 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
In this work, anthocyanin of rose residue was microencapsulated, using spray-drying (1:1 (w/w) of GA to MD, the inlet air temperature was170°C, the drying airflow rate was 5.0 m3/min, feed rate was 0.36 L/h) and freeze-drying (processing 48 h at −52°C, vacuum degree was 0.45 mbar) with gum arabic and maltodextrin as external materials. The total phenolic content (TPC), anthocyanin content (ACN), antioxidant activity, moisture content, water activity (Aw), solubility, hygroscopicity, colorimetric property, particle micromorphology and size distribution of the microencapsulated anthocyanin were measured. Thermal degradation kinetic and thermodynamic parameters of ACN extracts and microcapsules in the accelerated model were determined. The spray-dried powder (SDP) presented a spherical shape while the freeze-dried powders (FDP) were in indefinite and laminated structures with more homogeneous distribution. After microencapsulation, the retention rate of TPC and ACN was 86.00% and 75.85% for SDP, 91.44% and 95.12% for FDP, respectively. Both of spray-dried and freeze-dried strategy provided efficient preservation for rose residues, enabling them to have lower moisture content, water activity, significantly higher solubility (P < .05) compared with the rose anthocyanin extracts (RAE), and have high-fidelity color that was similar to RAE. Meanwhile, the antioxidant activity of the microcapsules decreased with the deteriorating of bioactive compounds. In the degradation kinetic study of anthocyanin at the temperature 70, 80, 90°C, both FDP and SDP had longer half-life duration, lower absolute value of activation entropy, and better effect of freeze-drying embedding method compared to RAE. Therefore, microencapsulation, especially by freeze-drying method, could efficiently enhance the stability of anthocyanin in rose residue during thermal processing and storage, and thus greatly facilitate the utilization of by-product of rose essential oil.
Abbreviations: RAE: rose anthocyanin extracts; GA: gum arabic; MD: maltodextrin; SDP: spray-dried powders; FDP: freeze-dried powders; TPC: total phenolic content; ACN: anthocyanin content
Introduction
In recent years, the rose essential oil has drawn much attention due to its special fragrance and physiological functions, and thus has developed greatly in medicine, food and chemical sectors, for which 6000 to 8000 tons of rose flowers are used annually in China, leaving much rose residue disposed as agricultural wastes. However, the residue, abundant with bioactive compounds, including phenolics and anthocyanins, shows excellent antioxidant[Citation1], antibacterial[Citation2] and anti-inflammatory[Citation3] properties to prevent people from cardiovascular diseases, neurodegenerative diseases, and diabetes.[Citation4] Furthermore, the anthocyanin, typically in attractive red, has great potential in food industry as a natural colorant. The reprocessing of by-products from rose essential oil, especially anthocyanin, is one of the key factors to improve the commercial value and trigger the development in rose industry. This study was aimed to improve the stability and expand the application of anthocyanin.
Anthocyanin is an attractive natural colorant with broad applications in the future for its pleasant color and diverse physiological activities. However, it is vulnerable to degradation during the process and storage, which restricts its applications. Degradation is a complex process, and many factors, such as temperature, light, oxygen, pH, metal complexing and enzymes, make bioactive compounds be decolored and impaired.[Citation4,Citation5] There are some methods that can defer the degradation, such as co-pigmentation and cryopreservation. However, most of the existing methods remain costly and are not friendly to storage. This, combined with the complexity of food processing, requires a comprehensive and deep understanding of this degradation process.
Microencapsulation is a novel technology whereby active compounds are encased by a shell coating or embedded in a homogeneous or heterogeneous matrix, formulating tiny droplets or particles, so as to efficiently enhance the stability of active compounds and adapt them to various processing conditions.[Citation6] Spray-drying and freeze-drying are the two most commonly used microencapsulation methods.[Citation5] Moreover, appropriate encapsulating agents are prerequisite for microencapsulation, and should be chosen according to the film-forming ability, biodegradability, viscosity, and acceptable cost.[Citation3,Citation6] Among the encapsulating agents, gum arabic (GA) and maltodextrin (MD) are most commonly used due to their favorable emulsifying property, dispersibility, high solubility, and low viscosity.
In this study, GA and MD were chosen as encapsulating agents to probe into the implication of spray- and freeze-drying methods on the microencapsulation of anthocyanin from the perspective of physicochemical properties. Furthermore, the degradation kinetic models were adopted to study the influence of heating on anthocyanins, as heading is typically considered as a predominant factor resulting in the instability of anthocyanins. The Arrhenius model based on the classic approach of chemical reactions and the theoretical Eyring model based on the transition state theory were applied to correlate the degradation reaction rate to temperature.[Citation2,Citation4] Thus, this study aimed to improve the stability of rose anthocyanins by microencapsulation and to evaluate the effectiveness of this protecting ability supported by chemometric methods.
Materials and methods
Samples' preparation and chemicals
The residue of rose was obtained from a local processing factory (Chengdu, China). After oven-dried and ground, the residue was extracted in 55% acidified ethanol aqueous solution (pH = 3.0) assisted by ultrasound, the solid–liquid ratio was 1:20 (m/V), immersed for 60 min at 55°C, then concentrated in a rotary evaporator (RV 10 digital V, IKA®, Staufen, Germany) at 45°C to remove ethanol. Resultant aqueous fraction (RAE) was kept at −18°C until further analysis.
Cyanidin 3-O-glucoside was purchased from Sigma-Aldrich (St. Louis, Missouri, United States). Gum Arabic (GA), maltodextrin (MD, DE of 4.0–17.0), 2,2-diphenyl-1-picrylhydrazyl (DPPH), and gallic acid were obtained from Aladdin Biochemical Co. Ltd. (Shanghai, China). Other reagents were analytical grade and purchased from Kelong Chemical Co. Ltd. (Chengdu, China).
Preparation of microencapsulated powders
Microencapsulated powders were prepared according to the early work with a few modifications,[Citation6,Citation7] a ratio of 1:1 (w/w) of GA to MD was selected to formulate encapsulating agents; then, 10 g of agents were added to 100 mL anthocyanin aqueous solution with continuous stirring at 35°C for 30 min. The spray-drying process was performed using a spray dryer (SD-1500, TRIOWIN Tech. Co. Ltd, Shanghai, China), with a feed nozzle diameter of 1.0 mm. The operating conditions were as follows: the temperature of inlet air was set to be 170°C, and that of outlet air temperature was about 80°C, the flow rate of drying air was 5.0 m3/min, feed rate was 0.36 L/h by feeding with a peristaltic pump. For freeze-drying process, the dispersions were kept in a freezer at −18°C for 24 h for complete hydration, then dried in a freeze dryer (FD-1E-50, BoYiKang Experimental Instrument Co. Ltd, Beijing, China) for 48 h at −52°C, vacuum degree was 0.45 mbar. Then, the resultant freeze-dried cakes were triturated using a mortar and pestle. Finally, the spray-dried powders (SDP) and freeze-dried powders (FDP) were stored in a desiccator containing silica for further analyses. The microencapsulation efficiency of the bioactive compounds was (69.83 ± 2.28)% for SDP and (72.15 ± 3.01)% for FDP.
Physical properties of the microencapsulated powders
Moisture content of the microcapsules and RAE was calculated based on the weight loss, according to the method of AOAC (2012). Water activity (Aw) of the sample was measured by direct reading in an electronic meter (LabMaster-aw, NOVASINA, Switzerland). The solubility of samples was determined according to the method described by Kuck et al.[Citation7] with some modification. One gram of sample was dissolved in 100 mL ultrapure water with continuous stirring at 25°C for 5 min, immediately centrifuged at 3000 rpm for 15 min. A 25 mL aliquot of the supernatant was transferred to a weighing bottle and oven-dried at 105°C for 5 h. The solubility (%) was calculated by weight difference. Hygroscopicity of samples was measured according to the pervious literature with a modification. Samples were weighed accurately and placed in an airtight desiccator with saturated sodium chloride solution (RH of 75.3%), stored at 25°C for 1 week. The hygroscopicity was expressed as g of adsorbed moisture per 100 g of dry solids (g/100 g). The colorimetric analysis was based on the CIELAB (L*, a*, b*) and the CIELCH (H°, C) space system, a spectrophotometer (CM-5, Konica Minolta Co. Ltd, Osaka, Japan) was used to measuring the color parameters.
Particle micromorphology and size distribution
The surface micromorphology of microparticles was evaluated in a scanning electron microscope (SEM, JSM-6510LV, Jeol, Tokyo, Japan) with an accelerating voltage of 15 kV and magnification of 1000–5000× . The size distribution was determined by a laser particle analyzer (JL-6000, Jingxin Powder Testing Equipment Co. Ltd, Chengdu, China). The samples were analyzed as dry dispersions. The volume moment means (D[4,3]), surface area moment means (D[3,2]) and the equivalent cumulative volume diameters D10, D50 and D90 were measured. Refer to the literature,[Citation8] the particle size distribution in the powders (span) was calculated as [(D90-D10)/D50].
Determination of bioactive compounds and antioxidant activity
The SDP, FDP, and RAE were dissolved in acidified ultrapure water (pH = 3.0) to appropriate concentrations according to the need of each analysis. UV-1800 BPC spectrophotometer (Mapada Instruments Co. Ltd, Shanghai, China) was used for spectral analysis.
The anthocyanin contents (ACN) were determined according to the earlier method[Citation9] with some modifications. The ACN were calculated with a regression function fitted by the standard curve of cyanidin 3-O-glucoside solutions (0–50 μg/mL): A = 0.01708c-0.0044, where A represented absorbance value, c represented the concentration of cyanidin 3-O-glucoside (μg/mL). The wavelength with maximum absorption was 518 nm. The results were expressed in mg cyanidin 3-O-glucoside equivalent (CyaE)/g DW.
The total phenolic contents (TPC) were subjected using the Folin-Ciocalteu reagent. The total phenolic contents were calculated by a standard curve of gallic acid solutions (0–70 μg/mL) and were expressed in mg gallic acid equivalent (GAE)/g DW: TPC = c× V/m, where c is the phenolic contents corresponded with standard curve, V is the volume of the sample, and m is the mass of the sample.
The DPPH radical scavenging capacity was measured according to the method described by Teng[Citation10] with a modification. Sample solutions (2 mL) were mixed with 2 mL DPPH solution (0.1 mM, dissolved in 95% ethanol), then incubated at 25°C for 30 min. The absorbance of the mixture was measured at 517 nm. The antioxidant capacity of samples was calculated as (1 – Asample 517/Acontrol 517) × 100% and expressed as EC50 value (μg/mL).
The hydroxyl radical scavenging capacity was determined according to the literature[Citation10] with some modifications. Briefly, sample solutions (1 mL) were mixed completely with ferrous sulfate solution (1 mL, 3 mM) and hydrogen peroxide solution (1 mL, 3 mM). After incubated at 37°C for 10 min, 1 mL of salicylic acid solution (3 mM, dissolved in ethanol) was added and kept at 37°C for 30 min. The absorbance of resulted solution was measured at 510 nm. The scavenging capacity in percent was calculated as (1 – Asample 510/Acontrol 510) × 100% and expressed as EC50 value (μg/mL).
The reducing power was performed according to the previous method with some modifications.[Citation10] Two and a half milliliters of sample solutions, 2.5 mL of phosphate-buffered saline (PBS, 0.2 M, pH 6.6), and 2.5 mL of potassium ferricyanide solution (1%, w/v) were mixed and incubated at 50°C for 20 min. Then, 2.5 mL of trichloroacetic acid solution (10%, w/v) was added and centrifuged at 3000 rpm for 10 min. Subsequently, a 2.5 mL aliquot of the supernatant was mixed with 2.5 mL ultrapure water and 0.5 mL ferric chloride (0.1%, w/v), the absorbance was measured at 700 nm. The reducing power was calculated from a standard curve prepared of ascorbic acid (10–60 μg/mL) and the result was expressed in mg ascorbic acid equivalent (AAE)/g DW.
Thermal degradation kinetic studies of anthocyanin
The thermal degradation of rose anthocyanin was investigated at 70°C, 80°C, and 90°C based on an accelerated model, in which temperature would not occur the phase transition.[Citation2] Samples were heated at specific temperatures in a thermostatic bath, with an accuracy of ± 1°C. Aliquot of 5 mL of the samples were removed at intervals, immediately cooled by plunging them into an ice water bath, and measured residual ACN contents. The time for the sample solution to reach the setup temperature could assumptive to be disregarded.[Citation4] The ACN retention (%) of different samples was considered by compared with the initial ACN content (C0) prepared for each product.
Previous studies[Citation11] showed that the thermal degradation of anthocyanins fitted a first-order reaction kinetics. The kinetic model could be expressed as the following equations:
where C0 is the initial ACN contents, Ct is the ACN contents after a certain time (t min) at the given temperature, k is the first-order kinetic constant (min−1), and t1/2 is the half-life time (h). The temperature dependence degradation rate constants of anthocyanins could be described by the Arrhenius equation[Citation11]:
where k0 is the frequency factor (min−1), Ea is the activation energy (J·mol−1), R is the universal gas constant (8.314 J·mol−1·K−1), T is the absolute temperature (in Kelvin, K). The theoretical Eyring model is also considered as a reference,[Citation4,Citation11] and the model’s parameters were calculated through following equations:
where ΔG is the free activation enthalpy (J·mol−1), ΔH is the activation enthalpy (J·mol−1), ΔS is the activation entropy (J·mol−1·K−1), kB is the Boltzmann constant (1.381 × 10−23 J·K−1), and h is the Planck constant (6.626 × 10−34 J·s). In addition, the coefficient Q10 (temperature coefficient) was used to determine the degradation rate by an increase in 10°C.[Citation11] It was calculated as the following equation:
Statistical analysis
All analyses were performed in triplicate and the results were expressed as mean ± standard deviation. Analysis of variance (ANOVA) and Tukey’s test were performed to determine the significant differences (P < .05) among the samples using the SPSS 19.0 (IBM, USA) software.
Results and discussion
Microstructure and particle size distribution of the microcapsules
It is necessary to evaluate the microstructure of the microcapsules, which governs the protection capacity of polymeric wall materials, especially the degree of integrality and porosity of powders.[Citation6] shows the microstructure of two microcapsules: the majority of SDP presented a spherical structure without cracks and agglomeration, and the rest were flat in appearance. The extensive concavities and wrinkles on the surface made the microcapsules look like erythrocytes (, . While FDP showed an indefinite and laminated structure that the compact brittle textures were distributed with prominently sharp edges (, . A similar morphology was also observed by other authors[Citation8,Citation12] through spray-drying microcapsules produced with GA and MD. In the spray-drying process, high temperature (~170°C) induced rapid water evaporation from atomized droplets, leading to the formation of spherical particles with shrinkage on the surface. Moreover, the encapsulation agents also affected the appearance of particles. For example, molecules with longer chain blocked the passing of water and then resulted in concavities and wrinkles on the surface of particles.[Citation13] However, water in materials was sublimated instantly in the form of ice in the process of freeze-drying, resulting in a slight change in volume and structure without shrinkage and collapse, and the remaining hollow was occupied by air.[Citation7]
Figure 1. Micrographs of rose anthocyanin microcapsules with GA and MD by spray-drying and freeze-drying. (a): spray-dried powders (magnification of 2000×); (b): spray-dried powders (magnification of 5000×); (c): freeze-dried powders (magnification of 1000×); (d): freeze-dried powders (magnification of 2000×)
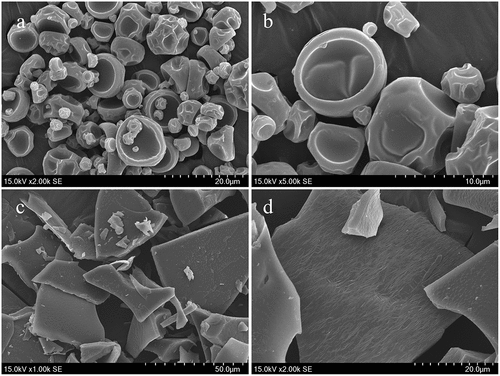
The particle sizes are summarized in . The results showed that the mean sizes (D[4,3], D[3,2],,) of SDP were 19.05 ± 1.02 and 1.54 ± 0.20 μm, respectively, while those of FDP (D[4,3], D[3,2]) were 25.89 ± 0.92 and 6.10 ± 0.71 μm. The force was not strong enough to change the surface or frozen droplets at low processing temperature during the freeze-drying process[Citation14], and the irreversible links were formed at the beginning of agglomeration process (frozen to a cake), so they led to a larger size of FDP.[Citation15] While the particle sizes during the spray-drying process were smaller as samples started with a liquid dispersion. Moreover, the grinding process after freeze-drying was manually done, which also influenced the particle sizes.[Citation7] FDP had a smaller span value of particle size distribution (2.24 ± 0.36) compared with SDP’s (4.73 ± 0.72). The small span value indicated a more homogeneous distribution.[Citation8] Both microcapsules had desirable homogeneousness and powder flowability.[Citation15]
Table 1. Average volume diameter, average surface area diameter and particle size distribution (Span) of the rose anthocyanin microcapsules with GA and MD by spray-drying and freeze-drying
Physical properties of the extracts and microencapsulated powders
Stable and pleasant color is the paramount element for the potential alternative of artificial colorants in food industry. The CIELAB color system and the CIELCH color system are generally used to evaluate color properties. The CIELAB color system is based on Cartesian polar coordinates, while the CIELCH color system is represented as cylindrical coordinates.[Citation3]
As shown in , both SDP and FDP were apparently lighter (P < .05) with L* values between 66.83 ± 0.91 to 77.57 ± 0.34 than RAE (L* = 27.39 ± 0.88), due to the white color of the encapsulation agents the former two used. With the light scattering properties considered, the empty spaces after sublimation of ice crystals resulted in darker appearance of FDP.[Citation7] Both a* value and b* value of all samples were positive, indicating the color of the samples was in red and yellow, and this demonstrated the presence of anthocyanins. The H° (H °= cot b*/a*) values varied from 1.83 ± 0.23 to 16.71 ± 0.52, indicating the powders were in red (H °= 0). This suggested the color of SDP was closer to that of RAE, according to the H° values. The parameters chroma represented the intensity of the color deviation from neutral gray. The C value of RAE (26.60 ± 0.23) was similar to the a* value (26.01 ± 1.08). It demonstrated that the C value may be in proportion to the a* value, indicating the impact of the volume of anthocyanins on color.[Citation3,Citation16]
Table 2. Physical properties of the rose anthocyanin extracts and microcapsules with GA and MD by spray-drying and freeze-drying
The moisture content, water activity (Aw), and hygroscopicity are the key factors to the storage stability of powder product. The solubility is important to evaluate the re-dissolving effect of powders which influences their application in aqueous systems.[Citation17] The microcapsules were characterized and summarized in . The powders obtained by different methods contained similar moisture contents (6.65 ± 0.34%-7.00 ± 0.12%), significantly lower than RAE (8.26 ± 0.28%) (P < .05). The Aw values varied from 0.220 ± 0.011 to 0.310 ± 0.006, and that of RAE was the highest. The lowest moisture content and Aw value were found in FDP. The two microcapsules and RAE could be considered microbiologically stable because the Aw value of them was all lower than the minimum value (0.6) required for the multiplication of microorganisms.[Citation17] Similarly, Rezende[Citation6] obtained similar moisture content (3.12%-7.05%) and lower Aw (0.07–0.26), after encapsulating extracts of acerola by spray- and freeze-drying. In addition, the hygroscopicity value of microcapsules and RAE was quite low, in the range of 11.68 ± 0.18–13.63 ± 0.22 g/100 g, indicating which was in compliance with other encapsulated products.[Citation18] The low hygroscopicity led to an effective conservation of anthocyanins and lengthened the powders’ shelf life.
The solubility of microcapsules was (88.64 ± 2.19)–(92.76 ± 3.08)%, significantly higher than that of RAE (67.42 ± 3.92%) (P < .05). Encapsulation with GA and MD improved solubility of ACN greatly, due to the high water solubility and better dispersibility of GA and MD.[Citation5,Citation18] In this study, the aqueous solubility of FDP was the highest, because freeze-drying process generated a porous brittle structure with cracks and led to a greater surface area available for hydration.
Anthocyanin content, total phenolic content, and antioxidation activity
There were significant differences (P < .05) observed among the three samples in terms of bioactive compounds and antioxidation activity, shown in . RAE contained the highest level of ACN and TPC, at (66.29 ± 2.05) mg CyaE/g DW and (619.09 ± 1.97) mg GAE/g DW, respectively, higher than the level of ACN and TPC in Rosa gallica, at 6 mg/g and 300 mg/g.[Citation19] This was because RAE was a highly-concentrated anthocyanin extract. The retention rate of ACN was 75.85% during SDP and 95.12% during FDP, while the retention rate of TPC was higher, at 86.00% during SDP and 91.44% during FDP, indicating that anthocyanins were more unstable than other phenolic compounds. Although the bioactive compounds suffered some losses after microencapsulation, there were considerable bioactive substances in microcapsules. Kuck[Citation7] encapsulated grape skin extracts with GA by spray-drying and freeze-drying, obtained 17.07–21.15 mg malvidin-3,5-diglucoside/g of ACN and 21.37–26.26 mg GAE/g of TPC, with the retention rate of 80.75%-99.58% for ACN and that of 81.4%-95.3% for TPC. Li[Citation20] reported the concentration of plum phenolics (296.19 mg GAE/g), only remained 23.51%-27.24% after encapsulation with GA and MD by spray-drying.
Table 3. Anthocyanin contents (ACN), total phenolic contents (TPC), and antioxidant activity (by scavenging DPPH radical, OH radical and reducing Fe3+) of the rose anthocyanin extracts and microcapsules with GA and MD by spray-drying and freeze-drying
Oxygen content and processing temperature were considered as the key factors to the degradation of bioactive compounds. In the spray-drying process, the surface areas exposed to oxygen and heat increased along with the atomization of sample solutions and the formation of misty droplets.[Citation14] Nevertheless, the exposure to the high inlet temperature (170°C) was short-lived, according to Santiago[Citation3], so the degradation caused by heat could be considered insignificant compared to other conventional thermal processes. During the freeze-drying process, the grinding after the powders were dried increased the chance of contact between the powders and the air, causing oxidation reaction – one of the main factors leading to the degradation of the active substance.[Citation18] In addition, the concavities and fissures on the surface tended to cause a premature release of the encapsulated compounds.[Citation21] The smaller losses of ACN and TPC during the freeze-drying process may be related to vacuum and low temperature that protect the active property effectively.[Citation21]
The antioxidant activities of anthocyanin and other phenolic compounds in food materials were evaluated using different methods: the scavenging of DPPH free radical and hydroxyl radical assays based on the electron transfer reaction, while the reduction of metal ions was based on redox reaction.[Citation17] It could be observed in that the antioxidant activities during SDP and FDP decreased significantly (P < .05) compared to the initial antioxidant activities in RAE. The decreasing degree of DPPH free radical activities was higher than other methods, because the DPPH free radical scavenging and processing methods were less related with the categories of materials.[Citation6] The correlation between ACN/TPC and antioxidant activities was evaluated. The Pearson’s correlation coefficient (R2) of antioxidant activity with ACN was at 0.622 (DPPH radical), 0.856 (OH radical) and 0.953 (reducing power), while the R2 of antioxidant activity with TPC was at 0.556 (DPPH radical), 0.774 (OH radical) and 0.979 (reducing power). In general, the correlation between antioxidant activity and ACN is higher except for the reducing power; in other words, ACN is the major contributor to the antioxidant activity.[Citation6]
Thermal degradation kinetics of anthocyanin
In order to evaluate the stability of anthocyanins during unit operation and storage process, the thermal degradation kinetic model was generally employed as a fast, objective, and economic tool.[Citation4] As shown in , the degradation of ACN increased logarithmically along with a linear rise of temperature, which indicated that the decay of ACN with time fitted a first-order equation (high regression coefficients at 0.957–0.984). The most rapid degradation was observed visually in RAE, followed by SDP and FDP, in the range of the test temperature.
Figure 2. Thermal degradation kinetics of rose anthocyanins vs temperature. (a): spray-dried powders with GA and MD; (b): freeze-dried powders with GA and MD; (c): rose anthocyanin extracts. Standard deviations were evaluated in triplicate with standard error <5%
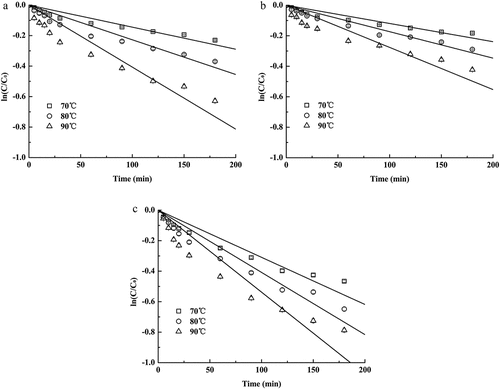
The kinetic parameters (k, t1/2, Ea) based on the Arrhenius model are summarized in . The kinetic constant k increased with the increase in temperature, which demonstrated that the degradation of ACN was related to the change in time and temperature. k was ranged from (1.19 ± 0.08)×103 to (2.76 ± 0.27)×103 min−1 at 70–90 °C for FDP, lower than (3.09 ± 0.26)×103 min−1 at 70°C for RAE. These data revealed that RAE was easier to be affected by heat than SDP, while FDP was the most stable. Correspondingly, microencapsulation lengthened the half-life period of ACN (2.15 ± 0.16–3.74 ± 0.31 h) significantly: 2–3 times longer for FDP (4.19 ± 0.41–9.71 ± 0.69 h) and 1–2 times longer for SDP (2.84 ± 0.22–7.97 ± 0.48 h). The better thermostability of FDP may be attributed to the vacuum operating environment with low oxygen content.[Citation4]
Table 4. Kinetic parameters of anthocyanin degradation during heat treatment
The activation energy (Ea) indicated the energy needed to achieve the active state of a reaction. The higher Ea value of RAE (144.38 ± 2.16 kJ·mol−1) compared with that of blueberry (119.22 kJ·mol−1)[Citation2] showed that the rose anthocyanin was more difficult to be activated and was more susceptible to the rising of temperature[Citation22], hence conservation measures were necessary. The activation energy surprisingly decreased after encapsulation, which was in contrast to tendencies of k and t1/2. This was probably because the experiment was assumed in an isothermal status, while the actual degradation process of anthocyanin in solid or semi-solid food matrix is usually not isothermal.[Citation22] In addition, the non-enzymatic browning occurred at high temperature could not be explained by the Arrhenius model.[Citation2] That may result in an unexpected decrease of the activation energy.
Further consideration of the thermodynamic parameters (ΔG, ΔH, ΔS) based on the theoretical Eyring model was conducive to look into the physical and chemical phenomena occurred in the reactions. The similar positive value of ΔG (100.93 ± 0.24–107.33 ± 0.29 kJ·mol−1) between RAE and microcapsules showed that ACN degradation was a nonspontaneous reaction. ΔG is a fundamental criterion to judge the spontaneity of a reaction.[Citation22] The activation enthalpy (ΔH) is defined as an energy barrier of the transformation from reagent to activated complex. As shown in , the differences of ΔH at a certain range of temperature were not significant (P < .05) during the same process, owing to the disrelation between the maximum value of energy barrier and temperature. The positive ΔH values in the experiment revealed an endothermic state in the conversion from reactant to activated complex, and proved that rose anthocyanin decays with the increasing of temperature. The ΔH of FDP (91.66 ± 1.78–91.83 ± 0.61 kJ·mol−1) was higher than that of SDP (74.07 ± 1.69–74.24 ± 1.53 kJ·mol−1), maybe due to the upraising of energy barrier in the vacuum environment.[Citation22] The activation entropy (ΔS) is defined as a disorder degree of changed molecules. The positive ΔS values of RAE indicated that the entropy increased when reaching the transition state.[Citation2] In contrast, the negative ΔS of FDP and SDP declared that the structural freedom of reactant was higher than activated complex. Part of the reaction system rearranged from the initial structure and arrived its own thermodynamic equilibrium, resulting in low absolute value of ΔS for FDP (34.46 ± 2.11–43.13 ± 2.23 J·mol−1·K−1). Therefore, encapsulation by freeze-drying formed a stable balanced system. A higher temperature coefficient (Q10) was found at the range of 80–90°C, which illustrated that the ACN was more susceptible to heat and was easier to decay at these temperatures. While the Q10 of three samples had no significant differences (P < .05) at the temperature of 70–80°C.
Conclusion
Spray-drying and freeze-drying processes were employed to microencapsulate rose anthocyanins with gum arabic and maltodextrin. Microcapsules exhibited better physical properties with lower moisture content, Aw and higher solubility than RAE. The micromorphology of particles of SDP and FDP showed a spherical structure and an indefinite, laminated structure, respectively. According to the a*, H° and C values, the color of SDP was closer to that of RAE, which showed a typically attractive red. In general, the stable physical properties resulted in a high retention rate of bioactive compounds and antioxidant activity. In addition, FDP possessed the lowest k and highest t1/2, indicating a more preferable thermostability than SDP and RAE. In summary, microencapsulation is beneficial to protect anthocyanin, especially the freeze-dried microcapsules, and provides developing room for rose anthocyanin in food additives industry so as to enhance the economic value of rose residue.
References
- Nogueira, G. F.; Soares, C. T.; Martin, L. G. P.; Fakhouri, F. M.; de Oliveira, R. A. Influence of Spray Drying on Bioactive Compounds of Blackberry Pulp Microencapsulated with Arrowroot Starch and Gum Arabic Mixture. J. Microencapsulation. 2019, 1–34. DOI:10.1080/02652048.2019.1693646.
- Celli, G. B.; Dibazar, R.; Ghanem, A.; Brooks, M. S.-L. Degradation Kinetics of Anthocyanins in Freeze-dried Microencapsulates from Lowbush Blueberries (Vaccinium Angustifolium Aiton) and Prediction of Shelf-life. Drying Technol. 2016, 34(10), 1175–1184.
- Santiago, D. A. M. C.; Nogueira, R. I.; Paim, D. R. S. F.; Gouvêa, A. C. M. S.; Godoy, R. L. D. O.; Peixoto, F. M.; Pacheco, S.; Freitas, S. P. Effects of Encapsulating Agents on Anthocyanin Retention in Pomegranate Powder Obtained by the Spray Drying Process. LWT - Food Sci. Technol. 2016, 73, 551–556. DOI: 10.1016/j.lwt.2016.06.059.
- Lee, J.; Park, G.; Chang, Y. H. Nutraceuticals and Antioxidant Properties of Lonicera Japonica Thunb. As Affected by Heating Time. Int. J. Food Prop. 2019, 22(1), 630–645.
- Çam, M.; İçyer, N. C.; Erdoğan, F. Pomegranate Peel Phenolics: Microencapsulation, Storage Stability and Potential Ingredient for Functional Food Development. LWT - Food Sci. Technol. 2014, 55(1), 117–123.
- Rezende, Y.; Nogueira, J. P.; Narain, N. Microencapsulation of Extracts of Bioactive Compounds Obtained from Acerola (Malpighia Emarginata DC) Pulp and Residue by Spray and Freeze Drying: Chemical, Morphological and Chemometric Characterization. Food Chem. 2018, 254, 281–291. DOI: 10.1016/j.foodchem.2018.02.026.
- Kuck, L. S.; Noreña, C. P. Z. Microencapsulation of Grape (Vitis Labrusca Var. Bordo) Skin Phenolic Extract Using Gum Arabic, Polydextrose, and Partially Hydrolyzed Guar gum as Encapsulating Agents. Food Chem. 2016, 194, 569–576. DOI: 10.1016/j.foodchem.2015.08.066.
- Fernandes, R. V.; Borges, S. V.; Botrel, D. A. Gum Arabic/starch/maltodextrin/inulin as Wall Materials on the Microencapsulation of Rosemary Essential Oil. Carbohydr. Polym. 2014, 101, 524–532. DOI: 10.1016/j.carbpol.2013.09.083.
- Moo-Huchin, V. M.; Moo-Huchin, M. I.; Estrada-León, R. J.; Cuevas-Glory, L.; Estrada-Mota, I. A.; Ortiz-Vázquez, E.; Betancur-Ancona, D.; Sauri-Duch, E. Antioxidant Compounds, Antioxidant Activity and Phenolic Content in Peel from Three Tropical Fruits from Yucatan, Mexico. Food Chem. 2015, 166, 17–22. DOI: 10.1016/j.foodchem.2014.05.127.
- Teng, H.; Fang, T.; Lin, Q.; Song, H.; Liu, B.; Chen, L. Red Raspberry and Its Anthocyanins: Bioactivity beyond Antioxidant Capacity. Trends Food Sci. Technol. 2017, 66, 153–165. DOI: 10.1016/j.tifs.2017.05.015.
- Wu, H.-Y.; Yang, K.-M.; Chiang, P.-Y. Roselle Anthocyanins: Antioxidant Properties and Stability to Heat and pH. Molecules. 2018, 23(6), 1357.
- Nunes, G. L.; Boaventura, B. C. B.; Pinto, S. S.; Verruck, S.; Murakami, F. S.; Prudêncio, E. S.; de Mello Castanho Amboni, R. D. Microencapsulation of Freeze Concentrated Ilex Paraguariensis Extract by Spray Drying. J. Food Eng. 2015, 151, 60–68. DOI: 10.1016/j.jfoodeng.2014.10.031.
- Bhusari, S. N.; Muzaffar, K.; Kumar, P. Effect of Carrier Agents on Physical and Microstructural Properties of Spray Dried Tamarind Pulp Powder. Powder Technol. 2014, 266, 354–364. DOI: 10.1016/j.powtec.2014.06.038.
- Saikia, S.; Mahnot, N. K.; Mahanta, C. L. Optimisation of Phenolic Extraction from Averrhoa Carambola Pomace by Response Surface Methodology and Its Microencapsulation by Spray and Freeze Drying. Food Chem. 2015, 171, 144–152. DOI: 10.1016/j.foodchem.2014.08.064.
- Rezvankhah, A.; Emam-Djomeh, Z.; Askari, G.. Encapsulation and Delivery of Bioactive Compounds Using Spray and Freeze-drying Techniques: A Review. Drying Technology. 2019, 1–24.
- Guofang, X.; Xiaoyan, X.; Xiaoli, Z.; Yongling, L.; Zhibing, Z. Changes in Phenolic Profiles and Antioxidant Activity in Rabbiteye Blueberries during Ripening. Int. J. Food Prop. 2019, 22(1), 320–329.
- Ostroschi, L. C.; Brito de Souza, V.; Echalar-Barrientos, M. A.; Tulini, F. L.; Comunian, T. A.; Thomazini, M.; Baliero, J. C. C.; Roudaut, G.; Genovese, M. I.; Favaro-Trindade, C. S. Production of Spray-dried Proanthocyanidin-rich Cinnamon (Cinnamomum Zeylanicum) Extract as a Potential Functional Ingredient: Improvement of Stability, Sensory Aspects and Technological Properties. Food Hydrocolloids. 2018, 79, 343–351. DOI: 10.1016/j.foodhyd.2018.01.007.
- Hussain, S. A.; Hameed, A.; Nazir, Y.; Naz, T.; Wu, Y.; Suleria, H.; Song, Y. Microencapsulation and the Characterization of Polyherbal Formulation (PHF) Rich in Natural Polyphenolic Compounds. Nutrients. 2018, 10(7), 843.
- Lee, M.-H.; Nam, T. G.; Lee, I.; Shin, E. J.; Han, A.-R.; Lee, P.; Lee, S.-Y.; Lim, T.-G. Skin Anti-inflammatory Activity of Rose Petal Extract (Rosa Gallica) through Reduction of MAPK Signaling Pathway. Food Sci. Nutr. 2018, 6(8), 2560–2567.
- Yinbin, L.; Wu, L.; Weng, M.; Tang, B.; Lai, P.; Chen, J. Effect of Different Encapsulating Agent Combinations on Physicochemical Properties and Stability of Microcapsules Loaded with Phenolics of Plum (Prunus Salicina Lindl.). Powder Technol. 2018, 340, 459–464. DOI: 10.1016/j.powtec.2018.09.049.
- Ramírez, M. J.; Giraldo, G. I.; Orrego, C. E. Modeling and Stability of Polyphenol in Spray-dried and Freeze-dried Fruit Encapsulates. Powder Technol. 2015, 277, 89–96. DOI: 10.1016/j.powtec.2015.02.060.
- Zhou, M.; Chen, Q.; Bi, J.; Wang, Y.; Wu, X. Degradation Kinetics of Cyanidin 3-O-glucoside and cyanidin 3-O-rutinoside during Hot Air and Vacuum Drying in Mulberry (Morus Alba L.) Fruit: A Comparative Study Based on Solid Food System. Food Chem. 2017, 229, 574–579. DOI: 10.1016/j.foodchem.2017.02.131.
