?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Safety and quality of the final product depend on the oil quality that is normally used for the frying purpose. In current study, sunflower oil blend with extra virgin olive oil (EVOO) and palm olein was optimized by D-optimal design and the best blend was chosen for multiple frying of frozen potato fries. Ten frying were performed and free fatty acid (FFA), peroxide (PV) and iodine (IV) values including Fourier transform–infrared (FT-IR) spectral signatures were recorded after each frying. The spectral fingerprints were preprocessed with standard normal variate and then principle component analysis was applied, which showed clear differentiation for various frying. Similarly, partial least square regression was used to predict the FFA, PV and IV which showed high coefficient of determination (R2) 0.83, 0.92 and 0.85, respectively. It can be concluded that FT-IR can be used as an innovative tool for rapid and nondestructive evaluation of frying oils.
KEYWORDS:
Introduction
Frying is one of the oldest techniques used for the processing of foods, usually at temperature between 120°C and 190°C, for increased palatability and ease in preparation.[Citation1] Although air and steam frying are getting common, deep fat frying is still first choice of the consumer and industry to prepare the food that not only improves sensory attributes but also provides food with a crispy texture and specific flavor without considering the absorption of a large amount of oil into the food.[Citation2] Therefore, it is necessary to ensure the quality of oil as high temperature during frying process and the presence of air and water from the food products may result in the quality deterioration due to oxidation and hydrolysis fat contents.
Pure vegetable oils have little use in cooking and frying due to their poor stability against oxidative and hydrolytic changes that can cause serious cardiovascular diseases and obesity.[Citation3] Blending of different oils is an easy, viable and cost-effective way of altering the oil’s physicochemical and nutritional properties to provide oil with a balanced fatty acid profile and higher stability against oxidation due to improved antioxidant and bioactive compounds’ profile.[Citation4] As reported, the amount of cholesterol and triacylglycerol in the serum and liver has been reduced by the optimized oil blends.[Citation5] Generally, oils enriched with natural antioxidants helps to decrease the oxidation rate during storage and frying of oil blends when combined with major proportion of vegetable oils.[Citation6] Keeping in view all above considerations, the study was planned to optimize blend of sunflower oil, extra virgin olive oil (EVOO) and palm olein for better stability against frying process.
Sunflower (Helianthus annuus L.), a widely cultivated crop, is preferred by the food industry for cooking and frying purposes due to light color, mild taste, and high smoke point of its oil.[Citation7] Sunflower oil, relatively with high PUFA content, is susceptible to thermo-oxidative degradation, which leads to color change, poor taste and rancidity.[Citation8] For the techno-economic advantages, usually, a blend of sunflower oil with palm olein is used at domestic as well as at commercial level. Palm olein is the liquid fraction of palm oil that can be obtained when the oil is extracted by a process called fractionation and is predominantly composed of low FFA contents and high amounts of total tocopherols.[Citation9] To further increase the stability of sunflower oil blend, EVOO was used. In the Mediterranean diet, EVOO is used as a source of fat due to its chemical profile as it contains mono-unsaturated (oleic-acid), polyunsaturated fatty acids as major and α-tocopherol and phenolic as minor components that guarantee its high stability against oxidation.[Citation10] Due to its high nutritional and functional capabilities, it has high oxidative stability, excellent digestive ability and potential to prevent heart and vascular diseases even when it is used for cooking.[Citation11]
At high temperatures, the oil comes into contact with the air (oxygen) and food (water) during deep fat frying which results in hydrolytic, oxidative and thermal alterations in the oil either as pure or as blend form.[Citation12] Currently, food industries and legislative authorities are looking for solutions for rapid and noninvasive measurement tools to analyze such degradative changes during and/or after processing. The combination of FT-IR and chemometrics, as adopted in this study, can be a step forward to meet this objective. FT-IR is a rapid, nondestructive and novel technology that has shown its versatile use in the development of new analytical methods for various food applications.[Citation13] It has been widely employed to determine the physicochemical parameters of various types of oils but it has never been used for characterization of optimized sunflower oil blend that not only shows better stability but also provides pronounced health benefits. To determine the quality of the oil blends for frying is still need to be explored.
Based on the literature available and background of the current study, objectives of this study were to determine the stability of blend during frying and to optimize blended oil based on free fatty acids, peroxide and iodine values. In addition, FT-IR spectroscopy was used to explore and to develop predictive models to monitor characteristics and behavior of frying.
Materials and methods
Raw material
Sunflower oil, EVOO, and frozen potato fries were bought from commercial market of Faisalabad-Pakistan. Palm olein (degummed and neutralized) was acquired from the oil processing industry, Faisalabad-Pakistan.
Formulation of oil blends
Sunflower oil (55–80%) was blended with EVOO (15–30%) and palm olein (5–15%) in different ratios as per D-optimal mixture design () using Design Expert 7.0 (Stat-Ease Inc., Minneapolis, MN, USA) software, keeping in view that final volume of each blend remained same. Briefly, D-optimal mixture design is used as an effective method to find out the best blend of various mixture components.[Citation14] Optimized formulation of oil blend based on the chemical analyses was further subjected to frying performance and characterization using FT-IR.
Table 1. Different blends of sunflower oil using D-optimal mixture design along with response variables
Frying of frozen potato fries
Frying experiments were carried out in a 2.5 L capacity “Cool Touch Electric Deep Fryer” (Anex, AG-2012) with controlled temperature. Fourteen (14) formulations of sunflower oil blends were prepared as per D-optimal design using software. For each, oil blend (1 L) was pre-heated and frozen potato fries (50 g) were placed into fryer at set temperature of 180°C for 4 min. After each frying, oil samples were collected separately, cooled at room temperature and quickly analyzed for FFA, PV and IV to avoid any further changes in the oil. After obtaining the best formulation using D-optimal design, the best oil blend was then further subjected to multiple frying (10) using the same protocol.
Chemical analysis of oil
Free fatty acid (FFA)
FFA was determined by following the AOCS Official Methods Ca 5a-40.[Citation15] For this, 10 g of cooled sunflower oil blend was taken into 250-ml conical flask, and heated ethanol (100 ml) and 1 ml of phenolphthalein solution (1 ml) as indicator were added. The solution was boiled for about 4–5 min and titrated with 0.1 N potassium hydroxide till the color of solution changed to pink for at least 30 sec. The value of FFA was calculated using the following formula:
where 56.1 = molecular weight of potassium hydroxide, V = Volume of potassium hydroxide used, N = Normality of potassium hydroxide solution, W = Weight of the sample
Peroxide value (PV)
Peroxide value is defined as milli-equivalents of oxygen per kg of oil (meq O2/kg of oil) and was determined by following the AOCS Official Methods Ca 5a-40.[Citation15] Approximately, 5 g of sunflower oil blend was taken in 250-ml conical flask fitted with a ground glass stopper and mixed with 30 ml of glacial acetic acid, 20 ml of chloroform and 0.5 ml of potassium iodide solution. A change in color was noticed. The mixture was further diluted with 30 ml of distilled water and left for a minute while swirling the flask occasionally. Starch (0.5 ml) was added to mixture as indicator which turned the solution to dark blue color. Titration process was performed with 0.01 M of sodium thiosulfate solution and continued till the dark blue color turned into a colorless solution. A blank test was run without the oil sample and PV was calculated using the following formula:
where Vs = Volume of sodium thiosulfate titrated, Vb = Volume of sodium thiosulfate used in a blank test, N = Normality of sodium thiosulfate solution, W = Weight of sample
Iodine value (IV)
IV was determined by following the AOCS Official Methods Ca 5a-40.[Citation15] A 0.25 g of sunflower oil blend was weighed into 500-ml titration flask with glass stopper and mixed with 25 ml of carbon tetrachloride. The mixture was kept in dark place for half an hour after adding 25 ml of Wij’s solution. After this time, 50 ml of potassium iodide solution (10%) was added and diluted with 100 ml of distilled water. Titration of mixture was performed with standardized sodium thiosulfate solution (0.1 N) using starch (0.5%) as indicator. This process was ended when blue color of starch solution totally disappeared after thorough shaking with the stopper on. The same procedure was adopted to conduct blank test and value was calculated as:
where B = Volume of standard sodium thiosulfate solution required for the blank, S = Volume of standard sodium thiosulfate solution required for the sample, N = Normality of standard sodium thiosulfate solution, W = Weight of sample.
Fourier transform infrared spectroscopy (FT-IR)
The Fourier Transform Infrared (FT-IR) technique is a powerful tool for analyzing chemical changes in edible oils, both qualitatively and quantitatively, using low amount of sample and thus reducing the chances of error.[Citation16] Spectroscopic data from the frying oil samples were attained by an infrared spectrometer (Alpha II FT-IR, Bruker, USA) equipped with an attenuated total reflection (ATR) accessory without a temperature controller. The samples were subjected to preconditioning by putting them in a water bath for 10 min at 40°C to evade any uncertainty in the samples. A small amount of the oil sample was uniformly deposited on the crystal surface of the ATR accessory equipped with horizontal ZnSe ATR crystal and crystal surface was cleaned with cellulose tissue, which was dipped in hexane, and rinsing was done with acetone after each spectral fingerprint. Spectrum related to each sample was recorded in transmission mode range from 4000 cm−1 to 650 cm−1. Time averaged related to each spectrum based on 16 scans at a resolution of 4 cm−1 using the Bruker FTIR-ATR spectrometer, which is well organized with the latest OPUS software version 7.0. For oil samples after each frying, spectra were taken in triplicate and averaged. Average spectral data of 12 samples were exported by the OPUS software and were then imported to Unscrambler software (CAMO, Oslo, Norway) for chemometrics.
Statistical and chemometric data analyses
The statistical software Design Expert 7.0 (Stat-Ease Inc., Minneapolis, MN, USA) was applied to generate the formulations of sunflower oil blends using D-optimal design and find out the best formulation based on the chemical analyses.
Preprocessing of spectral data
After optimization, the best blend was subjected for multiple frying of the frozen potato fries. Three replicates of the optimized oil blend were used for frying up to ten times and included in the model. The average of three spectral data taken after each frying sample were preprocessed using standard normal variate (SNV) to remove the abnormalities in the dataset for obtaining accurate and robust chemometric models. In SNV preprocessing method, every value of the spectrum is normally scaled with reference to the standard deviation of spectrum.[Citation17]
Principle components analysis (PCA)
Principle components analysis (PCA) is an exploratory tool that provides the pattern recognition and tells about the similarities and dissimilarities among various samples. It was applied to SNV preprocessed data using nonlinear iterative partial least squares (NIPALS) algorithm with full cross-validation method.[Citation18]
Partial least square regression (PLSR)
PLSR is one of the most widely used predictive models for various types of parameters in food analytics. In this study, FTIR spectral signatures were used to predict the FFA, PV and IV using five latent variables with leave one out cross-validation method. In this method, the dataset was divided into eleven groups, which include ten numbers of frying and oil without frying. In calibration model, ten groups were used to build PLSR model and eleventh group was used for prediction. In the similar pattern, all the groups were left once to build calibration model so that all groups may be used in the prediction.
Correctness of fit in PLSR model
The root mean square error of cross validation (RMSECV) and coefficient of determination (R2) are the parameters that were used to determine the accuracy of the PLSR models. The RMSECV and R2 can be calculated by using the EquationEquation 4(4)
(4) and Equation5
(5)
(5) , respectively.
Here n, mi, pi and stands for number of samples, measured, predicted and mean value, respectively.
Results and discussion
Optimization of oil blend
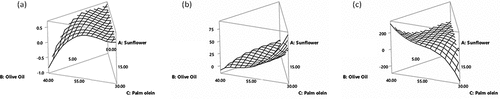
D-optimal design played an important role in optimization of best sunflower oil blend based on FFA value, PV and IV as responses to ensure the safety and keeping quality of frying oil. The amount of FFA is a hydrolytic rancidity indicator that is caused by a combination of high temperature during frying and moisture from food. For instance, low FFA value (~0.31%) was witnessed in blends B7 and B13 (sunflower 55%, EVOO 30%, palm olein 15%) while higher FFA value (~0.61%) was observed for B5 and B14 (sunflower 80%, EVOO 15%, palm olein 5%) as shown in . Graphical representation as response surface plots is also showing that every component in the blend is affecting the response parameters (). De Marco, Savarese[Citation19] reported a lower increment in FFA value in the blend of sunflower and palm oil (65:35) as compare to pure palm oil which might be attributed to the improved saturated fatty acid profile. Similarly, a blend of canola and olive oils with 20% palm olein was recommended for deep-frying principally due to lower ratio of unsaturated fatty acids to saturated ones.[Citation20] In addition, ternary blends have shown better performance during frying as compared to binary blends.[Citation21] As concerned with peroxide value (PV), similar pattern was observed as that of FFA value. Abbas Ali, Bamalli Nouruddeen[Citation22] concluded that sunflower oil had higher peroxide value as compared to the blends. Adding palm olein to sunflower oil sufficiently reduced the escalation in the sunflower oil peroxide value which could be due to a decrease in the amount of linoleic acids present in the blends. The results of this study was concordant with Hashem, Shahat[Citation23] who reported that presence of palm olein with oil blends acted as more stable oil having low formation of peroxide value in comparison to individual oils and other oil blends which were free from palm olein. In addition, the stabilization of oil against oxidation can be enhanced by mixing high-oleic acid contents or high antioxidant’s levels which are particularly present in EVOO.[Citation24] IV is measure of degree of unsaturation of fats and oils and is considered an important parameter to characterize their quality. Higher iodine value (113.1 g of I2/100 g oil) was observed in the blend B7 (). A previous study showed that blending palm oil with sunflower oil produced oleins with a much higher iodine value which are prone to oxidation and polymerization. The decrease in IV was also reported to be parallel to the decrease in the double bonds of unsaturated fatty acids. This could resultantly keep oliens in clear form for long time span.[Citation25] Another report shown 101.98% free fatty acid by blending of 40% palm olein with 60% sunflower oil.[Citation22] Martínez-Pineda, Ferrer-Mairal[Citation26] reported gradual decline in IV values such as, sunflower oil, high oleic acid sunflower oil (HOSO) and olive oil while frying occurred. Perhaps it is caused by decline of unsaturation of the oil samples. The dropping of unsaturation happened in all types of oils during frying. Resultantly multifarious physiochemical changes formed that shows decline in oxidation rate.[Citation27]
Figure 1. Response surface plots representing changes in free fatty acid (a), peroxide value (b) and iodine value (c).
The numerical optimization provided 14 different blends with different desirability indices. The optimized blend was based on the minimum values of stability parameters such as FFA and PV while maximum value of IV. For statistical interpretation, the blend with the minimum desirability index of ~0.99 was selected based on the studied chemical analyses and the best blend obtained after interpretation of linear, quadratic and cubic models in statistical design was on average 59.73% sunflower oil, 28.57% EVOO and 11.69% palm olein which were round off to 59:29:12, respectively. The optimized blend can be correlated with previously studied best blends based on various physicochemical parameters.[Citation22,Citation28] Moreover, the results of chemical analyses of predicted blends showed less than 5% deviation from their actual values, which indicate the validity of D-optimal design.
Chemical changes during multiple frying
The optimized blend (sunflower oil 59%, EVOO 29%, palm olein 12%) used for further multiple frying was further characterized for chemical analyses and FT-IR technique, and validated by applying chemometrics. During frying, the amount of FFA and PV increased and IV decreased slowly and did not surpass the upper cap as set by European regulations, even after 10th frying (). This stability during frying process might be related to reduced losses of oleic and linoleic acids and less formation of FFA and carbonyl compounds due to presence of EVOO and palm olein.[Citation29] The degree of degradation of the oil during frying is generally due to lipid hydrolysis reactions, cleavage, and double bond oxidation of unsaturated fatty acids. As the result of heat, light, and oxidation, the FFAs are further converted into a variety of other polar molecules.[Citation23] FFA formation is attributed to the cleavage and oxidation of double bonds to form carbonyl compounds, which are subsequently oxidized to fatty acids of low molecular masses.[Citation30] The presence of unsaturated fatty acids will easily react with oxygen to form peroxides.[Citation20] Under strong thermostatic conditions, decomposition and polymerization of oil occurred which lead carbonyl mixtures to their decay which generates rotten smell and unwanted taste.[Citation31] Thus, the greater the PV, the faster will the oxidation of the oil occur.[Citation32] The higher the unsaturation, namely high IV, the faster the oil, especially during heating and frying applications, tends to be oxidized. A reduction in IV is consistent with a decrease in the number of oxidized double bonds during the frying process.[Citation28]
Table 2. Physicochemical analysis of best oil blend after multiple frying
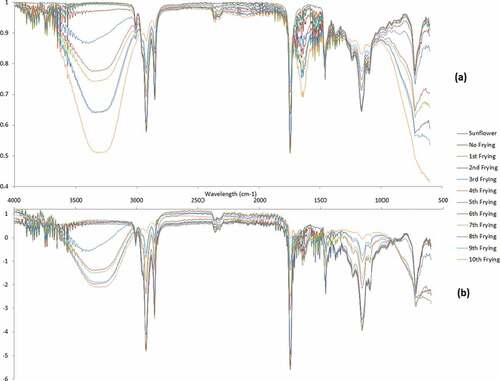
These chemical changes have also been observed with FT-IR spectroscopy (). The absorption band of spectra can be correlated with previous studies.[Citation33] The spectral peaks near to 1150 cm−1 and 1750 cm−1 can be assigned to C–O and carbonyl (C = O) bonds of aliphatic esters, respectively. Moving further, two strong peaks near to 2850 and 2930 cm−1 can be designated to symmetrical and asymmetrical C–H bonds of CH2 structures. All of these absorption bands were depleted as the number of frying increased and new absorption bands can be seen near to 1620 and 3300 cm−1 which indicated the formation of saturated bond (C–O) and free fatty acids.[Citation34] The shifting of peak at 1750 cm−1 to 1620 cm−1 is particularly linked with the hydrolysis of ester groups during frying, while appearance of band at 3300–3400 cm−1 corresponds to the formation of hydroxyl groups (-OH) during thermal oxidation. In addition to this, the multiple frying with different intervals increase may enhance the solubility of oxygen upon cooling the frying oil that may result such peaks in the edible oils. Although the spectroscopic data was showing the visible differences between different frying, it is necessary to apply multivariate calibration to validate the information from such complex and overlapping spectral information.
Chemometrics data evaluation
Impact of SNV preprocessing on spectral signatures
SNV is one of the most widely used preprocessing methods to remove the non-targeted factors from the spectral data. represents the raw and SNV preprocessed FT-IR spectral signatures taken for oil after multiple frying. In the raw spectra, one can see overlapping among the spectral fingerprints. There was overlapping between 1000 cm−1 to 1200 cm−1 in the raw spectral data but after preprocessing one can see clear differentiation among the different spectra taken for oil obtained after multiple frying. Similarly, there was overlapping between 2200 cm−1 to 2600 cm−1 in the raw spectral data but after preprocessing one can see clear differentiation among the different spectra taken for oil obtained after multiple frying that may be helpful for more robust chemometric models which can be seen in the loading plot of PCA model (). In addition to this, there are pronounced peaks in the range of 3000 cm−1 to 3600 cm−1 for 5th to 10th frying that reduce due to the application of SNV preprocessing. These peaks are due to the thermal oxidation of free fatty acids that ultimate generate hydroxyl group which is considered to be important for better differentiation of multiple frying using PCA. It can be inferred from the above discussion that SNV can be used to eliminate the spectral abnormalities that generate due to scattering or the particle size distribution.
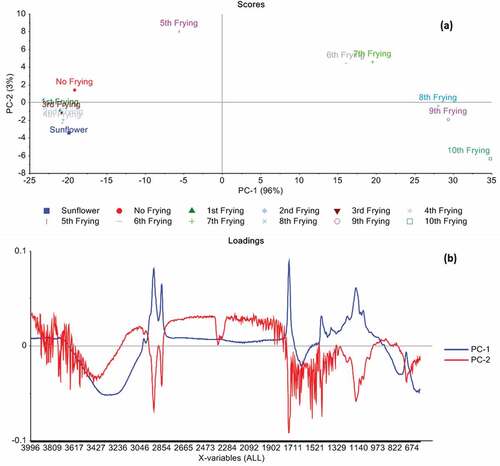
PCA on spectral fingerprints for multiple frying
PCA was applied on SNV preprocessed spectral dataset, which was taken during the multiple frying of sunflower oil blend (). In case of SNV preprocessed spectral fingerprints, PC1 and PC2 elaborate the 96% and 3%, respectively (). Against PC1, the maximum score was recorded for 10th frying whereas the lowest score were observed for 1st frying. Similarly, PC2 shows highest scores for 5th frying, whereas lowest score were recorded for the 10th frying. Hence, it can be seen from the score plot for SNV preprocessed spectra that up to fourth frying there are fewer changes. But the further frying operation shows a clear demarcation among various fryings that can easily tell the structural and chemical changes taken place during the multiple frying of oil. The generation of hydroxyl group due to the thermal oxidation of oil resulted in peaks in the range of 3000 cm−1 to 3600 cm−1 for 5th to 10th frying may considered to be important for differentiation of the multiple frying operation as presented in . Similarly, illustrates the loadings for PC1 and PC2, which show the peaks in different regions. These peaks correlate with the findings of score plot as well as with raw and SNV preprocessed spectra (), which differentiate among multiple frying. This differentiation after multiple frying may be related to different chemical changes such as lipid hydrolysis reactions, cleavage, and double-bond oxidation of unsaturated fatty acids etc. taken place during the frying operation that are pronounced at high frying temperature. It was also found that the oil up to fourth frying show minimal changes but after 5th frying, more pronounced changes takes place that results in clear differentiation in oil obtained from multiple frying. It can be inferred from the above result that PCA can be an important tool that can show chemical and structural changes taken place during the multiple frying operation from FTIR spectral signatures.
PLS Model
PLSR was applied to predict FFA, PV and IV of oil obtained from multiple frying from the spectral signature (). For prediction of FFA, five number of latent variables were used that resulted in R2 = 0.83 with RMSECV 0.18. FFA in the deep frying oil increases due to hydrolysis of lipids molecules and is of major concern for ensuring the quality and safety of the food product. In the previous study, the free fatty acids in oils obtained from street vendors using FTIR spectroscopy and found R2 in range of 0.79–0.99 which is in line with the findings of the present research findings.[Citation35] In addition to this FFA in kalvanji oil was also predicted and found R2 0.99 that are comparable with the current findings.[Citation36]
Figure 4. PLSR prediction models for in free fatty acid (a), peroxide value (b) and iodine value (c).
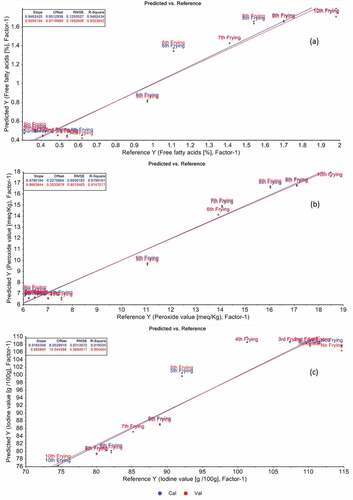
Peroxide value (PV) is an important criterion that determines the quality and safety of the fried food products. Peroxide value increases due to the oxidation of the fats and oil that ultimately deteriorated the quality of the final fried product.[Citation37] Therefore, its prediction is important to ensure the quality of the final product. In this study, PV was predicted where R2 was settled at 0.92 with RMSECV 1.15 using only one latent variable. The PV for the red fruit oil was also predicted and found R2 of 0.99, which is comparable with the findings of the present study[Citation38] the PV for the red fruit oil was also predicted and found R2 of 0.99, which is comparable with the findings of the present study. In addition to this, IV was also predicted using PLSR model with one latent variable that shows 5.77 RMSECV with R2 0.85. The results of the present study are in line with the previous finding of[Citation39] who determine the IV using PLS models on pretreated FT-IR spectra and found lower RMSECV that determine the authenticity of the developed models.
Conclusion
Single oil used for frying operation is liable to deterioration therefore sunflower oil blend was prepared using palm olein and olive oil that enhanced thermal stability of the oil. FTIR spectroscopy coupled with PCA shows clear demarcation of oil samples obtained after multiple frying. Similarly, FFA, PV and IV were predicted using PLSR model. All these parameters changed during the frying operation due to the oxidation of oil, breakdown of double bond and hydrolysis of lipids. All these parameters are essential to assess the quality of oil that finally leads to the production of good quality fried product having long shelf life with better sensory and textural attributes. Previously, these parameters were determined using laborious and time-consuming chemical based methods that also need hectic sample preparation. The prediction of these parameters with FTIR results in development of rapid and nondestructive technologies for industrial uses, which may pave the way toward sensor development.
Acknowledgments
Authors acknowledge Dr. Sanaullah Iqbal from University of Veterinary and Animal Sciences, Lahore-Pakistan for giving access to the laboratory for FT-IR spectroscopy. The authors declare that there are no conflicts of interest regarding the publication of this paper.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Erickson, M. D. Deep Frying: Chemistry, Nutrition, and Practical Applications; Cambridge, Massachusetts, United States: Elsevier, 2015.
- Oke, E.; Idowu, M. A.; Sobukola, O. P.; Adeyeye, S. A. O.; Akinsola, A. O.; Frying of Food: A Critical Review. J. Culinary Sci. Technol. 2018, 16(2), 107–127.
- Sebastian, A.; Ghazani, S. M.; Marangoni, A. G. Quality and Safety of Frying Oils Used in Restaurants. Food Res. Int. 2014, 64, 420–423. DOI: 10.1016/j.foodres.2014.07.033.
- Tiwari, M.; Tiwari, K.; Toliwal, S. Studies on Thermal Stability of Palm-sesame Oil Blends during Deep Fat Frying. J. Scient. Indust. Res. 2014, 73,153–156.
- Jan, K.-C., Huang, Mei-Ying, Chang, Chun-Ju, Liu, Tristan C.; Hypolipidemic Effect of Blended Oil in Hamster: Biochemical Analysis and Gene Expression Profiling. J. Food Nutr. Res. 2016, 4(1), 26–32.
- Sunil, L.; Reddy, P. V.; Krishna, A. G. G.; Urooj, A.; Retention of Natural Antioxidants of Blends of Groundnut and Sunflower Oils with Minor Oils during Storage and Frying. J. Food Sci. Technol. 2015, 52(2), 849–857.
- Sahranavard Azartamar, F.; Ghadimzadeh, M.; Darvishzadeh, R. Genetic Diversity and Structure Analysis of Oily Sunflower (Helianthus Annuus L.) Based on Microsatellite Markers. J. Plant Genet. Res. 2016, 2(2), 15–32. DOI: 10.29252/pgr.2.2.15.
- Upadhyay, R.; Sehwag, S.; Mishra, H. N. Chemometric Approach to Develop Frying Stable Sunflower Oil Blends Stabilized with Oleoresin Rosemary and Ascorbyl Palmitate. Food Chem. 2017, 218, 496–504. DOI: 10.1016/j.foodchem.2016.09.105.
- Ismail, R. Palm Oil and Palm Olein Frying Applications. Asia Pac. j. clin. nutr. 2005, 14(4), 414–419.
- Condelli, N.; Caruso, M. C.; Galgano, F.; Russo, D.; Milella, L.; Favati, F; Prediction of the Antioxidant Activity of Extra Virgin Olive Oils Produced in the Mediterranean Area. Food Chem. 2015, 177, 233–239. DOI: 10.1016/j.foodchem.2015.01.001.
- Guillaume, C.; Ravetti, L. Shelf-life Prediction of Extra Virgin Olive Oils Using an Empirical Model Based on Standard Quality Tests. J. Chem. 2016, 2016.
- Velasco, J.; Marmesat, S.; Dobarganes, M. C. Chemistry of Frying Adv. deep-fat frying foods. 2009,Boca Raton, FL: CRC press, 33–51.
- Valand, R.; Tanna, S.; Lawson, G.; Bengtström, L.; A Review of Fourier Transform Infrared (FTIR) Spectroscopy Used in Food Adulteration and Authenticity Investigations. Food Addit. Contam. 2020, 37(1), 19–38.
- Mohamad Zen, N. I.; Abd Gani, S. S.; Shamsudin, R.; Fard Masoumi, H. R.; The Use of D-Optimal Mixture Design in Optimizing Development of Okara Tablet Formulation as a Dietary Supplement. Sci. World J. 2015, 2015, 684319. DOI: 10.1155/2015/684319.
- Firestone, D. Official Methods and Recommended Practices of the AOCS; United States: AOCS, 2009.
- Xu, L.; Yu, X.; Liu, L.; Li, M.; Zhang, R.; A Rapid Method for Evaluating the Edible Oil Oxidative Stability during Ambient Storage by FTIR Spectroscopy Using A Mesh Cell. Anal. Methods. 2016, 8(25), 5117–5122.
- Ahmad, M. H.; Nache, M.; Waffenschmidt, S.; Hitzmann, B.; A Fluorescence Spectroscopic Approach to Predict Analytical, Rheological and Baking Parameters of Wheat Flours Using Chemometrics. J. Food Eng. 2016, 182, 65–71. DOI: 10.1016/j.jfoodeng.2016.03.006.
- Ahmad, M. H.; Nache, M.; Waffenschmidt, S.; Hitzmann, B.; Characterization of Farinographic Kneading Process for Different Types of Wheat Flours Using Fluorescence Spectroscopy and Chemometrics. Food Control. 2016, 66, 44–52. DOI: 10.1016/j.foodcont.2016.01.029.
- De Marco, E.; Savarese, M.; Parisini, C.; Battimo, I.; Falco, S.; Sacchi, R.; Frying Performance of a Sunflower/palm Oil Blend in Comparison with Pure Palm Oil. Eur. J. Lipid Sci. Technol. 2007, 109(3), 237–246.
- Roiaini, M.; Ardiannie, T.; Norhayati, H. Physicochemical Properties of Canola Oil, Olive Oil and Palm Olein Blends. Int. Food Res. J. 2015, 22(3), 1227.
- Farhoosh, R.; Kenari, R. E.; Poorazrang, H. Frying Stability of Canola Oil Blended with Palm Olein, Olive, and Corn Oils. J. Am. Oil Chem. Soc. 2009, 86(1), 71–76. DOI: 10.1007/s11746-008-1315-x.
- Abbas Ali, M.; Bamalli Nouruddeen, Z.; Idayu Muhamad, I.; Abd Latip, R.; Hidayu Othman, N.; Effect of Palm Olein Addition on the Quality Characteristics of Sunflower Oil during Deep Fat Frying. Acta Aliment. 2014, 43(2), 288–296.
- Hashem, H., Shahat, M.; El-Behairy, S.A.; Sabry, A.; Use of Palm Olein for Improving the Quality Properties and Oxidative Stability of Some Vegetable Oils during Frying Process. Middle East J. Appl. Sci. 2017, 7(1), 68–79.
- Abdel-Razek, A. G.; El-Shami, Safinaz M.; El-Mallah, M. Hassan; Hassanien, Minar Mahmoud M.; Blending of Virgin Olive Oil with Less Stable Edible Oils to Strengthen Their Antioxidative Potencies. Aust. J. Basic Appl. Sci. 2011, 5(10), 312–318.
- Nor Aini, I.; Hasmadi, M.; Mamot, S.; Radzuan, J.; Palm Oil and Sunflower Oil: Effect of Blend Composition and Stirrer Types during Fractionation on the Yield and Physicochemical Properties of the Oleins. J. Food Lipids. 2005, 12(1), 48–61.
- Martínez-Pineda, M.; Ferrer-Mairal, A.; Vercet, A.; Yagüe, C.; Physicochemical Characterization of Changes in Different Vegetable Oils (Olive and Sunflower) under Several Frying Conditions Caracterización Fisicoquímica de Los Cambios En Diferentes Aceites Vegetales (Oliva Y Girasol) Bajo Varias Condiciones de Fritura. CyTA-J. Food. 2011, 9(4), 301–306.
- Sumnu, S. G.; Sahin, S. Advances in Deep-fat Frying of Foods; Boca Raton, Florida, United States: CRC Press, 2008.
- Alireza, S., Tan, Chin Ping, Mirhosseini, Hamed, Man, Y.B. Che; Effect of Frying Process on Fatty Acid Composition and Iodine Value of Selected Vegetable Oils and Their Blends. Int. Food Res. J. 2010, 17(2), 295–302.
- Akil, E.; Castelo-Branco, V. N.; Costa, A. M. M.; Do Amaral Vendramini, A. L.; Calado, V.; Torres, A. G.; Oxidative Stability and Changes in Chemical Composition of Extra Virgin Olive Oils after Short-term Deep-frying of French Fries. J. Am. Oil Chem. Soc. 2015, 92(3), 409–421.
- Latha, R. B. Effect of Heat on Physico-chemical and Thermo-oxidative Stability of Repeatedly Heated Rice Bran Oil (RBO). Int. J. Food Nutritional Sci. 2016, 5(2), 49.
- Kaleem, A.; Aziz, S.; Iqtedar, M. Investigating Changes and Effect of Peroxide Values in Cooking Oils Subject to Light and Heat. FUUAST J. Biol. 2015, 5(2), 191–196.
- Omara, T.; Kigenyi, E.; Laker, F.; Adokorach, M.; Otim, G.; Kalukusu, R.; Musau, B.; Kagoya, S.; Nakabuye, B. V.; Effects of Continuous Deep Fat Frying on the Physical and Chemical Properties of Assorted Brands of Edible Cooking Oils Sold in Metropolitan Kampala Asian J Applied Chem. Res. 2019, 3(2), 1–13.
- Chen, J. Y.; Zhang, H.; Ma, J.; Tuchiya, T.; Miao, Y.; Determination of the Degree of Degradation of Frying Rapeseed Oil Using Fourier-Transform Infrared Spectroscopy Combined with Partial Least-Squares Regression. International Journal of Analytical Chemistry 2015,2015, 185367. DOI: 10.1155/2015/185367.
- Al-Degs, Y. S.; Al-Ghouti, M.; Salem, N. Determination of Frying Quality of Vegetable Oils Used for Preparing Falafel Using Infrared Spectroscopy and Multivariate Calibration. Food Anal. Methods. 2011, 4(4), 540–549. DOI: 10.1007/s12161-011-9201-9.
- Shen, Y.; Chen, S.; Du, R.; Xiao, Z.; Huang, Y.; Rasco, B. A.; Lai, K.; Rapid Assessment of the Quality of Deep Frying Oils Used by Street Vendors with Fourier Transform Infrared Spectroscopy. J. Food Meas. Charact. 2014, 8(4), 336–342.
- Mahesar, S. A.; Kandhro, A. A.; Khaskheli, A. R.; Talpur, M. Y.; Sherazi, S. T. H.; SB-ATR FTIR Spectroscopic Monitoring of Free Fatty Acids in Commercially Available Nigella Sativa (Kalonji) Oil. J. Spectrosc. 2014, 2014, 510890. DOI: 10.1155/2014/510890.
- Gotoh, N.; Wada, S. The Importance of Peroxide Value in Assessing Food Quality and Food Safety. J. Am. Oil Chem. Soc. 2006, 83(5), 473–474. DOI: 10.1007/s11746-006-1229-4.
- Andina, L.; Riyanto, S.; Rohman, A. Determination of Peroxide Value of Red Fruit Oil by FTIR Spectroscopy and Multivariate Calibration. Int. Food Res. J. 2017, 24(6), 2312–2316.
- Meng, X.; Ye, Q.; Nie, X.; Jiang, L.; Iodine Value Determination of Edible Oils Using ATR-FTIR and Chemometric Methods. Eur. J. Lipid Sci. Technol. 2017, 119(9), 1600323.