 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
In this study, the safety and quality of commercial cooking oils were evaluated. The emphasis of this evaluation was on the presence of oxidation and polymerization products in fresh oils, although the analyses were often conducted on used frying fats and oils. This was because polymerized triacylglycerols (PTGs) and monomeric oxidized triacylglycerols (oxTAGs) have been proposed as potential indicators of the adulteration of palm olein. The oil quality was evaluated based on PTG content, the presence of epoxy, keto, and hydroxy acids, fatty acid composition, and smoke point. Principal component analysis (PCA) was conducted to identify the relationships among the analytical parameters. The total polar compound content of all fresh oil samples was within the safety limit for human consumption (< 25% polar compounds). TAG oligomers or epoxy, keto, or hydroxy acids were not detected in any of the fresh oil samples. Most of the packet oils had lower smoke point (< 200 °C) and linoleic acid content than the bottled oils. The pure palm olein samples were found to be better in terms of overall oil quality, as indicated by the PCA biplots of all analytical parameters.
Abbreviations: ANOVA: analysis of variance; BPO: blended palm olein; DAG: diacylglycerol; FAME: fatty acid methyl ester; FFA: free fatty acid; HPLC: high-performance liquid chromatography; HPSEC: high-performance size exclusion chromatography; PC: principal component; PO: packet pure palm olein; PPO: pure palm olein; PCA: principal component analysis; SPE: solid-phase extraction; TAG: triacylglycerol; TPC: total polar compound
Introduction
Polymerized triacylglycerols, which are of high molecular weight, are normally abundantly formed in cooking oil during heat treatment. These byproducts are normally nonvolatile, more polar than the unaltered triacylglycerols, and their presence reduces the nutritional value of the oil.[Citation1,Citation2] The detection of the isolated polar compound and its constituents by high-performance size-exclusion chromatography provides a comprehensive evaluation of the impact of the three common degradation pathways that occur in oils during heat treatment, namely, polymerization, oxidation, and hydrolysis.[Citation3,Citation4] Therefore, it is vital to evaluate the quality of cooking oils, even fresh ones, as the presence of these compounds will subsequently affect the quality of fried food products.
Polar fatty acids such as epoxy, keto, and hydroxy acids are relatively stable final products that are formed from the decomposition of hydroperoxides.[Citation5] These oxygenated compounds are reported to be present at high levels, especially in thermally oxidized foods.[Citation6] These oxidized fatty acids, particularly long-chain epoxy fatty acids that can act as protoxins, are claimed to have toxic or adverse effects. This term ‘protoxin’ indicates that a compound only becomes a toxin when it is altered during metabolism.[Citation7] Therefore, the safety issues related to the presence of these oxygenated fatty acids in the oil cannot be underestimated.
Polymerized triacylglycerols and monomeric oxidized triacylglycerols have been proposed as potential indicators of the adulteration of palm olein. Because their molecular weights are generally higher, they are more difficult to remove during the refining process. The aim of this work was to determine the presence of oxidative polymerization products in fresh commercial cooking oils, although these analyses were often conducted on used frying fats and oils. This was considered part of the safety evaluation of commercial cooking oils available in Malaysia, as these compounds should not be present in fresh oil. Besides, the relationships between the analytical parameters were studied and the quality of commercial cooking oil samples from different categories, for instance, bottled pure palm olein, blended palm olein, and packet pure palm olein were evaluated and compared. The safety parameters of commercial cooking oils must be closely monitored because these oils are widely used for culinary purposes, e.g., in deep frying, cooking, and baking.
Materials and methods
Materials
A total of 23 commercial cooking oil samples were acquired from a local supermarket (Selangor, Malaysia); the samples were categorized into three main groups, namely, pure palm olein, blended palm olein, and packet pure palm olein. Pure palm olein is denoted PPO1 to PPO9, blended palm olein is denoted BPO1 to BPO3, and packet pure palm olein is denoted PO1 to PO11. The blended palm olein is a mixture consisting of major percentage of palm olein and minimal amount of several vegetable oils such as soybean, sunflower, sesame, and peanut oils.
Chemicals
All solvents and chemicals used in the analyses were of analytical grade. For column chromatography, silica gel 60 (0.063–0.200 mm) was purchased from Merck (Darmstadt, Germany). Tetrahydrofuran (HPLC grade), which was used as the mobile phase for high-performance size-exclusion chromatography (HPSEC), and platinum (IV) oxide hydrate were acquired from Thermo Fisher Scientific (Waltham, Massachusetts, USA). Analytical standards for the analysis of monomeric oxidized triacylglycerols such as methyl heneicosanoate, methyl 12-hydroxystearate, and methyl 12-oxostearate were purchased from Sigma-Aldrich (Darmstadt, Germany), while methyl trans-9,10-epoxystearate was purchased from Santa Cruz Biotechnology, Inc. (Dallas, Texas, USA). The Supelco 37 component FAME mix and methyl octanoate used in fatty acid composition analysis were purchased from Sigma-Aldrich (Darmstadt, Germany).
Physical property
Determination of smoke point: The smoke point of all commercial cooking oil samples was determined according to the official analytical method (Cc 91-48) of the American Oil Chemists’ Society.[Citation8]
Chemical properties
Total polar compounds and their distribution: An amount of 0.5 g commercial cooking oil samples were separated into two fractions, a polar fraction and a nonpolar fraction, by conventional silica gel column chromatography. The separation method was conducted according to a group of European researchers but with slight modifications.[Citation6] A total of 10 g of silica was adjusted to a moisture content of 5% (w/w) and was placed in a glass column (40 cm × 1 cm I.D.). First, 75 mL of n-hexane-diethyl ether (90:10, v/v) was used to elute the unaltered TAGs (the nonpolar fraction). Our interest in this study was the polar fraction, which was eluted with 75 mL of diethyl ether, a solvent of higher polarity than the previous eluent. The polar fraction collected in diethyl ether was evaporated under reduced pressure using a rotary evaporator at 40 °C for 10 min. After evaporation, the round-bottom flask containing the residue was placed in an oven at 105 °C to ensure that the residual solvent was fully evaporated. The collected polar fraction was then accurately weighed and dissolved in tetrahydrofuran (THF) at a concentration of 50 mg/ml. The sample was filtered and analyzed by high-performance size-exclusion chromatography using a refractive index detector (HPLC-SEC/RI).
High-performance size-exclusion chromatography (HPSEC): The distribution of total polar compounds in the commercial cooking oils was analyzed using HPSEC. TAG oligomers, TAG dimers, monomeric oxidized triacylglycerols, DAG, and FFA were quantitated using a Shimadzu HPLC equipped with a SIL-10AD injector, an LC-20AD pump, and a RID-10A refractive index detector. Two 100-Å and 500-Å phenogel columns (30 cm × 0.78 cm I.D., film thickness 5 µm) with porous, highly cross-linked styrene-divinyl benzene copolymers (Phenomenex, Torrance, CA, USA) as the stationary phase were connected in series to improve the peak resolution. Tetrahydrofuran was used as the mobile phase, and the flow rate was set to 1 mL/min. Each group of the polar fraction distributions was quantified using the following equations:
where PTPC, Poligomer, Pdimer, PoxTAG, PDAG, and PFFA represent the percentages of total polar compound, TAG oligomers, TAG dimers, monomeric oxidized triacylglycerols, diacylglycerols, and free fatty acids found in the polar fraction of the oil sample respectively; Aoligomer, Adimer, AoxTAG, ADAG, and AFFA represent the peak area of each specific fractions; and ∑A represents the sum of all peak areas.[Citation6]
Epoxy, keto, and hydroxy fatty acids: The commercial cooking oil samples were derivatized with sodium methoxide to produce fatty acid methyl esters (FAMEs), which are more volatile and more suitable for GC analysis. A 300-mg oil sample was weighed followed by the addition of 3 mL of tert-butyl methyl ether (TBME) and 1.5 mL of 0.2 M sodium methoxide in methanol. The mixture was vortexed for 1 min and allowed to stand at room temperature for 2 min. For the neutralization step, 0.1 mL of 0.5 M sulfuric acid was added, and the mixture was vortexed for 5 s. Subsequently, 3 mL of ultrapure water was added, and the mixture was vortexed for 10 s and centrifuged for 5 min at 4000 rpm. After centrifugation, the supernatant was transferred to a separate test tube and evaporated to dryness under nitrogen.[Citation6]
Solid-phase extraction (SPE): SPE is an important sample pretreatment step that is used to fractionate FAMEs into polar and nonpolar fractions using eluents with different polarities. A silica-based SPE cartridge containing 1 g sorbent (Phenomenex, Torrance, CA, USA) was used in the analysis. After preconditioning, 100 mg of FAMEs dissolved in 2 mL of n-hexane-diethyl ether (98:2; v/v) were transferred into the SPE cartridge. Fifteen milliliters of n-hexane-diethyl ether (98:2; v/v) was used to elute the nonpolar fraction. The separation was followed by elution of the polar fraction using 25 mL of diethyl ether. A total of 1 mL of methyl heneicosanoate (500 µg/mL) dissolved in TBME solution was added to the polar fraction as an internal standard. The mixture was vortexed, and the solvent was evaporated to dryness under nitrogen.[Citation6]
Hydrogenation: A total of 2 mL of methanol and platinum (IV) oxide hydrate, which acted as the metal catalyst for the hydrogenation process, was added to the collected polar FAMEs. The hydrogenation process was conducted at room temperature by bubbling hydrogen gas into the sample for 10 min. After hydrogenation, the methanol was evaporated under nitrogen. The sample was dissolved in 1.5 mL of diethyl ether, filtered, and injected into a gas chromatograph equipped with a flame ionization detector (GC-FID).[Citation6]
Gas chromatography: An Agilent 6890 series chromatography system (Agilent Technologies, Santa Clara, CA, USA) equipped with a split-splitless injector was used to analyze the epoxy, keto, and hydroxy acids. The injector was operated at 250 °C, and the split ratio was set to 20:1. The separation was performed using a J&W DB-Wax fused-silica capillary column (60 m × 0.25 mm internal diameter and a film thickness of 0.25 µm) (J&W Scientific, Folsom, CA, USA). The flame ionization detector temperature was set to 250 °C. The detector flow rates for hydrogen, air, and nitrogen were 40 mL/min, 450 mL/min, and 45 mL/min, respectively. The column flow rate of nitrogen as the carrier gas was 1 mL/min. The analysis was performed under isothermal conditions at 240 °C for 30 min.[Citation6] The signal-to-noise ratios of each peaks were monitored to ensure the reliability of the quantified data. The monomeric oxidized triacylglycerols contents were determined from the calibration curve constructed by plotting the concentration of standards versus the corresponding peak areas.
Fatty acid composition: A base-catalyzed method was used to derivatize the fatty acids at room temperature. A 100-mg sample of each commercial cooking oil was accurately weighed. Each sample was dissolved in 2 mL of hexane containing 1 mg/mL methyl tridecanoate (C13:0), followed by the addition of 0.1 mL of 2 M potassium hydroxide in methanol. The mixtures were vortexed for 15 s and centrifuged at 4000 rpm for 5 min. The organic layers were filtered and analyzed using a gas chromatograph equipped with a flame ionization detector (GC-FID).[Citation9]
Gas chromatography: An Agilent 6890 Series chromatography system (Agilent Technologies, Santa Clara, CA, USA) equipped with a split-splitless injector was used to analyze the FAMEs. The split mode was set at 20:1, the split ratio was set at 250 °C, and the flame ionization detector was set at 280 °C. An SGE BPX70 column (25 m × 0.32 mm internal diameter and 0.25 µm film thickness) was used in this analysis. The hydrogen gas flow was 40 mL/min, the air flow was 450 mL/min, and the flow of nitrogen, which acted as the auxiliary gas, was 25 mL/min. The carrier gas, which was nitrogen, was fixed at 10 psi. The oven temperature program was as follows: hold at 100 °C for 5 min, ramp to 240 °C at 4 °C/min, and hold for 20 min. A calibration curve for caprylic acid (R2 = 0.99) was constructed separately to quantify its concentration.
Statistical analysis
All the measurement data were analyzed using one-way ANOVA, and p-values < 0.05 were considered significant. Data analyses were conducted to identify any significant differences in the collected data and were performed using Minitab software (Minitab Version 17.1, Minitab Pty Ltd, Sydney, NSW, Australia). Principal component analysis (PCA) was also performed to study the relationships among analytical parameters and at the same time to evaluate the interaction between analytical parameters and types of commercial cooking oil samples. The PCA evaluation was performed using XLSTAT software (version 2018, Addinsoft, New York, USA).
Results and discussion
Smoke point
Smoke point is one of the important physical parameters that is used to gauge the stability of oils. The smoke point is generally related to the accumulation of decomposition products. The generation of smoke marks the beginning of both flavor and nutritional degradation of the oil.[Citation10] A low smoke point is generally related to low thermal stability.[Citation11] In other words, a higher smoke point indicates that the oil is of better quality and contains lower levels of FFA. The results presented in show that most of the packet oils (pure palm olein) had lower smoke points (< 200°C) than the bottled oils (pure palm olein and blended palm olein). Generally, these packet oils are sold at the lowest retail prices and are considered low-cost products. The use of low-quality crude palm oil with relatively high FFA content as the input for the refining process might explain the lower smoke point of the refined packet oils compared to bottled pure palm olein and blended palm olein. In addition, the process used to refine the packet oils might not be well monitored and controlled; refining is an important step in removing extraneous impurities and volatile trace constituents such as free fatty acids that reduce the smoke point of the oil.
Table 1. Determination of the smoke points of commercial cooking oils
Total polar compounds (TPC) and their distribution
Determination of the level of total polar compounds is the most reliable measure of fat deterioration and quality control for fats and oils.[Citation12] This is because it provides information on the total amount of new compounds formed upon various heat treatments. This gravimetric method is precise and accurate because it isolates polar compounds based on adsorption chromatography.[Citation13] summarizes the content of total polar compounds and their constituents such as triacylglygerol (TAG) dimers, monomeric oxidized TAG, diacylglycerols (DAG), and free fatty acids in the oil samples.
Table 2. Determination of the polar fraction content and composition of commercial cooking oils
Polymerized TAG, the major decomposition products of cooking oil, are nonvolatile and relatively stable.[Citation14] These compounds are separated based on their molecular weights using high-performance size-exclusion chromatography; in this method, TAG oligomers of the highest molecular weights will be eluted first, followed by TAG dimers, monomeric oxidized TAG, DAG, and, lastly, free fatty acids. Because palm olein contains a high amount of DAG, the percentage of total polar compounds in palm olein is slightly higher than that in other soft oils.[Citation15] Therefore, determination of the distribution of polar compounds is very important.
The total polar compounds for all fresh oil samples fell within the safety limit for human consumption (< 25% polar compounds) set in many European countries.[Citation16] No TAG oligomers were detected in any of the commercial oil samples. The TPC content in PPO ranged from 6.96% to 8.47%, BPO ranged from 7.31% to 9.67%, and PO ranged from 6.84% to 8.35%. High TPC is unfavorable because it accelerates the oil oxidation process, increases the oil’s viscosity, reduces heat transfer, causes a foaming reaction, produces undesirable color in the fried food, and increases the rate of oil absorption by foods.[Citation14] The TAG dimer levels ranged from 0.08% to 0.76% in all fresh commercial oil samples, which were far below the rejection limit (12–13%).[Citation17]
The presence of TAG dimers in fresh cooking oils is normal. This is because dimerization of triacylglycerols occurs during the deodorization step of the oil refining process due to the high-temperature processing. Acyclic dimers of triacylglycerols, such as oxygenated dimers (C-O-C), and nonpolar dimers (C-C bridges), can be formed when the oil is subjected to extreme conditions.[Citation14] These dimers could possibly lead to the formation of polyaromatic hydrocarbons or cyclic compounds.[Citation18]
The fresh commercial cooking oils also possessed a relatively high amount of free fatty acids (FFA) compared to the values reported in the literature.[Citation19] In fact, FFA are volatile, and they are mostly removed by steam during the deodorization process.[Citation20] FFA, which possess both hydrophilic and hydrophobic groups in the same molecule, act as pro-oxidants.[Citation21] FFA normally remain at the surface of cooking oils and decrease the surface tension of the cooking oil. Thus, they help increase the rate of diffusion of oxygen from the headspace into the oil and thereby accelerate oil oxidation.[Citation20] Therefore, a good-quality crude palm oil with low FFA is a prerequisite for the production of good-quality refined palm olein as well as for minimization of refining losses. Refined oils with high FFA levels might also result from poor vacuum conditions during the deodorization process, inadequate steam sparging, or leakage of air.
Epoxy, keto, and hydroxy fatty acids
Based on the results shown in , epoxy, keto, and hydroxy fatty acids were not detected in any of the 23 commercial fresh cooking oil samples. The analysis of epoxy, keto, and hydroxy fatty acids is normally conducted on thermally oxidized oils because they are reported to form in high amounts during heat treatment. However, one study reported that epoxy fatty acids were detected in amounts ranging from 0.03 to 2 mg g−1 even in fresh oil that was high in polyunsaturated fatty acids.[Citation7] According to literature, the major precursors of these oxygenated fatty acids are polyunsaturated fatty acids.[Citation7]. The formation of epoxy fatty acids was related to the concentration of unsaturated fatty acids present in the oil and not to the relative oxidizability rates. Therefore, palm olein, which is high in monounsaturated fatty acids (oleic acid), might also contribute to the formation of these oxygenated fatty acids during heat treatment. These compounds are nonvolatile, relatively stable and remain in the oil after their formation. Therefore, to ensure the standard quality and safety of the commercial cooking oils, it is also important to evaluate whether or not the fresh commercial cooking oils contain these oxygenated compounds.
Table 3. Quantitative determination of epoxyFAMEs, ketoFAMEs and hydroxyFAMEs (g/100 g) in commercial cooking oils
Fatty acid composition
Determination of fatty acid composition is a pivotal issue in the field of food research, especially in lipid studies.[Citation22] shows the fatty acid compositions of 23 commercial cooking oil samples available in the Malaysian market. The results of the analysis of cooking oil packaged in plastic bottles were in accordance with the findings of the Malaysian Palm Oil Board.[Citation23] Sample BPO3 contained a significantly high (p < 0.05) percentage of oleic and linoleic acid, likely because this sample was blended with sesame oil and groundnut oil, both of which are high in oleic and linoleic acid. Most of the packet oils showed significantly low (p < 0.05) linoleic acid content compared with the bottled oils.
Table 4. Determination of the fatty acid composition of commercial cooking oils
There was also a possibility that the palm oil was not well fractionated, especially in the packet oils, as some fat solids were noticeable in the liquid fraction despite the fact that the oil samples were carefully maintained at ambient temperature. This might explain the higher content of myristic and palmitic acid in packet oils compared with bottled oils; an exception was sample PO8, which had significantly low (p < 0.05) percentages of palmitic, stearic, oleic, and linoleic acids but a significantly high (p < 0.05) percentage of myristic acid.
Caprylic (C8:0) acid was quantified separately because it is commonly detected in palm kernel oil but not in palm olein.[Citation24] Short-chain fatty acids such as caprylic acid might be formed in small amounts during the thermal decomposition of fatty acid hydroperoxides. One study showed that all the major unsaturated fatty acids, including oleic, linoleic, and linolenic acids, are capable of forming caprylic acid from their 9-hydroperoxides.[Citation25] Caprylic acid was detected in residual amounts ranging from 0.07 to 2.13 ppm in some of the fresh palm olein samples.
Principal component analysis (PCA)
Due to the large number of samples (variables) with multiple responses, the use of PCA yields more robust and reliable results than univariate approaches, especially with respect to the correlations among analytical parameters.[Citation26] Based on the PCA plot (), the first and second principal components (PCs) had eigenvalues greater than 1 and described 48.39% and 20.70% of the variance, respectively. Together, the first two PCs represented 69.10% of the total variability. The loading plot of the two PCs provides information on the correlations among the analytical parameters. The levels of TAG dimers and the FFAs of the commercial cooking oil samples were found to be negatively correlated with other measured properties such as smoke points, TPC, DAGs, and oxTAGs along the PC1 axis.
Figure 1. Principal component (a) score plot and (b) biplot based on quality of commercial cooking oil samples (PPO = pure palm olein; BPO = blended palm olein; PO = packet oil)
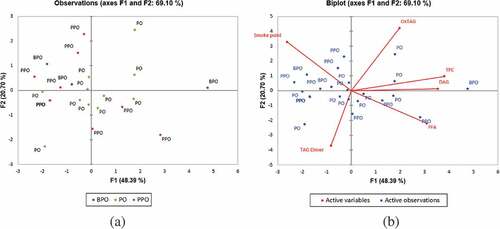
The primary axis was represented by the TPCs, DAGs, smoke point, and FFAs, whereas the secondary axis was related to the TAG dimers and oxTAGs of the cooking oil samples. The TPCs, oxTAGs, and DAG levels were strongly intercorrelated as these parameters were located in the same quadrant (right upper quadrant). In this series, the emphasis focused on the quality of different categories of cooking oils. A biplot is a combination of a score plot and a loading plot. Each of the dots in the score plot ()) represents a different type of cooking oil. The biplot ()) indicated that most of the packet oils contained high TPC, oxTAGs, DAGs, and FFA content. In addition, most of the pure palm oleins had higher smoke points than the blended oils and packet oils. In contrast to the packet oils, the pure palm olein samples were found to be better in overall quality as indicated by the biplot of all analytical parameters.
Conclusion
In this study, the safety and quality of 23 commercial cooking oils from different categories available in a Malaysian market were successfully evaluated and compared. The presence of the potential markers for the detection of recycled oil in the commercial oil samples was also determined. Correlations among analytical parameters were also evaluated through principal component analysis. Most of the packet oils (pure palm olein) had lower smoke points (< 200°C) than the bottled oils (pure palm olein and blended palm olein). The total polar compound content of all samples fell within the recommended safety limits for human consumption (< 25% polar compounds). Unsurprisingly, no TAG oligomers or epoxy, keto, or hydroxy acids were detected in the fresh oil samples. This result shows that the freshness of oil was well maintained and that the quality of cooking oils available in Malaysia is satisfactory. This finding also suggests that the presence of TAG oligomers and epoxy, keto, and hydroxy acids in fresh oil might serve as a potential indicator of the adulteration of palm olein. The reason is because the molecular weights of these compounds are high, making them more difficult to remove during the refining process. The pure palm olein samples were found to be superior to the other types of commercial oil samples in terms of overall oil quality. The results of this study provide useful insight into the quality of the commercial cooking oils available in the Malaysian market. This issue is of considerable concern because cooking oil is widely used during daily meal preparation.
Acknowledgments
The work was supported by the Putra Grant, Universiti Putra Malaysia (Project number UPM/700-1/2/GIPP/2017/9532400). The authors have declared no conflict of interest.
Additional information
Funding
References
- Endo, Y. Analytical Methods to Evaluate the Quality of Edible Fats and Oils: The JOCS Standard Methods for Analysis of Fats, Oils and Related Materials (2013) and Advanced Methods. J. Oleo Sci. 2018, 67(1), 1–10. DOI: 10.5650/jos.ess17130.
- Sayyad, R.; Jafari, S.; Ghomi, M. Thermoxidative Stability of Soybean Oil by Natural Extracted Antioxidants from Rosemary (Rosmarinus Officinalis L.). Int. J. Food Prop. 2017, 20(2), 436–446. DOI: 10.1080/10942912.2016.1166127.
- Arslan, F. N.; Şapçı, A. N.; Duru, F.; Kara, H. A Study on Monitoring of Frying Performance and Oxidative Stability of Cottonseed and Palm Oil Blends in Comparison with Original Oils. Int. J. Food Prop. 2017, 20(3), 704–717. DOI: 10.1080/10942912.2016.1177544.
- Feng, H. X.; Sam, R.; Jiang, L. Z.; Li, Y.; Cao, W. M. High-Performance Size-Exclusion Chromatography Studies on the Formation and Distribution of Polar Compounds in Camellia Seed Oil during Heating. J. Zhejiang Univ. Sci. B. 2016, 17(11), 882–891. DOI: 10.1631/jzus.B1600173.
- Velasco, J.; Marmesat, S.; Márquez-Ruiz, G.; Dobarganes, M. C. Formation of Short-Chain Glycerol-Bound Oxidation Products and Oxidised Monomeric Triacylglycerols during Deep-Frying and Occurrence in Used Frying Fats. Eur. J. Lipid Sci. Technol. 2004, 106(11), 728–735. DOI: 10.1002/ejlt.v106:11.
- Marmesat, S.; Velasco, J.; Dobarganes, M. C. Quantitative Determination of Epoxy Acids, Keto Acids and Hydroxy Acids Formed in Fats and Oils at Frying Temperatures. J. Chromatogr. A. 2008, 1211(1–2), 129–134. DOI: 10.1016/j.chroma.2008.09.077.
- Mubiru, E.; Shrestha, K.; Papastergiadis, A.; De Meulenaer, B. Improved Gas Chromatography-Flame Ionization Detector Analytical Method for the Analysis of Epoxy Fatty Acids. J. Chromatogr. A. 2013, 1318, 217–225. DOI: 10.1016/j.chroma.2013.10.025.
- AOCS. Official Methods and Recommended Practices; American Oil Chemists’ Society (Method Cc91-48): Champaign, 2013
- Márquez-Ruiz, G.; Dobarganes, C. Short-Chain Fatty Acid Formation during Thermoxidation and Frying. J. Sci. Food Agric. 1996, 70(1), 120–126. DOI: 10.1002/(ISSN)1097-0010.
- Yen, G. C.; Shao, C. H.; Chen, C. J.; Duh, P. D. Effects of Antioxidant and Cholesterol on Smoke Point of Oils. LWT - Food Sci. Technol. 1997, 30(7), 648–652.
- Alvarenga, B. R.; Xavier, F. A. N.; Soares, F. L. F.; Carneiro, R. L. Thermal Stability Assessment of Vegetable Oils by Raman Spectroscopy and Chemometrics. Food Anal. Methods. 2018, 11(7), 1969–1976. DOI: 10.1007/s12161-018-1160-y.
- Wang, F.; Jiang, L.; Zhu, X.; Hou, J. Effects of Frying on Polar Material and Free Fatty Acids in Soybean Oils. Int. J. Food Sci. Technol. 2013, 48(6), 1218–1223. DOI: 10.1111/ijfs.12080.
- Marmesat, S.; Velasco, J.; Márquez-Ruiz, G.; Dobarganes, M. C. A Rapid Method for Determination of Polar Compounds in Used Frying Fats and Oils. Grasas Y Aceites. 2007, 58(2), 179–184.
- Choe, E.; Min, D. B. Chemistry of Deep-Fat Frying Oils. J. Food Sci. 2007, 72(5), 77–86. DOI: 10.1111/j.1750-3841.2007.00352.x.
- Rodrigues Machado, E.; Marmesat, S.; Abrantes, S.; Dobarganes, C. Uncontrolled Variables in Frying Studies: Differences in Repeatability between Thermoxidation and Frying Experiments. Grasas Y Aceites. 2007, 58(3), 6.
- Marmesat, S.; Rodrigues, E.; Velasco, J.; Dobarganes, C. Quality of Used Frying Fats and Oils: Comparison of Rapid Tests Based on Chemical and Physical Oil Properties. Int. J. Food Sci. Technol. 2007, 42(5), 601–608. DOI: 10.1111/j.1365-2621.2006.01284.x.
- Kalogeropoulos, N.; Salta, F. N.; Chiou, A.; Andrikopoulos, N. K. Formation and Distribution of Oxidized Fatty Acids during Deep- and Pan-Frying of Potatoes. Eur. J. Lipid Sci. Technol. 2007, 109(11), 1111–1123. DOI: 10.1002/(ISSN)1438-9312.
- Erickson, D. R. Edible Fats and Oils Processing: Basic Principles and Modern Practices; American Oil Chemists’ Society: Champaign, 1990.
- Aniołowska, M. A.; Kita, A. M. The Effect of Frying on Glycidyl Esters Content in Palm Oil. Food Chem. 2016, 203, 95–103. DOI: 10.1016/j.foodchem.2016.02.028.
- Choe, E.; Min, D. B. Mechanisms and Factors for Edible Oil Oxidation. Compr. Rev. Food Sci. Food Saf. 2006, 5(4), 169–186. DOI: 10.1111/crfs.2006.5.issue-4.
- Miyashita, K.; Takagi, T. Study on the Oxidative Rate and Prooxidant Activity of Free Fatty Acids. J. Am. Oil Chem. Soc. 1986, 63(10), 1380–1384. DOI: 10.1007/BF02679607.
- Rohman, A.; Triyana, K.; Sismindari,; Erwanto, Y. Differentiation of Lard and Other Animal Fats Based on Triacylglycerols Composition and Principal Component Analysis. Int. Food Res. J. 2012, 19(2), 475–479.
- MPOB. The Usual Range of Major Fatty Acids in Commercial Cooking Oils; Official Portal of Malaysian Palm Oil Board. http://www.mpob.gov.my/faqs#item27, 2010.
- Lai, O. M.; Tan, C. P.; Akoh, C. C. Palm Oil: Production, Processing, Characterization, and Uses; AOCS Press: Urbana, Illinois, 2012.
- Brühl, L. Fatty Acid Alterations in Oils and Fats during Heating and Frying. Eur. J. Lipid Sci. Technol. 2014, 116(6), 707–715. DOI: 10.1002/ejlt.v116.6.
- Waghmare, A.; Patil, S.; LeBlanc, J. G.; Sonawane, S.; Arya, S. S. Comparative Assessment of Algal Oil with Other Vegetable Oils for Deep Frying. Algal Res. 2018, 31, 99–106. DOI: 10.1016/j.algal.2018.01.019.
