?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Leukemia is a type of dangerous cancer in humans. Many efforts have been done to find effective drugs to treat leukemia. One of the suitable options is the antioxidant molecules. Thus, the discovery of novel metabolites against cancer cells is of interest in current biomedicine. HMG-CoA Reductase activity was determined according to the method described by Takahashi S. et al. The MTT protocol was applied to determine the anti-leukemia potentials of 4-Butylresorcinol. Because of the 4-Butylresorcinol study, IC50 of HMG-CoA reductase enzyme: 182.04 micromolar was obtained, for urease IC50 was 91.44 micromolar. Molecular docking calculations were made to learn the theoretical activities of the 4-Butylresorcinol against the 3-hydroxy-3-methylglutaryl Coenzyme A (HMG-CoA) reductase reductase (PDB ID: 4I6A) and urease enzyme (PDB ID: 4H9M). Afterward, ADME/T calculations were made so that the molecule could be used as a drug. Additionally, the anticancer effects of molecule samples against the leukemia cells (KASUMI-1, HL-60, THP-1, K-562, RS4;11, and MOLT-4) were investigated. The aim of this study is to find a candidate compound for drug design that we recorded 4-Butylresorcinol as a good inhibitor and as an anticancer potential.
Introduction
These days, leukemia is one of the diseases with high death rates in the world and it affects a wide range of people of different ages and races. Chemotherapy and radiotherapy can be mentioned among the usual and common methods of leukemia treatment, which are mostly used as the chosen method of treatment in most parts of the world.[Citation1] Despite the widespread use of these methods, they have many disadvantages and complications that affect patients. One of the disadvantages of the common methods of leukemia treatment is that in these methods, both healthy and cancerous cells are affected by the drug and undergo changes, which causes the insufficient drug to reach the leukemia cells and increases the excretion of those drugs; In this way, it is necessary to increase the amount of drug injection, which will not be possible from an economic point of view and depending on the patient’s general condition and tolerance.[Citation1–3] The aim of modern medical knowledge is to minimize these problems and unpleasant side effects for leukemic patients so that they can go through the course of their illness and treatment with less pain and damage. In the last decade, following research and obtaining more information about tumors and types of leukemia, a new method called biotechnology had been proposed to treat leukemia. Recently, researchers have indicated the anti-leukemia effects of the antioxidant molecules.[Citation2,Citation3]
4-Butylresorcinol (), called 4-n-butylresorcinol, is a chemical molecule used to treatment of hyperpigmentation. Different chemicals recorded to inhibit tyrosinase production, like arbutin, hydroquinone, and kojic acid, have been discovered to be powerful inhibitors by an extensive margin.[Citation2–4]
The 27-carbon structure of cholesterol may seem complex, all carbon atoms come from a single precursor, acetate. Cholesterol synthesis consists of four steps. In the first step, acetoacetyl coenzyme A is formed by condensing acetyl coenzyme A and β-hydroxy-β-methylglutaryl-coenzyme A (HMG-CoA) is formed by combining with another acetyl coenzyme A. After this stage, mevalonic acid is synthesized from HMG-CoA thanks to the HMG-CoA reductase enzyme, the rate-limiting enzyme of cholesterol synthesis. In the third step, the formation of squalene from mevalonic acid and finally the final product cholesterol occurs. HMG-CoA reductase catalyzes the important reaction that HMG-CoA is converted to mevalonate molecule via the mevalonate molecule pathway in cholesterol molecule biosynthesis. Additionally, this enzyme is the rate-limiting enzyme that controls cholesterol molecule synthesis in the human body.[Citation5,Citation6] Indeed, inhibition of this enzyme is the major goal for lowering the serum cholesterol level. Also, statin molecules used as inhibitor compounds of the enzyme were first developed by Kurado and Endo in the 1970s. Additionally, statin molecules are drugs that play a significant role in adjusting cholesterol amounts in the body. However, long-term use of statins causes serious side effects such as myopathy, rhabdomyolysis, impaired liver enzyme functions, sexual dysfunctions and hepatotoxicity. Due to the serious side effects of statin group drugs, there is still a need for the discovery of new, effective and safer HMG-CoA reductase inhibitors.[Citation7,Citation8]
Urease is a nickel-containing hyperactive metalloenzyme that catalyzes the hydrolysis of urea to ammonia and CO2 and is responsible for an organism’s use of urea as a nitrogen source, as shown in the following reaction. Many bacteria produce urease, for example; Proteus spp., Klebsiella spp. and Staphylococcus spp. responsible for urinary tract infections. Helicobacter pylori is one of these microorganisms.[Citation9] Urease, which hydrolyses urea to form CO2 and ammonia, causes the pH to rise. As a result, it allows H. pylori to colonize effectively in the acidic environment of the gastric mucosa.[Citation10] On the other hand, urease plays an important role in the nitrogen metabolism of plants. Urease, which is found in excess in the soil, causes a rapid breakdown of urea used as fertilizer, leading to phytopathic effects and ammonia loss. Therefore, urease inhibitors, which slow down the rate of ammonia production by reducing the rate of the first hydrolysis step, provide farmers with an additional means of retaining nitrogen in their root zones, which shows agronomic and environmental benefits. Thus, they help reduce ammonia emissions into the atmosphere.[Citation11,Citation12]
Theoretical calculations are one of the quick and easy ways to predict the activities of molecules.[Citation13] With this method, the activities of molecules against enzymes were compared. Active sites of molecules can be determined by molecular docking calculations.[Citation14] With this method, more active molecules are used to synthesize. In this study, the activities of the 4-Butylresorcinol against the HMG-CoA reductase enzyme (PDB ID: 4L6A)[Citation15] and urease enzyme (PDB ID: 4H9M)[Citation16] were compared. Afterward, ADME/T (Distribution, Metabolism, Absorption, Excretion and Toxicity) calculations of the 4-Butylresorcinol were made.
Materials and methods
Enzymes
HMG-CoA Reductase activity according to the method described by Takahashi S. et al.; (Due to DL-HMG-CoA + 2NADPH + 2 H+ → (R)-mevalonate + 2NADP+ + CoASH reaction, it was measured spectrophotometrically using the absorbance change of NADPH at 340 nm.[Citation17] Reagents are: (i) HMG CoA: 0.30 mM, (ii) NADPH: 3 mm, (iii) NaN3: 2 M; (iv) Phosphate buffer: pH: 7.2; 50 mm.
After the spectrophotometer was adjusted to zero against distilled water at 340 nm, phosphate buffer, NaN3, NADPH and sample were added to the test tubes separately as blank and sample for each sample and mixed thoroughly and the change in absorbance was followed for 1 minute. Then HMG-CoA was added to the cuvette and the change in absorbance was followed for 3 minutes. HMG CoA activity was calculated using the ε coefficient of NADPH. The results are given as IU/L using the ε value of NADPH (ε: 6.22 × 103 L/mol.cm.).[Citation18]
Mobley’s[Citation19] method was used to determine urease inhibition activity. For urease enzyme inhibition activity, urea was used as a substrate after the extracts interacted with urease enzyme and ammonia formed as a result of the reaction was determined spectroscopically and this assay determined according to previous studies.[Citation20,Citation21] Thiourea compound was used as a positive control.[Citation22,Citation23]
Theoretical methods
Molecular modeling calculations to compare the activities of the compound against enzymes were calculated by the Maestro Molecular docking platform (version 12.8) by Schrödinger.[Citation24] Several preliminary preparations are required to prepare the enzymes and the 4-Butylresorcinol for calculations. For this preliminary preparation, the protein preparation module was utilized to determine the active sites of the studied proteins and to prepare the proteins for interactions. Then the LigPrep module[Citation25] was used for many conformers and flexibility of the 4-Butylresorcinol. The Glide ligand docking module was utilized to interact with the prepared molecules and enzymes. OPLS4 method was used for the preparation and interaction of compound and enzymes. Then, ADME/T analysis (distribution, absorption, excretion, metabolism, and toxicity) was performed in this work. The Qik-prop module[Citation26] of the Schrödinger software was used for this process.
Cancer study
In this work, the anticancer impacts of molecule samples against the leukemia cells (HL-60, THP-1, KASUMI-1, K-562, RS4;11, and MOLT-4) were investigated. To culture cancer cells, an RPMI medium (GIBCO, USA) was used. To provide a complete culture medium, 90 ml RPMI, 10 ml FBS (GIBCO, USA) and 1 ml penicillin/streptomycin (GIBCO, USA) were mixed. Then, the cell lines were kept in an incubator at 37 degrees Celsius, 5% CO2 and 95% humidity.
The MTT test (3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) is based on the MTT conversion into formazan crystals using living cells, which assesses mitochondrial activities. In this method, the toxicity of 4-Butylresorcinol was investigated to compare its effect on cancer cells. For this purpose, 96-well plates and about 5000 cells were cultured in each well. Cells were counted using trypan blue dye and hemocytometer slides. After the culture, the plate was incubated and after 24 hours, a new medium including different concentrations of 4-Butylresorcinol was added and re-incubation was done for 48 hours. The experiment was repeated three times for each concentration of 4-Butylresorcinol. In the next step, after removing the plates from the incubator and draining the supernatant, 20 microliters of MTT solution were added to each well and the plates were incubated for four hours at 37 degrees. In the next step, the culture medium and MTT were removed and 100 microliters of DMSO were added to each of the wells. DMSO caused the color of the produced formazan crystals to change to a purple solution, and the color intensity was evaluated by an ELISA plate reader at 570 nm wavelength. Finally, the survival rate of cells in each concentration was calculated by the following formula[Citation27]:
The control formula, it indicates the amount of optical absorption of formazan created in the negative control. Finally, by drawing a two-dimensional curve, the percentage of cell survival versus the concentration of 4-Butylresorcinol is determined, and this curve is used to calculate IC50 (the concentration at which 50% of cells are healthy and 50% of cells are dead).
Antioxidant study
DPPH (1,1-diphenyl-2-picryl-hydrazyl) is a way of measuring the antioxidant property based on electron transfer, in which a violet-colored solution is produced in ethanol and has a maximum absorption at the wavelength of 517 nm. To perform this test, DPPH powder was obtained from Sigma-Aldrich, USA. First, 0.1 mM DPPH solution was prepared in 96% ethanol, and in the next step, it was mixed with 4-Butylresorcinol and the standard antioxidant combination under BHT. After 45 minutes, the samples absorbance was read at a wavelength of 517 nm. To calculate the concentration required to inhibit 50% of antioxidant activity (IC50) for nanoemulsion and BHT, the experiment was carried out in eleven different concentrations (1–1000 µg/ml) of these two substances. Each experiment was done in three repetitions and the average values obtained were used for calculations. DPPH radical inhibition percentage was obtained by the following formula[Citation27]:
The results were evaluated as Mean ± SE using SPSS software version 12 and statistical tests of variance of completely randomized block design. Drawing graphs in Excel software was performed and the significance level of the differences was considered p < .01.
Result and discussion
Enzymes results
HMG-CoA reductase inhibitor compounds are good factors that have powerful blood cholesterol impacts and also are utilized for secondary and primary prevention in atherosclerosis. Also, studies have recorded that its mortality-reducing impacts in atherosclerosis cannot be explained solely by its lipid-lowering effects.[Citation28] For this reason, it has been shown in the studies that these agents have immunomodulatory, anti-inflammatory, corrective effects on endothelial dysfunction, and also effects on procoagulant activity and platelet functions. HMG-CoA reductase inhibitors also suppress C–reaktif protein synthesis.[Citation29,Citation30] Because of 4-Butylresorcinol study, IC50 of HMG-CoA reductase enzyme: 182.04 micromolar was obtained, for urease IC50 was 91.44 micromolar.
The use of urease inhibitor compounds in agricultural applications has long been explored as one of the mechanisms to maintain the food supply in adequate quantities.[Citation31] This is because urea, one of the most widely used nitrogen (N) fertilizers worldwide, is rapidly hydrolyzed with urease on the soil surface, resulting in a loss of 70% N to the environment.[Citation32] Ureases also cause the formation of kidney stones and infections, as well as hepatic coma. Studies on urease inhibitors are very important for the development of drugs to be used in the treatment of diseases caused by urease-containing pathogens in humans and animals.[Citation33] Urease inhibition study on natural compounds is important, and Urease activities on plants have been studied in recent years.[Citation34] For instance; Nabati et al. (2012)[Citation35] examined medicinal plants commonly used against urease activity in Iran. As a result of the study, they reported that plant extracts of Sambucus ebulus (IC50:57 µg/ml) and Rheum ribes (IC50:92 µg/ml) showed an inhibitory effect on urease enzyme. In another literature, Shabana et al.[Citation36] They investigated the urease inhibition of 125 kinds of plants and vegetables collected from Osaka, Japan, in different solvents, and reported that five samples (mung beans, pineapple, cinnamon, avocado peel and guava) showed high activity against urease in the range of 71–100%.
Molecular modeling results
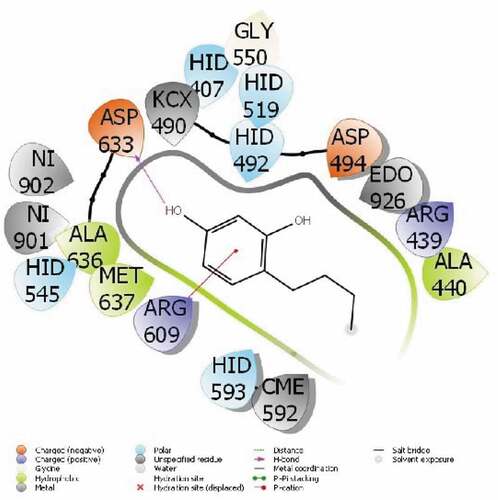
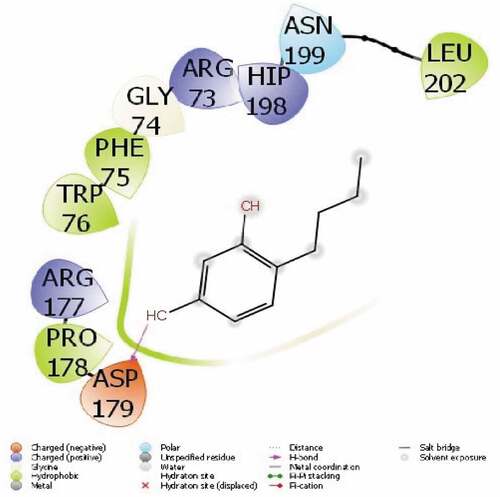
It is a common method used to compare the activity of the molecule with theoretical calculations. Among these methods, the most used and known are molecular docking calculations. With these calculations, the interactions of molecules with enzyme proteins are examined and their activities are predicted. It is predicted that as the interactions of molecules increase, their activities increase. If these chemical interactions occur; hydrogen bonds, polar and hydrophobic interactions, π-π and halogen.[Citation37] These interactions are given in . All parameters obtained as a result of this interaction are given in .
Table 1. Numerical values of the docking parameters of molecule against enzymes (Unit: Kcal/mol).
The parameter that predicts the activities of the molecules is the docking score, the molecule with the most negative numerical value. The parameter that predicts the activities of the molecules is the docking score, which is the molecule with the most negative numerical value.[Citation38] The proteins used in this study are 3-hydroxy-3-methylglutaryl Coenzyme A (HMG-CoA) reductase reductase (PDB ID: 4I6A) and urease enzyme (PDB ID: 4H9M).
The interactions between the proteins of the HMG-CoA reductase reductase enzyme and the 4-Butylresorcinol are as follows: A pi-cation interaction occurred between the ring of the 4-Butylresorcinol molecule and the ARG 609 protein in . It was observed that the H-Bound interaction occurred between the hydroxyl group attached to the ring of the 4-Butylresorcinol molecule and the ASP 633 protein of urease enzyme in . The activity of the molecule are calculated with the docking score parameter from Maestro Molecular modeling calculations. Interactions between molecule and proteins change the numerical value of the docking score. It is seen that the more chemical interactions between them increase, the more the activity of the molecules. These chemical interactions consist of many interactions, like hydrophobic interactions, polar, hydrogen bonds, π-π and halogen.
After examining the interaction of the molecule with the enzymes, the toxic effects and biological effects of the molecules should be examined for them to be used as drugs. For this, ADME/T analysis of the molecule should be performed. With this analysis, it is theoretically possible to examine all the effects molecules can have on human metabolism. All parameters found for ADME/T calculations are given in . In these parameters, the chemical properties of the molecule were examined first, and then its effects on human metabolism were examined. As a result of these calculations, RuleOfFive[Citation39] and RuleOfThree[Citation40] parameters are expected to be zero so that it can be a drug. This parameter is the condition for a molecule to be a drug.
Table 2. ADME properties of molecule.
Cancer results
Biological molecules with their emerging and unique properties, despite being widely used in industry and medicine, have adverse effects on biological systems. Various mechanisms have been proposed to justify the damaging processes of biological molecules. But in the meantime, increasing the level of ROS is more important.[Citation40,Citation41] Reactive oxygen species directly interact with DNA, protein and cellular lipids and disrupt the vital functions of cellular components such as the nucleus, mitochondria and lysosome. Antioxidants in the body’s defense system play an important role in maintaining the body’s immunity by balancing oxidation and reduction reactions against ROS.[Citation40] Oxidative stress is induced as a result of ROS overpowering antioxidants in the cell and by affecting the signaling pathways, it can lead to cell death in cancer cells. Toxicology studies on the toxic mechanisms of biological molecules are investigated in two ways: ex vivo in the laboratory environment and in vivo experiments in model animals and humans.[Citation41] The biological capability of living cells after contact with molecules changes with two general mechanisms dependent on ROS production and independent of reactive oxygen species.[Citation1,Citation41] The response intensity of cellular surfaces and biological systems is different to biological molecules, with different sizes, shapes and surface characteristics. Active species or oxygen radicals tend to react with other chemical compounds due to having a pair of free electrons. Therefore, ROS production is one of the biological molecules the cytotoxicity most important mechanisms.[Citation1,Citation40] In living cells, after exposure to biological molecules, due to chemical reactions and physical interactions of particles with cellular components involved in oxidation and reduction biological processes, reactive oxygen species with strong oxidant properties are produced at a high level. These compounds act by the oxidative attack in the cascade of biochemical changes of molecules controlling cell proliferation, inflammatory processes and cell death.[Citation1,Citation40,Citation41]
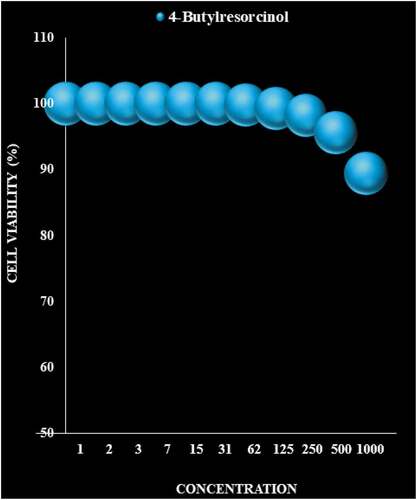
The compound of 4-Butylresorcinol was screened in vitro for their anticancer activities against leukemia cells (KASUMI-1, HL-60, THP-1, K-562, RS4;11, and MOLT-4). As can be seen, 4-Butylresorcinol exerted little toxicity on the normal cell line. The survival rate of cancer cells in the concentration of 50 μg/ml has been significantly reduced. Toxicity increases with increasing concentration, thus 4-Butylresorcinol reduce cell survival in a dose-dependent manner. The toxicity applied to cancer cells is higher than that of normal cells. In this part, between this molecule, 4-Butylresorcinol exhibited the most potent growth inhibition in the six leukemia cells (; ).
Figure 4. The anti-leukemia properties (Cell viability (%)) of 4-Butylresorcinol (Concentrations of 0–1000 µg/mL) against KASUMI-1 and HL-60 cell lines.
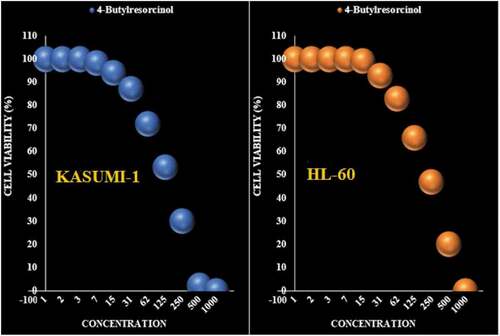
Figure 5. The anti-leukemia properties (Cell viability (%)) of 4-Butylresorcinol (Concentrations of 0–1000 µg/mL) against THP-1 and K-562 cell lines.
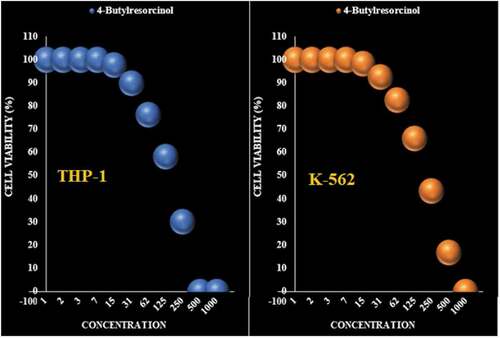
Figure 6. The anti-leukemia properties (Cell viability (%)) of 4-Butylresorcinol (Concentrations of 0–1000 µg/mL) against RS4;11 and MOLT-4 cell lines.
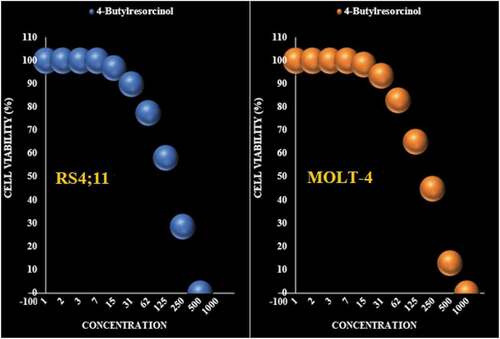
Table 3. The IC50 of 4-Butylresorcinol in the anti-leukemia test.
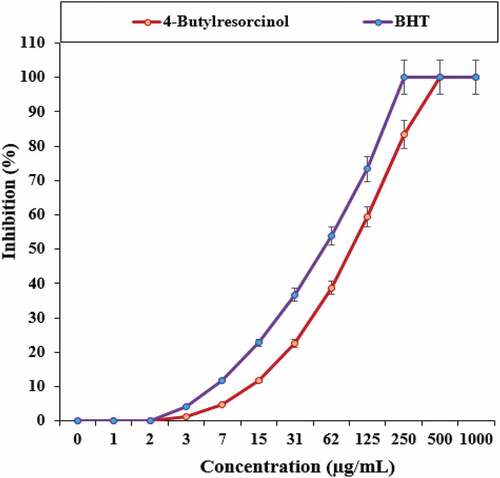
and show the activity of 4-Butylresorcinol in removing DPPH free radicals compared to BHT as a positive control. As can be seen, 4-Butylresorcinol acts in the removal of DPPH free radicals in a concentration-dependent manner; so the increase in the concentration of 4-Butylresorcinol, the activity of radical removal also increases. 50% of DPPH free radicals were inhibited at about 95 µg/ml 4-Butylresorcinol (IC50 = 95 µg/ml). At the final concentrations of 250, 500 and 1000 μg/ml, 100% of the radicals were removed.
Table 4. The IC50 4-Butylresorcinol and BHT in antioxidant test.
Conclusion
In this study, among the molecule that we successfully investigated, compound 4-Butylresorcinol had a strong an electron-withdrawing group in the phenyl ring with the highest cytotoxic activity against the cancer cell lines tested. On the other hand, the four molecule had anticancer potential, and it may used as anti- HMG-CoA reductase, and anti-urease drugs design. As a result of the theoretical calculations of the 4-Butylresorcinol molecule, by looking at the numerical values of the molecular docking parameters, comments are made on the activity of the enzymes counter-molecule. The 4-Butylresorcinol molecule has a docking score of −3.17 against the HMG-CoA reductase enzyme; on the other hand, 4-Butylresorcinol molecule has a docking score of −4.96 against the urease enzyme. However, ADME/T calculations were made to investigate the molecule’s use as a drug. These theoretical calculations will be an important guide for future in vitro and in vivo experiments.
Authors’ contributions
All authors have had the same role in preparing, designing, doing experiments, analyzing, writing, and submitting the recent manuscript.
Disclosure statement
There isn’t any conflict of Interest.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- Fuchs, D.; Daniel, V.; Sadeghi, M.; Opelz, G., and Naujokat, C.; ; , et al. Salinomycin overcomes ABC transporter-mediated multidrug and apoptosis resistance in human leukemia stem cell-like KG-1a cells. Biochem. Biophys. Res. Commun 2010, 394, 1098–1104. DOI: 10.1016/j.bbrc.2010.03.138.
- M. She, X. Niu, X. Chen, J. Li, M. Zhou, Y. He,Y. Le, K. Guo Resistance of leukemic stem-like cells in AML cell line KG1a to natural killer cell-mediated cytotoxicity Cancer Lett., 318 (2012), pp. 173–179.
- S. Neelakantan, S. Nasim, M.L. Guzman, C.T. JordanP.A., Crooks Aminoparthenolides as novel anti-leukemic agents: Discovery of the NF-kappaB inhibitor, DMAPT (LC-1) Bioorg. Med. Chem. Lett., 19 (2009), pp. 4346–4349.
- Katagiri T, Okubo T, Oyobikawa M et al. Inhibitory action of 4-n-butylresorcinol on Melanogenesis and its skin whitening effect. J Soc Cosmet Chem Jpn 2001; 35: 42– 49.
- Reiss, A. B.; Wirkowski, E. Role of HMG-CoA Reductase Inhibitors in Neurological Disorders. Drugs. 2007, 67, 2111–2120. DOI: 10.2165/00003495-200767150-00001.
- Farmer, J. A.; Torre-Amione, G. Comparative Tolerability of the HMG-CoA Reductase Inhibitors. Drug Saf. 2000 Sep, 23(3), 197–213. doi:10.2165/00002018-200023030-00003.
- Ucar, M.; Mjorndal, T.; Dahlqvist, R. HMG-CoA Reductase Inhibitors and Myotoxicity. Drug Saf. 2000 Jun, 22(6), 441–457. doi:10.2165/00002018-200022060-00003.
- Youssef, S.; Stuve, O.; Patarroyo, J. C.; Ruiz, P. J.; Radosevich, J. L.; Hur, E. M.; Bravo, M.; Mitchell, D. J.; Sobel, R. A.; Steinman, L., et al. The HMG-CoA Reductase Inhibitor, Atorvastatin, Promotes a TH2 Bias and Reverses Paralysis in Central Nervous System Autoimmune Disease. Nature. 2002Nov7, 420(6911), 78–84. DOI: 10.1038/nature01158.
- Abid, O.-U.-R.; Babar, T. M.; Ali, F. I.; Ahmed, S.; Wadood, A.; Rama, N. H.; Uddin, R.; Zaheer-ul-Haq; Khan, A.; Choudhary, M. I., et al. Identification of Novel Urease Inhibitors by high-throughput Virtual and in Vitro Screening. ACS Med. Chem. Lett. 2010, 1(4), 145–149.
- Aslam, M. A.; Mahmood, S. U.; Shahid, M.; Saeed, A.; Iqbal, J., et al. Synthesis, Biological Assay in Vitro and Molecular Docking Studies of New Schi Ff Base Derivatives as Potential Urease Inhibitors. Eur. J. Med. Chem. 2011, 46(11), 5473–5479.
- Abbas, A.; Ali, B.; Khan, K. M.; Ur Rahman, S.; Ur Rahman, S.; Perveen, S.; Perveen, S.; Perveen, S. Synthesis and in Vitro Urease Inhibitory Activity of Benzohydrazide Derivatives, in Silico and Kinetic Studies. Bioorg. Chem. 2019, 82, 163–177. DOI: 10.1016/j.bioorg.2018.09.036.
- Asadi, L.; Gholivand, K.; Zare, K. Phosphorhydrazides as Urease and Acetylcholinesterase Inhibitors: Biological Evaluation and QSAR Study. J. Iran. Chem. Soc. 2016, 13(7), 1213–1223. DOI: 10.1007/s13738-016-0836-8.
- Pyne, S.; Gayathri, P. Geometric Methods in Molecular Docking. Bioinform India J. 2005, III, 11–12.
- Girija, C. R.; Karunakar, P.; Poojari, C. S.; Begum, N. S.; Syed, A. A. Molecular Docking Studies of Curcumin Derivatives with Multiple Protein Targets for Procarcinogen Activating Enzyme Inhibition. J. Proteomics Bioinform. 2010, 3, 200–203. DOI: 10.4172/jpb.1000140.
- Steussy, C. N.; Critchelow, C. J.; Schmidt, T.; Min, J. K.; Wrensford, L. V.; Burgner, J. W.; Stauffacher, C. V.; Stauffacher, C. V. A Novel Role for Coenzyme A during Hydride Transfer in 3-hydroxy-3-methylglutaryl-coenzyme A Reductase. Biochemistry. 2013, 52(31), 5195–5205. DOI: 10.1021/bi400335g.
- Begum, A.; Choudhary, M. I.; Betzel, C. (2012). The First Jack Bean Urease (Canavalia Ensiformis) Complex Obtained at 1.52 Resolution.
- Takahashi, M.; Kusumi, K.; Shumiya, S.; Nagase, S. Plasma Lipid Concentrations and Enzyme Activities in Nagase Analbuminemia Rat (NAR. Exp Anim (Tokyo) 1983, 32, 39–46. DOI: 10.1538/expanim1978.32.1_39.
- Rosenson, R. S.;. Pluripotential Mechanisms of Cardioprotection with HMG-CoA Reductase Inhibitor Therapy. Am J Cardiovasc Drugs. 2001, 1(6), 411–420. DOI: 10.2165/00129784-200101060-00001.
- Mobley, H. L.; Hausinger, R. P. Microbial Ureases: Significance, Regulation, and Molecular Characterization. Microbiol Rev 1989, 53, 85–108. DOI: 10.1128/mr.53.1.85-108.1989.
- Arshad, M.; Jadoon, M.; Iqbal, Z.; Fatima, M.; Ali, M.; Ayub, K.; Qureshi, A. M.; Ashraf, M.; Arshad, M. N.; Asiri, A. M. Synthesis, Molecular Structure, Quantum Mechanical Studies and Urease Inhibition Assay of Two New Isatin Derived Sulfonylhydrazides. J. Mol. Struct. 2017, 1133, 80–89.
- Ahmed, M.; Qadir, M. A.; Hameed, A.; Arshad, M. N.; Asiri, A. M.; Muddassar, M. Azomethines, Isoxazole, N-substituted Pyrazoles and Pyrimidine Containing Curcumin Derivatives: Urease Inhibition and Molecular Modeling Studies. Biochem. Biophys. Res. Commun. 2017, 490(2), 434–440. DOI: 10.1016/j.bbrc.2017.06.059.
- Taha, M.; Shah, S. A. A.; Khan, A.; Arshad, F.; Ismail, N. H.; Afifi, M.; Imran, S.; Choudhary, M. I. Synthesis of 3, 4, 5-trihydroxybenzohydrazone and Evaluation of Their Urease Inhibition Potential. Arab. J. Chem. 2019, 12(8), 2973–2982. DOI: 10.1016/j.arabjc.2015.06.036.
- Rauf, A.; Shahzad, S.; Bajda, M.; Yar, M.; Ahmed, F.; Hussain, N.; Akhtar, M. N.; Khan, A.; Jończyk, J. Design and Synthesis of New barbituric-and Thiobarbituric Acid Derivatives as Potent Urease Inhibitors: Structure Activity Relationship and Molecular Modeling Studies. Bioorg. Med. Chem. 2015, 23(17), 6049–6058. DOI: 10.1016/j.bmc.2015.05.038.
- Schrödinger Release 2021-3: Maestro; Schrödinger, LLC: New York, NY, 2021.
- Schrödinger Release 2021-3: LigPrep; Schrödinger, LLC: New York, NY, 2021.
- Schrödinger Release 2021-3: QikProp; Schrödinger, LLC: New York, NY, 2021.
- Wang, W.; Elizabeth, R.; Rayburn, J. H.; Zhao, Y.; Wang, H.; Zhang, R. Anti-lung Cancer Effects of Novel Ginsenoside 25-OCH3-PPD. Lung Cancer. 2009, 65(3), 306–311. DOI: 10.1016/j.lungcan.2008.11.016.
- Schonbeck, U.; Libby, P. Inflammation, Immunity, and HMG-CoA Reductase Inhibitors: Statins as Antiinflammatory Agents? Circulation. 2004, 109(suppl 1), II18–II26. DOI: 10.1161/01.CIR.0000129505.34151.23.
- Waldman, A.; Kritharides, L. The Pleiotropic Effects of HMG-CoA Reductase Inhibitors. Drugs. 2003, 63, 139–152. DOI: 10.2165/00003495-200363020-00002.
- Chung, Y. S.; Lee, M. D.; Lee, S. K., et al. HMG-CoA Reductase Inhibitors Increase BMD in Type 2 Diabetes Mellitus Patients. J. Clin. Endocrinol. Metab. 2000, 85(3), 1137–1142.
- Mobley, H. L.; Island, M. D.; Hausinger, R. P. Microbiol. Rev. 1995, 59, 451–480.
- Khan, K. M.; Iqbal, S.; Lodhi, M. A.; Maharvi, G. M.; Zia- Ullah; Choudhary, M. I.; Atta-ur- Rahman; Perveen, M. I.; Ullah, Z.; Rahman, A.-U. Biscoumarin: New Class of Urease Inhibitors; Economical Synthesis and Activity. Bioorg. Med. Chem. 2004, 12(8), 1963–1968. DOI: 10.1016/j.bmc.2004.01.010.
- Menteşe, E.; Bektaş, H.; Sokmen, B. B.; Emirik, M.; Çakır, D.; Kahveci, B. Synthesis and Molecular Docking Study of Some 5, 6-dichloro-2-cyclopropyl-1H-benzimidazole Derivatives Bearing Triazole, Oxadiazole, and Imine Functionalities as Potent Inhibitors of Urease. Bioorg. Med. Chem. Lett. 2017, 27, 3014–3018. DOI: 10.1016/j.bmcl.2017.05.019.
- Isaac, I. O.; Al-Rashida, M.; Rahman, S. U.; Alharthy, R. D.; Asari, A.; Hameed, A.; Khan, K. M.; Iqbal, J. Acridine-based (Thio) Semicarbazones and Hydrazones: Synthesis, in Vitro Urease Inhibition, Molecular Docking and in-silico ADME Evaluation. Bioorg. Chem. 2019, 82, 6–16. DOI: 10.1016/j.bioorg.2018.09.032.
- Nabati F, Mojab F, Habibi-Rezaei M, Bagherzadeh K, Amanlou M, Yousefi B. Large scale screening of commonly used Iranian traditional medicinal plants against urease activity. Daru. 2012 Oct 31;20(1):72. DOI: 10.1186/2008-2231-20-72.
- Shabana, S.; Kawaı, A.; Kaı, K.; Akıyama, K.; Hayashı, H. Inhibitory Activity against Urease of Quercetin Glycosides Isolated from Allium Cepa and Psidium Guajava. Biosci., Biotechnol., Biochem. 2010, 74(4), 878–880. DOI: 10.1271/bbb.90895.
- Alyar, S.; Şen, T.; Özmen, Ü. Ö.; Alyar, H.; Adem, Ş.; Şen, C. Synthesis, Spectroscopic Characterizations, Enzyme Inhibition, Molecular Docking Study and DFT Calculations of New Schiff Bases of Sulfa Drugs. J. Mol. Struct. 2019, 1185, 416–424. DOI: 10.1016/j.molstruc.2019.03.002.
- Banuppriya, G.; Sribalan, R.; Padmini, V. Synthesis and Characterization of curcumin-sulfonamide Hybrids: Biological Evaluation and Molecular Docking Studies. J. Mol. Struct. March 5, 2018, 1155, 90e100. DOI: 10.1016/j.molstruc.2017.10.097.
- Lipinski, C. A.;. Lead-and drug-like Compounds: The rule-of-five Revolution. Drug Discov Today Technol. 2004, 1(4), 337–341. DOI: 10.1016/j.ddtec.2004.11.007.
- Lipinski, C. A.; Lombardo, F.; Dominy, B. W.; Feeney, P. J. Experimental and Computational Approaches to Estimate Solubility and Permeability in Drug Discovery and Development Settings. Adv. Drug Delivery Rev. 1997, 23, 3–25. DOI: 10.1016/S0169-409X(96)00423-1.
- Zochbauer-Muller, S.; Fong, K. M.; Virmani, A. K.; Geradts, J.; Gazdar, A. F.; Minna, J. D. Aberrant Promoter Methylation of Multiple Genes in non-small Cell Lung Cancers. Cancer Res. 2001, 61, 249–255.