ABSTRACT
In millennia, berries have captured great attention owing to their broad spectrum of functional as well as therapeutic activities, which is due to presence of their bioactive compounds. All edible forms of berries show important nutritional properties leading to their therapeutic potential and are considered as safe functional foods. Recently, various researches for further exploration of berries concerning nutritional and bioactive profiles as well as potential health benefits are on the way. This review highlights the latest research on bioactive compounds of all edible forms of berries and their related nutritional activities in humans and animals against different diseases. The current article revealed that berries have an array of bioactive moieties including phenolics, anthocyanins and ellagitannins with strong antioxidant potential contributing to their anti-cancer, anti-diabetic, anti-inflammatory and cardio-protective roles. The demand and consumption trend is increasing day by day, due to their therapeutic effects as mentioned above. C-1: English and clarity of this paper must be checked by an expert, otherwise I will asked for professional editing
Introduction
Berries are small fleshy, commercially cultivated fruits, which are present in many fresh and processed forms such as strawberry, blueberry, blackberry, cranberry, black raspberry and red raspberry.[Citation1,Citation2] Among these commonly consumed forms of berries, the strawberry is considered as most important fruit. Strawberry (Fragaria ananassa), which belongs to Ericaceae family, is a spherical-shaped dark red and tasty fruit that is a native Mediterranean specie and cultivated in other regions of Eastern Europe as well.[Citation3] These fruits are mostly consumed in processed forms such as alcoholic beverages, jams, jellies and marmalades and are rarely eaten in fresh forms.[Citation4,Citation5] As a rich source of phenolic compounds (most importantly anthocyanin), vitamin E, vitamin C and carotenoids, these are used as antiseptics, laxatives and diuretics in folk medicine and in the treatment of hypertension and diabetes.[Citation6–8]
Another widely cultivated forms of berries are blueberry (Vaccinium corymbosum), which belongs to the family of Ericaceae and has almost 450 species, which are native to North America and Europe.[Citation9–11] Just like other soft fruits, blueberries also have a single epidermis layer which provides a protective sheet against external factors such as desiccation, infections by pathogenic bacteria and insects and weather conditions and also controls the water uptake and chemical substances into fruit. This positive role of epicuticular layer increases the economic value of blueberry. Like other berries, blueberry is also acted as important health-promoting food because of its array of bioactive compounds such as anthocyanins, flavonoids, phenolic acids and tannins and is used in the treatment of cancer, diabetes, cardiovascular and neurodegenerative diseases.[Citation2,Citation12–15]
As far as blackberries (Rubus spp.) are concerned, these also belong to the family of Ericaceae and are native to North America, Europe, Asia, South America, Africa and Oceania Central America. This berry is considered an aggregate fruit and weight of this berry vary from 3 to 12 gram.[Citation16–18] Blackberries can be consumed in fresh and processed forms such as dietary supplements, ice cream, marmalade, jam and other confectionaries.[Citation19] Moreover, it is a rich source of anthocyanins and ellagitannins and other important phenolic compounds which increase its antioxidant activity and make it more prone to many diseases such as mouth and eye infections, obesity, coronary heart disease, degenerative conditions and many types cancer.[Citation12,Citation20–22]
Cranberry (Vaccinium macrocarpon), which belongs to the family of Ericaceae, is a smaller and healthier fruit that is native to North America and Europe. This fruit contributes to the color, flavor, nutritional value and functionality of products. It is consumed in fresh and processed forms such as juice cocktails. Like other soft fruits and berries, it also contains anthocyanins, flavones, tannins (ellagitannins and proanthocyanidins) and phenolic derivatives,[Citation23] which are associated with important health benefits against several diseases such as cancer, inflammation, cardiovascular diseases and many age-related disorders and contributes to the fruit’s organoleptic qualities.[Citation24–26]
Chemical composition
The chemical composition of berries depends on various factors such as varieties, ripening stage, growing, harvest and storage conditions.[Citation27]Among different forms of berries, strawberry is considered an important fruit owing to its nutritional value. It is a rich source of vitamins C, folates and many phenolic compounds.[Citation28–32] Blueberry contains water (84%), carbohydrates (9.7%), proteins (0.6%), fat (0.4%), dietary fiber (3–3.5% of fruit weight) and vitamin C (10 mg/100 g fruit). Moreover, the cell wall of blueberry contains alcohol-insoluble solids (3.4 g in 100 g of immature blueberries and 2.4 g in 100 g of ripe blueberries), lignin (27% of alcohol-insoluble solids), cellulose (16% of alcohol-insoluble solids) and neutral non-cellulosic polysaccharides.[Citation33]
Furthermore, blackberries contain several essential vitamins and minerals, carbohydrates, sugars (glucose, fructose, sucrose), organic acids (malic acid, citric acid, isocitric acid, shikimic acids, fumaric acid, succinic acid, ascorbic acid), anthocyanins and phenolic acids.[Citation19,Citation34] As far as the quantity of these components is concerned, blackberry contains about 3.2 to 4.88 and 0.81 to 1.17 g/100 g of fresh weight (FW) glucose to fructose, 0.24 g/100 g FW sucrose content. These contents vary with cultivars.[Citation35]
Bioactive compounds
Berries have captured greater attention owing to the presence of a broad spectrum of bioactive compounds such as polyphenols (anthocyanins, phenolic acids, tannins and carotenoids.[Citation36–38]Among these berries, strawberries are well known for the presence of many nutritive (sugars, vitamins and minerals) and non-nutritive compounds and bioactive compounds such as polyphenols like phenolic compounds, flavonoids, anthocyanins, flavones, ellagitannins and derivatives of hydroxycinnamic acid.[Citation28,Citation31] These compounds are of greater interest due to their antioxidant properties and synergistic effect on human health promotion and prevention of diseases. The bioavailability of these compounds in blood is low (i.e. anthocyanins 1–3%). Similarly, blueberries have higher levels of anthocyanins and phenolics. Their level is different in different fruits and depends upon various factors such as genetics, growing location, degree of maturation and cultivar type.[Citation38] Owing to the presence of these compounds, blueberries are used for the treatment of biliary disorders, coughs, diabetes, visual disorders and tuberculosis.[Citation39–42]
Likewise, blackberries have large contents of anthocyanins and phenolic compounds such as flavones and ellagitannins. The content of these compounds is affected by various factors such as genetics, maturation and growing conditions. Owing to the presence of these functional ingredients, blackberries possess high antioxidant capacity and other biological activities. They have anti-obesity, antidiabetic, antimicrobial, neurodegenerative and anti-inflammatory effects.[Citation43] Likely, cranberries have gained greater interest due to the high concentration of anthocyanins (galactosides, arabinosides of cyanidin and peonidin), flavone’s, tannins (ellagitannins and proanthocyanidins), flavan-3-ols and phenolic acid derivatives.[Citation44] Some anthocyanins are present in a negligible amount in cranberries such as cyanidin 3-O-glucoside, delphinidin 3-O-arabinoside, delphinidin 3-O-galactoside, peonidin 3-O-glucoside and delphinidin 3-O-glucoside.[Citation45]
Polyphenols
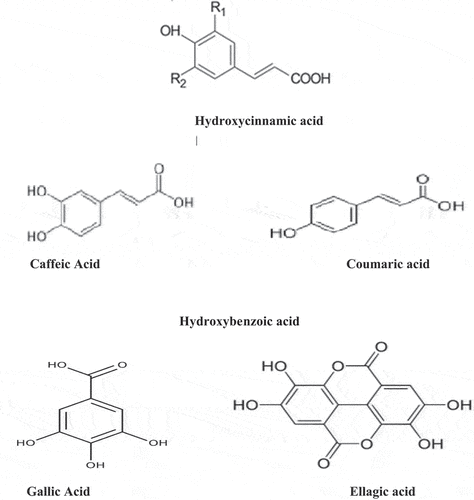
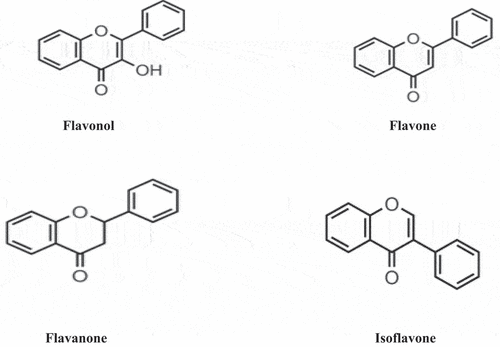
A wide number of phytochemicals like procyanidins, ellagitannins and anthocyanins and nutrients are present in strawberries which are absorbed and metabolized in the body to form different phenolic conjugates and metabolites within the body to reduce the risk of diseases. Structures of different polyphenol classes are shown in . A wide investigation described that red color of strawberry flash is because of phenols and the dominant compounds are pelargonidin with pelargonidin-3-glucoside and Cyanidin.[Citation45–49] While the main flavonols are glucuronides and glucosides of kaempferol and quercetin.[Citation50] Along with the identification of caffeic acid and glucosides, p-coumaroylglucoside, a primary cinnamic acid derivative is also described.
The main source of ellagic acid is blackberries and raspberries along with strawberries in human diet.[Citation51] There is variation regarding its content composition of it like, it is ellagic acid strictly, while from other reports it is ellagic acid with ellagic acid glycosides and ellagitannins. Glucose esterified named Ellagitannins are found in strawberries, which are cored with hexahydroxydiphenic acid, and one is reported as casuarictin (HHDP).[Citation52] Moreover, the leaves of strawberries have dimer sanguiin H-6along with its two monomers named potentillin and casuarictin, as well as pedunculagin. The functions performed by phenolics components of Strawberry are protection and repair of DNA damage, detoxification of free radicals and their propagation and the modulation of genetic metabolic expressions.
Noticing the health importance of phytochemical content of blueberries, the leading content is anthocyanins in the field of research and is considered responsible for health benefits. The polyphenols of Blackberries are including ellagitannins (ETs), flavonols, anthocyanins, flavan-3-ols, and procyanidins. Phenolic acids are found at high levels while lignans are at low levels. The phenolic range is from 114 to 1056 mg/100FW. Anthocyanins are the main cyanidin derivatives which are rutinose, xylose, and glucose attached at C3.[Citation53]
Cranberry also consists of phenolic contents like hydroxycinnamic and hydroxybenzoic acids[Citation54,Citation55] and much lower contents of o-hydroxybenzoic acids, 2,4-dihydroxybenzoic, p-hydroxybenzoic. The main contents of hydroxycinnamic acids present in cranberry are sinapic, p-coumaric, ferulic acids and caffeic acid. Of course, there is no specification of these phenolic acids with cranberries. It is difficult to compare the phenolic components of cranberries with other berry fruit. Mainly in abundance, flavonols are found in glycosides of quercetin, myricetin, and kaempferol. Quercetin 3-galactoside present in lesser extent. The leading active terpenes in cranberry need more attention. In American cranberry, Ursolic acid is abundant at 46–109 mg/100 g FW.
Anthocyanin
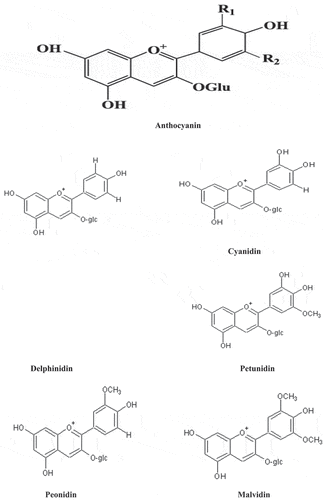
A major group of flavonoids named Anthocyanins is water-soluble pigment ranging from red-blue in seeds, fruits, plants, vegetal tissues and other flowers like strawberries (Fragaria ananassa)[Citation56,Citation57] açaí (Euterpeoleracea), elderberry,[Citation58] chokeberry (Aroniamelanocarpa). Shortly, almost all angiosperms species are found with anthocyanins. Their molecular structure is made up of an interlinked benzoic ring to a non-benzoic ring with an oxygen atom while another benzoic ring is attached to those first two by a carbon-carbon bond (C–C), naming this structure as 2-phenyl benzopyryliumcation or flavylium ion.[Citation59] More than 300 reported anthocyanidins[Citation60;] while up to 8000 diverse options of anthocyanins, like types of anthocyanidins and the glycosylated.[Citation61] Structures of different classes of anthocyanins are shown in .
Strawberries are consumed globally and are the main source of dietary anthocyanins. They consist of many important antioxidants like phytochemicals, carotenoids, vitamin C and phenolic compounds including pelargonidin-3-glucoside (Pel-glc) and anthocyanin.[Citation62,Citation63] Quantitatively, anthocyanin is the most important polyphenol compounds in strawberries.[Citation51] Water-soluble phenolic compounds include anthocyanins which are the main cause of many color range in fruits and juices like purple, red and blue.[Citation64] Moreover, they are also linked with a wide range of medical health benefits along with the reduced risk of cardiovascular diseases and coronary heart disease,[Citation21,Citation65] improved neuro function,[Citation66,Citation67] low risk of cancer,[Citation21,Citation68] and safeguard activity of the brain tissue against hypoxic-ischemic injury.[Citation69,Citation70] Moreover, visionary improvement in memory,[Citation71] and control of weight gain too that are attributed to anthocyanins.[Citation72] High antioxidant activity of anthocyanin shown in many in vitro cases regarding health benefits and also in vivo.[Citation73–75]
Blueberries consist of a broader range of anthocyanidins as compared to other barriers like dominant in malvidin and petunidin, cyanidin, pelargonidin and delphinidin.[Citation76,Citation77] In blueberries, glycosidic forms also present named galactosides, arabinosides and glucosides with changes originating in at least some cultivars, while relative proportions vary widely with chances of complete absence together. Various sugar residues like acetyl esters, primarily the p-coumaroyl on C-6 of the blueberries.[Citation20] Acylated species vary in different cultivars, with lower amounts hardly detected through respective methods of Nicoue et al.[Citation76] and Scalzo et al. .[Citation77] Furthermore, blueberries are a good source of antioxidants and are consumed increasing ex vivo serum antioxidant status.[Citation74,Citation78,Citation79] In grapes juice, Anthocyanins reduced in vitro oxidation of human low-density lipoprotein (LDL).[Citation80]
Anthocyanins are in high quantity, in cranberry, play a role in the color of fruits and are associated with derived foodstuff along with potential health effects on humans. A rare presence of glycosides in American cranberry: such as petunidin, cyanidin, pelargonidin, delphinidin, peonidin, and malvidin. With the ripening, cranberry anthocyanin content increases depending on the size of the fruit and cultivar.[Citation71,Citation81] In some other studies, it is said that there is only a report of total anthocyanin content than the individual amounts of anthocyanins. There is a chance of variation in this approach seems to affect the health properties and bioavailability of anthocyanins.[Citation53]
Ellagitannins
Ellagitannins are distinctive aggregates of hexahydroxydiphenic and gallic acid in combination with glucose, available with an extensive variety of chemical structures, for example, monomers (such as ellagic acid glycosides), oligomers (such as sanguiin H-6, the common ellagitannins present in the strawberry) and multiplex polymers. A combination of ellagitannins and gallotannins is known as hydrolyzable tannins, and ellagic acid is produced as a result of hydrolysis, except ellagic acid other metabolites can also be synthesized.[Citation82] These hydrolyzable tannins are dissimilar to those of solitary ellagitannins such as gallagic acid. Recently have been described ellagitannins combinations and substances as a constituent of food products, although ellagitannins have regularly been distinguished as the dynamic principles in restorative plants.[Citation83] In Finland, an examination of 33 normally devoured food products was screened and investigated for ellagitannin content by Koponen et al.[Citation83] About 21.7 to 83.2 mg/100 g (FW) of ellagitannins contents were observed only in berries from the Rosaceae family that included raspberry, sea buckthorn, strawberry and rose hip. Contrary to this extended from 0.7 to 4.3 mg/100 g (FW) were free ellagic acids moieties that include non-tannin ellagic acid glycosides. While other studies reveal about 25 to 59 mg/100 g ellagitannins compounds were detected in fresh samples of strawberries. Regardless of these studies, the ellagitannins compounds have been evaluated and distinguished by a few examinations. Sanguiin H-6 is the main component of ellagitannins compounds present in strawberries and raspberries. Further researchers have also investigated the occurrence of galloyl-hexahydroxydiphenoyl-glucose that was earlier found in genus Rubus of the flowering plant. This compound is a fundamental constituent of various ellagitannins including lambertianin C and sanguiin H-6 be composed of commonly 2 and 3 U.[Citation52]
More studies should be committed to this field because of its significance to human well-being. To heal the basic health issues in both the developed and underdeveloped countries, these ellagitannins which are the imperative class of phytochemicals and are enclosed in various fruits and edible plants are prescribed by the conventional medicine of different cultural societies. Ellagitannins compounds have acknowledged escalating consideration over the last few years because of their pharmaceutical and biological significance. Researchers have investigated the various pharmacological activities of ellagitannins compounds,[Citation84] and amongst these clinically significant activities, the anti-carcinogenic, anti-proliferative and chemo-preventive activities are getting more consideration and importance.
Health claims
In recent era, berries have gained much attention owing to the presence of a broad spectrum of phytochemicals mainly polyphenols which have the potential to combat multiple diseases such as cancer, cardiovascular diseases, diabetes, obesity, metabolic syndromes and other degenerative disorders. The potential of phytochemicals to fight against different disorders is attributed to their antioxidant capacity. Health benefits of all edible forms of berries are shown in . Pharmaceutical activities of strawberries include the prevention of cancer, cardiovascular diseases, obesity, diabetes and oxidative stress.[Citation21,Citation70,Citation97–104] Some positive health effects attributable to phenolics are anti-atherosclerotic, anti-inflammatory and anti-proliferative effects. These bioactive functions are associated with many functional ingredients present in strawberries such as minerals, vitamins, and polyphenols (mainly anthocyanin).[Citation105] One more factor that contributes to their medicinal importance is the degree of transformation during digestion.
Table 1. Health perspectives related to all edible forms of berries.
The literature revealed that the uptake of bioactive moieties in the gastrointestinal tract can be affected and their chemical nature can be changed through specific gastrointestinal conditions, enzymatic activity and local microbiota action.[Citation99,Citation106–109] One most commonly consumed form of berries is blueberry and it has a lot of health perspectives.[Citation110] In earlier and recent in vitro, in-vivo and clinical studies, it has been revealed that the blueberry is considered the most important edible form of berries with an array of bioactive functions such as anti-carcinogenic, anti-allergic, anti-inflammatory, anti-oxidant and anti-ulcer. These activities are associated with higher content of phenolics present in blueberries such as flavonoids, anthocyanin, ellagitannins and proanthocyanidins. Blueberries perform their role as anticancer fruit by reducing DNA damage and cancer cell proliferation and increasing apoptosis.[Citation111] Moreover, flavonoids present in blueberry play their role as anti-inflammatory, anti-carcinogenic, anti-allergic, anti-ulcer, and protective against oxidant stress.[Citation112] Prevention of lipid and protein oxidation to improve the food quality through berry phenolics is on trend these days owing to its antioxidant effect.[Citation113]
As far as the health perspectives of blackberries are concerned, it is revealed from the literature that blackberry is in the third position after strawberry and black raspberry in terms of antioxidant activity. This activity is associated with the higher content of acylated anthocyanins and cyanidin 3-glucoside present in blackberries. In recent era, blackberries have gained a pronoun position in Ericaceae family due to broad spectrum of health claims that are associated with the presence of various types of phytochemical constituents such as polyphenols, anthocyanins, ellagic acid, salicylic acid, soluble dietary fiber, insoluble dietary fiber, alkaloids, tannins, saponins, flavonoids, glycosides, terpenoids, sterols, carbohydrates, ascorbic acid, organic acids and volatile oils.[Citation114–116] The possible health benefits include anticancer, antioxidant, antimicrobial, anti-diabetic, anti-inflammation and anti-ulcer effects.[Citation117]
Along with strawberry, blackberry and blueberry, the most form of berries are cranberries which are considered healthy and nutritious owing to the presence of higher content of antioxidant phenolic compounds which help to protect the body from damage caused by oxidative stress (induced by reactive oxygen species) and support the natural antioxidant defense system. A long list of protective activities is associated with the consumption of cranberry that includes anti-atherosclerosis, anti-cancer, anti-bacterial, and antioxidant activities, which are due to the high anthocyanin content in cranberry. Cranberry also helps in protecting the body from urinary tract bacterial infections, gastric mucosa infections and oral cavity infections.[Citation118]
Antioxidant effect
A serious imbalance between the production of reactive oxygen species and their control results in damage to the body, nucleic acid, cell membrane and other parts of the human body. Many serious issues develop in the human body such as cancer, cardiovascular and other problems.[Citation119] For combating such issues, the trend of using antioxidant plants is increasing day by day like fruits, vegetables and many important herbs as well. Various researchers have analyzed and extracted numerous bioactive compounds related to antioxidant capacity in various sources through different methods. In a study, Tulipani et al.[Citation63] claimed that different bioactive compounds such as phenolic compounds and vitamin C which are known as strong oxygen radical scavengers are gaining importance owing to the antioxidant capacity of fruits. These results revealed that berries are at the top position for their total phenolics and total antioxidant capacity.[Citation120] The levels of total phenolics and antioxidant capacity in berries are four times, ten times and forty times their levels in other fruits, vegetables and cereals respectively.[Citation91]
Among different forms of berries, strawberries are considered the most important berries owing to their total antioxidant capacity that is associated with high content of anthocyanins. The total antioxidant capacity of strawberries is two to eleven folds than the antioxidant capacity of grapes, peaches, oranges, kiwifruit, apples, pears, or tomatoes.[Citation121] Moreover, in fruits, different phytochemicals contribute to the total antioxidant capacity in different proportions. For instance, in different strawberry cultivars, 30% contribution was due to vitamin C and 25–40% contribution was owing to the anthocyanin and rest of the antioxidant capacity was related to flavonols and ellagic acid (EA) derivatives.[Citation122]
Blackberry is enriched with antioxidant characteristics due to the anthocyanin content that varies along with environmental stress, genotype and other factors.[Citation123] Studies on the direct and indirect activities of reactive oxygen species revealed that fruits that are having anthocyanin have a great role in the signaling pathways and regulation of multiple genes expression. Blackberry juice has a high concentration of cyanidin-3-o-glucoside (C3G) is less than may also have a 5% of the total soluble phenolics. Various types of extraction methods are employed for the extraction of blueberry anthocyanin, the most important of which is ultrasound-assisted extraction (UAE), which is far famous because of time and solvent consumption with high temperature but it works with high contact of solvent and targeted compounds.[Citation124] Oxygen radical scavengers, such as vitamin C and phenolic compounds are closely related to the antioxidant power of fruits.[Citation120]
Besides blackberry, blueberry is also the most important form of edible berries of their antioxidant potential against many complications and diseases.[Citation125] The antioxidant potential of blueberry is much higher than other fruits and juices.[Citation126] The antioxidant potential of blueberry is more in raw form but when they are dried; their activity is reduced to 66%.[Citation92] In recent era, many in-vivo and in-vitro studies have been done to probe the antioxidant activity of blueberry extracts. In the study of Drozdz et al.,[Citation92] the comparative effect of antioxidant activity of blueberry and lingonberry was evaluated and it was found that blueberry was more effective against multiple oxidative stress-induced complications than lingonberry owing to the presence of higher content of phytochemicals which exhibited higher antioxidant activity measured by both assays comparison to lingonberry extracts.
As far as the antioxidant activity of cranberry is concerned, cranberry is important due to the presence of functional ingredients associated with possible health concerns of cranberry and other fruits. Among bioactive compounds, phenolic compounds with high antioxidant capacity are present in cranberry. This antioxidant potential of cranberry is associated with the treatment of many complications such as diabetes, hypertension, atherosclerosis and all other diseases linked with oxidative stress.[Citation118]
Anti-inflammatory effect
A prolonged pro-inflammatory condition is a prevailing factor in the growth, succession and obstacle of ordinary sustained illnesses. This inflammatory condition can be neutralized by dietary antioxidants which are considered a proficient instrument. Earlier studies revealed that a lot of studies have been done that demonstrated a strong connection between various morphologies related to inflammation and a diet that corresponds to excellent sources of indispensable vitamins, nutritional fibers and valuable biological active ingredients especially effluents in vegetables and fruits.[Citation102,Citation127,Citation128] Under immense nutritious components present in fruits, strawberries are considered as most beneficial for health subsistence such as fatty acids, fibers, vitamins and minerals, in addition to an extensive variety of polyphenol compounds including lignans, flavonoids, tannins and phenolic acids.[Citation32,Citation100]
Recent studies revealed that the bioactivities of phenolic compounds present in strawberries are extended to various other routes including cellular survival and cellular growth, even though they are recognized primarily for their antioxidant and anti-inflammatory diseases.[Citation102] In cells treated with lipopolysaccharide, research demonstrated that pre-treatment with strawberry decreases the apoptotic rate, level of reactive intracellular oxygen species, enhanced mitochondria functionality and carcinogenic defense. Incitement of the Nrf2 pathway and hindrance of NF-kB signaling pathway with AMPK-dependent mechanism is responsible for these defensive biological activities of strawberry. These research outcomes affirm the medicinal advancements of strawberries in inhibition of oxidative stress situations in lipopolysaccharide-treated cells and also against the anti-inflammatory activity.[Citation127]
Phenolics separated from strawberries have also been determined for in vitro studies of anti-inflammatory activity. For instance, the most favorable strawberry phenolic like fistein that showed bioactivity against inflammatory diseases in several promising ways including by decreasing the pro-inflammatory cytokines, in macrophages prepared with LPS nitric oxide synthesis, in humans and rats phagocytes and suppressing the pro-inflammatory molecular pathway. These study results illustrated the pharmaceutical potential of strawberries and their separated phenolic compounds toward anti-inflammatory activity by the modulation of concerning molecular pathways and the regulation of the proportion of the cellular pro-anti-inflammatory cytokine excretion. A lot of research has been conducted on in vivo berry impacts against inflammatory diseases, though very less studies carried out to study the inducing effects of strawberries in the inflammatory process and its phytology-related processes.[Citation112]
A research survey was done in a mouse model of diet-induced obesity, reduction in C-reactive protein (CRP), IL-6, and tumor necrosis factor alpha (TNF-α) as a consequence of routine utilization of strawberries, had positive impacts in regulating numerous features of inflammation and upkeep of blood glucose.[Citation93] After strawberry utilization, exposure of 1.5 gamma radiations of 56 Fe particles was done in decisive areas of the brain of rats, results indicated a decrease in inflammation and pro-oxidant load through a decrease in ROS synthesis and reduction in inflammatory indicators including NF-B and cyclooxygenase-2 (COX-2).[Citation129,Citation130] Neuroprotective activity of the phenolic compound fistein in a stroke mouse model was detected as a result of inflammatory response and inhibition of intracerebral resistant cell activation. Contrarily, when examined paw mice for inflammation activity induced by carrageenan results indicated failing to exhibit the activity and enrichment in activation of MAPK.37 furthermore, for two months of consumption of strawberry on Wistar rats processed with LPS due to cytokine gene expression in the liver, refurbish the plasma biomarkers of liver damage and also by a reduction in nitric oxide synthesis, showed satisfactory anti-inflammatory activity.
Concurrently, prevention against protein and lipid oxidation was observed by the consumption of strawberries, contrary to this improves the isolated liver mitochondria performance and enhanced antioxidant enzyme reserve, combating actively against inflammation. Certainly, an intense and prolonged human crossover experimental model has been done to observe the effect of strawberry utilization. Some experiments were carried out in corpulent persons providing refrigerated-dried powder[Citation105,Citation131] and others were executed on obese adults with exalted serum lipids and abdominal adiposity has given strawberries in the form of milk-based liquor.[Citation132–134] Results illustrated that intake of strawberries as a nutritional tool reduces fatness-related diseases and also wields satisfactory effects on insulin sensitivity problems, oxidation of lipids, also on postprandial inflammatory reaction and decreasing core roots of inflammation such as IL-6 and CRP.
Polyphenols such as anthocyanins are major constituents of blackberries.[Citation135] Pharmaceutical activities of polyphenols are playing a vital role in human health-related issues. Hull blackberries collected from Kentucky, their anthocyanin-rich extract examined and characterized for its composition, polymeric color and total antioxidant and anthocyanin activities. Experiments were carried out on In vitro cell culture by considering the concentration-dependent procedure of about 49.2 g of total anthocyanins/mL blackberry extract, results showed up to 66% inhibition toward HT-29 cell growth at 72 hours, and studies were done on HT-29 colon tumor cell growth. Similarly, in continuation of this procedure, mouse bone marrow-derived dendritic cells release lipid A-induced interleukin-12 by taking total anthocyanin extract concentrations in the range of 0–40 g/mL. Results indicated the satisfactory anti-proliferative, anti-inflammatory and antioxidant activities of Hull blackberry extract (HBE) further demonstrating that the products synthesized with HBE also possessed potential against inflammatory and carcinogenic diseases.[Citation21] Blackberries are composed of hydrolyzed tannins such as gallotannins and ellagitannins.[Citation136] Biological active compounds extracted from blackberries in case they deplete at appropriate levels are considered promising candidates against inflammatory diseases.
Recently a lot of in vitro trials and clinical research indicated that gastrointestinal diseases related to Helicobacter pylori virus[Citation137] and urinary tract health issues[Citation138,Citation139] pacify with the consumption of cranberry fruits. Studies also acknowledged their activity toward anti-inflammation, antioxidant and antiviral (against influenza and rotaviruses) effects.[Citation140–142] Present research demonstrated that polyphenols available in cranberries including flavonols, anthocyanins and flavonols are responsible for the regulation of inflammatory effects and decrease of free radicals.[Citation143] Consumption and metabolism procedures indicate the presence of their bioactivity.
Many in vitro researches were carried out to demonstrate the effects of bioactive compounds of cranberry and results indicated that bioactivities reduce the macrophage activity and T cells when resolved to compatible pro-inflammatory stimulus.[Citation144,Citation145] Particularly, cranberry juice was recommended to devote positive effects against periodontal ailments.[Citation138] When anthocyanins of cranberry are exposed to pro-inflammatory cytokines, perform to dull the appearance of monocyte chemotactic protein 1 and intercellular adhesion molecule 1 (ICAM-1).[Citation146] Various research studies are available that determine cranberry bioactivities against inflammatory diseases. Reduction in circulating adhesion molecules was examined by Ruel et al.[Citation147] in dormant middle-aged men with liability features by the utilization of cranberry juice. Utilization of anthocyanins in about 320 mg/d pure mixture results in the reduction of CRP, plasma IL-1b in sick person with hypercholesterolemia ailment and soluble vascular cell adhesion molecule 1 (sVCAM-1) were reported by Zhu et al.[Citation146] On the contrary, various research studies are unable to explain the biological effects on other inflammation-causing factors such as adhesion molecules and CRP.[Citation144] In some studies, distinguished features among background medication and participant population results be deficient toward anti-inflammatory effects in the case of plasma lipids, however more studies are required to elucidate these conflicting conclusions.
Anti-diabetic effect
Diabetes mellitus is one of the most common clinical diseases that is characterized by defects in insulin secretion and insulin action, resulting in disturbance in the metabolism of carbohydrates, proteins and fats. The worldwide burden of type 2 diabetes, one of the main causes of morbidity and mortality, has increased rapidly in tandem with increases in obesity. Now a day, it is prevalent among almost 150 million people worldwide. Important risk factors for this aberration are a sedentary lifestyle, obesity and consumption of an energy-rich diet.[Citation148] As a result of its increasing prevalence, various therapeutic approaches have been applied to treat this disease, unless no particular treatment yet been discovered. Emerging management strategies for this disorder involve insulin and oral antidiabetic agents, dietary modification and lifestyle management. Medicinal therapies are effective but toxic as well, whereas diet therapy is considered a nontoxic strategy.[Citation149] In this context, fruits and vegetables rich in fiber are usually recommended. In the fruit category, berries are considered important to cope with type II diabetes mellitus owing to the presence of an array of bioactive compounds such as flavonoids, anthocyanins and other phenolic compounds. These berries play important role in the treatment of type II diabetes by fighting related complications due to their antioxidant capacity.
Among berries, strawberry has gained greater interest for their antioxidant capacity owing to the presence of functional ingredients. A lot of literature exists to defend the functional importance of different forms of berries against diabetes mellitus and its related complications. Chang et al.[Citation150] probed the effect of strawberry juice on diabetes mellitus and its risk factors and found that free radicals and ox-low-density lipoprotein-induced proliferation were reduced significantly in rat aortic smooth muscle cells. Wolfe et al.[Citation151]claimed that strawberries are considered best for their contribution to cellular antioxidant activity among all fruits. Yi et al.[Citation152] worked on the treatment of diabetic mellitus through strawberries and concluded that strawberries reduce oxidative stress and atherosclerotic lesion owing to a functional ingredient i.e. ellagic acid. In another study, Pinto et al.[Citation95]explored the anti-hyperglycemic and anti-hypertensive effects of strawberries and found that ellagic acid present in strawberries can inhibit the activities of carbohydrate and lipid-related enzymes such as α-amylase, α-glucosidase, and angiotensin I-converting enzyme. Moreover, Moazen et al.[Citation96] examined that freeze-dried strawberries improved glycemic control and antioxidant status, and reduced lipid peroxidation and inflammatory response in patients with type II diabetes owing to the presence of phenolic compounds mainly anthocyanin and flavonoids. Many other types of research were conducted to check the anti-diabetic effect of strawberries and found positive results.[Citation153]
Cardioprotective effect
Cardiovascular disorder is a chronic aberration and leading etiology of morbidity and death worldwide.[Citation154,Citation155] According to the World Health Organization (WHO) statistics, an estimated 17.5 million people died from cardiovascular diseases in 2007, representing 31% of all global deaths.[Citation156] Cardiovascular disease includes coronary heart disease and diseases related to cerebral vessels.[Citation157] It can be treated in three different ways i.e. diet, medicine and lifestyle modification. Among these approaches, nutrition (diet) is the most preferred way to cure cardiovascular diseases because it has no harmful effects. For dietary approaches, fruits and vegetables are considered important treatments in the prevention of cardiovascular diseases.[Citation158]
Among fruits, berries have the potential to fight against various risk factors of cardiovascular disease such as hypertension, obesity and type II diabetes mellitus owing to the presence of various functional ingredients such as polyphenols (flavonoids), fiber, magnesium, potassium and folate.[Citation159,Citation160] The mechanism behind the cardioprotective effect of berries includes an enhancement in plasma lipid level, antioxidant activity and endothelial function by inserting anti-inflammatory effects.[Citation88]
Among berries, now a day, strawberries are gaining much attention for their cardioprotective effect owing to the presence of high content of folate, vitamin C and phenolic compounds (anthocyanins and ellagitannins). Among these functional components, anthocyanins and ellagitannins have the highest antioxidant capacity that can mitigate the risk factors of cardiovascular disease such as obesity, stress, hypertension and type II diabetes mellitus.[Citation161] The literature revealed that consumption of strawberries could improve the plasma lipid profile, biomarkers of antioxidant status, antihemolytic defenses and platelet function in healthy subjects, encouraging further evaluation of a population with higher cardiovascular disease risk.[Citation99] Cassidy et al.[Citation88] reported that strawberries have the potential to treat cardiovascular diseases due to three major positive effects such as antioxidant, anti-atherosclerotic and antihypertensive effects. Along with strawberry, blueberry also has potential to treat cardiovascular diseases and related risk factors owing to the presence of functional compounds with antioxidant capacity.[Citation89] Same as other types of berries, cranberry also has a cardio-protective effect mainly due to the presence of proanthocyanidins.[Citation90]
Anti-metabolic syndrome activity
Similar to cardiovascular disease and obesity, the metabolic syndrome also known as insulin resistance syndrome or syndrome X is a worldwide prevalent chronic aberration with multiple complications.[Citation94] As the incidence of complications associated with this syndrome has risen day by day, there is a need to properly combat these complications through different ways: medicine, diet, and lifestyle modification. Now a day, the dietary approach to this syndrome has captured greater attention due to increasing awareness in the public regarding its positive effects. In food category, the trend of fruit consumption for the management of metabolic syndrome is increasing day by day.
Among fruits, berries (in all forms) are important fruits to combat risk factors of metabolic syndrome such as hypertension, impaired fasting glucose, dyslipidemia and cardiovascular diseases.[Citation94,Citation157] Strawberry is one of the most important forms of berries and its consumption can lower blood glucose levels owing to its high anthocyanin content.[Citation162] Moreover, strawberries can overcome the main features of metabolic syndrome such as hypertension and hyperglycemia by deactivating α- glucosidase which is a carbohydrate digestive enzyme, and α-amylase which is lipid-related enzymes.[Citation155]
Similarly, much research was done to probe the anti-metabolic effect of strawberries and it was noticed that strawberry supplementation can improve postprandial metabolism and lowered postprandial hyperglycemia which are the prominent features of metabolic syndrome. Moreover, the postprandial need for insulin can be reduced through the regular intake of strawberries, which in turn lowers the risk of type II diabetes and metabolic syndrome and is suggested for persons with a higher risk of type II diabetes and metabolic syndrome.[Citation88] The main functional ingredient in strawberries for the anti-metabolic syndrome effect is anthocyanin. Some researchers reported that ellagitannins is also found effective in this sense. So it is concluded that anthocyanin is the only compound that is effective against features and risk factors of metabolic syndrome.[Citation159]
Anti-cancer activity
In recent years, various in-vitro and in-vivo studies have been conducted to probe the anti-carcinogenic effect of different edible forms of berries and it is concluded that phytochemicals present in berries may reduce the risk of cancer. The main mode of action behind the anti-carcinogenic property of berries is the prevention of cancer cells activated by tumor and protease activity, tumor angiogenesis reduction, stimulation of cell cycle apprehension and upgrade of apoptosis.[Citation160] In recent years, various in-vitro and in-vivo studies have been conducted to probe the anti-carcinogenic effect of different edible forms of berries and it is concluded that phytochemicals present in berries may mitigate cancer risk. The most important phytochemical compound concerning the anti-carcinogenic effect is anthocyanin which may inactivate the phase I enzymes induced carcinogenicity through phase II enzymes and protect human cells from damage and DNA damage.[Citation89,Citation90]
Among the most beneficial edible forms of berries, strawberries have captured greater attention for their multiple health-related activities such as anti-carcinogenic, anti-oxidative and geno-protective capacities in in-vivo and in-vitro studies,[Citation94] but rare data is present on the effect of strawberries concerning anti-carcinogenic properties in human. The mode of action behind this anti-carcinogenic activity of strawberries is the purification of carcinogens, reactive oxygen species sifting and oxidative DNA damage, protein 1 and NF-ĸb activator, Wnt signaling, TNF-α 46 and angiogenesis, cancer cell proliferation reduction through apoptosis and cell-cycle arrest.[Citation89,Citation94,Citation163]
Moreover, as far as the anti-carcinogenic effects of blueberries are concerned, they have gained much importance due to many phytochemicals and polyphenols especially anthocyanins such as cyanidin, delphinidin, malvidin, peonidin, and petunidin.[Citation164] These phytochemicals are responsible for the anti-cancer activity of blueberries, which include aid in the prevention of various types of cancer such as breast cancer, colon cancer, lung cancer and many other important types of cancer. Phytochemicals in blueberries are well known for their anti-carcinogenic effects.[Citation85,Citation86]
Nowadays, blackberries are well known for their anti-carcinogenic property owing to high phenolics and bioflavonoids. Feng et al.[Citation165] claimed that consumption of fresh blackberry extract helps prevent cancer promoted through tumor and signaling of cells. The main mechanism behind this anti-carcinogenic effect of blackberry extract is the blockage of AP-1 reconciled through reactive oxygen species and protein kinase enzyme activation through mitogen.
Breast cancer is prevalent in 2.6 million people in U.S. Dietary anthocyanins especially cyanidin, malyidin, peonidin, peunidin, delphinidin and pelargonidin have been noticed.[Citation166] In recent era, berries have fetched great attention in reducing the breast cancer risk.[Citation167] Phenolic compounds especially anthocyanins possess anti-inflammatory properties and may help to suppress the growth, size and proliferation of breast cancer cells. The most important form of berries for anti-carcinogenic activity is blueberry which contains about five major anthocyanins such as cyanidin, delphinidin, malvidin, peonidin, and petunidin.[Citation87]
Prostate cancer affects one-out-of-six men during their span in the US. Berries have gained interest in reducing the androgen levels in men with prostate cancer owing to the presence of higher content of ellagitannins and flavonoid-enriched fractions. Berries phytochemicals also affect hormonal levels and may influence cancer progression. Laboratory studies suggest that blueberry could protect against prostate cancer through the anti-inflammatory effects of its ellagitannins, its flavonoids promotion of apoptosis and perhaps through its phenolic content.[Citation168]
Another important type of cancer is colorectal cancer which is the third upcoming etiology of death in women and men. Diet is the most important factor to combat this type of cancer. 70–90% of colorectal cancer chances are reduced through dietary modification. Berries are considered as most important fruits to inhibit colon cancer owing to a broad spectrum of phenolics.[Citation169,Citation170] In the study of Hakansson et al.,[Citation171] rat study was performed to probe the effect of blueberry husks and probiotics on colorectal cancer and it was found that blueberry husks and probiotics are quite helpful in interrupting the liver injuries and activity of colon carcinogens.
Conclusion
Conclusively, all edible forms of berries especially strawberries, blueberries, blackberries and cranberries are rich sources of bioactive moieties mainly anthocyanins. These compounds are antioxidants and they have strong potential to combat various chronic diseases such as cancer, diabetes, obesity, inflammation, cardiovascular diseases and many related complications. These biological applications have opened the way to the utilization of berries and their bioactive compounds in the food and pharmaceutical industry like pharmaceuticals, nutraceuticals and dietary supplements.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Raudonė, L.; Liaudanskas, M.; Vilkickytė, G.; Kviklys, D.; Žvikas, V.; Viškelis, J.; Viškelis, P. Phenolic Profiles, Antioxidant Activity and Phenotypic Characterization of Lonicera Caerulea L. Berries, Cultivated in Lithuania. Antioxidants. 2021, 10(1), 1–15. DOI: 10.3390/antiox10010115.
- Seeram, N. P. Berry Fruits: Compositional Elements, Biochemical Activities and the Impact of Their Intake on Human Health, Performance and Disease. J. Agri. Food Chem 2008, 56(3), 627–629. DOI: 10.1021/jf071988k.
- Ayaz, F. A.; Kucukislamoglu, M.; Reunanen, M. Sugar Non-volatile and Phenolic Acids Composition of Strawberry Tree (Arbutus Unedo L. Var.ellipsoidea) Fruits. J. Food Comp. Anal. 2000, 13(2), 171–177. DOI: 10.1006/jfca.1999.0868.
- Alarcao-E-Silva, M.; Leitao, A. E. B.; Azinheira, H. G.; Leitao, M. C.-A. The Arbutus Berry: Studies on Its Color and Chemical Characteristics at Two Mature Stages. J. Food Comp. Anal. 2001, 14(1), 27–35. DOI: 10.1006/jfca.2000.0962.
- Pallauf, K.; Rivas-Gonzaloa, J. C.; Del Castilloc, M. D.; Canob, M. P.; de Pascual-Teresa, S. Characterization of the Antioxidant Composition of Strawberry Tree (Arbutus Unedo L.) Fruits. J. Food Comp. Anal. 2008, 21(4), 273–281. DOI: 10.1016/j.jfca.2007.11.006.
- Bnouham, M.; Merhfour, F. Z.; Legssyer, A.; Mekhfi, H.; Maallem, S.; Ziyyat, A. Antihyperglycemic Activity of Arbutus Unedo, Ammoides Pusilla and Thymelaea Hirsuta. Die. Pharmazie. 2007, 62, 630–632.
- Pawlowska, A. M.; De Leo, M.; Braca, A. Phenolics of Arbutus Unedo L. (Ericaceae) Fruits: Identification of Anthocyanins and Gallic Acid Derivatives. J. Agri. Food Chem 2006, 54, 10234–10238. DOI: 10.1021/jf062230o.
- Males, Z.; Plazibat, M.; Vundac, V.-B.; Zuntar, I. Qualitative and Quantitative Analysis of Flavonoids of the Strawberry tree-Arbutus Unedo L. (Ericaceae) Acta Pharmaceutica. 2006, 56, 245–250.
- Luby, J. J.; Wildung, D. K.; Stushnoff, C.; Munson, S. T.; Read, P. E.; Hoover, E. E. Northblue’, ‘Northsky’ and ‘Northcountry’ Blueberries. Hartsci. 1986, 21, 1240–1242.
- Lyrene, P. M.; Vorsa, N.; Ballington, J. R. Polyploidy and Sexual Polyploidization in the Genus Vaccinium. Euphytica. 2003, 133, 27–36. DOI: 10.1023/A:1025608408727.
- Tran, P. H.; Tran, T. T. Blueberry Supplementation in Neuronal Health and Protective Technologies for Efficient Delivery of Blueberry Anthocyanins. Biomolecules. 2021, 11(1), 102. DOI: 10.3390/biom11010102.
- Joseph, J. A.; Denisova, N. A.; Arendash, G.; Gordon, M.; Diamond, D.; Shukitt-Hale, B.; Morgan, D. Blueberry Supplementation Enhances Signaling and Prevents Behavioral Deficits in an Alzheimer Disease Model. Nutr. Neurosci 2003, 6(3), 153–162. DOI: 10.1080/1028415031000111282.
- Katsube, N.; Iwashita, K.; Tsushida, T.; Yamaki, K.; Kobori, M. Induction of Apoptosis in Cancer Cells by Bilberry (Vaccinium Myrtillus) and the Anthocyanins. J. Agri. Food Chem 2003, 51(1), 68–75. DOI: 10.1021/jf025781x.
- Krikorian, R.; Shidler, M. D.; Nash, T. A.; Kalt, W.; vinqvist-Tymchuk, M. R.; Shukitt-Hale, B.; Joseph, J. A. Blueberry Supplementation Improves Memory in Older Adults. J. Agri. Food Chem 2010, 58(7), 3996–4000. DOI: 10.1021/jf9029332.
- Smith, M. A. L.; Marley, K. A.; Seigler, D.; Singletary, K. W.; Meline, B. Bioactive Properties of Wild Blueberry Fruits. J. FoodSci. 2000, 65(2), 352–356.
- Tatar, M.; Varedi, M.; Naghibalhossaini, F. Epigenetic Effects of Blackberry Extract on Human Colorectal Cancer Cells. Nutr. Cancer. 2021, 74(4), 1–11. DOI: 10.1080/01635581.2022.2000788.
- Strik, B.C. A Review of Nitrogen Nutrition of Rubus. Acta Hortic. 2008, 777, 403–410. DOI: 10.17660/ActaHortic.2008.777.61
- Strik, B. C.; Clark, J. R.; Finn, C. E., and Banados, P. Worldwide Production of Blackberries, 1995 to 2005 and Predictions for growth. HortTech. 2007, 17(2), 205–213. https://doi.org/10.21273/HORTTECH.17.2.205.
- Kafkas, E.; Kosar, M.; Turemis, N.; Baser, K. H. C. Analysis of Sugars, Organic Acids and Vitamin C Contents of Blackberry Genotypes from Turkey. Food Chem 2006, 97, 732–736. DOI: 10.1016/j.foodchem.2005.09.023.
- Cho, M. J.; Howard, L. R.; Prior, R. L.; Clark, J. R. Flavonoid Glycosides and Antioxidant Capacity of Various Blackberry, Blueberry and Red Grape Genotypes Determined by high-performance Liquid Chromatography/ Mass Spectrometry. J. Sci. Food Agri. 2004, 84, 1771–1782. DOI: 10.1002/jsfa.1885.
- Dai, J.; Patel, J. D.; Mumper, R. J. Characterization of Blackberry Extract and Its anti-proliferative and anti-inflammatory Properties. J. Med. Food. 2007, 10(2), 258–265. DOI: 10.1089/jmf.2006.238.
- Kaparapu, J.; Pragada, P. M., and Geddada, M. N. R. Fruits and Vegetables and Its Nutritional Benefits. In Functional Foods and Nutraceuticals Book Subtitle Bioactive Components, Formulations and Innovations, eds Chukwuebuka Egbuna and Genevieve Dable Tupas; Springer: Cham, 2020; pp 241–260. https://doi.org/10.1007/978-3-030-42319-3.
- Girard, K. K.; Sinha, N. K. Cranberry, Blueberry, Currant, and Gooseberry. In Handbook of Fruits and Fruit Processing; Hui, Y. H., Ozsef Barta, J., Pilar Cano, M., Gusek, T. W., Sidhu, J. S., Sinha, N. K., Eds.; Blackwell Publishing Professional: Iowa, USA, 2006; pp 369–390.
- Baby, B.; Antony, P.; Vijayan, R. Antioxidant and Anticancer Properties of Berries. Crit. Rev. Food Sci. Nutr 2018, 58(15), 2491–2507. DOI: 10.1080/10408398.2017.1329198.
- Kren, V.; Martinkove, L. Glycosides in Medicine: The Role of Glycosidic Residue in Biological Activity. Curr. Med. Chem 2001, 8, 1303–1328. DOI: 10.2174/0929867013372193.
- Willet, W. C. Eat, Drink, and Be Healthy: The Harvard Medical School Guide to Healthy Eating; Simon and Schuster: New York, 2001.
- Talcott, S. T. Chemical Components of Berry Fruits. In Berry Fruit: Value-added Products for Health Promotion; Zhao, Y., Ed.; CRC press – Taylor & Francis Group: New York, 2007; pp 1–72.
- Aaby, K.; Skrede, G.; Wrolstad, E. R. Phenolic Composition and Antioxidant Activities in Flesh and Achenes of Strawberries (Fragaria Ananassa). J. Agri. Food Chem 2005, 53, 4032–4040. DOI: 10.1021/jf048001o.
- Battino, M.; Beekwilder, J.; Denoyes-Rothan, B.; Laimer, M.; McDougall, G. J.; Mezzetti, B. Bioactive Compounds in Berries Relevant to Human Health. Nutr. Rev 2010, 67(1), 145–150. DOI: 10.1111/j.1753-4887.2009.00178.x.
- Buendia, B.; Gil, M. I.; Tudela, J. A.; Gady, A. L.; Medina, J. J.; Soria, C.; Lopez, J. B.; Tomas-Barberan, F. A. HPLC-MS Analysis of Proanthocyanin Oligomers and Other Phenolics in 15 Strawberry Cultivars. J. Agri. Food Chem 2010, 58, 3916–3926. DOI: 10.1021/jf9030597.
- Giampieri, F.; Alvarez-Suarez, J. M.; Mazzoni, L.; Romandini, S.; Bompadre, S.; Diamanti, J.; Capocasa, F.; Mezzetti, B.; Quiles, J. L.; Ferreiro, M. S., et al. The Potential Impact of Strawberry on Human Health. Nat. Prod. Res. 2013, 27(4–5), 448–455. DOI: 10.1080/14786419.2012.706294.
- Giampieri, F.; Tulipani, S.; Alvarez-Suarez, J. M.; Quiles, J. L.; Mezzetti, B.; Battino, M. The Strawberry: Composition, Nutritional Quality, and Impact on Human Health. Nutr. 2012, 28(1), 9–19. DOI: 10.1016/j.nut.2011.08.009.
- Hamauzu, Y.; Mizuno, Y. Non-extractable Procyanidins and Lignin are Important Factors in the Bile Acid Binding and Radical Scavenging Properties of Cell Wall Material in Some Fruits. Plant Foods Hum. Nutr 2010, 66(1), 70–77. DOI: 10.1007/s11130-010-0207-z.
- King, E. S., and Bolling, B. W. Composition, Polyphenol Bioavailability, and Health Benefits of Aronia Berry: A Review. J. Food Bioact . 2020, 11, 13–30. https://doi.org/10.31665/JFB.2020.11235.
- Fan-Chiang, H. J.; Wrolstad, R. E. Sugar and Nonvolatile Acid Composition of Blackberries. J. AOAC Int. 2010, 93(3), 956–965. DOI: 10.1093/jaoac/93.3.956.
- Kresty, L. A.; Morse, M. A.; Morgan, C.; Carlton, P. S.; Lu, J.; Gupta, A.; Blackwood, M.; Stoner, G. D. Chemoprevention of Esophageal Tumorigenesis by Dietary Administration of Lyophilized Black Raspberries. Cancer Res 2001, 61, 6112–6119.
- Pineli, L. L. O.; Moretti, C. L.; Santos, M. S.; Campos, A. B.; Brasileiro, A. V.; Cordova, A. C.; Chiarello, M. D. Antioxidants and Other Chemical and Physical Characteristics of Two Strawberry Cultivar at Different Ripeness Stages. J. Food Comp. Anal. 2011, 24, 11–16. DOI: 10.1016/j.jfca.2010.05.004.
- Ain, H. B. U.; Saeed, F.; Barrow, C. J.; Dunshea, F. R., and Suleria, H. A. R. Food Processing Waste: A Potential Source for Bioactive Compounds. In Bioactive Compounds in Underutilized Fruits and Nuts. 2020, 625–649. https://doi.org/10.1007/978-3-030-30182-8_45.
- Martineau, L. C.; Couture, A.; Spoor, D.; Benhaddou-Andaloussi, A.; Harris, C.; Meddah, B.; Leduc, C.; Burt, A.; Vuong, T.; Le, P. M., et al. Anti-diabetic Properties of the Canadian Lowbush Blueberry Vaccinium Angustifolium Ait. Phytomed. 2006, 13, 612–623. DOI: 10.1016/j.phymed.2006.08.005.
- Valentova, K.; Ulrichova, J.; Cvak, L., and Simanek, V. Cytoprotective Effect of a Bilberry Extract against Oxidative Damage of Rat Hepatocytes. Food Chem. 2007, 101, 912–917.
- Zadernowski, R.; Naczk, M.; Czaplicki, S.; Rubinskiene, M.; Szalkiewicz, M. Composition of Phenolic Acids in Sea Buckthorn (Hippophae Rhamnoides L.) Berries. J. Am. Oil Chemists’ Soc. 2005, 82(3), 175–179. DOI: 10.1007/s11746-005-5169-1.
- Gür, M.; Gür, M.; Engin, M. S.; Avci, E. Antidiabetic and Antioxidant Properties of Bilberry (Vaccinium Myrtillus Linn.) Fruit and Their Chemical Composition. Environmental Monitoring and Assessment. 2018, 190. DOI: 10.1007/s10661-018-6829-6.
- Kaume, L.; Howard, L. R.; Devareddy, L. The Blackberry Fruit: A Review on Its Composition and Chemistry, Metabolism and Bioavailability, and Health Benefits. J. Agri.Food Chem. 2012, 60(23), 5716–5727. DOI: 10.1021/jf203318p.
- Neto, C. C. Cranberry and Blueberry: Evidence for Protective Effects against Cancer and Vascular Diseases. Mol. Nutr.Food Res. 2007, 51(6), 652–664. DOI: 10.1002/mnfr.200600279.
- Lin, L. Z.; Harnly, J. M. A Screening Method for the Identification of Glycosylated Flavonoids and Other Phenolic Compounds Using A Standard Analytical Approach for All Materials. J. Agri. Food Chem 2007, 55, 1084–1096. DOI: 10.1021/jf062431s.
- Bakker, J.; Bridle, P.; Bellworthy, S. J. Strawberry Juice Colour: A Study of the Quantitative and Qualitative Pigment Composition of Juices from 39 Genotypes. J. Sci. Food Agri. 1994, 64, 31–37. DOI: 10.1002/jsfa.2740640106.
- Hong, V.; Wrolstad, R. E. Characterization of Anthocyanin Containing Colorants in Fruit Juices by HPLC-photodiode Array Detection. J. Agri. Food Chem 1990, 38, 698–707. DOI: 10.1021/jf00093a025.
- Mazza, G.; Miniati, E. Anthocyanins in Fruits, Vegetables and Grains; CRC Press Inc: Boca Raton, FL, 1993.
- Wang, S. Y.; Lin, H. S. Antioxidant Activity in Fruits and Leaves of Blackberry, Raspberry and Strawberry Varies with Cultivar and Developmental Stage. J. Agri. Food Chem 2000, 48, 140–146. DOI: 10.1021/jf9908345.
- Ryan, J. J. Flavonol Glycosides of the Cultivated Strawberry. J. Food Sci 1971, 36, 867–870. DOI: 10.1111/j.1365-2621.1971.tb15547.x.
- Clifford, M. N.; Scalbert, A. Ellagitannins—nature, Occurrence and Dietary Burden. J. Sci. Food Agri. 2000, 80, 1118–1125. DOI: 10.1002/(SICI)1097-0010(20000515)80:7<1118::AID-JSFA570>3.0.CO;2-9.
- Maatta-Riihinen, K. R.; Kamal-Eldin, A.; Torronen, A. Identification and Quantification of Phenolic Compounds in Berries of Fragaria and Rubus Species (Family Rosaceae). J. Agri. Food Chem 2004, 52, 6178–6187. DOI: 10.1021/jf049450r.
- Wu, X.; Beecher, G. R.; Holden, J. M.; Haytowitz, D. B.; Gebhardt, S. E.; Prior, R. L. Concentrations of Anthocyanins in Common Foods in the United States and Estimation of Normal Consumption. J. Agri. Food Chem 2006, 54(11), 4069–4075. DOI: 10.1021/jf060300l.
- Wang, C.; Zuo, Y. Ultrasound-assisted Hydrolysis and Gas chromatography–mass Spectrometric Determination of Phenolic Compounds in Cranberry Products. Food Chem 2011, 128, 562–568. DOI: 10.1016/j.foodchem.2011.03.066.
- Zhang, K.; Zuo, Y. GC–MS Determination of Flavonoids and Phenolic and Benzoic Acids in Human Plasma after Consumption of Cranberry Juice. J. Agri. Food Chem 2004, 52, 222–227. DOI: 10.1021/jf035073r.
- Alvarez-Suarez, J. M.; Giampieri, F.; Tulipani, S.; Casoli, T.; Di Stefano, G.; Gonzalez-Paramas, A. M.; Santos-Buelga, C.; Busco, F.; Quiles, J. L.; Cordero, M. D., et al. One-month strawberry-rich Anthocyanin Supplementation Ameliorates Cardiovascular Risk, Oxidative Stress Markers and Platelet Activation in Humans. J. Nutr. Biochem 2014, 25(3), 289–294. DOI: 10.1016/j.jnutbio.2013.11.002.
- Ma, Y.; Ma, X.; Gao, X.; Wu, W.; Zhou, B. Light Induced Regulation Pathway of Anthocyanin Biosynthesis in Plants. Int. J. Mol. Sci 2021, 22(20), 11116. DOI: 10.3390/ijms222011116.
- Curtis, P. J.; Kroon, P. A.; Hollands, W. J.; Walls, R.; Jenkins, G.; Kay, C. D.; Cassidy, A. Cardiovascular Disease Risk Biomarkers and Liver and Kidney Function are Not Altered in Postmenopausal Women after Ingesting an Elderberry Extract Rich in Anthocyanins for 12 Weeks. J. Nutr 2009, 139(12), 2266–2271. DOI: 10.3945/jn.109.113126.
- Andersen, O. M.; Jordheim, M.; Byamukama, R.; Mbabazi, A.; Ogweng, G.; Skaar, I.; Kiremire, B. Anthocyanins with Unusual Furanose Sugar (Apiose) from Leaves of Synadenium Grantii (Euphorbiaceae). Phytochem. 2010, 71, 1558–1563. DOI: 10.1016/j.phytochem.2010.05.025.
- Doughty, J.; Aljabri, M.; Scott, R. J. Flavonoids and the Regulation of Seed Size in Arabidopsis. Biochem. Soc. Transact. 2014, 42, 364–369. DOI: 10.1042/BST20140040.
- Mouradov, A.; Spangenberg, G. Flavonoids: A Metabolic Network Mediating Plants Adaptation to Their Real Estate. Front. Plant Sci 2014, 5, 620. DOI: 10.3389/fpls.2014.00620.
- Messaoudi, O.; Gouzi, H.; El-Hoshoudy, A. N.; Benaceur, F.; Patel, C.; Goswami, D.; Bendahou, M.; Bendahou, M. Berries Anthocyanins as Potential SARS-CoV–2 Inhibitors Targeting the Viral Attachment and Replication; Molecular Docking Simulation. Egypt. J. Pet. 2021, 30(1), 33–43. DOI: 10.1016/j.ejpe.2021.01.001.
- Tulipani, S.; Mezzetti, B.; Capocasa, F.; Bompadre, S.; Beekwilder, J.; de Vos, C. H. R.; Capanoglu, E.; Bovy, A.; Battino, M. Antioxidants, Phenolic Compounds, and Nutritional Quality of Different Strawberry Genotypes. J. Agri. Food Chem 2008, 56(3), 696–704. DOI: 10.1021/jf0719959.
- Garzon, G. A.; Riedl, K. M.; Schwartz, S. J. Determination of Anthocyanins, Total Phenolic Content, and Antioxidant Activity in Andes Berry (Rubus Glaucus Benth). J. Food Sci 2009, 74, 227–232. DOI: 10.1111/j.1750-3841.2009.01092.x.
- Rechner, A. R.; Kroner, C. Anthocyanins and Colonic Metabolites of Dietary Polyphenols Inhibit Platelet Function. Thromb. Res 2005, 116, 327–334. DOI: 10.1016/j.thromres.2005.01.002.
- Hartman, R. E.; Shah, A.; Fagan, A. M.; Schwetye, K. E.; Parsadanian, M.; Schulman, R. N.; Finn, M. B.; Holtzman, D. M. Pomegranate Juice Decreases Amyloid Load and Improves Behavior in a Mouse Model of Alzheimer’s Disease. Neurobio. Dis. 2006, 24, 506–515. DOI: 10.1016/j.nbd.2006.08.006.
- Joseph, J. A.; Shukitt-Hale, B.; Casadesus, G. Reversing the Deleterious Effects of Aging on Neuronal Communication and Behavior: Beneficial Properties of Fruit Polyphenolic Compounds. Am. J.Clin. Nutr. 2005, 81, 313–316. DOI: 10.1093/ajcn/81.1.313S.
- Ding, M.; Feng, R.; Wang, S. Y.; Bowman, L.; Lu, Y.; Qian, Y.; Castranova, V.; Jiang, B. H.; Shi, X. Cyanidin 3-glucoside, a Natural Product Derived from Black Berry, Exhibits Chemopreventive and Chemotherapeutic Activity. J. Bio. Chem. 2006, 281, 17359–17368. DOI: 10.1074/jbc.M600861200.
- Loren, D. J.; Seeram, N.-P.; Schulman, R. N.; Holtzman, D. M. Maternal Dietary Supplementation with Pomegranate Juice Is Neuroprotective in an Animal Model of Neonatal hypoxic–ischemic Brain Injury. Pediatric. Res. 2005, 57, 858–864. DOI: 10.1203/01.PDR.0000157722.07810.15.
- West, T.; Atzeva, M.; Holtzman, D. M. Pomegranate Polyphenols and Resveratrol Protect the Neonatal Brain against hypoxic–ischemic Injury. Developmental Neurosci. 2007, 29, 363–372. DOI: 10.1159/000105477.
- Vilkickyte, G.; Motiekaityte, V.; Vainoriene, R.; Liaudanskas, M.; Raudone, L.; Niki, E.; Capanoglu, E.; Sieniawska, E. Development, Validation, and Application of UPLC-PDA Method for Anthocyanins Profiling in Vaccinium L. Berries. J. Berry Res. 2021, 11(4), 583–599. DOI: 10.3233/JBR-200658.
- Tsuda, T. Regulation of Adipocyte Function by Anthocyanins; Possibility of Preventing the Metabolic Syndrome. J. Agri, Food Chem.2008, 56, 642–646.
- Matsumoto, H.; Nakamura, Y.; Hirayama, M.; Yoshiki, Y.; Okubo, K. Antioxidant Activity of Black Currant Anthocyanin Aglycons and Their Glycosides Measured by Chemiluminescence in a Neutral pH Region and in Human Plasma. J. Agri. Food Chem 2002, 50, 5034–5037. DOI: 10.1021/jf020292i.
- Proteggente, A. R.; Pannala, A. S.; Paganga, G.; Buren, L. V.; Wagner, E.; Wiseman, S.; Put, F. V. D.; Dacombe, C.; Rice-Evans, C. A. The Antioxidant Activity of Regularly Consumed Fruit and Vegetables Reflects Their Phenolic and Vitamin C Composition. Free Rad. Bio.Med. 2002, 36, 217–233. DOI: 10.1080/10715760290006484.
- Ramirez-Tortosa, C.; Andersen, O. M.; Gardner, P. T.; Morrice, P. C.; Wood, S. G.; Duthie, S. J.; Collins, A. R.; Duthie, G. G. Anthocyanin-rich Extract Decreases Indices of Lipid Peroxidation and DNA Damage in Vitamin E-depleted Rats. Free Rad. Bio. Med. 2001, 31, 1033–1037. DOI: 10.1016/S0891-5849(01)00618-9.
- Nicoue, E. E.; Savard, S.; Belkacemi, K. Anthocyanins in Wild Blueberries of Quebec: Extraction and Identification. J. Agri. Food Chem 2007, 55, 5626. DOI: 10.1021/jf0703304.
- Scalzo, J.; Politi, A.; Pellegrini, N.; Mezzetti, B.; Battino, M. Plant Genotype Affects Total Antioxidant Capacity and Phenolic Contents in Fruit. Nutr. 2005, 21(2), 207–213. DOI: 10.1016/j.nut.2004.03.025.
- Vendrame, S.; Klimis-Zacas, D. Potential Factors Influencing the Effects of Anthocyanins on Blood Pressure Regulation in Humans: A Review. Nutrients. 2019, 11(6), 1431. DOI: 10.3390/nu11061431.
- Velioglu, Y. S.; Mazza, G.; Gao, L.; Oomah, B. D. Antioxidant Activity and Total Phenolics in Selected Fruits, Vegetables, and Grain Products. J. Agri. Food Chem 1998, 46, 4113–4117. DOI: 10.1021/jf9801973.
- Celik, H.; Ozgen, M.; Serce, S.; Kaya, C. Phytochemical Accumulation and Antioxidant Capacity at Four Maturity Stages of Cranberry Fruit. Scientia Horticult. 2008, 117, 345–348. DOI: 10.1016/j.scienta.2008.05.005.
- Brown, P. N.; Murch, S. J.; Shipley, P. Phytochemical Diversity of Cranberry (Vaccinium Macrocarpon Aiton) Cultivars by Anthocyanin Determination and Metabolomic Profiling with Chemometric Analysis. J. Agri. Food Chem 2012, 60, 261–271. DOI: 10.1021/jf2033335.
- Aristri, M. A.; Lubis, M. A. R.; Iswanto, A. H.; Fatriasari, W.; Sari, R. K.; Antov, P.; Gajtanska, M.; Papadopoulos, A. N.; Pizzi, A. Bio-Based Polyurethane Resins Derived from Tannin: Source, Synthesis, Characterisation, and Application. Forests. 2021, 12(11), 1516. DOI: 10.3390/f12111516.
- Koponen, J. M.; Happonen, A. M.; Mattila, P. H.; Torronen, A. R. Contents of Anthocyanins and Ellagitannins in Selected Foods Consumed in Finland. J. Agri. Food Chem 2007, 55, 1612–1619. DOI: 10.1021/jf062897a.
- Quideau, S.; Deffieux, D.; Douat-Casassus, C. L.; Pouysequ, L. Plant Polyphenols: Chemical Properties, Biological Activities, and Synthesis. Angewandte Chemie Int. Ed. 2011, 50, 586–621.
- Chen, C.; Li, Y.; Xu, Z. Chemical Principles and Bioactivities of Blueberry. Yao Xue Xue Bao= Acta Pharmaceutica Sinica. 2010, 45(4), 422–429.
- Johnson, S. A.; Arjmandi, B. H. Evidence for anti-cancer Properties of Blueberries: A mini-review. Anti-cancer Agents Med. Chem. 2012, 13(8), 1142–1148. DOI: 10.2174/18715206113139990137.
- Aqil, F.; Munagala, R.; Jeyabalan, J.; Vadhanam, M. V. Bioavailability of Phytochemicals and Its Enhancement by Drug Delivery Systems. Cancer Lett 2013, 334(1), 133–141. DOI: 10.1016/j.canlet.2013.02.032.
- Cassidy, A.; Mukamal, K. J.; Liu, L.; Franz, M.; Eliassen, A. H.; Rimm, E. B. High Anthocyanin Intake Is Associated with a Reduced Risk of Myocardial Infarction in Young and middle-aged Women. Circul. 2013, 27(2), 188–196. DOI: 10.1161/CIRCULATIONAHA.112.122408.
- Nyberg, S.; Gerring, E.; Giellan, S.; Vergara, M.; Lindstrom, T.; Nystrom, F. H. Effects of Exercise with or without Blueberries in the Diet on cardio-metabolic Risk Factors: An Exploratory Pilot Study in Healthy Subjects. Upsala. J. Med. Sci. 2013, 118(4), 247–255. DOI: 10.3109/03009734.2013.825348.
- Blumberg, J. B.; Basu, A.; Krueger, C. G.; Lila, M. A.; Neto, C. C.; Novotny, J. A.; Reed, J. D.; Rodriduez-Mateos, A.; Toner, C. D. Impact of Cranberries on Gut Microbiota and Cardiometabolic Health: Proceedings of the Cranberry Health Research Conference 2015. Adv. Nutr 2016, 7(4), 759–770. DOI: 10.3945/an.116.012583.
- Halvorsen, B. L.; Carlsen, M. H.; Phillips, K. M.; Bohn, S. K.; Holte, K.; Jacobs, D. R.; Blomhoff, R. Content of redox-active Compounds (Ie, Antioxidants) in Foods Consumed in the United States. Am. J. Clin. Nutr 2006, 84(1), 95–135. DOI: 10.1093/ajcn/84.1.95.
- Drozdz, P.; Seziene, V.; Pyrzynska, K. Phytochemical Properties and Antioxidant Activities of Extracts from Wild Blueberries and Lingonberries. Plant Foods Hum. Nutr 2017, 72(4), 360–364. DOI: 10.1007/s11130-017-0640-3.
- Joseph, S. V.; Edirisinghe, I.; Burton-Freeman, B. M. Berries: Anti-inflammatory Effects in Humans. J. Agri. Food Chem 2014, 62(18), 3886–3903. DOI: 10.1021/jf4044056.
- Basu, A.; Lyons, T. J. Strawberries, Blueberries and Cranberries in the Metabolic Syndrome: Clinical Perspectives. J. Agri. Food Chem 2011, 60(23), 5687–5692. DOI: 10.1021/jf203488k.
- Pinto, S. A.; Bohland, E.; Coelho Cde, P.; Morgulis, M. S.; Bonamin, L. V. An Animal Model for the Study of Grapeseed in Stress and Depression: Pilot Study. Homeopathy. 2008, 97(3), 141–144. DOI: 10.1016/j.homp.2008.04.001.
- Moazen, S.; Amani, R.; Homayouni Rad, A.; Shahbazian, H.; Ahmadi, K.; Taha Jalali, M. Effects of freeze-dried Strawberry Supplementation on Metabolic Biomarkers of Atherosclerosis in Subjects with Type 2 Diabetes: A Randomized double-blind Controlled Trial. Ann. Nutr. Metab. 2013, 63(3), 256–264. DOI: 10.1159/000356053.
- Ismail, T.; Calcabrini, C.; Diaz, A. R.; Fimognari, C.; Turrini, E.; Catanzaro, E.; Akhtar, S.; Sestili, P. Ellagitannins in Cancer Chemoprevention and Therapy. Toxins (Basel). 2016, 8(5), 151. DOI: 10.3390/toxins8050151.
- Alvarez-Suarez, J. M.; Mazzoni, L.; Forbes-Hernandez, T. Y.; Gasparrini, M.; Sabbadini, S.; Giampieri, F. The Effects of pre-harvest and post-harvest Factors on the Nutritional Quality of Strawberry Fruits: A Review. J. Berry Res. 2014, 4, 1–10. DOI: 10.3233/JBR-140068.
- Da Silva Pinto, M.; de Carvalho, J. E.; Lajolo, F. M.; Genovese, M. I.; Shetty, K. Evaluation of Antiproliferative, anti-type 2 Diabetes, and Antihypertension Potentials of Ellagitannins from Strawberries (Fragaria × Ananassa Duch.) Using in Vitro Models. J. Med. Food. 2010, 13, 1027–1035. DOI: 10.1089/jmf.2009.0257.
- Fang, J. Bioavailability of Anthocyanins. Drug Metab. Rev. 2014, 46, 508–520. DOI: 10.3109/03602532.2014.978080.
- Forbes-Hernandez, T. Y.; Gasparrini, M.; Afrin, S.; Bompadre, S.; Mezzetti, B.; Quiles, J. L.; Giampieri, F.; Battino, M. The Healthy Effects of Strawberry Polyphenols: Which Strategy behind Antioxidant Capacity? Crit. Rev. Food Sci. Nutr 2016, 56, 1–14. DOI: 10.1080/10408398.2015.1051919.
- Forbes-Hernandez, T. Y.; Giampieri, F.; Gasparrini, M.; Mazzoni, L.; Quiles, J. L.; Alvarez-Suarez, J. M.; Battino, M. The Effects of Bioactive Compounds from Plant Foods on Mitochondrial Function: A Focus on Apoptotic Mechanisms. Food Chem. Toxico. 2014, 68, 154–182.
- Kosinska-Cagnazzo, A.; Diering, S.; Prim, D.; Andlauer, W. Identification of Bioaccessible and Uptaken Phenolic Compounds from Strawberry Fruits in in Vitro digestion/Caco-2 Absorption Model. Food Chem 2015, 170, 288–294. DOI: 10.1016/j.foodchem.2014.08.070.
- Liu, C. J.; Lin, J. Y. Anti-inflammatory Effects of Phenolic Extracts from Strawberry and Mulberry Fruits on Cytokine Secretion Profiles Using Mouse Primary Splenocytes and Peritoneal Macrophages. Int. Immunopharmaco. 2013, 16, 165–170. DOI: 10.1016/j.intimp.2013.03.032.
- López, J.; Vera, C.; Bustos, R.; Florez-Mendez, J. Native Berries of Chile: A Comprehensive Review on Nutritional Aspects, Functional Properties, and Potential Health Benefits. J. Food Meas. Charact 2021, 15(2), 1139–1160. DOI: 10.1007/s11694-020-00699-4.
- Giampieri, F.; Alvarez-Suarez, J. M.; Battino, M. Strawberry and Human Health: Effects beyond Antioxidant Activity. J. Agri. Food Chem 2014, 62, 3867–3876. DOI: 10.1021/jf405455n.
- Zunino, S. J.; Parelman, M. A.; Freytag, T. L.; Stephensen, C. B.; Kelley, D. S.; Mackey, B. E.; Woodhouse, L. R.; Bonne, E. L. Effects of Dietary Strawberry Powder on Blood Lipids and Inflammatory Markers in Obese Human Subjects. Br. J. Nutr 2012, 108, 900–909. DOI: 10.1017/S0007114511006027.
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J. P.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (Poly)phenolics in Human Health: Structures, Bioavailability, and Evidence of Protective Effects against Chronic Diseases. Antioxi. Redox Signaling. 2013, 18, 1818–1892. DOI: 10.1089/ars.2012.4581.
- Henning, S. M.; Zhang, Y.; Rontoyanni, V. G.; Huang, J.; Lee, R. P.; Trang, A.; Nuernberger, G.; Heber, D. Variability in the Antioxidant Activity of Dietary Supplements from Pomegranate, Milk Thistle, Green Tea, Grape Seed, Goji, and Acai: Effects of in Vitro Digestion. J. Agri. Food Chem 2014, 62, 4313–4321. DOI: 10.1021/jf500106r.
- Wang, Z.; Li, Y.; Chen, L.; Xin, X.; Yuan, Q. A Study of Controlled Uptake and Release of Anthocyanins by Oxidized Starch Microgels. J. Agri. Food Chem 2013, 61, 5880–5887. DOI: 10.1021/jf400275m.
- Folmer, F.; Basavaraju, U.; Jaspars, M.; Hold, G.; El-Omar, E.; Dicato, M., and Diederich, M. Anticancer Effects of Bioactive Berry Compounds. Phytochem. rev. 2014, 13(1), 295–322 .
- Sher, H.; Al-Yemeni, M. N.; Leonard, W.; Shah, A. J. Ethnopharmaceutically Important Medicinal Plants and Its Utilization in Traditional System of Medicine, Observation from the Northern Parts of Pakistan. J. Med. Plants Res 2010, 4, 1853–1864.
- Diaconeasa, Z.; Leopold, L.; Rugina, D.; Ayyaz, H.; Socaciu, C. Antiproliferative and Antioxidant Properties of Anthocyanin Rich Extracts from Blueberry and Blackcurrant Juice. Int. J. Mol. Sci 2015, 16, 2352–2365. DOI: 10.3390/ijms16022352.
- Heinonen, M. Antioxidant Activity and Antimicrobial Effect of Berry phenolics–a Finnish Perspective. Mol. Nutr. Food Res 2007, 51(6), 684–691. DOI: 10.1002/mnfr.200700006.
- Martini, S.; D’Addario, C.; Colacevich, A.; Focardi, S.; Borghini, F.; Santucci, A.; Figura, N.; Rossi, C. Antimicrobial Activity against Helicobacter Pylori Strains and Antioxidant Properties of Blackberry Leaves (Rubus ulmifolius) and Isolated Compounds. Int. J. Antimicrob. Agent. 2009, 34(1), 50–59. DOI: 10.1016/j.ijantimicag.2009.01.010.
- Riaz, M.; Ahmad, M.; Rahman, N. Antimicrobial Screening of Fruit, Leaves, Root and Stem of Rubusfruticosus. J. Med. Plants Res 2011, 5(24), 5920–5924.
- Jakobsdottir, G.; Blanco, N.; Xu, J.; Ahrne, S.; Molin, G.; Sterner, O.; Nyman, M. Formation of Short-Chain Fatty Acids, Excretion of Anthocyanins, and Microbial Diversity in Rats Fed Blackcurrants, Blackberries, and Raspberries. J. Nutr. Metab. 2013, 202534, 12.
- Souza, V. R.; Pereira, P. A. P.; Silva, T. L. T.; Lima, L. C. O.; Pio, R.; Queiroz, F. Determination of the Bioactive Compounds, Antioxidant Activity and Chemical Composition of Brazilian Blackberry, Red Raspberry, Strawberry, Blueberry and Sweet Cherry Fruits. Food Chem 2014, 156, 362–368. DOI: 10.1016/j.foodchem.2014.01.125.
- Juan, C. A.; Pérez de la Lastra, J. M.; Plou, F. J.; Pérez-Lebeña, E. The Chemistry of Reactive Oxygen Species (ROS) Revisited: Outlining Their Role in Biological Macromolecules (DNA, Lipids and Proteins) and Induced Pathologies. Int. J. Mol. Sci 2021, 22(9), 4642. DOI: 10.3390/ijms22094642.
- Gonzalez, E. M.; de Ancos, B.; Cano, M. P. Relation between Bioactive Compounds and Free radical-scavenging Capacity in Berry Fruits during Frozen Storage. J. Sci. Food Agri. 2003, 83, 722–726. DOI: 10.1002/jsfa.1359.
- Wang, H.; Cao, G.; Prior, R. L. Oxygen Radical Absorbing Capacity of Anthocyanins. J. Agri. Food Chem 1997, 45, 304–309. DOI: 10.1021/jf960421t.
- Baranowska, M. Bartoszek, A. Antioxidant and Antimicrobial Properties of Bioactive Phytochemicals from Cranberry. Postepy Higieny I Medycyny Doswiadczalnej (Online). 2016, 70,1460–1468.
- Pap, N.; Fidelis, M.; Azevedo, L.; Do Carmo, M. A. V.; Wang, D.; Mocan, A.; Granato, D.; Xavier-Santos, D.; Sant’Ana, A. S.; Yang, B. Berry Polyphenols and Human Health: Evidence of Antioxidant, anti-inflammatory, Microbiota Modulation, and cell-protecting Effects. Curr. Opin. Food Sci 2021, 42, 167–186. DOI: 10.1016/j.cofs.2021.06.003.
- Phan, K.; Raes, K.; Van Speybroeck, V.; Roosen, M.; De Clerck, K.; De Meester, S. Non-food Applications of Natural Dyes Extracted from agro-food Residues: A Critical Review. J. Clean. Prod 2021, 301, 126920. DOI: 10.1016/j.jclepro.2021.126920.
- Hui, X. D.; Wu, G.; Han, D.; Gong, X.; Wu, X. Y.; Tang, S. Z.; Brennan, C. S.; Brennan, C. S. The Effects of Bioactive Compounds from Blueberry and Blackcurrant Powder on Oat Bran Pastes: Enhancing in Vitro Antioxidant Activity and Reducing Reactive Oxygen Species in Lipopolysaccharide-Stimulated Raw264. 7 Macrophages. Antioxidants. 2021, 10(3), 388. DOI: 10.3390/antiox10030388.
- Casas-Forero, N.; Orellana-Palma, P.; Petzold, G. Influence of Block Freeze Concentration and Evaporation on Physicochemical Properties, Bioactive Compounds and Antioxidant Activity in Blueberry Juice. Food Sci. Technol 2020, 40, 387–394. DOI: 10.1590/fst.29819.
- Reque, P. M.; Steffens, R. S.; Jablonski, A.; Flores, S. H.; Rios, A. O.; Jong, E. V. Cold Storage of Blueberry (Vaccinium Spp.) Fruits and Juice: Anthocyanin Stability and Antioxidant Activity. J, Food Comp. Anal. 2014, 33(1), 111–116. DOI: 10.1016/j.jfca.2013.11.007.
- Gasparrini, M.; Forbes-Hernandez, T. Y.; Giampieri, F.; Afrin, S.; Alvarez-Suarez, J. M.; Mazzoni, L.; Mezzetti, B.; Quiles, J. L.; Battino, M. Anti-inflammatory Effect of Strawberry Extract against LPS-induced Stress in RAW 264.7 Macrophages. Food Chem. Toxico. 2017, 102, 1–10. DOI: 10.1016/j.fct.2017.01.018.
- Parelman, M. A.; Storms, D. H.; Kirschke, C. P.; Huang, L.; Zunino, S. J. Dietary Strawberry Powder Reduces Blood Glucose Concentrations in Obese and Lean C57BL/6 Mice, and Selectively Lowers Plasma C-reactive Protein in Lean Mice. Br. J. Nutr 2012, 108, 1789–1799. DOI: 10.1017/S0007114512000037.
- Poulose, S. M.; Bielinski, D. F.; Carrihill-Knoll, K. L.; Rabin, B. M., and Shukitt-Hale, B. Protective Effects of Blueberry- and Strawberry Diets on Neuronal Stress following Exposure to (56) Fe Particles, Brain Res 2014, 1593, 9–18.
- Shukitt-Hale, B.; Lau, F. C.; Cheng, V.; Luskin, K.; Carey, A. N.; Carrihill-Knoll, K.; Rabin, B. M.; Joseph, J. A. Changes in Gene Expression in the Rat Hippocampus following Exposure to 56 Fe Particles and Protection by Berry Diets. Cent. Nerv. Sys. Agents Med. Chem. 2013, 13, 36–42. DOI: 10.2174/1871524911313010006.
- Basu, A.; Nguyen, A.; Betts, N. M.; Lyons, T. J. Strawberry as a Functional Food: An evidence-based Review. Crit. Rev. Food Sci. Nutr 2014, 54, 790–806. DOI: 10.1080/10408398.2011.608174.
- Edirisinghe, I.; Banaszewski, K.; Cappozzo, J.; Sandhya, K.; Ellis, C. L.; Tadapaneni, R.; Kappagoda, C. T.; Burton-Freeman, B. M. Strawberry Anthocyanin and Its Association with Postprandial Inflammation and Insulin. Br. J. Nutr 2011, 106, 913–922. DOI: 10.1017/S0007114511001176.
- Ellis, C. L.; Edirisinghe, I.; Kappagoda, T.; Burton-Freeman, B. Attenuation of meal-induced Inflammatory and Thrombotic Responses in Overweight Men and Women after 6-week Daily Strawberry (Fragaria) Intake. A Randomized placebo-controlled Trial. J. Atheroscl.Thromb. 2011, 18, 318–327. DOI: 10.5551/jat.6114.
- Moraes, D. P.; Lozano-Sánchez, J.; Machado, M. L.; Vizzotto, M.; Lazzaretti, M.; Leyva-Jimenez, F. J. J.; Barcia, M. T.; Ries, E. F.; Barcia, M. T. Characterization of a New Blackberry Cultivar BRS Xingu: Chemical Composition, Phenolic Compounds, and Antioxidant Capacity in Vitro and in Vivo. Food Chem 2020, 322, 126783. DOI: 10.1016/j.foodchem.2020.126783.
- Huang, W. W.; Chiu, Y. J.; Fan, M. J.; Lu, H. F.; Yeh, H. F.; Li, K. H.; Chen, P. Y.; Chung, J. G.; Yang, J. S. Kaempferol Induced Apoptosis via Endoplasmic Reticulum Stress and Mitochondria‐dependent Pathway in Human Osteosarcoma U‐2 OS Cells. Mol. Nutr. Food Res 2010, 54, 1585–1595. DOI: 10.1002/mnfr.201000005.
- Seeram, N. P.; Henning, S. M.; Zhang, Y. M.; Suchard, M.; Li, Z.; Heber, D. Pomegranate Juice Ellagitannin Metabolites are Present in Human Plasma and Some Persist in Urine for up to 48 Hours. J. Nutr 2006, 136(10), 2481–2485. DOI: 10.1093/jn/136.10.2481.
- Bodet, C.; Chandad, F.; Grenier, D. Cranberry Components inhibit IL-6, IL-8 and PGE2 production by lipopolysaccharide-activated Gingival Fibroblasts. Euro. J. Oral Sci. 2007, 115, 64–70. DOI: 10.1111/j.1600-0722.2007.00415.x.
- Huang, Y.; Nikolic, D.; Pendland, S.; Doyle, B. J.; Locklear, T. D.; Mahady, G. B. Effects of Cranberry Extracts and Ursolic Acid Derivatives on P-fimbriated Escherichia Coli, COX-2 Activity, pro-inflammatory Cytokine Release and the NF-κβ Transcriptional Response in Vitro. Pharmaceut. Bio. 2009, 47(1), 18–25. DOI: 10.1080/13880200802397996.
- Burger, O.; Ofek, I.; Tabak, M.; Neeman, I.; Sharon, N.; Neeman, I. A High Molecular Mass Constituent of Cranberry Juice Inhibits Helicobacter Pylori Adhesion to Human Gastric Mucus. FEMS Immun. Med. Microbio. 2001, 29(4), 295–301. DOI: 10.1111/j.1574-695X.2000.tb01537.x.
- de Liano, D. G.; Esteban-Fernandez, A.; Sanchez-Patan, F.; Martib-Alvarez, P. J.; Moreno-Arribas, M. V.; Bartolome, B. Anti-Adhesive Activity of Cranberry Phenolic Compounds and Their Microbial-Derived Metabolites against Uropathogenic Escherichia Coli in Bladder Epithelial Cell Cultures. Int. J. Mol. Sci 2015, 16(6), 12119–12130. DOI: 10.3390/ijms160612119.
- Denis, M. C.; Desjardins, Y.; Furtos, A.; Marcil, V.; Dudonne, S.; Montoudis, A.; Garofalo, C.; Delvin, E.; Marette, A.; Levy, E. Prevention of Oxidative Stress, Inflammation and Mitochondrial Dysfunction in the Intestine by Different Cranberry Phenolic Fractions. Clin. Sci.(Long). 2015, 128(3), 197–212. DOI: 10.1042/CS20140210.
- Maki, K. C.; Kaspar, K. L.; Khoo, C.; Derrig, L. H.; Schild, A. L.; Gupta, K. Consumption of a Cranberry Juice Beverage Lowered the Number of Clinical Urinary Tract Infection Episodes in Women with a Recent History of Urinary Tract Infection. Am. J. Clin. Nutr 2016, 103(6), 1434–1442. DOI: 10.3945/ajcn.116.130542.
- Anhe, F. F.; Pilon, G.; Roy, D.; Desjardins, Y.; Levy, E.; Marette, A. Triggering Akkermansia with Dietary Polyphenols: A New Weapon to Combat the Metabolic Syndrome? Gut Micro. 2016, 7(2), 146–153. DOI: 10.1080/19490976.2016.1142036.
- Duffey, K. J.; Sutherland, L. A. Adult Consumers of Cranberry Juice Cocktail Have Lower C-reactive Protein Levels Compared with Nonconsumers. Nutr. Rev 2015, 35(2), 118–126.
- Zhu, M.; Hu, J.; Perez, E.; Phillips, D.; Kim, W.; Ghaedian, R.; Napora, J. K.; Zou, S. Effects of Long-Term Cranberry Supplementation on Endocrine Pancreas in Aging Rats. J. Gerontol. Series A Bio. Sci. Med.Sci. 2011, 66(11), 1139–1151. DOI: 10.1093/gerona/glr105.
- Ruel, G.; Pomerleau, S.; Couture, P.; Lemieux, S.; Lamarche, B.; Couillard, C. Favourable Impact of low-calorie Cranberry Juice Consumption on Plasma HDL-cholesterol Concentrations in Men. Br. J. Nutr 2006, 96, 357–364. DOI: 10.1079/BJN20061814.
- Youdim, K. A.; McDonald, J.; Kalt, W.; Joseph, J. A. Potential role of Dietary Flavonoids in Reducing Microvascular Endothelium Vulnerability to Oxidative and Inflammatory Insults. J. Nutr. Biochem 2002, 13, 282–288. DOI: 10.1016/S0955-2863(01)00221-2.
- George, S.; Rochford, J. J.; Wolfrum, C.; Gray, S. L.; Schinner, S.; Wilson, J. C.; Soos, M. A.; Murgatroyd, P. R.; Williams, R. M.; Acerini, C. L., et al. A Family with Severe Insulin Resistance and Diabetes Mellitus Due to A Missense Mutation in AKT2. Sci. 2004, 304(5675), 1325–1328. DOI: 10.1126/science.1096706.
- Chang, W. C.; Yu, Y. M.; Chiang, S. Y., and Tseng, C. Y. Ellagic Acid Suppresses Oxidised low-density lipoprotein-induced Aortic Smooth Muscle Cell Proliferation: Studies on the Activation of Extracellular signal-regulated Kinase 1/2 and Proliferating Cell Nuclear Antigen Expression. Brit. J. Nutr. 2008. 99, 709–714.
- Wolfe, K. L.; Kang, X.; He, X.; Dong, M.; Zhang, Q.; Liu, R. H. Cellular Antioxidant Activity of Common Fruits. J. Agri. Food Chem 2008, 56, 8418–8426. DOI: 10.1021/jf801381y.
- Yi, W.; Fischer, J.; Krewer, G.; Akoh, C. C. Phenolic Compounds from Blueberries Can Inhibit Colon Cancer Cell Proliferation and Induce Apoptosis. J. Agri. Food Chem 2005, 53(18), 7320–7329. DOI: 10.1021/jf051333o.
- Abdulazeez, S. S.; Ponnusamy, P. Report: Antioxidant and Hypoglycemic Activity of Strawberry Fruit Extracts against Alloxan Induced Diabetes in Rats. Pak. J. Pharm. Sci. 2016, 29(1), 255–260.
- McCullough, M. L.; Peterson, J. J.; Patel, R.; Jacques, P. F.; Shah, R.; Dwyer, J. T. Flavonoid Intake and Cardiovascular Disease Mortality in a Prospective Cohort of US Adults. Am. J. Clin. Nutr 2012, 95(2), 454–464. DOI: 10.3945/ajcn.111.016634.
- Rosamond, W.; Flegal, K.; Furie, K.; Go, A.; Greenlund, K.; Haase, N.; Hailpern, S. M.; Ho, M.; Howard, V.; Kissela, B. Heart Disease and Stroke Statistics-2008 Update: A Report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circul. 2008, 117(4), e25–146.
- WHO. Prevention of Cardiovascular Disease: Guidelines for Assessment and Management of Total Cardiovascular Risk. In Vol. 2007; World Health Organization: Geneva, 2007, 1-30.
- Mendis, S.; Lindholm, L. H.; Anderson, S. G.; Alwan, A.; Koju, R.; Onwubere, B. J.; Kayani, A. M.; Abeysinghe, N.; Duneas, A.; Tabagari, S., et al. Total Cardiovascular Risk Approach to Improve Efficiency of Cardiovascular Prevention in Resource Constrain Settings. J. Clin. Epid. 2011, 64(12), 1451–1462. DOI: 10.1016/j.jclinepi.2011.02.001.
- Zurbau, A.; Au‐Yeung, F.; Blanco Mejia, S.; Khan, T. A.; Vuksan, V.; Jovanovski, E.; Leiter, L. A.; Kendall, C. W.; Jenkins, D. J.; Sievenpiper, J. L. Relation of Different Fruit and Vegetable Sources with Incident Cardiovascular Outcomes: A Systematic Review and Meta‐analysis of Prospective Cohort Studies. J. Am. Heart Assoc. 2020, 9(19), e017728. DOI: 10.1161/JAHA.120.017728.
- Dauchet, L.; Amouyel, P.; Dallongeville, J. Fruits, Vegetables and Coronary Heart Disease. Nat. Rev. Cardio. 2009, 6, 599–608. DOI: 10.1038/nrcardio.2009.131.
- Djousse, L.; Lee, I. M.; Buring, J. E.; Gaziano, J. M. Alcohol Consumption and Risk of Cardiovascular Disease and Mortality in Women: Potential Mediating Mechanisms. Circul. 2015, 120(3), 237–244. DOI: 10.1161/CIRCULATIONAHA.108.832360.
- Scalzo, J.; Currie, A.; Stephens, J.; McGhie, T.; Alspach, P. The Anthocyanin Composition of Different Vaccinium, Ribes and Rubus Genotypes. BioFact. 2009, 34, 13. DOI: 10.1002/biof.5520340103.
- Deayu Putri, M.; Wiboworini, B.; Dirgahayu, P. The Effect of Strawberry on Type 2 Diabetes Mellitus: A Review. Inter. J. Nutr. Sci 2020, 5(1), 1–6.
- Lee, I. T.; Chan, Y. C.; Lin, C. W.; Lee, W. J., and Sheu, W. H. Effect of Cranberry Extracts on Lipid Profiles in Subjects with Type 2 Diabetes. Diab Med. 2008, 25(12), 1473–1477.
- Erlund, I.; Koli, R.; Alfthan, G.; Marniemi, J.; Puukka, P.; Mustonen, P.; Mattila, P.; Jula, A. Favorable Effects of Berry Consumption on Platelet Function, Blood Pressure, and HDL Cholesterol. Am. J. Clin. Nutr 2008, 87(2), 323–331. DOI: 10.1093/ajcn/87.2.323.
- Feng, R.; Bowman, L. L.; Lu, Y.; Leonard, S. S.; Shi, X.; Jiang, B. H.; Castranova, V.; Vallyathan, V.; Ding, M. Blackberry Extracts Inhibit Activating Protein 1 Activation and Cell Transformation by Perturbing the Mitogenic Signaling Pathway. Nutr. Cancer. 2004, 50(1), 80–89. DOI: 10.1207/s15327914nc5001_11.
- Mazza, G.; Cacace, J. E.; Kay, C. D. Methods of Analysis for Anthocyanins in Plants and Biological Fluids. J. AOAC Int 2004, 87, 129–145. DOI: 10.1093/jaoac/87.1.129.
- Devasagayam, T. P.; Tilak, J. C.; Boloor, K. K.; Sane, K. S.; Ghaskadbi, S. S.; Lele, R. D.Free Radicals and Antioxidants in Human Health: Current Status and Future Prospects. J Assoc Phys India. 2004, 52, 794–804.[.
- Matchett, M. D.; MacKinnon, S. L.; Sweeney, M. I.; Gottschall-Pass, K. T.; Hurta, R. A. Inhibition of Matrix Metalloproteinase Activity in DU145 Human Prostate Cancer Cells by Flavonoids from Lowbush Blueberry (Vaccinium Angustifolium): Possible Roles for Protein Kinase C and mitogen-activated protein-kinase-mediated Events. J. Nutr. Biochem 2006, 17(2), 117–125. DOI: 10.1016/j.jnutbio.2005.05.014.
- Pericleous, M.; Mandair, D.; Caplin, M. E. Diet and Supplements and Their Impact on Colorectal Cancer. J. Gastroint. Oncol. 2013, 4(4), 409–423.
- Han, Y.; Huang, M.; Li, L.; Cai, X.; Gao, Z.; Li, F.; Rakariyatham, K.; Song, M.; Tomé, S. F.; Xiao, H. Non-extractable Polyphenols from Cranberries: Potential anti-inflammation and anti-colon-cancer Agents. Food Funct 2019, 10(12), 7714–7723. DOI: 10.1039/C9FO01536A.
- Hakansson, F.; Hogdall, E. V.; Nedergaard, L.; Lundvall, L.; Engelholm, S. A.; Pedersen, A. T.; Hartwell, D.; Danish, H. C. Risk of Malignancy Index Used as a Diagnostic Tool in a Tertiary Centre for Patients with a Pelvic Mass Acta Obstetricia et Gynecologica Scandinavica. Acta obstetricia et gynecologica Scandinavica.2012, 91(4), 496–502. DOI: 10.1111/j.1600-0412.2012.01359.x.