ABSTRACT
This study aims to evaluate the effects of different softening methods on the texture and quality of abalones (Haliotis discus hannai × Diversicolor diversicolor). We monitored the hardness, meat weight, pH, size, and appearance from the sterilized abalones pre-treated with alkaline soaking and enzymatic injection/soaking, with either vacuum orbital shaking or ultrasonic processing. The hardness of the abalones was reduced by alkaline and enzymatic treatments, and combined vacuum orbital shaking or ultrasound treatment and this could increase their tendering effects. The softest abalone was obtained by bromelain comparing to papain injection, and the TVBN measurements indicated that the abalones remained in a fresh state under all treatments. Both enzymatic treatments showed a dose-dependent effects on hardness and pH of sterilized abalones. The abalones pre-treated with injecting enzyme exhibited significant softening effect than soaking method. Histological observation indicated that severe fracturing and non-uniform large spaces between muscle fibers were observed in the abalones injected with bromelain, and the abalones subjected to NaHCO3 soaking showed distorted muscle fibers and small uniform spaces. SDS-PAGE further indicated the decrease of paramyosin in the abalones injected with bromelain. Sensory evaluation using senior panelists without chewing difficulties indicated that NaHCO3 soaking before sterilization exhibited the highest overall acceptability, possibly because this method could slightly soften abalone foot while retaining its palatability. This study should benefit the food industry in terms of developing and processing food for senior citizens.
Introduction
Abalone is a luxury food ingredient in Chinese cuisine traditionally reserved for weddings and celebrated meals. It has high nutritional value and it contains high amounts of protein, fat, glycogen, vitamins and many trace elements.[Citation1,Citation2] The nutritional value and high digestibility of water-soluble fraction and abundant potassium content from abalone muscle can be claimed as a high-quality food source.[Citation3] Abalone contains a high amount of myofibrillar proteins (tropomyosin and paramyosin), [Citation4] making its meat hard to chew and tough compared to that of fish.[Citation5] This has made it difficult to adapt abalone into a softer desirable delicacy for senior. Chewing capacity in older adults may influence nutritional status by altering food choices, and it has been reported dietary changes owing to chewing problems might increase the risks for metabolic syndrome[Citation6] and cardiovascular diseases.[Citation7] Foods prepared by softening methods do not maintain its original appearance, and they are not appetizing to patients with impaired mastication or their families.[Citation8]
Meat can be tenderized by various treatments. Sodium hydrogen carbonate (NaHCO3) has been used as a tenderizer for increasing meat tenderness and juiciness for decades.[Citation9] The sodium hydrogen carbonate treated pork ham exhibited a looser tissue structure and induced the formation of a number of small cavities.[Citation10] Bromelain and papain have been used as collagenous and myofibrillar protein tenderizers and they are generally recognized as safe.[Citation11] The use of ultrasonic treatment is a low-polluting, inexpensive, and efficient process of extracting and restructuring muscle tissue. Appropriate intensity and frequency levels of ultrasonic treatment has been shown it can increase enzyme activity[Citation12] and shorten the time of alkaline soaking necessary for physical weakening muscle tissue[Citation13] due to favorable structural alternation in protein molecules but do not change its conformational integrity of the meat.
Senior increased protein consume could improve the amounts of dietary nutrition intake of energy and protein, limit and help to treat the age-related physiological changes accompanied declining muscle strength, function, and mass in combination with exercise.[Citation14] Increased hydration and decreased hardness protein brought about by soaking beef and pork in increasing concentrations of sodium bicarbonate were highly correlated with senior’s ease in consuming the meat product.[Citation15] Chicken breast showed enhanced texture and palatability by soaking in 0.1–0.4 M NaHCO3.[Citation9] However, there are limited ability of softening methods to tenderized abalone, and the time and effort involved in the preparation are considered problematic.
The objectives of this study was to understand the hardness and quality of abalone flesh processed by different softening treatments. The meat of this mollusk treated with proteases injecting or soaking, and alkaline solution with or without vacuum orbital shaking or ultrasonic processing was compared to untreated control for the development of tenderized ready-to eat abalone for elderly people. Hardness, total volatile basic nitrogen (TVBN), tissue structure, pH, SDS-PAGE protein profiles, and sensory evaluation of abalone by seniors were recorded and analyzed in order to develop better strategies for softening methods and conditions have not been evaluated for abalone muscle before.
Materials
Raw materials and controls
Cultured hybride abalones (Haliotis discus hannai × Diversicolor diversicolor) was purchased and collected from October 2018 to January 2019 in Gonglian district of New Taipei City, Taiwan. The cultured abalones were starved for 1 day before collection. Raw abalones were blanched in boiling water for 1 min and then frozen and kept in freezer at −18°C. The frozen abalone was placed in running tap water until they had completely thawed, whereupon their shell, and viscera were removed. The foot length of abalone varies in size from 3.77 to 4.47 cm, with a foot weight of 6.27 to 8.42 g and an average foot content of 39.1%. All chemicals used in this study were of analytical grade. The foot of abalones was soaked in a polyethylene bag in either 2% NaHCO3 or 2% NaOH solution, or in deionized water as a control, for 4 days at 4°C (1:1 ratio of sample weight in g to solution volume in ml). After soaking, foots were washed with tap water until the surface of the muscle was no longer slippery.[Citation9] Another set of similarly soaked foots was loaded into an ultrasonic cleaner (DH200H, Yuantuo Technology, Taichung, Taiwan) or subjected to an ultrasonic processor at frequency of 40 KHz at a power of 200 W for 20 and 30 min.[Citation13] Because abalone foot soaked in 2% NaOH had a strong, fishy and alkaline off-odor, it could not be used in the sensory evaluation test described below; accordingly, most of the other trials described below only used the 2% NaHCO3 samples.
Experimental abalone samples
Abalone foots prepared as above were soaked in 0.1%, 0.25%, 0.5%, and 1.0% papain and bromelain solutions (1:1 ratio of enzyme solution volume in ml to abalone foot mass in gr) and transferred into a 4°C refrigerator for 6, 8 and 10 h. Orbital shaking in vacuum was carried out using a vacuum desiccator (P14-1000240, Hondwen Co. Ltd, Taipei, Taiwan) with a polycarbonate top and polypropylene bottom enclosing a cylindrical chamber 240 mm wide and 311 mm deep, placed atop an MS orbital shaker (MS-NOR 30, Major Science, Saratoga, CA, USA). Shaken at 120 rpm and unshaken (control) samples were kept for 10 h at 4°C and then immersed in boiling water for 1 min .[Citation16] Other abalone foots that had been soaked in a proportionally 4 times greater volume of the same enzyme solutions were loaded into an ultrasonic processor (UP500, ChromTech, New Taipei City, Taiwan) and subjected to a frequency of 40 KHz at a power of 250 W for 30 min at 4°C.[Citation13] The sonicated abalone foots were then immersed in boiling water for 1 min to inactivate all enzymes. Other thawed abalone foots were injected with the two above-mentioned enzymes at a dosage of 2 ml enzyme solution volume of 0.1%, 0.25%, 0.5% or 1.0% papain or bromelain solution per 10 g of foot (eight experimental treatments altogether), the solution being delivered perpendicular to the direction of foot muscle at sites approximately 0.5 cm apart. Injected and control samples were kept for 24 hrs at 4°C .[Citation17] There are four concentrations of each enzyme treated abalone for 1 min boiling water treatment to inactivate all enzymes. For mixed treatments using both enzymes and mechanical stimuli, abalone foots were soaked in 1.0% papain or bromelain solutions in a beaker at a ratio of 1 g of abalone foot per 1 ml of enzyme solution and shaken as above for 6, 8, or 10 h, then immersed in boiling water for 1 min to inactivate the enzyme.[Citation16] Other samples of abalone foot were similarly soaked in enzyme solutions and then loaded into the above-mentioned UP500 ultrasonic processor and subjected to a frequency of 40 KHz at a power of 250 W for 30 min. Again, enzymes were deactivated by immersion of the foot in boiling water for 1 min.
pH and total volatile basic nitrogen (TVBN)
Abalone foots were homogenized in 10 times their volume of deionized water in a disperser (T18 basic, IKA, Staufen, Germany). The pH of the homogenate was measured with a pH meter (pH 510, Thermo Eutech, Singapore) .[Citation18] Microdiffusion analysis of volatile nitrogen[Citation19] was done using 1 ml of trichloroacetic acid extract of abalone foot in a Conway microdiffusion dish with 1 ml saturated K2CO3 as the releasing agent and 1 ml 10% H3BO3 as the trapping agent with an indicator dye (methyl red). After diffusion for 90 min at 37°C, the trapping agent was titrated to the original methyl red color with 0.1 N HCl. The volatile nitrogen was calculated following the method of Cobb et al.[Citation19] All analyses were conducted in triplicate.
Light microscopy of abalone foot muscle tissue
A number of 2–3 mm thick foots of abalone subjected to different softening techniques as outlined above were fixed with 10% formalin at 4°C for 24 h. Dehydration and embedding were performed according to Hu et al..[Citation13] Sample preparative procedures for abalone foot muscle tissue with a compound photomicroscope (Olympus BX 53, Olympus, Melville, NY, USA) at 400x equipped with a digital camera (Olympus BX 53, Olympus, Melville, New York, USA) following the method of Bello et al. .[Citation20]
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE)
Abalone foot muscle protein was extracted according to Huang et al.[Citation21] Chopped abalone foot muscle (0.15 g) was homogenized in 3 ml of 0.5 M Tris-HCl at pH 6.8 with 0.2 g β-mercaptoethanol, 0.2 g sodium dodecylsulfate, and 0.024 g urea (Merck, Hoenebrunn, Germany) and centrifuged at 15,000 × g for 30 sec followed by continuous shaking for 24 h at 100 rpm in 4°C. Insoluble material in the homogenates was removed by centrifugation at 12,000 rpm for 10 min at 4°C. The supernatant was analyzed for myofibrillar protein degradation by SDS-PAGE. An aliquot of 10 μl from each treated sample was subjected to SDS-PAGE under reducing conditions. After electrophoresis (Gene Power Supply SPS 200/400 apparatus, Pantech, Taipei, Taiwan), the proteins in the gels were stained with Coomassie Brilliant Blue R-250 .[Citation21]
Instrumental texture analysis
Hardness of experimental and control abalone foot samples was measured using a texture analyzer (TA-XT2, Stable Micro Systems, Godalming, UK) equipped with a 10 mm diameter cylinder probe (P/0.5) (pretest speed: 2.0 mm/sec; test speed: 2.0 mm/sec; posttest speed: 2.0 mm/sec; strain: 50%; trigger force: 10 g). The compressive force rises with sample hardness. Tests were performed with three replicates of each sample centered under the probe.[Citation5]
Sensory evaluation
Sensory evaluation of pouch samples of 250 g of sterilized (heated at 121°C for 20 min) abalone foot was carried out to obtain subjective information on appearance, flavor, hardness, fishy odor and overall acceptability from untrained panelists on the basis of preference tests and an index of overall acceptability. Before serving, the pouches were reheated by immersion in boiling water for 5 min. The 49 panelists consisted of 13 male and 36 female students and staffs of the General Education Center of National Taiwan Ocean University ranging in age from 31 to 82 (mean age 66), 21 of them being over 70. Three experimental and control samples representing all of the treatments described above were coded with three random digits and supplied to the panelists in random order. Panelists were instructed to evaluate each of the above-mentioned attributes by ranking it from “1 = extremely dislike” to “9 = extremely like”.[Citation22]
Statistical analysis
The data were analyzed with the SPSS statistics package for Windows, version 12 (SPSS Inc., Chicago, IL, USA). One-way analysis of variance (ANOVA) and Duncan’s multiple range tests in order to detect differences between treatments at a 5% significance level (p < .05).
Results and discussions
Effects of alkali soaking and ultrasonic cleaning on hardness, meat weight and pH of abalone muscle
Hardness is an important texture characteristic of the consumer acceptance of ready-to eat abalone products.[Citation5] As shown in , the hardness and pH of the samples were monitored before (raw) and after different treatments (after sterilization). The control group (no treatment) showed an increase hardness after sterilization. Gao et al.[Citation23] reported the denaturation temperatures of actin, collagen and myosin in abalone were 68.3°C, 57.5°C, and 51.3°C, respectively; however, further shrinkage and denaturation of the myofibrils and gelatination of the collagens over 70°C might increase the hardness of abalone. Zhu et al.[Citation5] also reported the hardness of the edible abalone meat showed a high correlation with the abundance of the collagens, and protein denaturation caused by the heat of sterilization increased the proportion of contracted muscle fiber in the tissue and decreased its water-holding capacity, resulting in a harder texture. Treatments of soaking abalones in NaHCO3 solution (SB group) and NaOH solution (SH group) reduced their hardness, and the samples soaked in 2% NaOH was softer than the ones soaked in 2% NaHCO3 and control (p < .05) (). The pH of abalone foot soaked in 2% NaOH (9.93) was significantly higher than that of samples soaked in 2% NaHCO3 (9.01) or a control sample (7.27). The isoelectric point (PI) of meat is in the pH of 5.0–5.4, and during which the meat has the lowest the water-holding capacity .[Citation9]
Table 1. Hardness, meat weight and pH value of abalones soaked in alkaline solution with ultrasound treatment
The volume available within the myofibrils for holding water increased with higher pH because most of the acidic side-chain groups are negatively charged and are thus expelled from the fibers. Myofibrils’ capacity for holding water also increased with exposure to NaHCO3 due to a charge imbalance. Any increase in pH away from the isoelectric point will increase the water-holding capacity of the myofibrils and make the abalone foot muscle not only more tender, but also juicier. However, the abalone foot protein dissolves into 2% NaOH solution after 4 days soaking at 4°C and sterilization. Weight loss of sterilized abalone foot in sodium bicarbonate treatment is less than that of sodium hydroxide treatment. It was caused by water loss, drip of gelatin converted from collagen, squeeze out of water-soluble components, [Citation5] and the pH of NaOH treated abalone foot was away from isoelectric point and increased the water-holding capacity of protein and dissolved the protein into water causing more weight loss after sterilization. Furthermore, a significant softening effect was also found when alkali soaking was combined with ultrasonic cleaning as compared with the control and the abalone treated only with alkaline soaking. The SBU20 and SBU30 groups (NaHCO3 soaking + ultrasound treatment for 20 and 30 min, respectively) showed reduced hardness of the abalones as compared with the SBC group (NaHCO3 soaking for 30 min), and similar results were observed in the SHU20 and SHU30 groups (NaOH soaking + ultrasound treatment for 20 and 30 min, respectively) as compared with the SHC group (NaOH soaking for 30 min). Our data indicated that ultrasonic cleaning is a time-saving adjunct to the process of softening abalone muscle in alkali soaking. A previous reported indicated that ultrasonic treatment could tenderize squid muscle and concluded that a significant decrease in hardness was associated with damage to squid mantle muscle fibers[Citation13]; In addition, ultrasonic treatment could degrade large-Dalton muscle protein components including collagen, but did not alter the pH of squid muscle.[Citation13]
As for the meat weight, alkaline treatment except the SB group (NaOH soaking for 4 days) did not seem to have a significant impact on the change of the meat weight after sterilization, and all the tested groups showed a rate of change in the range of 43.6% to 62.37%. The weight loss in the samples treated with alkaline soaking was attributed to increase gelatination and solubilization of the collagen and partial rupture and shrinkage of abalone myofibrils.
Effects of Enzymatic Treatment and Ultrasonic Cleaning on Hardness, Meat Weight, and pH of the Abalone Muscle
The effects of enzymatic treatments on hardness abalone were evaluated, as shown in . The pre-treatment of injection of proteolytic enzymes could decrease the hardness of abalone foot muscle in a concentration manner. The abalones pre-treated with injection of 1.00% papain (P 1.00%) could reduce the hardness by 46.69% after sterilization, and the abalones injected with 1% bromelain (B 1.00%) exhibited the maximum reduced hardness of 89.42% after sterilization (). Bromelain injection rather than papain injection showed a better effect to decrease the hardness of the abalones, indicating that hydrolysis of myofibrillar protein by bromelain will induce a high degree of protein hydrolysis and softness in muscle cells, thus resulting in meat tenderization. Both enzymatic treatments showed a dose-dependent effects on hardness and pH of the pretreated abalones after sterilization. As for the meat weight change of the abalones treated with bromelain and papain injection, the weight loss of the abalone foot injected with both papain and bromelain was slightly higher than the weight loss of the control group (). As for the pH change of the abalones treated with bromelain and papain injection, the former samples seemed to have a lower pH (). A lower pH has been recorded for squid samples treated with papain or bromelain, as compared with the control.[Citation24] This may be due to a release of peptides and free amino acids following exposure of abalone foot muscle to these proteolytic enzymes. Lowered pH values have also been reported in enzyme-treated pork, giant catfish, and chicken.[Citation25]
Table 2. Hardness, meat weight, and pH value of the abalones injected with the enzyme solution
Table 3. Hardness, meat weight, and pH value of the abalones soaked in enzyme solution with vacuum orbital shaking
We further investigated the effects of bromelain enzyme soaking on the hardness of the abalones. As shown in , the lowest hardness of 17.4 N/m2 (decreased by 28.34%) was obtained when abalone foot was soaked in 1.0% bromelain solution and agitated in a vacuum orbital shaker for 10 h at 4°C (V10 group), whereas the VControl (Soaked in 1% bromelain solution without vacuum orbital shaking) failed to decrease the hardness. This results indicated that the combined uses of enzymatic soaking with vacuum orbital shaking could decrease the hardness of abalone, although enzymatic soaking processes exhibited a smaller effect on hardness of abalone foot than enzyme injection, possibly because only the surface of the test foot edge (epipodium) of abalone is affected in the soaking processes (). The effects of combined uses of enzymatic soaking and ultrasound treatment on hardness abalone were also investigated. It is shown that the hardness of abalone was decreased by 16.32% of the UB group (1% bromelain soaking + ultrasound for 30 min), whereas the UControl group exhibited an increased hardness after sterilization (). The cavitation effect of the ultrasonic processor used at 20 KHz on abalone foot muscle injected in 1.0% bromelain (UB) was favorable for enzyme conformational changes, resulting in an enhancement of enzyme activity. At higher ultrasound frequencies, such as 40 KHz at 200 W, bromelain activity might have been inhibited by the violent collapse of too many cavitation bubbles in the enzyme solution and the resulting generation of excessive heat (data not shown).[Citation12] As for the meat weight change of the abalones treated with bromelain soaking along with the vacuum orbital shaking and ultrasound treatment, both the vacuum orbital shaking and ultrasound treatment to be used in combination with bromelain soaking seemed to have no obvious effects on the meat weight changes and pH, as shown in . The softening effects of 2% sodium bicarbonate soaking is less effective comparing to bromelain injection (). The pH of abalone foot after sterilization sodium bicarbonate soaking will increase, nevertheless, the pH of that of bromelain injection will decrease (). Abalone weight loss (%) after sterilization with enzyme treatment is higher than that of 2% sodium bicarbonate soaking ().
Various treatments on the size, appearance and TVBN of the abalones
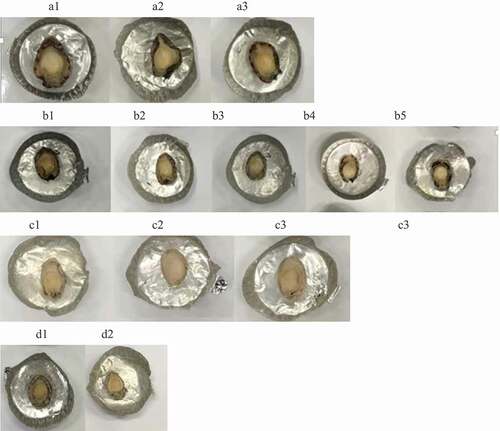
The changes in the length, width, and height in the abalones were monitored in different treatments before sterilization, such as alkaline and enzymatic treatments, and our data indicated that different treatment methods did not seem to cause significant differences in sizes of the abalones (data not shown) and weight of abalone after sterilization (). We also looked into the appearance of the treated abalones, as shown in , and found that the 2% sodium bicarbonate soaking (A1 group in ) with ultrasound treatment (A2 and A3 groups in ), bromelain injection (B2-B5 groups in ) and bromelain soaking with ultrasound (D groups in ) did not have a significant impact on the physical appearances of the treated abalones as compared with the control (B1); in addition, the abalones treated with bromelain soaking with vacuum orbital shaking (C groups in ) seemed to have a lighter color. It was due to the epipodium part (black color meat) of the abalone foot was hydrolyzed during soaking and shaking process.
Figure 1. Appearance of the sterilized abalones pre-treated with various methods. A1, 2% sodium bicarbonate soaking for 30 min; A2, 2% sodium bicarbonate soaking with ultrasound treatment for 20 min; A3, 2% sodium bicarbonate soaking with ultrasound treatment for 30 min; B1, untreated sample, B2, injection with 0.10% bromelain solution; B3, injection with 0.25% bromelain solution; B4, injection with 0.50% bromelain solution; B5, injection with 1.00% bromelain solution; C1, 1% bromelain soaking for 10 h; C2, 1% bromelain soaking with vacuum orbital shaking for 6 h; C3, 1% bromelain soaking with vacuum orbital shaking for 10 h; D1, 1% bromelain soaking for 30 min; D2, 1% bromelain soaking with ultrasound for 30 min
TVBN has been used as an indicator of fish quality or freshness.[Citation26] The TVBN for the abalone foot soaked in 2% NaHCO3 for 4 days, or injected with 1.0% bromelain solution and stored for 24 h at 4°C, or soaked in 1.0% bromelain solution in a vacuum orbital shaker at 4°C for 10 h increased from 1.58 mg/100 g sample (control) to 2.46, 2.16, and 1.69 mg/100 g, respectively. This concluded that all treatments resulted in values for TVBN below 25 mg/100 g, below which showed the freshness of seafood. In an earlier study[Citation24] using thawed neritic squid (Uroteuthis edulis), higher TVBN values (6.73 to 7.87 mg/100 g) were associated with higher volatile nitrogen compounds in squid species than in abalone. These TVBN values are still under fresh condition. Therefore, any of these treatments will keep abalone foot in acceptable fresh condition.
Histological observations by light microscopy
Histological microphotographs of control (raw) and variously softened abalone foot () showed differences. The transversal section of raw abalone foot exhibited muscles with compact fibers of regular shape, uniform size, narrow intervals, and thin diameter. The intracellular material was compact and the cells were in tight contact with each other (), although collagen fibers were not able to be identified under the image. The increase of hardness in untreated sterilized abalone could be attributed to the combined effect of denaturation and shrinkage of compact abalone myofibrils, and gelatination and solubilization of collagen.[Citation5] After injection with 1.0% bromelain, the tissue showed severe fracturing, damage, and non-uniform large spaces between loose broken muscle fibers, and large interfibrillar gap, large holes, and broken fibers were observed (). The abalone foot injected with 1% bromelain was the softest sample produced during this study ( and ), and the significant decrease of hardness might be due to rupture of the intrinsic structure and of the components of abalone including myofibril, sarcoplasmic proteins and connective tissues. The abalone foot soaked in 1.0% bromelain with ultrasound treatment for 30 min was shown in , where uniform spaces between the thicker fibers were observed, and the tissue showed no damage in the form of fractures within muscle fibers. Abalone foot muscle soaked in 1.0% bromelain and subjected to vacuum orbital shaking process for 10 h was shown in , where the muscles were still visible but more spaces and distorted myofibrils.
Figure 2. Histological sections of the abalone muscles by different softening treatments
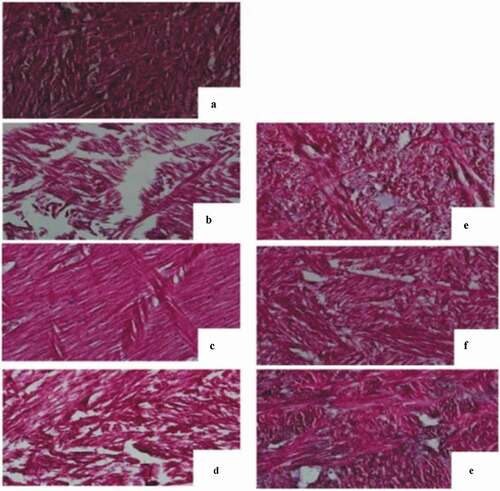
The images concluded that 1% bromelain injection could cause severe fracture damage and non-uniform spaces between the loose broken muscle fibers, which were not observed in the control (2A) and soaking treatments (2 C and 2D). These data were consistent with our previous data on hardness of the abalones (). As compared with the ones treated only with NaHCO3 soaking (), the abalone foot subjected to 2% NaHCO3 treatment combined with ultrasound treatment showed more void spaces and loose, distorted muscle fibers (). It is shown that microstructure of muscle tissue in did not have severe gap and large void structure, as shown in ; however, plenty of small uniform spaces were distributed among fiber muscles ( and ), and many tiny granular materials were found in this compacted tissue (). These densely precipitating particles among the myofibrils were proposed to be disintegration and denaturation of sarcolemmic, sarcoplasmic, and contractile coagulated proteins.[Citation27] The cells were twisted, fibers showing different directions of striation crossed each other, and disordered, denatured myofibrillar protein was observed when 1% bromelain was used ( and D).
SDS-PAGE
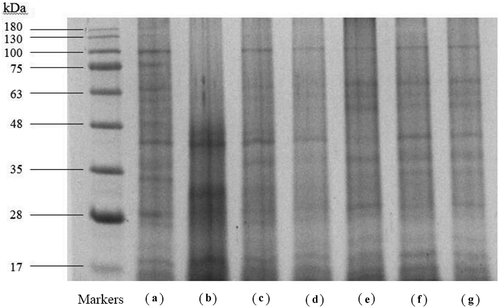
The protein components of untreated and variously softened samples of abalone foot muscle were analyzed by using SDS-PAGE (). The bands of myosin heavy chains (200 kDa) were not observable on this SDS-PAGE, and there was no protein band greater than 180 kDa to be found. These results might indicate no aggregated myofibrillar proteins were formed during different treatments and soluble myosin heavy chains were degraded after sterilization at 121°C for 20 min.[Citation5] The myofibrillar protein paramyosin (97 kDa) and actin (46 kDa) from abalone foot muscle were identifiable and predicted almost in all samples as shown in , although tropomyosin (38 kDa) was not clearly visible. Paramyosin is a specific protein for invertebrates and it is the core of the myosin-containing thick filaments.[Citation28] Intriguingly, decrease of paramyosin and increase of low molecular weight protein bands were observed in the samples injected with 1% bromelain and (band B) (), possibly indicating the abalones softening by injection of bromelain. The disappearance of paramyosin band is consistent with our data on the hardness of the abalones treated with various methods, where bromelain injection reduced the hardness of abalones the most. Suzuki et al.[Citation4] proposed that paramyosin was the second most significant allergen in mollusks. The injection of 1% bromelain not only softened the abalone but also degraded paramyosin for reducing potential allergic responses for elder people.
Figure 3. SDS-PAGE analysis of proteins in abalone minced meat by different softening treatments. Markers, Accupuler RGB prestained protein ladder; (A), control (untreated); (B), the abalones injected with 1% bromelain; (C), the abalones soaked in 1% bromelain with vacuum massage for 10 h. (D), the abalones soaked in 1% bromelain with ultrasound treatment for 30 min. (E), the abalones soaked in 2% sodium bicarbonate for 30 min; (F), the abalones soaked in 2% sodium bicarbonate solution with ultrasound treatment for 30 min; (G), the abalones soaked in 2% sodium hydroxide solution with ultrasound treatment for 30 min
Sensory evaluation
Previous data indicated that the abalone foot injected into 1% bromelain was indeed tenderized to obtain the hardness less than the class 1 (less than 5 × 104 N/m2) of the Japan Care Food Conference. We further used sensory evaluation to measure and analyze people’s responses to the abalones treated with enzymatic and alkaline treatments. As shown in , the best sensory evaluation score belongs to the abalone samples soaked in 2% NaHCO3 with an appearance score of 7.78 ± 1.39, a flavor score of 7.27 ± 1.65, a hardness score of 7.14 ± 2.02, a fishy odor score of 4.82 ± 2.65, and an overall acceptability score of 7.39 ± 1.88. The abalone sample treated with sodium bicarbonate soaking had significantly higher ratings for appearance, flavor, hardness, and overall acceptability than the abalone treated with bromelain injection (), which was the method to give the softest abalones. The abalone samples soaked in 2% NaHCO3 is higher than the control in hardness and overall acceptability, although not statistically, and the reason might be NaHCO3 soaking could avoid increasing hardness after sterilization, as mentioned in . In this study, all panelists did not have chewing or swallowing difficulties; they indicated that the texture of the bromelain treated abalone was mushy, and they preferred chewy abalone over mushy abalone. This might explain that the abalones treated with NaHCO3 soaking obtained the best score in sensory evaluation. However, it would be also valuable to check whether elderly people with difficulties in eating and swallowing would prefer softener abalone.
Table 4. Sensory evaluation of abalones
In our study, the hardness of the abalones muscles could be reduced by pre-treated with alkaline soaking, enzymatic injection, and enzymatic soaking. Both vacuum orbital shaking and ultrasound can increase the tendering effects of alkaline and enzymatic treatments on abalone and reduce the processing time. For the costumers without chewing and swallowing difficulties, the treatment of NaHCO3 soaking before sterilization appeared to be the most feasible process for softening abalone foot while retaining palatability.
Conclusion
We pre-treated the sterilized abalone foot with practical chemical and/or mechanical softening methods, such as alkaline and enzymatic treatments, along with the exposure to an ultrasonic cleaner or ultrasonic processor and orbital shaking in order to evaluate the effects of these treatments on the hardness and overall quality of abalone foot. We monitored the hardness, meat weight, pH, size, appearance and TVBN, and used histological observation and SDS-PAGE to indicate the changes of muscle fiber structure from the abalones subjected to various treatments. The epipodium of the abalone foot in black color muscle could be removed with bromelain soaking with vacuum orbital shaking. The abalones pre-treated with injection of 1% bromelain exhibited the maximum reduced hardness after sterilization. A sensory evaluation with senior panelists having no chewing and swallowing difficulties was also presented for preferring the sodium bicarbonate soaked softening processed abalones. Ultrasonic treatment proved to be a time-saving and inexpensive adjunct to the process of softening abalone muscle while retaining a similar hardness and overall acceptability in alkali solution. All these treatments will keep abalone foot in acceptable fresh condition. Such information may benefit the food industry in terms of developing and processing food for senior citizens.
Acknowledgments
The authors are grateful for the funding provided by the Fisheries of the Republic of China (Taiwan) and the Center of Excellence for the Oceans, National Taiwan Ocean University from The Featured Areas Research Center Program within the framework of the Higher Education Sprout Project by the Ministry of Education (MOE) in Taiwan and the edition of this manuscript by Mark J. Grygier, Center of Excellence for the Oceans at National Taiwan Ocean University.
Additional information
Funding
References
- Dong, J.; Fang, X.; Wang, H.; Zhang, X.; Tao, X. Abalone Muscle Texture Evaluation and Prediction Based on TPA Experiment. J. Food Qual. 2017, 2, 1–9. DOI: https://doi.org/10.1155/2017/2069470.
- Wang, Y.;. Analysis Study of Trace Elements in Abalone and Sea Cucumber. Spectrosc. Spectral Anal. 2009, 29(2), 511–514.
- Shi, L.; Hao, G.; Chen, J.; Ma, S.; Weng, W. Nutritional Evaluation of Japanese Abalone (Haliotis Discus Hannai Ino) Muscle: Mineral Content, Amino Acid Profile and Protein Digestibility. Food Res. Int. 2020, 129, 108876. DOI: https://doi.org/10.1016/j.foodres.2019.108876.
- Suzuki, M.; Kobayashi, Y.; Hiraki, Y.; Nakata, H.; Shiomi, K. Paramyosin of the Disc Abalone Haliotis Discus Discus: Identification as a New Allergen and Cross-reactivity with tropomyosin. Food Chem. 2011, 124(3), 921–926. DOI: https://doi.org/10.1016/j.foodchem.2010.07.020.
- Zhu, B.; Dong, X.; Sun, L.; Xiao, G.; Chen, X.; Murata, Y.; Yu, C. Effect of Thermal Treatment on the Texture and Microstructure of Abalone Muscle (Haliotis Discus). Food Sci. Biotechnol. 2011, 20(6), 1467–1473. DOI: https://doi.org/10.1007/s10068-011-0203-6.
- Friedlander, A. H.; Weinreb, J.; Friedlander, I.; Yagiela, J. A. Metabolic Syndrome: Pathogenesis, Medical Care and Dental Implications. J. Am.Dental Associat. 2007, 138(2), 179–187. DOI: https://doi.org/10.14219/jada.archive.2007.0134.
- Hung, H. C.; Colditz, G.; Joshipura, K. J. The Association between Tooth Loss and the Self-reported Intake of Selected CVD-related Nutrients and Foods among US Women. Comm. Dentist. Oral Epidemiol. 2005, 33(3), 167–173. DOI: https://doi.org/10.1111/j.1600-0528.2005.00200.x.
- Higashiguch, T.;. Novel Diet for Patients with Impared Mastication Evaluated by Consumption Rate, Nutrition Intake, and Questionnaire. Nutrition. 2013, 29(6), 858–864. DOI: https://doi.org/10.1016/j.nut.2012.12.016.
- Tabe, K.; Kim, Y. J.; Ohnuma, S.; Ogoshi, H.; Suzuki, A.; Nishiumi, T. Improvement of Texture and Palatability of Chicken Breast: Effect of High Hydrostatic Pressure and Sodium Hydrogen Carbonate. High Press Res. 2013, 33(2), 348–353. DOI: https://doi.org/10.1080/08957959.2013.780053.
- Kim, Y. J.; Nishiumi, T.; Fujimura, S.; Ogoshi, H.; Suzuki, A. Combined Effects of High Pressure and Sodium Hydrogen Carbonate Treatment on Pork Ham: Improvement of Texture and Palatability. High Pressure Res. 2013, 33(2), 354–36. DOI: https://doi.org/10.1080/08957959.2013.783029.
- Sullivan, G. A.; Calkins, C. Application of Exogenous Endogenous Enzymes to Beef Muscle of High- and Low-connective Tissue. Meat Sci. 2010, 85(4), 730–734. DOI: https://doi.org/10.1016/j.meatsci.2010.03.033.
- Nadar, S. S.; Rathod, V. K. Ultrasound Assisted Intensification of Enzyme Activity and Its Properties; a Mini-review. World J. Microbiol. Biotechnol. 2017, 33(170), 1–12. DOI: https://doi.org/10.1007/s11274-017-2322-6.
- Hu, Y.; Yu, H.; Dong, K.; Yang, S.; Ye, X.; Chen, S. Analysis of the Tenderization Jumbo Squid (Dosidicus Gigas) Meat by Ultrasonic Treatment Using Response Surface Methodology. Food Chem. 2014, 160, 219–222. DOI: https://doi.org/10.1016/j.foodchem.2014.01.085.
- Boirie, Y.;. Physiopathological Mechanism of Sarcopenia. J. Nutrit. Health Aging. 2009, 13(8), 717–723. DOI: https://doi.org/10.1007/s12603-009-0203-x.
- Takahashi, T.; Saitoh, A.; Kawano, A.; Asaga, K.; Wada, K.; Ogoshi, H. Influence of Tenderizing by Sodium Hydrogen Carbonate Soaking on the Hardness and Sensory Evaluation of Beef and Pork. J. Home Econ. Japan. 2002, 53, 347–354.
- Gokoglu, N.; Topuz, O. K.; Gokoglu, M.; Tokay, F. G. Characterization of Protein Functionality and Texture of Tumbled Squid, Octopus and Cuttlefish Muscles. J. Food Meas. Charact. 2017, 11(4), 1699–1705. DOI: https://doi.org/10.1007/s11694-017-9550-1.
- Eom, S. H.; Lee, S. H.; Chun, Y. G.; Kim, B. K.; Park, D. J. Texture Softening of Beef and Chicken by Enzyme Injection Process. Korean J. Food Sci. Animal Resour. 2015, 35(4), 486–493. DOI: https://doi.org/10.5851/kosfa.2015.35.4.486.
- Cruz-romero, M.; Kelly, A. L.; Kerry, J. P. Effects of High-pressure and Heat Treatments on Physical and Biochemical Characteristics of Oysters (Crassostrea Gigas). Innovative Food Sci. Emerging Technol. 2007, 8(1), 30–38. DOI: https://doi.org/10.1016/j.ifset.2006.05.002.
- Cobb, B. F.; Alaniz, I.; Thompson, C. A. Biochemical and Microbial Studies on Shrimp: Volatile Nitrogen and Amino Nitrogen Analysis. J. Food Sci. 1973, 38(3), 431–436. DOI: https://doi.org/10.1111/j.1365-2621.1973.tb01447.x.
- Bello, R. A.; Luft, J. H.; Pigott, G. M. Improved Histological Procedure for Microscopic Demonstration of Related Changes in Fish Muscle Tissue Structure during Holding and Freezing. J. Food Sci. 1981, 46(3), 733–737. DOI: https://doi.org/10.1111/j.1365-2621.1981.tb15337.x.
- Huang, B. B.; Lin, H. C.; Chang, Y. W. Analysis of Proteins and Potential Bioactive Peptides from Tilapia (Oreochromis Spp.) Processing Co-products Using Proteomic Techniques Coupled with BIOPEP Database. J. Funct. Foods. 2015, 19, 629–640. DOI: https://doi.org/10.1016/j.jff.2015.09.065.
- Jouki, M.; Yazdi, F. T.; Mortazavi, S. A.; Koocheki, A.; Khazaei, N. Effect of Quince Seed Mucilage Edible Films Incorporated with Oregano or Thyme Essential Oil on Shelflife Extension of Refrigerated Rainbow Trout Fillets. Int. J. Food Microbiol. 2014, 174, 88–97. DOI: https://doi.org/10.1016/j.ijfoodmicro.2014.01.001.
- Gao, X.; Ogawa, H.; Tashiro, Y.; Iso, N. Rheological Properties and Structural Changes in Raw and Cooked Abalone Meat. Fish. Sci. 2001, 67(2), 314–320. DOI: https://doi.org/10.1046/j.1444-2906.2001.00232.x.
- Grygier, M. J.; Fan, Y. W.; Sung, W. C. Effects of Different Softening Processes on the Hardness and Quality of Thawed Neritic Squid (Uroteuthis Edulis) Muscle. Processes. 2020, 8(2), 135. DOI: https://doi.org/10.3390/pr8020135.
- Rawdkuen, S.; Benjakul, S. Physicochemical Properties and Tenderness of Meat Samples Using Proteolytic Extract for Calotropis Procera Latex. Afr. J. Biotechnol. 2012, 11(76), 14088–14095.
- Vega-Gálvez, A.; Miranda, M.; Clavería, R.; Quispe, I.; Vergara, J.; Uribe, E.; Di Scala, K. Effect of Air Temperature on Drying Kinetics and Quality Characteristics of Osmo-treated Jumbo Squid (Dosidicus Gigas). LWT-Food Sci. Technol. 2011, 44(1), 16–23. DOI: https://doi.org/10.1016/j.lwt.2010.06.012.
- Gao, X.; Tashiro, Y.; Ogawa, H. Rheological Properties and Structural Changes in Steamed and Boiled Abalone Meat. Fish. Sci. 2002, 64, 643–647.
- Watabe, S.; Hartshorne, D. J. Paramyosin and the Catch Mechanism. Comp. Biochem. Physiol. B. 1990, 96(4), 639–646. DOI: https://doi.org/10.1016/0305-0491(90)90207-A.
