ABSTRACT
The aim of this study was to evaluate the effect of gelatin coating in combination with propolis extract (PE) on the microbiological (total mesophilic bacteria and psychrotrophic count), physicochemical (total volatile basic nitrogen (TVBN), 2-thiobarbituric acid reactive substances (TBARS), and free fatty acid (FFA)), and sensory characteristics in Saurida tumbil fillets during refrigerated storage (4 ± 1°C). The results showed that the gelatin–PE samples reduced the extent of lipid oxidation, as judged by TBARS and FFA, suggesting that there was a synergistic effect between gelatin coating and propolis. Moreover, the application of gelatin coatings with PE improved the TVBN and pH values of the Saurida tumbil samples significantly as compared to untreated sample, thus extending the shelf life of Saurida tumbil fillet approximately 4 days. Gelatin coating with PE was effective in inhibiting bacterial growth. The effect on the TVBN and pH indexes during storage was in accordance with bacterial activity. The gelatin–PE coatings had a positive effect on sensory acceptance of the fillets during storage. Thus, gelatin coatings enriched with PE had a better impact on the sensory characteristics and had more durability (4 days) than control. These results confirmed that gelatin coating enriched with PE can be an improved method of reducing deterioration of refrigerated Saurida tumbil fillets.
Introduction
Propolis or bee glue is a resin-like material made by honey bees (Apis mellifera) from the substances collected from parts of plants, buds, and exudates.[Citation1,Citation2] The main components of propolis include aromatic acids, aromatic esters, volatile compounds, aromatic compounds, hydrocarbons, steroids, enzymes, flavonoids, acids, micro- and macronutrients, vitamins, and essential oils.[Citation3,Citation4] Propolis, as a natural product, is the major source of bioactive compounds including antimicrobial properties[Citation1,Citation5,Citation6] and antioxidative properties[Citation4,Citation7,Citation8] that, in the last few decades, has gained wide acceptance across numerous countries as a natural preservative enhancing health and preventing disease in food products.[Citation9,Citation10] There are three methods for processing of soaking crumbled propolis including ethanol, glycerol, and/or water. Propolis extract (PE) can be added directly to foods, food can be immersed directly in PE[Citation11], or food can be coated with specifically developed coats based on polymers containing PE.[Citation12–Citation15] However, the application of PE in food products, including volatile aroma compounds, can be influenced by the deterioration of the flavor and odor.[Citation16] The results suggested that minimal addition of PE of various food products was ≤0.5% for sensory acceptance.[Citation17,Citation18] Several researchers have tried to use biopolymer coatings as a quality preservation material for fish products.[Citation19,Citation20] Among these biopolymers, gelatin is hydrophilic one with good affinity and compatibility,[Citation21] but gelatin coatings have poor biological characteristics, such as antimicrobial and antioxidant properties. Gelatin coatings supplemented with other natural preservations possess better quality-enhancing abilities than gelatin coatings alone. PE combined with biopolymer coatings has also been reported to possess antimicrobial and antioxidant functions for food packaging.[Citation12,Citation13,Citation15,Citation22] There are several published studies which deal with the possibility of using PE to extend the fresh fish shelf life.[Citation11,Citation23,Citation24] However, no study targeting the effects of gelatin coatings incorporated with PE of refrigerated fish has been reported to date. Fish and fish products are highly perishable compared with other fresh commodities, even under chilled storage.[Citation25] After harvest, initial deterioration of fish quality is caused primarily by autolytic changes, and then, loss of quality and spoilage occur as a result of bacteriological activity.[Citation26] A mixture of fish gelatin in combination with PE would seem to be suitable to protect seafood products. Therefore, the aim of the present study is to investigate in a comparative basis antimicrobial and antioxidant effect of gelatin coating enriched with PE on quality of Saurida tumbil fillets in refrigerated condition (4 ± 1°C).
Materials and methods
Preparation of propolis extract
The crude propolis sample was collected from honey bee colonies situated at the apiaries of Khorramabad, Lorestan Province, Iran. The sample was cut into small pieces, and 1 g of ground propolis was extracted with 10 ml of 80% ethanol by shaking at 150 RPM at 25°C for 48 h. The ethanolic extract of propolis was filtered (Whatman N0. 4 filter paper) and concentrated under vacuum with a rotary evaporator (IKA, RV 3 V, Germany). The samples were stored in the dark at 4°C until further analysis.[Citation27]
Sample preparation
The dry cold water fish skin gelatin (Sigma Chemical Co.) was dissolved in water (2% w/v), first being allowed to swell at 7°C for 15 min and then warmed to 55°C for 30 min. Glycerol was added at 0.75 ml/g concentration as a plasticizer and stirred for 10 min.[Citation28] The freeze-dried ethanolic extract of propolis was added to the coating at 0.1% concentrations and homogenized for 10 min by a magnetic stirrer.
The samples were filleted. The average weight of each fillet was 200.25 ± 0.52 g. Fillet samples were randomly assigned into four treatment lots consisting of one control lot (uncoated), two lots coated in PE, three lots coated in gelatin, and four lots treated with gelatin coating in combination with PE. To create the coating, fillets were soaked in a coating solution containing 0.1% PE for 30 s, then they were taken out, and after 2 min at ambient temperature (20°C), they were soaked in the solution for 30 s again.[Citation28] The coated samples were allowed to drain for 5 min under a biological safety cabinet. All samples were placed in polyethylene bags and stored at 4 ± 1°C for 16 days. Chemical, microbiological, and sensorial analyses were performed at 4-day intervals.
Microbiological analysis
Samples were collected aseptically. The samples (25 g) were placed in a Stomacher bag (Stomacher® 400 Circulator, West Sussex, UK) containing 225 mL of 0.85% saline water. After mixing for 1 min in a Stomacher blender, further serial dilutions were done using the same diluent. Thereafter, 0.1 mL of the appropriate dilution was used for microbiological analysis using the spread plate method. The media and conditions used were plate count agar (Merck, Darmstadt, Germany) incubated for total psychrotrophic count at 4°C for 10 days and for total mesophilic count at 30°C for 24–48 h. The microbial count was expressed as log10 CFU/g.[Citation2]
Physicochemical analysis
Total volatile basic nitrogen (TVBN) values were determined using the method of Goulas and Kontominas.[Citation30] The measurement of pH was carried out on 10 g of sample homogenized (T 10 basic ULTRA-TURRAX®, IKA, Staufen, Germany) in distilled water (1/10 sample/water). The pH value of the sample was determined using a digital pH meter (913 pH meter, Metrohm, Herisau, Switzerland).[Citation31] Thiobarbituric acid reactive substances (TBARS) was determined using the method of Siripatrawan and Noipha.[Citation32] Briefly, the free fatty acid (FFA) value was determined in the lipid extract using the method of procedures of Woyewoda et al.[Citation33]
Sensory evaluation
Sensory analysis of raw Saurida tumbil fillets was carried out using the quality index method as shown in by 13 panelists.[Citation34] Scores were given for each quality attributes according to descriptions, ranging from 0 to 5. On the first and last days of storage (day 0 and day 16), Saurida tumbil received a freshly QI (quality index) = 0 and completely deteriorate QI = 5. A preference score of 3 was viewed as the threshold for acceptable quality.
Table 1. Sensory evaluation criterions of Saurida tumbil fillets by gelatin coating incorporated with propolis extract
Statistical analyses
All experiments were performed in triplicate, and a completely randomized design was used. Analysis of variance was performed, and mean comparisons were done by Duncan̕s multiple range tests. The analysis was performed using an SPSS package (SPSS 11.0 for windows, SPSS Inc., Chicago, IL, USA). p Values less than 0.05 were considered statistically significant.
Results and discussion
Changes in total volatile basic nitrogen (TVBN) content
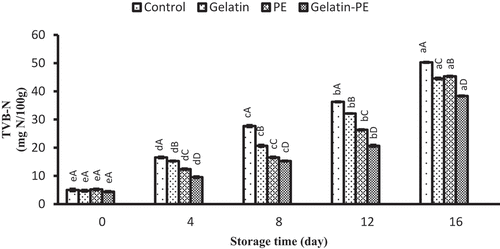
The production of total volatile basic nitrogen is related to the activity of spoilage bacteria and endogenous enzymes during fish storage and is used as an index to assess the deterioration of seafood productions.[Citation35] Changes in TVBN content of all samples during the storage are shown in . The initial TVBN of Saurida tumbil varied from 4.40 to 5.20 mg N/100 g. The TVBN level of Saurida tumbil fillets increased progressively with storage time in all the treatments. At day 16 of storage, the TVBN value of control was higher than the others, and the lowest value was 38.33 mg N/100 g in the samples coated with gelatin–PE at the end of the storage. The longer storage period of gelatin–PE-treated samples compared to other samples may have been due to lower bacteria populations which breakdown compounds, which leads to a decrease in the basic nitrogen fraction. According to Payandan et al.[Citation11], Bodini et al.[Citation22], and Hassanin and El-Daly[Citation23], the decrease in the microbial count is a plausible explanation for the slower increasing trend in the presence of PE. It can be concluded that coating in combination with PE is more effective than coating alone in controlling TVBN of fillets, suggesting that gelatin coating demonstrated synergism in retarding of TVBN value when used in combination with PE. Few information was found in the literature on the effect of PE on TVBN production in seafood products.[Citation24] Jafari et al.[Citation13] found that chitosan coating solution in combination with ethanolic extract of propolis could retard the increase in the TVBN content compared to other treatments. A level of 25 mg N/100 g muscle has been considered the highest acceptable level.[Citation36] At day 12 of storage, TVBN level of gelatin–PE was <25 mg N/100 g muscle, indicating that Saurida tumbil fillets were of good quality during storage. However, at the same time, the amounts of TVBN in control and gelatin- or PE-coated samples were higher than 25 mg N/100 g of muscle.
Figure 1. Combined effect of gelatin and propolis extract on TVBN content of Saurida tumbil during storage at refrigerator. Mean values and standard errors from the three replicates are presented. The different capital letters in the same columns within the same storage time indicate the significant differences (p < 0.05). The different small letters in the same rows within the same treatment indicate the significant differences (p < 0.05)
Changes in pH content
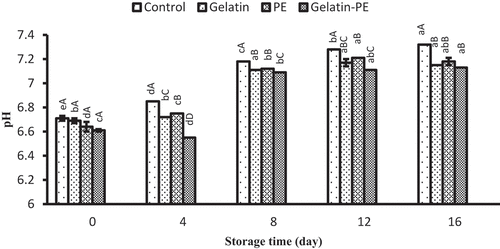
Changes in pH of Saurida tumbil muscle during refrigerated storage are shown in . The initial pH of Saurida tumbil samples was between 6.61 and 6.71. The pH of all the samples increased linearly during storage at refrigerator. The pH value of control (uncoated samples) reached to the maximum value (7.32) at the end of storage. The increase in pH might be attributed to the accumulation of alkaline compounds (such as biogenic amines and ammonia) generated from both endogenous enzymes and enzymatic actions of psychrotrophic bacteria.[Citation37] There was no significant difference (p> 0.05) in pH values among coated samples (gelatin, PE, and gelatin–PE) at the end of the storage. Piedrahíta Márquez et al.[Citation24] showed that the pH value of refrigerated cachama (Piaractus brachypomus) vacuum-packed fish fillets had a slower increase in the coated samples compared to the uncoated samples. Thaker et al.[Citation38] and Alparslan et al.[Citation39] showed that gelatin alone and in combination with natural additives could notably decrease pH value, due to reduction in the production of amine compounds with delay in the oxidative ability of bacteria. However, Sun et al.[Citation4] and Kaewprachu et al.[Citation40] reported that gelatin with food additives had no significant difference in the pH value of food products. The pH value is positively correlated with TVBN as increase in volatile amine leads to an increase of pH which is showed in this research.
Figure 2. Combined effect of gelatin and propolis extract on pH content of Saurida tumbil during storage at refrigerator. Mean values and standard errors from the three replicates are presented. The different capital letters in the same columns within the same storage time indicate the significant differences (p < 0.05). The different small letters in the same rows within the same treatment indicate the significant differences (p < 0.05)
Changes in TBARS content
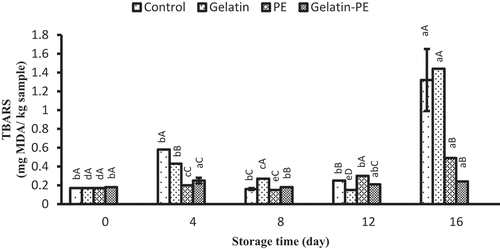
TBARS has proved a valuable indicator to assess the degree of lipid oxidation during the chilled storage. Changes in TBARS value of Saurida tumbil are shown in . The result indicated that TBARS levels increased from 0.17 mg malonaldehyde/kg sample on 0 day to 1.32, 1.44, 0.49, and 0.24 mg malonaldehyde/kg sample on 16th day for control, gelatin, PE, and gelatin–PE, respectively. Gelatin–PE had the lowest TBARS among other treatments, and no significant difference was presented in PE and gelatin–PE samples after 16 days. According to Antoniewski et al.[Citation41], gelatin coating on seafood products may reduce lipid oxidation due to hydrogen bonds in gelatin as a barrier to oxygen. However, in this study, there was no significant difference in the TBARS content of fillet between control and gelatin-coated samples. The addition of PE to gelatin-coated samples can increase the antioxidant activity of the gelatin. The antioxidant mechanism of propolis could be through radical scavenging capacities with antioxidant agent (polyphenolic compounds such as flavonoids and cinnamic acid derivatives) and/or protection of antioxidant food ingredients through forming a biodegradable coating (oxygen barrier properties)-treated samples during storage.[Citation41,Citation42,Citation43] Hassanin and El-Daly et al.[Citation23] observed the tilapia fillet coated with propolis stored at −18°C had a significant effect in preventing lipid oxidation. Jafari et al.[Citation13] observed a significant effect of the treatment in preventing lipid oxidation of chicken fillet coated with gelatin–PE stored at 4°C for 12 days in comparison to coating alone in gelatin. A lower TBARS content for rainbow trout fillets treated by PE was reported by Adnani et al.[Citation44] Spinelli et al.[Citation45] added 5% spray-dried propolis to a suitable fish-burger formula and showed lower lipid oxidation than the control because of higher phenol content and higher sequestrating activity on 2,2-diphenyl-1-picrylhydrazyl. In another study, the lipid oxidation of the burger meat was reduced by using microencapsulation PE.[Citation46] According to our study, similar effects were observed. TBARS values of 1 to 2 mg malonaldehyde/kg muscle are an acceptable sensory limit.[Citation47] In the current study, TBARS values for all samples were less than 2 mg malonaldehyde/kg muscle at the end of the storage. This indicates that the PE with or without gelatin coatings is able to reduce the lipid oxidation, resulting in lower rancidity compared with other samples.
Figure 3. Combined effect of gelatin and propolis extract on TBARS content of Saurida tumbil during storage at refrigerator. Mean values and standard errors from the three replicates are presented. The different capital letters in the same columns within the same storage time indicate the significant differences (p < 0.05). The different small letters in the same rows within the same treatment indicate the significant differences (p < 0.05)
Changes in FFA content
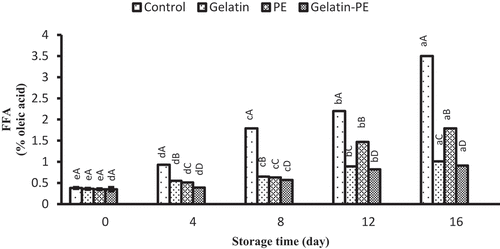
The initial FFA value was from 0.35% to 0.38% of oleic acid (). FFA content gradually increased in all samples, but gelatin–PE-coated fillet slowed the formation of FFA during refrigerated storage. From the result, gelatin–PE had the highest antioxidant effect for Saurida tumbil fillet, as it was seen for psychrotrophic bacteria population. The production of lipase and phospholipase with psychrotrophic bacteria, especially Pseudomonas spp.,[Citation37] can be reduced by antibacterial activity of propolis in samples coated with gelatin–PE. There is a significant relationship between the phenolic compounds and free radical scavenging activity of PE.[Citation41] As it was concluded that from delaying the oxidative changes of lipids (TBARS), PE added to gelatin coating protects Saurida tumbil fillets so would reduce the production of FFAs.
Figure 4. Combined effect of gelatin and propolis extract on FFA content of Saurida tumbil during storage at refrigerator. Mean values and standard errors from the three replicates are presented. The different capital letters in the same columns within the same storage time indicate the significant differences (p < 0.05). The different small letters in the same rows within the same treatment indicate the significant differences (p < 0.05)
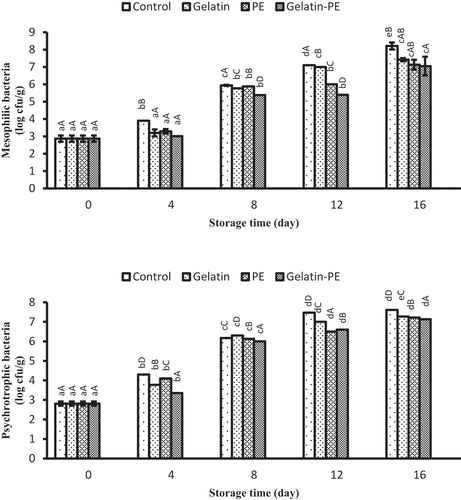
Changes in microbiological analysis
Variations in the value of total mesophilic bacteria (TMB) and psychrotrophic (PTC) counts of Saurida tumbil during the storage at refrigerator are presented in . The initial TMB and PTC (log10 CFU/g) in all the samples of Saurida tumbil fillet were 2.87 and 2.81 log10 CFU/g, respectively, indicating a good quality of seafood products.[Citation47,Citation48] All of the treatments led to a dramatic reduction in TMB and PTC in Saurida tumbil fillet compared to the control samples. By the day 12 of storage, TMB and PTC in Saurida tumbil fillet were reached to about 7 log10 CFU/g for control samples and gelatin samples, which is higher than the maximal recommended limit in raw fish[Citation29] while that of samples treated with PE and/or gelatin+PE achieved this count at the end of the storage time. The increase in TMB and PTC of Saurida tumbil fillet treated by gelatin and PE was significantly less than that treated by gelatin or PE coating at the 16 days of storage, might be attributed to the immediate antimicrobial effect of gelatin and PE. Payandan et al.[Citation11] and Hassanin and El-Daly[Citation23] reported that PE can be effective as antimicrobial agents on inhibition of the growth of spoilage bacteria of fishery products. Thus, the incorporation of PE in gelatin coating can remarkably delay the growth of bacteria. Jafari et al.[Citation13] showed that chitosan edible coating containing PE could extend the shelf life of chicken fillet during storage at refrigerator. The antimicrobial activity of PE has been ascribed to the presence of bioactive compounds such as flavonoids (e.g., tectochrysin, pinobanksin, pinocembrin, chrysin, galangin, apigenin, and kaempferol), which show antibacterial effect.[Citation42] The in vitro and in vivo antimicrobial activity of propolis against bacteria has been reported by Seibert et al.[Citation49] and Khodabakhshi et al.[Citation50] Duman and Özpolat[Citation17] stated how bacterial growth in PE-coated Barbus grypus reached less than that in uncoated samples. The result of the present study indicated that the PE used in fresh shibuta fillet coated with gelatin leads to a reduction in microbial contamination during long storage time.
Figure 5. Combined effect of gelatin and propolis extract on microbiology properties (mesophilic and psychrotrophic bacteria) of Saurida tumbil during storage at refrigerator. Mean values and standard errors from the three replicates are presented. The different capital letters in the same columns within the same storage time indicate the significant differences (p < 0.05). The different small letters in the same rows within the same treatment indicate the significant differences (p < 0.05)
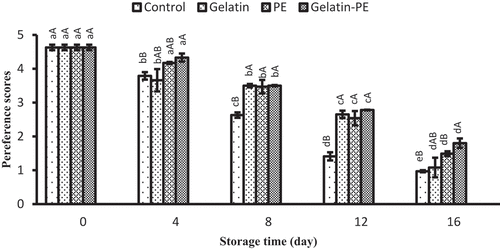
Sensory analysis
In , the results of the preference scores are presented for the fillets with or without gelatin and PE, along the storage time. The results indicate that the sensory analysis of all treatments decreased during the storage (p < 0.05). In general, gelatin+PE-coated samples presented lower changes in the color, odor, and overall appearance throughout the storage time. The increasing off-odor of uncoated samples could be smelt from 8th days backward, and the fish fillet was unaccepted after 12 days of storage (score = 1). However, samples coated with PE–gelatin obtained higher scores than PE- or gelatin-coated fillets during storage, suggesting that PE and gelatin can improve the organoleptic quality of refrigerated fillets. The deterioration of the organoleptic quality was correlated with the microbial spoilage and physicochemical analysis, caused the change of surface of color of samples.[Citation51] The antioxidant and antimicrobial effects of gelatin and PE have been shown to extend the shelf life of fish approximately 4 days for PE-/gelatin–PE-coated samples. This result was in agreement with that of TMB changes and TVBN values, which suggested that gelatin–PE coating was effective to delay sensory changes of fish fillet.
Figure 6. Combined effect of gelatin and propolis extract on sensory properties of Saurida tumbil during storage at refrigerator. Mean values and standard errors from the three replicates are presented. The different capital letters in the same columns within the same storage time indicate the significant differences (p < 0.05). The different small letters in the same rows within the same treatment indicate the significant differences (p < 0.05)
Conclusion
The results presented in this study indicated that gelatin coating incorporated with PE of Saurida tumbil fillet effectively retarded the growth of mesophilic bacteria and psychrotrophic bacteria. This may be the result of the protective effect of gelatin coating with PE against bacterial growth. In this study, uncoated Saurida tumbil fillets have a rather short shelf life (4 days) indicated by physicochemical (TVBN and pH) parameters and microbiological (TMB and PTC) and sensory acceptance (color, odor, and overall appearance) properties. PE or gelatin–PE coatings had a longer shelf life than gelatin coating/or control samples. Therefore, gelatin coating incorporated with PE provides a type of packaging that can be utilized as a safe preservative for fish under refrigerated storage.
References
- Bankova, V.; Galabov, A. S.; Antonova, D.; Vilhelmova, N.; Di Perri, B. Chemical Composition of Propolis Extract ACF® and Activity against Herpes Simplex Virus. Phytomedicine. 2014, 21, 1432–1438. DOI: 10.1016/j.phymed.2014.04.026.
- Pellati, F.; Orlandini, G.; Pinetti, D.; Benvenuti, S. HPLC-DAD and HPLC-ESIMS/MS Methods for Metabolite Profiling of Propolis Extracts. J. Pharm. Biomed. Anal. 2011, 55(5), 934–948. DOI: 10.1016/j.jpba.2011.03.024.
- Kubiliene, L.; Laugaliene, V.; Pavilonis, A.; Maruska, A.; Majiene, D.; Barcauskaite, K.; Kubilius, R.; Kasparaviciene, G.; Savickas, A. Alternative Preparation of Propolis Extracts: Comparison of Their Composition and Biological Activities. BMC Complementary Altern. Med. 2015, 15, 156–162. DOI: 10.1186/s12906-015-0677-5.
- Sun, C.; Wu, Z.; Wang, Z.; Zhang, H. Effect of Ethanol/Water Solvents on Phenolic Profiles and Antioxidant Properties of Beijing Propolis Extracts. In Evidence-based Complementary and Alternative Medicine, Hindawi Publishing Corporation, 2015; pp 1–9.
- Das Neves, M. V. M.; Da Silva, T. M. S.; Lima, E. O.; Cunha, E. V. L.; Oliveira, E. J. Isoflavone Formononetin from Red Propolis Acts as a Fungicide against Candida Sp. Braz. J. Microbiol. 2016, 47, 159–166. DOI: 10.1016/j.bjm.2015.11.009.
- Nolkemper, S.; Reichling, J.; Sensch, K. H.; Schnitzler, P. Mechanism of Herpes Simplex Virus Type 2 Suppression by Propolis Extracts. Phytomedicine. 2010, 17, 132–138. DOI: 10.1016/j.phymed.2010.04.005.
- Azemin, A.; Md-Zin, N. B.; Mohd-Rodi, M. M.; Kim-Chee, A. S.; Zakaria, A. J.; Mohd, K. S. Application of Metabolite Profiling and Antioxidant Activity in Assessing the Quality of Processed and Unprocessed Stingless Bee’ Propolis. J. Fundam. Appl. Sci. 2017, 9(2S), 637–660.
- Mello, C. B. S.; Hubinger, M. D. Antioxidant Activity and Polyphenol Contents in Brazilian Green Propolis Extracts Prepared with the Use of Ethanol and Water as Solvents in Different pH Values. Int. J. Food Sci. Technol. 2012, 47, 2510–2518. DOI: 10.1111/j.1365-2621.2012.03129.x.
- Ali, F. H.; Kassem, G. M.; Atta-Alla, O. A. Propolis as a Natural Decontaminant and Antioxidant in Fresh Oriental Sausage. Veterinaria Ital. 2010, 46(2), 167–172.
- Luis-Villaroya, A.; Espina, L.; García-Gonzalo, D.; Bayarri, S.; Pérez, C.; Rafael Pagán, R. Bioactive Properties of a Propolis-based Dietary Supplement and Its Use in Combination with Mild Heat for Apple Juice Preservation. Int. J. Food Microbiol. 2015, 205, 90–99. DOI: 10.1016/j.ijfoodmicro.2015.03.020.
- Payandan, E.; Zayyed-Alangi, S. Z.; Shamloofar, M.; Koohsari, H. Study of Chemical Composition and Efficacy of Different Extracts of Iranian Propolis on the Microbiological and Sensory Parameters of Minced Cyprinus Carpio Meat at 4°C Storage. J. Aquat. Food Prod. Technol. 2017, 26(5), 593–603. DOI: 10.1080/10498850.2016.1240281.
- Costa, S. S.; Druzian, J. I.; Machado, B. A. S.; de Souza, C. O.; Guimaraes, A. G. Bi-functional Biobased Packing of the Cassava Starch, Glycerol, Licuri Nanocellulose and Red Propolis. PLoS One. 2014, 9(11), 1–10. DOI: 10.1371/journal.pone.0112554.
- Jafari, N. J.; Kargozari, M.; Ranjbar, R.; Rostami, H.; Hamedi, H. The Effect of Chitosan Coating Incorporated with Ethanolic Extract of Propolis on the Quality of Refrigerated Chicken Fillet. J. Food Process. Preserv. 2017, 42(1), e13336. DOI: 10.1111/jfpp.13336.
- Rollini, M.; Mascheroni, E.; Capretti, G.; Coma, V.; Musatti, A. Propolis and Chitosan as Antimicrobial and Polyphenols Retainer for the Development of Paper Based Active Packaging Materials. Food Pack. Shelf Life. 2017, 14, 75–82. DOI: 10.1016/j.fpsl.2017.08.011.
- Shavisi, N.; Khanjari, A.; Basti, A. A.; Misaghi, A.; Shahbazi, Y. Effect of PLA Films Containing Propolis Ethanolic Extract, Cellulose Nanoparticle and Ziziphora clinopodioides Essential Oil on Chemical, Microbial and Sensory Properties of Minced Beef. Meat Sci. 2017, 124, 95–104. DOI: 10.1016/j.meatsci.2016.10.015.
- Narbona, E.; García- García, E.; Vázquez-Araújo, L. V.; Carbonell-Barrachina, Á. A. Volatile Composition of Functional ‘a La Piedra’ Turrón with Propolis. Int. J. Food Sci. Technol. 2010, 45, 569–577. DOI: 10.1111/j.1365-2621.2009.02167.x.
- Duman, M.; Özpolat, E. Effects of Water Extract of Propolis on Fresh Shibuta (Barbus grypus) Fillets during Chilled Storage. Food Chem. 2015, 189, 80–85. DOI: 10.1016/j.foodchem.2014.08.091.
- El-Mossalami, H.; Abdel-Hakeim, Y. A. Using of Propolis Extract as a Trial to Extend the Shelf-life and Improving the Quality Criteria of Fresh Egyptian Sausage. Assiut Vet. Med. J. 2013, 59(139), 23–33.
- Kakaei, S.; Shahbazi, Y. Effect of Chitosan-gelatin Film Incorporated with Ethanolic Red Grape Seed Extract and Ziziphora clinopodioides Essential Oil on Survival of Listeria monocytogenes and Chemical, Microbial and Sensory Properties of Minced Trout Fillet. LWT Food Sci. Technol. 2016, 72,432–438.
- Nowzari, F.; Shabanpour, B.; Ojagh, S. M. Comparison of Chitosan-gelatin Composite and Bilayer Coating and Film Effect on the Quality of Refrigerated Rainbow Trout. Food Chem. 2013, 141, 1667–1672. DOI: 10.1016/j.foodchem.2013.03.022.
- Pereda, M.; Ponce, A. G.; Marcovich, N. E.; Ruseckaite, R. A.; Martucci, J. F. Chitosan-gelatin Composites and Bi-layer Films with Potential Antimicrobial Activity. Food Hydrocolloids. 2011, 25, 1372–1381. DOI: 10.1016/j.foodhyd.2011.01.001.
- Bodini, R. B.; Sobral, P. J. A.; Favaro-Trindade, C. S.; Carvalho, R. A. Properties of Gelatin-based Films with Added Ethanolepropolis Extract. LWT Food Sci. Technol. 2013, 51, 104–110. DOI: 10.1016/j.lwt.2012.10.013.
- Hassanin, S. I. A.; El-Daly, E. S. A. Effect of Propolis and Garlic on Nile Tilapia Oreochromis niloticus Fillets during Frozen Storage. J. Arabian Aquacult. Soc. 2013, 8(1), 237–248.
- Piedrahíta Márquez, D. G.; Fuenmayor, C. A.; Mahecha, H. S. Effect of Chitosan‐propolis Edible Coatings on Stability of Refrigerated Cachama (Piaractus brachypomus) Vacuum‐packed Fish Fillets. Packag. Technol. Sci. 2019, 32, 1–11.
- Vásconez, M. B.; Flores, S. K.; Campos, C. A.; Alvarado, J.; Gerschenson, L. N. Antimicrobial Activity and Physical Properties of Chitosan–Tapioca Starch Based Edible Films and Coatings. Food Res. Int. 2009, 42, 762–769. DOI: 10.1016/j.foodres.2009.02.026.
- Arashisar, X.; Hisar, O.; Kaya, M.; Yanik, T. Effects of Modified Atmosphere and Vacuum Packaging on Microbiological and Chemical Properties of Rainbow Trout (Oncorynchus mykiss) Fillets. Int. J. Food Microbiol. 2004, 97, 209–214. DOI: 10.1016/j.ijfoodmicro.2004.05.024.
- Yaghoubi, M.; Satari, R. Antimicrobial Activity of Iranian Propolis and Its Chemical Composition. DARU J. Pharm. Sci. 2007, 15(1), 45–48.
- Ojagh, S. M.; Rezaei, M.; Razavi, S. H.; Hosseini, S. M. H. Effect of Chitosan Coatings Enriched with Cinnamon Oil on the Quality of Refrigerated Rainbow Trout. Food Chem. 2010, 120, 193–198. DOI: 10.1016/j.foodchem.2009.10.006.
- Sallam, K. I.;. Antimicrobial and Antioxidant Effects of Sodium Acetate, Sodium Lactate, and Sodium Citrate in Refrigerated Sliced Salmon. Food Control. 2007, 18, 566–575. DOI: 10.1016/j.foodcont.2006.02.002.
- Goulas, A. E.; Kontominas, M. G. Effect of Salting and Smoking-method on the Keeping Quality of Chub Mackerel (Scomber japonicus): Biochemical and Sensory Attributes. Food Chem. 2005, 93, 511–520. DOI: 10.1016/j.foodchem.2004.09.040.
- Suvanich, V.; Jahncke, M. L.; Marshall, D. L. Changes Selected Chemical Quality Characteristics of Channel Catfish Frame Mince during Chill and Frozen Storage. Food Sci. 2000, 65(1), 24–29. DOI: 10.1111/j.1365-2621.2000.tb15950.x.
- Siripatrawan, U.; Noipha, S. Active Film from Chitosan Incorporating Green Tea Extract for Shelf Life Extension of Pork Sausages. Food Hydrocolloids. 2012, 27, 102–108. DOI: 10.1016/j.foodhyd.2011.08.011.
- Woyewoda, A. D.; Shaw, S. J.; Ke, P. J.; Burns, B. G. (1986). Recommended Laboratory Methods for Assessment of Fish Quality. Canadian Technical Report of Fish and Aquatic Science, 1448.
- Zhu, Z. W.; Ruan, Z.; Li, B. S.; Meng, M. Y.; Zeng, Q. X. Quality Loss Assessment of Crisp Grass Carp (Ctenopharyngodon idellus C. Et V) Fillets during Ice Storage. J. Food Process. Preserv. 2013, 37, 254–261. DOI: 10.1111/jfpp.2013.37.issue-3.
- Benjakul, S.; Seymour, T. A.; Morrissey, M. T.; AN, H. Physicochemical Changes in Pacific Whiting Muscle Proteins during Iced Storage. J. Food Sci. 1997, 62, 729–733. DOI: 10.1111/jfds.1997.62.issue-4.
- Kilincceker, O.; Dogan, I. S.; Kucukoner, E. Effect of Edible Coatings on the Quality of Frozen Fish Fillets. LWT - Food Sci. Technol. 2009, 42, 868–873. DOI: 10.1016/j.lwt.2008.11.003.
- Nirmal, N. P.; Benjakul, S. Retardation of Quality Changes of Pacific White Shrimp by Green Tea Extract Treatment and Modified Atmosphere Packaging during Refrigerated Storage. Int. J. Food Microbiol. 2011, 149, 247–253. DOI: 10.1016/j.ijfoodmicro.2011.07.002.
- Thaker, M.; Hanjabam, M. D.; Gudipati, V.; Kannuchamy, N. Protective Effect of Fish Gelatin-based Natural Antimicrobial Coatings on Quality of Indian Salmon Fillets during Refrigerated Storage. J. Food Process Eng. 2017, 40, e12270. DOI: 10.1111/jfpe.2017.40.issue-1.
- Alparslan, Y.; Baygar, T.; Baygar, T.; Hasanhocaoglu, J. Effects of Gelatin-based Edible Films Enriched with Laurel Essential Oil on the Quality of Rainbow Trout (Oncorhynchus mykiss) Fillets during Refrigerated Storage. Food Technol. Biotechnol. 2014, 52, 325–333.
- Kaewprachu, P.; Amara, C. B.; Oulahal, N.; Gharsallaoui, A.; Joly, C.; Tongdeesoontorn, W.; Rawdkuen, S.; Degraeve, P. Gelatin Films with Nisin and Catechin for Minced Pork Preservation. Food Pack. Shelf Life. 2018, 18, 173–183. DOI: 10.1016/j.fpsl.2018.10.011.
- Antoniewski, M. N.; Barringer, S. A.; Knipe, C. L.; Zerby, H. N. Effect of a Gelatin Coating on the Shelf Life of Fresh Meat. J. Food Sci. 2007, 72, 382–387. DOI: 10.1111/j.1750-3841.2007.00430.x.
- Pobiega, K.; Kraśniewska, K.; Gniewosz, M. Application of Propolis in Antimicrobial and Antioxidative Protection of Food Quality – A Review. Trends Food Sci. Technol. 2019, 83, 53–62. DOI: 10.1016/j.tifs.2018.11.007.
- Al-Qurashi, A. D.; Awad, M. A. Postharvest Ethanolic Extract of Propolis Treatment Affects Quality and Biochemical Changes of ‘hindi-besennara’ Mangos during Shelf Life. Sci. Hortic. 2018, 233, 520–525. DOI: 10.1016/j.scienta.2017.12.030.
- Adnani, A.; Darvishi, S.; Mohammadi, K. Evaluation of the Effects of Extraction of Kurdistan Propolis on Biochemical and Microbiological Parameters of Rainbow Trout (Oncorhynchus mykiss). Iran. Sci. Fish. J. 2017, 25(4), 41–52.
- Spinelli, S.; Conte, A.; Lecce, L.; Incoronato, A. L.; Del Nobile, M. A. Microencapsulated Propolis to Enhance the Antioxidant Properties of Fresh Fish Burgers. J. Food Process Eng. 2015, 38(6), 527–535. DOI: 10.1111/jfpe.2015.38.issue-6.
- Reis, A. S.; Diedrich, C.; Moura, C.; Pereira, D.; de Florio Almeida, C.; Silva, L. D.; Plata-Oviedo, M. S. V.; Tavares, R. A. W.; Carpes, S. T. Physico-chemical Characteristics of Microencapsulated Propolis Co-product Extract and Its Effect on Storage Stability of Burger Meat during Storage at −15°C. LWT Food Sci. Technol. 2017, 76, 306–313. DOI: 10.1016/j.lwt.2016.05.033.
- Shakila, R.; Jeyasekaran, G.; Vijayalakshmi, S. Effect of Vacuum Packaging on the Quality Characteristics of Seerfish (Scomberomorus commersonii) Chunks during Refrigerated Storage. J. Food Sci. Technol. 2005, 42, 438–443.
- Sikorski, Z. E.; Kolakowska, A.; Burt, J. R. Post Harvest Biochemical and Microbial Changes Seafood. In Resources Nutritional Composition and Preservation; Sikorski, Z. E., Ed.; CRC Press Inc: Boca Raton, 1990; pp 55–75.
- Seibert, J. B.; Bautista-Silva, J. P.; Amparo, T. R.; Patita, A.; Pervier, P.; Dos Santos, J. C.; Azevedo, M. C.; Silveira, B. M.; Brandão, G. C.; de Souza, G. H. B.; et al. Development of Propolis Nanoemulsion with Antioxidant and Antimicrobial Activity for Use as a Potential Natural Preservative. Food Chem. 2019, 287, 61–67. DOI: 10.1016/j.foodchem.2019.02.078.
- Khodabakhshi, D.; Eskandarinia, A.; Kefayat, A.; Rafienia, M.; Navid, S.; Karbasi, S.; Moshtaghian, J. In Vitro and In Vivo Performance of a Propolis-coated Polyurethane Wound Dressing with High Porosity and Antibacterial Efficacy. Colloids Surface B. 2019, 178, 177–184. DOI: 10.1016/j.colsurfb.2019.03.010.
- Hui, G. H.; Liu, W.; Feng, H. L.; Li, J.; Gao, Y. Y. Effects of Chitosan Combined with Nisin Treatment on Storage Quality of Large Yellow Croaker (Pseudosciaena crocea). Food Chem. 2016, 203, 276–282. DOI: 10.1016/j.foodchem.2016.01.122.
