ABSTRACT
Purification of peroxidase POII from date palm cv. Agwa fruits were carried out. The molecular mass of POII was 43 kDa. The broad pH optimum of POII at pH’s 6.0–7.0 was detected. The maximum activity of POII was detected at 60°C and the enzyme was thermally stable up to 60°C. POII oxidized phenolic compounds such as guaiacol, o-phenylenediamine, o-dianosidine, p-aminoantipyrine, pyrogallol and ABTS in presence of H2O2 as second substrate. The Km values of POII were found to be 6 mM for H2O2 and 25 mM for guaiacol. The enzyme activity of POII was enhanced by Fe+3, Cu+2, and Co+2. In conclusion, POII oxidized some of phenolic compounds, which caused the browning color in tamer stage of date cv. Agwa.
Introduction
Peroxidases (EC 1.11.1.X) are enzymes that catalyze the oxidation of a wide variety of phenolic compounds by using H2O2. They are very wide in animals, plants, algae, fungi, and bacteria.[Citation1] They are involved in several physiological processes of plants such as lignification, suberization, auxin metabolism and protect the plants against pathogens and senescence.[Citation2,Citation3]
Plant peroxidases are used in medicinal applications such as enzyme immunoassay kits and treatment of cancer.[Citation4,Citation5] The environment and industrial applications of plant peroxidases included decolorization of dyes, treatment of wastewater containing phenolic compounds, removal of peroxides from industrial wastes, preparation of biosensor for determination of H2O2 and synthesis of different aromatic chemicals.[Citation6–Citation10]
Several peroxidases were purified and characterized by leaves and trees of date palms.[Citation11–Citation16] The purification of peroxidases from other sources such as radish species and guinea grass was detected.[Citation17,Citation18] Very little information was reported for the purification and characterization of peroxidases from date fruits.[Citation19] Dates can be consumed at three stages of their development mainly bisir, rutab and tamer depending on cultivar characteristics especially soluble tannin level, climatological conditions, and market demand.[Citation20] The very commercial date fruit consumed in Saudi Arabia is cv. Agwa in tamer stage. The tamer stage is characterized by browning. The enzymatic browning by oxidoreductases such as peroxidase may be essential to appear the brown color of the dates in tamer stage. Therefore, peroxidase as one of the oxidoreductases from fruits of date palm cv. Agwa was purified and characterized.
Methods
Plant
Date palm cv. Agwa fruit at tamer stage was purchased from local market of Jeddah, Saudi Arabia.
Peroxidase assay and protein determination
Peroxidase activity was estimated by procedure of Miranda et al.[Citation21] The reaction mixture contained 8 mM H2O2, 40 mM guaiacol, 50 mM sodium acetate buffer, pH 5.5 and 0.1 ml crude extract and completed to 1 ml with distilled water. One unit of peroxidase activity was defined as the amount of enzyme which increases the O.D. 1.0 at 470 nm per min under standard assay conditions. Protein concentrations were measured following the procedure of Bradford.[Citation22]
Crude enzyme extract
Ten gram of date fruit cv. Agwa was extracted with 50 mM Tris-HCl buffer, pH 7.5 using mortar. The extract was centrifuged at 12,000 rpm and 4°C for 10 min. The supernatant was designated as a crude enzyme extract and stored at −20°C until further analysis.
Peroxidase purification
The purification of peroxidase from date cv. Agwa was done through chromatography of crude enzyme extract on anion exchange DEAE-Sepharose column (10 × 1.2 cm i.d.) using 50 mM Tris-HCl buffer pH 7.2 and stepwise gradient of NaCl ranged from 0.0 to 0.3 M for elution of the enzyme. Four isoforms of peroxidase were eluted at 0.0 M, 0.1 M, 0.2 M, and 0.3 M NaCl, respectively. The second step of purification is chromatography of fraction eluted by 0.1 M on Sephacryl S-200 column (90 × 1.6 cm i.d.) using 50 mM Tris-HCl buffer pH 7.2 for elution of the enzyme. The flow rate of the column is 1ml/min and 3 ml of fraction. The molecular mass of purified peroxidase was determined by Sephacryl S-200 column and SDS-PAGE.[Citation23]
Characterization of peroxidase
The studies of peroxidase characterization were determined in accordance with pH optimum (pH 4.5–9.0), temperature optimum (30–80°C), effect of temperature on stability of enzyme (30–80°C), effect of different substrates (guaiacol, o-phenylenediamine, o-dianosidine, p-aminoantiprine, pyrogallo, ABTS), km values (guaiacol, H2O2) and effect of different metals (Fe3+, Cd2+, Co2+, Ni2+, Cd2+, Zn2+, Hg2+).
Statistical analysis
The data were statistically analyzed by a one-way ANOVA. The data were considered as means ± S.E. (n = 3).
Results
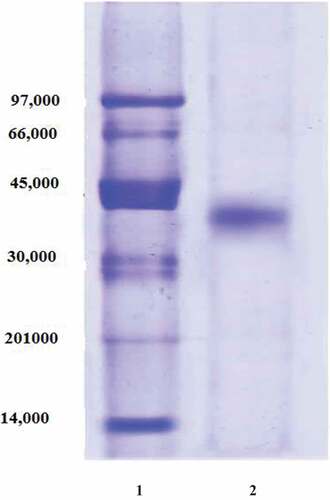
The results of purification of peroxidase from date cv. Agwa fruits were shown in . The crude extract of peroxidase possessed 845 units, 18 mg protein and specific activity (S.A.) 47 units/mg protein. By chromatography on DEA-Sepharose, four isoforms of peroxidase POI, POII, POIII, and POIV were eluted at 0.0 M, 0.1 M, 0.2 M and 0.3 M NaCl, respectively (). POII with the highest S.A. was chromatography on Sphacryl S-200 column. After this step, the S.A. and fold purification of POII increased to become 625 units/mg protein and 13.2, respectively (). The homogeneity of POII was detected by SDS-PAGE and only single band appeared with molecular mass 43 kDa (). This molecular mass was also proved by Sphacryl S-200 column.
Table 1. Purification scheme for date palm cv. Agwa peroxidase
Figure 1. Chromatography of date palm cv. Agwa on DEAE-Sepharose column. (•___•) Absorbant at 280 nm (x --- x) units/fraction
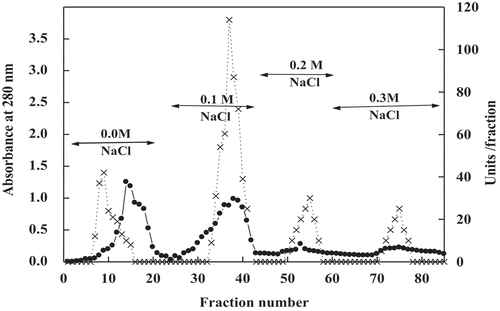
Figure 2. Chromatography of date palm cv. Agwa POII DEAE-Sepharose fraction on Sephacryl S-200 column. (•___•) Absorbant at 280 nm (x --- x) units/fraction
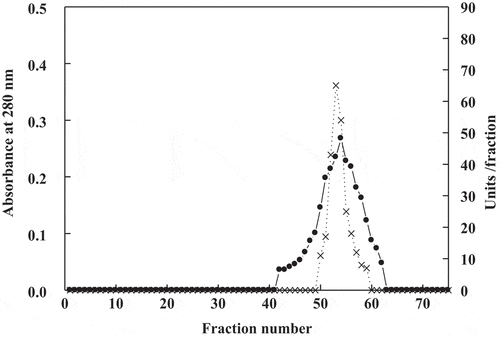
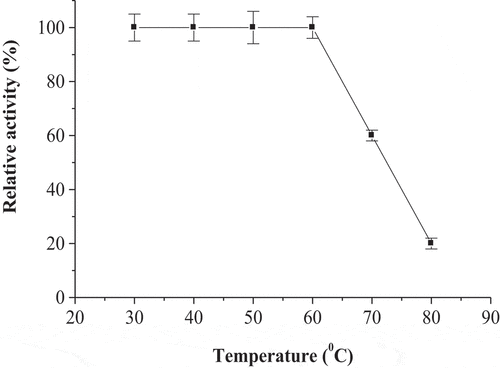
The broad pH optimum of POII at pH’s 6.0–7.0 was detected (). The activity of enzyme at acidic pH’s (4.5–5.5) was higher than that at alkaline pH’s (7.5–9.0). The maximum activity of POII was detected at 60°C (). The enzyme activity was greatly dropped at 80°C. The enzyme was thermal stable up to 60°C after incubation for 1 h (). The enzyme was also lost of most activity at 80°C.
Figure 4. pH optimum of date palm cv. Agwa POII. Each point represents the mean of three experiments ± S.E
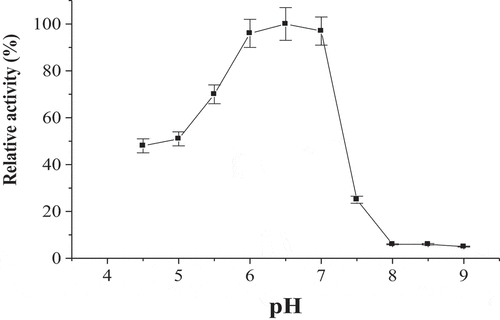
Figure 5. Temperature optimum of date palm cv. Agwa POII. Each point represents the mean of three experiments ± S.E
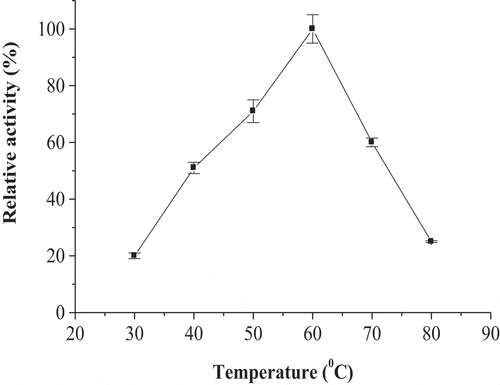
Figure 6. Effect of temperature on the thermal stability of date palm cv. Agwa POII. Each point represents the mean of three experiments ± S.E
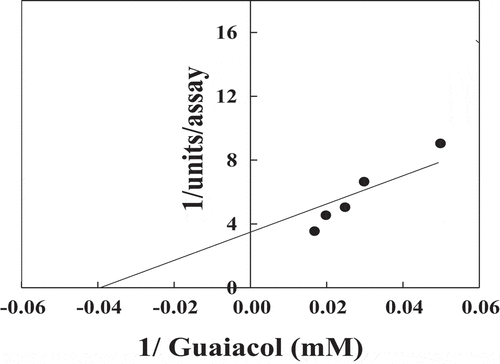
The different substrates oxidized by POII in presence of H202 were studied (). The relative activities % were 100, 76, 58, 35, 22 and 7 for guaiacol, o-phenylenediamine, o-dianosidine, p-aminoantipyrine, pyrogallol, and ABTS, respectively. The Km values of POII were found to be 6 mM for H2O2 () and 25 mM for guaiacol ().
Table 2. Relative activities of date cv. Agwa POII toward different substrates
Figure 7. Reciprocal of Lineweaver–Burk plot relating date palm cv. Agwa POII reaction velocities to H2O2.
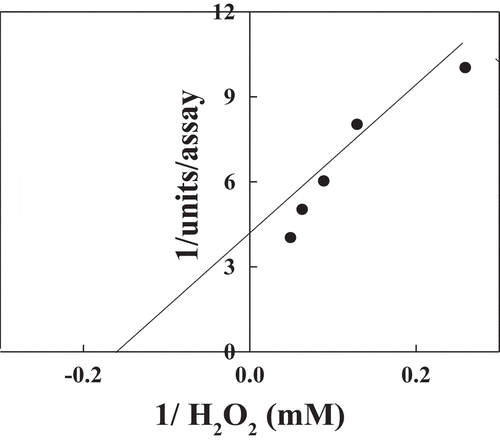
Figure 8. Reciprocal of Lineweaver–Burk plot relating date palm cv. Agwa POII reaction velocities to guaiacol
The effect of metal ions on the activity of POII was tested (). The enzyme activity of POII was enhanced by Fe3+, Cu2+, and Co2+ with 501%, 303%, and 198% relative activity, respectively. Ni2+ had no effect on the activity of POII. The enzyme activity was inhibited by Ca2+, Zn2+ and Hg2+ with 36%, 27%, and 22% relative activity, respectively.
Table 3. Effect of 5 mM metal cations on date cv. Agwa POII
Discussion
The purification of enzymes is very essential for the characterization study. In this study, the peroxidase from date cv. Agwa was purified. By DEAE-Sepharose column, non-adsorbed peroxidase was eluted by 0.0 M NaCl, which may be overload of the amount of peroxidase loaded on the column or it could be cationic peroxidases. In addition, three isoforms of adsorbed peroxidases POII, POIII, and POIV were detected, which designated as anionic peroxidases. The large number of isolated isoforms indicated that these enzymes belonging to plant class III peroxidases.[Citation24,Citation25] Several isolated peroxidases were reported for olive (four cationic and four anionic peroxidases)[Citation26], Citrus jambhiri cv. Adalia (four anionic and one cationic peroxidases)[Citation27], horseradish cv. Balady (four anionic peroxidases).[Citation28] Peroxidases from class-III implicated in several functions such as lignin synthesis, cell elongation, seed germination, and stress defense.[Citation29,Citation30] The last step of purification was performed by applying POII with the highest peroxidase activity on Sephacryl S-200 column. The POII became purified and homogenized by applied on SDS-PAGE, where one band of enzyme was detected. The molecular mass of POII was found to be 43 kDa. The different molecular weights of 55 and 68 kDa were reported for peroxidases from date palm leaves.[Citation11,Citation16] The similar molecular weight was detected for peroxidase from Raphanus sativus (44 kDa).[Citation31]
The pH optimum of the enzyme is very important for enzyme activity, where the active site changed by changing the ionic state of amino acids of enzyme.[Citation32] The broad pH optimum of POII from date palm cv. Agwa at pH’s 6.0–7.0 was determined. Kalsoom et al. reported that the optimum pH of the most of the plant peroxidases ranged from 3.5 to 5.5.[Citation32] Similar to POII, peroxidases from peels of Citrus reticulata var. and red cabbage showed pH optimum at pH 6.0 and 7, respectively.[Citation33,Citation34] For date palm leaves, the peroxidase exhibited broad pH optimum at pH 5–6.[Citation16]
Plant peroxidases exhibited different temperature optima and thermal stabilities based on the structure and amino acid composition of the enzymes.[Citation32] The optimum temperature of POII from date palm cv. Agwa was found to be 60°C. A peroxidase from leaves of Jatropha curcas exhibited the same optimum temperature at 60°C.[Citation35] The similar optimum temperature (55°C) was detected for peroxidase from date palm leaves.[Citation16] The two peroxidases from buckwheat seed had low optimum temperatures at 30°C and 10°C.[Citation36] The thermal stability of peroxidase is a very important character for applying in several applications.[Citation14] The POII was also thermally stable up to 60°C after incubation for 1 h. The peroxidase from date palm leaves retained its initial activity at 60–70°C for 1 h incubation.[Citation16] Peroxidase from Raphanus sativus was stable up to 40°C for 60 min incubation.[Citation37] The low thermal stability was detected for two peroxidases from buckwheat seed and the enzymes denatured at 60°C and 50°C.[Citation36]
The activity of purified date palm cv. Agwa POII toward tested hydrogen donors in the presence of H2O2 was detected. POII shows different activities toward various substrates based on the chemical structure of the substrates. POII had affinity toward substrates in the order of guaiacol > o-phenylenediamine > o-dianosidine > p-aminoantipyrine > pyrogallol > ABTS suggesting the enzyme related to class III peroxidases.[Citation24,Citation25] The similar substrate specificity was detected for peroxidase from date palm leaves.[Citation16] Phenolic compounds of plants played a dual role as antioxidants and substrates for oxidative browning enzymes, namely, polyphenoloxidases and peroxidases.[Citation38–Citation40] Therefore, POII is very important in tamer stage of date cv. Agwa, which characterized by browning color.
The higher Km values of POII from date palm cv. Agwa fruits were found to be 6 mM H2O2 and 25 mM guaiacol. The higher Km values (3.3 mM H2O2 and 16.4 mM guaiacol) of peroxidase from horseradish cv. Balady roots were also detected.[Citation28] Two peroxidases, mPOD-I and mPODII, from Metroxylon sagu had higher Km values for guaiacol (32.2 and 22.9 mM) and lower Km values for H2O2 (0.08 and 0.06 mM), respectively.[Citation41] Peroxidase from date palm leaves showed low Km values of 0.77 mM H2O2 and 0.045 mM guaiacol.[Citation16]
Generally, peroxidases required metal ions as prosthetic groups in the structure of the enzymes.[Citation29,Citation32] The enzyme activity of POII from date palm cv. Agwa was enhanced by Fe3+, Cu2+, and Co2+ and inhibited by Ca2+, Zn2+, and Hg2+. The similar effect of metals was detected for peroxidases from date palm leaves[Citation6], horseradish cv. Balady roots[Citation28] and Jatropha curcas leaves[Citation35] were also detected. The enhancement of the enzyme activity of POII by Fe3+ indicating that the presence of heme group in the structure of enzyme as reported by horseradish peroxidase.[Citation42]
Conclusion
The purified peroxidase POII from date palm cv. Agwa fruits characterized by broad pH optimum at pH’s 6.0–7.0, maximum activity at 60°C and thermal stable up to 60°C. The enhancement of the enzyme activity of POII by Fe3+ indicated the presence of heme group in the structure of enzyme. POII oxidized some of the phenolic compounds, which caused the browning color in tamer stage of date cv. Agwa.
Acknowledgments
This project was funded by the Deanship of Scientific Research (DSR) at King Abdulaziz University, Jeddah, under grant No. (31210). The author, therefore, acknowledges with thanks DSR for technical and financial support.
Additional information
Funding
References
- Pandey, V.; Awasthi, M.; Singh, S.; Tiwari, S.; Dwivedi, U. A Comprehensive Review on Function and Application of Plant Peroxidases. Biochem. Anal. Biochem. 2017, 6, 1000308. DOI: 10.4172/2161-1009.
- Monica, Q.; Consuelo, G.; Miguel, A. B.; Araceli, B.; Iraida, A.; Marıa, I. M.; Francisco, J. A.; Silvia Milrad, D. F.; Horacio, T.; Victoriano, V. A Tomato Peroxidase Involved in the Synthesis of Lignin and Suberin. Plant Physiol. 2000, 122, 1119–1127. DOI: 10.1104/pp.122.4.1119.
- Herder, J. D.; Lievens, S.; Rombauts, S.; Holsters, M.; Goormachtig, S. A Symbiotic Plant Peroxidase Involved in Bacterial Invasion of the Tropical Legume Sesbania Rostrate. Plant Physiol. 2007, 144, 717–772. DOI: 10.1104/pp.107.098764.
- Abdel-Aty, A. M.; Hamed, A. B.; Gad, A. M.; El-Hakim, A. E.; Mohamed, S. A. Ficus Sycomorus Latex: An Efficient Alternative Egyptian Source for Horseradish Peroxidase in Labeling with Antibodies for Immunodiagnostic Kits. Veterin. World. 2018, 11, 1364–1370. DOI: 10.14202/vetworld.2018.1364-1370.
- Jeong, Y. M.; Oh, M. H.; Kim, S. Y.; Li, H.; Yun, H. Y.; Baek, K. J.; Kwon, N. S.; Kim, W. Y.; Kim, D. S. Indole-3-acetic acid/horseradish Peroxidase Induces Apoptosis in TCCSUP Human Urinary Bladder Carcinoma Cells. Pharmazie. 2010, 65, 122–126.
- Hamid, M.;. Potential Applications of Peroxidases. Food Chem. 2009, 115, 1177–1186. DOI: 10.1016/j.foodchem.2009.02.035.
- Regaldo, C.; Garcia-Almendarez, B. E.; Duarte-Valzquez, M. A. Biotechnological Applications of Peroxidases. Phytochem. Rev. 2004, 3, 243–256. DOI: 10.1023/B:PHYT.0000047797.81958.69.
- Abdel-Aty, A. M.; Hamed, M. B.; Fahmy, A. S.; Mohamed, S. A. Comparison of the Potential of Ficus Sycomorus Latex and Horseradish Peroxidases in the Decolorization of Synthetic and Natural Dyes. J. Gen. Eng. Biotechnol. 2013, 11, 95–102. DOI: 10.1016/j.jgeb.2013.07.001.
- Mohamed, S. A.; Al-Harbi, M. H.; Almulaiky, Y. Q.; Ibrahim, I. H.; El-Shishtawy, R. M. Immobilization of Horseradish Peroxidase on Fe3O4 Magnetic Nanoparticles. Electr. J. Biotechnol. 2017, 27, 84–90. DOI: 10.1016/j.ejbt.2017.03.010.
- Xu, S.; Qi, H.; Zhou, S.; Zhang, X.; Zhang, C. Mediatorless Amperometric Bienzyme Glucose Biosensor Based on Horseradish Peroxidase and Glucose Oxidase Cross-linked to Multiwall Carbon Nanotubes. Microchim. Acta. 2014, 181, 535–541. DOI: 10.1007/s00604-014-1175-z.
- Al-Bagmi, M. S.; Khan, M. S.; Ismael, M. A.; Al-Senaidy, A. M.; Bacha, A. B.; Husain, F. M.; Alamery, S. F. An Efficient Methodology for the Purification of Date Palm Peroxidase: Stability Comparison with Horseradish Peroxidase (HRP). Saudi J. Biol. Sci. 2019, 26, 301–307. DOI: 10.1016/j.sjbs.2018.04.002.
- Wantanabe, L.; De Moura, P. R.; Bleicher, L.; Nascimento, A. S.; Zamorano, L. S.; Calvete, J. J.; Sanz, L.; Perez, A.; Bursakov, S.; Roig, M. G. Crystal Structure and Statistical Coupling Analysis of Highly Glycosylated Peroxidase from Royal Palm Tree (Roystonea regia). J. Struct. Biol. 2010, 169, 226–242. DOI: 10.1016/j.jsb.2009.10.009.
- Sakharov, I. Y.; Castillo, J.; Areza, J.; Galaev, I. Y. Purification and Stability of Peroxidase of African Oil Palm Elaies Guineensis. Biosepar. 2000, 9, 125–132. DOI: 10.1023/A:1008117217885.
- Sakharov, I. Y.; Galaev, I. Y.; Sakharova, I.; Pletjushkina, O. Y. Peroxidase from Leaves of Royal Palm Tree Roystonea Regia: Purification and Some Properties. Plant Sci. 2001, 161, 853–860. DOI: 10.1016/S0168-9452(01)00466-6.
- Caramyshev, A. V.; Firsova, Y. N.; Slastya, E. A.; Tagaev, A. A.; Potapenko, N. V.; Lobakova, E. S.; Pletjushkina, O. Y.; Sakharov, I. Y. Purification and Characterization of Windmill Palm Tree (Trachycarpus fortunei) Peroxidase. J. Agric. Food Chem. 2006, 54, 9888–9894. DOI: 10.1021/jf0615193.
- Al-Senaidy, A. M.; Ismael, M. A. Purification and Characterization of Membrane-bound Peroxidase from Date Palm Leaves (Phoenix Dactylifera L.). Saudi J. Biol. Sci. 2011, 18, 293–298. DOI: 10.1016/j.sjbs.2011.04.005.
- Oztekin, A.; Almaz, Z.; Gerni, S.; Erel, D.; Kocak, S. M.; Sengül, M. E.; Ozdemir, H. Purification of Peroxidase Enzyme from Radish Species in Fast and High Yield with Affinity Chromatography Technique. J. Chromatogr. B. 2019, 1114–1115, 86–92.
- Centeno, D. A.; Solan, X. H.; Castillo, J. J. A New Peroxidase from Leaves of Guinea Grass (Panicum maximum): A Potential Biocatalyst to Build Amperometric Biosensors. Bioelectrochem. 2017, 116, 33–38. DOI: 10.1016/j.bioelechem.2017.03.005.
- Haider, M. S.; Khan, I. K.; Jaskani, M. J.; Naqvi, S. A.; Khan, M. M. Biochemical Attributes of Dates at Three Maturation Stages. Emir. J. Food Agric. 2014, 26, 953–962. DOI: 10.9755/ejfa.
- Awad, M. A.;. Increasing the Rate of Ripening of Date Palm Fruit (Phoenix Dactylifera L.) Cv. ‘helali’ by Preharvest and Postharvest Treatments. Postharvest Biol. Technol. 2007, 43, 121–127. DOI: 10.1016/j.postharvbio.2006.08.006.
- Miranda, M. V.; Fernandez Lahor, H. M.; Cascone, O. Horseradish Peroxidase Extraction and Purification by Aqueous Two-phase Partition. Appl. Biochem. Biotechnol. 1995, 53, 147–154. DOI: 10.1007/BF02788604.
- Bradford, M. M.;. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Using the Principle of Protein-dye Binding. Anal. Biochem. 1976, 72, 248–254. DOI: 10.1016/0003-2697(76)90527-3.
- Laemmli, U. K.;. Cleavage of Structural Proteins during the Assembly of the Head Bacteriophage T4. Nature. 1970, 227, 680–685. DOI: 10.1038/227680a0.
- Boucoiran, C. F. S.; Kijne, J. W.; Recourt, K. Isolation and Partial Characterization of a Thermostable Isoperoxidases from Potato (Solanum Tuberosum L.) Tuber Sprouts. J. Agric. Food Chem. 2000, 48, 701–707. DOI: 10.1021/jf981352g.
- Tognolli, M.; Penel, C.; Greppin, H.; Simon, P. Analysis and Expression of the Class III Peroxidase Large Gene Family in Arabidopsis Thaliana. Gene. 2002, 288, 129–138. DOI: 10.1016/S0378-1119(02)00465-1.
- Saraiva, J. A.; Nunes, C. S.; Coimbra, M. A. Purification Ad Characterization of Olive (Olea Europaea L.) Peroxidase–Evidence for the Occurrence of Pectin Binding Peroxidase. Food Chem. 2007, 101, 1571–1579. DOI: 10.1016/j.foodchem.2006.04.012.
- Mohamed, S. A.; El-Badry, M. O.; Drees, E. A.; Fahmy, A. S. Properties of a Cationic Peroxidase from Citrus Jambhiri Cv. Adalia. Appl. Biochem. Biotechnol. 2008, 150, 127–137. DOI: 10.1007/s12010-008-8142-2.
- Mohamed, S. A.; Abulnaja, K. O.; Ads, A. S.; Khan, J. A.; Kumosani, T. A. Characterisation of an Anionic Peroxidase from Horseradish Cv. Balady. Food Chem. 2011, 128, 725–730. DOI: 10.1016/j.foodchem.2011.03.096.
- Shigeto, J.; Tsutsumi, Y. Diverse Functions and Reactions of Class III Peroxidases. New Phytol. 2015, 209, 1395–1402. DOI: 10.1111/nph.13738.
- Veitch, N. C.;. Horseradish Peroxidase: A Modern View of A Classic Enzyme. Phytochem. 2004, 65, 249–259. DOI: 10.1016/j.phytochem.2003.10.022.
- Kim, S. S.; Lee, D. J. Purification and Characterization of a Cationic Peroxidase Cs in Raphanus Sativus.. J. Plant Physiol. 2005, 162, 609–617. DOI: 10.1016/j.jplph.2004.10.004.
- Kalsoom, U.; Bhatti, H. N.; Asgher, M. Characterization of Plant Peroxidases and Their Potential for Degradation of Dyes: A Review. Appl. Biochem. Biotechnol. 2015, 176, 1529–1550. DOI: 10.1007/s12010-015-1674-3.
- Nouren, S.; Bhatti, H. N.; Bhatti, I. A.; Asghe, M. Kinetic and Thermal Characterization of Peroxidase from Peels of Citrus Reticulata Var. Kinnow. J. Animals Plant Sci. 2013, 23, 430–435.
- Somtürk, B.; Kalınand, R.; Özdemir, N. Purification of Peroxidase from Red Cabbage (Brassica Oleracea Var. Capitata F. rubra) by Affinity Chromatography. Appl. Biochem. Biotechnol. 2014, 173, 1815–1828. DOI: 10.1007/s12010-014-0968-1.
- Cai, F.; OuYang, C.; Duan, P.; Gao, S.; Xu, Y.; Chen, F. Purification and Characterization of a Novel Thermal Stable Peroxidase from Jatropha Curcas Leaves. J. Molec. Cataly. B: Enzym. 2012, 77, 59–66. DOI: 10.1016/j.molcatb.2011.12.002.
- Suzuki, T.; Honda, Y.; Mukasa, Y.; Kimb, S.-J. Characterization of Peroxidase in Buckwheat Seed. Phytochem. 2006, 67, 219–224. DOI: 10.1016/j.phytochem.2005.11.014.
- Aruna, N.; Lali, A. Purification of a Plant Peroxidase Using Reversibly Soluble Ion Exchange Polymer. Process Biochem. 2001, 37, 431–437. DOI: 10.1016/S0032-9592(01)00226-6.
- Robards, K.; Prenzler, D. P.; Tucker, G.; Swatsitang, P.; Glover, W. Phenolic Compounds and Their Role in Oxidative Processes in Fruits. Food Chem. 1999, 66, 401–436. DOI: 10.1016/S0308-8146(99)00093-X.
- Abdel-Aty, A. M.; Salama, W. H.; Fahmy, A. S.; Mohamed, S. A. Impact of Germination on Antioxidant Capacity of Garden Cress: New Calculation for Determination of Total Antioxidant Activity. Sci. Hort. 2019, 246, 155–160. DOI: 10.1016/j.scienta.2018.10.062.
- Mohamed, S. A.; Awad, M. A.; Al-Qurashi, A. D. Antioxidant Activity, Antioxidant Compounds, Antioxidant Andhydrolytic Enzymes Activities of ‘barhee’ Dates at Harvest and Duringstorage as Affected by Pre-harvest Spray of Some Growth Regulators. Sci. Hort. 2014, 167, 91–99. DOI: 10.1016/j.scienta.2014.01.003.
- Onsa, G. H.; Bin Saari, N.; Selamat, J.; Bakar, J. Purification and Characterization of Membrane Bound Peroxidases from Metroxylon Sagu. Food Chem. 2004, 85, 365–376. DOI: 10.1016/j.foodchem.2003.07.013.
- Isaac, I. S.; Dawson, J. H. Haem Iron-containing Peroxidases. Essays Biochem. 1999, 34, 51–69. DOI: 10.1042/bse0340051.