ABSTRACT
The objective of this paper was to examine the total polyphenols, vitamin C and capsaicin contents of pepper (varieties: Garam F1, Alma, Poseidon, Promontor, Slovakia, Claudius, Bhut Jolokia, Serrano, and Candlelight) and their correlation with the antioxidant activity by using the high performance liquid chromatography and Folin-Ciocalteu method. The limit of detection for capsaicin was 0.09 µg.g−1.Vitamin C and capsaicin ranged from 95.76 to 2,139 and from 44.40 to 2,224 μg.g−1 dry matter (DM), respectively. The determined total polyphenol content (TPC) ranged from 4,230 to 9,939 mg GAE.kg−1 DM and the values of antioxidant activity (AA) determined by using DPPH •, FRAP and ABTS • + methods were in range 19.48–91.81, 19.08–61.46 and 15.72–65.70 μmol TE.g−1 DM, respectively. Based on the statistical evaluation, there was a high correlation between vitamin C content and antioxidant activity of peppers. The obtained results suggest that due to the composition of bioactive compounds, especially antioxidants, the consumption of pepper fruits is an ideal choice for the prevention of the cell damage.
Introduction
Pepper fruit is one of the most important food commodities in the world, and also in Slovakia. It is not only of the economic importance but pepper is considered to be a very important and attractive type of vegetable for a wide range of consumers. It has a unique taste and aroma. Thanks to its sensory properties, it is consumed worldwide in both fresh and technologically modified form. Pepper (Capsicum L.) belongs to the family Solanaceae. It comes from the tropical and humid zones of the Central and South America and includes more than 200 varieties that vary in size, shape and taste. Chili peppers could be the oldest crops, as they were cultivated by Native Americans between 5 200 and 3 400 BC. The content of capsaicinoids (mainly capsaicin) in peppers is responsible for the spiciness and piquant taste of the varieties.[Citation1] Capsicum is popular not only for its organoleptic character but also for its antibacterial properties, as the members of this genus are able to kill or inhibit up to 70% of bacteria.[Citation2] Pepper is a rich and excellent source of chemoprotective substances, which are largely studied because of their health benefits.[Citation3] These phytochemicals include vitamin C, capsaicin, carotenoids, and polyphenolic compounds, which act to prevent the development of various diseases of civilization such as cancer, diabetes, cardiovascular disease, and obesity.[Citation4,Citation5]
Polyphenol compounds cannot be produced by the human body and must be taken in through a daily diet. Flavonols, flavone glycosides as well as hydroxycinnamic acids are the most common phenolic compounds found in peppers.[Citation4] Quercetin, luteolin and kaempferol are the most abundant aglycones identified in peppers.[Citation6] These flavonoids have an effect on cells, which may be mediated by their interactions with specific proteins.[Citation7]
These healthy compounds are characterized by the antioxidant properties, they scavenge free radicals, protect against oxidative cell damage, which can cause Alzheimer’s and Parkinson’s disease, and also they prevent the oxidation of the essential fats in brain cells, which is considered to be a necessary process for its optimal functioning.[Citation8,Citation9] The antiradical activity of phenolic compounds results from their redox properties, their structure, as well as the number and position of hydroxyl groups present.[Citation10] Their anticancer role is attributed to their ability to act as scavengers of singlet molecular oxygen, reactive oxygen species (ROS), peroxyl radicals, and reactive nitrogen species (RNS). Capsaicinoids intake effectively the reduced triacyclglycerols, plasma total cholesterol (PTC), and low density lipoprotein cholesterol, and thereby it helps in the prevention of cardiovascular ailments.[Citation5]
Several studies point to the antihypercholesterolemic properties of capsaicin as well as its beneficial effect on lipid metabolism.[Citation11] Natural capsaicinoids from chili peppers have the notable properties. They are characterized by a high biological activity and high pharmacological and neurological activity. Capsaicin and dihydrocapsaicin, in particular, have antimutagenic, antitumor and antioxidant properties.[Citation12] They are formed from the derivatives of phenylpropanoid compounds.[Citation13]
These compounds are used as topical painkillers in the form of creams and patches.[Citation14] Capsaicin is also known for its digestive properties, it stimulates saliva production, and affects gaseous amylase activity and bile secretion.[Citation15] The production of healthy substances in peppers is influenced by several factors, such as the variety itself, soil quality, macro and microelement content, presence of heavy metals, use of organic fertilizers, soil pH, climatic factors as well as ripening, post-harvest handling and storage conditions.[Citation16,Citation17]
The aim of this study was to determine the selected chemical parameters (vitamin C content, capsaicin content, total polyphenol content and antioxidant activity) of various species of the Capsicum L. cultivated mostly in the southern Slovakia, and also to determine which variety is most suitable for the cultivation in terms of quality parameters.
High performance liquid chromatography (HPLC) was used for the determination of vitamin C and capsaicin content. HPLC is an analytical technique utilized to separate, identify or quantify each component in a mixture. The mixture is separated by using the basic principle of column chromatography and then identified and quantified by spectroscopy.[Citation18] HPLC has become the method used for the determination of various bioactive compounds in foods as well as in drugs.[Citation19–21] The countless efforts has been made to increase, modify and improve the separation efficiency of the various stationary phases utilized in the separation methods and especially in chromatographic techniques.[Citation22–24] In the recent years HPLC has become the method most frequently used for analysis of capsaicinoids because of its rapidity and reliability.[Citation25]
UV-Vis spectrometry was used for the determination of total polyphenols and antioxidant activity. In a UV-Vis spectroscopic measurement, light absorption as a function of wavelength provides information about electronic transitions occurring in the material. The fraction of light transmitted is described by the Beer-Lambert law, which states that the fraction of the light measured after the interaction with the sample (usually measured as transmittance or reflectance) versus the incident intensity is dependent on the path length of light through the sample, the absorption cross section of the transition, and the difference in the population of the initial state and final state of the initial and final electronic energy levels. UV Vis spectrometry has an important place in the food analysis as well as in the analysis of bioactive substances, due to simple instrumentation, wide application possibilities and relatively high sensitivity.[Citation26]
Materials and methods
A small parcel trial was carried out in the village Imeľ. Imeľ is located in the south of Slovakia, on the Danubian Lowland between the rivers Nitra and Žitava. The cadaster of the village has an elongated shape in the east-west direction. The territory of the village lies at the altitude of 108–121 m. Imeľ is characterized by the warm and dry climate zone, the average annual air temperature is 9.9°C, and the average annual rainfall is 550 mm. Soils are typically fluvial plains – black soils (floodplain soils), modal carbonates, black earth soils, gley black soils. In flooded areas, fluvials are modal to gley. In the village there are sandy soils suitable for growing peppers.
Plant samples
Eight samples of Capsicum annuum L. fruits, sweet varieties (Promontor, Slovakia, Claudius), spicy varieties (Garam F1, Alma, Poseidon), chili varieties (Serrano, Candlelight), and one sample of Capsicum chinense L. fruit, chili variety Bhut Jolokia, were obtained directly in this area and then brought to the workplace and cleaned. The investigated Capsicum L. cultivars were conventionally cultivated in the same locality and under the same conditions. About 3 kg of pepper fruits were taken from the sampling sites for each cultivar.
Chemicals
The chemicals used for all analysis were MetOH (99,8%), MetOH (80%), DPPH (2,2ʹ-diphenyl-1-picrylhydrazyl), Trolox (2,5,7,8-tetramethylchroman-2-carboxylic acid), ABTS (2,2ʹ-azino-bis (3-ethylbenzthiazoline-6-sulfonic) acid, gallic acid (p.a.), TPTZ (2,4,6-tri (2-pyridyl) -s-triazine), Na2CO3, K2S2O8, including all HPLC standards (purity range 98.0–99.9%): methanol (HPLC grade), acetonitrile (gradient HPLC grade), and phosphoric acid (ACS grade) were purchased from Sigma-Aldrich (Sigma-Aldrich, USA). Folin-Ciocalteau reagent used for the determination of total phenolic content was purchased from Merck (Germany). Double deionized water (ddH2O) was treated (0.054 μS.cm−1) in a Simplicity 185 purification system (Millipore, UK).
Preparation of samples
The samples of nine tested cultivars of genus Capsicum were taken in state of full ripeness, in September 2020. One sample consisted of four samplings from four random sites. All pepper fruits were washed (first with drinking water and then with distilled water) and cut into slices with a thickness of 2–3 mm. The samples were mixed (Grindomix GM 2000 Retsch, 2000 rpm, 30 sec) and homogenized on the grinder IKA A10 (IKA GmBH, Germany).
Preparation of extracts
25 g of homogenized sample was steeped by 50 mL of 80% methanol at the laboratory temperature and extracted by the horizontal shaker (Unimax 2010, Heidolph Instrument GmbH, Germany) for 12 hours. The sample was filtered through Munktell No. 390 paper (Munktell & Filtrac, Germany) and stored in the closed 50 mL vial tubes.
Vitamin C
The concentrations of ascorbic acid in 9 varieties of pepper were determined by high performance liquid chromatography (HPLC) system.[Citation27] Washed peppers without seeds were chopped into small pieces and homogenized (Grindomix GM 2000 Retsch, 2000 rpm, for 30 sec.). 5 g of homogenate was weighted and 50 mL of 3% meta-phosphoric acid (MPA) was added. The extracts of peppers samples were subjected for 5 minutes long ultrasonic bath (Bandelin Sonorex Digitec DT 510 F, Fisher, Ltd., Slovak Republic), centrifuged (Hettich® Universal 320/320 R centrifuge, Sigma-Aldrich, USA) and then decanted through 55 mm Whatman filter papers (Sigma-Aldrich, USA). Prior to analysis the supernatants were filtered through 0.45 µM membrane filters (Millipore, Bedford, MA).
The reverse phase of liquid chromatography method was used for the determination of vitamin C content by isocratic elution by wavelength 254 nm. The separations were carried out on column Cortecs 18 (C18) 2.7 µm, 4.6 mm x 150 mm (Waters Corporation, MA, U.S.A.). The mobile phases comprise methanol and water from Purification system (Simplicity 185; Millipore SAS, Molsheim, France) which was used to provide double deionized water (ddH2O, 18.2 MΩ/cm, 20°C (5:95, v/v) and volume of injection was 10 µl. Temperature of the column during the analyses was adjusted at 25°C and the retention times were app. 5 minutes.
The stock solution of vitamin C standard as external standard was prepared with the concentration 1 mg.mL−1. The various concentrations of vitamin C ranged from 0.5 mg.mL−1 to 200 mg.mL−1 based on 10 calibration points. Due to the losses of vitamin C in all of the standards and extracted solutions of samples the flasks were kept in the aluminum packages.
Capsaicin
The content of capsaicin was determined by using of high performance liquid chromatography (HPLC) method, the system Waters Separations Module 2695 with UV detector 2996 (Waters, MA, USA).[Citation28] The clean pieces of peppers without seeds were ground by Grindomix GM 2000 Retsch, 2000 rpm, for 30 sec and homogenized on the grinder IKA A10 (IKA GmBH, Germany). 5 g of homogenate was weighted and 25 mL of methanol was added. Each sample had five sub-samples. The supernatants were ultrasonicated for 15 minutes (Bandelin Sonorex Digitec DT 510 F, Fisher, Ltd., Slovak Republic) and then filtrated through 0.45 µM membrane filters (Millipore, Bedford, MA).
The separation was performed with column Cortecs C18 (150 × 4,6 mm; 2,7 µm) (Waters Corporation, MA, U.S.A.), gradient elution with mobile phase comprised 0,1% acetic acid (more than 95% of purity, Sigma-Aldrich, USA) and methanol (HPLC gradient grade, Sigma-Aldrich, USA). The limit of detection (LOD) for capsaicin was 0.09 µg.g−1 and limit of quantification (LOQ) was 0.30 µg.g−1. The solvents, including meta-phosphoric acid (MPA), were used of high degree of purity or HPLC grade and were purchased from Sigma-Aldrich (USA). Ascorbic acid and capsaicin were purchased from Sigma Aldrich, St. Louis, MO, certified ACS grade (more than 95% purity).
Total polyphenol content
Total polyphenol content was determined by using Folin-Ciocalteau reagent (Merck, Germany), 20% Na2CO3 (Sigma-Aldrich, USA), and distilled water.[Citation29] 0.2 mL of sample extract, 2.5 mL Folin-Ciocalteau reagent and 5 mL H2O were added to a 50 mL flask. 5 mL of 20% Na2CO3 were added to the flask after 5 minutes, and the volume was then made up to 50 mL with distilled water and left to stay at the laboratory temperature for 2 hours. The absorbance was measured against blank solution at 765 nm wave length on the spectrophotometer Shimadzu UV-VIS 1800 (Shimadzu, Japan). The total polyphenol content was expressed as gallic acid equivalents (GAE) in mg.kg−1 FW (fresh weight) and DM (dry matter). The linearity range was determined at 0–150 μg.mL−1 (R2 = 0.9948).
DPPH radical scavenging assay
Free radical scavenging activity was determined by using 2,2-diphenyl-1-picrylhydrazyl radical (DPPH•) and methanol.[Citation30] To prepare stock solution, 0.025 g of DPPH• was diluted with methanol to 100 mL. Before the analysis, 1:10 dilution of the stock with methanol was prepared as a working solution. For the analysis, 3.9 mL of the DPPH• working solution was added to a cuvette and the absorbance was measured at 516 nm (A0), with Shimadzu UV-VIS 1800 spectrophotometer (Shimadzu, Japan). Then, 0.1 mL of the extract was added to the cuvette with DPPH• solution, stirred, and after 10 min the absorbance was measured against a blank solution (A10). The total antioxidant activity was expressed in μmol Trolox equivalent (TE).g−1 FW and DM based on the calibration curve (R2 = 0.9905).
ABTS•+ scavenging ability assay
The free radical scavenging activity was determined by using 2,2´-azino-bis (3-ethylbenzthiazoline-6-sulfonic) acid (ABTS•+ radical cation), potassium persulfate, and acetate buffer (pH = 4.3)[Citation31] ABTS•+ (Sigma-Aldrich, USA) was dissolved in water to 7 mM concentration. ABTS•+ radical cation was produced through the reaction between ABTS•+ stock solution. 2.45 mM potassium persulfate and acetate buffer. 50 μl of the sample extract was pipetted into 3 mL of diluted ABTS•+ solution, stirred, and after 20 min. the absorbance was measured against a blank solution at 734 nm wavelength, using Shimadzu UV-1800 spectrophotometer. The total antioxidant activity was expressed in μmol Trolox equivalent (TE).g−1 FW and DM based on the calibration curve (R2 = 0.9583).
Ferric reducing antioxidant power (FRAP) assay
The free radical scavenging activity was determined by using Fe3+-(2,4,6-tris(2-pyridyl)-S-triazine) (TPTZ), FeCl3 · 6 H2O, and acetate buffer (pH = 3.5)[Citation32] The FRAP reagent was prepared by mixing acetate buffer, TPTZ and FeCl3 · 6 H2O (Sigma-Aldrich, USA). 50 μl of extract was pipetted into 3 mL of the FRAP reagent to cuvettes, stirred, and after 20 min. the absorbance was measured against a blank solution at 593 nm wavelength, using Shimadzu UV-1800 spectrophotometer. The total antioxidant activity was expressed in μmol Trolox equivalent (TE).g−1 FW and DM based on the calibration curve (R2 = 0.9896).
Statistical analysis
All the tested variables did not follow the normal distribution according to the Shapiro-Wilk test and Kolmogorov-Smirnov test, therefore Kruskal-Wallis and Wilcoxon tests were performed to find the significant differences between the tested variables. For a better understanding and interpretation of the results, each variety was compared with the median value (horizontal line) using the Wilcoxon test. The Spearman correlation test at the significance level α = 0.05 was used to analyze the relationships between the variables. The principal component analysis was performed to summarize and visualize the information in a data set. Descriptive statistics, normality tests, and the principal component analysis were performed by using the MS Excel with the XLSTAT package.[Citation33] Kruskal-Wallis and Wilcoxon tests were performed in RStudio software, version 1.2.5033.[Citation34]
Results and discussion
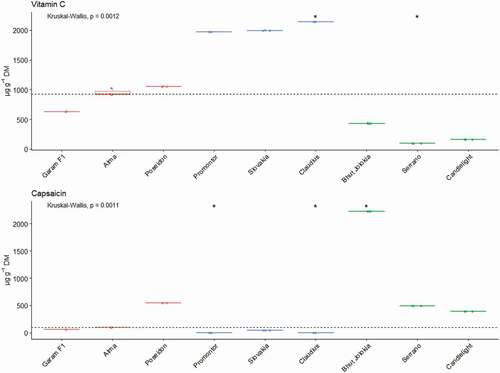
In the present article we dealt with the analysis of important chemo protective substances that are found in pepper fruits. Genus Capsicum contains a large amount of antioxidants, which by their properties help to scavenge free radicals, thus preventing the development of oxidative stress and preventing the development of diseases of civilization.[Citation35,Citation36] Pepper fruit is one of the most important sources of vitamin C, the content of vitamin C in the pepper fruit is higher than in citrus.[Citation37,Citation38] The nine different pepper cultivars contained the different contents of vitamin C, capsaicin and total polyphenols ().
Table 1. Vitamin C content, capsaicin content, and total polyphenol content in analyzed samples
Table 2. Antioxidant activity of analyzed samples
Vitamin C
Our results showed that sweet pepper varieties are the richest in vitamin C content, followed by spicy pepper varieties and chili pepper varieties contained the least vitamin C. The highest value of vitamin C content was recorded in the sweet variety Claudius – 2139 μg.g−1 DM (228.49 μg.g−1 FW) and the lowest value was found in chili variety Serrano – 95.76 μg.g−1 DM (23.39 μg.g−1 FW). The highest content of vitamin C from the monitored chili varieties was determined in the pungent variety Bhut Jolokia and represented the value 433.26 μg.g−1 DM. Bhut Jolokia, grown in the northeast India, contained higher levels of vitamin C.[Citation39]
The determination of vitamin C content was also considered by Patrick et al.[Citation40] The obtained values ranged from 106 μg.g−1 to 276 μg.g−1 FW. Similar values of vitamin C content in peppers are reported by Chávez-Mendoza et al.[Citation41] We can confirm that the specified range of values corresponds to our range of values. Hamed et al.[Citation42] and Gomes et al.[Citation43] dealt with the content of bioactive components in several pepper varieties. The authors state the values of vitamin C content ranged from 2230 to 10250 μg.g−1 DM as well as from 346 to 1,100 μg.g−1 FW, which are higher values in comparison with our obtained results. In contrast, Loizzo et al.[Citation6] report lower values of vitamin C content compared to our results. The content of vitamin C in the genus Capsicum is influenced by several factors. Vitamin C production depends mainly on plant metabolism, nutrient supply, water content and soil quality, as well as storage conditions and time. Soil microbes can also affect vitamin C production.[Citation44] The amount of vitamin C increases during the ripening of the fruit. Also, the content of vitamin C depends on the climatic conditions and it is significantly influenced by the variety itself, which is attributed to the variable water content in individual varieties.[Citation45] Higher levels of ascorbic acid in the mature stage of crops are caused by higher light intensity as well as higher levels of glucose, which are precursors of ascorbic acid.[Citation46]
Capsaicin
Another important parameter that we monitored in the selected varieties of the genus Capsicum is capsaicin. Capsaicin is a compound with a pungent taste, it belongs to the capsaicinoids and the largest amounts are found mainly in spicy varieties of peppers.[Citation47] Capsaicin and dihydrocapsaicin represent about 75–90% of the capsaicinoids in pepper fruits and have significant chemo preventive and antioxidant properties.[Citation48] The capsaicin content of pepper fruits showed a wide variation among genotypes (from 44.4 μg.g−1 DM to 2, 224.36 μg.g−1 DM). The highest content of capsaicin was recorded in the chili variety Bhut Jolokia (C. chinense). Sarpras et al.[Citation49] also report higher capsaicin content in this variety, which correlates with higher expression of the capsaicin regulatory gene. There was an undetectable amount in the analyzed varieties Promontor and Claudius. The determination of the capsaicin content in pepper fruits was discussed by Ionică et al.[Citation50] Their measured values ranged from 24.85 μg.g−1 DM to 229.81 μg.g−1 DM, which is comparable to our results. Alothman et al. determined capsaicin content in various Capsicum samples in the range from 26.2 μg.g−1 DM to 4,795.5 μg.g−1 DM. Similar results were reported by other authors.[Citation42,Citation43,Citation51] The findings from several studies suggest that the variability in capsaicin content in the different pepper fruit varieties can also be attributed to the intrinsic genetic factors of each variety, also depending on the growing conditions, environmental conditions and storage conditions.[Citation49,Citation52] In addition to these factors, the accumulation of capsaicin is also affected by the activity of the enzymes capsaicin synthase and peroxidase ().[Citation42]
Higher levels of vitamin C were found in sweet peppers. A significantly higher content was observed in the Claudius variety. On the other hand, lower levels of vitamin C were observed in the chili type. A significantly lower vitamin C content was observed in the Serrano variety. The analysis of the capsaicin confirmed the highest contents in the chili type. A significantly higher content was observed in the variety Bhut Jolokia. Variety Poseidon had a capsaicin content comparable to chili type. The fact that sweet peppers have a significantly lower content of capsaicin compared to the spicy and chili types has been confirmed.
Total polyphenol
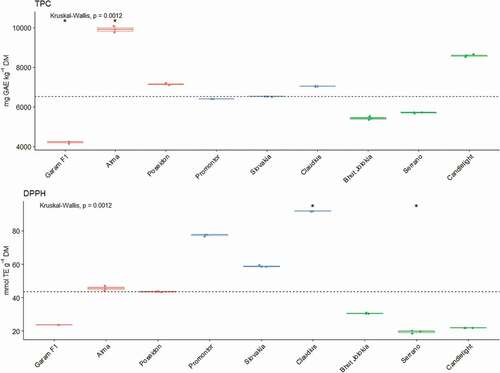
Genus Capsicum is an excellent source of the bioactive compounds, including polyphenols. These phenolic compounds are secondary plant metabolites with a strong antioxidant effect, which is attributed to their ability to scavenge free radicals in vitro and in vivo in biological systems.[Citation53] There are numerous studies dealing with content of these chemprotective substances in various food commodities.[Citation54–56]
Based on the results, we can state that there were no significant differences in the content of total polyphenols between the individual observed spicy, sweet and chili varieties of peppers. The lowest TPC value was determined in the Garam F1 variety (4230 mg GAE.kg−1 DM) and the highest TPC value was recorded in the Alma variety (9939 mg GAE.kg−1 DM). Our obtained values of polyphenols in the selected pepper varieties correspond to most of the data reported in the literature. Similar values are reported by other authors.[Citation16,Citation57] Our results are comparable also with TPC values 2096-7689 mg GAE.kg−1 DM determined by Hamed et al.[Citation42] Denev et al.[Citation58] stated the content of total polyphenols in their varieties in the range from 420–2660 mg GAE.kg−1 FW. Our results are comparable with the results of these authors.
Lower values of 1500–3500 mg GAE.kg−1 DM are reported by Caruso et al.[Citation59] The highest TPC (9,727–14,140 mg GAE.kg−1 DM) was determined by Hervert-Hernández et al.[Citation60] in spicy pepper varieties. A wide range of TPC values in pepper fruits can be attributed by several factors. These differences could be explained by the differences in chemical forms of the analyzed compounds, preparation of samples and their extraction, quantification methods, diversity of genotypes and varieties, maturity stage, and the use of fresh or dehydrated fruits.[Citation61] The biosynthesis of polyphenolic compounds is also significantly affected by the maturation process as well as the presence of nitrates (NO3−) and phosphates (PO43-), which contributes to the production of polyphenolic substances.[Citation62]
Antioxidant activity
Antioxidant activity is a parameter that evaluates the nutritional value of the quality of fruits and vegetables. Several studies have been performed to investigate the antioxidant properties of the genus Capsicum and several methods have been used.
DPPH assay
The use of DPPH • radical is a standard method of measuring the antioxidant activity of fruits and vegetables that are rich in antioxidant. The determined values of AA using DPPH • method in the selected pepper varieties were in the range 19.48–91.81 μmol TE.g−1 DM, the highest value being recorded in the sweet variety Claudius. Our results correspond to the values reported by Gomes et al.[Citation34] (50.50–72.90 μmol TE.g−1 DM.) Slightly lower values are given by Ionică et al.[Citation50] (5.26–20.04 μmol TE.g−1 DM.) Dinu et al.[Citation63] determined the values in the range from 3.36 to 17.86 μmol TE.g−1 FW.
ABTS assay
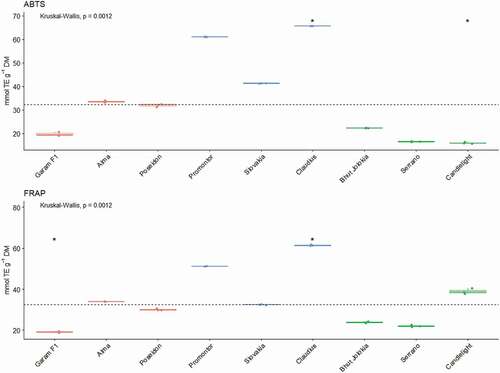
There has been a current interest in the use of ABTS radical assay in evaluating the radical scavenging capabilities of several plants extracts because of the different report on phenolic antioxidants’ ability to scavenge ABTS+ either by electron transfer or by donation of hydrogen atom, or a combination of both. The values determined by this method ranged from 15.7 to 65.7 μmol TE.g−1 DM. By using this method, we also determined the highest antioxidant activity in the sweet variety Claudius. The highest AA values determined by all methods in this sweet variety may also be related to the highest content of vitamin C in this monitored variety. Arslan et al.[Citation64] reported antioxidant activity in the genus Capsicum in the range 44.31–55.64 μmol TE.g−1 DM. Hervert-Hernandez et al.[Citation60] reported values of antioxidant activity in the range from 26.6–44.4 μmol TE.g−1 DM. Similar values have been reported by other authors.[Citation8] Slightly higher AA values determined by ABTS, 72–157 μmol TE.g−1 DM are reported by Hamed et al.[Citation42]
FRAP assay
The antioxidant activity of the extracts was also measured by using the FRAP assay. The FRAP is based on the reduction of metal complexes by antioxidants while DPPH is based on the measurement of the scavenging capacity of antioxidants toward the free radical neutralization.[Citation65] The determined values of antioxidant activity ranged from 19.09 to 61.46 μmol TE.g−1 DM. Again, we can say that the highest value was recorded in the extract of the sweet variety Claudius. Similar values of 44.31–55.64 μmol TE.g−1 DM are given by other authors.[Citation60,Citation64] Gomes et al.[Citation43] and Sora et al.[Citation66] reported the values of antioxidant activity measured by FRAP in a wider range from 3.99–84.67 μmol TE.g−1 DM and from 40.5–185.50 μmol TE.g−1 DM.
The results of this study suggest that the studied varieties of the genus Capsicum showed strong antioxidant activities. The antioxidant activity of the genus Capsicum is higher compared to other vegetables consumed. The evaluation of antioxidant activity provides valuable information on the functional quality of plants and it can be used for a better characteristics of plant species.[Citation67] The content of compounds with bioactive antioxidant properties is influenced by many factors as well as the genotype, whereby the values may also differ between the varieties of the studied vegetable.[Citation37] Agrochemical and climatic factors are also essential factors influencing the antioxidant activity. The differences between the individual methods (DPPH, FRAP, ABTS) of antioxidant activity determination could also be explained by the variation of sample preparation, extraction and quantification of samples of the given method, chemical form of analyzed compounds, stage of maturity, storage conditions as well as variety and genotype diversity ().
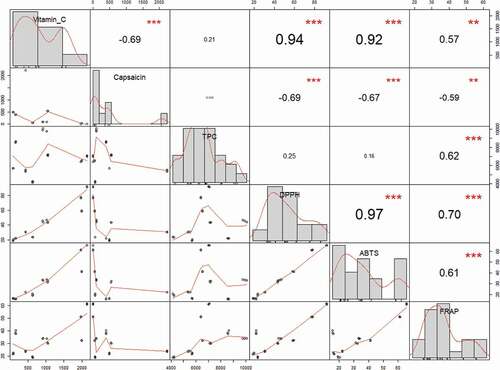
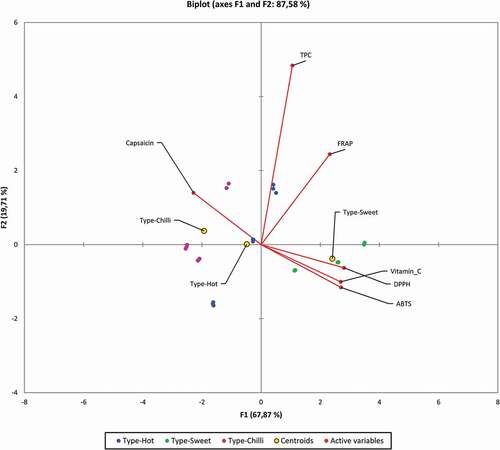
In the case of TPC, the statistically significant differences were found only in the Alma variety. Alma is characterized by a high content of total polyphenols. In the Claudius variety, a significantly higher antioxidant activity was found measured by DPPH, FRAP and ABTS methods. In general, it can be stated that the chili varietes have a lower antioxidant activity and total polyphenol content compared to other types of peppers (). The relationships between the tested parameters are shown as a Spearman correlation matrix. Very strong positive relationships were found between Vitamin C and DPPH (r = 0.94) and ABTS (r = 0.92). A strong positive relationship was also observed between FRAP and all tested parameters except Capsaicin (r = −0.59). Capsaicin as the only parameter tested showed an inverse relationship with all other parameters except TPC, where the relationship was no significant. In the case of TPC, a positive significant relationship was found only with FRAP (r = 0.62).
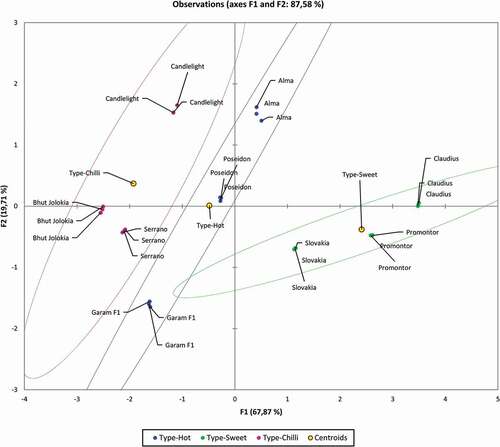
Spearman type of Principal Component Analysis was performed to find the hidden patterns between the tested parameters. The result of the Bartlett’s test of sphericity indicates that the data was likely factorable (Chi-square (observed = 206.73), Chisquare (critical = 31.41), P ≤ 0.0001). The principal component analysis revealed that 87.58% of the total variability embodied in 6 parameters could be effectively condensed into and explained by the first two principal components, with eigenvalues of 4.07 and 1.18, respectively. The remaining principal components had eigen values below 1, therefore they were not suitable for PCA analysis. The F1 is accounting for 67.87% of inertia, represented by vitamin C, DPPH and ABTS contents. F2 is explaining 19.71% of the inertia represented by TPC content. The tested variables clustered around the centroids can be seen as a 2D map in . The observations are represented by their projections, the variables are represented by their correlations in . As can be seen in , the sweet type of pepper can be clearly separated from other types. The sweet type of pepper is characterized by a higher content of vitamin C and antioxidant parameters DPPH and ABTS. The chili type can also be clearly separated from other types, and it is characterized by a high content of capsaicin. The most important for varieties Alma and Candlelight is the total polyphenol content. The PCA method has proven to be a suitable method for characterizing individual types of peppers.
Conclusion
Genus Capsicum is an important component of the daily diet thanks to its properties such as a high content of bioactive compounds with a high value of antioxidant activity, which increases the gastronomic value. Based on our results, we can state that the most valuable source of bioactive compounds from the spicy varieties was the Alma variety and from the sweet varieties the Claudius variety. Out of the chili varieties, we evaluate very positively the Candlelight variety, which was characterized by the highest content of polyphenols, and the Bhut Jolokia variety, which was characterized by the highest content of vitamin C. The acquired knowledge could be used in the agricultural as well as in the breeding area, to cultivate such varieties in the territory of the southern Slovakia, which are currently of commercial interest for the functional food sector.
Disclosure statement
There are no conflicts to declare.
Additional information
Funding
References
- Meghvansi, M.; Siddiqui, S.; Khan, M. H.; Gupta, V.; Vairale, M.; Gogoi, H.; Singh, L. Naga Chilli: A Potential Source of Capsaicinoids with Broadspectrum Ethnopharmacological Applications. J. Ethnopharmacol. 2010, 132, 1–14. DOI: https://doi.org/10.1016/j.jep.2010.08.034.
- Omolo, M. A.; Wong, Z. Z.; Mergen, A. K.; Hastings, J. C.; Le, N. C.; Reiland, H. A.; Case, K. A.; Baumler, D. J. Antimicrobial Properties of Chili Peppers. Infect Dis Ther. 2014, 2, 145–153.
- Salehi, B.; Hernández-Álvarez, A. J.; Del Mar Contreras, M.; Martorell, M.; Ramírez-Alarcón, K.; Melgar-Lalanne, G.; Sharifi-Rad, J. Potential Phytopharmacy and Food Applications of Capsicum Spp.: A Comprehensive Review. Nat. Prod. Commun. 2018, 13(11), 1543–1556.
- Morales-Soto, A.; Gómez-Caravaca, A. M.; García-Salas, P.; Zegura-Carretero, A.; Fernández-Gutiérrez, A. High-performance Liquid Chromatography Coupled to Diode Array and Electrospray Time-of-flight Mass Spectrometry Detectors for a Comprehensive Characterization of Phenolic and Other Polar Compounds in Three Pepper (Capsicum Annuum L.) Samples. Food Res. Int. 2013, 51, 977–984. DOI: https://doi.org/10.1016/j.foodres.2013.02.022.
- Imran, M.; Butt, M. S.; Suleria, H. A. R. Capsicum Annuum Bioactive Compounds: Health Promotion Perspectives. In Bioactive Molecules in Food; edited by J-M. Mérillon and K.G. Ramawat. Springer: Cham, 2018; pp 1–22.
- Loizzo, M. R.; Bonesi, M.; Serio, A.; Chaves-López, C.; Falco, T.; Paparella, A.; Tundis, R. Application of Nine Air-dried Capsicum Annum Cultivars as Food Preservative: Micronutrient Content, Antioxidant Activity, and Foodborne Pathogens Inhibitory Effects. Int. Food Prop. 2016, 20(4), 899–910. DOI: https://doi.org/10.1080/10942912.2016.1188310.
- Williams, R. J.; Spencer, J. P. E.; Rice-Evans, C. Flavonoids: Antioxidants or Signalling Molecules? Free Radical Biol. Med. 2004, 36, 838–849. DOI: https://doi.org/10.1016/j.freeradbiomed.2004.01.001.
- Blanco-Ríos, A. K.; Medina-Juarez, L. A.; González-Aguilar, G. A.; Gamez-Meza, N. Antioxidant Activity of the Phenolic and Oily Fractions of Different Sweet Bell Peppers. J. Mex. Chem. Soc. 2013, 57, 137–143.
- Oboh, G.; Rocha, T. B. J. Distribution and Antioxidant Activity of Polyphenols in Ripe and Unripe Tree Pepper (Capsicum Pubescens). J. Food Biochem. 2007, 31, 456–473. DOI: https://doi.org/10.1111/j.1745-4514.2007.00123.x.
- Lu, J.; Papp, L. V.; Fang, J.; Rodriguez, N. S.; Zhivotovsky, B.; Holmgren, A. Inhibition of Mammalian Thioredoxin Reductase by Some Flavonoids: Implications for Myricetin and Quercetin Anticancer Activity. Cancer Res. 2006, 66(8), 4410–4418. DOI: https://doi.org/10.1158/0008-5472.CAN-05-3310.
- Huang, W.; Cheang, W. S.; Wang, X.; Lei, L.; Liu, Y.; Ma, K. Y.; Zheng, F.; Huang, Y.; Chen, Z.-Y. Capsaicinoids but Not Their Analogue Capsinoids Lower Plasma Cholesterol and Possess Beneficial Vascular Activity. J. Agric. Food Chem. 2014, 62, 8415–8420. DOI: https://doi.org/10.1021/jf502888h.
- Korkutata, N. F.; Kavaz, A. A Comparative Study of Ascorbic Acid and Capsaicinoid Contents in Red Hot Peppers (Capsicum Annum L.) Grown in Southeastern Anatolia Region. Int. J. Food Prop. 2015, 18(4), 725–734. DOI: https://doi.org/10.1080/10942912.2013.850507.
- Perucka, I.; Materska, M. Phenylalanine Ammonia-lyase and Antioxidant Activities of Lipophilic Fraction of Fresh Pepper Fruits Capsicum Annuum L. Innov. Food Sci. Emerg. Technol. 2001, 2, 189–192. DOI: https://doi.org/10.1016/S1466-8564(01)00022-4.
- Srinivasan, K. Biological Activities of Red Pepper (Capsicum Annuum) and Its Pungent Principle Capsaicin: A Review. Crit. Rev. Food Sci. Nutr. 2016, 56, 1488–1500. DOI: https://doi.org/10.1080/10408398.2013.772090.
- Maji, A. K.; Banerji, P. Phytochemistry and Gastrointestinal Benefits of the Medicinal Spice, Capsicum Annuum L. (Chilli): A Review. J. Complement Integr. Med. 2016, 13, 97–122. DOI: https://doi.org/10.1515/jcim-2015-0037.
- Ghasemnezhad, M.; Sherafati, M.; Payvast, G. A. Variation in Phenolic Compounds, Ascorbic Acid and Antioxidant Activity of Five Coloured Bell Pepper (Capsicum Annum) Fruits at Two Different Harvest Times. J. Funct. Foods. 2011, 3(1), 44–49. DOI: https://doi.org/10.1016/j.jff.2011.02.002.
- Zayed, M. S.; Hassanein, M.; Esa, N. H.; Abdallah, M. Productivity of Pepper Crop (Capsicum Annuum L.) As Affected by Organic Fertilizer, Soil Solarization, and Endomycorrhizae. Ann. Agric. Sci. 2013, 58, 131–137. DOI: https://doi.org/10.1016/j.aoas.2013.07.011.
- Aryal, S. High-performance Liquid Chromatography. Microbe Notes. 2018. https://microbenotes.com/high-performance-liquid-chromatography-hplc/.
- Wabaidur, S. M.; AlAmmari, A.; Aqel, A.; AL-Tamrah, S. A.; Alothman, Z. A.; Ahmed, A. Y. B. H. Determination of Free Fatty Acids in Olive Oils by UPHLC–MS. J Chromatog B. 2016, 1031, 109–115. DOI: https://doi.org/10.1016/j.jchromb.2016.07.040.
- Abdullah AlFaris, N.; Zaidan ALTamimi, J.; Alothman, Z. A.; Fahad Al Qahtani, S.; Wabaidur, S. M.; Ghfar, A. A.; Saleh Aldayel, T. Analysis of Aflatoxins in Foods Retailed in Saudi Arabia Using Immunoaffinity Column Cleanup and High-performance Liquid Chromatography-fluorescence Detection. J. King Saud Univ. Sci. 2020, 32(2), 1437–1443. DOI: https://doi.org/10.1016/j.jksus.2019.11.039.
- AlFaris, N. A.; Wabaidur, S. M.; Alothman, Z. A.; Altamimi, J. Z.; Aldayel, T. S. Fast and Efficient Immunoaffinity Column Cleanup and Liquid Chromatography–tandem Mass Spectrometry Method for the Quantitative Analysis of Aflatoxins in Baby Food and Feeds. J. Sep. Sci. 2020, 43(11), 2079–2087. DOI: https://doi.org/10.1002/jssc.201901307.
- Khan, M. R.; Alothman, Z. A.; Naushad, M.; Ghfar, A. A.; Wabaidur, S. M. Simultaneous Analysis of Vitamin C and Aspirin in Aspirin C Effervescent Tablets by High Performance Liquid Chromatography–photodiode Array Detector. J Liq Chromatogy Relat Technol. 2012, 35(17), 2454–2461. DOI: https://doi.org/10.1080/10826076.2011.633679.
- Wabaidur, S. M.; Alothman, Z. A.; Khan, M. R. A Rapid Method for the Simultaneous Determination of L-ascorbic Acid and Acetylsalicylic Acid in Aspirin C Effervescent Tablet by Ultra Performance Liquid Chromatography–tandem Mass Spectrometry. Spectrochim. Acta A. 2013, 108, 20–25. DOI: https://doi.org/10.1016/j.saa.2013.01.070.
- Alothman, Z. A.; Wabaidur, S. M. Application of Carbon Nanotubes in Extraction and Chromatographic Analysis: A Review. Arab. J. Chem. 2019, 12(5), 633–651. DOI: https://doi.org/10.1016/j.arabjc.2018.05.012.
- Alothman, Z. A.; Wabaidur, S. M.; Khan, M. R.; Ghafar, A. A.; Habila, M. A.; Ahmed, Y. B. H. Determination of Capsaicinoids in Capsicum Species Using Ultra Performance Liquid Chromatography-mass Spectrometry. J. Sep. Sci. 2012, 35(21), 2892–2896. DOI: https://doi.org/10.1002/jssc.201200459.
- Chen, Z.; Deutsch, T. G.; Dinh, H. N.; Domen, K.; Emery, K.; Forman, A. J.; Turner, J. UV-Vis Spectroscopy. In Photoelectrochemical Water Splitting, edited by Z. Chen, H. N. Dinh and E. Miller, Springer: New York, 2013; pp 49–62.
- Odriozola-Serrano, I.; Hernández-Jover, T.; Martín-Belloso, O. Comparative Evaluation of UV-HPLC Methods and Reducing Agents to Determine Vitamin C in Fruits. Food Chem. 2007, 105(3), 1151–1158. DOI: https://doi.org/10.1016/j.foodchem.2007.02.037.
- González-Zamora, A.; Sierra-Campos, E.; Luna-Ortega, J. G.; Pérez-Morales, R.; Ortiz, J. C. R.; García-Hernández, J. L. Characterization of Different Capsicum Varieties by Evaluation of Their Capsaicinoids Content by High Performance Liquid Chromatography, Determination of Pungency and Effect of High Temperature. Molecules. 2013, 18(11), 13471–13486. DOI: https://doi.org/10.3390/molecules181113471.
- Lachman, J.; Proňek, D.; Hejtmánková, A.; Pivec, V.; Faitová, K. Total Polyphenol and Main Flavonoid Antioxidanrs in Different Onion (Allium Cepa L.) Varieties. Hortic Sci Prague. 2003, 30(4), 142–147. DOI: https://doi.org/10.17221/3876-HORTSCI.
- Brand-Williams, W.; Cuvelier, M. E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT. 1995, 28(1), 214–222. DOI: https://doi.org/10.1016/S0023-6438(95)80008-5.
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26(9–10), 1231–1237.
- Pedersen, C. B.; Kyle, J.; Jenkinson, A.; Gardner, P. T.; McPhail, D. B.; Duthie, G. G. Effects of Blueberry and Cranberry Juice Consumption on the Plasma Antioxidant Capacity of Healthy Female Volunteers. Eur. J. Clin. Nutr. 2000, 54(5), 405–408. DOI: https://doi.org/10.1038/sj.ejcn.1600972.
- Addinsoft XLSTAT. Analyse de donneés et statistique avec MS Excel; Addinsoft: New York, 2014.
- RStudio Team. RStudio: Integrated Development for R; RStudio, Inc.: Boston, 2005. http://www.rstudio.com/.
- Kaur, G.; Kathariya, R.; Bansal, S.; Singh, A.; Shahakar, D. Dietary Antioxidants and Their Indispensable Role in Periodontal Health. J. Food Drug Anal. 2016, 24(2), 239–246. DOI: https://doi.org/10.1016/j.jfda.2015.11.003.
- Thuphairo, K.; Sornchan, P.; Suttisansanee, U. Bioactive Compounds, Antioxidant Activity and Inhibition of Key Enzymes Relevant to Alzheimer’s Disease from Sweet Pepper (Capsicum Annuum) Extracts. Prev. Nutr. Food Sci. 2019, 24(3), 327. DOI: https://doi.org/10.3746/pnf.2019.24.3.327.
- Olatunji, T. L.; Afolayan, A. J. Comparison of Nutritional, Antioxidant Vitamins and Capsaicin Contents in Capsicum Annuum and C. Frutescens. Int. J. Veg. Sci. 2019, 26(2), 190–207. DOI: https://doi.org/10.1080/19315260.2019.1629519.
- Finger, F. L.; Lannes, S. D.; Schuelter, A. R.; Doege, J.; Comerlato, A. P.; Gonçalves, L. S. A. Genetic Diversity of Capsicum Chinense (Solanaceae) Accessions Based on Molecular Markers and Morphological and Agronomic Traits. Genet. Mol. Res. 2010, 9(3), 1852–1864. DOI: https://doi.org/10.4238/vol9-3gmr891.
- Mena, E.; Warade, S. D.; Ansari, M. T.; Ramjan, M. D. Evaluation of Capsaicin, Ascorbic Acid, α-carotene and β-carotene in Bhut Jolokia (Capsicum Chinense Jacq.) Genotypes from North East India Ephilo. J. Pharm. Innov. 2018, 7(7), 93–97.
- Patrick, A. O.; Fabian, U. A.; Peace, I. C.; Fred, O. O. Determination of Variation of Vitamin ‘C’ Content of Some Fruits and Vegetables Consumed in Ugbokolo after Prolonged Storage. J. Environ. Sci. Toxicol. Food Technol. 2016, 10(7), 17–19.
- Chávez-Mendoza, C.; Sanchez, E.; Muñoz-Marquez, E.; Sida-Arreola, J.; Flores-Cordova, M. Bioactive Compounds and Antioxidant Activity in Different Grafted Varieties of Bell Pepper. Antioxidants. 2015, 4(2), 427–446. DOI: https://doi.org/10.3390/antiox4020427.
- Hamed, M.; Kalita, D.; Bartolo, M. E.; Jayanty, S. S. Capsaicinoids, Polyphenols and Antioxidant Activities of Capsicum Annuum: Comparative Study of the Effect of Ripening Stage and Cooking Methods. Antioxidants. 2019, 8(9), 364. DOI: https://doi.org/10.3390/antiox8090364.
- Gomes, G. P.; Constantino, L. V.; Erpen-Dalla Corte, L.; Riger, C. J.; Chaves, D. S. A. Gonçalves LSA Characterization of Biochemical Compounds and Antioxidant Activity of “Dedo-de-moça” Chili Pepper Accessions. Hortic Bras. 2019, 37, 429–436. DOI: https://doi.org/10.1590/s0102-053620190411.
- Chauhan, A. K.; Das, A.; Kharkwal, H.; Kharkwal, A. C.; Varma, A. Impact of Microorganisms on Environment and Health. In Microbes: Health and Environment, edited by A.K. Chauhan and A. Varma I.K. International Publishing House Pvt. Ltd.: New Delhi, India, 2006; pp 1–12.
- Teodoro, A. F. P.; Alves, R. D. B.; Ribeiro, L. B.; Reis, K.; Reifschneider, F. J. B.; Fonseca, M. E. D. N.; Da Silva, J. P.; Agostini-Costa, T. D. S. Vitamin C Content in Habanero Pepper Accessions (Capsicum Chinense). Hortic Bras. 2013, 31(1), 59–62. DOI: https://doi.org/10.1590/S0102-05362013000100009.
- Mozafar, A. Plant Vitamins: Agronomic, Physiological and Nutritional Aspects; CRC Press: Boca Raton, FL, USA, 1994.
- Popelka, P.; Jevinová, P.; Šmejkal, K. Roba P Determination of Capsaicin Content and Pungency Level of Different Fresh and Dried Chilli Peppers. Folia Vet. 2017, 61(2), 11–16. DOI: https://doi.org/10.1515/fv-2017-0012.
- Zhuang, Y.; Chen, L.; Sun, L.; Cao, J. Bioactive Characteristics and Antioxidant Activities of Nine Peppers. J. Funct. Foods. 2012, 4(1), 331–338. DOI: https://doi.org/10.1016/j.jff.2012.01.001.
- Sarpras, M.; Gaur, R.; Sharma, V.; Chhapekar, S. S.; Das, J.; Kumar, A.; Yadava, S. K.; Nitin, M.; Brahma, V.; Abraham, S. K. Comparative Analysis of Fruit Metabolites and Pungency Candidate Genes Expression between Bhut Jolokia and Other Capsicum Species. PLoS ONE. 2016, 11, e0167791.
- Ionică, M. E.; Nour, V.; Trandafir, I. Bioactive Compounds and Antioxidant Activity of Hot Pepper Fruits at Different Stages of Growth and Ripening. J. Appl. Bot. Food Qual. 2017, 90, 232–237.
- Islam, M. A.; Sharma, S. S.; Sinha, P.; Negi, M. S.; Neog, B.; Tripathi, S. B. Variability in Capsaicinoid Content in Different Landraces of Capsicum Cultivated in North-eastern India. Sci. Hortic. 2015, 183, 66–71. DOI: https://doi.org/10.1016/j.scienta.2014.12.011.
- Usman, M. G.; Rafii, M. Y.; Ismail, M. R.; Malek, M. A.; Latif, M. A. Capsaicin and Dihydrocapsaicin Determination in Chili Pepper Genotypes Using Ultra-fast Liquid Chromatography. Molecules. 2014, 19(5), 6474–6488. DOI: https://doi.org/10.3390/molecules19056474.
- Perez-Jimenez, J.; Neveu, V.; Vos, F.; Scalbert, A. Identification of the 100 Richest Dietary Sources of Polyphenols: An Application of the Phenol-Explorer Database. Eur. J. Clin. Nutr. 2010, 64, 112–120. DOI: https://doi.org/10.1038/ejcn.2010.221.
- Badjah Hadj Ahmed, A. Y.; Obbed, M. S.; Wabaidur, S. M.; Alothman, Z. A.; Al-Shaalan, N. H. High-Performance Liquid Chromatography Analysis of Phenolic Acid, Flavonoid, and Phenol Contents in Various Natural Yemeni Honeys Using Multi-walled Carbon Nanotubes as a Solid-Phase Extraction Adsorbent. J. Agric. Food Chem. 2014, 62(24), 5443–5450. DOI: https://doi.org/10.1021/jf5011758.
- Wabaidur, S. M.; Ahmed, Y. B. H.; Alothman, Z. A.; Obbed, M. S.; AL-Harbi, N. M.; AL-Turki, T. M. Ultra High Performance Liquid Chromatography with Mass Spectrometry Method for the Simultaneous Determination of Phenolic Constituents in Honey from Various Floral Sources Using Multiwalled Carbon Nanotubes as Extraction Sorbents. J. Sep. Sci. 2015, 38(15), 2597–2606. DOI: https://doi.org/10.1002/jssc.201500386.
- Wabaidur, S. M.; Obbed, M. S.; Alothman, Z. A.; Alfaris, N. A.; Badjah-Hadj-Ahmed, A. Y.; Siddiqui, M. R.; Altamimi, J. Z.; ALDAYEL, T. S. Total Phenolic Acids and Flavonoid Contents Determination in Yemeni Honey of Various Floral Sources: Folin-Ciocalteu and Spectrophotometric Approach. Food Science and Technology. 2020, 40(2), 647–652. DOI: https://doi.org/10.1590/fst.33119.
- Gougoulias, N.; Wogiatzi, E.; Vagelas, I.; Giurgiulescu, L.; Gogou, I.; Ntall, M. N.; Kalfountzos, D. Comparative Study on Polyphenols Content, Capsaicin and Antioxidant Activity of Different Hot Peppers Varieties (Capsicum Annuum L.) Under Environmental Conditions of Thessaly Region, Greece Carpath. J. Food Sci. Technol. 2017, 9(1), 109–116.
- Denev, P.; Todorova, V.; Ognyanov, M.; Georgiev, Y.; Yanakieva, I.; Tringovska, I.; Grozeva, S.; Kostova, D. Phytochemical Composition and Antioxidant Activity of 63 Balkan Pepper (Capsicum Annuum L.) Accessions. Journal of Food Measurement and Characterization. 2019, 13(4), 2510–2520. DOI: https://doi.org/10.1007/s11694-019-00171-y.
- Caruso, G.; Stoleru, V. V.; Munteanu, N. C.; Sellitto, V. M.; Teliban, G. C.; Burducea, M.; TENU, I.; MORANO, G.; BUTNARIU, M. Quality Performances of Sweet Pepper under Farming Management. Notulae Botanicae Horti Agrobotanici Cluj-Napoca. 2018, 47(2), 458–464. DOI: https://doi.org/10.15835/nbha47111351.
- Hervert-Hernández, D.; Sáyago-Ayerdi, S. G.; Goñi, I. Bioactive Compounds of Four Hot Pepper Varieties (Capsicum Annuum L), Antioxidant Capacity, and Intestinal Bioaccessibility. J. Agric. Food Chem. 2010, 58(6), 3399–3406. DOI: https://doi.org/10.1021/jf904220w.
- Deepa, N.; Kaur, C.; George, B.; Singh, B.; Kapoor, H. C. Antioxidant Constituents in Some Sweet Pepper (Capsicum Annuum L) Genotypes during Maturity. LWT. 2007, 40, 121–129. DOI: https://doi.org/10.1016/j.lwt.2005.09.016.
- Menichini, F.; Tundis, R.; Bonesi, M.; Loizzo, M. R.; Conforti, F.; Statti, G.; De Cindio, B.; Houghton, P. J.; Menichini, F. The Influence of Fruit Ripening on the Phytochemical Content and Biological Activity of Capsicum Chinense Jacq Cv Habanero. Food Chem. 2009, 114, 553–560. DOI: https://doi.org/10.1016/j.foodchem.2008.09.086.
- Dinu, M.; Soare, R.; Hoza, G.; Bãbeanu, C. Changes in Phytochemical and Antioxidant Activity of Hot Pepper Fruits on Maturity Stages, Cultivation Areas and Genotype. South-west J Hortic Biol Environ. 2018, 9(2), 65–76.
- Arslan, D.; Özcan, M. M. Dehydration of Red Bell-pepper (Capsicum Annuum L): Change in Drying Behavior, Colour and Antioxidant Content. Food Bioprod Proc. 2011, 89(4), 504–513. DOI: https://doi.org/10.1016/j.fbp.2010.09.009.
- Alam, M. N.; Bristi, N. J.; Rafiquzzaman, M. Review on in Vivo and in Vitro Methods Evaluation of Antioxidant Activity. Saudi Pharm. J. 2013, 21, 143–152. DOI: https://doi.org/10.1016/j.jsps.2012.05.002.
- Sora, G. T. S.; Haminiuk, C. W. I.; Silva, M. V.; Zielinski, A. A. F.; Goncalves, G. A.; Bracht, A.; Peralta, R. M. A Comparative Study of the Capsaicinoid and Phenolic Contents and in Vitro Antioxidant Activities of the Peppers of the Genus Capsicum: An Application of Chemometrics. J. Food Sci. Technol. 2015, 52, 8086–8094. DOI: https://doi.org/10.1007/s13197-015-1935-8.
- Sharma, K.; Assefa, A. D.; Kim, S.; Ko, E. Y.; Lee, E. T.; Park, S. W. Evaluation of Total Phenolics, Flavonoids and Antioxidant Activity of 18 Korean Onion Cultivars: A Comparative Study. J. Sci. Food Agric. 2014, 94(8), 1521–1529. DOI: https://doi.org/10.1002/jsfa.6450.