ABSTRACT
Ethylene plays an important role in kiwifruit ripening. However, it is unclear whether there are differences in ethylene production in different parts of kiwifruit during postharvest ripening. Kiwifruit were treated with 0.5 μL L−1 1-MCP for 12 h at 20°C and stored at 20 ± 1°C. The results showed the firmness, soluble sugar, total soluble solids (TSS), and AcACS1 and AcETR2 gene expressions were the highest in the core, and starch, AcACO2, and AcACO3 gene expressions were the highest in the seedless pulp at harvest. Starch content and firmness decreased significantly, and soluble sugar content, TSS, AcACO2, and AcETR2 gene expressions increased significantly in all parts during postharvest ripening. On the other hand, 1-MCP can significantly inhibit these changes in the fruits during ripening. The respiration rate and AcACS1 gene expression increased first and then reduced in all parts and were suppressed by 1-MCP. Ethylene was detected in the core and seeded pulp at 3 and 9 d of storage, respectively, but not in the seedless pulp. Overall, the results suggested there are differences in softening, ethylene production, and related gene expression in different parts of kiwifruit at harvest and during postharvest ripening, and 1-MCP inhibited softening and ethylene production of kiwifruit during postharvest ripening.
Introduction
Kiwifruit is popular because of its nutritional composition and antioxidant properties, and it is a typical climacteric fruit. Exogenous ethylene or chilling injury can induce endogenous ethylene synthesis in kiwifruit, strengthened by supplementing with continuous treatment with methyl jasmonate, since methyl jasmonate treatment also promotes ethylene synthesis.[Citation1–4] Ethylene plays an important role in the ripening of kiwifruit. Ethylene or propylene treatment before or after harvest can rapidly induce the increase of endogenous ethylene by up-regulating AcACS1, AcACO1, AcACO2, AcSAM1, and AcSAM2, respiration, and soluble solids concentration (SSC), and the decrease in firmness, and fruit softening.[Citation5–8] On the one hand, low concentration ethylene (0.01 ml L−1) promotes fruit softening and shortens shelf life during commercial storage.[Citation9,Citation10] However, ASA, H,2 and 1-MCP treatments inhibit fruit ripening mainly by interfering directly with ethylene biosynthesis and perception.[Citation11,Citation12] On the other hand, ethylene is used to regulate fruit ripening for commercial purposes and maintain good quality.[Citation13,Citation14] Adequate postharvest ripening could preserve the shelf quality after transferring from cold storage by reducing the ethylene release rate, increasing the antioxidant enzymatic activity, and maintaining the reactive oxygen metabolism balance.[Citation15]
Early work suggested that fruit softening, SSC increase, and decrease in titratable acidity (TA) occurs faster in fruit at 5°C, 10°C, or 15°C compared with 0°C and 20°C in the absence of any detectable ethylene, and up–regulates AdACO1, AdACO2, AcACO3, AdETR2, AdETR3, AcMADS2, AcNAC3, and AcNAC5,[Citation3,Citation8,Citation16–18] and restores levels of aroma-related esters before ripening after cold storage (1.5°C) for two or 4 months.[Citation19]
Ethylene inhibitor 1-methylcyclopropene (1-MCP) is commonly used to inhibit climacteric fruit ripening. 2 μL L−1 of 1-MCP treated fruit respired less and evolved less ethylene, and could be stored for 18 days under ambient conditions without much loss of fruit quality.[Citation20,Citation21] Pre-storage treatment with 0.5 μL L−1 of 1-MCP inhibited endogenous ethylene biosynthesis, maintained aroma development, and delayed kiwifruit ripening during shelf life at room temperature and in the short and medium term after cold storage, but had no effect during shelf-life after long-term cold storage.[Citation22–24] 1.0 μL L−1 of 1-MCP delayed kiwifruit softening and prolonged storage to 180 d at 0°C.[Citation25] However, 1-MCP treatment could not inhibit the low temperature-modulated fruit ripening during low-temperature storage independent of ethylene.[Citation17,Citation26–28]
Our previous study found there were significant differences in nutritional components and their changes in different parts of kiwifruit postharvest. Nutrients were metabolized and transferred to different parts during postharvest ripening, affecting the quality of kiwifruit.[Citation29] However, it is unclear whether there are differences in ethylene production rate and expression of ethylene-related genes in different parts of the kiwifruit. Therefore, to understand the changes of ethylene and expressions of ethylene-related genes in different parts of kiwifruit during postharvest ripening, the effects of 0.5 μL L−1 of 1-MCP on kiwifruit were investigated. In this study, we analyzed the changes in respiration rate, firmness, TSS, starch content, soluble sugar content, ethylene production rate, and expression of ethylene-related genes in different parts of kiwifruit by 1-MCP treatment during storage at 20°C for 9 days. This study is helpful for understanding the ethylene synthesis in different parts of kiwifruit during postharvest ripening, and provides a theoretical basis for improving the storage and transportation technology of kiwifruit.
Materials and methods
Plant materials and treatment with 1-MCP
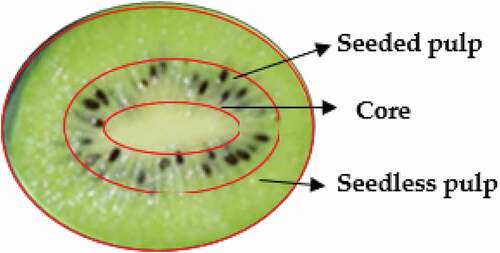
Kiwifruit (Actinidia chinensis cv. ‘Guichang’) was hand-harvested from a commercial orchard in Xiuwen County, Guizhou, China, with a mean TSS of 12.0°Brix. Fruits without physical defects and with uniformity were selected and divided into two treatments. Treatment was performed, namely L−1 1-MCP for 12 h at 20°C and the control of air. All treatments used a mini fan to maintain air circulation. After treatment, each treatment was divided into 12 groups, with three replicates in each group, 60 fruits in each replicate, and stored at 20 ± 1°C with 85 ± 5% RH. The respiration rate, ethylene production, firmness, and TSS were analyzed at 0,3, 6, and 9 d of storage. Samples of seedless pulp, seeded pulp, and core () were immediately frozen in liquid nitrogen and stored at – 80°C for analysis of starch content, soluble sugar starch, and gene expression.
Evaluation of respiration rate and ethylene production
The respiration rate and ethylene production of kiwifruit were determined according to Xie et al. (2020), and expressed as mg kg−1 h−1 CO2 and mg kg−1 h−1 C2H4, respectively.[Citation30] The core, seeded pulp, and seedless pulp of 40 fruits were collected, and the remaining 20 fruits were taken as the whole fruit with a thickness of 2 cm in the middle of the core, seeded pulp, and seedless pulp (as shown in ).
Determination of firmness and total soluble solids
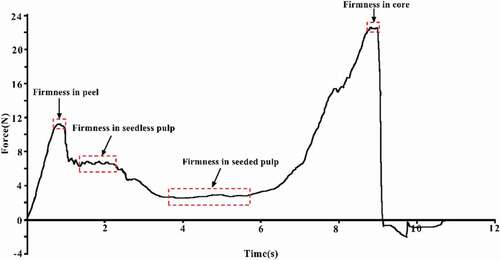
The force–time curves of kiwifruits were determined using a P/2 of TA. XT Plus texture profile analyzer (Stable Micro Systems Ltd., UK). The force was measured with the following instrumental settings: 2 mm s−1 of pretest speed, 1 mm s−1 of test speed, 10 mm s−1 of posttest speed, 6 mm of depth, and an auto-force trigger of 5.0 g. The firmness of the seedless pulp, seeded pulp, and core are shown in . Fifteen fruits were tested per replicate, and expressed as N. TSS was measured by a previously described method, and expressed as °Brix.[Citation31]
Determination of starch content and soluble sugar content
Starch content and soluble sugar content was measured by using a starch content kit and plant soluble sugar content test kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China), respectively, and expressed as g kg−1 and %, respectively.
RNA extraction and qRT-PCR assays
The expressions of ethylene-related genes (AcACS1, AcACO2, AcACO3, and AcETR2) were detected in kiwifruit samples. Total RNA extraction and qRT-PCR assays were carried out according to a previous method.[Citation32] Primers were designed by Beacon Designer 7.9 and are listed in . Samples from day 0 in the core of kiwifruit (assigned to an arbitrary quantity of “1”) were used as a calibrator to calculate the relative quantity of the results.
Table 1. Genes and sequences of primers used for qRT-PCR analysis
Statistical analysis
The results were reported as the mean ± standard error. Statistical tests were performed using IBM SPSS Statistics 25 (International Business Machines Corporation, Armonk, New York, USA). The significant differences were measured by Duncan’s multiple-range tests at 0.05 probabilities (P < .05).
Results
Effects of 1-MCP on respiration rate and ethylene production rate in different parts of kiwifruit during postharvest ripening
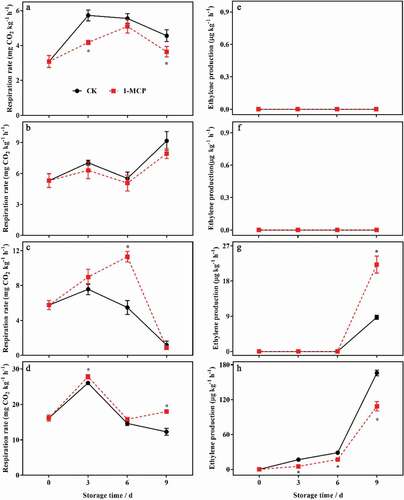
There were significant differences in the respiration rates of the whole fruit and different parts of the kiwifruit during postharvest ripening. The respiration rate in the core was the highest and that of the whole fruit was the lowest. The respiration rate of the whole fruit and all parts increased first and then decreased, and reached a peak at 3 d of storage, but the respiration rate of the seeded pulp increased significantly at 9 d of storage. 1-MCP inhibited the respiration rate of the whole fruit and the seedless pulp, and delayed its peak at day 6 d of storage, but significantly promoted the respiration rate in the seeded pulp and the core (–d). Ethylene production was not detected in the whole fruit or the seedless pulp during postharvest ripening. However, ethylene production was detected in the core at 3 d of storage, and then increased dramatically, which was induced by 1-MCP. Ethylene production was also detected in the seeded pulp at 9 d of storage, which was lower than that of the seeded pulp, and 1-MCP significantly promoted its increase (P < .05) (–g).
Effects of 1-MCP on firmness and total soluble solids in different parts of kiwifruit during postharvest ripening
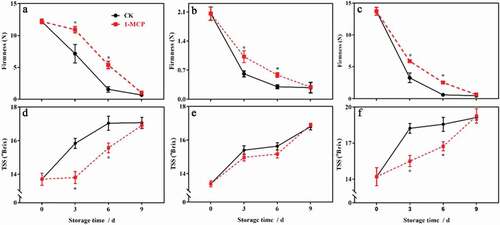
There were significant differences in firmness and TSS in different parts of the kiwifruit at harvest, and the firmness and TSS in the core was significantly higher than that in the seeded pulp (P < .05). Firmness in the whole fruit and different parts of the kiwifruit decreased sharply during storage, but TSS increased significantly, and the biggest change occurred at 3 d of storage. 1-MCP significantly inhibited the decrease in firmness of the whole fruit and the different parts, and significantly inhibited the increase of TSS in the seedless pulp and the core before 6 d of storage (P < .05). However, the firmness and TSS in the whole fruit and different parts of the control and 1-MCP treatment reached the same level at 9 d of storage ().
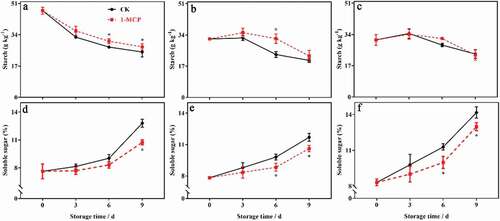
Effects of 1-MCP on starch content and soluble sugar content in different parts of kiwifruit during postharvest ripening
The starch content in different parts of the kiwifruit decreased significantly, while the soluble sugar content increased significantly during storage. 1-MCP significantly inhibited the decrease in starch content in the seedless pulp and the seeded pulp, and inhibited the increase in soluble sugar content in different parts ().
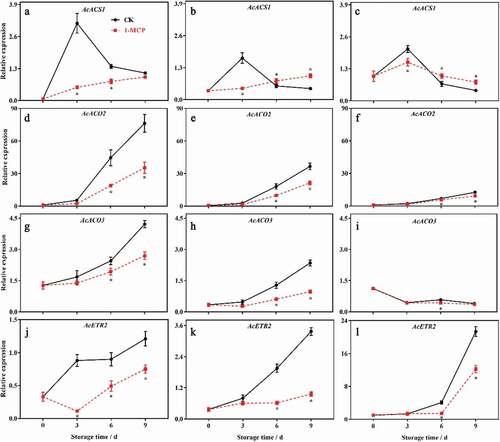
Effects of 1-MCP on ethylene related genes of in different parts of kiwifruit during postharvest ripening
The expression of ethylene-related genes in all parts of the kiwifruit was significantly different. The expression of AcACS1 was significantly higher in the core than in the seedless pulp and the seeded pulp at harvest (P < .05), but was significantly higher in the seedless pulp than in other parts during storage (P < .05). The expression of AcACS1 in different parts increased first and then decreased during postharvest ripening, and 1-MCP inhibited its expression significantly (P < .05) (–c). The expression of AcACO2 and AcACO3 in the seeded pulp was significantly lower than that in other parts of the kiwifruit at harvest (P < .05). The expression of AcACO2 and AcACO3 in other parts of the kiwifruit increased except the expression of AcACO3 in the core was down-regulated during postharvest ripening. 1-MCP significantly inhibited the expression of AcACO2 and AcACO3 in different parts of the kiwifruit (P < .05) (–i). The expression of AcETR2 in the core was significantly higher than in other parts at harvest and during postharvest ripening (P < .05), and increased during postharvest ripening. 1-MCP significantly inhibited the expression of AcETR2 in all parts (P < .05) (–l).
Discussion
Kiwifruit is a typical climacteric fruit, and ripening is induced by ethylene or low temperature.[Citation6] During storage at low temperature (≤15°C), although ethylene was not detected during the softening process of kiwifruit,[Citation17,Citation26] ripening related genes, including AcACS1, AcACO2, AcACO3, AdETR2, and AdETR3, were significantly up-regulated.[Citation8,Citation27,Citation28] Furthermore, low concentration of ethylene (0.01 ml L−1) promotes fruit softening and shortens shelf life in commercial storage,[Citation9,Citation10] which suggests that ethylene plays an important role in kiwifruit ripening. Most storage technology, including ASA, H2, 1-MCP, and Adequate postharvest ripening, is to extend its storage and shelf life of kiwifruit by regulating ethylene.[Citation11,Citation12,Citation15] Preliminary study found there were significant differences in nutritional components and their changes in different parts of kiwifruit during postharvest ripening, and they metabolized and transferred in different parts.[Citation29] However, it was unclear whether there were differences in ethylene production rates and expressions of ethylene-related genes in different parts of kiwifruit. In this study, our main purpose was to compare the effects of 0.5 μL L−1 of 1-MCP on changes of ethylene and expression of ethylene-related genes in different parts of kiwifruit during postharvest ripening.
It has been reported that the respiratory peak of kiwifruit is 4–15 d at room temperature, which is greatly affected by variety and harvest maturity,[Citation1,Citation29] and significantly suppressed by 1-MCP.[Citation20,Citation33] In this work, we found there were significant differences in the respiration rate of the whole fruit and different parts of kiwifruit, among which the core had the highest and the whole fruit had the lowest. The respiration rate of the whole fruit and all parts reached a peak at 3 d of storage, but that of the seeded pulp increased significantly at 9 d of storage. 1-MCP inhibited the respiration rate of the whole fruit and the seedless pulp, and delayed its peak to day 6 d of storage, consistent with other reports.[Citation20,Citation33] However, 1-MCP significantly promoted the respiration rate in the seeded pulp and the core, which may be related to ethylene production, and further research is needed. Ethylene has been detected or not even detected during postharvest storage, and is greatly affected by variety, harvest maturity, and storage temperature,[Citation1,Citation3,Citation4,Citation17,Citation34,Citation35] and is significantly suppressed by 1-MCP.[Citation20,Citation35] In this work, ethylene was not detected in the whole fruit or the seedless pulp during storage, consistent with previous reports.[Citation3,Citation5] However, ethylene was detected in the core and the seeded pulp at 3 and 9 d of storage, and ethylene production in core was significantly higher than that in the seeded pulp. We also found that 1-MCP significantly inhibited ethylene production in the core, but promoted ethylene production in the seeded pulp.
In this work, the starch content decreased significantly, and soluble sugar content increased significantly in all parts of kiwifruit during storage, consistent with reports on the whole fruit.[Citation35,Citation36] 1-MCP significantly inhibited the decrease in starch content in the seedless pulp and the seeded pulp, and inhibited the increase in soluble sugar content in all parts, consistent with previous reports.[Citation35,Citation37]
The decrease of firmness and the increase of TSS during storage leads to the softening of kiwifruit. In this work, there were significant differences in firmness and TSS in the whole fruit and different parts at harvest, and the firmness and TSS in the core was significantly higher than that of the seeded pulp. Firmness in the whole fruit and different parts decreased sharply during storage, but TSS increased significantly, consistent with previous reports.[Citation29,Citation36] 1-MCP significantly inhibited the decrease in firmness of the whole fruit and different parts, and significantly inhibited the increase of TSS in the seedless pulp and the core at the early stage of storage. However, the firmness and TSS in the whole fruit and different parts of the control and 1-MCP treatment reached the same level at 9 d of storage. These changes in the whole fruit are consistent with other reports.[Citation21,Citation33]
Ethylene biosynthesis was increased with the increase in ACC content, ACS, and ACO activities during ripening,[Citation1,Citation26] AdACS1, AdACO2, and AdACO2 are considered to be the key genes of ethylene biosynthesis in kiwifruit.[Citation4,Citation5,Citation8,Citation27,Citation28,Citation35] In this work, we found that the expression of ethylene-related genes in different parts of kiwifruit were significantly different. The expression of AcACS1 was significantly higher in the core than in other parts at harvest, but was significantly higher in the seedless pulp than in other parts during storage. The expression of AcACS1 in different parts increased first and then decreased during storage. The expression of AcACO2 and AcACO3 in the seeded pulp was significantly lower than that in other parts of kiwifruit at harvest, and the expression of AcACO2 and AcACO3 in the seedless pulp and the seeded pulp continued to increase, except the expression of AcACO3 in the core was down regulated during storage. 1-MCP significantly suppressed the expression of AcACO2 and AcACO3 in different parts. The expression of ethylene-related genes was suppressed by 1-MCP, consistent with other reports on the whole fruit.[Citation5,Citation35] However, the expression of AcETR2 in all parts continued to increase during storage, and was significantly suppressed by 1-MCP. On the contrary, the expression of AcETR2 in the core was significantly higher than that in the seeded and seedless pulp. We speculate that precursors may be synthesized in the seedless or seeded pulp, and then transported to the core to synthesize ethylene, which needs further verification.
Conclusion
The effects of 1-MCP on ethylene production and related genes in different parts of kiwifruit during postharvest ripening were evaluated. The firmness, soluble sugar, TSS, and AcACS1 and AcETR2 gene expressions were the highest in the core, and starch, and AcACO2 and AcACO3 gene expressions were the highest in the seedless pulp at harvest. The starch content and firmness decreased significantly, and soluble sugar content, TSS, AcACO2, and AcETR2 gene expressions increased significantly in all parts during postharvest ripening; these changes were significantly inhibited by 1-MCP. The respiration rate and AcACS1 gene expressions increased first and then decreased in all parts, and were suppressed by 1-MCP. Ethylene was detected in the core and the seeded pulp at 3 and 9 d of storage, respectively, but not in the whole fruit or the seedless pulp. Overall, the results suggested that there were differences in softening, ethylene production, and related gene expression in different parts of kiwifruit during harvest and during postharvest ripening, and 1-MCP inhibited ripening and ethylene production of kiwifruit during postharvest storage.
Acknowledgments
Authors’ contributions are as follows: Conceptualization, G.X.; methodology, N.L.; formal analysis, N.L.; investigation, Y.C., C.Y. and P.Z.; data curation, N.L.; writing – original draft preparation, N.L.; writing—review and editing, G.X.; project administration, G.X.; funding acquisition, G.X. The authors declare no conflict of interest.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Park, Y. S.; Jung, S. T.; Gorinstein, S. Ethylene Treatment of ‘Hayward’ Kiwifruits (Actinidia Deliciosa) during Ripening and Its Influence on Ethylene Biosynthesis and Antioxidant Activity. Sci. Hortic. 2006, 108(1), 22–28. DOI: https://doi.org/10.1016/j.scienta.2006.01.001.
- Minas, I. S.; Tanou, G.; Karagiannis, E.; Belghazi, M.; Molassiotis, A. Coupling of Physiological and Proteomic Analysis to Understand the Ethylene- and Chilling-induced Kiwifruit Ripening Syndrome. Front. Plant Sci. 2016, 7, 120. DOI: https://doi.org/10.3389/fpls.2016.00120.
- Mitalo, O. W.; Tokiwa, S.; Kondo, Y.; Otsuki, T.; Galis, I.; Suezawa, K.; Kataoka, I.; Doan, A. T.; Nakano, R.; Ushijima, K.; et al. Low Temperature Storage Stimulates Fruit Softening and Sugar Accumulation without Ethylene and Aroma Volatile Production in Kiwifruit. Front. Plant Sci. 2019a, 10, 888. DOI: https://doi.org/10.3389/fpls.2019.00888.
- Wu, Y. Y.; Liu, X. F.; Fu, B. L.; Zhang, Q. Y.; Tong, Y.; Wang, J.; Wang, W. Q.; Grierson, D.; Yin, X. R. Methyl Jasmonate Enhances Ethylene Synthesis in Kiwifruit by Inducing NAC Genes that Activate ACS1. J. Agr. Food. Chem. 2020, 68(10), 3267–3276. DOI: https://doi.org/10.1021/acs.jafc.9b07379.
- Mworia, E. G.; Yoshikawa, T.; Yokotani, N.; Fukuda, T.; Suezawa, K.; Ushijima, K.; Nakano, R.; Kubo, Y. Characterization of Ethylene Biosynthesis and Its Regulation during Fruit Ripening in Kiwifruit, Actinidia Chinensis ‘Sanuki Gold.’ Postharvest Biol. Tec. 2010, 55, 108–113. DOI: https://doi.org/10.1016/j.postharvbio.2009.08.007.
- Mworia, E. G.; Yoshikawa., T.; Salikon, N.; Chisato, O.; Asche, W. O.; Yokotani, N.; Daigo, A.; Ushijima, K.; Nakano, R.; Kubo, Y. Low-temperature-modulated Fruit Ripening Is Independent of Ethylene in ‘Sanuki Gold’ Kiwifruit. J. Exp. Bot. 2012, 63(2), 963–971. DOI: https://doi.org/10.1093/jxb/err324.
- Kongsuwan, A.; Hiromi, I.; Takanori, S.; Katsyua, O.; Hitoshi, O.; Staoru, K. Effects of Pre-harvest Application of Ethephon or Abscisic Acid on ‘Kohi’ Kiwifruit (Actinidia Chinensis) Ripening on the Vine. Sci. Hortic. 2016, 209, 255–260. DOI: https://doi.org/10.1016/j.scienta.2016.06.026.
- Mitalo, O. W.; Asiche, W. O.; Kasahara, Y.; Tosa, Y.; Tokiwa, S.; Ushijima, K.; Nakano., R.; Kubo, Y. Comparative Analysis of Fruit Ripening and Associated Genes in Two Kiwifruit Cultivars (‘sanuki Gold’ and ‘Hayward’) at Various Storage Temperatures. Postharvest Biol. Tec. 2019b, 147, 20–28. DOI: https://doi.org/10.1016/j.postharvbio.2018.08.017.
- Pranamornkith, T.; East, A.; Heyes, J. Influence of Exogenous Ethylene during Refrigerated Storage on Storability and Quality of Actinidia Chinensis (Cv. Hort16A). Postharvest Biol. Tec. 2012, 64, 1–8. DOI: https://doi.org/10.1016/j.postharvbio.2011.09.011.
- Jabbar, A.; East, A. R. Quantifying the Ethylene Induced Softening and Low Temperature Breakdown of ‘Hayward’ Kiwifruit in Storage. Postharvest Biol. Tec. 2016, 113, 87–94. DOI: https://doi.org/10.1016/j.postharvbio.2015.11.002.
- Yin, X. R.; Zhang, Y.; Zhang, B.; Yang, S. L.; Yang, S. N.; Ferguson, L. B.; Chen, K. S. Effects of Acetylsalicylic Acid on Kiwifruit Ethylene Biosynthesis and Signaling Components. Postharvest Biol. Tec. 2013, 83, 27–33. DOI: https://doi.org/10.1016/j.postharvbio.2013.03.012.
- Hu, H.; Zhao, S.; Li, P.; Shen, W. Hydrogen Gas Prolongs the Shelf Life of Kiwifruit by Decreasing Ethylene Biosynthesis. Postharvest Biol. Tec. 2018, 135, 123–130. DOI: https://doi.org/10.1016/j.postharvbio.2017.09.008.
- Lim, S.; Lee, J. G.; Lee, E. J. Comparison of Fruit Quality and GC-MS-based Metabolite Profiling of Kiwifruit ‘Jecy Green’: Natural and Exogenous Ethylene-induced Ripening. Food Chem. 2017, 234, 81–92. DOI: https://doi.org/10.1016/j.foodchem.2017.04.163.
- Shi, X. Y.; Xie, G. F.; Wang, X. B.; Li, X.; Zhao, Z. B.; Liu, Y. L. Effect of Exogenous Ethylene on the Shelf Quality of ‘Guichang’ Kiwifruit. Food Ind. 2018, 39(6), 74–76. DOI: CNKI:SUN:SPGY.0.2018-06-019.
- Liu, N.; Long, J. Y.; He, W. C.; Zhao, Z. B.; Xie, G. F. Effect of Adequate Postharvest Ripening on the Shelf Quality of Kiwifruits with Cold Storage Treatment. Food Ferment. Ind. 2021, 47(4), 27–32. DOI: https://doi.org/10.13995/j.cnki.11-1802/ts.025157.
- Yin, X. R.; Allan, C. A.; Zhang, B.; Wu, R. M.; Burdon, J.; Wang, P.; Ferguson, I. B.; Chen, K. S. Ethylene-related Genes Show a Differential Response to Low Temperature during ‘Hayward’ Kiwifruit Ripening. Postharvest Biol. Tec. 2009, 52(1), 9–15. DOI: https://doi.org/10.1016/j.postharvbio.2008.09.016.
- Asiche, W. O.; Mitalo, O. W.; Kasahara, Y.; Tosa, Y.; Mworia, E. G.; Ushijima, K.; Nakano, R.; Kubo, Y.; Effect of Storage Temperature on Fruit Ripening in Three Kiwifruit Cultivars. Horticult. J. 2017, 86(3), 403–410. DOI:https://doi.org/10.2503/hortj.OKD-028.
- Afshar-Mohammadian, M.; Fallah, S. F.; Rezadoost, M. H. Different Expression of Kiwifruit Ethylene-related Genes during Low Storage Temperatures. J. Consum. Prot. Foods. 2019, 14, 113–120. DOI: https://doi.org/10.1007/s00003-018-1205-6.
- Günther, C. S.; Marsh, K. B.; Winz, R. A.; Harker, R. F.; Wohlers, M. W.; White, A.; Goddard, M. R. The Impact of Cold Storage and Ethylene on Volatile Ester Production and Aroma Perception in ‘Hort16a’ Kiwifruit. Food Chem. 2015, 169, 5–12. DOI: https://doi.org/10.1016/j.foodchem.2014.07.070.
- Jhalegar, M. J.; Sharma, R. R.; Pal, R. K.; Arora, A. D.; Ahuja, A.; Analysis of Physiological and Biochemical Changes in Kiwifruit (Actinidia Deliciosa Cv. Allison) after the Postharvest Treatment with 1-methylcyclopropene. J. Plant Biochem. Biot. 2011, 20(2), 205–210. DOI:https://doi.org/10.1007/s13562-011-0047-4.
- Jhalegar, J.; Sharma, R. R.; Pal, R. K.; Sharma, S.; Effect of 1-MCP on Shelf-life and Quality of Kiwifruit Stored under Ambient Conditions. Indian J. Hortic. 2012, 69(2), 258–262. DOI:https://doi.org/10.1016/j.scienta.2012.04.022.
- Koukounaras, A.; Sfakiotakis, E. Effect of 1-MCP Prestorage Treatment on Ethylene and CO2 Production and Quality of ‘Hayward’ Kiwifruit during Shelf-life after Short, Medium and Long Term Cold Storage. Postharvest Biol. Tec. 2007, 46(2), 174–180. DOI: https://doi.org/10.1016/j.postharvbio.2007.05.002.
- Minas, I. S.; Tanou, G.; Krokida, A.; Karagiannis, E.; Belghazi, M.; Vasilakakis, M.; Papadopouou, K. K.; Molassiotis, A. Ozone-induced Inhibition of Kiwifruit Ripening Is Amplified by 1-methylcyclopropene and Reversed by Exogenous Ethylene. BMC Plant Biol. 2018, 8(1), 358. DOI: https://doi.org/10.1186/s12870-018-1584-y..
- Chen, H. J.; Zhang, J. Y.; Li, S.; Jiang, T. J.; Shen, S. I.; Zheng, X. I. Effect of 1-methylcyclopropene Treatment on Quality, Volatile Production and Ethanol Metabolism in Kiwifruit during Storage at Room Temperature. Sci. Hortic. 2020, 265, 109266. DOI: https://doi.org/10.1016/j.scienta.2020.109266.
- Quillehauquy, V.; Fasciglionr, M. G.; Moreno, A. D.; Monterubbianesi, M. G.; Casanovas, E. M.; Sánchez, E. E.; Yommi, A. K. Effects of Cold Storage Duration and 1-MCP Treatment on Ripening and ‘Eating Window’ of ‘Hayward’ Kiwifruit. J. Berry Res. 2020, 10, 419–435. DOI: https://doi.org/10.3233/JBR-190492.
- Antunes, M.; Sfakiotakis, E. M.; Chilling Induced Ethylene Biosynthesis in ‘Hayward’ Kiwifruit following Storage. Sci. Hoortic. 2002, 92(1), 29–39. DOI:https://doi.org/10.1016/S0304-4238(01)00278-3.
- Asiche, W. O.; Mitalo, O. W.; Kasahara, Y.; Tosa, Y.; Mworia, E. G.; Owino, W. O.; Ushijima, K.; Nakano, R.; Yano, K.; Kubo, Y. Comparative Transcriptome Analysis Reveals Distinct Ethylene–independent Regulation of Ripening in Response to Low Temperature in Kiwifruit. BMC Plant Biol. 2018, 18(1), 47. DOI: https://doi.org/10.1186/s12870-018-1264-y.
- Mitalo, O. W.; Asiche, W. O.; Kasahara, Y.; Tosa, Y.; Owino, W. O.; Mworia, E. G.; Ushijima, K.; Nakano, R.; Kubo, Y.; Characterization of Ripening-related Genes Involved in Ethylene-independent Low Temperature-modulated Ripening in ‘Rainbow Red’ Kiwifruit during Storage and On-vine. Horticult. J. 2018, 87(3), 421–429. DOI:https://doi.org/10.2503/hortj.okd-035.
- Liu, N.; Deng, Q. Q.; Chai, Y. Q.; Zhao, Z. B.; Xie, G. F.; Nutrients Changes in Different Parts of Kiwifruit during Postharvest Ripening. Food Fermen. Ind. 2020, 46(19), 181–185. DOI:https://doi.org/10.13995/j.cnki.11-1802/ts.024111..
- Xie, G. F.; Wang, L. R.; Fan, K. X.; Liu, N.; Liu, Y. L.; Zhao, B.; Postharvest 1-methylcyclopropene Treatments Maintain the Quality of Rosa Sterilis D. Shi during Storage. Food Sci. Tech. 2020, 40(1), 89–94. DOI:https://doi.org/10.1590/fst.32818.
- Xie, G. F.; Tan, S. M. Effect of Cultivar on Quality of the Common Bean during Storage. Int. Agric. Eng. J. 2015, 24(2), 69–78.
- Xie, G. F.; Yang, C.; Fei, Y.; Ma, L. Z. Physiological and Proteomic Analyses of 1-MCP Treatment on Lignification in Fresh Common Bean (Phaseolus Vulgaris L) during Storage. Postharvest Biol. Tec. 2020, 160, 111041. DOI: https://doi.org/10.1016/j.postharvbio.2019.111041.
- Huan, C.; Zhang, J.; Jia, Y.; Li, S.; Jiang, T. J.; Shen, S. L.; Zheng, X. L. Effect of 1-methylcyclopropene Treatment on Quality, Volatile Production and Ethanol Metabolism in Kiwifruit during Storage at Room Temperature. Sci. Hortic. 2020, 265, 109266. DOI: https://doi.org/10.1016/j.scienta.2020.109266.
- Zhang, A. D.; Xiong, H.; Kuang, S.; Ge, H.; Yin, X. R.; Chen, K. S. Isolation, Classification and Transcription Profiles of the Ethylene Response Factors (Erfs) in Ripening Kiwifruit. Sci. Hortic. 2016, 199, 209–215. DOI: https://doi.org/10.1016/j.scienta.2015.12.055.
- Zhang, A. D.; Wang, W. Q.; Yang, T.; Li, M. J.; Grierson, D. Transcriptome Analysis Identifies a Zinc Finger Protein Regulating Starch Degradation in Kiwifruit. Plant Physiol. 2018, 178(2), 850–863. DOI: https://doi.org/10.1104/pp.18.00427.
- Hu, X.; Kuang, S.; Zhang, A. D.; Zhang, W. S.; Chen, M. J.; Yin, X. R.; Chen, K. S. Characterization of Starch Degradation Related Genes in Postharvest Kiwifruit. Int. J. Mol. Sci. 2016, 17(12), 2112. DOI: https://doi.org/10.3390/ijms17122112.
- Chen, J. D.; Xu, F.; Chen, W.; Yang, Z. F.; Starch Degradation and Analysis of Key-gene Expression during Postharvest Kiwifruit Softening. J. Nucl. Agric. Sci. 2018, 32(2), 236–243. DOI:https://doi.org/10.11869/j..100-8551.2018.02.0236..