?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Fortification of edible oil with retinyl palmitate (vitamin A) is a significant advancement to fulfill the nutritional deficiency of major population. The growing interest in the enrichment of foods warrants a simple and accurate method to analyze fortified products. This study developed an easy and reliable strategy based on normal-phase HPLC analysis to determine the edible oil’s retinyl palmitate. We also validated the method according to the International Conference on Harmonization (ICH) guidelines. The analysis was performed with a DB silica column (250 cm × 4.6 mm; 5 μm), with a mobile phase composed of n-heptane and isopropyl alcohol at a ratio of 75:25 v/v and a flow rate of 1.0 ml/min. The detection was performed at a wavelength of 326 nm, and the retention time of vitamin A was around 8 min, with a total run time of 10 min. The calibration plot gave a linear relationship (R2 = 0.9998) over the concentration range of 3.93–63 μg/ml. The LOD and LOQ were 0.029 and 0.096 μg/ml, respectively. The accuracy of the proposed method was determined by recovery studies and was found with a mean recovery of >95%. RSD% of the determination of precision was <2%. The results of robustness and solutions stability studies were within acceptable limits. The proposed method showed excellent linearity, accuracy, precision, specificity, robustness, LOD, LOQ, and system suitability results within the acceptable criteria.
Introduction
Vitamin A is considered one of the most vital elements of our regular diet.[Citation1] The sources of vitamin A are usually different colored foodstuffs containing beta carotene, consisting of two retinol molecules.[Citation2] Deficiency of vitamin A may lead to several diseases, including impaired vision, cellular differentiation, anemia, lower resistance to infections, and even death. In fact, vitamin A deficiency is a severe health risk and concern in developing and impoverished nations. Approximately 44–50% of under 5 children in South Asia are affected by severe vitamin A deficiency.[Citation3] Malnutrition and severe vitamin A deficiency induced death among neonates and under 5 children in India and Bangladesh constituted one-third of the global mortality rate.[Citation4] According to the reports of the WHO NLiS country profile, the sub-clinical vitamin A deficiency among pre-school age children was highest in Pakistan (51.5%) followed by Afghanistan (50.4%), Bangladesh (20.5%), India (17.6%), and Nepal (11.11%).[Citation5] Even with numerous vitamin A supplementation and dietary changes, this health issue could not be solved entirely.[Citation6] Fortification of vegetable oil is one of the cheapest and easiest strategies to combat vitamin A deficiency. Two commercial forms of vitamin A are available as food fortificants, e.g., retinyl palmitate and retinyl acetate. Among them, retinyl palmitate was found to be more stable in vegetable oils.[Citation7] Many countries have chosen the edible oil fortification technique as a convenient medium to deliver vitamin A.[Citation8] In order to decrease the frequency of vitamin A inadequacy, many developing nations have made it essential to fortify cooking oils with vitamin A. For example, the fortification status of cooking oils must be 1.5–3.0 mg/100 g, 20 IU/g, and 33 IU/g of oil in Bangladesh, India, and Pakistan, respectively.[Citation7,Citation9] In Bangladesh, vegetable oil is considered one of the most convenient vehicles of vitamin A fortification due to several advantages, e.g., centrally processed, widely distributed, and highly consumed per day.[Citation10] After that, the Government of Bangladesh passed a relevant law, National Edible Oil Fortification Law, 2013,[Citation11] to initiate and implement the vitamin A fortification program. Determination of vitamin A has always been of great interest and concern to food sciences, pharmaceuticals, and biochemistry.[Citation12] Numerous studies conducted in Bangladesh, where they have found that the amount of vitamin A in fortified edible oils was lower than the Bangladesh Standards and Testing Institution (BSTI) set standards. Most of the analyses used the reversed-phase high-performance liquid chromatography (RP-HPLC) technique.[Citation13–15] HPLC is usually the primary technique to determine vitamin A.[Citation16] Conventional analytical methods for investigating vitamin A in food products entail complicated and time-consuming sample preparation steps (vitamin A extraction and purification). Specifically, the reverse-phase HPLC methods require saponification of the samples which is more intricate and laborious. Besides, in some cases, interference from other ingredients in the sample raises questions about the method’s sensitivity, accuracy, and precision.[Citation12] Several normal-phase HPLC methods were also applied for analysis of retinyl palmitate from different food stuff. Many of those methods offered a higher retention time[Citation17,Citation18] as well as a higher extraction time.[Citation18,Citation19] Therefore, a highly straightforward, time-saving, and workable method for simultaneous determination of retinyl palmitate (vitamin A) in fortified edible oil using a normal-phase HPLC system was developed and validated.
Material and methods
Instrumentation
An analytical method was developed with a normal-phase High-Performance Liquid Chromatograph (Shimadzu Corporation, Japan) system using a solvent delivery module (LC-20AT), system controller (CBM-20A/20Alite), a column oven with 20 μL injector loop (for Shimadzu LC-6A HPLC system), two high-pressure pumps, a degasser, a “Restek” Pinnacle DB silica column, USA (250 × 4.6 mm; 5 μM particle size), and a UV detector (SPD-20A/20AV) operated at 326 nm. Lab solution software was used to perform data processing and calculation (version 5.30 SPI).
Procurement of chemicals, reagents, and fortified edible oil sample
A HPLC grade sample of retinyl palmitate (Type IV, ~1,800,000 USP units/g, oil; assigned purity 99.7%) was obtained from SIGMA-ALDRICH, Com. Germany. HPLC grade n-heptane and isopropyl alcohol were purchased from Merck (Merck, Germany). The latest production of fortified soybean, sunflower, and palm oil samples was collected from oil dealers and kept in black plastic bags placed in cardboard (25 ± 5°C) to ensure protection from sunlight, avoid contamination, and guarantee good storage conditions until analysis.
Chromatographic conditions
The mobile phase was consisted of 0.2% 2-propanol in n-heptane (25% for A pump) and 100% n-heptane (75% for B pump), and Pinnacle DB silica column was used as the stationary phase. Before using the mobile phase, it was filtered by using 0.22 µM membrane filters, and also sonicated for 5 min for degassing. Pinnacle DB silica column, USA (250 × 4.6 mm; 5 μM particle size) was used as a stationary phase, and detection operation was conducted at 326 nm wavelength. The flow rate was constant at 1.0 mL/min while maintaining the column temperature at 40°C. A minimum volume of 20 μL samples was injected into the HPLC system with a run time of 10 min.
Preparation of standard solution
An approximate quantity of retinyl palmitate (~1 mg) was dissolved in 50 mL of n-heptane (mobile phase), then labeled as a stock solution with standard concentration against its total volume (e.g., µg/mL). The solution was mixed thoroughly for 5 min using a vortex (TRXH-C, Oscillator, China), followed by sonication (Digital Ultrasonic Bath, UBT-580) for 5 min. After thorough mixing, serial dilution was made according to convenience.
Extraction of vitamin A (sample preparation)
One drop of fortified edible oil sample (~1.00 mg) was weighed by a digital balance machine and taken into a 10 mL volumetric flask. HPLC grade n-heptane was then added to the sample for up to 10 mL. The volumetric flask was labeled with sample concentration (e.g., mg/mL). The sample solution was vortexed (TRXH-C, Oscillator, China) for 5 min, followed by sonication (Digital Ultrasonic Bath, UBT-580) for another 5 min. After mixing the sample and n-heptane, the sample solution was filtered with a PTEF syringe filter (0.45 µM) using a 5 mL disposable syringe. The filtered sample was transferred in a microcentrifuge.
Method validation
The proposed analytical method was validated according to the guidelines of the International Conference on Harmonization (ICH)[Citation20] comprising system suitability, linearity and range, accuracy, specificity, limit of detection (LOD), and limit of quantification (LOQ), precision, and robustness.
Specificity
The specificity of the proposed method was assessed by injecting a volume of 20 µL of retinyl palmitate standard solution, prepared sample, and blank solution separately to distinguish between the target analyte and the other constituents in the prepared samples.[Citation21]
System suitability
To ensure the system’s performance, the system suitability was evaluated regarding theoretical plate count, resolution (peak separation), retention time, tailing factor, and relative standard deviation percentage (%RSD). Each suitability parameter was analyzed using the data generated from the three consecutive injections of retinyl palmitate standard and prepared sample solutions. The system performance was considered optimum when the suitability parameters such as theoretical plate counts were above 5000, the tailing factor was not more than 2, and the relative standard deviation percentage (%RSD) was calculated below 2%.[Citation22]
Linearity and range
The stock solution of retinyl palmitate standard was diluted with n-heptane (mobile phase) at various concentrations, e.g., 3.93, 7.87, 15.75, 31.5, and 63 μg/mL, which cover 50%, 100%, 200%, 400%, and 800% of the target concentration correspondingly. Each of the standards was injected thrice with similar operative conditions and analyzed. The linearity of the calibration curve was analyzed with the linear regression analysis method.
Sensitivity (LOD and LOQ)
The limit of detection and quantitation of retinyl palmitate were assessed by measuring the signal-to-noise ratio. The LOD and LOQ are the concentrations that give a signal-to-noise ratio of nearly 3:1 and 10:1, respectively, along with the %RSD (n = 3) of less than 2%.[Citation23] LOD and LOQ of the standard and sample were based on the following equation.[Citation24]
Recovery of retinyl palmitate (standard)
The recovery percentage of the target analyte evaluated the accuracy of the proposed method at different concentration levels (e.g., 50%, 100%, and 200%) such as 3.93, 7.87, and 15.75 μg/mL and each of the concentrations was injected thrice. The recovery percentage of the added retinyl palmitate and %RSD was analyzed for every replicate sample.
Recovery of the sample (spiking)
For the vitamin A analysis, three samples of edible oil (1 mL for each recovery/spiked sample) with retinyl palmitate concentrations of 0.049 μg/mL were spiked separately with 1 mL of 7.87, 15.75, and 31.5 μg/mL (3 recoveries/spiked samples) standard solutions. The spiked samples were analyzed, and the results obtained were used to calculate recoveries. Both recoveries of the standard and sample were based on the following equation.[Citation24]
Precision
Repeatability and reproducibility were studied to determine the precision of the proposed method. It was carried out by both inter and intraday repeatability of responses after the consecutive injections of replicates. The percentage of the relative standard deviations among the responses was calculated by using the typical formula of RSD calculation.[Citation25] In the current study, considering the validation protocols, the proposed method’s precision was analyzed by three replicate analyses at the concentration of 21.62 µg/mL of retinyl palmitate standard solution.
Stability of analytical solutions
The analytical solution’s stability (both standard and sample) was determined by analyzing the retinyl palmitate standard and sample solutions at 0 h and after 24 h in light, dark (at room temperature 28–30°C), and refrigerator.
Robustness
This parameter was studied by some deliberate modifications of different HPLC experimental conditions and considering the output of each of the experimental condition set-ups, such as (i) Flow rate: ±0.2 mL/min, (ii) Wavelength: ±3 nm, and (iii) The mobile phase composition, organic composition ±5%. Minor changes were made to assess the effects of the changes on the method. Acquired results for each case were estimated by analyzing the % of RSD and recovery.[Citation23]
Data analysis
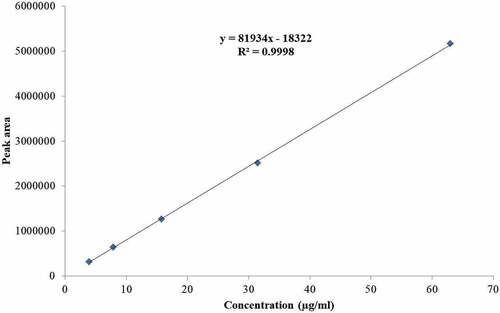
In vitro data analysis and graphical presentation were performed using Microsoft Excel (Office version 19.0). The HPLC results were calculated using linear regression, according to the equation: Y = 81,934x − 18,322, where Y is the area under the peak and X is the concentration of retinyl palmitate. The % RSD was calculated for all values.
RESULTS
Optimization of chromatographic conditions for retinyl palmitate
Retinyl palmitate is soluble and well dissolved in organic solvent[Citation26] having lowest polarity in polarity index, such solvents are n-hexane (0.1), n-heptane (0.1), cyclopentane (0.1), and so on,[Citation27] and even soluble in slightly more polar solvent such as iso-propyl alcohol (3.9), chloroform (4.1), and so on.[Citation26] In the proposed method, we have used the mobile phases consisting of 100% n-heptane and 0.2% isopropanol in n-heptane using retinyl palmitate standard. Since comprehensive separation of the peaks was not possible with a sole nonpolar solvent,[Citation28] we have lowered the quantity of isopropanol, yielding 0.2% in n-heptane. Later, we encountered the best resolution and effective separation of chromatographic peaks, both in standard and in extracted samples. A similar experiment was done by Tan and Brzuskiewicz[Citation29] where a mobile phase consisting of 99% hexane and 1% isopropanol was used as polar modifier to optimize a solvent system for amino and silica-normal phase columns. Many researches have been reported on the usage of isopropyl alcohol for the determination of retinyl palmitate as well.[Citation17,Citation30–32] The suitable flow rate (1.0 ml/min) of the mobile phase was selected after reviewing numerous research studies where different forms of vitamin A were determined with such flow rate along with other chromatographic conditions.[Citation30–36] Usually the detection wavelength range for retinyl palmitate is between lambda max 325–328 nm[Citation26] and the most appropriate wavelength for the highest absorbance was determined from the robustness parameter (). In normal-phase chromatography, a widely used stationary phase is silica column and it has been used for excellent chromatographic separation in numerous research studies.[Citation28–30,Citation37] Therefore, in the current study, Pinnacle DB silica column (250 × 4.6 mm; 5 μM particle size) with excellent theoretical plate count (93,347 plates/meter) has been used for complete chromatographic separation. Lastly, the final selections of major chromatographic conditions were confirmed upon findings of the robustness study.
Table 1. System suitability and validation parameters.
Table 2. Linearity of response for vitamin A standard (retinyl palmitate).
Table 3. LOD and LOQ of retinyl palmitate standard.
Table 4. Recovery data for retinyl palmitate standard.
Table 5. Recovery data for spiked sample.
Table 6. Solutions stability data of the proposed HPLC method.
Table 7. Precision data of retinyl palmitate standard (21.6 µg/mL).
Table 8. Robustness data of the proposed HPLC method (28 µg/mL of retinyl palmitate standard).
Method validation
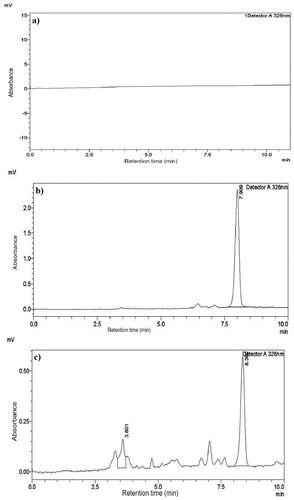
Specificity: The proposed HPLC method’s specificity is shown in , which displays the complete identical chromatographic figures of blank, vitamin A standard (retinyl palmitate), and vitamin A in the edible oil sample. The retention times of both the sample and standard peaks were comparable enough. Besides, the blank solution did not have any interfering peaks on its chromatogram.
Figure 1. Specificity of analytical solutions; chromatogram of (a) blank solution, (b) standard solution, and (c) retinyl palmitate in fortified edible oil.
System suitability: summarizes all the data regarding the system suitability of the proposed method. The peaks due to all the analytes were symmetrical as the observed tailing factors were less than two and the resolution of the retinyl palmitate peaks were satisfactory (>2). In contrast, the relative standard deviation was below 2%. The observed system suitability parameters of the present method were within the acceptable ranges set by the United States Food and Drug Administration (FDA) regulations,[Citation22] which indicate the suitability of the system.
Linearity and Range: The calibration curve () was attained by plotting the average peak areas against corresponding concentrations. It shows a linear relationship over the concentration range of 3.93–63 μg/mL for retinyl palmitate ( and ). A linear equation was attained: y = 81,934x – 18,322 and the correlation coefficient (R2) was found to be 0.9998 through the regression analysis. It also indicates a linear association between the concentration of analyte and the area under the peak.
Limit of detection and Limit of quantification
reveals that the limit of detection and quantification of retinyl palmitate standard are 0.029 and 0.096 µg/mL, respectively. It interprets that the proposed HPLC method is capable of detecting and quantifying retinyl palmitate at a minimum concentration of 0.029 and 0.096 µg/mL.
Recovery/accuracy
Recovery of standard
The recovery data () exhibited the accuracy of the proposed method. It was analyzed at three altered concentrations of retinyl palmitate standard, and the value was calculated as recovery percentage between the concentrations found and injected for the standard. The average percentage of recovery was found to be 104.99%, 102.32% and 99.71% for 3.93 µg/mL, 7.87 µg/mL, and 15.75 µg/mL, respectively (). The final average recovery percentage for the proposed HPLC method was 102.34% with a percentage of relative standard deviation (RSD) 0.10%.
Recovery of spiked sample
shows the data of recovery percentage for edible oil sample with mean relative standard deviation percentage. The average recovery percentage was found to be 101.55%, 96.81%, and 93.04% for three spiked edible oil samples, respectively. The final average recovery percentage for the proposed HPLC method for the sample was calculated as 97.13% with a relative standard deviation (RSD) of 0.44%.
Solution stability
exhibits that the stability of standard retinyl palmitate was highest at 0 h and after 24-h dark storage, confirmed by the recovery percentages as 100.45% and 99.46%, respectively. The standard solution was found to be comparatively less stable after 24-h storage at light and refrigerator.
also demonstrates vitamin A stability in edible oil sample. It showed that sample solution was more stable at 24-h storage in dark (recovery 102.5%) and refrigerator (recovery 82.5%) than 24-h storage at light (recovery 72.5%).
Precision
Both intra-day and inter-day precision data are presented in , where a standard solution of 21.62 µg/mL concentration was analyzed for 3 days consecutively. The precision is expressed in %RSD and was found satisfactory and less than 2%. Mean recovery was in the range of 93.92–101.54% (intra-day) and 84.31–101.54% (inter-day). It was also noticed that the mean recovery percentage decreased with an increase in storage time.
Robustness
summarizes the results of robustness testing and exhibits that small changes in the HPLC analytical conditions, such as mobile phase compositions, wavelength, and flow rate, are fairly robust and also within satisfactory limits. The results also revealed that the proposed method can separate and recover vitamin A more efficiently with mobile phase composition of 75/80% (A pump), 25/20% (B pump), 326 nm wavelength, and 1 mL/min flow rate.
In all alterations, extraction and good separation of vitamin A (retinyl palmitate) were attained, and it was significant that the recovery percentage was within the acceptable ranges (90% or above) and the percentage of RSD was also within satisfactory limits (<2.0%).
Application
The proposed method was applied to determine the vitamin A (retinyl palmitate) contents of three kinds of fortified edible oils (soybean, sunflower, and palm oil) (). The identification was made by the retention time, which was very reproducible in this study. It is obvious that the content of vitamin A varied from oil types to types. Vitamin A content of fortified edible oils was analyzed with the proposed method where vitamin A was detected and quantified with an average content of 1.01 ± 0.008, 1.15 ± 0.03, and 0.98 ± 0.002 mg/100 g oils for soybean, sunflower, and palm oil, respectively. For all types of fortified oil, the RSD was within the acceptable limits (2%).
Table 9. Vitamin A content in different fortified edible oils.
Discussion
In this study, a normal-phase HPLC method was developed to analyze retinyl palmitate in fortified edible oils, without laborious and time-wasting sample preparation. The oil samples were simply dissolved into the extracting solvent (mobile phase) and directly injected into the HPLC system. The sample preparation before the final injection into the HPLC system takes only 10 min. Whereas, many studies subjected oil samples to saponification with an average of 2–3 h per sample (based on the AOAC method 2001.13).[Citation38] This laborious saponification time can be lessened to many extents by simply dissolving them into extracting solvent. Similar extracting solvent was used by Noll and Becker[Citation19] where they used n-heptane as a major extracting solvent with the addition of slight polar tert-butyl methyl ether solvent. However, the retention time of the above normal-phase and many reverse-phase HPLC methods for retinyl palmitate determination is lower than the proposed method. But considering the overall procedure, including sample preparation, operational steps etc., the proposed method offered lower experimental duration than other methods. The overall retinyl palmitate analysis through this current method took only 20 min. Various normal phase studies reported that analytical values (recovery rate) from the direct solvent extraction method are higher than values from the saponification method.[Citation30] The potential mechanism might be the loss of retinyl palmitate during the saponification process. An approximate 10% loss of retinyl palmitate was observed through saponification by Ishimaru et al.[Citation36] Excluding the saponification step did not affect the recovery rate in this study also, which was more than 93.0%. The recoveries are comparable with other normal phase analysis including Ishimaru et al. (88.94–103.82%)[Citation36] where the samples were subjected to saponification. The recoveries are also similar to the previous recovery rates of reverse phase analysis of retinyl palmitate conducted by Wang and Huang (85–96%),[Citation33] Wielinski and Olszanowski (99.2%)[Citation12] and Davydova et al. (98.0%).[Citation39] A very common silica column was used for chromatographic separation (stationary phase) of retinyl palmitate with UV detector. Usually, when any lipid portion is directly injected into the HPLC system, there is always a risk of declining separation efficiency as well as the lifespan of the HPLC column.[Citation40] The proposed validation showed that the lipid portion did not deteriorate the column’s separation power and lifetime. The precise choice of mobile phase ensured better column performance. Moreover, column washing after each sample injection was not essential at all. The method was fully validated and also compared with other normal and reverse phase methods of retinyl palmitate determination regarding various validation parameters. The linearity range of standard concentration was 3.93–63 µg/mL which gave an excellent linear line during regression analysis. The r2 (0.9998) value was comparable with previous findings of Wang and Huang[Citation33] and Lee et al.[Citation30] indicating quantification of retinyl palmitate with the developed method is highly reproducible even than Johnson-Davis et al.[Citation41] and Ishimaru et al.[Citation36] The precision level of the proposed method was found below 2% (Intra-day: 0.28–0.37%; Inter-day: 0.35–1.28%) which is similar to the value reported by Wielinski and Olszanowski[Citation12] (Intra-day: 1.34%; Intra-day: 0.82–1.1%) and Davydova et al.[Citation39] (Intra-day: 0.6–1.2%; Intra-day: 0.4–1.5%). According to McMahon et al.[Citation42] the RSD values must be below 2%. The RSD values of the current study indicated more precision in comparison with other RSD values of similar normal phase validation results.[Citation31,Citation42] The proposed method’s LOD and LOQ were recorded at 0.029 and 0.096 µg/mL, respectively. Whereas, nearly values were also reported in a reverse phase (0.015, 0.02 µg/mL) by Lisa et al.[Citation43] and normal phase (0.003, 0.15 µg/mL) by Carafa et al.[Citation18] Regarding the application of the suggested method, it provided pretty similar results to the previously applied methods (both reverse phase and normal phase). The analysis of retinyl palmitate in fortified edible oils showed that all the samples were below the fortification standards (1.5–3.0 mg/100 g) and recorded for soybean oil (1.01 ± 0.008 mg/100 g), sunflower oil (1.15 ± 0.03 mg/100 g) and palm oil (0.98 ± 0.002 mg/100 g). The results of the applications of the proposed method are in agreement with the previous findings of Lisa et al.[Citation13] where most of the oil samples had much lower vitamin A (0.17–0.28 mg/100 g) than the standard value. Vitamin A content of fortified soybean oil was also analyzed by Ahmed et al.[Citation14] and it was reported that oil samples had lower vitamin A content than the standard value and is consistent with the current study’s findings. GAIN and ICDDRB[Citation15] analyzed that 58% of market oil samples did not comply with the minimum fortification standards. Remarkably, all of the above results were analyzed by applying reverse-phase HPLC methods and are consistent with the results of the proposed normal-phase HPLC method.
Conclusion
The optimized normal-phase HPLC method with UV detector for the detection and quantification of vitamin A (retinyl palmitate) in fortified edible oils has been established and laboratory validated according to ICH guidelines. It involves a simple procedure for the preparation of the samples and injection. The method is efficient, accurate, and reliable in terms of rapidity, simplicity (direct sample extraction), high selectivity, and reliable sensitivity. Notably, the high recovery percentage data showed that the analytical method is free from interference. Additionally, the significant features of the developed method are short run time, retention time, and analysis completion time. The method is robust enough to reproduce precise results under diverse chromatographic conditions and was successfully applied to simultaneously determine retinyl palmitate in fortified edible oils. Finally, all the results obtained with the proposed method confirmed the method’s suitability for the analysis of retinyl palmitate from fortified edible oils.
Disclosure statement
The authors declare no potential conflict of interest.
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Fragoso, Y. D.; Campos, N. S.; Tenrreiro, B. F.; Guillen, F. J. Systematic Review of the Literature on Vitamin A and Memory. Dement. Neuropsychol. 2012, 6(4), 219–222. DOI: 10.1590/S1980-57642012DN06040005.
- Bendich, A.; Olson, J. A. Biological Actions of Carotenoids. FASEB J. 1989, 3(8), 1927–1932. DOI: 10.1096/fasebj.3.8.2656356.
- WHO. Global Prevalence of Vitamin A Deficiency in Populations at Risk 1995-2005: WHO Global Database on Vitamin A Deficiency. Geneva: World Health Organization, 2009.
- Nair, H.; Arya, G.; Vidnapathiranad, J.; Tripathi, S.; Talukder, S. H.; Srivastava, V. Improving Neonatal Health in South-East Asia. Public Health. 2012, 126(3), 223–226. DOI: 10.1016/j.puhe.2011.12.006.
- WHO. 2019. Nutrition Landscape Information System (NLIS) Country Profile. Nutrition and nutrition-related Health and Development Data. Geneva: World Health Organization. Available from: https://www.who.int/data/nutrition/nlis/country-profile
- West, K. P.; Darnton-Hill, I. Vitamin A Deficiency. In Nutrition and Health in Developing Countries, 2nd ed.; Semba, R. D., Bloem, M. W., Eds.; Humana Press, Inc: Totowa, NJ, 2008; pp 377–433.
- Bagriansky, J.; Ranum,P.Vitamin A fortification of P. L. 480. Washington D.C: Vegetable Oil, SUSTAIN.[cited 2022 Aug11]. Available form: https://pdf.usaid.gov/pdf_docs/PNACK055.pdf. 1998.
- Riskesdas,; (Riset Kesehatan Dasar). 2010. National Report on Basic Health Research by National Institute of Health Research and Development. Indonesia: Ministry of Health.
- BSTI. Bangladesh Standards Specification for Vegetable Oils. A. BDS 1769: 2014. (Fortified Soybean Oil), B. BDS 1773: 2016 (Fortified Sunflower Oil), C. BDS 1770: 2014 (Fortified Edible Palm Oil), D. BDS 1886: 2014 (Fortified Edible Rice Bran Oil), E. BDS 25. 2014.
- Laillou, A.; Panagides, D.; Garrett, G. S.; Moench-Pfanner, R. Vitamin A Fortified Vegetable Oil Exported from Malaysia and Indonesia Can Significantly Contribute to Vitamin A Intake Worldwide. Food Nutr. Bull. 2013, 34(2), 72–80. DOI: 10.1177/15648265130342S109.
- Unicef, F.;. Fortification of Edible Oil in Bangladesh. [cited 2022 Aug 15]. https://gapssbd.com/2014-02-10-19-06-10/2014-02-10-19-06-39/fortification-and-mal-nutrition.html. 2014.
- Wieliński, S.; Olszanowski, A. Development and Validation of HPLC Method for Simultaneous Determination of Fat-soluble Vitamins in Capsules. J. Liq. Chromatogr. Relat. Tech. 2001, 24(2), 201–213. DOI: 10.1081/JLC-100001482.
- Lisa, S. A.; Khan, S.; Kabir, M. A.; Islam, F.; Mohajan, S.; Chowdhury, K. Quality and Vitamin A Status Assessment of Different Commercial Edible Oil. Bangladesh J. Sci. Ind. Res. 2019, 54(1), 11–20. DOI: 10.3329/bjsir.v54i1.40726.
- Ahmed, T.; Sarwar, N.; Ahmad, M.; Sharmin, K. N. Evaluation of Quality and Vitamin a Fortification Level of Soybean Oil Available in Bangladesh. Bangladesh J. Vet. Ani. Sci. 2019, 7(2), 38–46.
- GAIN (Global Alliance for Improved Nutrition) and ICDDRB (International Centre for Diarrheal Diseases and Research, Bangladesh). Assessment of Presence of Edible Oil Brands in Bangladesh and Their Content of Fortification; Geneva, Switzerland. https://www.gainhealth.org/resources/reports-and-publications/assessment-presence-edible-oil-brands-bangladesh-and-their, 2017.
- Gąsior, R.; Pieszka, M. Validation of a Rapid Method for Simultaneous Determination of Vitamins A and E in Milk Using HPLC. Polish J. Food Nutr. Sci. 2007, 57(4), 151–155.
- Kittagaa, M.; Hosotanii, K. Improved Method of Determining Retinol and Retinyl Palmitate in Rat Liver and Serum by High-Performance Liquid Chromatography. J Nutr Sci Vitamirnol. 2000, 46(1), 42–45. DOI: 10.3177/jnsv.46.42.
- Carafa, M.; Marianecci, C.; Salvatorelli, M.; Di Marzio, L.; Cerreto, F.; Lucania, G.; Santucci, E. Formulations of Retinyl Palmitate Included in Solid Lipid Nanoparticles: Characterization and Influence on Light-induced Vitamin Degradation. J. Drug Del. Sci. Tech. 2008, 18(2), 119–124. DOI: 10.1016/S1773-2247(08)50019-0.
- Noll, G. N.; Becker, C. High-performance Liquid Chromatography of Non-polar Retinoid Isomers. J. Chromatogr. A. 2000, 881(1–2), 183–188. DOI: 10.1016/S0021-9673(00)00232-6.
- International conference on harmonization (ICH). Validation of analytical procedures: Text methodology, (Q2-R1) Geneva, 2005, 6–13.
- Batrawi, N.; Naseef, H.; Al-Rimawi, F. Development and Validation of a Stability-indicating HPLC Method for the Simultaneous Determination of Florfenicol and Flunixin Meglumine Combination in an Injectable Solution. J. Anal. Methods Chem. 2017, 2017, 01–07. DOI: 10.1155/2017/1529280.
- FDA (Food and Drug Administration), Center for Drug Evaluation and Research. Reviewer Guidance, Validation of Chromatographic Methods; FDA: Rockville, MD, USA; .[cited 2022 July 20]. Available from: www.fda.gov/media/75643/download , 1994.
- Naseef, H.; Moqadi, R.; Qurt, M. Development and Validation of an HPLC Method for Determination of Anti-diabetic Drug Alogliptin Benzoate in Bulk and Tablets. J. Anal. Methods Chem. 2018, 2018, 01–07. DOI: 10.1155/2018/1902510.
- Adewuyi, G. O.; Olatoye, O. I.; Abafe, A. O.; Otokpa, M. O.; Nkukut, N. K. High Performance Liquid Chromatographic Method for Evaluation of Two Antibiotic Residues in Liver and Muscle of Broilers in Ibadan City, Southern Nigeria. J. Pharm. Biomed. Sci. 2011, 11(11), 01–04.
- Kayesh, R.; Rahman, A.; Sultan, M. Z.; Uddin, M. G.; Aktar, F.; Rashid, M. A. Development and Validation of a RP-HPLC Method for the Quantification of Omeprazole in Pharmaceutical Dosage Form. J. Sci. Res. 2013, 5(2), 335–342. DOI: 10.3329/jsr.v5i2.12779.
- SCCS (Scientific Committee on Consumer Safety), Opinion on Vitamin A (Retinol, Retinyl Acetate, Retinyl Palmitate), SCCS/1576/16, 20 April 2016, Final Version of 6 October 2016, Corrigendum on 23 December 2016.
- Kowalska, T.; Kaczmarski, K.; Prus, W.; Sajewicz, M. Theory and Mechanism of Thin-Layer Chromatography: Chapter-2 “Adsorption Planar Chromatography in the Nonlinear Range: Selected Drawbacks and Selected Guidelines”. In Preparative Layer Chromatography. Kowalska, T. CRC Press: Boca Raton, USA, 2006, 29-30.
- Chávez-Servín, J. L.; Castellote, A. I.; Lóopez-Sabater, M. C. Simultaneous Analysis of Vitamin A and E in Infant Milk-based Formulae by Normal-phase High Performance Liquid Chromatography-Diode Array Detection Using A Short Narrow-bore Column. J. Chromatogr. 2006, 1122(1–2), 138–143. DOI: 10.1016/j.chroma.2006.04.059.
- Tan, B.; Brzuskiewicz, L. Separation of Tocopherol and Tocotrienol Isomers Using Normal- and Reverse-phase Liquid Chromatography. Anal. Biochem. 1989, 180(2), 368–373. DOI: 10.1016/0003-2697(89)90447-8.
- Lee, J.; Suknark, K.; Kluvitse, Y.; Phillips, R. D.; Eitenmiller, R. R. Rapid Liquid Chromatographic Assay of Vitamin E and Retinyl Palmitate in Extruded Weaning Foods. J. Food Sci. 1999, 64(6), 968–972. DOI: 10.1111/j.1365-2621.1999.tb12261.x.
- Woollard, D. C.; Bensch, A.; Indyk, H.; McMahon, A. Determination of Vitamin A and Vitamin E Esters in Infant Formulae and Fortified Milk Powders by HPLC: Use of Internal Standardisation. Food Chem. 2015, 15, 457–465. DOI: 10.1016/j.foodchem.2015.10.077.
- Mohan, M. S.; Jurat-Fuentes, J. L.; Harte, F. Binding of Vitamin A by Casein Micelles in Commercial Skim Milk. J. Dairy Sci. 2013, 96(2), 790–798. DOI: 10.3168/jds.2012-5777.
- Wang, L.; Huang, S. Determination of Vitamins A, D, E, and K in Human and Bovine Serum, and Carotene and Vitamin A Palmitate in Cosmetic and Pharmaceutical Products, by Isocratic HPLC. Chromatographia. 2002, 55(5/6), 289–296. DOI: 10.1007/BF02491661.
- Scalzo, M.; Santucci, E.; Cerreto, F.; Carafa, M. Model Lipophilic Formulations of Retinyl Palmitate: Influence of Conservative Agents on Light-induced Degradation. J. Pharm. Biomed. Anal. 2003, 34(5), 921–931. DOI: 10.1016/S0731-7085(03)00653-8.
- Carafa, M.; Marianecci, C.; Codecà, A.; Squillaci, P.; Scalzo, M.; Cerreto, F.; Santucci, E. Retinyl palmitate-loaded Vesicles: Influence on Vitamin light-induced Degradation. J. Drug Del. Sci Tech. 2006, 16(6), 407–412. DOI: 10.1016/S1773-2247(06)50080-2.
- Ishimaru, M.; Haraoka, M.; Hatate, H.; Tanaka, R. High-Performance Liquid Chromatography with Fluorescence Detection for Simultaneous Analysis of Retinoids (Retinyl Palmitate, Retinyl Acetate, and Free Retinol) and α-, β-, γ-, and δ-Tocopherols in Foods. Food Anal. Methods. 2017, 10(1), 92–99. DOI: 10.1007/s12161-016-0553-z.
- Abidi, S. L.; Mounts, T. L.; Liq, J. Chromatogr. Rel. Technol. 1996, 19, 509.
- AOAC International. 2001. Official Methods of Analysis of AOAC International (2011), AOAC International, Rockville (MD, USA). Official Method 2001.13: Vitamin A (Retinol) in Foods.
- Davydova, N.; Stippler, E.; Jin, P.; Giancaspro, G. Development and Validation of A Dissolution Test Method for Vitamin A in Dietary Supplement Tablets. J. Pharm. Biomed. 2010, 53(3), 295–301. DOI: 10.1016/j.jpba.2010.03.036.
- Rimkus, G. G.; Schubert, M.; Morgan, D.; Jungjohann, S. Rapid Direct Analysis of Retinyl Palmitate (Vitamin A) in Fortified Vegetable Oils by HPLC-FLD. Food Additives and Contaminants - Part A Chemistry, Analysis, Control. Exposure and Risk Assessment. 2022, 39(6), 1–11. DOI: 10.1080/19440049.2021.1977854.
- Johnson-Davis, K. L.; Moore, S. J.; Owen, W. E.; Cutler, J. M.; Frank, E. L. A Rapid HPLC Method Used to Establish Pediatric Reference Intervals for Vitamins A and E. Clin. Chim. Acta . 2009, 405(1–2), 35–38. DOI: 10.1016/j.cca.2009.03.058.
- McMahon, A.; Christiansen, S.; Shine, L.; Loi, C.; Dowell, D. Simultaneous Determination of 13-cis and all-trans Vitamin A Palmitate (Retinyl Palmitate), Vitamin A Acetate (Retinyl Acetate), and Total Vitamin E (α-Tocopherol and DL-α-Tocopherol Acetate) in Infant Formula and Adult Nutritionals by Normal Phase HPLC: First Action 2012.10. J. AOAC Int. 2013, 96(5), 1073–1081. DOI: 10.5740/jaoacint.13-103.
- Lisa, E. L. P.; Croitoru, O.; Coman, G.; Stefan, C. S.; Tutunaru, D.; Cuciureanu, R. Validation of HPLC Method for the Determination of Retinol in Different Dietary Supplements. Rom. Biotechnol. Lett. 2014, 19(6), 8975–8988.