyzed Transesterification to Produce Human Milk Fat Substitute. J. Agric. Food Chem. 2006, 54(14), 5175. DOI: 10.1021/jf060623h.
- Koohikamali, S.; Tan, C. P.; Ling, T. C. Optimization of Sunflower Oil Transesterification Process Using Sodium Methoxide. Sci. World J. 2012, 2012, 1–8. DOI: 10.1100/2012/475027.
- Korus, R. A.; Hoffman, D. S.; Bam, N.; Peterson, C. L.; Drown, D. C. Transesterification Process to Manufacture Ethyl Ester of Rape Oil. In Proceedings of the 1st Biomass Conference of the Americas: Energy, Environment, Agriculture, and Industry 1993, vol. 2, pp. 815–826. Retrieved from https://www.researchgate.net/publication/237625759_TRANSESTERIFICATION_PROCESS_TO_MANUFACTURE_ETHYL_ESTER_OF_RAPE_OIL.
- Marangoni, A. G.; Rousseau, D. Engineering Triacylglycerols: The Role of Interesterification. Trends Food Sci. Technol. 1995, 6(10), 329–335. DOI: 10.1016/S0924-2244(00)89167-0.
- Lai, O. M.; Ghazali, H. M.; Chong, C. L. Use of Enzymatic Transesterified Palm Stearin-Sunflower Oil Blends in the Preparation of Table Margarine Formulation. Food Chem. 1999, 64(1), 83–88. DOI: 10.1016/s0308-8146(98)00083-1.
- Fasdm, S.; Rcd, S.; Kcgd, S.; Lourenço, M. B.; Soares, D. F.; Gioielli, L. A. Effects of Chemical Interesterification on Physicochemical Properties of Blends of Palm Stearin and Palm Olein. Food Res. Int. 2009, 42(9), 1287–1294. DOI: 10.1016/j.foodres.2009.03.022.
- Pantzaris, T. P.;. Pocketbook of Palm Oil Uses. PORIM Inf. Ser. 1991, 15, 15–19.
- Aini, I. N.; Miskandar, M. S. Utilization of Palm Oil and Palm Products in Shortenings and Margarines. Eur. J. Lipid Sci. Technol. 2010, 109(4), 422–432. DOI: 10.1002/ejlt.200600232.
- Zhang, L. P.; Xu, A. J. Determination of Fatty Acid Compositions and Solid Fat Contents in Several Palm Oils with Different Melting Point. Cereals Oils. 2010, 05, 14–16.
- Senanayake, S. P. J. N.; Shahidi, F. Enzymatic Incorporation of Docosahexaenoic Acid into Borage Oil. J. Am. Oil Chem. Soc. 1999, 76(9), 1009–1015. DOI: 10.1007/s11746-999-0197-x.
- Chen, M. L.; Vali, S. R.; Lin, J. Y.; Ju, Y. H. Synthesis of the Structured Lipid 1,3-Dioleoyl-2-Palmitoylglycerol from Palm Oil. J. Am. Oil Chem. Soc. 2004, 81(6), 525–532. DOI: 10.1007/s11746-006-0935-2.
- Jimenez, M. J.; Esteban, L.; Robles, A. Production of Triacylglycerols Rich in Palmitic Acid at Sn-2 Position by Lipase-Catalyzed Acidolysis. Biochem. Eng. J. 2010, 51(3), 172–179. DOI: 10.1016/j.bej.2010.06.015.
- Nagao, T.; Shimada, Y.; Sugihara, A.; Murata, A.; Komemushi, S.; Tominaga, Y. Use of Thermostable Fusarium Heterosporum Lipase for Production of Structured Lipid Containing Oleic and Palmitic Acids in Organic Solvent-Free System. J. Am. Oil Chem. Soc. 2001, 78(2), 167–172. DOI: 10.1007/s11746-001-0238-7.
- Peng, L.; Song, Z. H.; Teng, C. Z.; Fan, S. G.; Wang, Y. Q.; Wang, X. G. Preparation of Medium/Long-Chain Structured Triglycerides by Sodium Methoxide Catalyzed Transesterification. China Oils Fats. 2011, 36, 5–9.
- Luis, E.; María, J. J.; Estrella, H.; Pedro, A. G.; Lorena, M.; Alfonso, R. Production of Structured Triacylglycerols Rich in Palmitic Acid at Sn-2 Position and Oleic Acid at Sn-1,3 Positions as Human Milk Fat Substitutes by Enzymatic Acidolysis. Biochem. Eng. J. 2011, 54(1), 62–69. DOI: 10.1016/j.bej.2011.01.009.
- Wang, X. S.; Chen, Y.; Zheng, L. Y.; Jin, Q. Z.; Wang, X. G. Synthesis of 1,3-Distearoyl-2-Oleoylglycerol by Enzymatic Acidolysis in a Solvent-Free System. Food Chem. 2017, 228, 420–426. DOI: 10.1016/j.foodchem.2017.01.146.
- Zhang, Y.; Wang, X. S.; Zou, S.; Xie, D.; Jin, Q. Z.; Wang, X. G. Synthesis of 2-Docosahexaenoylglycerol by Enzymatic Ethanolysis. Bioresour. Technol. 2017, 251, 251–334. DOI: 10.1016/j.biortech.2017.12.025.
- Niezgoda, N.; Wawrzeńczyk, C. An Efficient Method for Enzymatic Purification of Cis-9, Trans-11 Isomer of Conjugated Linoleic Acid. J. Mol. Catal. B Enzym. 2014, 100, 40–48. DOI: 10.1016/j.molcatb.2013.11.019.
- Xu, X.; Skands, A. R. H.; Høy, C. E.; Mu, H.; Balchen, S.; Adler-Nissen, J. Production of Specific-Structured Lipids by Enzymatic Interesterification: Elucidation of Acyl Migration by Response Surface Design. J. Am. Oil Chem. Soc. 1998, 75(9), 1179–1186. DOI: 10.1007/s11746-998-0309-z.
- Xu, Y. D.; Cui, H. L.; Zhang, Y. J.; Song, H. B. Low Calorie Oil Production through Transesterification Using Sodium Methoxide. J. Chin. Cereal. Oils Assoc. 2012, 28, 63–67. DOI: 10.4028/www.scientific.net/AMR.554-556.1577.
- National Health and Family Planning Commission of the People's Republic of China. National Standards for Food Safety Standards for the Use of Food Additives: GB2760-2014 [S]. Beijing: China Standard Press, 2010: 186.
- Lee, J. H.; Son, J. M.; Akoh, C. C.; Kim, M. R. Optimized Synthesis of 1, 3-Dioleoyl-2-Palmitoylglycerol-Rich Triacylglycerol via Interesterification Catalyzed by a Lipase from Thermomyces Lanuginosus. New Biotechnol. 2010, 27(1), 38–45. DOI: 10.1016/j.nbt.2009.10.006.
- Vikbjerg, A. F.; Mu, H.; Xu, X. Parameters Affecting Incorporation and By-Product Formation during the Production of Structured Phospholipids by Lipase-Catalyzed Acidolysis in Solvent-Free System. J. Mol. Catal. B Enzym. 2005, 36(1), 14–21. DOI: 10.1016/j.molcatb.2005.07.002.
- Xu, X.;. Engineering of Enzymatic Reactions and Reactors for Lipid Modification and Synthesis. Eur. J. Lipid Sci. Technol. 2010, 105(6), 289–304. DOI: 10.1002/ejlt.200390059.
- Du, W.; Xu, Y. Y.; Liu, D. H.; Li, Z. B. Study on Acyl Migration in Immobilized Lipozyme Tl-Catalyzed Transesterification of Soybean Oil for Biodiesel Production. J. Mol. Catal. B Enzym. 2005, 37, 68–71. DOI: 10.1016/j.molcatb.2005.09.008.
- Kousuke, K.; Yukihiro, Y.; Setsuko, H. Enzymatic Preparation of Human Milk Fat Substitutes and Their Oxidation Stability. J. Oleo Sci. 2015, 64(3), 275–281. DOI: 10.5650/jos.ess14254.
- Nagachinta, S.; Akoh, C. C. Production and Characterization of Dha and Gla-Enriched Structured Lipid from Palm Olein for Infant Formula Use. J. Am. Oil Chem. Soc. 2013, 90(8), 1141–1149. DOI: 10.1007/s11746-013-2255-7.
- National Health and Family Planning Commission of the People's Republic of China. National Food Safety Standard Food Nutrition Enhancer 1,3-Dioleic Acid-2-palmitic Acid Triglyceride: GB 30604-2015[S]. Beijing: China Standard Press, 2015.
- Shunyi, Y.; Minhua, Z.; Yun, L.; Ruhua, L. Cocoa Butter Substitute Production from Palm Oil Mid Fraction by Lipase-Catalyzed Interesterification in Organic Phase. Food Ferment. Ind. 1995, 2, 17–21.
- Pande, G.; Sabir, J. S. M.; Baeshen, N. A.; Akoh, C. C. Synthesis of Infant Formula Fat Analogs Enriched with DHA from Extra Virgin Olive Oil and Tripalmitin. J. Am. Oil Chem. Soc. 2013, 90(9), 1311–1318. DOI: 10.1007/s11746-013-2281-5.