ABSTRACT
Marula (Sclerocarya birrea, A. Rich) is an underutilized wild edible fruit tree species that grows naturally across large parts of sub-Saharan Africa. Almost every part of the tree, from leaves, bark, wood, roots and fruit has some use. The fruit is consumed fresh or processed. The fruit contains considerable amounts of dietary fiber, protein, vitamins (A, B3, C, E and carotene), minerals, amino acids, and fatty acids. The main structural classes of the marula fruit include polyphenols, flavonoids, condensed tannins and polysaccharides (pectin), these components can prevent chronic and degenerative diseases. The marula fruit is functional food because of its components that have beneficial properties on health and prevention of diseases. Different studies have demonstrated the utilization of the marula fruit in juice processing, alcohol based products, jams and jellies, fruit leather, vinegar and animal feed. Therefore, this article reviews the nutritional composition, polyphenolic compounds and biological activities of the marula fruit and its reported use in food applications.
Introduction
Most climate change models predict future increases in the frequency and severity of droughts in sub-Saharan Africa.[Citation1, Citation2] This will have deleterious effects on the production of conventional crops such as cereals, vegetables, and fruit crops thereby endangering the food and nutritional security, especially of poor rural households.[Citation3] There is therefore a need to identify alternative crops that can withstand harsh growing conditions to meet the increasing demand for food and nutrition. The cultivation and utilization of underutilized food sources, including wild edible species, has become a subject of intense research interest in climate change adaptation studies in recent years.[Citation4,Citation5] Mabhaudhi et al.[Citation6] state that wild edible species are plants that grow naturally in certain geographic areas and are often characterized by limited development comparative to their prospective.
Marula (Sclerocarya birrea, A. Rich) Hochst. subsp. caffra (Sond.), a member of the Anacardiaceae family, is an example of an underutilized wild edible fruit tree species that grows naturally across large parts of sub-Saharan Africa. Marula fruit has been a precious plant to rural communities of the African continent for many centuries.[Citation7,Citation8] It grows in open woodland and it is common right through the semi-arid and deciduous savannas of sub-Saharan Africa including South Africa, its neighboring countries as well as Madagascar.[Citation9,Citation10] The Sclerocarya genus consists of only two species which are birrea and gillettii. However, birrea has three subspecies, namely Birrea, Caffra and Multifoliolata.[Citation11] Marula fruit is found naturally or plowed in the Sahel, East and Southern Africa on the margins of the moist forest zone.[Citation12]
Marula trees grow in a wide range of altitudes varying from sea level up to 1 800 m above sea level.[Citation13–15] Temperatures varying from as low as 10°C are favorable for the growth of marula trees in high altitudes whereas they can grow in temperatures of up to 40°C in low-lying areas.[Citation16] Warm and frost-free climate is desirable for the growth of marula trees which is also tolerant to salt.[Citation17] High altitude areas with short and intermittent frosts also support marula growth although they are also widespread in warm, frost-free areas such as the lowveld region of South Africa.[Citation13,Citation18] Marula is very sensitive to frost and this is a critical environmental requirement which restricts its distribution. The species is moderately resistant to drought and wide temperature ranges of 27 to 37°C are good for the germination of marula seeds.[Citation19,Citation20] Higher marula populations in South Africa are found in the rainfall range of 400 to 1 000 mm per annum.[Citation10,Citation21]
Harvesting of marula fruits contribute to both household consumption of the fruit and their products and income generation.[Citation22] Mostly women harvest marula fruits by picking them from the ground where they ripen.[Citation18] The ripening process completes when the fruit has been detached from the tree for unclear reasons. Different authors have indicated that marula seeds germinate easy on the ground and vegetative propagation via utilization of cuttings is used to grow new trees.[Citation23] However, excessive picking of the fruit can have negative effects on the marula population dynamics in each area since the seeds are dispersed by animals which affects their distribution and germination and the sustainability of the species.[Citation24] Shackleton et al.[Citation9] and Shackleton et al.[Citation11] argue that picking marula fruit for human utilization might negatively affect the regeneration rates. Marula fruits are also prone to the Natal and Mediterranean fruit flies.
In terms of nutritional properties and phenolic compounds, marula fruit is rich in vitamin C and its extracts have antioxidant, antibacterial, antifungal, astringent anticonvulsant,[Citation25,Citation26] antihyperglycemic, anti-inflammatory, and antiatherogenic properties.[Citation27,Citation28] High content of polyphenolic compounds and antioxidant activities in the marula fruit could be the contributing factors to most of these properties.[Citation29,Citation30] Moreover, the marula fruit extracts might also be utilized to prevent the oxidation of unsaturated lipids in foods due to their high antioxidant activity.[Citation31] Previous studies have reported the availability of eleven phenolic compounds in the marula leaf extracts as well as pulp extracts.[Citation29,Citation32] The dietary intake of wild fruits such as marula fruit has a strong inverse relationship with the likelihood to develop cardiovascular diseases and cancer. Polyphenols and antioxidant vitamins are attributed to such antioxidant activity although polyphenols are the most effective.
Materials and methods
The following database were used to search an online literature from October – December 2021: Scopus, Web of Science, Google Scholar and Science Direct. The following key words were used: Marula fruit, nutritional composition, polyphenolic compounds, biological activities, volatile compounds and food applications. For this review, authors selected articles published from 1982 to 2021. About 280 articles were obtained through the search engines and only[Citation180] were cited in this review. Selection was based on relevance to the title of the manuscript and the literature was used to structure the document according to the sub-sections. The following research questions were formulated to assist authors to write the manuscript: (i) What is the body of knowledge around the nutritional composition, polyphenolic compounds and biological properties of marula fruit? (ii) What is the botanical, agronomic, morphology and physical characteristics of marula fruit? (iii) How are different parts of marula fruit utilized? (iv) What are the nutritional composition and health benefits of marula fruit? (v) What are the polyphenolic compounds, biological properties and volatile compounds of marula fruit? (vi) How is marula fruit utilized in food and feed applications?
Botanical and agronomic diversity of marula fruit
The marula tree () is one of the most recognized indigenous trees belonging to Anacardiaceae family.[Citation33] It is in the Anacardiaceae family because of its dioecy nature, resin canals in the outer covering as well as its production of plumpy fruits by female trees.[Citation34,Citation35] This family comprises more than 600 species and 73 genera.[Citation33] From its hard seed or nut, the genus Sclerocarya was derived from Greek words which are Sklero meaning hard and Karyon meaning nut.[Citation36,Citation37] The genus Sclerocarya has two species that originated in Africa namely; Sclerocarya birrea and Sclerocarya giletti.[Citation38] The current study focuses on the species Sclerocarya birrea (S. birrea). The species name birrea was obtained from the common name ‘birr’ of the tree in Senegal and other West African countries.[Citation39] The species S. birrea is further divided to three documented subspecies all over its dispersal that is; S. birrea ssp. cafra, and S. birrea ssp. multifoliata.[Citation40] Petjie[Citation36] notes that the subspecies are found in different parts of Africa with birrrea in North Africa, caffra in the Southern Africa and Mulitfoliata in Tanzania. Furthermore, the name caffra was derived from the word ‘kaffaria’ in the Eastern Cape of South Africa.[Citation36] Apart from its trade name (marula) other commonly names used in the northern part of South Africa include; elephant tree/ Jelly plum (English), mafula (Venda), nkanyi (Xitsonga), and morula (Sepedi).
Morphology and physical characteristics of marula fruit
Generally, marula tree is an average to large sized tree which can be about 9 m in height, however, given favorable growing conditions its height may double up to 18 m.[Citation34] It is a deciduous, and in few occasions, it has both male and female flowers in one tree, with one trunk about of 120 cm in diameter, condensed and scattering top.[Citation34,Citation35] Almost all the marula tree parts; the bark, fruits, nuts, leaves and stem are vital and their uses varies with various locations and tribes.[Citation34] The outermost layer of the trunk is coarse with a pale gray color whereas its inner layer is either red, pink or yellowish with darker lines.[Citation38] It peels off in a level round or oval disc shaped scales giving the trunk a mottled look while revealing the underlying light yellow tissues.[Citation36] Marula trees have grayish-green multiple leaves with steeply pointed leaflets, each leaflet is attached to the stalk by a long petiole.[Citation41] This is one of the main feature that distinguishes the marula from the false marula which has no petioles. The leaf petiole is in three to eight opposite pairs of leaflets and terminal one making them alternatively settled while packed adjacent at the end of branches.[Citation37,Citation42]
For the species S. berrea, each tree has staminate (male) or pistillate (female) flowers and mostly not both.[Citation36] Even though the marula tree is considered a dioecious, there are rare cases especially in the subspecies caffra were a tree is moecy, that is when a tree produces flowers of both sexes.[Citation40,Citation43,Citation44] Flowers are located differently, whether in one tree or different trees, in a bunch of about 5 to 8 cm in length at the branch terminal.[Citation34,Citation38] Flower sex is determined by its color and where they are located, with male flowers appearing dark red in the beginning, turning pink or white when open and located in racemes below newly formed leaves. The female flowers are blood red at first, change color to pink or white after opening and are located below the leaves on long pedicles and have four bending petals, many unproductive stamens and extended glossy ovaries.[Citation45] There are 15 to 25 productive stamens and vestigial ovary in each male flower and the female flowers contain 15 to 25 staminodes and the ovary is almost round with isolated stigmas.[Citation36,Citation41] Pollination is mainly influenced by insects.[Citation13] Flowering occurs from September to November (spring) when the trees are producing new leaves.
The fruits are produced by female trees and are cylinder like, drupe, plum sized, and may range from 3 to 4 cm in thickness, they are green colored before maturing and they turn yellow as they ripen.[Citation46,Citation47] After reaching full physiological maturity, they fall from the tree while still green and then turn yellow when already on the ground or after harvest.[Citation9,Citation16] The ripe fruits are aromatic with turpentine taste and white succulent pulp, which sticks tightly to the nut.[Citation34] The brown, firm, woody nut is about 2 to 3 cm in diameter and is divided into three or four hollows and each has one seed.[Citation38] Each hollow is protected by a small thin ‘lid’ which is separated when the nut is cracked. Venter and Venter[Citation42] indicate that, annually, individual marula trees may produce up to 500 kg of fruits with an average crop yield of about 30 000 fruits per tree. However, productivity is influenced by tree size; large tree can bear up to 70 000 fruits per tree.
Uses of different parts of marula trees
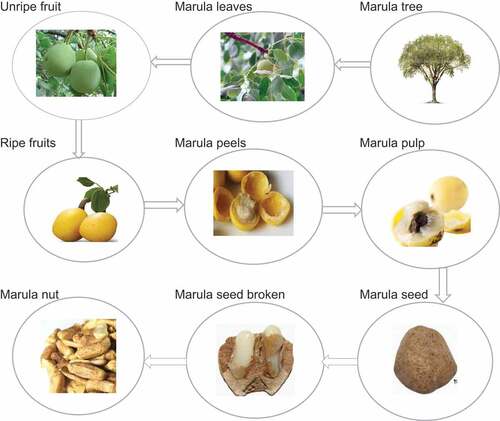
Marula trees are referred to as miracle trees in some communities because of their multiple uses. Almost every part of the tree, from the leaves, bark, wood, roots and fruit has some use as we demonstrate in this section.
Marula fruit
Marula fruit is either eaten fresh or fermented for marula beer brewing.[Citation10,Citation44] The fruit also has powerful alcoholic properties and this is partly attributed to twenty-nine different yeasts that are present in the skin.[Citation48] The marula fruit is also utilized during the production of collagen protein and acts as anti-aging agent. Collagen protein is necessary for building and maintaining the health of skin, joints, blood vessels and cartilage.[Citation21] The mesocarp of the fleshy juice has refreshing, an acceptable acidic and sour-taste, thirst-quenching, and enhances energy.[Citation49] The marula juice improves the utilization of nutrients in the gut and acts as stimulant since it improves the production of sperm.[Citation9,Citation50]
Marula fruit is utilized in producing products such as chutneys and pie fillings in small scale and the skin is normally boiled and drunk or burnt and utilized to replace coffee.[Citation51,Citation52] Marula fruit materials also have medicinal properties. The stem and bark contain antihistamines and can be steeped in boiling water and steam inhaled for cleansing purposes.[Citation53] Some communities in southern Africa crush the bark into powder and add it to sorghum or millet water, milk and drink for fever reduction while others produce pulp by crushing the bark, adding it to cold water and drink to treat dysentery, diarrhea, insect bites, burns and other disorders.[Citation21] Marula fruit contains important bioactive compounds such as polyphenols, flavonoids, tannins, triterpenoids and phytosterols.[Citation54] Moreover, different studies have demonstrated that marula fruit has functional properties such as anti-microbial, anti-diabetic, anti-diarrheal, anti-inflammatory, anti-hypertensive and anti-oxidant properties.[Citation51,Citation52]
Marula seeds
Marula seeds are rich in minerals and vitamins such as iron, magnesium, calcium, zinc, phosphorus, nicotinic acid and thiamine.[Citation55] The seeds are eaten fresh, dried or milled and incorporated to vegetables, meat, and soups, making the seeds creditable in imparting flavor to food.[Citation44,Citation48] The fresh seeds are also incorporated to porridge and boiled meat to enhance the flavor.[Citation36] Shone[Citation56] notes that the Venda people in South Africa, mill and shape marula seeds into meat-like cakes which are stored as Venda biltong for future utilization. Moreover, the VhaVenda use marula oil to preserve meat and meat products.[Citation44,Citation57] In Namibia, some communities process marula seeds into oil or consume them as a snack.
Marula leaves
The leaves are used for making compost and as animal feed together with stems or branches.[Citation9,Citation21] Most southern African communities collect and consume the larvae of mopane worm (Imbrasia belina) which hatch on marula leaves. Moreover, saturnalia caterpillars and larvae of cerambycid wood-boring beetle are also collected from marula leaves.[Citation50] Lastly, marula leaves are ecologically used as host by two parasitic mistletoe, causing the infection of Erianthemum dregei and Pedistylis galpinii.[Citation58]
Marula oil
Marula seeds contain oil that is a rich source of protein which combats stretch marks because of its anti-aging characteristics.[Citation51,Citation54] Marula oil possesses a high amount of mono-unsaturated oleic acid which can be beneficial in substituting sunflower oil for the biodiesel production.[Citation59] The oil is very stable to lipid oxidation and communities in southern Africa use it to preserve meat and its products.[Citation60] Elijah et al.[Citation59] indicate that the marula oil is ten times more stable to lipid oxidation compared to olive oil. Thus, the marula oil is also utilized in making various medicines, cosmetic and skin care products due to its chemical stability and slow oxidizing effect.[Citation52] However, the marula oil has low B-tocopherol and this results in oil having low vitamin E compared to other similar nut oils.[Citation61]
Nutritional composition and health benefits of marula fruit
The COVID-19 pandemic has made fruits very expensive due to high rates of unemployment especially in developing countries, and global recession.[Citation62] However, indigenous wild marula fruit is available seasonally free of charge and may play an important role in improving food, nutrition and security since it is rich in nutrients.[Citation63] Marula fruit is a rich source of nutrients such as vitamin C, carotenoids, amino acids and minerals.[Citation64,Citation65] Low consumption of fruits such as marula is associated with increased risk of chronic diseases such as cardiovascular diseases, diabetes mellitus, hypertension and high mortality rate.[Citation66–68] Moreover, marula fruit could be utilized for industrial processing of innovative foods products that can combat malnutrition and improve food security. shows the proximate composition of the marula fruit and the pulp contains high fresh mass of water, ash, carbohydrates and fiber than nut. Protein and lipids constituents are lower in pulp than in nut. Marula nut is rich in protein content while fat is insignificant. The nutritional composition of edible marula fruit provides a useful database information for future planning of research and innovation. Marula products can be commercialized and utilized in the prevention of poverty, hunger and in promoting food security in Africa.
Table 1. Proximate analysis of edible marula pulp and nut (g/100 g).
Marula fruit is a valuable source of water soluble and fats soluble vitamins and minerals (). Vitamin A found in marula fruit is used in anti‐aging products, including skin therapy for different skin disorders due to extracellular matrix production, oxidant/antioxidant, sunscreen influence and prevention of vitamin A deficiency caused by ultraviolet.[Citation79] Vitamin C is high in marula pulp ranging from 62 mg/100 g to above 400 mg/100 g in the fresh fruit.[Citation46,Citation69] The marula pulp and nut contain three major minerals including calcium, potassium, and magnesium which the body needs in high concentration. Calcium could be stored in the bones and is essential for muscles contraction and hormone secretion.[Citation73,Citation76] Marula nut contains high amounts of calcium and magnesium than the pulp. Potassium is an intracellular cation which regulates a cellular osmotic balance. Marula nut is also rich in minor essential minerals such as iron, copper and zinc.
Table 2. Vitamins and mineral composition of marula pulp and nut.
Copper is essential for the body constituents of redox enzymes while zinc found in the marula nut and pulp may eliminate zinc deficiency in the diet.[Citation76] Marula could be a valuable source of minerals that provides desirable health benefits and the consumption of its fruit does not pose health risks because of its low toxic minerals as set by the Food and Agricultural organization (FAO)/World Health Orginazation (WHO) and Food and Drug Administration (FDA). Moreover, the important minerals do not solely rely on nutritional physiological, activate different enzymes but also contribute to aroma and texture of fruit.[Citation73] Nonetheless, extensive research about vitamins and minerals analysis of the marula fruit, its products and by-products is still needed. There is insufficient data particularly about the effects of different geographical areas on the quality of the marula fruit.
Marula nuts contain essential amino acids which are below the recommendations of the FDA and WHO as shown in . Most of these amino acids have branched chain enzymatic steps in their pathway and are essential because they are only obtained from food since the human body cannot produce them.[Citation63,Citation81] Marula nuts contribute to consumers’ nutritional well-being by playing an important role in physiological processes such as metabolism of fatty acid, glucose transportation, growth, and immunity.[Citation84] They also provide fluidity of lipids that supplies energy substrates to maintain synthesis of protein.[Citation86] Moreover, the consumption of marula fruit, nut and their products can prevent deficiencies of propionic acidemia, osteoporosis, Barth syndrome and Costeff optic atrophy.[Citation81]
Table 3. Amino acids in marula nut and pulp.
Marula nuts provide essential fatty acids that contain fat-soluble vitamins.[Citation85] Lipids that are essential fatty acids are bioactive like essential amino acids and can only be obtained from food. The marula pulp and nut are rich in a specific fatty acid which may prevent many diseases that cause health problems as shown in . Ogbobe et al.[Citation92] reported that marula nut and pulp are rich sources of essential fatty acids. Glew et al.[Citation75] reported that hexadecanoic, octadecanoic, and arachidonic acids were the most dominant fatty acids in the marula nut and pulp. However, information on fatty acid composition of fruit skin and pulp is still lacking.[Citation102] Therefore, further research is still needed on fatty acids composition of pulp, skin and marula oil cake. The marula fruit is a rich source of essential fatty acids which can reduce the rate of neurological/neuropsychiatric disorders, mortality, prevent the risk of cancer and cardiovascular morbidity, vision, arthritis and diabetes mellitus.
Table 4. Fatty acid composition of marula fruit.
Understanding essential fatty acids in the marula fruit is a key to devising novel approaches to the treatment and prevention of many diseases. Clearly, the marula nut and pulp are good sources of essential fatty acids which are important to human health.
Polyphenolic compounds in marula fruit and human health
The research on the bioactive compounds of the marula fruit has concentrated on the leaves, bark, pulp and peel. Overall, various bioactive substances that include Vitamin C, phenolic acid, flavonoids, tannins, proanthocyanidins as well as other compounds have been reported but their presence significantly differs in various parts of the marula fruit (). The way in which phenolic compounds are distributed in fruits is useful in optimizing the yield of phenolic compounds of processed fruits and vegetables.[Citation107] From the nutritional point of view, polyphenolic compounds contribute to the beneficial health effects on humans such as antioxidants, anti-cancer, and anti-inflammation.[Citation108,Citation109] Based on their amounts, phenolic compounds have a double biological function in plants because they are antioxidant and pro-oxidant agents at low and high amounts.[Citation110]
Table 5. The distribution of bioactive substances in marula fruit.
Phenolic compounds play a major role in plant defense process against bacteria as well as other categories of environmental stress such as wound and uncontrolled light or ultra violet radiation.[Citation111,Citation112] Moreover, phenolic compounds have a variety of biological characteristics such as antioxidant activity, antimicrobial and antifungal properties.[Citation113,Citation114]
Polyphenols
The total phenolic content (TPC) of different parts of the marula fruit is shown in . The pulp contains large amounts of polyphenols compared to other parts of the fruit. The variation in the TPC of different parts of the marula fruit is due to the different methods of extraction as well as the quality of the fruits used.[Citation52] Factors such as environmental conditions and the nutritional characteristic of the plant also contribute to the variations in the TPC.[Citation103] Robards et al.[Citation110] indicate that the quantitative distribution of polyphenolic compounds differs between and within plant species as well as between various parts of the plant. This is corroborated by Gous et al.[Citation74] who state that for a period of three years the TPC of seven marula juice products from individual trees were high, ranging from 226–414 mg/100 ml tannic acid equivalence. The recovery of polyphenols depends on the type of fruit as well as the solution used to extract polyphenols.[Citation115] Therefore, some fruits might be successfully extracted with 100% methanol or acetone while 50% of the same solution might extract other fruits.[Citation106] Plants with high concentrations of polyphenols exhibit high antioxidant capacity. The redox characteristics of polyphenols is related to the antioxidant properties of wild plants such as the marula which warrant them to act as reducing agents, hydrogen donors and singlet oxygen quenchers.[Citation116]
Table 6. Polyphenolic compounds in marula fruit.
The peel of the marula fruit contains polyphenols such as vanillic acid, caffeic acid, ferulic acid, p-hydroxybenzaldehyde, p-hydroxybenzoic acid and p-coumaric acid whereas caffeic acid, ferulic acid and p-coumaric acid have been identified in the pulp.[Citation103] Different authors have reported the uneven distribution of polyphenols in the peels and pulps of fruits.[Citation117,Citation118] Polyphenols’ presence in wild fruits such as marula have antioxidant, pro-oxidant, anti-inflammatory properties and the bioavailability of nitric oxide in humans is also influenced by these polyphenols.[Citation119] Stoclet et al.[Citation120] and Mann et al.[Citation121] demonstrated that the reduction of nitric oxide by polyphenols leads to an increase in nitric oxide bioavailability which is an important step in decreasing the risk of cardiovascular diseases.
Flavonoids
The most common flavonoids in the marula fruit are procyanidins and galloyl derivatives of flavonoid glycosides. The leaf extracts exclusively contain flavonoid glycosides whereas the galloylated tannins are dominant in the bark extracts.[Citation105] However, Ndhlala et al.[Citation29] indicate that the most important principal source of flavonoids in the marula fruit is the pulp as it is twice richer in flavonoids than the peel (). Notably, in other fruits, the peel is the principal source of flavonoids. For example, fruits such as grape, apple and peach have high flavonoids content in the peel than the pulp.[Citation118] The antioxidant activity of wild fruits such as the marula is attributed to the presence of flavonoids.[Citation32] Marula leaves and young stem contain proanthocyanidins in the range of 1.25% and 1.16% while the pulp and the peel contain condensed tannin.[Citation103] The presence of tannins contributes to the antioxidant, antifungal, anti-inflammatory and healing characteristics of the marula fruit extracts.[Citation122] Moreover, lipoxygenase enzyme which is paramount in inflammation physiology can be inhibited by tannins during the metabolism of arachidonic acid.[Citation123] Flavonoids play a significant role in maintaining the cellular redox balance against cancer in human health. Consumption of fruits supplies various types of flavonoids that play a preventative role in human health by reducing the prevalence of cardiovascular diseases and cancer.[Citation124]
Biological activities of marula fruit
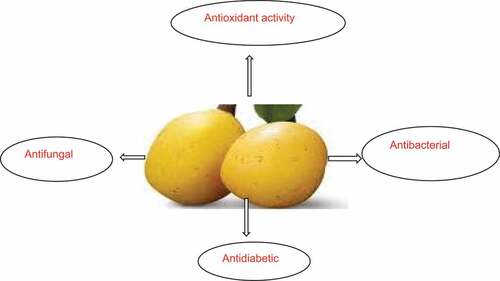
The biological activities of marula fruit are summarized in and have been reported by different researchers.
Antioxidant activity
Olsen et al.[Citation125] and Moyo et al.[Citation103] reported that the methanolic extract of the marula leaf has antioxidant activity which is higher or equal to that of butylated hydroxytoluene. Moyo et al.[Citation103] note that extracts of marula leaves, young stem and opercula have high antioxidant activity and possess high phenolic compounds, thereby demonstrating an impregnable association between phenolic compounds and antioxidant activity. Moreover, Mariod et al.[Citation126] demonstrated that the extracts of the marula bark and roots have high TPC than the leaves extract when using 60% aqueous. It is hypothized that high levels of TPC of sample extracts constitute the predominant source of antioxidant activity. Hillman et al.[Citation27] reported that marula juice has antioxidant capacity ranging from 141–440 mg/100 ml ascorbic acid equivalent. Borochov-neori et al.[Citation46] studied the antioxidant activity and nutritional composition of marula fruit juice. The juice contained a significant amount of phenolics (56 mg of pyrogallol equivalence) and potent antioxidant capacity of 382 mg of vitamin C equivalent. Moreover, the antioxidant activity of the juice was not destroyed by pasteurization although 14% was lost after four weeks of low temperature storage at −18°C. This is against traditional beliefs that heat treatment destroys the scavenging ability of individual antioxidants. Ryan and Prescott[Citation127] gave three reasons for the preservation of the antioxidants: firstly, the antioxidant activity of the juice increases after processing since more antioxidant compounds are liberated due to the disruptions of cell walls by heat treatment. Secondly, oxidative enzymes that usually destroy antioxidants are destroyed by heat treatment. Lastly, there are new structural groups that are generated during heat treatment which improve antioxidant potential.
Antibacterial
The presence of antimicrobial compounds in the marula fruit is hypothesized based on various ethnomedical uses. In South Africa, the VhaVenda use marula bark to treat fever, stomach ailments as well as ulcers. Marula root is utilized for various purposes such as curing sore eyes in Zimbabwe.[Citation128] Moreover, the root is raw material in alcoholic medicine that is used to treat an internal disease called kati whereas the bark is used to treat stomach disorders.[Citation129] Eloff[Citation26] also indicates marula leaves and bark extracts have antibacterial activity. The author tested the extracts against bacteria such as Pseudomonas aeruginosa, Escherichia coli, Escherichia faecalis and Staphylococcus aureus with minimum inhibitory concentration (MIC) values varying from 0.15 to 3 mg/ml, and reported that the inner bark extracts was the most potent followed by extracts of the outer bark and leaf. Based on the values of MIC, it seems that marula leaves can also treat bacterial infections.
Antifungal
Different research studies have reported the antifungal activity of the marula fruit. Hamza et al.[Citation130] state that methanolic extracts from marula roots with MIC value of 0.5 mg/ml roots prevent the growth of Candida albicans, Candida glabrata, Candida parapsilosis, Candida tropicalis, Candida kruseii and Cryptococcus neoformans. Masako et al.[Citation131] indicate that ethanolic extract of the marula plant is very active against all the tested molds. The most sensitive mold is Cryptococcus albidus, followed by Candida parapsilosis and Rhodotorula mucilaginosa with an average MIC value of 0.17, 028 and 0.43 mg/ml, respectively. Masako et al.[Citation131] further explain that three extracts which are methanol, ethanol and acetone exhibited significant total activity against Cryptococcus albidus followed by Candida parapsilosis after 48 h but Rhodotorula mucilaginosa was extremely resistant. Methanol had the highest total activity of 387 ml/g, ethanol (363 ml/g) and acetone (299 ml/g) bark extracts.
Antidiabetic
Marula fruit is generally used as traditional medicine in cases of diabetes in Africa.[Citation132] The extracts of the marula bark have hypoglycemic effects in animal models. For example, Ojewole[Citation53] and Dimo et al.[Citation133] demonstrated that acute administration of the marula bark extract decreases blood sugar levels in rats with streptozotocin-induced diabetes. Chronically, Gondwe et al.[Citation134] administered the potency of the marula bark extract for a period of five weeks and it had the same effect as the treatment of metformin since it decreased glycemia. The marula fruit contains phenolic compounds such as epicatechin-3-galloyl ester and boosts glucose tolerance in diabetic mice and human subjects.[Citation135,Citation136]
Volatile compounds in marula fruit
Volatiles compounds are divided into higher alcohols, esters, carbonyl compounds and sulfur containing compounds.[Citation137] Esters and hydrocarbons are the most superior compounds in marula fruit while the fruit pulp contains β-caryophyllene, α humulene and E germacrene D ().[Citation139] From the identified ester compounds, only isoamyl isovalerate and ethyl lactate are available in both peel and flesh while more compounds are present in the peel. Most of these compounds are described as having sweet fruity aroma qualities except (Z)-3-hexen-1-yl valerate which has green (unripe) fruity aroma.[Citation138] Pretorius et al.[Citation139] determined the volatile compounds of marula juice and identified sesquiterpene hydrocarbons as the ample constituents with β-caryophyllene being the major compound.
Table 7. Volatile compounds identified in marula fruit.
Moreover, few monoterpenes such as linalool, furanoid oxide and geraniol were also identified. Β-caryophyllene and α-humulene are the most major volatile compounds in the marula flavor profile and have sweet fruity odor characteristics although their odor strength is not strong.[Citation140] Therefore, the contribution of these compounds to marula flavor cannot be neglected considering their large contents. The traditional process of making marula alcohol based products involve throwing away marula peel. Since the marula peel shows that it is rich in aroma volatiles, it is recommended to use the peel during production process and optimize contact time of the peel with the fruit pulp to produce products with desirable sensory characteristics.[Citation52]
Utilization of marula fruit in food and feed application
Researchers have explored the possibility of using the marula fruit to develop new food products with improved nutritional composition and nutraceutical properties such juice, alcohol based products, jams and jellies, fruit leather, vinegar and animal feed.
Juice
Fresh and fully ripe marula pulp is used to prepare juice that is high in vitamin C content ranging from 62 mg/100 g to more than 400 mg/100 g in the fresh fruit.[Citation46,Citation69] The juice also contains minerals such as magnesium, calcium, potassium, iron, zinc and manganese. Marula juice is also produced traditionally whereby a cow horn is used to penetrate the leathery skin of the fruit and then hands are used to squeeze the juice out of the fruit and the seed is removed.[Citation141] The major challenge with the flavor of marula juice is the sourness as well as the lack of sweetness.[Citation140] Von Teichman[Citation142] states that the peel of the marula fruit contains high amounts of flavor compounds. Therefore, the non-inclusion of marula peels during juice processing contribute to the lack of sweetness.[Citation106] The sugar content of fresh marula juice is 4.9 mg/ml sucrose, 4.9 mg/ml glucose and 22 mg/ml fructose. Therefore, there might be a need to adjust the comparatively low sugar to acid ratios so that the juice is accepted for human consumption. In addition, several drawbacks have been identified during marula processing such as the thickness of the peel, removal of the pulp from the central pit and small percentages (around 20%) of flesh to skin and pit.[Citation74] Borochov-Neori et al.[Citation27] say that people consume the marula fruit juice because it protects against atherosclerosis since it contains antioxidant fractions such as catechins, tannins and hydroxycinnamic acid derivatives.
Alcohol based products
Traditionally, a clay pot is used to store the pulp during marula beer production and a fork or knife is used to separate the skin from the flesh.[Citation143] The pulp is squeezed by hands and the resulting juice is collected and kept in a clay pot. Water is normally added to the nuts that still have flesh to liberate any leftover juice and pulp.[Citation144] The fermentation of the pulp is spontaneous whereby the yeasts that are naturally found on the fruit are utilized. The fermentation process occurs between two to four days at an ambient temperature of 25°C.[Citation145] Non-fermenting yeast species such as H. guilliermondii as well as lactic acid bacteria such as L. brevis and L. plantarum and the Enterobacteriaceae are commonly found at the initial stages of fermentation, whereas S. cerevisiae and Acetic acid bacteria dominate the final stages of fermentation. The slurry is generated on top of the liquid during the fermentation period and brewers remove it once or twice daily.[Citation144] Afterward, a sieve is used to carefully filter the mixture, and then the beer would be ready to be served. Brix normally decrease from 11.8 for unfermented juice and 2.6% for marula beer and this is attributed to the disappearance of sugar. Moreover, sugar is metabolized by lactic acid bacteria into lactic acid which contributes to pH dropping from 4.38 before fermentation to 3.44 at the final stage of fermentation. The alcoholic content of marula beer ranges from 0.9% (v/v) for the initial stage and 5.5% (v/v) for the final stage of fermentation.[Citation106] The preparation of marula beer does not require additional ingredients such as brown sugar, maize, yeast and sorghum as opposed to other traditional beers.[Citation146] However, the problem with marula beer is its shelf life which ranges between two to four days. Shackleton and Shackleton [145 indicate that brewers can extend the shelf life (extra 2–3 days) of the beer by mixing it with fresh marula juice daily or by storing it in a refrigerator.
On a commercial scale, amarula cream liqueur is an alcohol beverage produced from the marula fruit. The beverage is manufactured by Distell in Stellenbosch, South Africa and it has popularized the marula fruit all over the world.[Citation146] The marula fruits are collected by communities during harvesting period and delivered to Distell plant in Phalaborwa for juice processing. Distell SA only uses 30% of the harvested marula fruits in their production facility. The pulp is transported to Distell in Stellenbosch and the fermentation process is similar to making grape wine.[Citation143] Copper pots are used for the double distillation process of marula wine after fermentation. The small casks of oak are used to mature the juvenile and immature liqueur for up to two years and the alcohol content is 17% by volume.
Fruit leather
Fruit leathers are known as fruit rolls and they are produced by drying fruit pulp/puree or a mixture of fruit juice concentrates added with other ingredients.[Citation147,Citation148] Fully ripe fruits are used to produce fruit leathers with no preservatives or additives added.[Citation149] The blanching method is used to pre-treat the puree and other ingredients such as sugar, lemon juice and preservative such as sodium metabisulphite or citric acid are added before drying to prevent enzymatic browning of puree and to inhibit spoilage and pathogenic bacteria.[Citation150] For the marula fruit leather, extraction of the pulp is the most important step because the pulp hold on to the seeds which results in repetition of extraction. Sodium metabisulphite is added to the pulp to preserv4 the color as well as inhibiting the growth of bacteria and sugar is added to improve the flavor.[Citation146] The challenge with the marula fruit leather is its hardness which is attributed to low moisture content during drying.[Citation151]
Jams and jellies
Postharvest loss of fresh produce is reduced by producing valued added products such as jams, jellies, pickles and chutneys.[Citation152] Jam or jelly is processed from marula fruits using traditional methods at a small scale.[Citation153] The jam or jelly making involves the utilization of pulp alone or by mixing pulp and juice whereby they are heated to obtain the necessary soluble solids content and afterward the final product is pasteurized prior to packaging and setting.[Citation153,Citation154]Hiwilepo van-Hal et al.[Citation106] reported that the taste of marula jam and jelly is acceptable by consumers and have attractive color of waxy yellow and no colorants are added during the manufacturing. However, undesirable changes in nutritional content, functional and sensory properties as well as the destruction of phenolic compounds usually take place during thermal processing of fruits.[Citation155,Citation156] Therefore, there is a need to carry out further research on the preservation or loss of phenolic compounds, nutrients and sensory characteristics during jam or jelly manufacturing.
Vinegar
Based on the raw materials used, vinegar is classified as grain or fruit vinegar which contains starch or sugar through the sequential fermentation of ethanol and acetic acid.[Citation157] Vinegar production consists of two fermentation process called submerged and surface culture fermentations.[Citation158] Commercially, the submerged culture fermentation is the most popular method. Marula by- products are utilized in vinegar production. In terms of organic acids in marula vinegar, the acetic acid accounts for more than 99% because of the presence of lactic acid bacteria in the vinegar. Lactic acid bacteria are known to generate acetic acid as a metabolic by-product.[Citation159] The color of the marula vinegar is light amber to intense amber. The color changes of vinegar from light to dark brown might be attributed to polyphenol oxidase enzymes which oxidize the phenolic compounds. During consumer acceptability tests of marula vinegar, panelist preferred it over other commercial vinegars. They recommended that the marula vinegar should be utilized in salad dressing or mayonnaise. The sweet taste of marula vinegar detected by the panelist might be related to the natural flavor of marula fruit.[Citation146]
Animal feed
Marula fruits are a source of food for cattle, goats and sheep when they are ripe and have fallen to the ground. After juice extraction from the ripe fruit by mostly rural people, the fruit skin and nuts are discarded as waste and livestock, especially ruminants, are often found on the dump sites feeding on the marula waste. The marula seed cake (MSC), a by-product of oil extraction from the marula fruit seed, is the most researched component of the fruit regarding its nutritive value as food material for livestock.[Citation160–163] shows the chemical composition of the marula seed cake and MSC has high dry matter ranging from 90–96.6% which makes easy to store. Mthiyane and Mhlanga[Citation162] produced the marula seed cake in eSwatini and it had a dry matter ranging from 90.1–94.6% and was lower than 96.6% reported by Phenya[Citation163] for MSC produced in South Africa. The differences could be attributed to level of dryness of the kernels before oil extraction and variety. Moreover, the MSC is rich in protein ranging from 36–47.3%. This is an indication that MSC can be used as a source of protein for livestock. Significantly, the marula seed cake produced in eSwatini has higher crude protein content with an average of 47% whereas that produced in South Africa has 36%. This is due to differences in locations and probably the differences in varieties of the marula trees from which the fruits were collected.
Table 8. Chemical composition of marula seed cake.
The ether extract of the MSC ranges from 28–63% with Mdziniso et al.[Citation161] reporting 28.96% and Phenya[Citation163] 63.2%. The wide range in ether extract values shows inconsistencies in the removal of oil from kernels although the products were subjected to the same cold press oil extraction method. The high lipid content of MSC could be beneficial to livestock as a source of energy, however, in ruminants, high oil content could have a negative effect on the proper functioning of rumen microbes.[Citation164] Therefore, the high lipid content of MSC could limit the inclusion levels of this cake in diets for ruminants.
The crude fiber content of MSC ranges from 4–6%. The 4.69% crude fiber content of MSC reported by Mdziniso et al.[Citation161] is comparable to 5.82% reported by Mthiyane and Mhlanga.[Citation162] The comparability of crude fiber content of MSC is because these researchers used the same eSwatini source. Mlambo et al.[Citation163] indicate that the MSC contains 19.4% of neutral detergent fiber which is significantly lower than 33.8% reported by Phenya.[Citation165] These differences are due to the shell removal process where at times quantities of small shell chips remain in kernels before oil extraction.
The acid detergent fiber of 13.1%[Citation160] is comparable to 14.74%[Citation161] all produced in eSwatini. However, these are lower than 16.8%[Citation166] produced in South Africa. The variation could be attributed to differences in the production inconsistencies between companies. However, the acid detergent fiber values show acceptable levels for both ruminants and non-ruminants feeding. The 6.61% ash content of MSC reported by Mdziniso et al.[Citation161] is comparable to 5.55% and 5.43% reported by Mlambo et al.[Citation160] Ash is a representative of mineral content of a feed material.
The marula seed cake is a protein source or protein supplement in the diets of growing beef cattle as its inclusion does not negatively affect the growth performance of animals. Mlambo et al.[Citation160] indicate that the MSC can supply animals with rumen microbes and protein due to its slow degradability in the rumen. The marula seed cake is highly degradable in the rumen, indicating that the cake can provide the necessary nutrient for the rumen microbes and subsequently the animal itself.[Citation163]
Future projections of marula fruit
Wild fruits products, including marula based products are of significant importance to the African economy, nutrition and food security. Areas that could be explored to improve the utilization of the marula fruit include using pulp and peels for jams, jelly or marmalades since peels are a rich source of flavonoids and phenolic acids.[Citation167] The production cost for jam is very low, easy to process and transport and does not require refrigeration. However, only pulp is used during jam production and this leads to by-products being thrown away. Research studies have demonstrated a positive influence in incorporating fruit peel in marmalade and jam.[Citation165,Citation166,Citation168] Younis et al.[Citation165] added sweet lemon peels in jam and the chewability and firmness were increased. The incorporation of sweet orange peel powder improved the phenolic compounds and antioxidant activity of marmalade, although the anthocyanin content decreased.[Citation168] Marula peels can also be utilized to produce pectin and as animal feed.[Citation169,Citation170] Moreover, the marula seed cake can be utilized in meat products such as patties, ground meat to extend their shelf life since it contains polyphenolic compounds.
Other areas of interest are the adoption of non-thermal technologies such as high pressure processing (HPP) and pulse electric field (PEF) in the marula juice processing. Marula juice is rich in vitamin C and is very sensitive to heat, and conventional thermal processing such as pasteurization may result in quality changes such as color and flavor of the product.[Citation171,Citation172] Consumers demand safe and nutritious food and there is a need for the food industry to develop novel processing technologies that utilize minimal heat, thus resulting in fresh like products of high nutritional value.[Citation173] HPP inactivates and prevents the growth of microorganism in fruit juices as well as activating or inactivating enzymes at refrigerated temperatures, while low molecular weight compounds such as vitamins and compounds associated with pigments and aroma are not altered.[Citation173,Citation174] Therefore, HPP retains quality parameters such as color, flavor and nutritional value of fruit juice because of its low effect on the covalent bonds of low molecular-weight compounds.[Citation74,Citation76] HPP has been utilized in different fruit juices such as orange, blackberry, strawberry, tomato puree and others and the vitamin C content of the fruit juice was not altered.[Citation171,Citation175,Citation176]
PEF is another novel technology that can be used in the marula juice processing since it inactivates microorganisms when combined with temperature of < 50°C. This makes PEF a potential alternate to conventional thermal processes for liquid foods that contain bioactive that are heat sensitive or volatile components such as fruit juices.[Citation177] PEF is effective in inhibiting the growth of spoilage microorganisms in liquid foods such as juices, thus improving the shelf life and quality of the product.[Citation178] PEF has been utilized in orange juice processing and it retains vitamin C, carotenoids, volatile compounds and it results better sensory properties of orange juice after manufacturing and during refrigerated storage compared to pasteurized orange juice.[Citation179,Citation180]
Conclusion
The marula fruit is rich in nutrients such as vitamin C which is beneficial to human health because of its antioxidant activity. It is also associated with good human health because its biological activities play an important role in preventing and treating diseases. Currently, there is still a great opportunity for the fruit processing industry to develop and utilize bioactive components of the marula fruit. The consumption of marula fruit and its processed products rich in vitami C and polyphenolic compounds is associated with low prevalence of cardiovascular diseases, diabetes, cancer as well as other chronic diseases. The health benefits of the marula fruit have been demonstrated but there is still limited knowledge on the antioxidant activity of processed marula products such as fermented juice. Therefore, further research is still need in this regard. Moreover, bioactive components in the marula fruit should be extracted and used as functional ingredients to be incorporated in different food products. This will add value in protecting human health as well as improving the ailing economy by commercializing marula products. The innovation of marula products as well as modifications to guarantee their sustainability must therefore be a continuous process.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Schulze, R. E.; Maharaj, M., and Ghile, Y. Climatic Zonation. In South African Atlas of Climatology and Agrohydrology, Ed.; Schulze, R. E.,1-3. Water Research Commission: Pretoria, RSA, 1997, WRC Report 1489/1/06. Section 3.3
- Jiménez, A.; Saikia, P.; Giné, R.; Avello, P.; Leten, J.; Liss Lymer, B.; Schneider, K.; Ward, R. Unpacking Water Governance: A Framework for Practitioners. Water. 2020, 12(3), 827. DOI: 10.3390/w12030827.
- Nkosi, N. M.; Mostert, T. H. C.; Dzikiti, S.; Ntuli, N. R. Prioritization of Indigenous Fruit Tree Species with Domestication and Commercialization Potential in KwaZulu-Natal, South Africa. Genet. Resour. Crop Evol. 2020, 67(3), 1567–1575. DOI: 10.1007/s10722-020-00932-5(0123456789.
- Ebert, A. Z. Potential of Underutilized Traditional Vegetables and Legume Crops to Contribute to Food and Nutritional Security, Income and More Sustainable Production Systems. Sustainability. 2014, 6(1), 319–335. DOI: 10.3390/su6010319.
- Padulosi, S.; Heywood, V.; Hunter, D.; Jarvis, A. Underutilized Species and Climate Change: Current Status and Outlook. Crop Adaptation to Climate Change, 1st ed; Yadav, S. S., Redden, R. J., Hatfield, J. L., Lotze-Campen, H., Hall, A. E. Eds.; John Wiley & Sons, Blackwell Publishing Ltd: New Jersey, U.S.A, 2011.
- Mabhaudhi, T.; Chimonyo, V. G. P.; Modi, A. T. Status of Underutilized Crops in South Africa: Opportunities for Developing Research Capacity. Sustainability. 2017, 9(9), 1569. DOI: 10.3390/su9091569.
- Gouwakinnou, G. N.; Kindomihou, V.; Assogbadjo, A. E.; Sinsin, B. Population Structure and Abundance of Sclerocarya Birrea (A. Rich) Hochst Subsp. Birrea in Two Contrasting land-use Systems in Benin. Int. J. Biodivers. Conserv. 2009, 1(6), 194–201.
- Helm, C. V.; Scott, S. L.; Witkowski, E. T. Reproductive Potential and Seed Fate of Sclerocarya Birrea Subsp. Cafra (Marula) in the Low Altitudes of South Africa. S. Afr. J. Bot. 2011, 77(3), 650–664. DOI: 10.1016/j.sajb.2011.02.003.
- Shackleton, S. E.; Shackleton, C. M.; Cunningham, A. B.; Lombard, C.; Sullivan, C. A.; Netshiluvhi, T. R. Knowledge on Sclerocarya Birrea Subsp. Caffra with Emphasis on Its Importance as A non-timber Forest Product in South and Southern Africa: A Summary. Part 1: Taxonomy, Ecology and Role in Rural Livelihoods. S. Afr. For. J. 2002, 194, 27–41. DOI: 10.1080/20702620.2002.10434589.
- Hamidou, A.; Iro, D. G.; Boube, M.; Malik, T. S.; Ali, M. Potential Germination and Initial Growth of Sclerocarya Birrea (A. Rich.) Hoscht, in Niger. J. Appl. Biosci. 2014, 76, 6433–6443. DOI:10.4314/jab.v76i1.13.
- Shackleton, S.; Sullivan, C.; Cunningham, T.; Leakey, R.; Laird, S.; Lombard, C. An Overview of Current Knowledge on Sclerocarya Birrea (A.rich.) Hochst. Subsp. Caffra (Sond.) Kokwaro with Particular Reference to Its Importance as a non-timber Forest Product (NTFP) in Southern Africa; SA-DC, INR, Ethnoecology Services. UK: South Africa, Namibia, 2001; pp 56. CEH, Rhodes University, CSIR, CRIAA
- Orwa, C.; Mutua, A.; Kindt, R.; Jamnadass, R.; Simons, A. Agroforest Tree Database: A Tree Reference and Selection Guide Version 4.0. 2009. http://www.worldagroforestry.org/af/treedb/. (accessed Nov 20, 2021).
- Ngorima, G. T. Towards sustainable use of marula (Sclerocarya birrea) in the Savannah woodlands of Zvishavane District. MSc Thesis. Faculty of Science, University of the Witwatersrand, Johannesburg, South Africa, 2006.
- Jacobs, O. S.; Biggs, R. The Impact of the African Elephant on Marula Trees in the Kruger National Park. S. Afr. J. Wildl. 2002, 32(1), 13–22.
- Bandeira, S. O.; Albano, G. G.; Barbosa, F. M. Diversity and Uses of Plant Species in Goba, Lebombo Mountains, Mozambique, with Emphasis on Trees and Shrubs. In African Plants: Biodiversity, Taxonomy and Uses; Timberlake, J., Kativu, S., Eds.; Kew: Royal Botanical Gardens: London, 1999; pp 429–439.
- Hall, J. B.; O’Brien, E. M.; Sinclair, F. L. Sclerocarya Birrea: A Monograph; School of Agriculture and Forest Sciences, University of Wales: Bangor, Cardiff, United Kingdom, 2000.
- Du Plessis, P.; Lombard, C.; den Adel, S. Marula in Namibia: Commercial Chain Analysis; CRIAA SA-DC: Namibia, 2002; pp 28.
- Neumann, R. P.; Hirsch, E. Commercialisation of non-timber Forest Products: Review and Analysis of Research; Centre for International Forestry Research: Bogor, Indonesia, 2000.
- Lawes, M.; Eelly, H.; Schackleton, C.; Geach, B. Indigenous Forests and Woodlands in South Africa: Policy, People and Practice; University of KwaZulu-Natal press: Scottsville, South Africa, 2004.
- Lewis, D. M. Fruiting Patterns, Seed Germination, and Distribution of Sclerocarya Caffra in an Elephant-Inhabited Woodland. Biotropica. 1987, 19(1), 50–56. DOI: 10.2307/2388459.
- Hall, J. B. Sclerocarya Birrea Record from Protabase; A. Rich.) Hochst In Oyen, L. P. A., Lemmens, R. H. M. J., Eds.; PROTA (Plant Resources of Tropical Africa) Programme. http://www.prota4u.org/search.asp. Accessed 20 Nov 2021. Wageningen, the Netherlands, 2002.
- Dlamini, C. S. Provenance and Family Variation in Seed Mass and Fruit Composition in Sclerocarya Birrea sub-species Caffra. J. Hortic. For. 2011, 3(9), 286–293.
- Maruzane, D.; Tapfumaneyi, L.; Matarirano, L. C. Marula Propagation, Uses and Marketing; World Agroforestry Centre: Nairobi, Kenya, 2002.
- Helm, C. V.; Witkowski, E. T. Continuing Decline of a Keystone Tree Species in the Kruger National Park, South Africa. Afr. J. Ecol. 2013, 51(2), 270–279. DOI: 10.1111/aje.12032.
- McGaw, L. J.; Van der Merwe, D.; Eloff, J. N. In Vitro Anthelmintic, Antibacterial and Cytotoxic Effects of Extracts from Plants Used in South African Ethnoveterinary Medicine. Vet. J. 2007, 173(2), 366–372. DOI: 10.1016/j.tvjl.2005.09.004.
- Eloff, J. N. Antibacterial Activity of Marula (Sclerocarya Birrea (A. Rich.) Hochst. Subsp. Caffra (Sond.) Kokwaro) (Anacardiaceae) Bark and Leaves. J. Ethnopharmacol. 2001, 76(3), 305–308. DOI: 10.1016/s0378-8741(01)00260-4.
- Borochov-Neori, H.; Judeinstein, S.; Greenberg, A.; Fuhrman, B.; Attias, J.; Volkova, N.; Hayek, T.; Aviram, M. Phenolic Antioxidants and Antiatherogenic Effects of Marula (Sclerocarrya Birrea Subsp. Caffra) Fruit Juice in Healthy Humans. J. Agric. Food Chem. 2008, 56(21), 9884–9891. DOI: 10.1021/jf801467m.
- Ndifossap, I. G. M.; Casimir, F. M.; Tsofack, F. N.; Dongo, E.; Kamtchouing, P.; Dimo, T.; Maechler, P. Sclerocarya Birrea (Anacardiaceae) stem-bark Extract Corrects Glycaemia in Diabetic Rats and Acts on-cells by Enhancing Glucose Stimulated Insulin Secretion. J. Endocrinol. 2010, 205(1), 79–86. DOI: 10.1677/JOE-09-0311.
- Ndhlala, A. R.; Kasiyamhuru, A.; Mupure, C.; Chitindingu, K.; Benhura, M. A.; Muchuweti, M. Phenolic Composition of Flacourtia Indica, Opuntia Megacantha and Sclerocarya Birrea. Food Chem. 2007, 103(1), 82–87. DOI: 10.1016/j.foodchem.2006.06.066.
- Lamien-Meda, A.; Lamien, C. E.; Compaore, M. M. Y.; Meda, R. N. T.; Kiendrebeogo, M.; Zeba, B.; Millogo, J. F.; Nacoulma, O. G. Polyphenol Content and Antioxidant Activity of Fourteen Wild Edible Fruits from Burkina Faso. Molecules. 2008, 3(3), 581–594. DOI: 10.3390/molecules13030581.
- Brunton, N. P.; Cronin, D. A.; Monahan, F. J. Volatile Components Associated with Freshly Cooked and Oxidized off-flavours in Turkey Breast Meat. Flavour Fragr. J. 2002, 17(5), 327–334. DOI: 10.1002/ffj.1087.
- Braca, A.; Politi, M.; Sanogo, R.; Sanou, H.; Morelli, I.; Pizza, C.; De Tommasi, N. Chemical Composition and Antioxidant Activity of Phenolic Compounds from Wild and Cultivated Sclerocarya Birrea (Anacardiaceae) Leaves. J. Agric. Food Chem. 2003, 51(23), 6689–6695. DOI: 10.1021/jf030374m.
- Viljoen, A. M.; Kamatou, G. P. P.; Başer, K. H. C. Head-space Volatiles of Marula (Sclerocarya Birrea Subsp. Caffra). S. Afr. J. Bot. 2008, 742, 325–326. DOI:10.1016/j.sajb.2007.10.005.
- Tsautage, A. Storage Temperature and Atmosphere Influenced Postharvest Quality of Marula (Sclerocarya Birrea Subspecies Caffra) Fruit. MSc Dissertation (Horticulture. Department of Crop and Soil Sciences. Botswana University of Agriculture and natural Resources. 2020.
- Taylor, F. W.; Butterworth, K. J., and Mateke, M. M. The Importance of Indigenous Fruit Trees in semi-arid Areas of Southern and Eastern Africa. In Paper Presented at the African Academy of Sciences Second Roundtable Discussion on non-wood/timber Products, Pretoria, South Africa: African Academic of Science, karen, Nairobi. Kenya, 1995.
- Petje, K. F. Determination of fruit yield and fruit quality in marula (Sclerocarya birrea subsp. caffra) selections. MSc Thesis, Departments of Plant Production and Soil Science, University of Pretoria, South Africa, 2008.
- Mkwezalamba, I.; Munthali, C. R. Y.; Missanjo, E. Phenotypic Variation in Fruit Morphology among Provenances of Sclerocarya Birrea (A. Rich.) Hochst. Int. J. For. Res. 2015. Article ID 735418. DOI: 10.1155/2015/735418.
- Gechemba, M. F. Molecular Characterization of Sclerocarya Birrea (Marula) Field Genebank Collections. MSc Dissertation. School of Pure and Applied Sciences. Kenyatta University: Nairobi, Kenya, 2018.
- Masarirambi, M. T.; Nxumalo, K. A. Post – Harvest Physiological Indicators on the Phenotypic Variation of Marula Fruits (Sclerocarya Birrea Subspp. Caffra) in Swaziland. Int. J. Biol. Pharm. Allied Sci. 2012, 1(8), 10251039.
- Nyoka, B. I. T. C.; Mng’omba, S. A.; Akinnifesi, F. K.; Sagona, W. Variation in Growth and Fruit Yield of Populations of Sclerocarya Birrea (A. Rich.) Hochst. Agrofor. Syst. 2015, 89, 397–407. DOI: 10.1007/s10457-021-00592-z.
- Palgrave, K. C. Trees of Southern Africa. Struik Publisher, Cape Town. South Africa. 1995, 65, 489–505.
- Venter, F.; Venter, F. Making the Most of Indigenous Trees; 2nd; Briza Publisher: Pretoria, South Africa, 2005; pp 274.
- Hyde, M. A., and Wursten, B. Flora of Zimbabwe: Species Information: Sclerocarya Birrea Subsp. In caffra. University of Zimbabwe, 1–5. Harare, Zimbabwe, 2010.
- Maroyi, A. Traditional Use of Medicinal Plants in south-central Zimbabwe: Review and Perspectives J. Ethnobiol. Ethnomed. 2013, 9(1), 31. DOI: 10.1186/17464269-9-31.
- Net Database, M. Sclerocarya birrea; Rich, A. Ed.; Hochst, Website, 2003, http//www.worldforestrycentre.org/sites/Tree-DBS/Marula/info.htm (accessed Nov 10, 2021).
- Hillman, Z.; Mizrahi, Y.; Beit-Yannai, E. Evaluation of Valuable Nutrients in Selected Genotypes of Marula (Sclerocarya Birrea Sspcaffra). Sci. Hortic. 2008, 1174, 321–328. DOI:10.1016/j.scienta.2008.05.008.
- Muhammad, S.; Hassan, L. G.; Dangoggo, S. M.; Hassan, S. W.; Umar, R. A.; Umar, K. J. Nutritional and Antinutritional Composition of Sclerocarya Birrea Peels. Int. J. Sci. Basic Appl Res. 2015, 21(2), 3948.
- Sybille, B.; Suarez, C.; Beckett, K. Marula Fruit: The Next Beverage Innovation. Nutraceutical Bus. Technol. 2012, 8(3), 34–37.
- Bille, P. G.; Shikongo- Nambabi, M., and Cheikhyoussef, A. 2013. Value Addition and Processed Products of Three Indigenous Fruits in Namibia. Afr. J. Food. Agric. Nutr. Dev. 13(1), 7192–7212.
- Marula Natural Products. Marula Products. [Online] www.marula.org.za/prodfruit.html.DBS/Marula/info.htm. (accessed Nov 06, 2021).
- Mawoza, T.; Ojewole, J. A.; Chiwororo, W. D.; Owira, P. M. Sclerocarya Birrea (A. Rich.) Hochst [Marula] (Anacardiaceae): A Review of Its Phytochemistry, Pharmacology and Toxicology and Its Ethnomedicinal Uses. Phytother Res. 2010, 24(5), 633–639. DOI: 10.1002/ptr.3080.
- Hiwilepo-van Hal, P. Processing of marula (Sclerocarya birrea subsp. caffra) fruits: a case study on health-promoting compounds in marula pulp. PhD thesis, Wageningen University, Wageningen, Netherlands, 2013.
- Ojewole, J. A. Hypoglycaemic Effect of Sclerocarya Birrea (A. Rich.) Hochst. [Anacardiaceae] stem-bark Aquous Extracts in Rats. Phytomedicine. 2003, 10(8), 675–681. DOI: 10.1078/0944-7113-00295.
- Todorov, S. D.; Dicks, L. M. Bacteriocin Production by Pediococcus Pentosaceus Isolated from Marula (Sclerocarya Birrea). Int. J. Food Microbiol. 2009, 132(2–3), 117–126. DOI: 10.1016/j.ijfoodmicro.2009.04.010.
- Mariod, A. A.; Abdelwahab, S. I. Sclerocarya Birrea (Marula), an African Tree of Nutritional and Medicinal Uses: A Review. Food Rev. Int. 2012, 28(4), 375–388. DOI: 10.1080/87559129.2012.660716.
- Shone, A. K. Notes on Marula. South African Department of Water Affairs and Forestry Bulletin 58, Pretoria ; South Africa, 58, 1–9, 1979.
- Duke, J. CRC Handbook of Nuts; CRC Press, Inc: Boca Raton, 1989.
- Dzerefos, C. M.; Shackleton, C. M.; Witkowski, E. T. Sustainable Utilization of woodrose-producing Mistletoes (Loranthaseae) in South Africa. Econ. Bot. 1999, 53, 439–447. DOI: 10.1007/BF02866724.
- Elijah, R.; Abdulkadir, L.; Adisa, B. Investigation of Extracted Sclerocarya Birrea Seed Oil as a Bioenergy Resource for Compression Ignition Engines. Int. J. Agric. Biol. Eng. 2012, 5(3), 59–69. DOI: 10.3965/j.ijabe.20120503.00.
- Enweremadu, C. C.; Rutto, H. L. Optimization and Modelling of Process Variables of Biodiesel Production from Marula Oil Using Response Surface Methodology. J. Chem. Soc. Pak. 2015, 37(2), 256–265. DOI: 10.13140/2.1.2186.8165.
- Eromosele, C. O.; Paschal, N. H. Characterization and Viscosity Parameters of Seed Oils from Wild Plants. Bioresour. Technol. 2003, 86(2), 203–205. DOI: 10.1016/S0960-8524(02)00147-5.
- FAO; IFAD; UNICEF; WFP; WHO. The State of Food Security and Nutrition in the World; Food and Agriculture Organization: Rome, Italy, 2020.
- Sibiya, N. P.; Kayitesi, E.; Moteetee, A. N. Proximate Analyses and Amino Acid Composition of Selected Wild Indigenous Fruits of Southern Africa. Plants. 2021, 10(4), 721. DOI: 10.3390/plants10040721.
- Sibiya, N. P.; Kayitesi, E.; Moteetee, A. Mineral Composition of Selected Southern African Fruits. S. Afr. J. Bot. 2020, 132, 87–94. DOI: 10.1016/j.sajb.2020.04.014.
- Iddir, M.; Brito, A.; Dingeo, G.; Del Campo, S. S. F.; Samouda, H.; La Frano, M. R. Strengthening the Immune System and Reducing Inflammation and Oxidative Stress through Diet and Nutrition: Considerations during the COVID-19 Crisis. Nutrients. 2020, 12(6), 1562. DOI: 10.3390/nu12061562.
- Wang, P. Y.; Fang, J. C.; Gao, Z. H.; Zhang, C.; Xie, S. Y. Higher Intake of Fruits, Vegetables or Their Fiber Reduces the Risk of Type 2 Diabetes: A meta-analysis. J. Diabetes Investig. 2016, 7(1), 56–69. DOI: 10.1111/jdi.12376.
- Wu, Y.; Zhang, D.; Jiang, X.; Jiang, W. Fruit and Vegetable Consumption and Risk of Type 2 Diabetes Mellitus: A dose-response meta-analysis of Prospective Cohort Studies. Nutr. Metab. Cardiovasc. Dis. 2015, 25(2), 140–147. DOI: 10.1016/j.numecd.2014.10.004.
- Achaglinkame, M. A.; Aderibigbe, R. O.; Hensel, O.; Sturm, B.; Korese, J. K. Nutritional Characteristics of Four Underutilized Edible Wild Fruits of Dietary Interest in Ghana. Foods. 2019, 8(3), 104. DOI: 10.3390/foods8030104.8030104.
- Eromosele, I. C.; Eromosele, C. O.; Kuzhkuzha, D. M. Evaluation of Mineral Elements and Ascorbic Acid Contents in Fruits of Some Wild Plants. Plant Foods Human Nutri. 1991, 41(2), 151–154. DOI: 10.1007/BF02194083.
- Jiang, B.; Tsao, R.; Li, Y.; Miao, M. Food Safety: Food Analysis Technologies/Techniques. In Book: Encyclopedia of Agric. Food Syst, Ed.; Neal, K.; Van Alfen, Elsevier: Netherlands, 2014; pp 273–288. DOI: 10.1016/B978-0-444-52512-3.00052-8.
- Attiogbe, F. K.; Abdul-Razak, T. Evaluation of the Physicochemical Properties of Northern Ghana Sclerocarya Birrea Seed Oil and Proximate Analysis of the Process Waste. Afr. J. Food Sci. 2016, 10(4), 48–53. DOI: 10.5897/AJFS2016.1425.
- Chiesa, G.; Kiriakov, S.; Khalil, A. S. Protein Assembly Systems in Natural and Synthetic Biology. BMC Biol. 2020, 18, 35. DOI: 10.1186/s12915-020-0751-4.
- Food Drug Administration. Interactive Nutritional Facts Label. Vitamin and Minerals Chart. 2020. www.FDA.gov.nutritioneducation. (accessed Nov 20, 2021).
- Gous, F.; Weinert, I. A. G.; Van Wyk, P. J. Selection and Processing of Marula Fruit (Sclerocarya Birrea subspCaffra). Lebensm. - Wiss. u. Technol. 1988, 21, 259–266.
- Glew, R. S.; VanderJagt, D. J.; Huang, Y. S.; Chuang, L. T.; Bosse, R., and Glew, R. H. Nutritional Analysis of the Edible Pit of Sclerocarya Birrea in the Republic of Niger (daniya, Hausa). J. Food Compos. Anal. 17(1), 99–111 .doi:10.1016/S0889-1575(03)00101-7 . 2004.
- Aganga, A. A.; Mosase, K. W. Tannin Content, Nutritive Value and Dry Matter Digestibility of Lonchocarpus Capassa, Zizyphus Mucronata, Sclerocarya Birrea, Kirkia Acuminata and Rhus Lancea Seeds. Anim Feed Sci. Technol. 2001, 91(1), 107–113. DOI: 10.1016/S0377-8401(01)00235-8.
- Wairagu, N. W.; Kiptoo, J.; Githiomi, J. K. Nutritional Assessment of Sclerocarya Birrea (Amarula) Fruit from Kenya. Int. J. Curr Res. 2013, 5(5), 1074–1078.
- Oyeleke, G. O.; Salam, M. A.; Aderoro, R. O. Some Aspects of Nutrients Analysis of Seed, Pulp and Oil of Baobab (Andasonia Digitata L.). J. Environ Sci. Toxicol. Food Technol. 2012, 1(4), 32–35. DOI: 10.9790/2402-0143235.
- Morales, J. O.; Valdés, K.; Morales, J.; Oyarzun-Ampuero, F. Lipid Nanoparticles for the Topical Delivery of Retinoids and Derivatives. Nanomedicine. 2015, 10(2), 253–269. DOI: 10.2217/nnm.14.159.
- Huxtable, R. J. The Metabolism and Functions of Methionine. In Biochemistry of Sulfur. Biochemistry of the Elements; Huxtable, R. J., Ed.; Springer: Boston, MA, USA, 1986; pp 61–120.
- Manoli, I.; Venditti, C. P. Disorders of Branched Chain Amino Acid Metabolism. Transl. Sci. Rare Dis. 2016, 1(2), 91–110. DOI: 10.3233/TRD-160009.
- Kohlmeier, M. P. In Food Science and Technology. In Nutrient Metabolism; Kohlmeier, M., Ed.; Academic Press: Cambridge, MA, USA, 2003; pp 314–321.
- Zhang, S.; Zeng, X.; Ren, M.; Mao, X.; Qiao, S. Novel Metabolic and Physiological Functions of Branched Chain Amino Acids: A Review. J. Anim. Sci. Biotechnol. 2017, 8, 10. DOI: 10.1186/s40104-016-0139-z.
- Gu, C.; Mao, X.; Chen, D.; Yu, B.; Yang, Q. Isoleucine Plays an Important Role for Maintaining Immune Function. Curr. Protein Pept. Sci. 2019, 20(7), 644–651. DOI: 10.2174/1389203720666190305163135.
- FAO. Fats and Fatty Acids in Human Nutrition. Report of an Expert Consultation; Food and Agriculture Organization of the United Nations: Rome, Italy, 91, 2008.
- Duan, Y.; Li, F.; Li, Y.; Tang, Y.; Kong, X.; Feng, Z.; Anthony, T. G.; Watford, M.; Hou, Y.; Wu, G., et al. The Role of Leucine and Its Metabolites in Protein and Energy Metabolism. Amino Acids. 2016, 48(1), 41–51. DOI: 10.1007/s00726-015-2067-1.
- Zimba, N.; Wren, S.; Stuck, A. Three Major Tree Nut Oils of Southern Central Africa: Their Uses and Future as Commercial Base Oils. Int. J. Aromather. 2005, 15(4), 177–182. DOI: 10.1016/j.ijat.2005.10.009.
- Aparna, V.; Dileep, K. V.; Mandal, P. M.; Karthe, P.; Sadasivan, C.; Haridas, M. Anti-inflammatory Property of n-hexadecanoic Acid: Structural Evidence and Kinetic Assessment. Chem. Biol. Drug Des. 2012, 80(3), 434–439. DOI: 10.1111/j.1747-0285.2012.01418.x.
- Matemu, A. O.; Adeyemi, D.; Nyoni, H.; Mdee, L.; Tshabalala, P.; Mamba, B.; Msagati, T. A. M. Fatty Acid Composition of Dried Fruits of Sclerocarya Birrea, Diospyros Blancoi and Landolphia Kirkii. Int. J. Environ. Res. Public Health. 2017, 14(11), 1401. DOI: 10.3390/ijerph14111401.
- Morse, N. Are Some Health Benefits of Palmitoleic Acid Supplementation Due to Its Effects on 5′ Adenosine monophosphate-activated Protein Kinase (AMPK)? Lipid Technol. 2015, 27(12), 278–281. DOI: 10.1002/lite.201500061.
- Osman, M. A. Chemical and Nutrient Analysis of Baobab (Adansonia Digitata) Fruit and Seed Protein Solubility. Plant Food Human Nutri. 2004, 59(1), 29–33. DOI: 10.1007/s11130-004-0034-1.
- Ogbobe, O. 1992. Physico-chemical Composition and Characterisation of the Seed and Seed Oil of Sclerocarya Birrea. Plant Foods Human Nutri. 42(3), 201–206. DOI: 10.1007/BF02193927.
- Wynberg, R.; Cribbins, J.; Leakey, R.; Lombard, C.; Mander, M.; Shackleton, S.; Sullivan, C. Knowledge on Sclerocarya Birrea Subsp. Caffra with Emphasis on Its Importance as a non-timber Forest Product in South and Southern Africa: A Summary. Part 2: Commercial Use, Tenure and Policy, Domestication, Intellectual Property Rights and benefit-sharing. J. S. Afr. For. 2002, 196, 67–77.
- Eteshola, E.; Oraedu, A. Fatty Acid Compositions of Tigernut Tubers (Cyperus Esculentus L.), Baobab Seeds (Adansonia Digitata L.), and Their Mixture. J. Am. Oil Chem.’ Soc. 1996, 73(2), 255–257. DOI: 10.1007/BF02523905.
- Greenfield, H., and Southgate, D. Sampling. In Food and Agriculture Organization of the United Nations, 2nd; Eds.; Burlingame, B., Charrondiere, U., Rome, Italy: FAO Publishing Management Service, 2003.
- Chivandi, E. M.; Erlwanger, K. H.; Davidson, B. C. Lipid Content and Fatty Acid Profile of the Fruit Seeds of Diospyros Mespiliformis. Int. J. Integr. Biol. 2009, 5(2), 121–124.
- Nagy, K.; Tiuca, L. Importance of Fatty Acids in Physiopathology of Human Body; Intechopen: Croatia, 1–18. 2017. DOI:10.5772/67407.
- Darnet, S. H.; Da Silva, L. H. M.; Rodrigues, A. M. D. C.; Lins, R. T. Nutritional Composition, Fatty Acid and Tocopherol Contents of Buriti (Mauritia Flexuosa) and Patawa (Oenocarpus Bataua) Fruit Pulp from the Amazon Region. Food Sci. Technol. Campinas. 2011, 31(2), 488–491. DOI: 10.1590/S0101-20612011000200032.
- Mariod, A. A.; Matthaüs, B.; Eichner, E. Fatty Acid, Tocopherol and Sterol Composition as Well as Oxidative Stability of Three Unusual Sudanese Oils. J. Food Lipids. 2004, 11(3), 179–189. DOI: 10.1111/j.1745-4522.2004.01131.x.
- Magaia, T.; Uamusse, A.; Sjöholm, I.; Skog, K. Proximate Analysis of Five Wild Fruits of Mozambique. Sci. World J. 2013, 2013, 1–6. Article ID 601435. DOI: 10.1155/2013/601435.
- Ezeagu, I. E. Baobab (Adansonia Digitata L.) Seed Protein Utilization in Young Albino Rats. Biochemical Ingredients and Performance Characteristics. Anim Res Int. 2005, 2(1), 240–245.
- Fernstrom, J. D.; Fernstrom, M. H. Tyrosine, Phenylalanine, and Catecholamine Synthesis and Function in the Brain. J. Nutri. 2007, 137(6), 1539S–1547S. DOI: 10.1093/jn/137.6.1539S.
- Moyo, M.; Ndhlala, A. R.; Finnie, J. F.; Van Staden, J. Phenolic Composition, Antioxidant and Acetylcholinesterase Inhibitory Activities of Sclerocarya Birrea and Harpephyllum Caffrum (Anacardiaceae) Extracts. Food Chem. 2010, 123(1), 69–76. DOI: 10.1016/j.foodchem.2010.03.130.
- Hilou, A.; Bougma, A.; Dicko, M. H. Phytochemistry and agro-industrial Potential of Native Oilseeds from West Africa: African Grape (Lannea Microcarpa), Marula (Sclerocarya Birrea), and Butter Tree (Pentadesma Butyracea). Agriculture. 2017, 7(3), 24. DOI: 10.3390/agriculture7030024(3).
- Russo, D.; Kenny, O.; Smyth, T. J.; Milella, L.; Hossain, M. B.; Diop, M. S.; Rai, D. K.; Brunton, N. P. Profiling of Phytochemicals in Tissues from Sclerocarya Birrea by HPLC-MS and Their Link with Antioxidant Activity. ISRN Chromatogr. 2013, 2013, 1–11. Article ID 283462. DOI: 10.1155/2013/283462.
- Hiwilepo-van Hal, P.; Bille, P. G.; Verkerk, R.; van Boekel, M. A. J. S.; Dekker, M. A Review of the Proximate Composition and Nutritional Value of Marula (Sclerocarya Birrea Subspcaffra). Phytochem. Rev. 2014, 13(4), 881–892. DOI:10.1007/s11101-014-9352-6.
- Hirota, S.; Shimoda, T.; Takahama, U. Tissue and Spatial Distribution of Flavonol and Peroxidase in Onion Bulbs and Stability of Flavonol Glucosides during Boiling of the Scales. J. Agric. Food Chem. 1988, 46(9), 497–3502. DOI: 10.1021/jf980294w.
- Can, Z.; Dincer, B.; Sahin, H.; Baltas, N.; Yildiz, O.; Kolayli, S. Polyphenol Oxidase Activity and Antioxidant Properties of Yomra Apple (Malus Communis L.) from Turkey. J. Enzyme Inhib. Med. Chem. 2014, 29(6), 829–835. DOI: 10.3109/14756366.2013.858144.
- Padua, T. A.; de Abreu, B. S.; Costa, T. E.; Nakamura, M. J.; Valente, L. M.; Henriques, M. D.; Siani, A. C.; Rosas, E. C. Anti-inflammatory Effects of Methyl Ursolate Obtained from a Chemically Derived Crude Extract of Apple Peel: Potential Use in Rheumatoid Arthritis. Arch. Pharm. Res. 2014, 37(11), 1487–1495. DOI: 10.1007/s12272-014-0345-1.
- Robards, K.; Prenzler, P. D.; Tucker, G.; Swatsitang, P.; Glover, W. Phenolic Compounds and Their Role in Oxidative Processes in Fruits. Food Chem. 1999, 66(4), 401–436. DOI: 10.1016/S0308-8146(99)00093-X.
- Harborne, J. B. The Flavonoids: Advances in Research since 1986; Chapman and Hall: London, UK, 1994.
- Wallace, G.; Fry, S. C. Phenolic Components of the Plant Cell Wall Int. Rev. Cytol. 1994, 151, 229–267. DOI: 10.1016/S0074-7696(08)62634-0
- Dohi, S.; Terasaki, M.; Makino, M. Acetylcholinesterase Inhibitory Activity and Chemical Composition of Commercial Essential Oils. J. Agric. Food Chem. 2009, 57, 4313–4318. DOI: 10.1021/jf804013j.
- Eldeen, I. M. S.; Elgorashi, E. E.; Van Staden, J. Antibacterial, anti-inflammatory, anti-cholinesterase and Mutagenic Effects of Extracts Obtained from Some Trees Used in South African Traditional Medicine. J. Ethnopharmacol. 2005, 102, 457–464. DOI: 10.1016/j.jep.2005.08.049.
- Alothman, M.; Bhat, R.; Karim, A. A. Antioxidant Capacity and Phenolic Content of Selected Tropical Fruits from Malaysia, Extracted with Different Solvents. Food Chem. 2009, 115(3), 785–788. DOI: 10.1016/j.foodchem.2008.12.005.
- Hakkim, F. L.; Shankar, C. C.; Girija, S. Chemical Composition and Antioxidant Property of Holy Basil (Ocimum Sanctum L.) Leaves, Stems, and Inflorescence and Their in Vitro Callus Cultures. J. Agric. Food Chem. 2007, 55(22), 9109–9117. DOI: 10.1021/jf071509h.
- Kim, K.; Tsao, R.; Yang, R.; Cui, S. W. Phenolic Acid Profiles and Antioxidant Activities of Wheat Bran Extracts and the Effect of Hydrolysis Conditions. Food Chem. 2006, 95(3), 466–473. DOI: 10.1016/j.foodchem.2005.01.032.
- Price, K. R.; Prosser, T.; Richetin, A. M. F.; Rhodes, M. J. C. A Comparison of the Flavanol Content and Composition in Dessert, Cooking and cider-making Apples; Distribution within the Fruit and Effect of Juicing. Food Chem. 1999, 66(4), 489–494. DOI: 10.1016/S0308-8146(99)00099-0.
- Li, Y. Antioxidants in Biology and Medicine: Essentials. In Advances and Clinical Applications. ed. Yunbo, Li; Nova Science Publishers: New York, 2011; pp 266–335.
- Stoclet, J. C.; Chataigneau, T.; Ndiaye, M.; Oak, M. H.; El Bedoui, J.; Chataigneau, M. Vascular Protection by Dietary Polyphenols. Eur. J. Pharmacol. 2004, 500, 299–313. DOI: 10.1016/j.ejphar.2004.07.034.
- Mann, G. E.; Rowlands, D. J.; Li, F. Y.; de Winter, P.; Siow, R. C. Activation of Endothelial Nitric Oxide Synthase by Dietary Isoflavones: Role of NO in Nrf2-mediated Antioxidant Gene Expression. Cardiovasc. Res. 2007, 75(2), 261–274. DOI: 10.1016/j.cardiores.2007.04.004.
- Araújo, T. A. S.; Alencar, N. L.; de Amorim, E. L. C.; De albuquerque, U. P. A New Approach to Study Medicinal Plants with Tannins and Flavonoids Contents from the Local Knowledge. J. Ethnopharmacol. 2008, 120(1), 72–80. DOI: 10.1016/j.jep.2008.07.032.
- Okuda, T. Systematics and Health Effects of Chemically Distinct Tannins in Medicinal Plants. Phytochem. 2005, 66(17), 2012–2031. DOI: 10.1016/j.phytochem.2005.04.023.
- Hollman, P. C. H.; Hertog, M. G. L.; Katan, M. B. Analysis and Health Effects of Flavonoids. Food Chem. 1996, 57(1), 43–46. DOI: 10.1016/0308-8146(96)00065-9.
- Olsen, P.; Meyer, O.; Bille, N.; Wurtzen, G. Carcinogenicity Study on Butylated Hydroxytoluene (BHT) in Wistar Rats Exposed in Utero. Food Chem. Toxicol. 1986, 24(1), 1–12. DOI: 10.1016/0278-6915(86)90256-5.
- Mariod, A. A.; Matthaus, B.; Hussein, I. H. Antioxidant Properties of Methanolic Extracts from Different Parts of Sclerocarya Birrea. Int. J. Food Sc. Technol. 2008, 43(5), 921–926. DOI: 10.1111/j.1365-2621.2007.01543.x.
- Ryan, L.; Prescott, S. L. Stability of the Antioxidant Capacity of twenty-five Commercially Available Fruit Juices Subjected to an in Vitro Digestion. Int. J. Food Sci. Technol. 2010, 45(6), 1191–1197. 02254.x. DOI: 10.1111/j.1365-2621.2010.
- Gelfand, M.; Mavi, S.; Drummond, R. B.; Ndemera, B. The Traditional Medical Practitioner in Zimbabwe; Mambo Press: Gweru, Zimbabwe, 1985.
- Kokwaro, J. O. Medicinal Plants of East Africa; East African Literature Bureau, Nairobi, Dar es Salaam: Kenya, 1976.
- Hamza, O. J. M.; van den Bout-van den Beukel, C. J. P.; Matee, M. I. N.; Moshi, M. J.; Mikx, F. H. M.; Selemani, H. O.; Mbwambo, Z. H.; Van der, J. A. M.; Verweij, P. E. Antifungal Activity of Some Tanzanian Plants Used Traditionally for the Treatment of Fungal Infections. J. Ethnopharmacol. 2006, 108(1), 124–132. DOI: 10.1016/j.jep.2006.04.026.
- Masoko, P.; Mmushi, T. J.; Mogashoa, M. M.; Mokgotho, M. P.; Mampuru, L. J.; Howard, R. L. In Vitro Evaluation of the Antifungal Activity of Sclerocarya Birrea Extracts against Pathogenic Yeasts. Afr. J. Biotechnol. 2008, 7(20), 3521–3526.
- Dieye, A. M.; Sarr A, A.; Diop, S. N.; Ndiaye, M.; Sy, G. Y.; Diarra, M.; Gaffary, I. R.; Sy, A. N.; Faye, B. Medicinal Plants and the Treatment of Diabetes in Senegal: Survey with Patients. Fundam. Clin. Pharmacol. 2008, 22(2), 211–216. DOI: 10.1111/j.1472-8206.2007.00563.x.
- Dimo, T.; Rakotonirina, S. V.; V. Tan, J. A.; Dongo, E.; Kamtchouing, P.; Cros, G. Effect of Sclerocarya Birrea (Anacardiaceae) Stem Bark Methylene chloride/methanol Extract on streptozotocin-diabetic Rats. J. Ethnopharmacol. 2007, 110(3), 434–438. DOI: 10.1016/j.jep.2006.10.020.
- Gondwe, M.; Kamadyaapa, D. R.; Tufts, M.; Chuturgoon, A. A.; Musabayane, C. T. Sclerocarya Birrea [(A. Rich.) Hochst.] [Anacardiaceae] stem-bark Ethanolic Extract (SBE) Modulates Blood Glucose, Glomerular Filtration Rate (GFR) and Mean Arterial Blood Pressure (MAP) of STZ-induced Diabetic Rats. Phytomedicine. 2008, 15(19), 699–709. DOI: 10.1016/j.phymed.2008.02.004.
- Galvez Peralta, J.; Zarzuelo, A.; Busson, R.; Cobbaert, C.; de Witte, P. (-)-epicatechin-3-galloyl Ester: A Secretagogue Compound from the Bark of Sclerocarya Birrea. Planta Med. 1992, 58(2), 174–175. DOI: 10.1055/s-2006-961423.
- Tsuneki, H.; Ishizuka, M.; Terasawa, M.; Wu, J. B.; Sasaoka, T.; Kimura, I. Effect of Green Tea on Blood Glucose Levels and Serum Proteomic Patterns in Diabetic (db/db) Mice and on Glucose Metabolism in Healthy Humans. BMC Pharmacol. 2004, 4, 18. DOI: 10.1186/1471-2210-4-18.
- Kobayashi, M.; Shimizu, H.; Shioya, S. Beer Volatile Compounds and Their Application to low-malt Beer Fermentation. J. Biosci. Bioeng. 2008, 106(4), 317–323. DOI: 10.1263/jbb.106.317.
- Viljoena, A. M.; Kamatoua, G. P. P.; Başer, K. H. C. Head-space Volatiles of Marula (Sclerocarya Birrea Subspcaffra). S. Afr. J. Bot. 2008, 74, 325–326. DOI: 10.1016/j.sajb.2007.10.005.
- Pretorius, V. E.; Rohmer, E.; Rapp, A.; Holtzhausen, L. C.; Mandery, H. Volatile Flavour Components of Marula Juice. Z. Lebensm. Unters. Forsch. 1985, 181, 458–461. DOI: 10.1007/BF01140947.
- Schäfer, G.; McGill, A. E. J. Flavour Profiling of Juice of the Marula (Sclerocarya Birrea Subsp. Caffra) as an Index for Cultivar Selection. Acta Hortic. 1986, 194, 215–222. DOI: 10.17660/ActaHortic.1986.194.23.
- Tam, C. U.; Yang, F. Q.; Zhang, Q. W.; Guan, J.; Li, S. P. Optimization and Comparison of Three Methods for Extraction of Volatile Compounds from Cyperus Rotundus Evaluated by Gas chromatography–mass Spectrometry. J. Pharm. Biomed. 2007, 44, 444–449. DOI: 10.1016/j.jpba.2006.10.026.
- von Teichman, I. Notes on the Distribution, Morphology, Importance and Uses of the Indigenous Anacardiaceae: 1. The Distribution and Morphology of Sclerocarya Birrea (The Marula). Trees in South Africa, Oct. – December. Pp. 35–41, 1983.
- Ndlovu, P. F. The Development of Indigenous Marula (Sclerocarya Birrea) Fruit Leather: Effect of Drying Temperature and Sugar Concentration on the Drying Characteristics, physico-chemical and Consumer Sensory Properties of Marula Fruit Leathers. MSc Thesis (Horticulture). School of Agricultural, Earth and Environmental Sciences. University of Kwazulu-Natal, Pietermaritzburg, South Africa, 2016.
- Shackleton, C. M.; Shackleton, S. The Importance of non-timber Forest Products in Rural Livelihood Security and as Safety Nets: A Review of Evidence from South Africa. S. Afr. J. Sci. 2004, 100(11–12), 658–664.
- Rampedi, I. T.; Olivier, J. Traditional Beverages Derived from Wild Food Plant Species in the Vhembe District, Limpopo Province in South Africa. Ecol. Food Nutr. 2013, 52(3), 203–222. DOI: 10.1080/03670244.2012.706131.
- Molelekoa, T. B. J.; Regnier, T.; da Silva, L. S.; Augustyn, W. A. Potential of Marula (Sclerocarya Birrea Subsp.) Waste for the Production of Vinegar through Surface and Submerged Fermentation. S. Afr. J. Sci. 2018, 114(11/12), 4874. DOI: 10.17159/sajs.2018/4874.
- Al-hinai, K. Z.; Guizani, N.; Singh, V.; Rahman, M. S.; AL-Subhi, L. Instrumental Texture Profile Analysis of Date-Tamarind Fruit Leather with Different Types of Hydrocolloids. Food Sci. Technol Res. 2013, 19(4), 531–538. DOI: 10.3136/fstr.19.531.
- Diamante, L. M.; Bai, X.; Busch, J. Fruit Leathers: Method of Preparation and Effect of Different Conditions on Qualities. Int. J. Food Sci. 2014, 2014, 1–12. Article ID 139890. DOI: 10.1155/2014/139890.
- Maskan, A.; Kaya, S.; Maskan, M. Hot Air and Sun Drying of Grape Leather (Pestil). J. Food Eng. 2002, 54(1), 81–88. DOI: 10.1016/S0260-8774(01)00188-1.
- Orrego, C.; Salgado, N.; Botero, C. Developments and Trends in Fruit Bar Production and Characterization. Crit. Rev. Food Sci Nutr. 2014, 54(1), 84–97. DOI: 10.1080/10408398.2011.571798.
- Gujral, H.; Oberoi, D.; Singh, R.; Gera, M. Moisture Diffusivity during Drying of Pineapple and Mango Leather as Affected by Sucrose, Pectin, and Maltodextrin. Int. J. Food Prop. 2013, 16(2), 359–368. DOI: 10.1080/10942912.2011.552016.
- Singh, S.; Jain, S.; Singh, S.; Singh, D. Quality Changes in Fruit Jams from Combinations of Different Fruit Pulps. J. Food Process. Preserv. 2009, 33(s1), 41–57. DOI: 10.1111/j.1745-4549.2008.00249.x.
- Saka, J. D. K.; Msonthi, J. D. Nutritional Value of Edible Fruits of Indigenous Wild Trees in Malawi. For. Ecol. Manag. 1994, 64(2–3), 245–248. DOI: 10.1016/0378-1127(94)90298-4.
- Bille, P. D.; Shikongo-Nambabi, M.; Cheikhyoussef, A. Value Addition and Processed Products of Three Indigenous Fruits in Namibia. Afr. J. Food Agric. Nutr. Dev. 2013, 13(1), 7121–7192. DOI: 10.18697/ajfand.56.11495.
- Igual, M.; García-Martínez, E.; Camacho, M. M.; Martínez-Navarrete, N. Physicochemical and Sensorial Properties of Grapefruit Jams as Affected by Processing. Food Bioprocess Technol. 2013, 6(1), 177–185. DOI: 10.1007/s11947-011-0696-2.
- Nayak, B.; Liu, R. H.; Tang, J. Effect of Processing on Phenolic Antioxidants of Fruits, Vegetables and grains—a Review. Crit. Rev. Food Sci. Nutr. 2015, 55(7), 887–918. DOI: 10.1080/10408398.2011.654142.
- FAO/WHO. Draft European Regional Standard for Vinegar. Codex Alimentarus Commission. 1982.
- Tan, S. C. Vinegar fermentation. MSc thesis. Louisiana State University, Baton Rouge, New Orleans, LA, Louisiana, 2005.
- Gullo, M.; Verzelloni, E.; Canonico, M. Aerobic Submerged Fermentation by Acetic Acid Bacteria for Vinegar Production: Process and Biotechnological Aspects. Process Biochem. 2014, 49(10), 1571–1579. DOI: 10.1016/j.procbio.07.003.
- Mlambo, V.; Dlamini, B. J.; Ngwenya, M. D.; Mhazo, N.; Beyene, S. T.; Sikhosana, J. L. N. In Sacco and in Vivo Evaluation of Marula (Sclerocarya Birrea) Seed Cake as a Protein Source in Commercial Cattle Fattening Diets. Livest. Res. Rural Dev. 2011, 23, 121.
- Mdziniso, P. M.; Dlamini, A. M.; Khumalo, G. Z.; Mupangwa, J. F. Effects of Feeding Marula (Sclerocarya Birrea) (L)] Seed Cake on Milk Yield and Composition for Lactating Dairy Cows. Br. J. Appl Sci. Technol. 2016, 18(1), 1–10. DOI: 10.9734/BJAST/2016/29682.
- Mthiyane, D. M. N.; Mhlanga, B. S. The Nutritive Value of Marula (Sclerocarya Birrea) Seed Cake for Broiler Chickens: Nutritional Composition, Performance, Carcass Characteristics and Oxidative and Mycotoxin Status. Trop. Anim. Health Prod. 2017, 49(4), 835–842. DOI: 10.1007/s11250-017-1269-9.
- Phenya, J. S. M. Evaluation of oil cakes from amarula (sclerocarya birrea), macadamia (integrifolia) and baobab (adansonia digitate l.) as protein supplements for ruminant diets. MSc dissertation, Department of Agriculture and Animal Health Univesity of South Africa, Pretoria, South Africa, 2018.
- Moran, J. Tropical Dairy Farming: Feeding Management for Small Holder Dairy Farmer in the Humid Tropics; Landlinks press: The Stables, Ford Road, Totnes, Devon, TQ9 5LE. United Kingdom. p. 312, 2005; pp 1–6.
- Younis, K.; Islam, R. U.; Jahan, K.; Yousuf, B.; Ray, A. Effect of Addition of Mosambi (Citrus Limetta) Peel Powder on Textural and Sensory Properties of Papaya Jam. Cogent Food. Agric. 2015, 1(1), 1023675. DOI: 10.1080/23311932.2015.1023675.
- Chacko, C. M.; Estherlydia, D. Sensory, Physicochemical and Antimicrobial Evaluation of Jams Made from Indigenous Fruit Peels. Carpath. J. Food Sci. Technol. 2013, 5(1), 69–75.
- Ademosun, A. O.; Oboh, G.; Passamonti, S.; Tramer, F.; Ziberna, L.; Boligon, A. A.; Athayde, M. L. Phenolic Composition of Orange Peels and Modulation of Redox Status and Matrix Metalloproteinase Activities in Primary (Caco-2) and Metastatic (Lovo and LoVo/ADR) Colon Cancer Eells. Eur. Food Res. Technol. 2016, 242, 949–1959. DOI: 10.1007/s00217-016-2694-0.
- Sicari, V.; Pellicanò, T. M.; Laganà, V.; Poiana, M. Use of Orange by-products (Dry Peel) as an Alternative Gelling Agent for Marmalade Production: Evaluation of Antioxidant Activity and Inhibition of HMF Formation during Different Storage Temperature. J. Food Process. Preserv. 2018, 42, e13429. DOI: 10.1111/jfpp.13429.
- Ruviaro, A. R.; Barbosa, P. D. P. M.; Macedo, G. A. Enzyme-assisted Biotransformation Increases Hesperetin Content in Citrus Juice by-products. Food Res. Int. 2019, 124, 213–221. DOI: 10.1016/j.foodres.2018.05.004.
- Favela-Hernández, J. M. J.; González-Santiago, O.; Ramírez-Cabrera, M. A.; Esquivel-Ferriño, P. C.; Camacho-Corona, M. D. R. Chemistry and Pharmacology of Citrus Sinensis. Molecules. 2016, 21, 247. DOI: 10.3390/molecules21020247.
- Polydera, A. C.; Galanou, E.; Stoforos, N. G.; Taoukis, P. S. Inactivation Kinetics of Pectin Methylesterase of Greek Navel Orange Juice as a Function of High Hydrostatic Pressure and Temperature Process Conditions. J. Food Eng. 2004, 62(3), 291–298. DOI: 10.1016/S0260-8774(03)00242-5.
- Cortes, C.; Esteve, M. J.; Frigola, A. Color of Orange Juice Treated by High Intensity Pulsed Electric Fields during Refrigerated Storage and Comparison with Pasteurized Juice. Food Control. 2008, 19(2), 151–158. DOI: 10.1016/j.foodcont.2007.03.001.
- Rastogi, N. K.; Raghavarao, K. S. M. S.; Balasubramaniam, V. M.; Niranjan, K.; Knorr, D. Opportunities and Challenges in High Pressure Processing of Foods. Crit. Rev. Food Sci. Nutr. 2007, 47(1), 69–112. DOI: 10.1080/10408390600626420.
- Saucedo-Reyes, D. A.; Marco-Celdrán, M. C.; Pina-Pérez, D. R.; Martínez-López, A. Modeling Survival of High Hydrostatic Pressure Treated Stationary- and exponential-phase Listeria Innocua Cells. Innov. Food Sci. Emerg. Technol. 2009, 10(2), 135–141. DOI: 10.1016/j.ifset.2008.11.004.
- Patras, A.; Brunton, N. P.; Da Pieve, S.; Butle, F. Impact of High Pressure Processing on Total Antioxidant Activity, Phenolic, Ascorbic Acid, Anthocyanin Content and Colour of Strawberry and Blackberry Purées. Innov. Food Sci. Emerg. Technol. 2009, 10(3), 308–313. DOI: 10.1016/j.ifset.2008.12.004.
- Rodrigo, D.; Jolie, R.; Van Loey, A.; Hendricx, M. Thermal and High Pressure Stability of Tomato Lipoxygenase and Hydroperoxide Lyase. J. Food Eng. 2007, 79, 423–429. DOI: 10.1016/j.jfoodeng.2006.02.005.
- Buckow, R.; Ng, S.; Toepf, S. Pulsed Electric Field Processing of Orange Juice: A Review on Microbial, Enzymatic, Nutritional, and Sensory Quality and Stability. Compr. Rev. Food Sci. Food Saf. 2013, 12(5), 455–467. DOI: 10.1111/1541-4337.12026.
- Min, S.; Evrendilek, G. A.; Zhang, H. Q. Pulsed Electric Fields: Processing System, Microbial and Enzyme Inhibition, and Shelf Life Extension of Foods. IEEE Trans. Plasma. Sci. 2007, 35(1), 59–73. DOI: 10.1109/TPS.2006.889290.
- Cortes, C.; Torregrosa, F.; Esteve, M. J.; Frigola, A. Carotenoid Profile Modification during Refrigerated Storage in Untreated and Pasteurized Orange Juice and Orange Juice Treated with high-intensity Pulsed Electric Fields. J. Agric. Food Chem. 2006, 54(17), 6247–6254. DOI: 10.1021/jf060995q.
- Elez-Mart´ınez, P.; Mart´ın-Belloso, O. Effects of High Intensity Pulsed Electric Field Processing Conditions on Vitamin C and Antioxidant Capacity of Orange Juice and Gazpacho, a Cold Vegetable Soup. Food Chem. 2007, 102(1), 201–209. DOI: 10.1016/j.foodchem.2006.04.048.