 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Prebiotics-based encapsulation aids in improving the structure of microbeads and the survivability of probiotics. The current study focused on the exploration of a prebiotic-based encapsulation system (alginate-inulin) to improve the viability of probiotics under in vitro and carrier food. Probiotic (L. acidophilus) was encapsulated by the ionotropic gelation method. Microbeads with inulin inclusion were found to be compact and smooth with the highest encapsulation efficiency (98.87%) among the rest of the treatments. Alginate-inulin-based microbeads showed the highest count (8.41log CFU) as compared to other treatment as well free cells under simulated gastrointestinal conditions. Furthermore, alginate-inulin encapsulation maintained recommended (107–108 log CFU/ml) probiotic viability in carrier food throughout the storage period. Probiotic encapsulation aids in controlling the post-acidification of the carrier product (yogurt). The results of this study indicated that the alginate-inulin-based encapsulation system has promising potential to ensure the therapeutic number of probiotics in vitro as well in carrier foods.
Introduction
Beyond nutritional impacts, food can be deemed ‘functional’ if it has beneficial effects on humans, to improve health and wellbeing by reducing chronic diseases. Functional foods are gaining popularity around the world and are becoming an important constituent of people’s diets. Therefore, scientists and the food industry have turned their attention toward functional foods that contain positive health benefits beyond basic nutrition.[Citation1] Nowadays, probiotic-based functional foods are gaining popularity. Yogurt is one of the most important dairy-based products for researchers due to its higher nutritional profile especially its advantages to using as a carrier for probiotics.[Citation2]
Prebiotics were recognized for manipulating the microflora of the host and improving human health.[Citation3] The ultimate goal of the addition of prebiotics is to improve health and therefore, reduce the risk of diseases.[Citation4] Probiotics along with prebiotics play a significant role in the modulation of intestinal microbiota which exerts a beneficial effect on consumer health.[Citation5] The efficiency of probiotic bacteria depends on their viability except for their survival in the gut. Probiotics are sensitive to the harsh environment, i.e., low pH, bile salt, high temperature, etc. The incorporation of prebiotics increases the viability of bacteria that are passing through Gastrointestinal tract GIT.[Citation6]
Various approaches are tried to improve the probiotic resistance against the extreme surrounding conditions such as the use of micronutrients (amino acids, peptides, and micro-encapsulation). Providing probiotic live cells with a physical barrier contrary to harmful environments is a method of interest that is now gaining a lot of attention.[Citation7] Microencapsulation of probiotics also provides a physical barrier and maintains a minimum viable count of probiotics to exert positive health benefits.[Citation8]
Microencapsulation is an alternative technique for probiotic protection, which serves as a convenient microenvironment for the encapsulated microbes that enhance the viability of probiotics.[Citation9] Survival of probiotics was suppressed from various factors including acidity, pH, environmental stress, and digestive system environment.[Citation10,Citation11] Alginate is the widely used encapsulation matrix, for both food-grade and non-food applications.[Citation12] Alginate is made up of different ratios of mannuronic and guluronic acid units connected by glycosidic linkages, hence known as anionic polysaccharide.[Citation13] Lack of mechanical strength leads to poor encapsulation of alginate and lowers the effectiveness and release of encapsulated materials in stomach’s low pH are some drawbacks of the alginate hydrogel matrix.[Citation14] However, incorporation of prebiotic matrix along with alginate coating may result in better protection of probiotics in food systems as well as in GIT due to synbiosis.[Citation15]
To confirm the high level of probiotics, count for the consumers, the addition of prebiotics along with probiotics (co-encapsulation) is highly recommended. In the current study, the prebiotic matrix was used to encapsulate the probiotic to prolong their viability under hostile conditions. After the encapsulation process, the encapsulated cells of probiotics were combined in the food system to further evaluate their viability and stability.
Materials and methods
All media and chemicals including inulin and sodium alginate used in this research study were purchased from Sigma Aldrich, Germany. The probiotic strain of L. acidophilus (ATTC 4356) was obtained from the National Institute for Biotechnology and Genetic Engineering (NIBGE). For yogurt preparation, raw cow milk was purchased from the local market of Faisalabad, Pakistan.
Activation of L. acidophilus
Pure culture in freeze-dried form, L. acidophilus (ATTC‐4356) was identified by following the method of Afzaal et al.[Citation16] The probiotic culture was activated under anaerobic conditions at 37°C for 24 hr in MRS (Man Rogosa Sharpe) broth. Centrifugation at 3000 g for 15 minutes using a centrifuge machine (Thermo Fisher Scientific Inc.705276 EA, USA) was done for the collection of cells of probiotics and used further for the research study.
Encapsulation of probiotics
L. acidophilus (ATTC‐4356) was coated in sodium alginate and inulin and separately by ionotropic gelation (IG) method[Citation16] with a slight modification. Sodium alginate solution (2.0% w/v) was prepared and sterilized at 121°C for 15 min. The sterile solutions of polysaccharides (sodium alginate, sodium alginate + inulin) were combined with 2.5 mL of 1010 CFU/mL probiotic cells present in an aqueous saline solution. Afterward, 0.1 M calcium chloride in an aqueous form was added to harden the beads. Microbeads were obtained by filtration after 40–45 minutes and washed twice using distilled water. Obtained beads were stored at 4°C for further use.
Characterization of beads
Encapsulation efficiency
The encapsulation efficiency for the formed beads was calculated by using the following formula as described by Prasanna et al.[Citation17]
where, CMC is the total number of microbeads, and CAA is the total cells added to the alginate and inulin-based microbead formulation.
Cell size
Determination for the size of the capsule is done by the method described by Prasanna et al.[Citation17] Briefly, different microbeads were observed for their size by using a Vernier caliper. For this purpose, 30–35 microbeads were selected on a random basis and their mean size was calculated.
Morphology
Cell morphology including surface and cross-sectional shape of the prepared beads were observed under the scanning electron microscope (SEM). Beads were mounted by using adhesive tape under a vacuum. Observations were performed by using the microscope with magnification varying from 25× – 9000 × .[Citation18]
In vitro study for artificial gastric and intestinal conditions
The encapsulated and unencapsulated culture of L. acidophilus was evaluated for viability under the artificial stomach and intestinal conditions by following the methods of Atraki and Azizkhani[Citation19] with some minor changes. For the evaluation, under artificial gastric conditions (AGC) a solution containing aseptic saline and pepsin (2.99 g/L) was dissolved and pH was adjusted to nearly 2.0 by adding 0.5 M of HCL. The solution was poured to different test tubes and encapsulated and un-encapsulated microbeads were added accordingly in a range of 1010 CFU/mL. Tubes were examined at 0 min, 30 min, 60 min, and 120 min and the mean results were recorded as log CFU/mL.
For viability under intestinal conditions, dipotassium hydrogen phosphate (13.5 g) was used along with 0.25 M NaOH solution (150 mL), 18 g trypsin, and 1.5 g of bovine salt. Distilled water was added to make a solution and pH was then adjusted to 7.5 by adding NaOH. After that, the prepared solution was up to 1 L and the solution was added to the test tubes. Prepared microbeads and free probiotics are added accordingly in a range of 1010 CFU/mL. Test tubes were examined after 30 minutes (0 min, 30 min, 60 min, and 120 min) intervals and the mean results were documented.
Product development
Probiotic yogurt was produced by using the method adopted by Afzaal et al.[Citation16] Briefly, the Ultra High Temperature (UHT) treated milk was purchased and heated till the temperature reached 45°C. After heating, the milk was cooled to 32°C and poured in four different containers for yogurt preparation. The milk yogurt culture was inoculated with Lactobacillus Bulgaricus and Streptococcus thermophilus) at 2.5% (w/v). Then, the sample was incubated at 42–43°C till the pH level reached at ~4.5. The milk in different containers were termed as Yo (control treatment), the next treatment was prepared with the addition of free probiotics in it along with yogurt culture and termed as Y1 (yogurt with free probiotics). Y2 is the yogurt treatment prepared in third container with probiotics encapsulated with alginate coating and Y3 is the yogurt treatment made with the addition of probiotics with alginate + prebiotic matrix. Probiotic culture (L. acidophilus), free and encapsulated cells was added in it in the range of 1010 log CFU/g and the samples were incubated for 6–8 hours. The yogurt was made in airtight food-grade storage cups and stored at 4°C for 21-day interval study. The sampling was carried out on weekly basis (0, 7, 14, and 21 days) for the analysis of the viable count of L. acidophilus.
Physicochemical analysis of yogurt
The pH for yogurt treatments was measured with a digital pH meter according to the method followed by Prasanna et al.[Citation20] pH meter (Eutech instruments pH 700) was used for pH determination of yogurt.
Probiotic enumeration of yogurt
The total viable probiotic count for yogurt was determined following the guidelines described by Ghasempour et al.[Citation21] with modifications. The yogurt sample was dissolved in 9 mL of peptone water and mixed thoroughly. The prepared sample solution was poured on the surface of MRS agar and incubated at 37°C and examined after every 7th day (0, 7, 14, and 21). The final colonies obtained were calculated using a colony counter.
Sensory evaluation
A scorecard was formed by following the guidelines of the American Daily Association, which includes the parameters as flavor, texture, appearance, and overall acceptability. According to which all the samples were organoleptically rated by using 9 points (9 = like extremely; 1 = dislike extremely). For sensory evaluation, 40 trained panelists were selected, out of which 20 were males (age between 20 and 40 years) and 20 females (20–40 between 20 and 40 years). All panelists were selected from the department of Food Science and Technology. In between the sensory evaluation, the evaluators were provided with water and unsalted crackers for neutralizing the taste buds of the evaluators.[Citation22]
Statistical analysis
The data obtained from all the experiments were analyzed statistically by using Statistix 8.1 software, using two-way analysis of variance (ANOVA) along with ±standard deviation at 5% the least significant difference
Results and discussions
Encapsulation efficiency
The wall materials used for the encapsulation showed a significant effect on the encapsulation efficiency. The results shown in illustrate that the addition of inulin along with alginate significantly improves the results regarding encapsulation efficiency. As in this study, the encapsulation efficiency for the Alginate was 92% but if we add the inulin along with alginate the encapsulation yield increases significantly to 96%. It has also been reported that the coating material helps to improve the stability of probiotics even in extreme conditions. Also, the effectiveness of the coating materials was closely related to the materials used for the coating, as the encapsulation efficiency was more in the case of inulin+ alginate matrix than with sodium alginate alone. The results of this study are in line with the findings of Wu and Zhang[Citation23] who encapsulated L. plantarum with SA and arabinoxylan and found that the efficiency for both added wall materials increased from 85%. From this high yield, it has been shown that the beads formed as a result of the inulin + alginate matrix are compatible with the entrapment of probiotic Bifidobacterium bifidum. The reason behind the higher for capsules containing prebiotic matrix may be bacteria inside capsules may utilize the prebiotic matrix as its food that increases the viability of probiotic and as a result, symbiotic relation might form between probiotics and prebiotic matrix inside the capsules and resulted in higher yield as compared to other treatments
Table 1. Size and encapsulation efficiency encapsulated cells. Size of cells shown with ± standard deviation. Values are expressed as means (n = 3) followed by the same lower-case letters on the columns differ significantly by LSD at 5% probability.
The results varied for the case of alginate and the case of inulin + alginate matrix. While it has been cleared from that the addition of inulin into the alginate beads results in lowering the size of the beads significantly. Moreover, it has been illustrated that the size of beads is smaller in which inulin (prebiotic) is used along with alginate as a coating material (3.41 ± 0.04 mm), however, the results for the size of alginate beads were found to be higher (3.51 ± 0.08 mm). The reason for lower capsule size is that with the addition of inulin into the alginate beads, the viscosity of the beads increased and resulted in more tightly bonded and intact structure of capsules/beads that bounded the probiotics more tightly than simple alginate coating. The results of our studies are consistent with the finding of Darjani et al.,[Citation24] who studied that the addition of prebiotic with coating material did not increase the size of the capsules.
A cross-sectional view of the beads showed each type of bead has its distinctive structure. It had been observed that the beads with alginate showed a porous structure. While the addition of inulin into the alginate beads results in a less porous structure with comparatively low visible pores. Inulin+ alginate beads are exposed to have a more compact structure; this may be due to the reason that the inulin made a compact structure with the alginate proteins. Which results in a lower size and more compact structure. The beads compact structure with milk and alginate matrix plays an important role during digestion as it protects the bacteria from being vulnerable in the harsh and extreme environmental conditions of the stomach.
Survival of free and encapsulated L. acidophilus in simulated gastric juice (SGJ)
The safe delivery of probiotics in the human gut is important to ensure potential health benefits. For this reason, wall materials play a very important role as they provide a safety barrier and resist environmental changes.[Citation25] The mean results obtained for simulated gastric juice (SGJ), showed a significant effect of wall materials which are shown in . Maximum results were noted for encapsulated probiotics, however, the least population was calculated for free probiotics. The peak probiotic growth was noted in treatment T3 (L. acidophilus encapsulated with sodium alginate+ inulin) with a minimum log reduction of 1.85 CFU/mL. However, 2.76 CFU/mL log reduction was obtained for probiotics that were encapsulated with sodium alginate after 120 minutes. The results with free probiotics reveal a rapid decline in the probiotic population with a reduction of 9.75 log CFU/mL.
Figure 1. Viability of un-encapsulated and encapsulated (sodium alginate, sodium alginate + inulin) probiotic microgels under simulated gastric fluid conditions during storage intervals (0, 30, 60, 90, and 120 minutes) compared with control. Each line represents mean value for viability of treatments. T1 (un-encapsulated probiotics), T2 (L. acidophilus encapsulated with sodium alginate) and T3 (L. acidophilus encapsulated with sodium alginate+inulin).
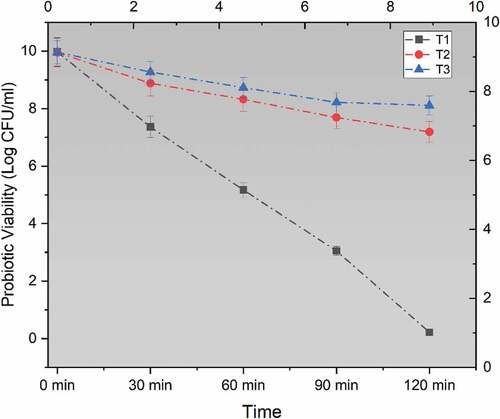
The reason behind this rapid fall in the bacterial population may depend on the fact that probiotics did not survive the severe acidic conditions of the stomach and died however sodium alginate provides a barrier against these extreme conditions. As inulin is a prebiotic thus the combination of inulin with the wall coating material showed compatibility and resulted in maximum survival of L. acidophilus. Similar studies were conducted by many researchers and a study by Wu and Zhang[Citation23] was conducted and they discover the addition of arabinoxylan along with alginate wall material results in the better viability of L. plantarum during gastric conditions. A study by Sathyabama et al.[Citation26] showed positive results for coencapsulation of different prebiotic matrix along with probiotics and suggested positive role of incorporation of prebiotic into alginate matrix during encapsulation under simulated gastric conditions. It was also suggested that the probiotics encapsulation with chicoru prebiotic matrix was more stable in intestinal conditions as well. Another study by Chavarri et al.[Citation27] concluded that co-encapsulation of probiotics with prebiotics helps the survival and enhance the probiotic enumeration even at lower gastric conditions. The fact for higher viability might be due to the inulin long chains, owing to its self-aggregation abilities, probably forms insoluble masses inside alginate matrix which that could possibly delay the penetration of H+ ions into microcapsules by blocking the pores.
Release of encapsulated L. rhamnosus in simulated intestinal fluid (SIF)
For obtaining maximum health benefits from probiotics, it is essential that they pass through the gut and reach the intestinal lining of the host and colonize there.[Citation28] The survival rate profile of probiotics differs according to the wall materials used in this study. The mean results for the viability of probiotics under SIF are shown in . A significant decline was noted for all treatments when subjected to SIF. Higher results were obtained for encapsulated probiotics however a sharp decline was obtained for non-encapsulated L. acidophilus. Maximum survivability rate was calculated for L. acidophilus that was encapsulated with sodium alginate along with inulin.
Figure 2. Viability of un-encapsulated and encapsulated (sodium alginate, sodium alginate + inulin) probiotic microgels in simulated intestinal fluid environment during storage intervals (0, 30, 60, 90, and 120 minutes) compared with control. Each line represents mean value for viability of treatments. T1 (un-encapsulated probiotics), T2 (L. acidophilus encapsulated with sodium alginate) and T3 (L. acidophilus encapsulated with sodium alginate+inulin).
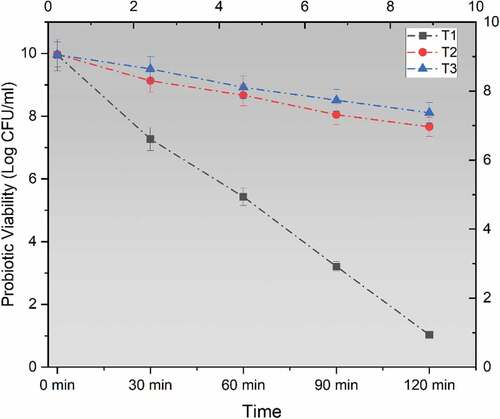
A minimum of 1.85 CFU/mL log reduction was obtained for L. acidophilus encapsulated with sodium alginate and inulin while a log reduction of 2.31 CFU/mL was noted for encapsulated L. acidophilus with sodium alginate. However, a maximum decline in the viability of free L. acidophilus was obtained and noted as 8.92 log CFU/mL at the end of 120 minutes. Rajam and Anandharamakrishnan[Citation29] in 2015 disclosed that the addition of Fructo-oligisaccharide (FOS) and whey protein isolate with coating materials results in better survival rates, however, FOS showed higher significant results. Silva et al.[Citation30] proposed that the addition of prebiotics with coatings such as alginate-gelatin and alginate-gelatin-FOS results in a better probiotic survival rate during intestinal digestion conditions. A study by Sathyabama et al.[Citation26] showed positive results for co-encapsulation and suggested that the probiotics encapsulation with chicory prebiotic matrix was more stable in intestinal conditions as well. This could be attributed to the enhancement of microcapsules structure and adhesion of bifidobacteria to starch granules via surface proteins.[Citation31] Similar findings were also reported by Chen et al.[Citation32] that suggested that co-encapsulation of probiotics along with fructo-oligosaccharides maximizes the probiotic survival by three log cycles. The effect of inulin on the enhanced survival of probiotics in high acidic environments has also been reported by Wang et al.[Citation33] Atia et al.[Citation18] reported the co-encapsulation of lactobacilli along with the addition of 50 mg/g inulin enhanced the viability and survival of probiotic bacteria in gastric and intestinal.
Physicochemical analysis
Mean results for the pH of yogurt shown in indicates their storage and a significant interaction imparted between them. The highest pH value was observed at 0 days in all treatments that decreases with time. Maximum pH was observed for yogurt with free probiotic bacteria on the 21st day (3.16 ± 0.03), respectively. However, the storage study showed the least decrease of pH in yogurt containing sodium alginate along with inulin 4.57 ± 0.01 during storage. From the results, it can be concluded that microencapsulation of probiotic bacteria along with prebiotic matrix results in the formation of a more stable product that can resist pH for a longer duration of time. The results of the present study are in line with the findings of.[Citation34]
Table 2. Effect of pH on different probiotic yogurt treatments. Values are expressed as means (n = 3) with ± standard deviation followed by the same lower-case letters on the columns that differ significantly by LSD at 5% probability.
Microbiological assay
Mean results regarding the viability of probiotic bacteria showed a significant decrease in the viable count of probiotics as time progresses during storage. From , it has been shown that the viability for Y1 (yogurt with free probiotics) was found to be the lowest at the end of the storage study due to a rapid decline of probiotics while Y2 and Y3 showed higher viability rates, respectively. However, the maximum probiotic viability was observed in the Y3 yogurt treatment that had the probiotics encapsulated with alginate together with inulin.
Figure 3. Effect of free (unencapsulated) and encapsulated (sodium alginate, sodium alginate + inulin) L. acidophilus on probiotic viability in Yogurt during storage intervals (0, 5, 10, and 15 days) compared with control. Each line represents mean value for viability of treatments. Y0 (control/ without L. acidophilus), Y1 (yogurt with free probiotics), Y2 (Yogurt having L. plantarum encapsulated with sodium alginate) and Y3 (Yogurt containing L. plantarum encapsulated with sodium alginate+inulin).
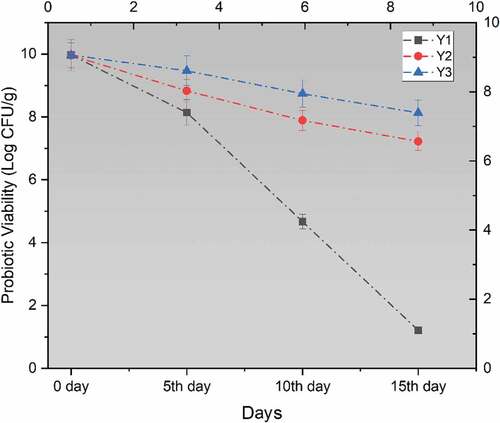
A maximum of 8.76 log CFU/g log reduction was noted in Y1 while a 1.84 log CFU/g reduction in viability was calculated for Y3 yogurt treatment. Hence, according to this research, coating materials improve the viability of probiotics in dairy products during storage.[Citation35] Qi et al.[Citation36] also suggest that the polymers enhance the chance of probiotic viability under adverse and in vitro situations.
Sensory evaluation
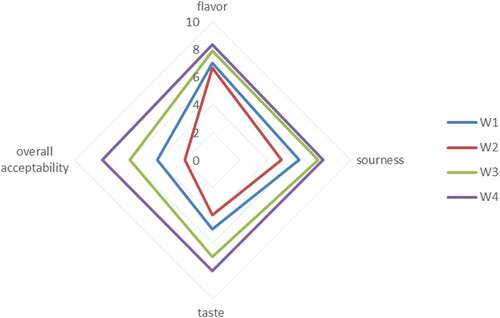
shows the mean significant results for sensory evaluation according to which it can be concluded that the yogurt sample with free probiotics showed the least acceptability in taste, flavor, sourness, and overall acceptability. However, yogurt samples with encapsulated probiotic cells showed higher acceptability scores from all sensory evaluators. From all the collected data, it had been observed that the sensory evaluation portrays an important role during the storage of all yogurt samples regarding taste, flavor, and texture. Over time, the carbohydrates present inside the yogurt were consumed by the bacteria which results in the production of organic acids which affects the organoleptic yogurt properties.
Figure 4. Effect of free (unencapsulated) and encapsulated (sodium alginate, sodium alginate + inulin) L. acidophilus on the sensory profile in Yogurt during storage intervals (0, 5, 10, and 15 days) compared with control. Each line represents mean value for viability of treatments. Y0 (control/ without L. acidophilus), Y1 (yogurt with free probiotics), Y2 (Yogurt having L. plantarum encapsulated with sodium alginate) and Y3 (Yogurt containing L. plantarum encapsulated with sodium alginate+inulin).
Conclusion
In the recent study, L. acidophilus was encapsulated using wall material (sodium alginate) along with prebiotic (inulin) to determine the effect of probiotic viability under simulated gastric and intestinal conditions. High encapsulation efficiency was obtained for probiotic cells having inulin with wall material with better overall structural stability. The cells showed better viability when subjected to simulated in vitro environments. Probiotic viability cells containing inulin in coating material showed better probiotic viability during storage. Hence, microencapsulation of probiotic bacteria along with prebiotic matrix is an efficient way to prolong the viability of probiotics to obtained potential health benefits.
Ethical approval
The study does not involve any human or animal testing.
Acknowledgments
Authors are thankful to Government College University, Faisalabad, for providing research and literature collection facilities. Authors are also thankful to Kampala International University, Kampala, for providing part of the facilities used in the research.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
The data that support the findings of this study are available from the corresponding author, upon request.
References
- Munekata, P. E.; Pateiro, M.; Zhang, W.; Dominguez, R.; Xing, L.; Fierro, E. M., and Lorenzo, J. M. Health Benefits, Extraction and Development of Functional Foods with Curcuminoids. J. Funct. Foods. 2021, 79, 104392. DOI: 10.1016/j.jff.2021.104392.
- Savaiano, D. A.; Hutkins, R. W. Yogurt, Cultured Fermented Milk, and Health: A Systematic Review. Nutr. Rev. 2021, 79(5), 599–614. DOI: 10.1093/nutrit/nuaa013.
- Li, R., Wang, Y., YiMing, L., HaiSu, S., Xu, C., Zhao, Y., & JunRui, W. Effect of adding different prebiotics on free amino acids and flavor of yogurt. Shipin Kexue / Food Sci. 2020, 41(20), 83–89. DOI: 10.7506/spkx1002-6630-20190903-03.
- Hadrich, J.; Wolf, C. A.; Lombard, J., and Dolak, T. M. Estimating Milk Yield and Value Losses from Increased Somatic Cell Count on US Dairy Farms. J. Dairy Sci. 2018, 101(4), 3588–3596. https://www.sciencedirect.com/science/article/pii/S0022030218300389 doi:10.3168/jds.2017-13840.
- Jokari, M. M.; Jafarpour, D. Comparison of the Effectiveness of Two Prebiotics Inulin and Green Banana Flour on the Survival of Lactobacillus Plantarum and Bacillus Coagulans in low-calorie Synbiotic Yogurt. Food Sci. Technol. 2021, 18(117), 49–63. DOI: 10.52547/fsct.18.117.49.
- Soh, J. I. X.; Wilian, M.; Yan, S. W. Inulin Enhances Nutritional, Sensorial and Technological Characteristics of Synbiotic Yogurt Drink. Br. Food J. 2021, 123(7), 2571–2581. DOI: 10.1108/BFJ-11-2020-1044.
- Kailasapathy, K. Encapsulation Technologies for Functional Foods and Nutraceutical Product Development. CABI Rev. 2009, 2009, 1–19. DOI: 10.1079/PAVSNNR20094033.
- Champagne, C. P.; Fustier, P. Microencapsulation for the Improved Delivery of Bioactive Compounds into Foods. Curr. Opin. Biotechnol. 2007, 18(2), 184–190.
- Li, H.; Liu, T.; Yang, J.; Wang, R.; Li, Y.; Feng, Y.; Liu, D.; Li, H., and Yu, J. Effect of a Microencapsulated Synbiotic Product on Microbiology, Microstructure, Textural and Rheological Properties of Stirred Yogurt. LWT. 2021, 152, 112302. DOI: 10.1016/j.lwt.2021.112302.
- Krasaekoopt, W.; Watcharapoka, S. Effect of Addition of Inulin and Galactooligosaccharide on the Survival of Microencapsulated Probiotics in Alginate Beads Coated with Chitosan in Simulated Digestive System, Yogurt and Fruit Juice. LWT Food Sci. Technol. 2014, 57(2), 761–766. DOI: 10.1016/j.lwt.2014.01.037.
- Hussein, Z. E. H.; Silva, J. M.; Alves, E. S.; Castro, M. C.; Ferreira, C. S. R.; Chaves, M. L. C.; da Silva Bruni, A. R., and Santos, O. O. Technological Advances in Probiotic Stability in Yogurt: A Review. Res. Soc.Dev. 2021, 10(12), e449101220646–e449101220646. DOI: 10.33448/rsd-v10i12.20646
- Dimitrellou, D.; Kandylis, P.; Lević, S.; Petrović, T.; Ivanović, S.; Nedović, V., and Kourkoutas, Y. Encapsulation of Lactobacillus Casei ATCC 393 in Alginate Capsules for Probiotic Fermented Milk Production. LWT. 2019, 116, 108501. DOI: 10.1016/j.lwt.2019.108501.
- Abka-khajouei, R.; Tounsi, L.; Shahabi, N.; Patel, A. K.; Abdelkafi, S., and Michaud, P. Structures, Properties and Applications of Alginates. Mar. Drugs. 2022, 20(6), 364.
- Nayak, A. K.; Pal, D. Development of pH-sensitive Tamarind Seed polysaccharide–alginate Composite Beads for Controlled Diclofenac Sodium Delivery Using Response Surface Methodology. Int. J. Biol. Macromol. 2011, 49(4), 784–793. DOI: 10.1016/j.ijbiomac.2011.07.013.
- Rashidinejad, A.; Bahrami, A.; Rehman, A.; Rezaei, A.; Babazadeh, A.; Singh, H., and Jafari, S. M. Co-encapsulation of Probiotics with Prebiotics and Their Application in functional/synbiotic Dairy Products. Crit. Rev. Food Sci. Nutr. 2022, 62(9), 2470–2494. DOI: 10.1080/10408398.2020.1854169
- Afzaal, M.; Saeed, F.; Saeed, M.; Ahmed, A.; Ateeq, H.; Nadeem, M. T., and Tufail, T. Survival and Stability of Free and Encapsulated Probiotic Bacteria under Simulated Gastrointestinal Conditions and in Pasteurized Grape Juice. J. Food Process. Preserv. 2020, 44(3), e14346.
- Prasanna, P.; Charalampopoulos, D. Encapsulation of Bifidobacterium Longum in alginate-dairy Matrices and Survival in Simulated Gastrointestinal Conditions, Refrigeration, Cow Milk and Goat Milk. Food Biosci. 2018, 21, 72–79. DOI: 10.1016/j.fbio.2017.12.002.
- Atia, A.; Gomaa, A.; Fliss, I.; Beyssac, E.; Garrait, G., and Subirade, M. A Prebiotic Matrix for Encapsulation of Probiotics: Physicochemical and Microbiological Study. J. Microencapsulation. 2016, 33(1), 89–101.
- Atraki, R.; Azizkhani, M. Survival of Probiotic Bacteria Nanoencapsulated within Biopolymers in a Simulated Gastrointestinal Model. Innovative Food Sci. Emerg. Technol. 2021, 72, 102750. DOI: 10.1016/j.ifset.2021.102750.
- Prasanna, S.R.V.S., Balaji, K., Pandey, S., & Rana, S. Metal oxide based nanomaterials and their polymer nanocomposites, in Nanomate. polym. nanocomposites. Karak, N. 2019, Elsevier. 123–144. https://doi.org/10.1016/B978-0-12-814615-6.00004-7
- Ghasempour, Z.; Javanmard, N.; Mojaddar Langroodi, A.; Alizadeh-Sani, M.; Ehsani, A., and Moghaddas Kia, E. Development of Probiotic Yogurt Containing Red Beet Extract and Basil Seed Gum; techno-functional, Microbial and Sensorial Characterization. Biocatal. Agric. Biotechnol. 2020, 29, 101785. DOI: 10.1016/j.bcab.2020.101785.
- Larmond, E.;. Laboratory Methods for Sensory Evaluation of Food; Ottawa, Ontario, Canada: Research Branch, Canada Department of Agriculture, 1977.
- Wu, Y.; Zhang, G. Synbiotic Encapsulation of Probiotic Latobacillus Plantarum by alginate-arabinoxylan Composite Microspheres. LWT. 2018, 93, 135–141. DOI: 10.1016/j.lwt.2018.03.034.
- Baral, K. C., Bajracharya, R., Lee, S. H., & Han, H. K. (2021). Advancements in the Pharmaceutical Applications of Probiotics: Dosage Forms and Formulation Technology. International journal of nanomedicine, 16, 7535–7556. https://doi.org/10.2147/IJN.S337427.
- Baral, K. C., et al. Advancements in the Pharmaceutical Applications of Probiotics: Dosage Forms and Formulation Technology. Int. J. Nanomed. 2021, 16, 7535. DOI: 10.2147/IJN.S337427.
- Sathyabama, S.; Vijayabharathi, R.; Bruntha Devi, P.; Vijayabharathi, R.; Brindha Priyadharisini, V. Co-encapsulation of Probiotics with Prebiotics on Alginate Matrix and Its Effect on Viability in Simulated Gastric Environment. LWT Food Sci. Technol. 2014, 57(1), 419–425. DOI: 10.1016/j.lwt.2013.12.024.
- Chávarri, M.; Marañón, I.; Ares, R.; Ibáñez, F.C.; Marzo, F.; and Villarána, M. C. Microencapsulation of a Probiotic and Prebiotic in alginate-chitosan Capsules Improves Survival in Simulated gastro-intestinal Conditions. Int. J. Food Microbiol. 2010, 142(1–2), 185–189. https://doi.org/10.1016/j.ijfoodmicro.2010.06.022.
- Sudheer, S.; Gangwar, P.; Usmani, Z.; Sharma, M.; Sharma, V. K.; Sana, S. S.; Almeida, F.; Dubey, N. K.; Singh, D. P.; Dilbaghi, N., et al. Shaping the Gut Microbiota by Bioactive Phytochemicals: An Emerging Approach for the Prevention and Treatment of Human Diseases. Biochimie. 2022, 193, 38–63. DOI: 10.1016/j.biochi.2021.10.010.
- Rajam, R.; Kumar, S. B.; Prabhasankar, P., and Anandharamakrishnan, C. Microencapsulation of Lactobacillus Plantarum MTCC 5422 in Fructooligosaccharide and Whey Protein Wall Systems and Its Impact on Noodle Quality. J. Food Sci. Technol. 2015, 52(7), 4029–4041.
- Silva, K. C. G.; Cezarino, E. C.; Michelon, M., and Sato, A. C. K. Symbiotic Microencapsulation to Enhance Lactobacillus Acidophilus Survival. LWT. 2018, 89, 503–509. DOI: 10.1016/j.lwt.2017.11.026.
- Crittenden, R.; Morris, L. F.; Harvey, M. L.; Tran, L. T.; Mitchell, H. L., and Playne, M. J. Selection of a Bifidobacterium Strain to Complement Resistant Starch in a Synbiotic Yoghurt. J. Appl. Microbiol. 2001, 90(2), 268–278.
- Chen, K. N.; Chen, M.-J.; Liu, J.-R.; Lin, C.-W., and Chiu, H.-Y. Optimization of Incorporated Prebiotics as Coating Materials for Probiotic Microencapsulation. J. Food Sci. 2005, 70(5), M260–M266.
- Wang, L.; Yu, X.; Xu, H.; Aguilar, Z. P., and Wei, H. Effect of Skim Milk Coated inulin-alginate Encapsulation Beads on Viability and Gene Expression of Lactobacillus Plantarum during freeze-drying. LWT Food Sci. Technol. 2016, 68, 8–13. DOI: 10.1016/j.lwt.2015.12.001.
- Qian Li, Hongyi Lin, Jing Li, Lu Liu, Jialu Huang, Yi Cao, Tiantian Zhao, David Julian McClements, Jun Chen, Chengmei Liu, Jiyan Liu, Peiyi Shen, Mengzhou Zhou. Improving probiotic (Lactobacillus casei) viability by encapsulation in alginate-based microgels: Impact of polymeric and colloidal fillers. Food Hydrocolloids, 2023, 134(108028). https://doi.org/10.1016/j.foodhyd.2022.108028.
- Homayouni, A.; Azizi, A.; Ehsani, M. R.; Yarmand, M. S., and Razavi, S. H. Effect of Microencapsulation and Resistant Starch on the Probiotic Survival and Sensory Properties of Synbiotic Ice Cream. Food Chem. 2008, 111(1), 50–55.
- Li, Q., et al. Improving Probiotic (Lactobacillus Casei) Viability by Encapsulation in alginate-based Microgels: Impact of Polymeric and Colloidal Fillers. Food Hydrocolloids. 2022, 108028.
