ABSTRACT
Chia and basil seeds have comparable nutritional profiles, bioactive components, and phytochemistry, which make it difficult for consumers and researchers to choose between the two. Thus, an in-depth study was conducted to compare the physiochemical, nutritional and bioactive profile of these seeds in order to obtain complete database regarding nutritional and phytochemical profiling of both seeds. The wonder seeds were analyzed for physico-chemical composition, fatty acid constituents, amino-acid profile and phytochemical screening. Fatty acid constituents were determined using GC-MS, while amino acids profile and identification and quantification of phytochemicals were performed using HPLC procedure. Physico-chemical evaluation of basil and chia seed revealed that basil was found superior to chia in terms of dietary fiber (40.85 g/100 g), fat content (33.10 g/100 g), vitamin A (1583 µg/100 g) and E (779 µg/100 g) while chia seed was rich in protein (21.54 g/100 g), iron (7.7mg/100 g), magnesium (335 mg/100 g) and phosphorus (860 mg/100 g). Amino acid profiling revealed the presence of high-quality protein in chia seeds comprising of almost all essential and non-essential amino acids in significant (p < .05) amounts as compared to basil seeds. Furthermore, higher proportion of α-linoleic acid (ALA) was detected in basil seeds (24.4g/100 g) as compared to chia seeds (5.25 g/100 g) as determined by GC-MS. Phytochemical screening showed that basil contained significantly (p < .05) higher amounts of total phenolic (17.66mgGAE/g of dry sample) and flavonoid content (0.57mgQE/100 g of dry sample) in comparison to chia seeds, which contributed to its higher anti-oxidant activity. Phenolic characterization revealed that basil seeds contained higher concentration of caffeic acid (4780.01 µg/g) followed by gallic acid (2330 µg/g). The inference drawn from the study concludes that both seeds can be put forth for enrichment of various food items or can be directly consumed as functional foods.
Introduction
Nutraceutical and functional foods play a vital role in alleviating many lifestyle-oriented diseases such as diabetes, obesity and cardiovascular diseases. Now-a-days a considerable interest in products of plant origin has arisen due to the presence of copious amount of health-promoting constituents. In particular, an immense interest has shifted toward medicinal seeds because of their rich biochemistry and phytochemistry, and prophylactic properties.[Citation1] Thus, they are considered as wonder foods/new gold or super nutrients and termed as super seeds, the seeds of the 21st century. Consuming these basil and chia seeds can alleviate various dreaded diseases such as obesity, cardiovascular disease, diabetes and some types of cancers.[Citation2] The rich bioactive composition of these super seeds (chia and basil seeds) is responsible for such health promoting effects. These bioactive compounds include, e.g., polyphenols, carotenoids, phytoestrogens, sterols, stanols, vitamins, dietary fiber, fatty acids, probiotics, prebiotics, and bioactive peptides.
Chia (Salvia hispanica), derived from the Nahuati word “chian” which means oily, is one such super seed belonging to flowering herbaceous plant of family Lamiaceae.[Citation3] The seed is flat, oval shaped and white to brownish in color and contains high proportions of the essential fatty acid, α-linolenic acid, which is associated with maintaining healthy cholesterol level, brain development and immune system. Besides rich fatty acid profile, it contains significant amount of dietary fiber, and the seed exudes a mucilaginous polysaccharide when placed in aqueous medium[Citation4]which promotes digestion. Therefore, both chia seeds and its mucilage are nutrient-rich food sources. .[Citation5] Moreover, chia is believed to be free of toxins, allergens and other anti-nutritional factors. The profuse amount of bioactive compounds in chia makes it as a suitable nutritional supplement. Besides, chia is also approved as a Novel Food by the European Parliament and Council of Europe in 2009.
Basil (Ocimum basilicum), mucilaginous endemic plant, is another herbaceous plant of mint family (Lamiaceae). Basil seeds, black ellipsoidal seeds, are often regarded as ‘king of herbs,’[Citation6] and are commonly added to beverages and ice cream besides being used as whole seed or milled into flour for use in bakery products so as to improve their flavor, nutritional status and textural characteristics as basil seeds have high content of hydrocolloids. Furthermore, it is considered as an essential ingredient in preparation of various delicacies due to the characteristic flavor it imparts.[Citation7] Besides exuberant flavor, basil seeds have been consumed from older times as functional food because of the many phytochemicals they contain, which provide significant nutritional and health benefits. Indian system of medicine use basil seeds for treatment of stomachache, vomiting, and urinary problems.[Citation8] In addition, it provides considerable amount of α-linolenic acid (ALA). Seed oil is also known to have strong antioxidant activity and has anticancer, antiviral and antimicrobial properties.[Citation6] On hydration, the black ellipsoid basil seed produces a gelatinous mass called mucilage. This mucilage has emulsifying, foaming, thickening, stabilizing, viscosity, and gelling properties.[Citation9]
These were earlier classified as a non-conventional seed, however, a pragmatic increase in consumption of these super seeds (chia and basil) has been observed due to their considerable nutritional composition consequent beneficial effects on human health. Some of most important applications of the chia and basil seeds include their use as nutritional supplements and as an ingredient in cereal bars, biscuits, pasta, bread, snacks and yogurt, among others that include their use even in cake formulations. Several research works have reported incorporation of these wonder seeds (chia and basil seeds) in different food products in order to increase their nutrition status.[Citation10,Citation11]
Although both chia and basil seeds are readily available in any departmental stores and super markets for direct consumption but the relatively similar nutritional profile, bioactive constituents and phytochemistry of these seeds make it difficult for the consumers and researchers to select among the two. Many studies have individually characterized chia and basil seeds for nutritional and biochemical aspects.[Citation8,Citation12] However, none of the research work has compared chia and basil seeds for nutritional, phytochemical and biochemical properties. Therefore, owing to the unexplored nutritional and economic potential of chia and basil seeds in the food industry, it was deemed important to conduct an in-depth study on the complete physiochemical, nutritional and bioactive profile of these seeds. Therefore, the present study was undertaken with an aim to characterize both the seeds for their lipid profile, amino acid composition, phytochemical screening, vitamins and mineral content in order to simplify the selection process for health-conscious consumers besides providing a database for researchers working on food fortification and development of functional foods.
Material and methods
Basil seeds (Ocimum basilicum var. thyrsiflora) of Himalayan origin were procured from farms of District Ganderbal, Jammu and Kashmir (J&K), India, located at 33.9606° N and 74.69° E. Chia seeds (Salvia hispanica L.) are not cultivated in J&K, therefore the seeds were procured from Utter Pradesh (UP) state of India. Chia is grown in some of the farms of UP (Amseruva and Siddhaur). In sandy soil at an altitude of 400 m above sea level having coordinates 26.76° N and 81.39° E. The chemicals used in the study were of analytical reagent grade and were purchased from Sigma.
Physico-chemical analysis of chia and basil seeds
Proximate composition
Moisture, protein, fat and ash contents were determined according to the standard methods laid down by AOAC.[Citation13] Carbohydrate was calculated by subtraction method by as per the following equation.[Citation12]
Carbohydrate (%) = 100 – (% moisture +% fat+ %protein + %Total dietary fiber + %ash).
Dietary fiber content
Dietary fiber content of seed samples was determined using Dietary Fiber system (fibraplus DF). Enzymatic Gravimetric method was followed to estimate the dietary fiber content of seed samples.[Citation13]
Mineral profile
Mineral estimation was done after dry ashing of samples using atomic absorption spectrophotometer (Labtronics, Model LT-2100). Total phosphorus was determined spectrophotometrically by a pH adjustment method. Acid oxidation of samples was carried out using mixture of ammonium molybdate and ammonium vanadate reagent followed by absorbance measurement at 440 nm.[Citation14,Citation15]
Vitamin analysis
Standard AOAC[Citation13] procedures were followed for estimation of Vitamin A, E, C and B complex. Vitamin A was determined spectrophotometrically by treating samples with acetic anhydride reagent and the absorbance was recorded at 620 nm after interval period of 15 and 30 seconds. 2,6-dichlorophenol indophenol was followed for estimation of ascorbic acid content of seed samples. Absorbance of the samples was spectrophotometrically recorded at 520 nm. Tocopherol content was spectrophotometrically analyzed by treating seed samples with dipyridyl and recording the absorbance at 520 nm. Thiamine, Riboflavin and niacin contents were analyzed using HPLC (YounglinR 930D) in accordance with the procedure given by Marzougui et al.[Citation16] A two pump reverse phase HPLC equipped with control panel, injector port of 20 µl and ultraviolet absorbance detector (254 mm) was employed for determination of thiamine, riboflavin and niacin content in chia and basil seeds. A Eurospher 100C-18.5 column with dimensions 250 ×4.6 mm and internal dia 5µm was used for vitamin determination. A mixture of methanol (9%), crystalline acetic acid (10 ml) and distilled water was used as mobile phase. Solvent added to sulfonic acid pentane and sulfonic acid octane was deaerated through agitation followed by filtration through 45 µm Teflon filter. Afterward, the solvent is pumped across the column with a constant flow rate between 0.5 and 2.1 ml/min. The obtained peaks were calculated using electronic integrator and developed into a chromatogram.
Fatty acid profile
For fatty acid estimation, standard AOCS[Citation17] was followed. Oil of the seeds was extracted by grounding seeds to fine powder and treating it with nonpolar solvent in Soxhlet apparatus AOAC.[Citation13] Saponifiable lipids were extracted by treating 100 ml of oil with 1 mL of 10% KOH in methanol. Non-saponifiable lipids were extracted with petroleum ether and later saponified with HCl. Extracted oil was used for quantification of different fatty acids (in the form of methyl esters) via gas chromatography-mass spectrometry (Shimadzu GCMS-QP2010 Plus). The suspension was then heated for 30 min at 85°C before injecting in GC-MS column. Fatty acids were transformed into methyl esters for quantification purpose. For methylation purpose, fatty acids extract was treated with 1 mL of boron triflouride (20% solution in methanol) for 45 min at 60°C. Fatty acid was quantified in GC-MS by injecting 1 mL hexane solution containing fatty acid methyl esters into the column.[Citation18] Temperature of the column was set around 140–260°C to carry out the separation. Helium as carrier (107.4 kPa) was flown at the rate of 4°C/min and FID was kept at 280°C. The m/z value was set from 40 to 650 for the mass spectrometric analysis. Identification of fatty acids was done by comparing retention times (RT) of fatty acids with those of authentic standards while for quantification, peak to area was determined and results were expressed in relative percentages (g/100 g) of each individual fatty acid .[Citation19]
Amino acid composition
Amino acid composition was quantified by reversed-phase HPLC after hydrolysis with 6 N HCl at 110°C under nitrogen for 24 h. Derivatization was done with AccQ-Fluor reagent kit (WAT052880-Waters Corporation, USA). To 20 μL of hydrolyzed samples, AccQ-Fluor borate buffer (60 μL) was added and samples were vortexed. After that, 20 μL of AccQ-Fluor reagent was added and again samples were vortexed for 30 seconds. The treated samples in vials were heated to 55°C before separation of amino acids. Separation of amino acids was done by injecting 10 μ L of sample into reversed phase AccQ Tag Silica-bonded Amino Acid Column C 18. The mobile phase used was AccQ Tag Eluent A diluted to 10% in Milli-Q water and 60% acetonitrile in a separation gradient with a flow rate of 1.0 mL/min. The amino acids were detected using photo diode array (PDA) detector at 254 nm with the column condition set at 37°C.[Citation20] A set of amino acid standards (Merck Germany) were also analyzed and peaks obtained were evaluated for quantification of amino-acids. A standard calibration curve at five concentrations 20, 40, 60, 80 and 100 mg/ml having 2.5 μ mol/ml of L-forms of Asp, Glu, Ser, Gly, Thr, Arg, Ala, Tyr, Val, Met, Phe, Ile, Leu, Lys, Csy, Tryp, Pro, His and 1.25 μ mol/ml of cystine in 0.1 N HCl were developed for calculating the concentration of amino acids in seeds. Amino acid assignments were visually checked to verify the peak assignment. Injections (10 μL) of 10, 20, 30, 40 and 50 mg/ml of amino acid standard produces a calibration curve. The proportional molar concentration for each amino acid was calculated based on the concentration of standard amino acids and expressed as mg amino acid/100 g sample. Identification of the amino acids in the samples was carried out by comparison with the retention times of the standards.[Citation21]
Phytochemical screening
Extraction of antioxidant compounds
Ground samples were dissolved with 80% ethanol[Citation22] and placed on shaker overnight. Afterward, the extracts were recovered by centrifugation at 6000 rpm for 15 min. The prepared seed extracts were used for estimation of total phenolic and total flavonoid content, characterization of phenolic compound and antioxidant activity.
Total phenolic content
Folin-Ciocalteu method as described by Gao et al.[Citation23] was followed for estimation of phenolic content. A known volume of Folin-Ciocalteu reagent (1.5 mL) was added to 200 μL of extract and afterward, 1.5 mL of sodium carbonate solution was added after an incubation period of 5 min. Then, absorbance of the solution was measured at 725 nm after a gap 0 f 90 min. Results were expressed as mg of gallic acid equivalents (GAE) per gram of sample (extract in dry weight).
Total flavonoid content
Flavonoid content was determined according to Yermakov et al.[Citation24] A known volume of extract (0.05 mL) (100 mg/mL) was treated with 1 mL of 2% aluminum trichloride (AlCl3) in ethanol. The absorption was read at 400 nm at 37°C. Flavonoid content was expressed in terms of mg of quercetin per gram of sample (extract in dry weight).
Determination of anti-oxidant activity
% DPPH inhibition
Antioxidant activity was estimated by DPPH radical scavenging activity. The DPPH radical scavenging activity was determined according to Singleton et al.[Citation25] About 3.9 mL of 105 mol/L DPPH solution was added to 100 μL of extract (100 mg/mL). Absorbance of the mixture was measured at 515 nm, after a resting period of 30 min. Antioxidant activity of seed extracts was measured as percent (%) inhibition of DPPH. Linear Regression analysis was used to calculate IC50 values of both seed extracts.
Ferric reducing antioxidant potential (FRAP)
Ferric reducing power of chia and basil samples was carried out according to the method reported by Dudonne et al.[Citation26] The working solution was prepared by mixing 1 volume of 10 mM TPTZ in 40 mM HCl with 1 volume of 20 mM FeCl3.4 H2O and 10 volumes of 300 mM acetate buffer, pH 3.6. A volume of 900 lL of the working solution was mixed with 90 lL of distilled water and 30 lL of the sample extract. The mixture was maintained at 37°C for 30 min and the absorbance was read at 595 nm. A standard curve was prepared by plotting the absorbance against concentration of Trolox (300–1500 lM). The results were expressed in µmolQE/g dw.
Identification and quantification of phenolic compounds
For quantification and identification of phenolic and flavonoids constituents (Gallic acid, caffeic acid, chlorogenic acid, ferulic acid, quercetin, kaempferol, epicatechin, rutin and p-coumaric acid) in the crude extracts of seed samples, procedure of high-performance liquid chromatography (HPLC) laid down by Martinez-Cruz and Parades-lopez[Citation27] was followed. Agilent 1290 infinity LC system (Agilent Technologies, Santa Clara, CA) equipped with a binary pump, a degasser and automatic purge valve, an autosampler, thermostatted column compartment and diode array detector was used for separation, identification and quantification of seed phenolic and flavonoids. ZORBAX RRHD Eclipse Plus C18 column with dimensions of 1.8 m ×50 mm × 2.1 mm i.d. (Agilent Technologies, Santa Clara, CA) were employed to carry out the separation process. For efficiency separation of phenolic and flavonoid constituents, binary mobile phase consisting of solvent A (2% acetic acid in water) and solvent B (2% acetic acid, 30% acetonitrile and68% water) was used. Prior to separation, all the solvents were filtered through a 0.45 membrane. Solvent were run in the HPLC system by following the gradient program: 0–4 min, 0–10% B; 4–6 min, 10–15% B; 6–8 min,15–40% B, 8–14 min, 40–100% B and finally 2 min, 0% B for equilibration of the column for the next run. Rate of solvent flow was kept constant at 0.4 mL/min for a total run time of 18 min. Equilibration was done by running column at initial conditions for 2 min. Chia and basil seed extract diluted to an injection volume of 1 µL, was fed to the head of column and absorbance was measured at 280, 325 and 260 nm. Standards were prepared in 80% ethanol and later diluted using distilled water to give serial concentrations in a range of 0.0004125–0.1650 mg/mL for phenolic acids, and for flavonoids standards in a range of 0.0001–0.1 mg/mL. HPLC system generated standard curve were prepared using the peak areas (uv. s, y-axis) of different concentrations (mg/mL, x-axis), and were expressed by the linear least-squares regression equation. All measurements were performed in triplicate for each assay, and the results were expressed as the µg/g of samples. Quantification and identification of phenolics in chia and basil seeds extract were determined by comparing retention time (RT) and area (A) under the peaks in chromatograms with RT and A of peaks characteristics for elution of ethanolic standards.
Statistical analysis
Experiments were conducted in triplicate and results presented are average of three replications ± standard deviation. Statistical significance of physico-chemical and anti-oxidant potential was determined by Students t-test using SPSS software. Mean values were compared by Duncan’s Multiple Range test at p <.05 level of significance
Results and discussion
Physico-chemical properties of chia and basil seeds
Proximate composition of chia and basil seeds is shown in . Moisture content of chia and basil seeds was recorded as 6.33 g/100 g and 8.50 g/100 g respectively. Chia seeds recorded significantly (p <.05) higher protein (21.54 g/100 g) and carbohydrate content (10.27 g/100 g) while significantly (p <.05) higher ash (5.40 g/100 g) and fat content (33.10 g/100 g) was recorded in basil seeds. Plant-based foods that provide more than 12% of their calorific value in protein are considered to be remarkable suppliers of protein.[Citation6 A significantly (p <.05) lower dietary fiber content (37.47 g/100 g) of chia seed as compared to basil seeds was recorded. Marineli et al.[Citation28] also reported lower protein and higher dietary fiber values for basil seeds as compared to chia seeds. The insoluble dietary fiber (IDF) and soluble dietary fiber (SDF) content of basil seeds were recorded as 21.68 g/100 g and 19.17 g/100 g respectively. Lignin, hemicellulose and cellulose constitute the fiber content of basil. Basil seeds recorded significantly (p <.05) higher SDF content while higher IDF content was recorded in chia seeds. SDF content of these super seeds is due to mucilaginous capsule formed when the seeds are soaked in water. Neutral sugars mainly constitute SDF which indicates the presence of diverse carbohydrates that form the structure of the mucilage.[Citation27] Ratio of IDF:SDF of basil seed is around 1.13 which is under the prescribed range (1.0–2.3) for fibers in order to exert the desirable physiological effects of both soluble and insoluble fractions. Thus, fiber composition of basil is in good proportion in order to deliver beneficial effects. Fat content of basil seed (33.10 g/100 g) was significantly (p <.05) higher than chia seeds (20.09 g/100 g). Basil seed oil is a rich source of polyunsaturated fatty acids (71.84%).[Citation29] Similar values for protein, ash, fat and fiber were reported by Kaur and Bains[Citation30] for chia seeds and Nazir and Ahmed[Citation8] for basil seeds.
Table 1. Physico-chemical composition of Chia and basil seeds.
Mineral and Vitamin analysis of chia and basil seeds
illustrates different minerals present in chia and basil seeds. Minerals form the inorganic constituents of plant materials and are considered vital for overall health and wellbeing of humans despite accounting for only 4% to 6% of the body weight. Macronutrients such as phosphorous, potassium, calcium, magnesium, and sodium acts as structural components of tissues, and function in the cellular and basal metabolism, and water and acid-base balance while micro-nutrients-viz-iron, zinc, manganese, copper and iodine are very important for hormones, vitamins, and enzyme activity.[Citation29] Chia reported significantly (p <.05) higher iron, magnesium, phosphorus, sodium and zinc while calcium, potassium, copper and manganese content of chia was statistically at par with that of basil seeds. Relatively similar mineral composition of chia and basil seeds resulted in its non-significant difference in ash content (). Calcium, potassium, copper and manganese content of chia was recorded as 631 mg/100 g, 407 mg/100 g, 0.9 mg/100 g and 2.7mg/100 g respectively. Chia and basil are high calcium, potassium and relatively low sodium crops as compared to certain nuts and dried fruits.[Citation31] Calcium and potassium content of basil seeds was recorded as 636 mg/100 g and 481 mg/100 g respectively. Magnesium content of chia and basil seeds were recorded as 335 mg/100 g and 31.55 mg/100 g respectively. According to the Food and Nutrition Board,[Citation32] the daily requirements of calcium, magnesium, and potassium for an adult are 310–400, 1000, and 2600–3400 mg/day, respectively. Calcium, phosphorus and magnesium content of chia seeds was sufficient to meet 70% of their RDA while basil seeds can supply 100% of the Ca, around 50% of Mg, and around 20% of K according to the requirements.[Citation32] In the present study, iron content was recorded as 7.7 mg/100 g for chia seeds and 2.27 mg/100 g for basil seeds. Concentration of iron found in chia is higher than that in liver and 2.23 times more than spinach.[Citation3] Thus, chia seeds can be explored as an option for treating of anemia in women due to its copious iron content. Similar values for mineral content were reported by Kulczynski et al.[Citation33] for chia seeds and Bravo et al.[Citation6] for basil seeds.
Vitamin content of chia and basil seeds is listed in . A significant (p <.05) difference in vitamin A, E, C, niacin and folate content of chia and basil seeds were recorded. Vitamin content of chia seeds was recorded as 54 µg/100 g while basil seeds recorded significantly (p <.05) higher vitamin A content (1583 µg/100 g). Vitamin A plays an essential role in normal vision, gene expression, growth and immune function and maintenance of epithelial cell functions. Vitamin E content of chia and basil seeds were recorded as 512 µg/100 g and 779 µg/100 g respectively. Nazir and Ahmad[Citation8] also reported that basil seed oil comprises of around 0.21% of γ-tocopherol. Basil seeds (1837 µg/100 g) recorded significantly (p <.05) higher ascorbic acid content as compared to chia seeds (1612 µg/100 g). Basil seeds recorded relatively higher thiamine (640 µg/100 g) and riboflavin content (380 µg/100 g)and significantly (p <.05) lower niacin (72 µg/100 g) and folate content (68 µg/100 g). Chia seeds are reported to meet the 50% of RDA for niacin and folate content.[Citation31] Niacin content (8839 µg/100 g) of chia seeds is higher than corn, soybeans and rice, while thiamine (607 µg/100 g) and riboflavin content (211 µg/100 g) of chia seed was found to be similar to rice and corn.[Citation29] Kulczynski et al.[Citation33] also reported similar values of thiamine, riboflavin and niacin in chia seeds. Fatty acid profile of chia and basil seeds
compares the composition of saturated, monounsaturated, polyunsaturated and omega-3 fatty acids of chia and basil seeds. A significantly (p <.05) higher proportions of saturated, monounsaturated and polyunsaturated fatty acids were recorded in basil seeds as compared to chia seeds. Significant (p <.05) difference in lipid content of chia and basil seeds might have resulted in statistical difference in their fatty acid profile. Chia seeds recorded significantly (p <.05) lower lauric, palmitic, steric, oleic, linoleic, linolenic and arachidic acids as compared to basil seeds. α-linoleic acid (ALA) is the major fatty acid in both seed oil however, basil seeds (51.5g/100 g) contained significantly (p <.05) higher percentage of ALA as compared to chia seed (19.55 g/100 g). ALA is believed to be a precursor of omega-3 PUFA eicosapentaenoic acid (EPA) and docosahexaenoic acid (DPA) fatty acids.[Citation6] These are categorized as essential fatty acids because of the inability of human body to produce them. EPA and DHA are necessary for normal working of brain besides being associated with several other health benefits.
Table 2. Fatty acid profile of chia and basil seeds.
From , it is evident that basil seeds contained predominantly unsaturated fatty acids followed by monounsaturated fatty acid and saturated fatty acid. The dominant saturated fatty acid found in basil seeds was palmitic acid. Bravo et al.[Citation6] also showed that palmitic and stearic acid as the major saturated fatty acid in basil. Furthermore, basil seeds recorded significantly (p <.05) higher percentage of omega-3 fatty acids as compared to chia seeds. Omega-3 fatty acids are essential for normal functioning of brain cells. Nazir and Ahmed[Citation8] also recorded higher concentration of alpha-linolenic acid, palmitic acid and stearic acid in basil seeds. PUFA such as linoleic, linolenic and arachidic fatty acid plays a major role in prevention of cardiovascular diseases and other health related ailments.
Amino-acid profile of chia and basil seeds
A comparison of amino acid profile of chia and basil seeds is depicted in . It was evident that all essential as well as non-essential amino acids were detected in chia seeds thereby depicting its excellent protein quality. Olivos-Lugo et al.[Citation34] reported that chia protein contains rich amino acid profile in comparison to other grains. All the amino acid present in chia seeds were found to be significantly (p <.05) higher than those present in basil seeds. Presence of almost all the essential and non-essential amino acids in chia seeds resulted in significantly (p <.05) higher protein in chia seeds (21.54 g/100 g) as compared to basil seeds (9.40 g/100 g) (). The predominant amino acid recorded in chia seed was glutamic acid followed by arginine while methionine and histidine were found to be limiting ones. Bueno et al.[Citation35] also reported that glutamic acid and arginine forms the major amino-acid while lysine and threonine are the limiting amino acids in chia seeds. Major amino acids recorded in basil seeds were arginine and glutamic acid while serine was found to be the limiting one. Furthermore, basil seeds were found to be deficient in tryptophan and cysteine. Similar amino acid profile of basil seeds was reported by Bravo et al.[Citation6]
Table 3. Amino acid profile of chia and basil seeds.
Gluten forming amino acids were recorded low in chia seeds () which makes chia a desirable functional food for patients suffering from celiac. Campos et. al.[Citation36] also reported gluten free nature of chia seeds. Methionine and cystine content of chia seed was found to be 0.59 g/100 g and 0.41 g/100 g respectively. Chia seeds contain higher proportion of essential sulfur containing amino-acids than certain cereal grains.[Citation37] The results for amino acid profile of chia seeds were in concomitance with those reported by Kulczynski et al.[Citation33] and Munoz et al.[Citation4]
Phytochemical analysis and anti-oxidant activity of chia and basil seeds
depicts the total phenolic, total flavonoid content and anti-oxidant activity of chia and basil seeds. Total phenolic and flavonoid content of chia seeds was significantly (p <.05) lower than basil seeds. Chia seeds recorded 1.65mgGAE/g and 0.35mgQE/g of total phenolic and flavonoids content respectively while significantly (p <.05) higher total phenolic content of basil seed was recorded (17.466mgGAE/g). Total phenolics content of basil seed is higher than raspberry and strawberry which are known for their rich phenolic composition.[Citation35] Phenolics are present as free or bonded to sugars by glycosidic linkages, which increases their solubility in water but decreases their availability. This might be the reason for low phenolic content of chia seed.
Table 4. Phytochemical screening of chia and basil seeds.
Anti-oxidant activity
A number of compounds with potent anti-oxidant activity have been identified in chia and basil seeds. Among them, the most important are phenolic compounds, ascorbic acid and tocopherols. These compounds are primary and synergic antioxidants and make a proportionally greater contribution to the antioxidant activity of chia and basil seeds. Basil seed recorded significantly (p <.05) higher DPPH radical scavenging activity (47.01%) and Ferric iron reducing anti-oxidant power (FRAP) (70.93 µmol trolox equivalent/kg of dry sample) as compared to chia seeds. Higher amount of phenolics (17.66mgGAE/g) and flavonoids (0.57 mgQE/g) in basil seeds might have resulted in its increased anti-oxidant activity as phenolic compounds such as gallic acid, chlorogenic acid have much stronger antioxidant properties than that of vitamin C (ascorbic acid) and vitamin E (α-tocopherol). Anti-oxidant activity recorded in basil seeds and chia seeds through DPPH radical scavenging method was comparatively low as compared to FRAP method. This may be attributed to the difference in anti-oxidant molecular size and difference in their abilities to mitigate peroxyl radicals and reduce DPPH free radical and ferric ion from the solution .[Citation38]
IC50, the anti-oxidant capacity of sample, measures the minimum concentration of samples required to inhibit 50% of DPPH free radicals in DPPH free radical scavenging method. IC50 value is inversely proportional to anti-oxidant property of sample.[Citation39] Basil seeds (2.14 mg/ml) recorded significantly low IC50 value as compared to chia seed (3.61 mg/ml). Thus, further validating the higher anti-oxidant potential of basil seeds in comparison to chia seeds. Scapin et al.[Citation40] also reported similar IC50 values for chia seed extract. Samples with IC50 values ranging from 10–50 mg/ml exhibit strong anti-oxidant activity while IC 50 values of 50–100 mg/ml implies that the samples possess intermediate anti-oxidant activity.[Citation41]
Phytochemical screening of chia and basil seeds
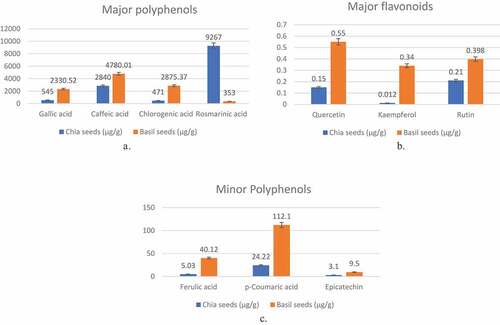
Major phenolic and flavonoid compounds identified and quantified in basil and chia seeds are depicted in . Phenolic compounds, widely distributed throughout animal kingdom, are the secondary metabolites found in plant. revealed that basil seeds consisted of significantly (p <.05) higher concentration of major phenolic compounds such as gallic acid (2330.52 µg/100 g), caffeic acid (4780.01 µg/100 g) and chlorogenic acid(2875.37 µg/100 g) while rosmarinic acid (9267.01 µg/100 g) was found in abundance in chia seeds. Cruz and Lopez[Citation42] also reported that rosmarinic acid was the major phenolic compound identified in chia seed extract. Several biological activities have been described for rosmarinic acid such as antioxidant, astringent, anti-inflammatory, antithrombotic, antimutagen, antibacterial and antiviral.[Citation41] Caffeic acid concentration in chia and basil seeds was found to be around 2840 µg/g and 4870 µg/g of seed weight respectively which is higher than some of the commonly consumed fruits such as mango (0.0077 mg/g), papaya (0.0159 mg/g), and blueberry (0.0216 mg/g).[Citation40] Martinez-Cruz and Parades-lopez[Citation27] also reported 0.0274 mg/g of caffeic acid in chia seed. Caffeic acid besides acting as potent antioxidant and enzyme inhibitor also exhibits significant binding activity with specific receptors. Moreover, caffeic acid is believed to inhibit LDL (low density lipoprotein) oxidation and thus protect against cardiovascular diseases. Concentration of gallic acid in chia and basil seeds were recorded as 545 µg/g and 2330.52 µg/g respectively which exceeds that of blueberry (0.0000028 mg/g). Higher percentage of gallic acid in basil seeds as compared to chia seeds resulted in its higher anti-oxidant activity. Chlorogenic acid content of chia seeds and basil seeds were found to be around 471 µg/g and 2875.37 µg/g respectively, which was comparable to that of tea polyphenols.[Citation4] Chlorogenic acid protect against free radicals and inhibit the peroxidation of fats. Among minor phenolics, basil seed recorded higher amount of p-coumaric acid (112.1 µg/100 g) followed by ferulic acid (40.12 µg/100 g) as compared to chia seeds. represents major flavonoids of chia and basil seeds determined through GC-MS. The quercetin, kaempferol and rutin content of basil was recorded as 0.55 µg/100 g, 0.34 µg/100 g and 0.398 µg/100 g which were higher than that found in chia seeds. The inference drawn from the phytochemical analysis of both the seeds concluded that basil seeds can be preferred functional food choice and can further be explored for development of nutraceuticals ().
Conclusion
The research work was based on nutritional and phytochemical characterization of basil and chia seeds. It concluded that basil was an excellent source of dietary fiber and α-linoleic acid while chia comprises high-quality protein than basil. In addition to rich unsaturated, fatty acid composition, basil also reported outstanding phytochemical composition. Furthermore, basil seeds contained higher proportion of phenolic and flavonoids, which yielded its enhanced anti-oxidant activity. Based on the results from physico-chemical composition, fatty acid constituents, amino-acid profile and phytochemical screening, it can be concluded that basil seeds can be an important non-conventional functional food source and can be used for development of nutraceuticals to be used in human diet while chia can be a viable functional food option for celiac patients to fulfil their nutritional requirements besides being an excellent nutritional supplement. However, a study to compare the functional behavior of these two wonder seeds can be conducted in future in order to determine which of the two seeds is better suited for incorporation into different food products in order to alleviate their nutritional status.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Author contribution
Tabeen Jan – Formal analysis and writing original draft
Tabasum Fatima – Visualization
Tahiya Qadri – Review and editing
Asima Rafiq – Resources and Project administration
Ajaz Malik - Resources
Bazila Naseer – Validation
Syed Zameer Hussain - Supervision
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Njeru, N. S.; Matasyoh, J.; Mawaniki, C. G.; Mwendia, K. G.; Kobia, J. A review of some phytochemicals commonly found in medicinal plants. Int. J. Med. Pl. 2013, 105, 135–140.
- Marcinek, K.; Krejpcio, Z. Rocz Panstw Zakl Hig. Roczniki Panstwowego Zakladu Higieny. 2017, 68(2), 123–129.
- Ullah, R.; Nadeem, M.; Khalique, A.; Imran, M.; Mehmood, S.; Javid, A. Nutritional and Therapeutic perespectives of chia (Salvia hispanica L.): A review. Jfst. 2016, 53(4), 1750–1758.
- Munoz, L. A.; Cobos, A.; Diaz, O.; Aguilera, J. M. Food Res. Int. 2013, 29, 394–408.
- Salgado-Cruz, M. D. L. P.; Beltrán-Orozco, M. D. C.; Cedillo, L. D. C. VII Congreso Nacional de Ciencias de los Alimentos. Ed. Revista Salud Pública y Nutrición: Guanaguato, Mexico, 2005.
- Bravo, C.; Céspedes, H. V.; Zura-Bravo, L.; Muñoz, L. A. Basil Seeds as A Novel Food, Source of Nutrients and Functional Ingredients with Beneficial Properties: A Review. Foods. 2021, 10(7), 1467. DOI: 10.3390/foods10071467.
- Agarwal, C.; Sharma, N. L.; Gaurav. S.s. Ind. J Fund. App. Life Sci. 2013, 3, 521–525.
- Nazir, S.; Ahmed, N. physico-chemical characterization of basil (Ocimum basilicum L.) seeds. J App. Res. Med. Aro. Pl. 2021, 22, 100295.
- Kim, S. Y.; Hyeonbin, O.; Lee, P.; Kim, Y. S. The Quality Characteristics, Antioxidant Activity, and Sensory Evaluation of reduced-fat Yogurt and Nonfat Yogurt Supplemented with Basil Seed Gum as a Fat Substitute. J. Dairy Sci. 2020, 103(2), 1324–1336. DOI: 10.3168/jds.2019-17117.
- Romankiewicz, D.; Hassoon, W. H.; Cacak-Pietrzak, G.; Sobczyk, M. B.; Wirkowska-Wojdyła, M.; Ceglińska, A.; Dziki, D. The effect of chia seeds (Salvina hispanica L.) addition on quality and nutritional value of wheat bread. J. Food Qua. 2017. DOI: 10.1155/2017/7352631.
- Ziemichód, A.; Wójcik, M.; Różyło, R. CyTA J. Food. 2019 Ocimum tenuiflorum seeds and Salvia hispanica seeds: mineral and amino acid composition, physical properties and use in gluten free bread , 17, 804–813.
- Barreto, A. D.; Gutierrez, E. M. R.; Silva, M. R.; Silva, F. O.; Silva, N. O. C.; Lacerda, I. C. A.; Labanca, R. A.; Araújo, R. L. B. Basil Seeds as A Novel Food, Source of Nutrients and Functional Ingredients with Beneficial Properties: A Review. Am. J Pl. Sci. 2016, 7(7), 2323–2337. DOI: 10.4236/ajps.2016.715204.
- AOAC International Official method of analysis. Agricultural Chemicals, Contaminants. Drugs. Gaitherburg: AOAC International. 2012, 16(1), 24–36.
- Wani, S. A.; Kumar, P. Developemnt and parameter optimization of health promising extrudates based on fenugreek, oats and pea. Food Biosci. 2016, 14(1), 34–40.
- Okalebo, J. R.; Gathua, K. W.; Woomer. P.l; Tropical Soil Biology and Fertility Programme: Nairobi, 1993.
- Marzougui, N.; Guasmi, F.; Mkaddem, M., Assessment of Tunisian Trigonella foenum graecum diversity using seed vitamin B6, B1, B9 and C contents. J Food, Agri. Environ. 2009, 7(1), 56–61.
- AOCS Official Method. Determination of composition of the sterol faction of animal and vegetable oils and fats by TLC and capillary GLC. In Methods and Recommended Practices of the AOCS; Sixth edition. Firestone, D.; Ed.; Champaign, IL: AOCS Press, 1997; 1–6.
- Shen, Y.; Zhen, L.; Ji, J.; Li, X.; Fu, J.; Wang, M.; Guan, Y.; Song, X. Phytochemical and Biological Characteristics of Mexican Chia Seed Oil. Molecules. 2018, 23(12), 3219. DOI: 10.3390/molecules23123219.
- Terán, C.; Wilman, M. C.; Carlos, C.; Mario, A.; Silva, M. As. J. Pharma Clin Res. 2018, 11. DOI: 10.22159/ajpcr.2018.v11i2.17096.
- Bhat, M. H.; Fayaz, M.; Kumar, A.; Dra, A. A.; Jain, A. K. Pharma. Sci. 2019, 25, 65–69.
- Hikmawanti, N. P. E.; Fatmawati, S.; Asri, A. W. IOP Conf. Ser.: Earth Environ. Sci. 2021, 755(1), 012060.
- Dhillon, A.; Kumar, M.; Gujar, P. A common HPLC -PDA method for amino acid composition in insects and plants. Indian J Exp. Bio. 2014 , 52, 73–77.
- Gao, L.; Wang, S.; Oomah, B. D.; Mazza, G. Wheatnquality: Anti-oxidant activity of wheat milstreams. In Wheat Quality Elucidation; Ng, P.; Wrigley, C. W.; Eds.; AACC International: St. Paul, M.N, 2002; 219–233.
- Yermakov, A. I.; Arasimov, V. V.; Yarosh, N. P. Method of biochemical analysis of plant (In Russian). Agropromizdat Leningrad. 1987, 2, 122–142.
- Singleton, V.; Orthofer, R.; Lamuela, R. Analysis of total phenols, oxidation substrates and anti-oxidants by means of folin-ciocalteu reagent. Methods in Enzymol. 1999, 299, 152–178.
- Dudonne, S.; Vitrac, J.; Coutiere, P.; Woillez, M.; Merillon, J. M. Comparative Study of Antioxidant Properties and Total Phenolic Content of 30 Plant Extracts of Industrial Interest Using DPPH, ABTS, FRAP, SOD, and ORAC Assays. J Agri. Food Chem. 2009, 57(5), 1768–1774. DOI: 10.1021/jf803011r.
- Martinez-Cruz, O.O; Parades-lopez. J Chroma. A. 2014, 1346, 43–48.
- Marineli, R.; daLenquiste, S. A.; Moraes, E. A.; Marostica, M. R. Food Res. Int. 2015, 76, 666–674.
- Caudillo, E. R.; Tecante, A.; Valdivia, M. A.; Lopez, L. dietary fibre content and anti-oxidant activity of phenolic compounds present in Mexican chia (Salvia hispanica L.) seeds. Food Chem. 2008, 107(2), 656–666.
- Kaur, S.; Bains, K. Nutri. Food Sci. 2019, 50(3), 463–479.
- Pachkore, G.; Dhale, D. Int. Sci. Innov. Discov. 2012, 2, 201–207.
- FoodandNutrition Board.Recommended Dietary Allowances and Adequate Intakes. Daily intake of Nutrients. 1997, 1, 21–33.
- Kulczynski, B.; Kobus-Cisowska, J.; Taczanowski, M.; Kmiecik, D.; Gramza-Michałowska, A. The Chemical Composition and Nutritional Value of Chia Seeds—Current State of Knowledge. Nutrients. 2019, 11(6), 1242. DOI: 10.3390/nu11061242.
- Olivos-Lugo, B. L.; Valdivia-Lopez, M. A.; Tecante, A. Thermal and physicochemical properties and nutritional value of the protein fraction of Mexican chia seed (Salvia hispanica L.). Food Sci.Technol. Int. 2010, 16(1), 89–96.
- Bueno, M.; Di Sapio, O.; Barolo, M.; Busilacchi, H.; Quiroga, M.; Severin, C. Quality tests of Salvia hispania L. (Lamiaceae) fruits marketed in the city of Rosario (Santa Fe province, Argentina). Boletin Latinoamericano yDel Caribe de Plantas Medicinales y Aromaticas. 2010, 9(3), 221–227.
- Campos, M. R. S.; Guerrero, L. A.; Ruelas, C. A. F.; Ancona, B. D. A. Chia seeds (Salvia hispanica L.). Nutritional and health benefits of edicinal plants. Springer: Boston, MA, 2016 .
- Paredes-Lopez, O.;.; Cervantes-Ceja, M. L.;.; Vigna-Pe´rez, M.;.; Hern´andez-P´erez, T. Pl Foods Human. Nutri. 2010, 65, 299–308.
- Shahinuzzaman, M.; Yaakob, Z.; Anuar, F. H.; Akhtar, P.; Kadir, N. H. A.; Hasan, A. K. M.; Sobayel, K.; Nour, M.; Sindi, H.; Amin, N., et al. Sci. Rep. 2020, 10, 10852.
- Jadid, N.; Hidayati, D.; Hartanti, S. R.; Arraniry, B. A.; Rachman, R. Y.; Wikanta, W. Anti-oxidant activities of different solvent extracts of Piper retrofractum Vahl. using DPPH assay. AIP Conference Proceedings, Indonesia. 2017, 1854.
- Scapin, G.; Schmidt, M. M.; Dornelles, P.; Rosa, R.; Claudia, N. Phenolics compounds, flavonoids and antioxidant activity of chia seed extracts obtained by different extraction conditions. Food res. Int. 2016, 23(6), 2341–2346.
- Phongpaichit, S.; Nikom, J.; Rungjindamai, N.; Sakayaroj, J.; Hutadilok-Towatana, N.; Rukachaisirikul, V.; Kirtikara, K. Biological activities of extracts from endophtic fungi isolated from garcinia plants. FEMS Immunol. Med. Microbiol. 2007, 51(3), 517–525. DOI: 10.1111/j.1574-695X.2007.00331.x.
- Cruz, M. O.; Lopez, P. O. Phytochemical Profile and Nutraceutical Potential of Chia Seeds (Salvia Hispanica L.) by Ultra High Performance Liquid Chromatography. J Chroma. A. 2014, 1346, 43–48. DOI: 10.1016/j.chroma.2014.04.007.