ABSTRACT
Existing evidence on the possible effects of probiotics on inflammatory status is inconclusive. Therefore, this systematic review and meta-analysis aimed to clarify the effects of probiotic consumption on inflammatory markers among athletes. A systematic literature search was performed in the databases PubMed, Scopus, ISI Web of science, Cochrane’s library, and Google Scholar (up to July 2020). Randomized controlled trials (RCTs) investigating the effects of probiotics on inflammatory markers in athletes were included. Jadad scale was used for quality assessment. In fixed-effects meta-analysis model, standard mean difference (SMD) and 95% confidence interval (CI) demonstrated the overall effect. Any possibility of publication bias was inspected through Egger’s and Begg’s statistics. A total of 14 RCTs with 393 participants were included. Probiotic consumption resulted in a significant decrease in plasma concentrations of interleukin-6 (SMD = −0.58; 95% CI, −0.87 to −0.28; P˂0.001) and tumor necrosis factor-α (SMD = −0.72; 95% CI, −1.11 to −0.33; P < .001), and significant increase in interferon-γ (SMD = 0.43; 95% CI: 0.09 to 0.76; P = .012). The effects were more pronounced when probiotics were consumed in Asian, male athletes, using a single strain capsule or when they were consumed for lower than 4 weeks. Probiotic supplements may be beneficial to improve inflammatory markers, including interleukin-6, tumor necrosis factor-α, and interferon-γ.
Introduction
Exercise training, based on its intensity, frequency, and volume, can have positive or negative upshots on overall health.[1] Athletes with exhausting physical activity levels are often prone to elevated levels of circulating reactive oxygen species (ROS). Only a single session of intense exercise is ample to yield significant amounts of ROS, which results in elevated levels of inflammatory markers. During exercise, oxygen consumption is 200 times higher in active muscle fibers compared to resting time, which leads to the production of inflammatory markers.[Citation2–Citation4] While inflammation appears to be an imperative factor of muscular adaptation to exercise, athletes under exhausting trainings or participating in tournaments may benefit from lower inflammation in order to support the performance of consequent bouts at maximal intensity.[Citation5] Previously, it has been revealed that dietary interventions such as antioxidant-rich foods are linked with reduced levels of inflammatory mediators and elevated plasma antioxidant concentrations in athletes, both at rest and following exercise.[Citation6] This finding encouraged scientists to look for other foods that improve athletes’ oxidative stress and reduce their inflammatory markers.
Probiotics are living microorganisms that positively affect the host body by adjusting its microbial balance.[Citation7] There is an increasing body of evidence supporting the potential health benefits of probiotics such as reduced oxidative stress and inflammation,[Citation8,Citation9] improved antitumor activity,[Citation10] higher immunity,[Citation11,Citation12] and reinforcement of gastrointestinal barrier.[Citation13] Probiotic consumption could act as a shield against oxidative damage in athletes to equip a suitable antioxidant barrier essential for preventing dangerous levels of oxidative stress and consequent inflammation.[Citation14]
Furthermore, many interventions have employed these dietary constituents to investigate their impact on inflammatory markers. However, the results of these studies are inconclusive.[Citation4,Citation5,Citation15-Citation22] Therefore, current study aimed to examine the effects of probiotic consumption on inflammatory markers, including interleukin-6 (IL-6), IL-10, tumor necrosis factor-alpha (TNF-α), and interferon-gamma (INF-γ) in athletes using a systematic review and meta-analysis of randomized controlled trials (RCTs).
Methods and materials
This study was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) statement.[Citation23]
Data source and search strategy
We searched databases including PubMed, Scopus, Cochrane Library, ISI web of sciences up to July 2020 to identify appropriate studies. The reference lists of the included articles were also assessed to detect additional eligible studies. The following search strategy was run in PubMed and tailored to each database as necessary: “Athlete” OR “Athletes” OR “exercise” OR “training” OR “sport” AND “fermented foods” OR “probiotic” OR “Lactobacillus” OR “Bifidobacterium” AND “Intervention studies” OR “Intervention” OR “Controlled trial” OR “Randomized” OR “Randomized” OR “Random” OR “Randomly” OR “Placebo” OR “Assignment.” No restrictions on language, publication time, and study design were considered.
Study selection
In order to systematically select the relevant studies, we defined our PICO (Population/intervention/comparison/outcome) components: P (adult athletes), I (probiotics (supplement or fortified food)), C (placebo or non-fortified food), O (inflammatory markers). To be included in this study, publications investigating the effects of probiotic consumption on inflammatory markers had to meet the following criteria:[1] original human RCTs with parallel or crossover design;[Citation2] which used probiotics (supplement or food) as intervention;[Citation3] examined the effect of probiotics on at least one of the inflammatory markers;[Citation4] published in English; and[Citation5] studies that recruited athletes as population. The study selection process was done independently by two investigators to minimize the potential error. If there was any disagreement, it was resolved by consensus or involving a third researcher.
Data extraction
For all included studies, two reviewers independently extracted information, including first author’s name, year of publication, country, sample size, participants’ age, gender, body mass index, RCT design, duration of intervention, dose and type of intervention in experimental and comparison groups, probiotics strains, and mean and standard deviation (SD) of outcome measures at baseline, post-intervention and if possible their change from the baseline. Corresponding authors were contacted by e-mail in case of any missing information.
Quality assessment
Jadad scale for reporting randomized clinical trial (RCT) was used to assess the quality of interventional studies. In the recent scale, papers are evaluated based on randomization (mentioned as randomized gets 1 point and mentioned randomization methods get another point), blinding (mentioned as double blind gets 1 point and mentioned blinding methods gets another point) and inclusion of participants (mentioned withdrawals and dropouts get 1 point). In this scale, studies with 3 points or more are ranked as high quality.[Citation24]
Statistical analysis
Statistical analyses were performed using STATA statistical program version 11.2 (Stata Corporation, College Station, TX, USA). If there was any standard error (SE) for variation of mean in a study, SD was calculated by the following formula: SE×√n. Effect sizes for the meta-analysis were defined as the standard mean difference (SMD; measurement at end trial minus the measurement at baseline) and 95% confidence interval (CI). When SD of difference was missing, it was imputed following the method of Follmann et al.,[Citation25] with a correlation coefficient of 0.5. The I2 index was evaluated to assess heterogeneity. Low, moderate, and high heterogeneity were defined as I2 index <40, 40–75, and >75%, respectively.[Citation26] Subgroup analyses based on the duration of the intervention (<4 weeks or ≥4 weeks), geographical location (Asian or non-Asian), and number of bacteria strains (single or multi) were done to check the sources of heterogeneity. Sensitivity analyses were conducted to assess the influence of individual RCTs on the overall meta-analysis results, using leave-one-out method. Publication bias was assessed using Egger’s and Begg’s statistics. A p-value <0.05 was considered as statistically significant.
Results
Search results
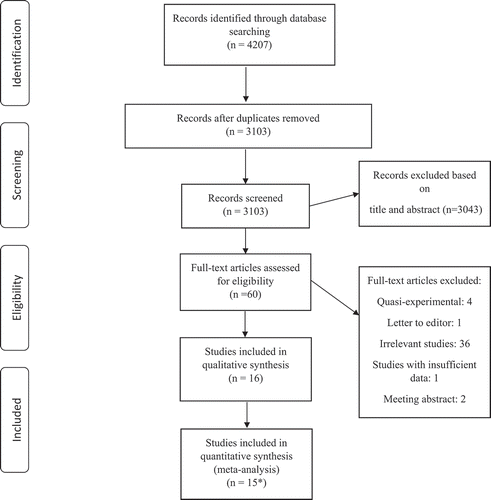
Our primary search through databases identified a total of 4207 papers. Omitting duplicates yielded 3103 articles, which were reviewed based on the title and abstract by two independent reviewers, and 3043 irrelevant studies were excluded at this stage. Sixty papers were retrieved and reviewed based on full text, and 14 articles met the inclusion criteria and were included in our systematic review and meta-analysis. The PRISMA flow diagram summarizes the results of the study selection process ().
Overview of included studies
The general characteristics of the included articles are shown in . A total of 14 studies involving 393 participants were included in our systematic review and meta-analysis with their sample sizes ranging from 7 to 84 subjects. Included studies were published between 2010 and 2019. Mean age of the participants ranged between 20.1 and 37.9 years. Four studies recruited both gender,[Citation17,Citation20,Citation21,Citation28] nine were conducted only in men,[Citation5,Citation15,Citation16,Citation18,Citation19,Citation22,Citation27,Citation29] and one used female subjects.[Citation4] All the included studies used supplements except for two,[Citation4,Citation17] which used probiotic foods as intervention. Three studies were conducted in the United States,[Citation5,Citation27,Citation28] two in UK,[Citation17,Citation21] two in Australia,[Citation15,Citation22] two in Taiwan,[Citation29] and the others were established from Iran,[Citation4] Israel,[Citation16] Serbia,[Citation20] Austria,[Citation19] and Malaysia.[Citation18] The total daily dose of probiotic consumption varied between 1 × 109 to 5 × 1019 colony-forming units (CFU), and the duration of administration varied between 3 and 16 weeks. Using Jadad scale, 12 studies [,Citation4,Citation15,Citation17-Citation22,Citation27-Citation29] were considered as high quality and 2 [,Citation5,Citation16] were of low quality.
Table 1. Characteristics of included trials.
Findings from the meta-analysis
Effect of probiotic consumption on IFN-γ
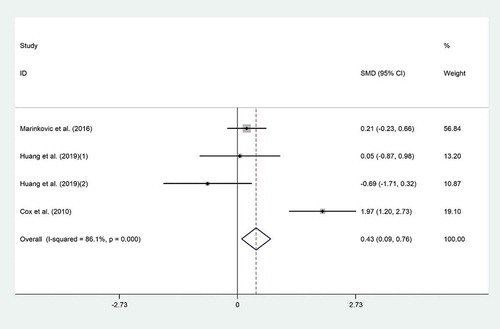
The effect of probiotic consumption on IFN-γ was examined in three trials.[Citation15,Citation20,Citation29] Overall, meta-analysis showed that probiotic consumption significantly increased IFN-γ (SMD = 0.43; 95% CI: 0.09 to 0.76; p = .012) with high heterogeneity (I2 = 86.1%, p˂0.001) (). Subgroup analysis suggested a more pronounced elevation in IFN-γ in studies with duration of less than 4 weeks (SMD = 1.001; 95% CI: 0.38 to 1.61; I2 = 94%). But the elevation in trials with duration ≥4 weeks was not significant (SMD = 0.18; 95% CI: −0.21 to 0.58; I2 = 0%) (). Findings from the sensitivity analysis revealed that exclusion of the Cox et al. study from the analysis changed the overall effect (SMD = 0.06; 95% CI, −0.3 to 0.43). No evidence of publication bias was observed (p = .49, Begg’s test and p = .99, Egger’s test).
Table 2. Subgroup analysis to assess the effect of probiotic consumption on lipid inflammatory markers.
Effect of probiotic consumption on IL-6
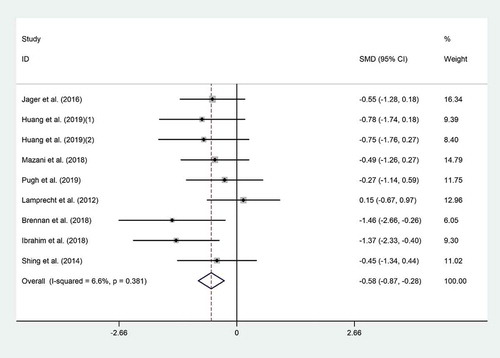
Eight trials reported the effect of probiotic consumption on IL-6.[Citation4,Citation5,Citation18,Citation19,Citation21,Citation22,Citation28,Citation29] Probiotics significantly reduced IL-6 (SMD = −0.58; 95% CI, −0.87 to −0.28; p˂0.001) compared to the controls (). A non-significant heterogeneity between the effect sizes of the included studies was observed (I2 = 6.6%, p = .38). Subgroup analysis revealed that probiotic effect was more prominent in Asian (SMD = −0.72; 95% CI: −1.13 to −0.32; I2 = 0.0%) and male athletes (SMD = −0.56; 95% CI: −0.92 to −0.20; I2 = 16.2%) (). Excluding individual studies did not result in a significant change in the overall meta-analysis results. No evidence of publication bias was observed (p = .09, Begg’s test and p = .057, Egger’s test).
Effect of probiotic consumption on IL-8
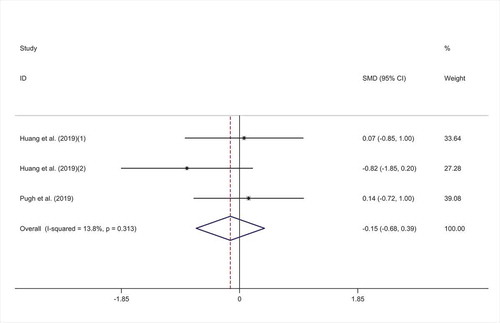
The overall result of meta-analysis of three studies [,Citation21,Citation29] for IL-8 did not show any significant efficacy for probiotic consumption (SMD = −0.15; 95% CI: −0.68 to 0.39; p = .594) with low heterogeneity (I2 = 13.8%, p = .313) (). Overall finding was not sensitive to exclusion of any individual studies. No evidence of publication bias was observed (p = .117, Begg’s test and p = .229, Egger’s test).
Effect of probiotic consumption on IL-10
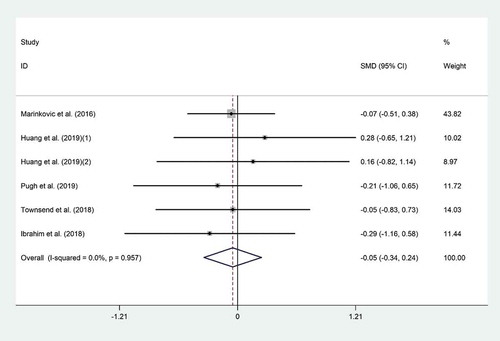
The meta-analysis of six trials [,Citation18,,Citation20,,Citation21,,Citation27,,Citation29] for the mean difference in IL-10 did not show any significant effect for probiotic consumption on IL-10 (WMD = 0.05 pg/ml; 95% CI, −0.34 to 0.24; p = .73) (). There was no evidence of significant heterogeneity among the included studies (I2 = 0.0%, p = .95). Subgroup analysis did not show any change in this result (). Overall meta-analysis result for IL-10 was not sensitive to individual studies. No evidence of publication bias was observed (p = .57, Begg’s test and p = .69, Egger’s test).
Effect of probiotic consumption on TNF-α
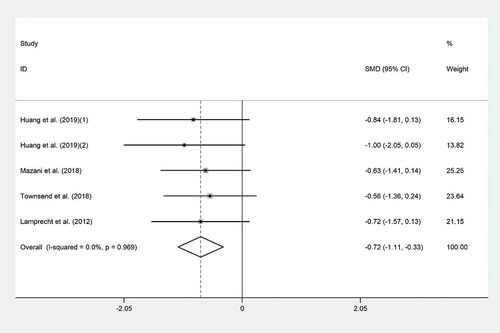
The effect of probiotic consumption on TNF-α was reported in four clinical trials.[Citation4,Citation19,Citation27,Citation29] Probiotic consumption significantly decreased TNF-α level (SMD = −0.72; 95% CI, −1.11 to −0.33; p < .001) compared to the controls (). Significant heterogeneity was observed (I2 = 0.0%, p = .96). The effect of probiotic consumption on TNF-α was more noticeable among male athletes (SMD = −0.74; 95% CI: −1.19 to −0.29; I2 = 0.0%) whom were supplemented with single-strain probiotics (SMD = −0.71; 95% CI: −1.15 to −0.27; I2 = 0.0%) (). Excluding individual studies did not result in any significant change in the overall meta-analysis results. There was evidence of publication bias (p = .05, Begg’s test and p = .01, Egger’s test); therefore, we did trim & fill analysis to find out probable missed studies, but this method could not add any studies to our included ones.
Discussion
This systematic review and meta-analysis about the effects of probiotic consumption on inflammatory markers in athletes revealed improvements in IFN-γ, IL-6, and TNF-α but not IL-8 and IL-10. The subgroup analyses suggested that a greater improvement in inflammatory markers may be expected when probiotics are consumed in Asian countries, by male athletes, using single strain capsule or when lower than 4 weeks of supplementation are implemented.
There are some inconsistencies among available literature, which should be taken into account when interpreting the results. The finding regarding the IFN-γ is inconsistent with the previous meta-analysis in a different population that found no significant effects for probiotic consumption.[Citation30–Citation32] Reduction in IL-6 levels is in agreement with Milajerdi et al.[Citation31] finding but inconsistent with others.[Citation30,Citation32] We found that probiotic consumption did not change IL-8, which has been approved by previous reports.[Citation30,Citation31] Our finding regarding IL-10 has not been reported in previous papers [,Citation31,Citation32] except in a study by Kazemi et al.[Citation30] Probiotic intake significantly reduced TNF-α, which is consistent with previous datasets.[Citation30–Citation32] A probable explanation for these inconsistencies might be related to the implementation settings and mythology of each study. Furthermore, the previous meta-analysis has been done among patients with different diseases, which is different from our population of athletes.
Regular training is associated with improved inflammatory status, especially when compared to sedentary lifestyle.[Citation33] It has been indicated that moderate exercise causes fluctuations in microbiota in humans, which differs based on body composition and exercise maintenance.[Citation34] Low-intensity training can upsurge the abundance of health-promoting micro-organisms, and low-to-moderate exercise may exert impacts on immune function, inflammation, and oxidative stress, with favorable upshots to the intestinal health.[Citation35–Citation37] Furthermore, high-intensity exercise, which is a part of routine trainings in athletes, includes the production of metabolites and inflammatory intercessors, and biological adjustment to exercise may lead to a decline in pro-inflammatory cytokines.[Citation33] Systemic release of pro-inflammatory cytokines as a result of bacterial translocation across the gastrointestinal barrier into the circulation leads to increased body temperature.[Citation38] It has been reported that, with increases in body temperature during exercise, progressively elevated plasma levels of IL-6 and TNF-α are observed.[Citation39,Citation40]
Alterations in serum IL-6 levels, a pro-inflammatory cytokine, are often used as an indicator of muscle recovery or a marker of training stress.[Citation41,Citation42] The pro-inflammatory cytokines (TNF-α, IL-6) are released after the physical activity of sufficient intensity, followed by the release of anti-inflammatory or regulatory cytokines (IL-4, IL-10, IL-1RA, and IL-13) that attenuate that response.[Citation43] The pro-inflammatory response caused by training promotes anti-inflammatory response. Though, if the pro-inflammatory cytokine response is lessened, this would also block the anti-inflammatory cytokine response as well. These conflicting responses are hard to elucidate but are consistent with the complexity linked with the cytokine response to muscle-damaging events.[Citation44,Citation45] Therefore, sufficient attention is needed to decrease inflammatory status and its undesirable effects among athletes.
Probiotics can help regulate inflammation in a number of ways. First, there are some mechanisms whereby certain probiotic strains can improve the structure and function of intestinal epithelial barriers. Of particular interest in this regard is the decrease in tumor necrosis factor-alpha, a pro-inflammatory cytokine that causes leaky barriers by directly impairing the integrity of the tight junctional complexes between epithelial cells.[Citation46,Citation47] Another indirect effect of probiotics on inflammation occurs via their ability to ferment certain types of fiber, thus increasing the production of short-chain fatty acids with anti-inflammatory properties (e.g. butyrate).[Citation48] In addition, enhanced synthesis and secretion of antimicrobial peptides might prove to be an additional indirect pathway whereby probiotics could help regulate an inflammatory response.[Citation49] Furthermore, probiotics and some of their secreted metabolic products can act as ligands for innate immune system receptors, directly influencing key pro-inflammatory pathways. They also stimulate the differentiation and activity of important immune cells (e.g., dendritic cells, T cells), and subsequently increase the production of important regulatory cytokines, including interleukin-10 (IL-10) and transforming growth factor-beta (TGF-β).[Citation50]
Another finding of the present review was that a greater improvement in inflammatory markers was observed when probiotics were consumed in Asian countries. It could be that additional complexity in developing individualized inflammation-regulating therapies using probiotics is created by the knowledge that different persons have unique endo-types of commensal microbiota as well as the recognition that numerous factors, including geography, can cause lasting shifts in commensal microbial ecology.[Citation51]
To our knowledge, the present study is the initial work to clarify the effect of probiotic consumption on inflammatory markers among athletes using a systematic review and meta-analysis. However, there are some limitations in this study that should be taken into account when interpreting the results. A significant heterogeneity was detected between included studies. While the source of heterogeneity was explored, other factors such as the dosage of probiotics, different exercise programs, different duration and sample size, baseline age of participants, study conditions, and diverse bacteria strains may have caused further heterogeneity.
Conclusion
This systematic review and meta-analysis suggested that probiotic consumption may be beneficial in reducing IFN-γ, IL-6, and TNF-α, but not IL-8 and IL-10. The subgroup analyses of this study suggested that a greater improvement in inflammatory markers may be expected when probiotics are consumed in Asian countries, by male athletes, as single strain capsule or when they are administered for lower than 4 weeks. Further interventions with better methodology are required to explore the effect and possible mechanism of the influence of probiotics on inflammation.
Acknowledgments
The authors have no conflicts of interests. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. H. H and A. A contributed to the conception of research. Z. F, M. N, and H. H searched databases, screened articles, and extracted data. H. H and A. A performed statistical analysis; and all authors contributed to the writing and revision of the manuscript.
References
- Pedersen, B. K.; Hoffman-Goetz, L. Exercise and the Immune System: Regulation, Integration, and Adaptation. Physiol. Rev. 2000, 80(3), 1055–1081. DOI: 10.1152/physrev.2000.80.3.1055.
- Deaton, C. M.; Marlin, D. J. Exercise-associated Oxidative Stress. Clini. Tech. Equine Pract. 2003, 2(3), 278–291. DOI: 10.1053/S1534-7516(03)00070-2.
- Halliwell, B.; Gutteridge, J. M. Free Radicals in Biology and Medicine; Oxford University Press: USA, 2015.
- Mazani, M.; Nemati, A.; Baghi, A. N.; Amani, M.; Haedari, K.; Alipanah-Mogadam, R. The Effect of Probiotic Yoghurt Consumption on Oxidative Stress and Inflammatory Factors in Young Females after Exhaustive Exercise. J. Pak. Med. Assoc. 2018, 68(12), 1748–1754.
- Jäger, R.; Purpura, M.; Stone, J. D.; Turner, S. M.; Anzalone, A. J.; Eimerbrink, M. J.; Pane, M.; Amoruso, A.; Rowlands, D.; Oliver, J.; et al. Probiotic Streptococcus Thermophilus FP4 and Bifidobacterium Breve BR03 Supplementation Attenuates Performance and Range-of-motion Decrements following Muscle Damaging Exercise. Nutrients. 2016, 8(10), 10.
- Koivisto, A. E.; Olsen, T.; Paur, I.; Paulsen, G.; Bastani, N. E.; Garthe, I.; Raastad, T.; Matthews, J.; Blomhoff, R.; Bøhn, S. K.; et al. Effects of Antioxidant-rich Foods on Altitude-induced Oxidative Stress and Inflammation in Elite Endurance Athletes: A Randomized Controlled Trial. PloS One. 2019, 14(6), e0217895–e.
- Fuller, R.;. Probiotics in Man and Animals. J. App. Bacteriol. 1989, 66(5), 365–378. DOI: 10.1111/j.1365-2672.1989.tb05105.x.
- Vitetta, L.; Coulson, S.; Thomsen, M.; Nguyen, T.; Probiotics, H. S. D–Lactic Acidosis, Oxidative Stress and Strain Specificity. Gut Microbes. 2017, 8(4), 311–322. DOI: 10.1080/19490976.2017.1279379.
- Borzabadi S, Oryan S, Eidi A, Aghadavod E, Kakhaki RD, Tamtaji OR, Taghizadeh M, Asemi Z. The effects of probiotic supplementation on gene expression related to inflammation, insulin and lipid in patients with Parkinsonâ s disease: A randomized, double-blind, placebo-controlled trial. Arch. Iran. Med. 2018, 21(7), 289–295.
- Ratnikova, I.; Gavrilova, N.; Bayakyshova, K.; Turlybaeva, Z. Z.; Kosheleva, L.; Utegenova, N. Antitumor Activity of Probiotic Bacteria and the Bacterial Association in the Tumor Growth Model and in Cell Culture. Biol. Bull. 2018, 45(2), 155–159. DOI: 10.1134/S1062359018020103.
- Khani, A. H.; Jazayeri, S. M. M.; Ebrahimi, E.; Younesi-Melerdi, E.; Farhadi, A. The Bifidobacterim Bifidum (BIB2) Probiotic Increased Immune System Factors in Men Sprint Athletes. Curr. Nutr. Food Sci. 2018, 14(4), 324–328. DOI: 10.2174/1573401313666170725114130.
- Salami, M.; Kouchaki, E.; Asemi, Z.; Tamtaji, O. R. How Probiotic Bacteria Influence the Motor and Mental Behaviors as Well as Immunological and Oxidative Biomarkers in Multiple Sclerosis? A Double Blind Clinical Trial. J. Funct. Foods. 2019, 52, 8–13. DOI: 10.1016/j.jff.2018.10.023.
- Bron, P. A.; Kleerebezem, M.; Brummer, R.-J.; Cani, P. D.; Mercenier, A.; MacDonald, T. T.; Garcia-Ródenas, C. L.; Wells, J. M. Can Probiotics Modulate Human Disease by Impacting Intestinal Barrier Function? Br. J. Nutr. 2017, 117(1), 93–107.
- Verdenelli, M. C.; Ghelfi, F.; Silvi, S.; Orpianesi, C.; Cecchini, C.; Cresci, A. Probiotic Properties of Lactobacillus Rhamnosus and Lactobacillus Paracasei Isolated from Human Faeces. Eur. J. Nutr. 2009, 48(6), 355–363. DOI: 10.1007/s00394-009-0021-2.
- Cox, A. J.; Pyne, D. B.; Saunders, P. U.; Fricker, P. A. Oral Administration of the Probiotic Lactobacillus Fermentum VRI-003 and Mucosal Immunity in Endurance Athletes. Br. J. Sports Med. 2010, 44(4), 222–226. DOI: 10.1136/bjsm.2007.044628.
- Gepner, Y.; Hoffman, J. R.; Shemesh, E.; Stout, J. R.; Church, D. D.; Varanoske, A. N.; Zelicha, H.; Shelef, I.; Chen, Y.; Frankel, H.; et al. Combined Effect of Bacillus Coagulans GBI-30, 6086 and HMB Supplementation on Muscle Integrity and Cytokine Response during Intense Military Training. J. Appl. Physiol. 2017, 123(1), 11–18.
- Gleeson, M.; Bishop, N. C.; Oliveira, M.; Tauler, P. Daily Probiotic’s (Lactobacillus Casei Shirota) Reduction of Infection Incidence in Athletes. Int. J. Sport Nutr. Exerc. Metab. 2011, 21(1), 55–64. DOI: 10.1123/ijsnem.21.1.55.
- Ibrahim, N. S.; Muhamad, A. S.; Ooi, F. K.; Meor-Osman, J.; Chen, C. K. The Effects of Combined Probiotic Ingestion and Circuit Training on Muscular Strength and Power and Cytokine Responses in Young Males. Appl. Physiol. Nutr. Metab = Physiologie Appliquee, Nutrition Et Metabolisme. 2018, 43(2), 180–186. DOI: 10.1139/apnm-2017-0464.
- Lamprecht, M.; Bogner, S.; Schippinger, G.; Steinbauer, K.; Fankhauser, F.; Hallstroem, S.; Schuetz, B.; Greilberger, J. F. Probiotic Supplementation Affects Markers of Intestinal Barrier, Oxidation, and Inflammation in Trained Men; a Randomized, Double-blinded, Placebo-controlled Trial. J. Int. Soc. Sports Nutr. 2012, 9(1), 45.
- Michalickova, D.; Minic, R.; Dikic, N.; Andjelkovic, M.; Kostic-Vucicevic, M.; Stojmenovic, T.; Nikolic, I.; Djordjevic, B. Lactobacillus Helveticus Lafti L10 Supplementation Reduces Respiratory Infection Duration in a Cohort of Elite Athletes: A Randomized, Double-blind, Placebo-controlled Trial. Appl. Physiol. Nutr. Metab = Physiologie Appliquee, Nutrition Et Metabolisme. 2016, 41(7), 782–789.
- Pugh, J. N.; Sparks, A. S.; Doran, D. A.; Fleming, S. C.; Langan-Evans, C.; Kirk, B.; Fearn, R.; Morton, J. P.; Close, G. L. Four Weeks of Probiotic Supplementation Reduces GI Symptoms during a Marathon Race. Eur. J. Appl. Physiol. 2019, 119(7), 1491–1501.
- Shing, C. M.; Peake, J. M.; Lim, C. L.; Briskey, D.; Walsh, N. P.; Fortes, M. B.; Ahuja, K. D. K.; Vitetta, L. Effects of Probiotics Supplementation on Gastrointestinal Permeability, Inflammation and Exercise Performance in the Heat. Eur. J. Appl. Physiol. 2014, 114(1), 93–103.
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L. A. Preferred Reporting Items for Systematic Review and Meta-analysis Protocols (PRISMA-P) 2015 Statement. Syst. Rev. 2015, 4(1), 1.
- Arab, A.; Mehrabani, S.; Moradi, S.; Amani, R. The Association between Diet and Mood: A Systematic Review of Current Literature. Psychiatry Res. 2019, 271, 428–437. DOI: 10.1016/j.psychres.2018.12.014.
- Follmann, D.; Elliott, P.; Suh, I.; Cutler, J. Variance Imputation for Overviews of Clinical Trials with Continuous Response. J. Clin. Epidemiol. 1992, 45(7), 769–773. DOI: 10.1016/0895-4356(92)90054-Q.
- Cumpston M, Li T, Page MJ, Chandler J, Welch VA, Higgins JP, Thomas J. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev. 2019 Oct 3;10:ED000142.
- Townsend JR, Bender D, Vantrease WC, Sapp PA, Toy AM, Woods CA, Johnson KD. Effects of probiotic (Bacillus subtilis DE111) supplementation on immune function, hormonal status, and physical performance in division I baseball players. Sports. 2018, 6(3), 70.
- Brennan, C. J.; Axelrod, C. L.; Paul, D.; Hull, M.; Kirwan, J. P. Effects of A Novel Probiotic on Exercise-Induced Gut Permeability and Microbiota in Endurance Athletes. Med. Sci. Sports Exercise. 2018, 50(5), 840. DOI: 10.1249/01.mss.0000538764.55259.9c.
- Huang, W. C.; Wei, C. C.; Huang, C. C.; Chen, W. L.; Huang, H. Y. The Beneficial Effects of Lactobacillus Plantarum PS128 on High-Intensity, Exercise-Induced Oxidative Stress, Inflammation, and Performance in Triathletes. Nutrients. 2019, 11, 2. DOI: 10.3390/nu11020353.
- Kazemi, A.; Soltani, S.; Ghorabi, S.; Keshtkar, A.; Daneshzad, E.; Nasri, F.; Mazloomi, S. M. Effect of Probiotic and Synbiotic Supplementation on Inflammatory Markers in Health and Disease Status: A Systematic Review and Meta-analysis of Clinical Trials. Clin. Nutr. 2020, 39(3), 789–819.
- Milajerdi, A.; Mousavi, S. M.; Sadeghi, A.; Salari-Moghaddam, A.; Parohan, M.; Larijani, B.; Esmaillzadeh, A. The Effect of Probiotics on Inflammatory Biomarkers: A Meta-analysis of Randomized Clinical Trials. Eur. J. Nutr. 2020, 59(2), 633–649.
- Maia, L. P.; YLdAS, L.; Do Prado RL, Dos Santos Santinoni C, Marsicano JA. Effects of Probiotic Therapy on Serum Inflammatory Markers: A Systematic Review and Meta-analysis. J. Funct. Foods. 2019, 54, 466–478.
- O’Sullivan, O.; Cronin, O.; Clarke, S. F.; Murphy, E. F.; Molloy, M. G.; Shanahan, F.; Cotter, P. D. Exercise and the Microbiota. Gut Microbes. 2015, 6(2), 131–136.
- Allen, J. M.; Mailing, L. J.; Niemiro, G. M.; Moore, R.; Cook, M. D.; White, B. A.; HOLSCHER, H. D.; WOODS, J. A. Exercise Alters Gut Microbiota Composition and Function in Lean and Obese Humans. Med. Sci. Sports Exerc. 2018, 50(4), 747–757.
- Bressa, C.; Bailén-Andrino, M.; Pérez-Santiago, J.; González-Soltero, R.; Pérez, M.; Montalvo-Lominchar, M. G.; Maté-Muñoz, J. L.; Domínguez, R.; Moreno, D.; Larrosa, M.; et al. Differences in Gut Microbiota Profile between Women with Active Lifestyle and Sedentary Women. PLoS One. 2017, 12(2), e0171352.
- Cook, M. D.; Allen, J. M.; Pence, B. D.; Wallig, M. A.; Gaskins, H. R.; White, B. A.; Woods, J. A. Exercise and Gut Immune Function: Evidence of Alterations in Colon Immune Cell Homeostasis and Microbiome Characteristics with Exercise Training. Immunol. Cell Biol. 2016, 94(2), 158–163.
- Sallam, N.; Laher, I. Exercise Modulates Oxidative Stress and Inflammation in Aging and Cardiovascular Diseases. Oxid. Med. Cell. Longev. 2016, 2016, 1–32. DOI: 10.1155/2016/7239639.
- Gupta, A.; Chauhan, N. R.; Chowdhury, D.; Singh, A.; Meena, R. C.; Chakrabarti, A.; Singh, S. B. Heat Stress Modulated Gastrointestinal Barrier Dysfunction: Role of Tight Junctions and Heat Shock Proteins. Scand. J. Gastroenterol. 2017, 52(12), 1315–1319.
- Philippou, A.; Maridaki, M.; Psarros, C.; Koutsilieris, M. Systemic Responses of Inflammation-Related Factors following Eccentric Exercise in Humans. Am. J. Sports Sci. 2018, 6(2), 32–37. DOI: 10.11648/j.ajss.20180602.11.
- Mohr, A. E.; Basile, A. J.; Meli’sa, S. C.; Sweazea, K. L.; Carpenter, K. C. Probiotic Supplementation Has A Limited Effect on Circulating Immune and Inflammatory Markers in Healthy Adults: A Systematic Review of Randomized Controlled Trials. J. Acad. Nutr. Diet. 2020, 120(4), 548–564. DOI: 10.1016/j.jand.2019.08.018.
- Fischer, C. P.;. Interleukin-6 in Acute Exercise and Training: What Is the Biological Relevance. Exercise Immunol. Rev. 2006, 12(6–33), 41.
- Welc, S. S.; Clanton, T. L. The Regulation of Interleukin‐6 Implicates Skeletal Muscle as an Integrative Stress Sensor and Endocrine Organ. Exp. Physiol. 2013, 98(2), 359–371. DOI: 10.1113/expphysiol.2012.068189.
- Gleeson, M.; Bishop, N. C.; Stensel, D. J.; Lindley, M. R.; Mastana, S. S.; Nimmo, M. A. The Anti-inflammatory Effects of Exercise: Mechanisms and Implications for the Prevention and Treatment of Disease. Nat. Rev. Immunol. 2011, 11(9), 607–615. DOI: 10.1038/nri3041.
- Paulsen, G.; Ramer Mikkelsen, U.; Raastad, T.; Peake, J. M. Leucocytes, Cytokines and Satellite Cells: What Role Do They Play in Muscle Damage and Regeneration following Eccentric Exercise? Exercise Immunol. Rev. 2012, 18, 42–97.
- Tidball, J. G.; Villalta, S. A. Regulatory Interactions between Muscle and the Immune System during Muscle Regeneration. Am. J. Physiol Reg Integr. Comp. Physiol. 2010, 298(5), R1173–R87. DOI: 10.1152/ajpregu.00735.2009.
- Donato, K. A.; Gareau, M. G.; Wang, Y. J. J.; Sherman, P. M. Lactobacillus Rhamnosus GG Attenuates interferon-γ and Tumour Necrosis factor-α-induced Barrier Dysfunction and Pro-inflammatory Signalling. Microbiology. 2010, 156(11), 3288–3297. DOI: 10.1099/mic.0.040139-0.
- Heyman, M.; Terpend, K.; Ménard, S. Effects of Specific Lactic Acid Bacteria on the Intestinal Permeability to Macromolecules and the Inflammatory Condition. Acta Pædiatrica. 2005, 94, 34–36. DOI: 10.1111/j.1651-2227.2005.tb02153.x.
- Vinolo, M. A.; Rodrigues, H. G.; Nachbar, R. T.; Curi, R. Regulation of Inflammation by Short Chain Fatty Acids. Nutrients. 2011, 3(10), 858–876. DOI: 10.3390/nu3100858.
- Schlee, M.; Harder, J.; Köten, B.; Stange, E.; Wehkamp, J.; Fellermann, K. Probiotic Lactobacilli and VSL# 3 Induce Enterocyte β‐defensin 2. Clin. Exp. Immunol. 2008, 151(3), 528–535. DOI: 10.1111/j.1365-2249.2007.03587.x.
- Thomas, C. M.; Versalovic, J. Probiotics-host Communication: Modulation of Signaling Pathways in the Intestine. Gut Microbes. 2010, 1(3), 148–163. DOI: 10.4161/gmic.1.3.11712.
- Lescheid, D. W.;. Probiotics as Regulators of Inflammation: A Review. Funct. Foods Health Dis. 2014, 4(7), 299–311. DOI: 10.31989/ffhd.v4i7.2.