?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Jujube has received more and more attention due to their nutritional value and pharmacological effects. This study evaluated the quality of different varieties of jujube by comparing the main active components and antioxidant activities of thirty-seven varieties of jujube through titration, UV spectrophotometry and High performance liquid chromatography (HPLC). The results revealed that there were differences in active components and antioxidant capacity among jujube cultivars. Among the thirty-seven varieties of jujube, Qingxuyuanzao (QXYZ) had the highest contents of total phenols and flavonoids, which were 16.33 mg GAE/g DW and 41.18 mg RE/g DW, respectively, and had the highest free radical scavenging (DPPH: 29.79 mg Vc/g DW; ABTS: 50.90 mg Trolox/g DW) and reducing abilities (62.13 mg Trolox/g DW). Correlation analysis indicated that the content of total flavonoids, total phenols and phenolic acid ((+)-catechin, caffeic acid, rutin) in jujube had high positive correlation with antioxidant capacity. According to principal component analysis (PCA), the comprehensive scores of different varieties of jujube were calculated. The top five jujube varieties with comprehensive scores are ‘QXYZ,’ Suyuanling (SYL), Xiangzao (XZ), Dunhuangdazao (DHDZ) and Meimizao (MMZ). In this paper, the main active components and antioxidant capacity of thirty-seven jujube varieties have been studied, which provides a theoretical basis for jujube breeding and the development of active nutrients for jujube.
Introduction
Jujube (Ziziphus jujuba Mill.) is a plant of the Rhamnaceae family, widely distributed in subtropical and tropical regions, especially in East Asia (China, Korea and India), the Middle East (Pakistan and Iran) and North African countries .[Citation1–3] Jujube originated in China and has a history of planting for more than 4,000 years .[Citation4] It is the world’s largest red date producer and only red date exporter, accounting for more than 90% of the world’s total red date output .[Citation5] In addition, it is also grown in Southeastern Europe (such as, Spain and Italy), Southwestern United States, Australia and Russia .[Citation6]
In the past decade, the interest and use of medicinal plant products have increased significantly .[Citation7] Therefore, more and more researchers begin to pay attention to a variety of bioactive substances in plants, fruits and vegetables and their importance to human health, such as Borago officinalis L. flower, [Citation8] Galanthus transcaucasicus, [Citation9] red cabbage, [Citation10] Clematis orientalis and Clematis ispahanica .[Citation11] Jujube have always been regarded as an important food or traditional medicinal material. Other parts of jujube, such as seeds, fruits, leaves and flowers, are also used as medicinal preparations .[Citation2,Citation12,Citation13] Among them, the edible part (pulp and peel) of jujube fruit is the focus of research, which contains most of the active active substances. Some studies have indicated that jujube contains a large number of active substances, such as polyphenol, flavonoid, triterpene, polysaccharide, ascorbic acid, cyclic adenosine monophosphate (cAMP) .[Citation14,Citation15] These active ingredients have antioxidant, antiinflammatory, antibacterial, liver protection, gastrointestinal protection, blood glucose reduction and anticancer effects .[Citation16–20] The nutritional value and beneficial effects of jujube on health, as well as people’s increasing attention to its antioxidant activity, indicate that it is an excellent health food with important research value. Therefore, it is necessary to select varieties with more relevant active ingredients according to the expected use of the fruit.
As far as we know, there are more than 700 varieties of jujube in China. However, the origin of its species is very confusing, and the nature of different varieties of jujube is still unclear. There are few studies on its active active ingredients and antioxidant activity. Therefore, in this study, the active components of thirty-seven Chinese jujube varieties were determined, and their antioxidant activities were quantitatively evaluated. In order to provide a theoretical basis for jujube breeding and the development of jujube active nutrients.
Materials and methods
Materials and chemicals
2, 2ʹ-diphenyl-1-picryhydrazyl (DPPH) from Shanghai Hualan Chemical Technology Co., Ltd (Shanghai, China). 2, 2ʹ-azinobis (3-ethylbenzothia zoline-6-sulfonic acid) diammonium salt (ABTS) from Hefei Bomei Biological Technology Co., Ltd (Anhui, China). Trifluoroacetic acid (TFA) from Shanghai McLean Biochemical Technology Co., Ltd (Shanghai, China) were purchased. Rutin, coumarin, caffeic acid, cinnamic acid, (+)-catechin, chlorogenic acid, and cAMP were obtained from Shanghai Yuanye Bio-Technology Co., Ltd (Shanghai, China). All other reagents used are analytical grade unless otherwise specified.
Thirty-seven jujube varieties () were collected from the red-ripe stage of Laoling city (Hundred jujube garden, latitude 37°81ʹ46, 70” N × longitude 117°31ʹ13, 16” E, 9 m above sea level), Shandong Province, China. The fruits of the similar sizes, without diseases, insect pests or mechanical damages, were randomly plucked from different tree species and brought to the laboratory on the same day and frozen at −20°C.
Table 1. Summary of tested cultivars of Ziziphus jujuba.
Sample preparation
The extraction solution was prepared by using the optimized conditions of the extraction process of jujube polyphenols obtained in the laboratory. The specific conditions are as follows. The 1.0 g freeze-dried jujube powder was extracted with 20 mL of aqueous ethanol (67%, v/v) under ultrasound at 275 W for 30 min. The extraction was done twice. The extracts were used for the determination of total phenols, total flavones, phenolic acids and antioxidant capacity.
Determination of polysaccharides
Jujube powder (0.5 g) and 12 mL (80%) ethanol were added into a 50 mL centrifuge tube and shaken evenly. Ultrasonic extraction lasted for 30 min and centrifugation lasted for 10 min. The insoluble substance was transferred to 50 mL centrifuge tube and 25 mL water was added. Ultrasonic extraction was performed for 30 min and repeated for 2 times. The solution was filled to a constant volume into 100 mL volumetric flask. The total polysaccharide content was measured using phenol sulfuric acid colorimetric method[Citation21] and slight modification. Simply, 0.5 mL standard glucose solution (0.04–0.2 mg/mL) or sample solution was added with 1 mL phenol and 5 mL concentrated sulfuric acid and mixed well with water at 30°C for 30 min. The absorbance was measured at 490 nm.
Determination of ascorbic acid
Ascorbic acid content was determined by 2, 6-dichloro-indophenol titration method[Citation22] and modified appropriately. Homogenized sample (2.0 g) was blended with 40 mL of oxalic acid solution (20 g/L). The 5 mL filtered solution was diluted with 20 g/L oxalic acid to 10 mL, and titrated with 2, 6-dichloro-indophenol (0.69 mmol/L) to pink, and the color was kept fade-free for 15 seconds. The content was expressed as mg/100 g FW.
Determination of cAMP
The content of cAMP was determined by HPLC[Citation23] and slightly modified. Jujube powder (0.1 g) was blended with 50 mL distilled water. Ultrasonic assisted extraction was performed for 30 min. The extract solution was centrifuged for 15 min and the supernatant was filtered through a 0.45 µm polytetrafluoroethylene (PTFE) filter. HPLC conditions were as follows: Type LC-20A, equipped with PDA detector; Inertsil ODS-3 C18 column (250 mm × 4.6 mm, 5 µm); Mobile phase consisted of 0.05 mol/L KH2PO4-methanol (volume ratio = 80:20) at a flow rate of 0.6 mL/min; isometric elution; Column temperature was maintained at 30°C, injection volume 10 µL, detection wavelength 254 nm. The content of cAMP was computed employing the formula obtained from the regression equation. The concentration was represented as milligrams per 100 g of DW.
Determination of total phenolic
The total phenol content was measured by Folin-Ciocalteu method[Citation24] and some adjustments. Simply, 0.5 mL gallic acid standard solution (0.04–0.20 mg/mL) or polyphenol solution into 10 mL colorimetric tube was poured, 0.5 mL forinol-phenol reagent (0.5 N) was added, and shook well. Let stand for 5 min, added 2.5 mL Na2CO3 (10% w/v) solution, added deionized water and made the volume reach 10 mL. The solution was shaken well and placed still in the dark for 1 h. The detection wavelength was 760 nm. The results were expressed as milligrams of gallic acid equivalent (GAE)/g DW.
Determination of total flavonoids
The content of total flavonoids in jujube fruit was determined by colorimetric method .[Citation25] Simply, 1.0 mL rutin standard solution (0.10–0.50 mg/mL) or extract sample solution into 10 mL colorimetric tube was absorbed, 0.3 mL of 5% NaNO2 and 0.3 mL of 10% Al(NO3)3 were added in turn, the solution was shaken well and then placed still for 5 min for settling. Then, 4.0 mL of 4% NaOH was added to the solution and diluted with 60% ethanol solution to scale. The solution was shaken well and placed still for 15 min. The detection wavelength was 510 nm. With rutin as the standard, the flavonoids content was expressed in rutin equivalent (RE.)/100 g DW.
Determination of triterpenic acid
Jujube powder (1.0 g) and 25 mL ethanol (70%) were ultrasonically extracted for 30 min at 60°C. The extract was extracted twice and the final volume was 100 mL. Sample solution (0.1 mL) or oleanolic acid standard solution (0.2–1.2 mg/mL) was poured into a test tube and evaporated till dryness in a water bath (T ≥ 85°C). Then, 0.2 mL of vanillin-acetic acid solution (5:95 w/v) and 0.8 mL HClO4 were added and shaken well. The mixture was placed in a water bath (T = 60°C) for 15 min before it was cooled to room temperature. Glacial acetic acid (5.0 mL) was added to it and blended. The absorbance was performed at 547 nm. The result was expressed as mg oleanolic acid equivalent/g DW (mg OAE/g DW) .[Citation26]
Qualitative and quantitative analysis of phenolic substances
The extracting solution was concentrated by rotary evaporation at 40°C, and diluted to 25 mL with chromatography grade methanol. The extract was filtered through 0.45 µm organic membrane. HPLC conditions were as follows: Type LC-20A, equipped with PDA detector; C18 analytical column (250 × 4.6 mm i.d.); Column temperature was maintained at 30°C, injection volume 10 µL, detection wavelength 280 nm; Mobile phase consisted of (A) 5% methanol (0.1% TFA) and (B) methanol at a flow rate of 0.8 mL/min. Gradient elution was as follows: 0–30 min (20% B), 30–35 min (20–35% B), 35–60 min (35–60% B), 60–65 min (60–100% B), 65–75 min (100% B), 75–80 min (100–20% B), and 80–85 min (20% B). The phenolic acid was quantitatively analyzed by external standard method, and their contents were represented as mg/100 g DW.
Antioxidant capability
DPPH scavenging assay
The ability of jujube extract to scavenge DPPH free radicals was measured according to the previously reported method, [Citation27] with appropriate modification. Extracting solution (0.1 mL) or the standard Vc solution (0–0.30 mg/mL) was blended with 5.0 mL ethanolic solution of DPPH (0.1 mmol/L). After 30 min, the absorbance value was measured at 517 nm. The DPPH clearance rate was calculated using the following EquationEq. (1)(1)
(1) . The results were expressed as mg Vc/g DW.
where A0 and At are the absorbances of the control sample and the sample solution, severally.
ABTS scavenging assay
The scavenging capacity of ABTS cationic radical was measured by a reported method[Citation28] with some modifications. Mix 7.0 mmol/L ABTS solution and 5 mL 2.45 mmol/L K2S2O8 solution in an equal proportion and leave for 12–16 h in the dark. On the day of testing, the absorbance of ABTS reserve solution diluted to 734 nm wavelength was 0.700 ± 0.050. The 0.2 mL extract or the standard solution (0–0.10 mg/mL of trolox) was blended with 5.0 mL ABTS solution. After 8 min, the absorbance was determined at 734 nm. The ABTS clearance rate was calculated using the following EquationEq. (2)(2)
(2) . The results were expressed as mg trolox/g DW.
where A0 and At are the absorbances of the control sample and the sample solution, severally.
FRAP reducing power assay
Extracting solution (0.6 mL) or the standard trolox solution (0–0.50 mg/mL), 2.5 ml phosphate buffer solution (0.2 mol/L) and 2.5 mL K3Fe(CN)6 solution (1% w/v) were successively added into the colorimetric tube. Mixture was then immersed in a water bath (T = 45°C) for 20 min. After cooling, 2.5 mL trichloroacetic acid (TCA) (10% w/v) was added, shaken, and centrifuged. Supernatant (2.5 mL) was absorbed into another colorimeter tube and then 2.5 mL deionized water and 0.5 mL FeCl3 (0.1% w/v) were added in turn .[Citation29] After 8 min, the absorbance was measured at 700 nm. The results were expressed as mg trolox/g DW.
Statistical analysis
The data in the tables were expressed as the mean ± standard deviation (SD). The significance of independent variables was compared using Duncan’s multiple range test and P < .05 indicated significant difference. The correlation of date was determined by Pearson correlation analysis. The data were also analyzed by Principal Component Analysis. Origin 2018 and SPSS 24 statistical software were used.
Results and discussion
Analysis of polysaccharides, ascorbic acid, and cAMP
Polysaccharide is an important biological component in jujube and has been shown to have antioxidant, anti-tumor, liver protection, blood glucose reduction, and other biological activities .[Citation20,Citation30] In our study, the polysaccharides content in jujube fruits ranged from 1.64 g/100 g DW of ‘LJZ’ to 16.48 g/100 g DW of ‘GTXZ’ (). The polysaccharide content of ‘GTXZ’ is significantly higher than that of other jujube varieties, so it may be an ideal research object of jujube polysaccharide.
Table 2. Total phenols, total flavonoids, total triterpene, polysaccharides, Vc and cAMP of thirty-seven jujube cultivars
Ascorbic acid, as a natural antioxidant, has a good scavenging ability on reactive oxygen species (ROS), and plays a role in protecting cells from oxidative damage .[Citation31] Jujube is rich in Vc, which has the reputation of “natural vitamin pill.” An average of 20 grams of jujube can meet the daily Vc requirement of adults .[Citation32] The content of Vc varied greatly among different jujube varieties. The ascorbic acid content of thirty-seven jujube fruits ranged from 120.14 mg/100 g FW of ‘DGZ’ to 359.22 mg/100 g FW of ‘YL1H,’ which was basically consistent with the results measured by Kou et al., [Citation33] who measured 1.67–4.24 mg/g FW. Jujube fruits were considered a good source of ascorbic acid in the diet.
cAMP is an important substance involved in the regulation of substance metabolism and biological function in cells, and is the second messenger of life information transmission .[Citation34,Citation35] cAMP is an important active substance in jujube, which has the effects of anti-oxidation, improving immunity, nerve protection, and anticancer properties .[Citation36] Studies have shown that jujube has the highest cAMP content among more than 180 kinds of natural plants .[Citation37] In our study, the highest content of cAMP measured in ‘LYX’ was 41.56 mg/100 g DW; however, it wasn’t detected in ‘GHDZ.’ Therefore, ‘LYX’ was a promising source of cAMP.
Analysis of secondary metabolites in jujube fruit
Flavonoid is a kind of natural phenolic compound that have been shown to have a wide range of pharmacological effects, such as antiviral, anti-inflammatory and anti-obesity effects .[Citation38–40] The total flavonoids content of the jujube fruit measured ranged from 7.07 mg/g DW of ‘JS1H’ to 41.18 mg/g DW of ‘QXYZ,’ which was higher than the content of flavonoids determined by Rashwan et al., [Citation2] which was 0.7–1.8 g/100 g DW. The total polyphenol content in jujube fruit ranged from 8.45 mg GAE/g DW of ‘JS4H’ to 16.33 mg GAE/g DW of ‘QXYZ,’ which was similar than 8.76–21.61 mg GAE/g determined by Ivanišová et al. .[Citation41] The difference of polyphenols content may be caused by the difference in jujube varieties and their origin .[Citation1]
Triterpenic acid has attracted much attention due to its anti-inflammatory, antibacterial, liver-protecting, and antioxidant effects .[Citation42] In our work, the total content of triterpenes in thirty-seven jujube varieties ranged from 24.97 mg/g DW of ‘SZDZ’ to 78.33 mg/g DW of ‘JS3H.’ In addition, the contents of triterpenoid acids in ‘LXMZ’ (75.61 mg OAE/g DW), ‘LLJXZ’ (74.94 mg OAE/g DW), ‘WDXZ’ (73.21 mg OAE/g DW) and ‘JZ’ (73.12 mg OAE/g DW) were also higher. Thus, ‘JS3H,’ ‘LXMZ,’ ‘LLJXZ,’ ‘WDXZ’ and ‘JZ’ are promising source of triterpenes. The growth conditions of all jujube varieties in this study are the same. The difference in composition between the varieties mentioned above may be caused by the genotype of jujube.
Composition and content of phenolic substances in different jujube fruit
Many studies have shown that polyphenols are a good source of natural antioxidants among the antioxidants consumed in the diet .[Citation43,Citation44] Some studies compared the total phenols and antioxidant activities of 62 kinds of fruits and found that jujube is rich in phenols and has high antioxidant activity, making it an excellent natural antioxidant food .[Citation45] In this study, six phenolic substances in jujube were determined by high performance liquid chromatography ( and ). In general, the content of polyphenols in different red jujube varieties is very different. It can be seen from that this kind of composition is found in the vast majority of date varieties, which is similar to the observations found by Zhao et al. .[Citation46] The contents of (+)-catechin, caffeic acid and rutin in jujube fruits of different varieties were relatively rich, which were higher than the other three phenolic compounds, and are obviously dominant. The contents of (+)-catechin, caffeic acid, and rutin in ‘QXYZ’ were the highest, which were 65.31 mg/100 g DW, 26.29 mg/100 g DW and 39.16 mg/100 g DW, respectively. A previous study[Citation33] reported the total phenolic content from fifteen jujube cultivars, but ignored the content of individual phenolic substances. As a matter of fact, the phenolic acid content measured in this work were higher than previously reported .[Citation24] The reason for this phenomenon may be due to differences in detection methods, varieties and regions .[Citation46]
Table 3. Contents of phenolic acids in extracts of thirty-seven jujube cultivars
Figure 1. HPLC of the phenolic extracts from jujube (a: Standard; b: ‘QXYZ.’)
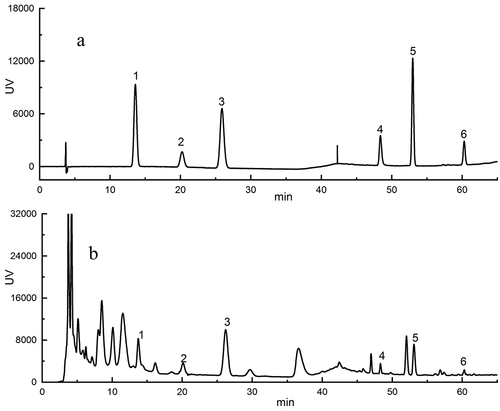
At present, many studies have shown that these monomeric phenols can prevent and treat many related diseases caused by oxidative stress. For example, (+)-catechin and caffeic acid have a wide range of antibacterial properties, [Citation47,Citation48] antiviral activity, [Citation49,Citation50] etc. Rutin can be transformed into quercetin in the body, which inhibits adipocyte differentiation and plays a role in alleviating obesity .[Citation51] Therefore, jujube as a high quality source of polyphenols, has important research and development value.
Antioxidant capacity
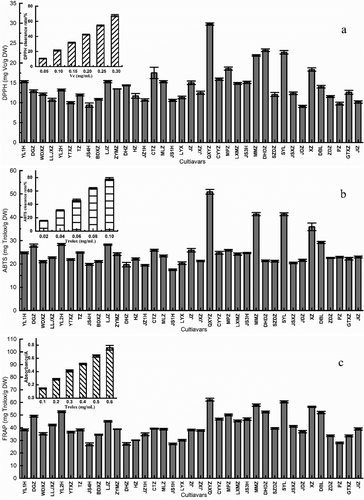
Free radicals are constantly produced in the body due to continuous contact with the outside world, including respiration (oxidation reaction), external pollution, radiation exposure, and other factors .[Citation52] Scientific research has indicated that cancer, aging or other diseases are mostly linked to the production of excess free radicals .[Citation53] The mechanism of antioxidant action is to directly act on free radicals or indirectly consume substances that are easy to generate free radicals to prevent further reactions. Therefore, in recent years, the natural antioxidants contained in food and their effects have become the focus of extensive research .[Citation54]
In this work, the antioxidant abilities of DPPH, ABTS and FRAP extracts from different jujube fruits were measured, and the results were shown in . The results of DPPH measurement showed that ‘QXYZ’ has high antioxidant activity with a value of 29.79 mg Vc/g DW, followed by ‘DHDZ.’ The ‘QXYZ’ was shown to have the highest antioxidant activity based on ABTS and FRAP assays, with values of 50.90 mg Trolox/g DW and 62.13 mg Trolox/g, followed by ‘SYL’ and ‘MMZ.’ The ABTS scavenging capacities of thirty-seven jujube species ranged from 17.50 mg Trolox/g DW of ‘JS1H’ to 50.90 mg Trolox/g DW of ‘QXYZ,’ and FRAP values ranged from 26.87 mg Trolox/g DW of ‘JS4H’ to 62.13 mg Trolox/g DW of ‘QXYZ,’ which were lower than the measured results (ABTS: 59.67–118.56 mg Trolox/g DW; FRAP: 44.20–87.15 mg Trolox/g DW) by Wojdyło et al. .[Citation55] This phenomenon may be caused by the variety and regional differences of jujube. Studies have shown that showed that the antioxidant capacity of jujube fruit decreased with the increase of maturity .[Citation5,Citation56,Citation57] Nonetheless, the ABTS free radical scavenging ability of jujube fruit was 2–3 times of that of common fruits, such as lemon, litchi, apple, avocado, cantaloupe, banana, caramel, durian, longan, papaya, etc .[Citation39] Thus, jujube fruit was a good source of natural antioxidants.
Correlation analysis
In order to investigate the effect of the main active ingredients on antioxidant capacity of jujube fruit, the correlation coefficients were calculated (). Our results showed that both total phenols and total flavonoids were significantly correlated with antioxidant capacity, which is similar with earlier report (DPPH: R = 0.565; FRAP: R = 0.871*) (DPPH: R = 0.654*; FRAP: R = 0.836**) .[Citation5] In addition, (+)-catechin, caffeic acid, rutin, and ascorbic acid were found to have a positive correlation with antioxidant capacity. However, the contents of cAMP and coumarin were also negatively correlated with the three types of antioxidant capacities. The content of total polysaccharides was negatively correlated with DPPH free radical scavenging capacity (DPPH, R = −0.052). Kou et al.[Citation33] reported similar result (DPPH, R = −0.041). The mechanism behind this outcome was unknown, as polysaccharides had been certified to have antioxidant capacity .[Citation58,Citation59]
Table 4. Correlation coefficients (R) between each antioxidant capacity method and the active ingredients of jujube fruits
Principal component analysis and hierarchical cluster analysis
It is distinctly that the active components and antioxidant activities of different varieties of jujube fruits are different. All the results were analyzed by Principal component analysis (PCA) and hierarchical clustering analysis (HCA). The results are shown in and . All the variables were divided into four principal components, accounting for 76.31% of the total variance. The first principal component explained 47.80% of the total variance, including total phenols, total flavonoids, Vc, (+)-catechin, caffeic acid, rutin, DPPH, ABTS, FRAP. The second principle component explains 10.77% of total variance with triterpene acid and chlorogenic acid. The third principal component covers cAMP and cinnamic acid can explain 9.82% of total variance, while the forth principal component possesses polysaccharide and coumarin with 8.54% of total variance.
Table 5. Eigenvalues, cumulative contribution and eigenvectors associated with four principal components for the jujube fruits
Figure 3. PCA of thirty-seven different jujube cultivars. (a) Loading plot of PCA, (b) scores scatter plot of PCA, and (c) Scores histogram of PCA
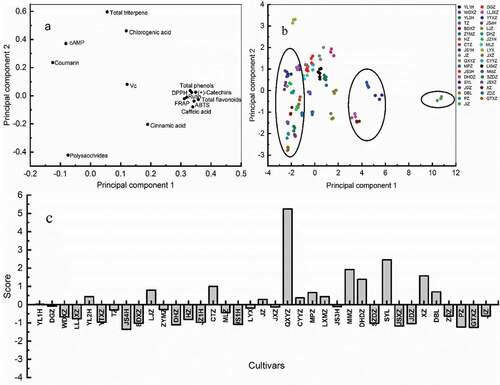
Thirty-seven kinds of jujube fruits were scored, as shown in ). The top five jujube varieties with comprehensive score were ‘QXYZ,’ ‘SYL,’ ‘MMZ,’ ‘XZ’ and ‘DHDZ.’ The results of our study indicated that ‘QXYZ’ had high total phenols, total flavonoids, FRAP, DPPH and ABTS scavenging activities, and was suitable for food production. This study provides important information for further research on jujube fruits and provides ideas for investigating the potential health benefits of jujube fruits.
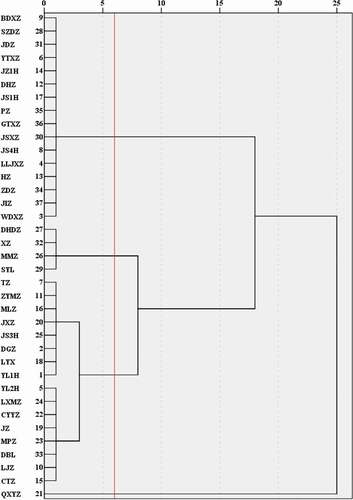
HCA was used to analyze the genetic relationship between different jujube species (). The clusters obtained from principal component analysis can be confirmed by the clusters obtained from HCA. From all the results, ‘QXYZ’ forms an independent cluster, which contains the highest active ingredients and antioxidant capacity. The main varieties in the second cluster are ‘SYL,’ ‘MMZ,’ ‘XZ,’ ‘DHDZ,’ and their active ingredients and antioxidant activities are relatively high. Sixteen jujube cultivars, such as ‘JS4H,’ ‘BDXZ,’ ‘DHZ’ and ‘SZDZ,’ formed a cluster, and the content of active ingredients and antioxidant capacity of them were the lowest. Therefore, according to its active ingredient content and antioxidant capacity, thirty-seven jujube varieties were distinguished.
Conclusion
In summary, this study conducted on thirty-seven red jujube varieties, including total phenols, total flavonoids, Vc, polysaccharides, triterpene acids, cAMP, six phenolic acids and antioxidant activity. The results exhibited that there were differences in active components and antioxidant capacity among jujube cultivars. Among them, the contents of total phenols (16.33 mg GAE/g DW), total flavones (41.18 mg RE/g DW), (+)-catechin (65.31 mg/100 g DW), caffeic acid (26.29 mg/100 g DW), rutin (39.16 mg/100 g DW) and cinnamic acid (0.33 mg/100 g DW) in ‘QXYZ’ were the highest. The highest contents of total triterpenes, polysaccharides, ascorbic acid, cAMP, chlorogenic acid, and coumarin were ‘JS3H,’ ‘GTXZ,’ ‘YL1H,’ ‘LYX,’ ‘CTZ,’ and ‘CYYZ,’ respectively. ‘QXYZ’ has the highest free radical scavenging ability and reducing ability among thirty-seven kinds of jujube. Among them, total phenols, total flavonoids, (+)-catechins, caffeic acid and rutin have a high positive correlation with antioxidant capacity. The results of principal component analysis showed that ‘QXYZ,’ ‘SYL,’ ‘MMZ,’ ‘XZ’ and ‘DHDZ’ were the top five jujubes, which contained more active components. Our research results can provide references for the selection of varieties in the planting and production of jujube and the development of active foods.
Acknowledgments
The authors declare that there is no conflict of interest.
Additional information
Funding
References
- Wang, B. N.; Liu, L. G.; Huang, Q. Y.; Luo, Y. Quantitative Assessment of Phenolic Acids, Flavonoids and Antioxidant Activities of Sixteen Jujube Cultivars from China. Plant Foods Hum. Nutr. 2020, 75(2), 154–160. DOI: https://doi.org/10.1007/s11130-020-00796-1.
- Rashwan, A. K.; Karim, N.; Shishir, M. R. I.; Bao, T.; Lu, Y.; Chen, W. Jujube Fruit: A Potential Nutritious Fruit for the Development of Functional Food Products. J. Funct. Foods. 2020, 75, 104205. DOI: https://doi.org/10.1016/j.jff.2020.104205.
- Zhang, R. T.; Sun, X.; Zhang, K. Q.; Zhang, Y. L.; Song, Y. R.; Wang, F. Z. Fatty Acid Composition of 21 Cultivars of Chinese Jujube Fruits (Ziziphus Jujuba Mill.). J. Food Meas. Charact. 2021(15), 1225-1240. DOI: https://doi.org/10.1007/s11694-020-00718-4.
- Zhang, L.; Liu, X. Q.; Wang, Y. J.; Liu, G. P.; Zhang, Z.; Zhao, Z. X.; Cheng, H. L. In Vitro Antioxidative and Immunological Activities of Polysaccharides from Zizyphus Jujuba cv.Muzao. Int. J. Biol. Macromol. 2017, 95, 1119–1125. DOI: https://doi.org/10.1016/j.ijbiomac.2016.10.102.
- Wang, B. N.; Huang, Q. Y.; Venkitasamy, C.; Chai, H. K.; Gao, H.; Cheng, N.; Cao, W.; Lv, X. G.; Pan, Z. L. Changes in Phenolic Compounds and Their Antioxidant Capacities in Jujube (Ziziphus Jujuba Miller) during Three Edible Maturity Stages. LWT Food Sci. Technol. 2016, 66, 56–62. DOI: https://doi.org/10.1016/j.lwt.2015.10.005.
- Reche, J.; Almansa, M. S.; Hernandez, F.; Carbonell-Barrachina, A. A.; Legua, P.; Amoros, A. Fatty Acid Profile of Peel and Pulp of Spanish Jujube (Ziziphus Jujuba Mill.) Fruit. Food Chem. 2019, 295, 247–253. DOI: https://doi.org/10.1016/j.foodchem.2019.05.147.
- Pilerood, S. A.; Prakash, J. Evaluation of Nutritional Composition and Antioxidant Activity of Borage (Echium Amoenum) and Valerian (Valerian Officinalis). J. Food Sci. Technol. 2014, 51(5), 845–854. DOI: https://doi.org/10.1007/s13197-011-0573-z.
- Karimi, E.; Oskoueian, E.; Karimi, A.; Noura, R.; Ebrahimi, M. Borago Officinalis L. Flower: A Comprehensive Study on Bioactive Compounds and Its Health-promoting Properties. J. Food Meas. Charact. 2018, 12(2), 826–838. DOI: https://doi.org/10.1007/s11694-017-9697-9.
- Karimi, E.; Mehrabanjoubani, P.; Homayouni-Tabrizi, M.; Abdolzadeh, A.; Soltani, M. Phytochemical Evaluation, Antioxidant Properties and Antibacterial Activity of Iranian Medicinal Herb Galanthus Transcaucasicus Fomin. J. Food Meas. Charact. 2018, 12(1), 433–440. DOI: https://doi.org/10.1007/s11694-017-9656-5.
- Ravanfar, S. A.; Karimi, E.; Mehrabanjoubani, P.; Ebrahimi, M. Enhancement of Phenolic and Flavonoids Compounds, Antioxidant and Cytotoxic Effects in Regenerated Red Cabbage by Application of Zeatin. Nat. Prod. Res. 2020, 34(6), 898–902. DOI: https://doi.org/10.1080/14786419.2018.1508145.
- Karimi, E.; Ghorbani Nohooji, M.; Habibi, M.; Ebrahimi, M.; Mehrafarin, A.; Khalighi-Sigaroodi, F. Antioxidant Potential Assessment of Phenolic and Flavonoid Rich Fractions of Clematis Orientalis and Clematis Ispahanica (Ranunculaceae). Nat. Prod. Res. 2018, 32(16), 1991–1995. DOI: https://doi.org/10.1080/14786419.2017.1359171.
- Song, L.; Liu, P.; Yan, Y.; Huang, Y.; Bai, B.; Hou, X.; Zhang, L. Supercritical CO2 Fluid Extraction of Flavonoid Compounds from Xinjiang Jujube (Ziziphus Jujuba Mill.) Leaves and Associated Biological Activities and Flavonoid Compositions. Ind. Crops Prod. 2019, 139, 111508. DOI: https://doi.org/10.1016/j.indcrop.2019.111508.
- Yang, T.; Fang, L.; Lin, T.; Li, J.; Zhang, Y.; Zhou, A.; Xie, J. Ultrasonicated Sour Jujube Seed Flavonoids Extract Exerts Ameliorative Antioxidant Capacity and Reduces Abeta-induced Toxicity in Caenorhabditis Elegans. J. Ethnopharmacol. 2019, 239, 111886. DOI: https://doi.org/10.1016/j.jep.2019.111886.
- Gao, Q. H.; Wu, C. S.; Wang, M. The Jujube (Ziziphus Jujuba Mill.) Fruit: A Review of Current Knowledge of Fruit Composition and Health Benefits. J. Agric. Food. Chem. 2013, 61(14), 3351–3363. DOI: https://doi.org/10.1021/jf4007032.
- Sun, X.; Gu, D. Y.; Fu, Q. B.; Gao, L.; Shi, C.; Zhang, R. T.; Qiao, X. G. Content Variations in Compositions and Volatile Component in Jujube Fruits during the Blacking Process. Food Sci. Nutr. 2019, 7(4), 1387–1395. DOI: https://doi.org/10.1002/fsn3.973.
- Lin, T. T.; Liu, Y.; Lai, C. J. S.; Yang, T. T.; Xie, J. B.; Zhang, Y. Q. The Effect of Ultrasound Assisted Extraction on Structural Composition, Antioxidant Activity and Immunoregulation of Polysaccharides from Ziziphus Jujuba Mill Var. Spinosa Seeds. Ind. Crops Prod. 2018, 125, 150–159. DOI: https://doi.org/10.1016/j.indcrop.2018.08.078.
- Zhan, R.; Xia, L.; Shao, J. H.; Wang, C.; Chen, D. F. Polysaccharide Isolated from Chinese Jujube Fruit (Zizyphus Jujuba Cv. Junzao) Exerts Anti-inflammatory Effects through MAPK Signaling. J. Funct. Foods. 2018, 40, 461–470. DOI: https://doi.org/10.1016/j.jff.2017.11.026.
- Cheng, D.; Zhu, C. Q.; Cao, J. K.; Jiang, W. B. The Protective Effects of Polyphenols from Jujube Peel (Ziziphus Jujube Mill) on Isoproterenol-induced Myocardial Ischemia and Aluminum-induced Oxidative Damage in Rats. Food Chem. Toxicol. 2012, 50(5), 1302–1308. DOI: https://doi.org/10.1016/j.fct.2012.01.026.
- Liu, G.; Liu, X.; Zhang, Y. Hepatoprotective Effects of Polysaccharides Extracted from Zizyphus Jujube Cv. Huanghetanzao. Int. J. Biol. Macromol. 2015, 76, 169–175. DOI: https://doi.org/10.1016/j.ijbiomac.2015.01.061.
- Ji, X. L.; Peng, Q.; Yuan, Y. P.; Shen, J.; Xie, X. Y.; Wang, M. Isolation, Structures and Bioactivities of the Polysaccharides from Jujube Fruit (Ziziphus Jujuba Mill.): A Review. Food Chem. 2017, 227, 349–357. DOI: https://doi.org/10.1016/j.foodchem.2017.01.074.
- Lu, L. F.; Luo, A. Y.; Lin, B. Y.; Xie, Z. L.; Gan, G. Y.; Huang, X. Z.; Lei, Z. D.; Chen, S. M.; Huang, S. Y. Optimization of Ultrasonic Extraction Process of Oenanthe Benghalensss Polysaccharide by Orthogonal Test. Med. Plant. 2018, 9(5), 23–26.
- Moo-Huchin, V. M.; Estrada-Mota, I.; Estrada-León, R.; Cuevas-Glory, L.; Ortiz-Vázquez, E.; Vargas, M. L. V.; Betancur-Ancona, D.; Sauri-Duch, E. Determination of Some Physicochemical Characteristics, Bioactive Compounds and Antioxidant Activity of Tropical Fruits from Yucatan, Mexico. Food Chem. 2014, 152, 508–515. DOI: https://doi.org/10.1016/j.foodchem.2013.12.013.
- Chen, K.; Fan, D. Y.; Fu, B.; Zhou, J. Z.; Li, H. R. Comparison of Physical and Chemical Composition of Three Chinese Jujube (Ziziphus Jujuba Mill.) Cultivars Cultivated in Four Districts of Xinjiang Region in China. Food Sci. Technol. 2019, 39(4), 912–921. DOI: https://doi.org/10.1590/fst.11118.
- Xie, P. J.; You, F.; Huang, L. X.; Zhang, C. H. Comprehensive Assessment of Phenolic Compounds and Antioxidant Performance in the Developmental Process of Jujube (Ziziphus Jujuba Mill.). J. Funct. Foods. 2017, 36, 233–242. DOI: https://doi.org/10.1016/j.jff.2017.07.012.
- Xie, G. F.; Xu, X. Y.; Zhou, X. L.; Liu, Y. L.; Zhao, Z. B. Changes in Phenolic Profiles and Antioxidant Activity in Rabbiteye Blueberries during Ripening. Int. J. Food Prop. 2019, 22(1), 320–329. DOI: https://doi.org/10.1080/10942912.2019.1580718.
- Song, L. J.; Zhang, L.; Xu, L.; Ma, Y. J.; Lian, W. S.; Liu, Y. G.; Wang, Y. H. Optimized Extraction of Total Triterpenoids from Jujube (Ziziphus Jujuba Mill.) And Comprehensive Analysis of Triterpenic Acids in Different Cultivars. Plants. 2020, 9(4), 412. DOI: https://doi.org/10.3390/plants9040412.
- Brand-Williams, W.; Cuvelier, M. E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. Lebensm. Wiss. Technol. 1995, 28(1), 28–30. DOI: https://doi.org/10.1016/S0023-6438(95)80008-5.
- Miller, N. J.; Rice-Evans, C. A. Factors Influencing the Antioxidant Activity Determined by the ABTS.+ Radical Cation Assay. Free Radical Res. 1997, 26(3), 195–199. DOI: https://doi.org/10.3109/10715769709097799.
- Lin, X. M.; Ji, X. L.; Wang, M.; Yin, S.; Peng, Q. An Alkali-extracted Polysaccharide from Zizyphus Jujuba Cv. Muzao: Structural Characterizations and Antioxidant Activities. Int. J. Biol. Macromol. 2019, 136, 607–615. DOI: https://doi.org/10.1016/j.ijbiomac.2019.06.117.
- Xie, J. H.; Tang, W.; Jin, M. L.; Li, J. E.; Xie, M. Y. Recent Advances in Bioactive Polysaccharides from Lycium Barbarum L., Zizyphus Jujuba Mill, Plantago Spp., And Morus Spp.: Structures and Functionalities. Food Hydrocolloids. 2016, 60, 148–160. DOI: https://doi.org/10.1016/j.foodhyd.2016.03.030.
- Asensi-Fabado, M. A.; Munne´-Bosch, S. Vitamins in Plants: Occurrence, Biosynthesis and Antioxidant Function. Trends Plant Sci. 2010, 15(10), 582–592. DOI: https://doi.org/10.1016/j.tplants.2010.07.003.
- Li, J. W.; Fan, L. P.; Ding, S. D.; Ding, X. L. Nutritional Composition of Five Cultivars of Chinese Jujube. Food Chem. 2007, 103(2), 454–460. DOI: https://doi.org/10.1016/j.foodchem.2006.08.016.
- Kou, X. H.; Chen, Q.; Li, X. H.; Li, M. F.; Kan, C.; Chen, B.; Zhang, Y.; Xue, Z. H. Quantitative Assessment of Bioactive Compounds and the Antioxidant Activity of 15 Jujube Cultivars. Food Chem. 2015, 173, 1037–1044. DOI: https://doi.org/10.1016/j.foodchem.2014.10.110.
- Leslie, S. N.; Nairn, A. C. cAMP Regulation of Protein Phosphatases PP1 and PP2A in Brain. BBA-Mol. Cell Res. 2019, 1866, 64–73.
- Tavares, L. P.; Negreiros-Lima, G. L.; Lima, K. M.; PMR, E. S.; Pinho, V.; Teixeira, M. M.; Sousa, L. P. Blame the Signaling: Role of cAMP for the Resolution of Inflammation. Pharmacol. Res. 2020, 159, 105030.
- Chen, C.; Li, H. Y.; Lv, X. Y.; Tang, J.; Chen, C.; Zheng, X. X. Application of near Infrared Spectroscopy Combined with SVR Algorithm in Rapid Detection of cAMP Content in Red Jujube. Optick. 2019, 194, 163063.
- Giannattasio, M.; Mandato, E.; Macchia, V. Content of 3′,5′ Cyclic AMP and Cyclic AMP Phosphodiesterase in Dormant and Activated Tissues of Jerusalem Artichoke Tubers. Biochem. Biophys. Res. Commun. 1974, 57(2), 365–371. DOI: https://doi.org/10.1016/0006-291X(74)90939-5.
- Wang, L.; Song, J.; Liu, A.; Xiao, B.; Li, S.; Wen, Z.; Lu, Y.; Du, G. Research Progress of the Antiviral Bioactivities of Natural Flavonoids. Natur. Prod. Bioprosp. 2020, 10(5), 271–283. DOI: https://doi.org/10.1007/s13659-020-00257-x.
- Owona, B. A.; Abia, W. A.; Moundipa, P. F. Natural Compounds Flavonoids as Modulators of Inflammasomes in Chronic Diseases. Int. Immunopharmacol. 2020, 84, 106498. DOI: https://doi.org/10.1016/j.intimp.2020.106498.
- Song, D.; Cheng, L.; Zhang, X.; Wu, Z.; Zheng, X. The Modulatory Effect and the Mechanism of Flavonoids on Obesity. J. Food Biochem. 2019, 43(8), e12954. DOI: https://doi.org/10.1111/jfbc.12954.
- Ivanišová, E.; Grygorieva, O.; Abrahamová, V.; Schubertova, Z.; Terentjeva, M.; Brindza, J. Characterization of Morphological Parameters and Biological Activity of Jujube Fruit (Ziziphus Jujuba Mill.). J. Berry Res. 2017, 7(4), 249–260. DOI: https://doi.org/10.3233/JBR-170162.
- Guo, S.; Duan, J. A.; Qian, D. W.; Tang, Y. P.; Wu, D. W.; Su, S. L.; Wang, H. Q.; Zhao, Y. N. Content Variations of Triterpenic Acid, Nucleoside, Nucleobase, and Sugar in Jujube (Ziziphus Jujuba) Fruit during Ripening. Food Chem. 2015, 167, 468–474. DOI: https://doi.org/10.1016/j.foodchem.2014.07.013.
- Ma, T. T.; Sun, X. Y.; Gao, G. T.; Wang, X. Y.; Liu, X. Y.; Du, G. R.; Zhan, J. C. Phenolic Characterisation and Antioxidant Capacity of Young Wines Made from Different Grape Varieties Grown in Helanshan Donglu Wine Zone (China). S. Afr. J. Enol. Vitic. 2014, 35(2), 321–331.
- Wang, L.; Sun, X.; Li, F.; Yu, D.; Liu, X.; Huang, W.; Zhan, J. Dynamic Changes in Phenolic Compounds, Colour and Antioxidant Activity of Mulberry Wine during Alcoholic Fermentation (Article). J. Funct. Foods. 2015, 18, 254–265. DOI: https://doi.org/10.1016/j.jff.2015.07.013.
- Fu, L.; Xu, B. T.; Xu, X. R.; Gan, R. Y.; Zhang, Y.; Xia, E. Q.; Li, H. B. Antioxidant Capacities and Total Phenolic Contents of 62 Fruits. Food Chem. 2011, 129(2), 345–350. DOI: https://doi.org/10.1016/j.foodchem.2011.04.079.
- Zhao, H. X.; Zhang, H. S.; Yang, S. F. Phenolic Compounds and Its Antioxidant Activities in Ethanolic Extracts from Seven Cultivars of Chinese Jujube. Food Sci. Hum. Well. 2014, 3(3–4), 183–190. DOI: https://doi.org/10.1016/j.fshw.2014.12.005.
- Bernal-Mercado, A. T.; Gutierrez-Pacheco, M. M.; Encinas-Basurto, D.; Mata-Haro, V.; Lopez-Zavala, A. A.; Islas-Osuna, M. A.; Gonzalez-Aguilar, G. A.; Ayala-Zavala, J. F. Synergistic Mode of Action of Catechin, Vanillic and Protocatechuic Acids to Inhibit the Adhesion of Uropathogenic Escherichia Coli on Silicone Surfaces. J. Appl. Microbiol. 2020, 128(2), 387–400. DOI: https://doi.org/10.1111/jam.14472.
- Li, Q.; He, Y. N.; Shi, X. W.; Kang, L. Y.; Niu, L. Y.; Wang, X. G.; Feng, W. Clerodens E-J, Antibacterial Caffeic Acid Derivatives from the Aerial Part of Clerodendranthus Spicatus. Fitoterapia. 2016, 114, 110–114. DOI: https://doi.org/10.1016/j.fitote.2016.08.021.
- You, H. L.; Huang, C. C.; Chen, C. J.; Chang, C. C.; Liao, P. L.; Huang, S. T. Anti-pandemic Influenza A (H1N1) Virus Potential of Catechin and Gallic Acid. J. Chin. Med. Assoc. 2018, 81(5), 458–468. DOI: https://doi.org/10.1016/j.jcma.2017.11.007.
- Langland, J.; Jacobs, B.; Wagner, C. E.; Ruiz, G.; Cahill, T. M. Antiviral Activity of Metal Chelates of Caffeic Acid and Similar Compounds Towards Herpes Simplex, VSV-Ebola Pseudotyped and Vaccinia Viruses. Antiviral Res. 2018, 160, 143–150. DOI: https://doi.org/10.1016/j.antiviral.2018.10.021.
- Selloum, L.; Reichl, S.; Muller, M.; Sebihi, L.; Arnhold, J. Effects of Flavonols on the Generation of Superoxide Anion Radicals by Xanthine Oxidase and Stimulated Neutrophils. Arch. Biochem. Biophys. 2001, 395(1), 49–56. DOI: https://doi.org/10.1006/abbi.2001.2562.
- Zhang, H.; Jiang, L.; Ye, S.; Ye, Y.; Ren, F. Systematic Evaluation of Antioxidant Capacities of the Ethanolic Extract of Different Tissues of Jujube (Ziziphus Jujuba Mill.) From China. Food Chem. Toxicol. 2010, 48(6), 1461–1465. DOI: https://doi.org/10.1016/j.fct.2010.03.011.
- Santos, P. H. D.; Neves, S. M.; Sant’Anna, D. O.; Oliveira, C.; Carvalho, H. D. The Analytic Hierarchy Process Supporting Decision Making for Sustainable Development: An Overview of Applications. J. Cleaner Prod. 2019, 212, 119–138. DOI: https://doi.org/10.1016/j.jclepro.2018.11.270.
- Niki, E. Antioxidant Capacity: Which Capacity and How to Assess It? J. Berry Res. 2011, 1(4), 169–176. DOI: https://doi.org/10.3233/JBR-2011-018.
- Wojdyło, A.; Carbonell-Barrachina, Á. A.; Legua, P.; Hernández, F. Phenolic Composition, Ascorbic Acid Content, and Antioxidant Capacity of Spanish Jujube (Ziziphus Jujube Mill.) Fruits. Food Chem. 2016, 201, 307–314. DOI: https://doi.org/10.1016/j.foodchem.2016.01.090.
- Zozio, S.; Servent, A.; Cazal, G.; Mbéguié-A-Mbéguié, D.; Ravion, S.; Pallet, D.; Abel, H. Changes in Antioxidant Activity during the Ripening of Jujube (Ziziphus Mauritiana Lamk). Food Chem. 2014, 150, 448–456. DOI: https://doi.org/10.1016/j.foodchem.2013.11.022.
- Cosmulescu, S.; Trandafir, I.; Nour, V.; Achim, G.; Botu, M.; Iordanescu, O. Variation of Bioactive Compounds and Antioxidant Activity of Jujube (Ziziphus Jujuba) Fruits at Different Stages of Ripening. Not. Bot. Horti. Agrobo. 2018, 46(1), 134–137. DOI: https://doi.org/10.15835/nbha46110752.
- Ji, X. L.; Hou, C. Y.; Yan, Y. Z.; Shi, M. M.; Liu, Y. Q. Comparison of Structural Characterization and Antioxidant Activity of Polysaccharides from Jujube (Ziziphus Jujuba Mill.) Fruit. Int. J. Biol. Macromol. 2020, 149, 1008–1018. DOI: https://doi.org/10.1016/j.ijbiomac.2020.02.018.
- Liu, X. X.; Liu, H. M.; Yan, Y. Y.; Fan, L. Y.; Yang, J. N.; Wang, X. D.; Qin, G. Y. Structural Characterization and Antioxidant Activity of Polysaccharides Extracted from Jujube Using Subcritical Water. LWT Food Sci. Technol. 2020, 117, 108645. DOI: https://doi.org/10.1016/j.lwt.2019.108645.