ABSTRACT
The effects of hydrothermal (HT)-calcium chloride (CaCl2) treatment on water loss, chlorophylls, L-ascorbic acid, total phenol, antioxidant capacity, malondialdehyde (MDA), peroxidase (POD), polyphenol oxidase (PPO), catalase (CAT), and phenylalanine ammonia lyase (PAL) of peppers were assessed for 32 days of storage at 8°C. The results showed two water populations corresponding to strongly immobilized water and weakly bound water were observed in all the peppers. Comparing with other treatments, HT-CaCl2 treatment restricted water mobility and maintained higher immobilized water content during storage. HT-CaCl2 treated peppers showed lower MDA content whereas presented higher chlorophylls, L-ascorbic acid, total phenol content, and stronger antioxidant capacities than those subjected to other treatments. These characteristics indicated HT-CaCl2 treatment improved storage quality of postharvest peppers. In addition, HT-CaCl2 treatment retained lower POD, PPO, and PAL activities and higher CAT activity in the peppers during storage than other treatments, respectively. Based on above results, the combination of HT and CaCl2 treatment showed positive and continuous effects on the quality attributes and related enzyme activities of peppers during storage.
Introduction
Pepper (Capsicum annuum L.), which belongs to the Solanaceae family, is planted worldwide and covers approximately 1.99 million ha of harvested area and an annual production of 36.1 million tonnes in 2017 according to FAO.[Citation1] Pepper fruit is rich in nutrients and bioactive compounds, including L-ascorbic acid, phenolic compounds, carotenoids, and capsaicin, which exhibit antioxidant activities and anti-inflammatory effects according to Ribes-Moya et al.[Citation2] Hence, pepper fruit is widely accepted by many consumers and sold worldwide. However, given that fresh pepper consists of approximately 90% water, it easily matures and decays, causing quality deterioration and reducing commodity value. Mohebbi et al.[Citation3] found that shrinkage percentage, firmness, and color of bell peppers were very sensitive to storage time, while storage temperature had the most effect on moisture reduction. Chitravathi et al.[Citation4] found that ascorbic acid, total chlorophylls, capsaicin contents, and total antioxidant activity tended to decrease during storage at 7–9°C (RH: 85%-95%). Hence, the physicochemical characteristic and antioxidant capacities of postharvest peppers decrease at some extent during storage, and the preservation technologies must be applied to retain the storage qualities. Current research about methods regulating the storage qualities of peppers focused on physical, chemical, and biological preservation, particularly through heating treatment,[Citation5] coating,[Citation3] salicylic acid, and calcium chloride (CaCl2) treatment.[Citation6] Thus, novel preservation methods must be constantly improved based on the needs of high efficiency, green, and safe.
Hydrothermal (HT) or CaCl2 treatment maintains the qualities of fruits and vegetables. Positively maintaining physicochemical quality, hot air or water at 45–65°C was used as HT treatment in the research of Glowacz et al.[Citation7] and Maxin et al.[Citation8] The quality deterioration of fruits and vegetables with HT treatment was less than that of untreated samples, and their texture was better than the control. Ravanfar et al.[Citation9] found that sour cherry immersed at 40°C hot water for 2 min showed improved water holding capacity and defense. Calcium plays a crucial role in reducing water desorption because it prevents or delays the loss of firmness. Calcium may diffuse within the cell wall structure by increasing the amount of endogenous calcium that can combine with pectin and form calcium bridge according to Ngamchuachit et al.[Citation10] Thus, calcium treatment poses a positive and continues effect on the retention of fruits and vegetables quality during storage. Ngamchuachit et al.[Citation10] found that fresh-cut Tommy Atkin mangos treated with 0.136 M CaCl2 at 10°C for 2.5 min maintained better texture and other qualities than control. Belge et al.[Citation11] found that cherry fruit with 3% CaCl2 treatment for 2 min showed lower water loss and slower delay than the control. Some advances had recently focused on the combination of HT and CaCl2 treatment in postharvest fruits and vegetables. Comparing with HT or CaCl2 treatment alone, a better co-effect is found in HT-CaCl2 treatment. Ayón-Reyna et al.[Citation12] subjected fresh-cut papaya to HT treatment at 49°C for 25 min with CaCl2 (1%) and dipping in chitosan (Chit; 1%, 3 min). The result showed that all treatments reduced the deterioration processes, maintained microbial, chemical and physical qualities, and extended the shelf life, but HT-CaCl2 treatment resulted in the best texture and qualities compared with other treatments. However, studies on the HT-CaCl2 treatment of peppers are rare, even on treatment using HT or CaCl2 alone. Hence, the effects of HT-CaCl2 treatment on the storage qualities of peppers should be studied and used as reference for the development of safe and effective preservation method for peppers.
In this study, postharvest peppers were preserved by HT-CaCl2 treatment, and the effects of HT, CaCl2, and HT-CaCl2 treatments on the qualities and related enzymes of peppers during storage were investigated. Then, water loss; chlorophyll a and b contents; L-ascorbic acid content; total phenol content; antioxidant capacity; malondialdehyde (MDA) content; and the activity of related enzymes, including peroxidase (POD), polyphenol oxidase (PPO), catalase (CAT), and phenylalanine ammonia lyase (PAL), in peppers stored at 8°C for up to 32 days were determined. This experiment will provide theoretical evidence with HT-CaCl2 treatment regulating the qualities and related enzymes of postharvest peppers during storage.
Materials and methods
Materials
Fresh green peppers cv xiangyan No. 16 were harvested from Yueyang, Hunan province, China, on 26th July 2018. All the peppers reached commercial maturity (about 78 maturity). After harvest, the peppers were delivered to the laboratory immediately. Thereafter, they were washed and drained. Peppers with uniformity of size, color, and weight, free from visible blemishes, disease and/or physical damage were selected as the experiment raw materials. Selected peppers were divided into four groups for treatment and storage.
Treatments
Based on previous research, the pre-experiments were carried out to explore suitable HT temperature (40°C, 45°C, 50°C for 2 min) and CaCl2 concentration (1.5%, 2.5%, 3.5% for 20 min) during storage of 32 days at 8°C (RH: 90–95%) for peppers. By comparing the changes of appearance qualities, such as color, firmness, shrinkage, and so on, we selected 45°C/2 min HT and 2.5%/20 min CaCl2 to treat peppers.
HT: the peppers were placed in the 45°C hot water for 2 min under atmospheric pressure; CaCl2 treatment: the peppers were placed in the 2.5% CaCl2 solution for 20 min at room temperature under atmospheric pressure; HT-CaCl2 treatment: the peppers were placed in the 45°C hot water for 2 min, and then placed in the 2.5% CaCl2 solution for 20 min at room temperature under atmospheric pressure; untreated samples was used as control. After the above treatments, all of the peppers were drained and cooled into room temperature. Treated samples (approximately 250 g each) were packaged into No.10 sealed bag (34 cm length × 24 cm width) before storage.
Storage condition
Sample storage was conducted at 8°C (RH: 90–95%). All treated and untreated samples were prepared in triplicate and stored at above conditions. The samples were considered and analyzed at 0 (untreated), 8, 16, 24, and 32 days during storage.
Water relaxation time measurement
Water relaxation time was determined using a low field nuclear magnetic resonance (NMR) MesoMR12-150H-I (Shanghai Niumag Corporation, China) with 12.7977 MHz with a modification of the method described by Bulut et al.[Citation13] The same pepper in every treatment was inserted in the NMR probe equipped with 0.5 T strength magnets at 32°C for different storage time. The transverse relaxation time (T2) was measured using a CPMG pulse sequence. A 90° pulse followed by a train of 180° pulses was contained in this sequence to refocus the NMR signal. Relaxation curves obtained from the CPMG sequence were analyzed using NMR software.
Chlorophyll a and b contents analysis
Chlorophyll extraction was performed according to the method described by Xie et al.[Citation14] In brief, 1 g lyophilization of freeze-dried pepper powder was mixed with 10 mL 80% (v/v) cold acetone solution, and centrifuged at 5300 × g for 10 min at 4°C. The supernatant was collected and filtered through a centrifugal filter before HPLC analysis.
HPLC analysis was performed using a Waters HPLC System (Waters 2695 Separations Module, Milford, USA) equipped with a photodiode array detector (Waters 2996, Milford, USA) according to the method of Teng and Chen et al.[Citation15] Chlorophylls were separated within 20 min by a C18 column (Cosmosil 5C18-AR-Ⅱ; 250 × 4.6 mm; i.d., 5 µm; Nacalai Tesque Inc., Japan) with a flow rate of 1.0 mL/min at 30°C, using a solvent system of acetonitrile/methanol/chloroform/n-hexane (75: 12.5: 7.5: 7.5, v/v/v/v) as the isocratic mobile phase. The injection volume was 20 µL. The identification of chlorophyll a and b was based on the comparison with peaks of standards according to the absorption spectrum at 432 nm. Results were expressed as mg g−1 DW.
L-ascorbic acid content measurement
L-ascorbic acid was determined using a method reported by Valdenegro et al.[Citation16] with some modifications. Peppers (20.0 ± 5.0 g) were smashed by shredding machine. All pulverized peppers were placed in a 100 mL beaker, and 50 mL of cold (4°C) 2.5% meta-phosphoric acid (Acros Organics, UK) was added. The processed peppers were immediately centrifuged at 5300 × g for 20 min at 4°C. Supernatants were filtered using Sep Pak filters (Phenomenex, UK), and 1.5 mL was collected in 100 mL volumetric flask. Samples were analyzed using an Agilent 1100.
HPLC (Agilent, UK) with a Luna 5 μm NH2 100 A column (250 mm × 4.6 mm) (Phenomenex, UK) at a flow rate of 0.5 mL min−1 with a pressure in the range of 70–80 bars at 30°C, using a solvent system of n-hexane/acetonitrile/methanol/chloroform/(75: 7.5: 12.5: 7.5, v/v/v/v) as the isocratic mobile phase. The identification of L-ascorbic acid was based on the comparison with peaks of standards according to the absorption spectrum at 254 nm. Results were expressed as mg 100 g−1 FW.
Total phenol content measurement
Total phenol content was measured using Folin–Ciocalteu method according to Deng et al.[Citation17] Approximately 0.5 g freeze-dried pepper powder was extracted using 80% methanol. After 30 min, 0.4 mL of extract was mixed with 2 mL of Folin-Ciocalteu reagent, then 3 mL of Na2CO3 (10%) was added to the mixture and incubated at room temperature for 60 min. The absorbance was measured at 760 nm. GAE was used as standard, and the result was expressed as µg GAE eq mg−1 DW.
Antioxidant capacities analysis
DPPH radical scavenging capacity
DPPH radical scavenging capacity was determined using a method according to Li et al.[Citation18] with some modification. DPPH solution (0.0552 g) was prepared using 100 mL of methanol solution. Approximately 0.5 g of freeze-dried pepper powder was extracted with 80% methanol. Approximately 0.4 mL of pepper extract was mixed with 3.5 mL of DPPH solution and was incubated at room temperature for 20 min. The absorbance was measured at 517 nm. Trolox was the standard substance, and the result was expressed as mg Trolox eq g−1 DW.
ABTS+ radical scavenging capacity
ABTS+ radical scavenging capacity was measured with a method reported by Lin et al.[Citation19] Approximately 0.5 g of freeze-dried pepper powder was extracted using 80% methanol. The reaction solution was mixed with 0.4 mL of pepper extract and 3.6 mL ABTS radical cation solution. After reacting at 25°C for 30 min, the reaction solution was immediately measured at 735 nm. Trolox was used for the calibration curves, and the result was expressed as mg Trolox eq g−1 DW.
FRAP
FRAP was performed using the method described by Anand et al.[Citation20] with some modification. Approximately 0.5 g of freeze-dried pepper powder was extracted using 80% methanol. The radical FRAP solution contained 2.5 mL of a 10 mM TPTZ solution in 40 mM HCl, 2.5 mL of 20 mM FeCl3, and 25 mL of acetate buffer (0.3 M, pH 3.6). The reaction solution consisted of 0.08 mL of the radical FRAP solution and 0.1 mL of pepper extract. The mixture was measured at 593 nm after the reaction in water bath at 37°C for 5 min. Trolox was used as the standard, and the result was expressed as mg Trolox eq g−1 DW.
MDA content measurement
MDA content was determined with a modified method described by Liu et al.[Citation21] In brief, 30 g of fresh peppers were homogenized in 50 mL of 100 g L−1 trichloroacetic acid solution and centrifuged at 5300 × g for 30 min at 4°C. The supernatant was then placed in a volumetric flask. The reaction mixture absorbance was measured at 450, 532, and 600 nm. The results were expressed as mmol 100 g−1 FW.
POD and PPO enzyme activities analysis
The solution of POD and PPO was extracted using the method described by Terefe et al.[Citation22] with some modifications. POD and PPO were extracted from peppers (30 g) with extraction solution (50 mL) consisting of 0.1 M sodium acetate buffer solution (pH 5.5) and 20 g L−1 crospolyvinylpyrrolidone (PVPP). The mixture was homogenized for 2 min at 1200 × g and then stewed at 4°C for 1 h. The mixture was then centrifuged at 4°C for 30 min at 5300 × g (Avanti J-26 XP, Beckman CoulterInc., USA). The supernatant of POD (200 µL) was mixed with 0.05 M guaiacol in 0.05 M sodium acetate buffer solution at pH 5.5 (10 mL). In the blank, the sample was replaced with the extraction solution. The absorbance of the reaction mixture at 20°C-22°C was measured at 470 nm every 30 s for 7 min (Agilent 8453 Spectrophotometer, Agilent Technologies, Wald-bronn, Germany). The supernatant of PPO (200 µL) was mixed with 0.01 M catechol in 0.05 M sodium acetate buffer solution at pH 5.5 (8 mL) and then placed in a water-bath at 37°C for 10 min. The blank was the same as the POD. The absorbance of the reaction mixture at 20°C-22°C was measured at 420 nm every 5 s for 100 s (Agilent 8453 Spectrophotometer, Agilent Technologies, Wald-bronn, Germany). Enzyme activity was determined in the linearrange (the first 3 min for POD and the first 30 s for PPO) and expressed as U g−1 FW.
CAT enzyme activity analysis
CAT activity was determined according to method of Wang et al.[Citation23] with the following modifications. The mixture was homogenized in extraction solution (50 mL) consisting of 0.05 M phosphate buffer (pH 7.5) containing 5 mM dithiothreitol and 20 g/L PVPP. The mixture was then stirred for 20 min at 4°C, and centrifuged for 20 min at 5300 × g at 4°C. The reaction mixture contained 1.5 mL of 0.05 mM H2O2 in 0.05 M phosphate buffer. The reaction started by the addition of 0.2 mL of enzyme extract. CAT activity was monitored at 240 nm for 5 min at room temperature by using a UV-vis spectrophotometer. CAT specific activity was reported as U g−1 FW.
PAL enzyme activity analysis
PAL activity was determined using a modified method by Chen et al.[Citation24] The peppers (30 g) were homogenized on ice with 50 mL of 100 mM sodium borate buffer (pH 8.8) containing 2 mM EDTA, 40 g L−1 PVPP, and 5 mM b-mercaptoethanol. The mixture was centrifuged for 30 min at 5300 × g at 4°C. The reaction mixture consisted of 0.5 ml of 20 mM L-phenylalanine, 0.9 mL of the crude extract, and 3 mL of 50 mM sodium borate buffer at pH 8.8. The mixture was incubated for 60 min at 37°C, and the reaction was stopped using 0.2 mL of 6 M HCl. PAL activity was monitored at 290 nm for 3 min at room temperature with a UV-vis spectrophotometer. PAL activity was expressed as U g−1 FW.
Statistical analysis
All the treatments were performed in triplicate. ANOVA was performed using the software Microcal Origin 8.0 (Microcal Software, Inc., Northampton, USA). The results were expressed or plotted as the mean value ± standard deviation.
Results and discussion
Appearance quality
The effects of HT-CaCl2 treatment on appearance quality are illustrated in . Commercial postharvest quality is important to practical use of peppers. As shown in , appearance quality of treated peppers was better maintained than that of untreated ones during storage. The shrinkage of all peppers increased with storage, especially for untreated ones. The upper and stem parts of untreated peppers had a notable shrinkage after 24 days. However, shrinkage rate of treated peppers was less than untreated peppers. For HT-CaCl2 treated peppers, they looked fuller than other three groups. Above results indicated that HT-CaCl2 treatment could effectively inhibit water loss and senescence of peppers during storage, further retained better appearance quality.
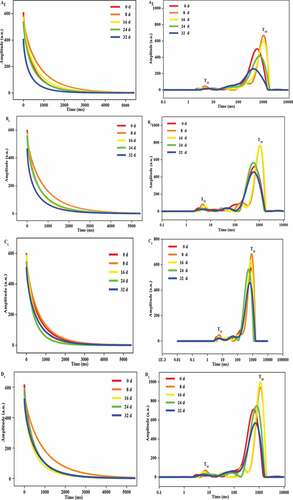
Water relaxation time
The water Carr–Purcell–Meiboom–Gill (CPMG) signals and relaxation time of all treatments are illustrated in . According to (A1, B1, C1, D1), the decay time showed a decreasing trend with storage time, which was related to water changes during storage. By comparing untreated and treated peppers, it was clear that untreated peppers decayed the fastest and the decayed time was less than 1000 ms. Whereas HT-CaCl2 treated peppers showed the longest decay time, more than 1000 ms. However, only from the CPMG decay curves, the water states were difficult to distinguish. To describe the water stages during storage, T2 spectra were obtained by the multi-exponential fitting of CPMG decay curves using an NMR analysis software. T2i represents the mobility of water and can be assigned to four water population, including T21, T22, T23, and T24. T21, T22, T23, and T24 refer to the transverse relaxation time of bound water, weakly bound water, immobilized water, and free water, respectively.[Citation25] As shown in (A2, B2, C2, D2), two peaks in untreated and treated peppers were observed. The highest peak, from 100 ms to 1000 ms, represented immobilized water (T23), and the lowest peak, from 0 ms to 10 ms, represented bound water (T21). The higher value of T23 showed higher water mobility at some extent. From (A2, B2, C2, D2), T23 values increased in the early storage and then decreased in the late storage. The result indicated that some of the immobilized water in peppers gradually transformed into other water forms during storage. However, T21 values were minimally changed. From the result of Wang et al.[Citation26], bound water was firmly associated with other components in peppers, such as protein, and complex structure was stable. Comparing untreated, HT-treated, and CaCl2-treated samples, the T23 value was similar with each other. This result demonstrated that the effects of HT or CaCl2 treatment on restricting water mobility were not evident. However, the T23 value in Figure 2D2 was approximately 1.5 times higher than that in Figure 2A2. This result indicated that HT-CaCl2 treatment could effectively restrict water mobility and maintain high immobilized water content. This finding was because Ca2+ could form clathrate hydrates with water by chemical bonding at a certain temperature and restrict water mobility.[Citation4]
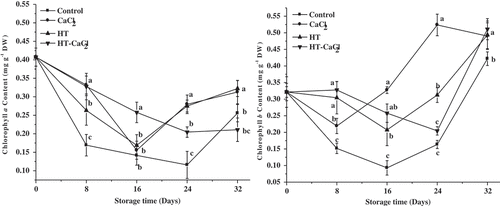
Chlorophyll a and b contents
The effects of HT-CaCl2 treatment on chlorophyll a and b contents are presented in . Chlorophyll a and b contents in fresh peppers were 0.41 and 0.32 mg g−1 DW, respectively. Chlorophyll a and b contents of all the peppers first decreased and then increased during storage. Since chlorophylls are sensitive to heat[Citation27], their decrease in the early storage period was probably due to respiration heat increase, which accelerated chlorophyll degradation. In the late storage, chlorophyll a and b contents increase could be ascribed to the accumulation of light harvest complex Ⅱ (LHCⅡ) under stress. Sato et al.[Citation28] found that both chlorophyll a and b were related to LHCII, especially for chlorophyll b. When preceding the degradation of LHCII, the chlorophylls in LHCII could be degraded. Horton et al.[Citation29] showed that variable amounts of LHCII could not be degraded under stress, but organized PSII into large super-complexes to capture light. Based on above researches, we speculated that LHCII could not be properly degraded and caused chlorophylls accumulation in the late storage, especially for chlorophyll b. Further explore needs to be focused on the changes of LHCII in peppers during storage. According to , the chlorophyll contents in CaCl2-treated peppers were the most comparing other treatments during storage. He et al.[Citation30] also found that CaCl2 treatment reduced the hypoxic damage of cucumber leaves and enhanced the stability of chlorophylls. However, HT and HT-CaCl2 treatments were related to temperature, and chlorophylls are sensitive to temperature, which accelerated chlorophylls degradation in some extent. Moreover, an interesting phenomenon was found, that is, the content of chlorophyll b was more than chlorophyll a in peppers after 24 days. This result was related to the accumulation of LHCII under stress. Nick et al.[Citation31] showed that a chlorophyll b-less mutant did not accumulate LHCII, and chlorophyll b metabolism was closely related to LHCII content. Meanwhile, sato et al.[Citation28] showed that chlorophyll cycle existed in land plant, which was believed to facilitate the regulation of the chlorophyll composition throughout developmental stages. However, rüdiger et al.[Citation32] found that chlorophyll cycle did not function as a complete cycle at one and the same time, and its function was believed to supply either chlorophyll a or chlorophyll b according to the particular physiological need. It was possible that there was a conversion between chlorophyll a and b in the late storage, and it resulted in the increase of chlorophyll b content in peppers. Above speculation needs to be further studied, basing on the changes of LHCII and enzymes activities related to chlorophyll cycle in pepper during storage.
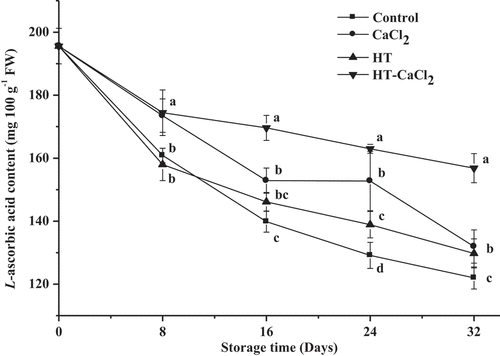
L-ascorbic acid content
Changes in L-ascorbic acid content of peppers with different pretreatments during storage are illustrated in . The initial content of L-ascorbic acid was 195.60 mg 100 g−1 FW. From , L-ascorbic acid content of all samples showed a decrease trendy with storage time and was evident in the first 16 days. Change in L-ascorbic acid content was probably related to external environment and internal factors. Gu et al.[Citation27] found that L-ascorbic acid degradation was probably caused by genetics, temperature, light, water content, and so on. Herbig et al.[Citation33] found that high PPO activity accelerated the degradation of L-ascorbic acid during early storage. The result about the variation of PPO activity further confirmed this view. Compared with the control, peppers with HT or CaCl2 treatment decreased slower, especially in the late storage. Kumar et al.[Citation34] explored the effects of different temperatures at different times for L-ascorbic acid content in citrus fruit and found that citrus heated at 50°C for 90 s delayed the decrease of L-ascorbic acid content. Hussain et al.[Citation35] also found that the total L-ascorbic acid content in apple with CaCl2 treatment was higher than that in the control. The above results showed that HT or CaCl2 treatment could improve the stability of L-ascorbic acid. As shown in , when combining HT and CaCl2 treatment, the decreasing rate was less and had a significant (P< .05) difference comparing with other groups, especially for untreated peppers. On the 16, 24 and 32 days, L-ascorbic acid content in HT-CaCl2 treated peppers could, respectively, reach 169.62, 162.97, and 156.84 mg 100 g−1 FW. Especially on the last day, L-ascorbic acid content in HT-CaCl2 treated peppers was still 1.29 times than that in untreated peppers. Meanwhile, because of antioxidant capacities of L-ascorbic acid, HT-CaCl2 treated peppers had a higher antioxidant capacity than other groups. The later conclusions for total phenol and antioxidant capacities of the peppers further confirmed this view.
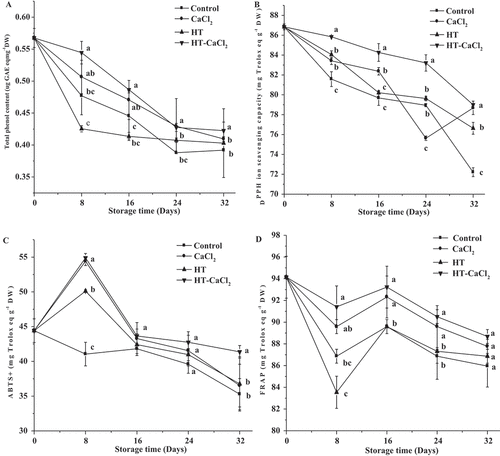
Total phenol content
Total phenol is important antioxidant components in fruits and vegetables.[Citation36] The effect of HT-CaCl2 treatment on total phenol content is presented in . The initial content of total phenol was 0.57 µg GAE eq mg−1 DW. As shown in , total phenol content in all samples decreased with the storage time. The decrease of total phenol content was related to PPO activity. Deng et al.[Citation17] found that faster water loss and higher PPO activity in litchi pericarp could accelerate the oxidation of phenolics during storage. The maximum decrease of total phenol was shown in the early 8 days but slowed in the late storage. The result was because high PPO activity accelerated total phenol degradation in the early storage. HT-CaCl2 treatment presented the highest total phenol content in all the treatments, and there was a significant (P< .05) difference between untreated and HT-CaCl2 treated peppers in the first 16 days. On the eighth day, total phenol content with HT-CaCl2 treatment was up to 0.54 µg GAE eq mg−1 DW and was 1.22 times higher than that of the control. According to above result, it showed that HT-CaCl2 treatment could positively affect the maintenance of total phenol content. This result had been proven in previous studies. Ayón-Reyna et al.[Citation12] found that fresh-cut papaya with HT (49°C, 25 min) containing CaCl2 (1%) followed by dipping in Chit (1%) could positively inhibit the reduction of total phenol. Aghdam et al.[Citation37] used 40, 60, and 80 mM CaCl2 to treat cherry and found that all the treatments could maintain high total phenol, especially for 80 mM CaCl2 treatment.
Antioxidant capacities
The antioxidant capacities of fruits and vegetables are affected by different antioxidant components.[Citation38] The effect of HT-CaCl2 treatment on antioxidant capacities is presented in –. In , there were decrease trend for DPPH, ABTS+ and FRAP radical scavenging capacity during storage, but the variation was different. For the DPPH radical scavenging capacity, the antioxidant capacities of untreated peppers decreased from 86.84 mg Trolox eq g−1 DW to 72.21 mg Trolox eq g−1 DW. After treatment, the decrease in the DPPH radical scavenging capacity was inhibited, especially for HT-CaCl2 treatment. On the final day, DPPH radical scavenging capacity in HT-CaCl2 treated peppers maintained the highest values, up to 79.00 mg Trolox eq g−1 DW, and had a significant (P< .05) difference comparing untreated peppers. The decrease of DPPH radical scavenging capacity could be ascribed to the loss of antioxidants, such as total phenol and L-ascorbic acid. Wojdylo et al.[Citation39] had shown that positive correlations were observed between DPPH radical scavenging capacity and total phenol or L-ascorbic acid. Above results indicated that HT-CaCl2 treatment could positively maintain DPPH radical scavenging capacity. ABTS+ and FRAP radical scavenging capacities also exhibited similar trends. In , more difference was found between HT-CaCl2 treated peppers and others in the early storage. On the eighth day, ABTS+ and FRAP radical scavenging capacities in HT-CaCl2 treatment were 1.34 and 1.05 times higher than that of the control, respectively. Antunes et al.[Citation40] found that kiwifruit treated with ascorbic acid or 2% CaCl2 positively affected DPPH and ABTS+ radical scavenging capacities maintenance mainly in the first 4 days, but CaCl2 treatment exhibited a continuous effect until the last day. Viña et al.[Citation41] also found that the antioxidant capacity of pre-cut celery could be better retained with 50°C for 90 s than that of untreated ones. Above results suggested that HT-CaCl2 treatment was available to maintain antioxidant capacities.
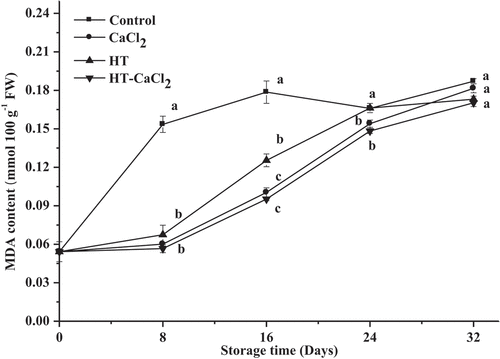
MDA content
MDA is one of the end products of membrane lipid oxidation and is a crucial indicator of membrane damage according to Yan et al.[Citation42] If an increased amount of MDA is produced in fruits and vegetables, cell will be severely damaged, and cause fruits and vegetables easily decay. As shown in , MDA content in fresh peppers was 0.05 mmol 100 g−1 FW. MDA content increased with storage time for all the peppers, which could be due to membrane lipid oxidation. Comparing with untreated and treated peppers, MDA content in untreated peppers was the highest during storage. Significant (P< .05) differences were shown between untreated and treated peppers on the eighth and sixteenth days. MDA content of the untreated peppers, respectively, increased into 0.15 and 0.18 mmol 100 g−1 FW at 8 and 16 days, more than treated peppers. For all treatments, HT-CaCl2 treatment could effectively inhibit the increase of MDA during storage, followed by HT or CaCl2 treatment, and the effect was more obvious in the first 16 days. Similar results were found in other researches. Wu et al.[Citation43] also found that calcium, chlorine dioxide, and heat treatment could reduce respiration production and MDA content of apricots stored at 20°C for 10 days. HT-CaCl2 treatment could positively inactivate oxidase enzymes and increase Ca2+ content in membrane, further sustaining membrane stability. Hence, HT-CaCl2 treatment was an effect of pretreatment for inhibiting the increase of MDA content in peppers and extending its shelf life.
Related enzyme activities
POD activity: POD is a physiological index of the ripening and senescence of fruits and vegetables. The effect of HT-CaCl2 treatment on POD activity in peppers during storage is shown in . POD activity decreased with storage time in HT, CaCl2, and HT-CaCl2-treated peppers. This phenomenon was probably caused by the decrease of the physiochemical metabolism in peppers. POD activity of untreated peppers showed a decreasing fluctuation trend. As shown in , POD activity in untreated peppers showed a higher value compared with treated peppers during storage. Especially on the 16th day, POD in control reached 1.16 U g−1 FW and was 1.63 times higher than in HT-CaCl2 treatment. However, Higher POD could cause numerous adverse physiological reactions. Liu et al.[Citation44] had proved POD catalyzed various oxidation reactions involving CAT and caused enzymatic browning with PPO. Kim et al.[Citation45] also showed that PPO and POD were crucial for the oxidation of phenolic compounds and resulted in enzymatic browning. Liu et al.[Citation44] also found that POD was related to anti-browning of fresh-cut potato slices during storage. When other physiochemical qualities were considered, POD played a key role in regulating enzymatic browning compared with oxidation resistance in our research. Hence, according to above results, increased POD activity accelerated oxidation reactions and caused enzymatic browning.[Citation46] As shown in , HT, CaCl2, HT-CaCl2 treatments could decrease POD activity during storage, especially for HT-CaCl2 treatment. This observation corresponded well with the result of Marszałek et al.[Citation47], who found that the POD activity of strawberries treated with 50°C hot water for 15 min was obviously inhibited than that of untreated ones. Reichel et al.[Citation48] also found that CaCl2 treatment could decrease the POD activity of litchi pericarp and showed improved cooperating effect with other chemical agent, such as cysteine.
Figure 7. POD, PPO, CAT, and PAL activities of the peppers treated with CaCl2, HT, and HT-CaCl2 for 32 days at 8°C. Each value is the mean of three replications, and vertical bar represents the standard error of the means (n = 3)
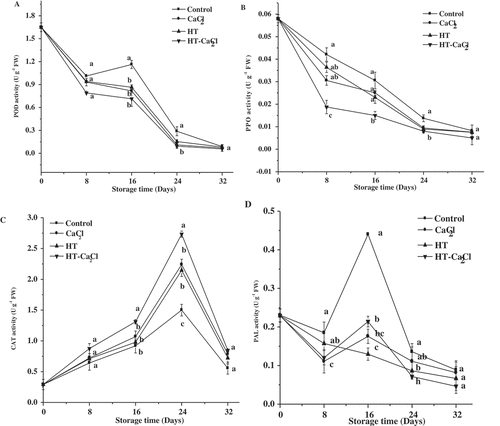
PPO activity: PPO is associated with the deposition of phenolic compounds into plant cell walls during resistance interactions.[Citation49] The effect of HT-CaCl2 treatment on PPO activity in peppers during storage is shown in . It was evident that PPO activity showed a decreasing trend in untreated and treated peppers, and reached a minimum value on the final day. Comparing four groups, PPO activity in untreated peppers was the highest, and the HT-CaCl2 treated peppers were the lowest during storage. From , there were significant (P< .05) differences between untreated and HT-CaCl2 treated peppers in the first 16 days. Especially on the eighth day, PPO activity of HT-CaCl2 treated peppers only up to 45% of that in untreated peppers. Above results showed that PPO activities were effectively inhibited by HT-CaCl2 treatment. Some previous researches have proved HT or CaCl2 treatment inhibited PPO activity. Vámos-Vigyázó et al.[Citation50] found that fruits and vegetables shortly exposed to temperatures from 70°C to 90°C showed lower activity of PPO than that of untreated ones. Youryon et al.[Citation51] also showed that 48 h 2% calcium gluconate treatment effectively retarded the PPO activity of pineapple. However, high PPO activity could cause adverse reaction. Bajwa et al.[Citation52] found that PPO was involved in enzymatic browning of fruits and vegetables, which could catalyze phenolic substances and various oxidation reactions involving CAT. Mrad et al.[Citation53] found that browning in pears was mainly caused by increasing PPO activity. Hence, High PPO activity was associated with L-ascorbic acid and chlorophyll degradation. According to above results, HT-CaCl2 treatment could decrease PPO activity and maintain some qualities.
CAT activity: CAT is related to oxidation resistance. According to Gao et al.[Citation54], high CAT activity is effective to remove ROS, especially O2- produced by metabolism. Otherwise, too much O2- could accelerate the membrane lipid oxidation and produce mass MDA. The test result of CAT activity is illustrated in . A sharp increasing trend for untreated and treated peppers in the first 24 days and thereafter decreased. Huang et al.[Citation55] proved high metabolism of postharvest fruits and vegetables increased the production of ROS in the early storage, and the accumulation of ROS further caused senescence. Hence, when CAT activity increased, O2- accumulated in peppers could easily be removed. Meanwhile, High CAT activity was beneficial for maintaining the oxidation resistance and delayed senescence of peppers in the early storage. The similar variation trend was found in the experiment of Wu et al.[Citation43], CAT activity of apricots slightly increased in the first 6 days and then decreased. After HT or CaCl2 treatment, CAT activity showed similar level but was higher than that in untreated peppers. Raseetha et al.[Citation56] showed that broccoli heated at 70°C for 10 min showed similar increasing trend for CAT activity. For CaCl2 treatment, Wu et al.[Citation43] found that CAT activity in apricots with 0.5% CaCl2 treatment at 20°C for 5 min increased and was higher than control. It was obvious shown that when treating with the combination of HT and CaCl2, peppers showed the highest CAT level in untreated and treated peppers during storage, ranging from 0.29 U g−1 FW to 2.73 U g−1 FW. On the 24th day, a significant (P< .05) difference was found between HT-CaCl2 treatment and other three groups, especially for untreated peppers. The CAT activity in HT-CaCl2 treated peppers reached up to 2.73 U g−1 FW on the 24th day, but the untreated peppers were only 1.5 U g−1 FW. Hence, HT-CaCl2 treatment was effective for maintaining CAT activity and improving resistance.
PAL activity: shows the effect of HT-CaCl2 treatment on PAL activity in peppers during storage. For the control, CaCl2, and HT-CaCl2 treatment, PAL activity decreased drastically in the first 8 days and increased from the eighth day to 16th day, thereafter decreased to the lowest level. However, PAL activity decreased with the storage time for HT treated peppers. Wulfkuehler et al.[Citation57] speculated that the reduced PAL activities in lettuce were correlated with delayed browning at cut edges, and perceived by sensory evaluation and stereo microscopy. Khan et al.[Citation58] also found that CaCl2 treatment exhibited a beneficial effect on inhibiting PAL activity. However, in this experiment, the effect of PAL activity on quality was not clear, so further research needs to be carried out. In , PAL activity in untreated peppers was higher than that in treated peppers during storage. On the 16th day, there was a significant (P< .05) difference between untreated and treated peppers. PAL activity was 2.44, 3.38, 2.10 times than CaCl2, HT and HT-CaCl2 treatments, respectively. Hence, HT, CaCl2 or HT-CaCl2 treatment had a better effect on inhibiting PAL activity.
Conclusion
Based on the results of our investigation, HT-CaCl2 treatment exhibited the most positive and continuous effect for quality attributes and related enzyme activities of peppers during storage in all the treatments. Two water populations corresponding to strongly immobilized water and weakly bound water were observed in all the peppers. The immobilized water content first increased considerably and then decreased with storage time. However, the bound water content was stable during storage. Compared with other treatments, HT-CaCl2 treatment could restrict water mobility and maintain higher immobilized water content during storage. HT-CaCl2-treated peppers exhibited lower MDA content, higher chlorophyll, L-ascorbic acid, and total phenol content, and antioxidant capacities than those of other treatments. For the related enzymes, HT-CaCl2 treatment retained low POD, PPO, and PAL activity and high CAT activity of peppers during storage. For the further verification of our results, the next research will focus on the effects of HT-CaCl2 treatment on physiological metabolism, including respiration, ethylene production, and so on.
Additional information
Funding
References
- FAO. 2017. http://www.fao.org/faostat/en/#data/QC.
- Ribes-Moya, A. M.; Raigón, M. D.; Moreno-Peris, E.; Fita, A.; Rodríguez-Burruezo, A. Response to Organic Cultivation of Heirloom Capsicum Peppers: Variation in the Level of Bioactive Compounds and Effect of Ripening. PLos One 2018, 13, 1–24. DOI: 10.1371/journal.pone.0207888.
- Mohebbi, M.; Amiryousefi, M. R.; Hasanpour, N.; Ansarifar, E. Employing an Intelligence Model and Sensitivity Analysis to Investigate Some Physiochemical Properties of Coated Bell Pepper during Storage. Int. J. Food Sci. Technol. 2012, 47, 299–305. DOI: 10.1111/j.1365-2621.2011.02839.x.
- Chitravathi, K.; Chauhan, O. P.; Raju, P. S. Influence of Modified Atmosphere Packaging on Shelf-life of Green Chilies (capsicum Annuum L.). Food Pack. Shelf Life 2015, 4, 1–9. DOI: 10.1016/j.fpsl.2015.02.001.
- Sgroppo, S. C.; Pereyra, M. V. Using Mild Heat Treatment to Improve the Bioactive Related Compounds on Fresh-cut Green Bell Peppers. Int. J. Food Sci. Technol. 2009, 44, 1793–1801. DOI: 10.1111/j.1365-2621.2009.01998.x.
- Rao, T. V. R.; Gol, N. B.; Shah, K. K. Effect of Postharvest Treatments and Storage Temperatures on the Quality and Shelf Life of Sweet Pepper (capsicum Annum L.). Sci. Hortic. 2011, 132, 18–26. DOI: 10.1016/j.scienta.2011.09.032.
- Glowacz, M.; Mogren, L. M.; Reade, J. P. H.; Cobb, A. H.; Monaghan, J. M. Can Hot Water Treatments Enhance or Maintain Postharvest Quality of Spinach Leaves? Postharvest. Biol. Technol. 2013, 81, 23–28. DOI: 10.1016/j.postharvbio.2013.02.004.
- Maxin, P.; Weber, R. W. S.; Pedersen, H. L.; Williams, M. Control of a Wide Range of Storage Rots in Naturally Infected Apples by Hot-water Dipping and Rinsing. Postharvest. Biol. Technol. 2012, 70, 25–31. DOI: 10.1016/j.postharvbio.2012.04.001.
- Ravanfar, R.; Niakousari, M.; Maftoonazad, N. Postharvest Sour Cherry Quality and Safety Maintenance by Exposure to Hot-water or Treatment with Fresh Aloe Vera Gel. J. Food Sci. Technol. 2014, 51, 2872–2876. DOI: 10.1007/s13197-012-0767-z.
- Ngamchuachit, P.; Sivertsen, H. K.; Mitcham, E. J.; Barrett, D. M. Effectiveness of Calcium Chloride and Calcium Lactate on Maintenance of Textural and Sensory Qualities of Fresh-cut Mangos. J. Food Sci. 2014, 79, 786–794. DOI: 10.1111/1750-3841.12446.
- Belge, B.; Goulao, L. F.; Comabella, E.; Graell, J.; Lara, I. Refrigerated Storage and Calcium Dips of Ripe ‘celeste’ Sweet Cherry Fruit: Combined Effects on Cell Wall Metabolism. Sci. Hortic. 2017, 219, 182–190. DOI: 10.1016/j.scienta.2017.02.039.
- Ayón-Reyna, L. E.; Tamayo-Limón, R.; Cárdenas-Torres, F.; López-López, M. E.; López-Angulo, G.; López-Moreno, H. S.; López-Cervántes, J.; López-Valenzuela, J. A.; Vega-García, M. O. Effectiveness of Hydrothermal-calcium Chloride Treatment and Chitosan on Quality Retention and Microbial Growth during Storage of Fresh-cut Papaya. J. Food Sci. 2015, 80, 594–600. DOI: 10.1111/1750-3841.12783.
- Bulut, M.; Bayer, Ö.; Kırtıl, E.; Bayındırlı, A. Effect of Freezing Rate and Storage on the Texture and Quality Parameters of Strawberry and Green Bean Frozen in Home Type Freezer. Int. J. Refrig. 2018, 88, 360–369. DOI: 10.1016/j.ijrefrig.2018.02.030.
- Xie, J.; Yao, S. X.; Ming, J.; Deng, L. L.; Zeng, K. F. Variations in Chlorophyll and Carotenoid Contents and Expression of Genes Involved in Pigment Metabolism Response to Oleocellosis in Citrus Fruits. Food Chem. 2019, 272, 49–57. DOI: 10.1016/j.foodchem.2018.08.020.
- Teng, S. S.; Chen, B. H. Formation of Pyrochlorophylls and Their Derivatives in Spinach Leaves during Heating. Food Chem. 1999, 65, 367–373. DOI: 10.1016/S0308-8146(98)00237-4.
- Valdenegro, M.; Fuentes, L.; Herrera, R.; Moya-León, M. A. Changes in Antioxidant Capacity during Development and Ripening of Goldenberry (physalis Peruviana L.) Fruit and in Response to 1-methylcyclopropene Treatment. Postharvest. Biol. Technol. 2012, 67, 110–117. DOI: 10.1016/j.postharvbio.2011.12.021.
- Deng, M.; Deng, Y. Y.; Dong, L. H.; Ma, Y. X.; Liu, L.; Huang, F.; Wei, Z. C.; Zhang, Y.; Zhang, M. W.; Zhang, R. F. Effect of Storage Conditions on Phenolic Profiles and Antioxidant Activity of Litchi Pericarp. Molecules. 2018, 23, 1–12. DOI: 10.3390/molecules23092276.
- Li, H. Y.; Yuan, Q.; Yang, Y. L.; Han, Q. H.; He, J. L.; Zhao, L.; Zhang, Q.; Liu, S. X.; Lin, D. R.; Wu, D. T.; et al. Phenolic Profiles, Antioxidant Capacities, and Inhibitory Effects on Digestive Enzymes of Different Kiwifruits. Molecules. 2018, 23, 1–16. DOI: 10.3390/molecules23112957.
- Lin, S.; Guo, H.; Gong, J. D. B.; Lu, M.; Lu, M. Y.; Wang, L.; Zhang, Q.; Qin, W.; Wu, D. T. Phenolic Profiles, β-glucan Contents, and Antioxidant Capacities of Colored Qingke (tibetan Hulless Barley) Cultivars. J. Cereal Sci. 2018, 81, 69–75. DOI: 10.1016/j.jcs.2018.04.001.
- Anand, S.; Pang, E.; Livanos, G.; Mantri, N. Characterization of Physico-chemical Properties and Antioxidant Capacities of Bioactive Honey Produced from Australian Grown Agastache Rugosa and Its Correlation with Colour and Poly-phenol Content. Molecules. 2018, 23, 1–12. DOI: 10.3390/molecules23010108.
- Liu, W.; Zhang, J. H.; Zhang, Q.; Shan, Y. Effects of Postharvest Chilling and Heating Treatments on the Sensory Quality and Antioxidant System of Daylily Flowers. Hortic. Environ. Biotechnol. 2018, 59, 671–685. DOI: 10.1007/s13580-018-0087-y.
- Terefe, N. S.; Yang, Y. H.; Knoerzer, K.; Buckow, R.; Versteeg, C. High Pressure and Thermal Inactivation Kinetics of Polyphenol Oxidase and Peroxidase in Strawberry Puree. Innovative Food Sci. Emerging Technol. 2010, 11, 52–60. DOI: 10.1016/j.ifset.2009.08.009.
- Wang, Y. S.; Tian, S. P.; Xu, Y. Effects of High Oxygen Concentration on Pro- and Anti-oxidant Enzymes in Peach Fruits during Postharvest Periods. Food Chem. 2005, 91, 99–104. DOI: 10.1016/j.foodchem.2004.05.053.
- Chen, Y. F.; Zhan, Y.; Zhao, X. M.; Guo, P.; An, H. L.; Du, Y. G.; Han, Y. R.; Liu, H.; Zhang, Y. H. Functions of Oligochitosan Induced Protein Kinase in Tobacco Mosaic Virus Resistance and Pathogenesis Related Proteins in Tobacco. Plant Physiol. Biochem. 2009, 47, 724–731. DOI: 10.1016/j.plaphy.2009.03.009.
- Lin, S. Y.; Yang, S. L.; Li, X. F.; Chen, F.; Zhang, M. D. Dynamics of Water Mobility and Distribution in Soybean Antioxidant Peptide Powders Monitored by LF-NMR. Food Chem. 2016, 199, 280–286. DOI: 10.1016/j.foodchem.2015.12.024.
- Wang, S. Q.; Lin, Z. Y.; Xia, K. X.; Li, Y.; Tan, M. Q. Dynamics of Water Mobility and Distribution in Sur Clam (mactra Chinensis) during Dehydration and Rehydration Processes Assessed by Low-field NMR and MRI. J. Food Meas. Charact. 2017, 11, 1342–1354. DOI: 10.1007/s11694-017-9512-7.
- Gu, F. L.; Huang, F. F.; Wu, G. P.; Zhu, H. Y. Contribution of Polyphenol Oxidation, Chlorophyll and Vitamin C Degradation to the Blackening of Piper Nigrum L. Molecules. 2018, 23, 1–12. DOI: 10.3390/molecules23020370.
- Sato, R.; Ito, H.; Tanaka, A. Chlorophyll B Degradation by Chlorophyll B Reductase under High-light Conditions. Photosynth. Res. 2015, 126, 249–259. DOI: 10.1007/s11120-015-0170-5.
- Horton, P.;. Optimization of Light Harvesting and Photoprotection: Molecular Mechanisms and Physiological Consequences. Philos. Trans. Royal Soc. B-Biol. Sci. 2012, 367, 3455–3465. DOI: 10.1098/rstb.2012.0069.
- He, L. Z.; Yu, L.; Li, B.; Du, N. S.; Guo, S. R. The Effect of Exogenous Calcium on Cucumber Fruit Quality, Photosynthesis, Chlorophyll Fluorescence, and Fast Chlorophyll Fluorescence during the Fruiting Period under Hypoxic Stress. BMC Plant Biol. 2018, 18, 1–10. DOI: 10.1186/s12870-018-1393-3.
- Nick, S.; Meurer, J.; Soll, J.; Ankele, E. Nucleus-encoded Light-harvesting Chlorophyll A/b Proteins are Imported Normally into Chlorophyll B-free Chloroplasts of Arabidopsis. Mol. Plant 2013, 6, 860–871. DOI: 10.1093/mp/sss113.
- Rüdiger, W.;. Biosynthesis of Chlorophyll B and the Chlorophyll Cycle. Photosynth. Res. 2002, 74, 187–193. DOI: 10.1023/A:1020959610952.
- Herbig, A. L.; Maingonnat, J. F.; Renard, C. M. G. C. Oxygen Availability in Model Solutions and Purées during Heat Treatment and the Impact on Vitamin C Degradation. LWT Food Sci. Technol. 2016, 85, 493–499. DOI: 10.1016/j.lwt.2016.09.033.
- Kumar, D.; Ram, L.; Kumar, S.; Khadse, A. Wet Heat Treatment of Nagpur Mandarin (citrus Reticulata) Fruits to Reduce Decay Loss. Indian J. Agric. Sci. 2018, 88, 112–116.
- Hussain, P. R.; Meena, R. S.; Dar, M. A.; Wani, A. M. Effect of Post-harvest Calcium Chloride Dip Treatment and Gamma Irradiation on Storage Quality and Shelf-life Extension of Red Delicious Apple. J. Food Sci. Technol. 2012, 49, 415–426. DOI: 10.1007/s13197-011-0289-0.
- Hernández-Ruiz, K. L.; Ruiz-Cruz, S.; Cira-Chávez, L. A.; Gassos-Ortega, L. E.; Ornelas-Paz, J. D. J.; Del-Toro-Sánchez, C. L.; Márquez-Ríos, E.; López-Mata, M. A.; Rodríguez-Félix, F. Evaluation of Antioxidant Capacity, Protective Effect on Human Erythrocytes and Phenolic Compound Identification in Two Varieties of Plum Fruit (spondias Spp.) By UPLC-MS. Molecules. 2018, 23, 1–14. DOI: 10.3390/molecules23123200.
- Aghdam, M. S.; Dokhanieh, A. Y.; Hassanpour, H.; Fard, J. R. Enhancement of Antioxidant Capacity of Cornelian Cherry (cornus Mas) Fruit by Postharvest Calcium Treatment. Sci. Hortic. 2013, 161, 160–164. DOI: 10.1016/j.scienta.2013.07.006.
- Podsedek, A.; Majewska, I.; Redzynia, M.; Sosnowska, D.; Koziolkiewicz, M. In Vitro Inhibitory Effect on Digestive Enzymes and Antioxidant Potential of Commonly Consumed Fruits. J. Agric. Food Chem. 2014, 62, 4610–4617. DOI: 10.1021/jf5008264.
- Wojdylo, A.; Nowicka, P.; Oszmianski, J.; Golis, T. Phytochemical Compounds and Biological Effects of Actinidia Fruits. J. Funct. Foods. 2017, 30, 194–202. DOI: 10.1016/j.jff.2017.01.018.
- Antunes, M. D. C.; Dandlen, S.; Cavaco, A. M.; Miguel, G. Effects of Postharvest Application of 1-MCP and Postcutting Dip Treatment on the Quality and Nutritional Properties of Fresh-cut Kiwifruit. J. Agric. Food Chem. 2010, 58, 6173–6181. DOI: 10.1021/jf904540m.
- Viña, S. Z.; Chaves, A. R. Effect of Heat Treatment and Refrigerated Storage on Antioxidant Properties of Pre-cut Celery (apium Graveolens L.). Int. J. Food Sci. Technol. 2008, 43, 44–51. DOI: 10.1111/j.1365-2621.2006.01380.x.
- Yan, R. X.; Wang, Z. W.; Zhou, J.; Gao, R. Y.; Liao, S. W.; Yang, H. F.; Wang, F. Gold Nanoparticle Enriched by Q Sepharose Spheres for Chemical Reaction Tandem SERS Detection of Malondialdehyde. Sens. Actuators B Chem. 2019, 281, 123–130. DOI: 10.1016/j.snb.2018.10.078.
- Wu, B.; Guo, Q.; Wang, G. X.; Peng, X. Y.; Wang, J. D.; Che, F. B. Effects of Different Postharvest Treatments on the Physiology and Quality of ‘xiaobai’ Apricots at Room Temperature. J. Food Sci. Technol. 2015, 52, 2247–2255. DOI: 10.1007/s13197-014-1288-8.
- Liu, X.; Yang, Q.; Lu, Y. Z.; Li, Y.; Li, T. T.; Zhou, B. Y.; Qiao, L. P. Effect of Purslane (portulaca Oleracea L.) Extract on Anti-browning of Fresh-cut Potato Slices during Storage. Food Chem. 2019, 283, 445–453. DOI: 10.1016/j.foodchem.2019.01.058.
- Kim, A. N.; Lee, K. Y.; Kim, H. J.; Chun, J.; Kerr, W. L.; Choi, S. G. Effect of Grinding at Modified Atmosphere or Vacuum on Browning, Antioxidant Capacities, and Oxidative Enzyme Activities of Apple. J. Food Sci. 2018, 83, 84–92. DOI: 10.1111/1750-3841.14013.
- Jia, B.; Zheng, Q. L.; Zuo, J. H.; Gao, L. P.; Wang, Q.; Guan, W. Q.; Shi, J. Y. Application of Postharvest Putrescine Treatment to Maintain the Quality and Increase the Activity of Antioxidative Enzyme of Cucumber. Sci. Hortic. 2018, 239, 210–215. DOI: 10.1016/j.scienta.2018.05.043.
- Marszałek, K.; Woźniak, Ł.; Skąpska, S. The Application of High Pressure-mild Temperature Processing for Prolonging the Shelf-life of Strawberry Purée. High Pressure Res. 2016, 36, 220–234. DOI: 10.1080/08957959.2016.1172072.
- Reichel, M.; Wellhöfer, J.; Triani, R.; Sruamsiri, P.; Carle, R.; Neidhart, S. Postharvest Control of Litchi (litchi Chinensis Sonn.) Pericarp Browning by Cold Storage at High Relative Humidity after Enzyme-inhibiting Treatments. Postharvest. Biol. Technol. 2017, 125, 77–90. DOI: 10.1016/j.postharvbio.2016.10.002.
- Palma-Orozco, G.; Ortiz-Moreno, A.; Dorantes-Álvarez, L.; Sampedro, J. G.; Nájera, H. Purification and Partial Biochemical Characterization of Polyphenol Oxidase from Mamey (pouteria Sapota). Phytochemistry. 2011, 72, 82–88. DOI: 10.1016/j.phytochem.2010.10.011.
- Vámos-Vigyázó, L.; Haard, N. F. Polyphenol Oxidases and Peroxidases in Fruits and Vegetables. Crit. Rev. Food Sci. Nutr. 1981, 15, 49–127.
- Youryon, P.; Supapvanich, S.; Kongtrakool, P.; Wongs-Aree, C. Calcium Chloride and Calcium Gluconate Peduncle Infiltrations Alleviate the Internal Browning of Queen Pineapple in Refrigerated Storage. Hortic. Environ. Biotechnol. 2018, 59, 205–213. DOI: 10.1007/s13580-018-0028-9.
- Bajwa, V. S.; Shukla, M. R.; Sherif, S. M.; Murch, S. J.; Saxena, P. K. Identification and Characterization of Serotonin as an Anti-browning Compound of Apple and Pear. Postharvest. Biol. Technol. 2015, 110, 183–189. DOI: 10.1016/j.postharvbio.2015.08.018.
- Mrad, N. D.; Boudhrioua, N.; Kechaou, N. Influence of Air Drying Temperature on Kinetics, Physicochemical Properties, Total Phenolic Content and Ascorbic Acid of Pears. Food Bioprod. Process. 2012, 90, 433–441. DOI: 10.1016/j.fbp.2011.11.009.
- Gao, H. Y.; Zeng, Q.; Ren, Z. N.; Li, P. Z.; Xu, X. X. Effect of Exogenous γ-aminobutyric Acid Treatment on the Enzymatic Browning of Fresh-cut Potato during Storage. J. Food Sci. Technol. 2018, 55, 5035–5044. DOI: 10.1007/s13197-018-3442-1.
- Huang, R. H.; Xia, R. X.; Hu, L. M.; Lu, Y. M.; Wang, M. Y. Antioxidant Activity and Oxygen-scavenging System in Orange Pulp during Fruit Ripening and Maturation. Sci. Hortic. 2007, 113, 166–172. DOI: 10.1016/j.scienta.2007.03.010.
- Raseetha, S.; Oey, I.; Burritt, D. J.; Heenan, S.; Hamid, N. Evolution of Antioxidant Enzymes Activity and Volatile Release during Storage of Processed Broccoli (brassica Oleracea L. Italic). LWT Food Sci. Technol. 2013, 54, 216–223. DOI: 10.1016/j.lwt.2013.05.024.
- Wulfkuehler, S.; Stark, S.; Dietz, J.; Schmidt, H.; Weiss, A.; Carle, R. Effect of Water Jet Cutting and Moderate Heat Treatment on Quality of Fresh-cut Red Oak Leaf Lettuce (lactuca Sativa L. Var. Crispa). Food Bioprocess. Technol. 2014, 7, 3478–3492. DOI: 10.1007/s11947-014-1360-4.
- Khan, Z. U.; Li, J. Y.; Khan, N. M.; Mou, W. S.; Li, D. D.; Wang, Y. S.; Feng, S. M.; Luo, Z. S.; Mao, L. C.; Ying, T. J. Suppression of Cell Wall Degrading Enzymes and Their Encoding Genes in Button Mushrooms (agaricus Bisporus) by CaCl2 and Citric Acid. Plant Foods Human Nutr. 2017, 72, 54–59. DOI: 10.1007/s11130-016-0588-8.