ABSTRACT
This study aimed to compare phytochemical content and antioxidant activities of eight diverse citrus cultivars grown in Jeju Island depending on their harvest time owing to their health benefits. Plant peels, flesh, and leaves were extracted, and their flavonoid contents were measured by HPLC. Flavonoid levels were high in peel followed by leaves and juices. It was highest in immature fruits and rapidly declined during ripening. While total phenolic content in all citrus peels and fleshes reduced during ripening, those of leaves did not significantly decrease. Antioxidant activity matched the total phenolic content. This indicates that immature citrus tissues exhibited the highest flavonoid and phenolic content with high antioxidant activity and can be potentially employed as a readily accessible natural antioxidant source.
Introduction
Citrus fruits are one of the most significant fruits in the global food industry.[Citation1] Jeju Island in Korea is unique as citrus plants are cultivated on a large scale in the island’s subtropical climate.[Citation2] Here, citrus cultivation commenced before 476 A.D., and 22 native varieties were cultivated however, they were gradually enhanced to new varieties due to poor quality and reproduction.[Citation3] Fruit quality (size, color, and ease of peeling), seedlessness, and harvest season extension are the key selection objectives for cultivars.[Citation4] Jeju citrus including Jinkyool (Citrus sunki), mandarin (Citrus reticulata), and tangor (Citrus reticulata x sinensis) are vastly cultivated. Jinkyool is a native citrus cultivated in Jeju Island and is not apt as a raw food hence, they are predominantly utilized as processed foods and herbal medicines.[Citation5] Mandarin (e.g., Byeonggam, Ilnam, Gungchenjosaeng) and tangor (e.g., Hwangkeumhyang, Redhyang, Hallabong, Chonhyehyang) are extensively cultivated for raw food. Citrus peels and leaves are generally regarded as waste, and making good use of waste materials is imperative. Citrus fruits and derived products have a beneficial effect on human health due to their nutritional and antioxidant attributes.[Citation6] Citrus plants are of great interest as their fruits and leaves accumulate high concentrations of flavonoid glycosides, whose aglycones are early intermediates in the flavonoid biosynthetic pathway.[Citation7] Flavonoids established in citrus fruits encompass more than sixty types, considering the five classes: flavones, flavanones, flavonols, flavans, and anthocyanins.[Citation8] Flavonoids are secondary metabolites, which reportedly have several health benefits in humans.[Citation9] They depict a strong antioxidant and radical scavenging activity[Citation10–12] and seem to be associated with reduced risk for certain chronic diseases,[Citation13,Citation14] prevention of cardiovascular disorders,[Citation15,Citation16] and certain cancerous processes.[Citation17–19] They also exhibit antiviral,[Citation20] antimicrobial,[Citation21] and anti-inflammatory activities,[Citation22,Citation23] useful effects on capillary fragility[Citation24] and capability to inhibit human platelet aggregation,[Citation25] antiulcer,[Citation26,Citation27] antiallergenic,[Citation28] and anti-obesity[Citation4] properties. Flavonoids have been found in citrus seeds, leaves, juice, stems, and peels. However, maximum studies have been performed on citrus flesh and peels, and studies on site-specific flavonoids and antioxidant activity over harvest days are inadequate. The objectives of this study were to explore and compare the flavonoid content, phenolic content, and radical scavenging activity of eight citrus species in peels, flesh, and leaves primarily produced and sold in Jeju Island.
Materials and methods
Chemicals and reagents
Rutin, naringin, hesperidin, quercetin, naringenin, and hesperetin standards were acquired from Sigma–Aldrich Chemical Co. (St. Louis, MO). The other reagents, sinensetin, nobiletin, and tangeretin, were purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). HPLC-grade solvents employed for extraction and as the mobile phase were obtained from Fisher Scientific Korea, Ltd. (Seoul, Korea). All other reagents were purchased from Sigma Chemical Co. unless otherwise noted.
Plant materials and extraction
Eight typical citrus varieties cultivated in Jeju Island were chosen for this research, including one native, three mandarin, and four tangor varieties (). Citrus peel, flesh, and leaves were obtained every month between July 2018 to March 2019 from Jeju Island, Republic of Korea. They were cleaned under running water and dried employing a hot air drier for 24 h at 60°C. Then they were pulverized to 74–140 microns powder utilizing a pulverizer. Peel, flesh, and leaves were extracted with 70% ethanol for 48 h at 25°C. Each extract was filtered and concentrated in a rotary evaporator (Buchi Korea, INC., Korea) at 40°C, and the aqueous phase was freeze-dried employing a vacuum freeze dryer (IlshinBioBase Co., Ltd., Gyeonggi, Korea). The samples were stored at −70°C until use.
Table 1. Eight citrus samples harvested in Jeju.
Flavonoid compounds
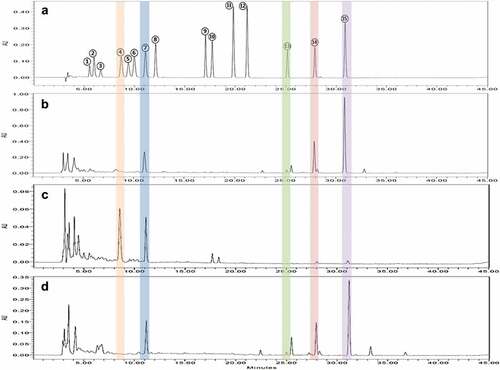
A Waters 2695 Alliance HPLC system which comprised two pumps, an autosampler, column oven, and Waters 2998 photodiode array (PDA) detector was employed to identify the 15 flavonoids (Waters, Milford, MA, USA). Instrument control and data acquisition were accomplished using Empower software (version 2.0; Waters). Flavonoid separation was attained utilizing a Sunfire C18 column (4.6 × 250 mm ID, 5 μm; Waters, Milford, MA, USA) and was monitored at 280 nm. The mobile phase encompassed acetonitrile containing 0.1% phosphoric acid (A) and 0.1% phosphoric acid aqueous solution (B) under the following conditions: 0.0–5.0 min, 20.0% A; 5.0–40.0 min, 70.0% A; 40.0–41.0 min, 100.0% A; 41.0–43.0 min, 100.0% A; 43–44 min, 20.0% A; and 44–45 min, 20.0% A. Separation was performed in a column oven at 40°C with 10 μL sample injection volume and 0.8 mL/min flow rate. Stock solutions of the 15 flavonoids (2 mg/mL) were prepared by dissolving them in ethanol/DMSO (1/1, v/v). Standard solutions (12.5, 25, 50, and 100 μg/mL) were prepared by diluting the stock solution with ethanol/DMSO and a standard curve was employed to verify the concentrations. Correlation coefficients of the standard curves exceeded 0.999. Citrus extract flavonoids were determined by comparing their retention times and ultraviolet spectra with those of the standard solutions (). Compound concentrations were calculated based on the integrated peak area of each sample and the corresponding standard.
Figure 1. HPLC chromatograms of 15 flavonoid standards (A), Jinkyool peel (B), Jinkyool flesh (C), and Jinkyool leaves extract (D). Peak 1) rutin, 2) eriocitrin, 3) neoeriocitrin, 4) narirutin, 5) rhoifolin, 6) naringin,7) hesperidin, 8) neohesperidin, 9) neoponcirin, 10) poncirin, 11) naringenin, 12) hesperetin, 13) sinensetin, 14) nobiletin, and 15) tangeretin. Peaks were detected at 280 nm.
Total phenolic (TP) contents
TP contents were estimated employing the Folin-Ciocalteu approach with some modifications.[Citation29] 0.5 mL of extract (1 mg/mL) in 1 mL of distilled water was mixed thoroughly with 0.5 mL of 2 N Folin-Ciocalteu reagent for 5 min, followed by the addition of 2 mL of 7% (w/v) sodium carbonate. The mixture was allowed to react for 90 min at 25°C and its absorbance was measured using an ELSIA reader (Bio-Tek, Winooski, VT, USA) at 750 nm. TP contents were computed based on the calibration curve and were expressed as mg of gallic acid (GAE) equivalent per g dry weight.
Antioxidant activity
2,2-diphenyl-1-picrylhydrazyl (DPPH) and 2,2’-azino-bis-3-ethylbenzothiazoline-6-sulfonic acid (ABTS) assays were conducted employing the method of Floegel et al.[Citation30] For the DPPH assay, 100 μL of the extracts (1 mg/mL) were distributed into 96-well plates and mixed with 100 μL of 0.4 mM DPPH solution. The plates were incubated in the dark for 30 min, and absorbance was measured at 517 nm. For ABTS assay, the extracts (100 μL) were dispensed into 96-well plates and equilibrated with 7 mM ABTS and 2.45 mM ammonium persulfate of the same volume. After standing for 10 min in the dark, using an ELSIA reader (Bio-Tek, Winooski, VT, USA) absorbance was estimated at 745 nm. DPPH and ABTS radical scavenging rates (%) were computed as follows: % radical scavenging rate = (control – sample)/control × 100.
Statistical analysis
Statistical analyses were conducted adopting SPSS version 21.0 for Windows (SPSS; Chicago, IL, USA). All the data are presented as means ± standard deviations (SDs). Differences between groups were inspected by Duncan’s multiple range test in one-way analysis of variance (ANOVA). A p-value < 0.05 was considered to indicate statistical significance.
Results and discussion
Flavonoid content of citrus
Based on the standard curve, flavonoid content of the harvest time peel, flesh, and leaves derived from eight citrus species are presented in . During cultivation from July 2018 to March in 2019 period, the concentration of narirutin was Byeonggam (1.99–6.38 mg/g), Ilnam no. 1 (26.17–118.18 mg/g), Gungchenjosaeng (38.62–92.90 mg/g), Hwangkeumhyang (15.47–59.33 mg/g), Redhyang (23.89–68.51 mg/g), Hallabong (17.76–69.81 mg/g), Chonhyehyang (79.14–257.63 mg/g) in the peel. In flesh, it was Jinkyool (2.39–8.42 mg/g), Byeonggam (1.47–10.40 mg/g), Ilnam no. 1 (5.82–41.53 mg/g), Gungchenjosaeng (5.58–26.11 mg/g), Hwangkeumhyang (1.59–12.10 mg/g), Redhyang (4.79–62.97 mg/g), Hallabong (3.47–34.16 mg/g), Chonhyehyang (8.40–135.73 mg/g), and was not detected in the leaves. Hesperidin was predominantly contained in peel and leaves. The hesperidin content of peel was Jinkyool (25.60–32.90 mg/g), Byeonggam (14.68–21.62 mg/g), Ilnam no. 1 (31.12–42.58 mg/g), Gungchenjosaeng (39.58–47.46 mg/g), Hwangkeumhyang (26.53–32.24 mg/g), Redhyang (29.50–46.10 mg/g), Hallabong (23.62–35.09 mg/g), Chonhyehyang (5.66–17.12 mg/g), and the flesh was Jinkyool (5.43–6.93 mg/g), Byeonggam (3.20–6.50 mg/g), Ilnam no. 1 (4.84–5.90 mg/g), Gungchenjosaeng (5.20–7.57 mg/g), Hwangkeumhyang (2.86–7.33 mg/g), Redhyang (4.57–16.48 mg/g), Hallabong (2.63–4.74 mg/g), Chonhyehyang (1.25–15.77 mg/g). In leaves, it was Jinkyool (15.61–26.44 mg/g), Byeonggam (26.48–27.36 mg/g), Ilnam no. 1 (31.50–43.75 mg/g), Gungchenjosaeng (39.73–51.54 mg/g), Hwangkeumhyang (33.57–37.45 mg/g), Redhyang (27.60–34.56 mg/g), Hallabong (22.45–38.99 mg/g), and Chonhyehyang (1.22–2.65 mg/g), respectively. PMFs in the flesh were lower or undetected. These results are consistent with those of a previous study.[Citation31–34] PMF contents were higher in Jinkyool, Byeonggam, Cheonhyehyang, and Hallabong than in other species, and were primarily detected in peel and leaves. Nobiletin and tangeretin were the key PMFs among the three evaluated components.[Citation4] The sinensetin content of peel and leaves was Jinkyool (2.99–5.55 mg/g), Byeonggam (0.91–1.80 mg/g), Redhyang (1.51–4.86 mg/g), Hallabong (0.84–5.21 mg/g), Chonhyehyang (6.74–8.63 mg/g). The highest flavonoid content was established in July and August in 2018 and the lowest in harvest season. Narirutin and hesperidin were high in most citrus peels, and PMFs were identified only in some citrus.[Citation6,Citation35] The proportion of flavonoid content may vary among diverse cultivars, climate conditions, and horticultural practices in the orchard.[Citation6,Citation31] A regular decline was observed until the harvest stage in all the citrus parts.[Citation31] Narirutin was undetected in Jinkyool peel and other citrus leaves and depicted the highest content between July and August in 2018, which is the immature period in peel and flesh. The flavonoid was principally contained in peels and leaves, and the content reduced as the peel and flesh matured, and the decline in leaves was small. Other flavonoid compounds content demonstrated lower tendencies from rutin, eriocitrin, and neoponcirin (data not shown). The key flavonoids were narirutin, hesperidin, sinensetin, nobiletin, and tangeretin and were predominantly contained in peel and leaves. Jinkyool, Byeonggam, and Chonhyehyang contained a high amount of PMFs with high physiological activity effects. In general, the flavonoid content of citrus is known to decrease as the harvest season approaches, but it has augmented in some citrus varieties.
Table 2. Major flavonoid content found in peel, flesh, and leaves of eight different citrus cultivars harvested in Jeju, Korea from July 2018 to March 2019.
TP contents
TP content of citrus peel, flesh, and leaves is represented in . Jeong et al., (2004) reported that the outer layers of plants including peels contain huge amounts of polyphenolic compounds to protect inner materials. The highest phenolic content was detected in the immature and the lowest in mature stages. Mandarin and tangor species had similar TP contents, hence there was no significant difference between the two groups. In the peel, the TP content ranged between 10.48 and 22.04 mg GAE/g, Byeonggam (22.04 mg GAE/g) had the highest TP content, followed by Jinkyool (21.85 mg GAE/g) and Chonhyehyang (19.78 mg GAE/g). Flesh presented the highest amounts in Byeonggam (20.03 mg GAE/g), and the other varieties displayed similar values, 18.27 mg GAE/g and 18.23 mg GAE/g for Jinkyool and Chonhyehyang, respectively. In leaves, Byeonggam (21.85 mg GAE/g) has the highest TP content, followed by Ilnam no. 1 (20.98 mg GAE/g) and Hwangkeumhyang (20.25 mg GAE/g). The reduction rate of TP content by region according to the cultivation period in peel, flesh, and leaves was 19.46–49.55%, 54.41–68.35%, and 20.68–42.42%, respectively. For peel, Chonhyehyang had the lowest value of 19.46%, while Byeonggam had the highest value of 49.55% in the reduction rate of TP content. Jinkyool and Byeonggam were reduced to 54.41% and 68.35%, respectively, in TP content of pulp, and Redhyang 20.68%, and Ilnam no. 1 42.42% in TP content of leaves. Total phenolic content in citrus peel and flesh reduced during ripening and the leaves did not decrease significantly. Regardless of cultivars, TP content significantly reduced with maturation, as reported previously.[Citation31,Citation36]
Antioxidant activity
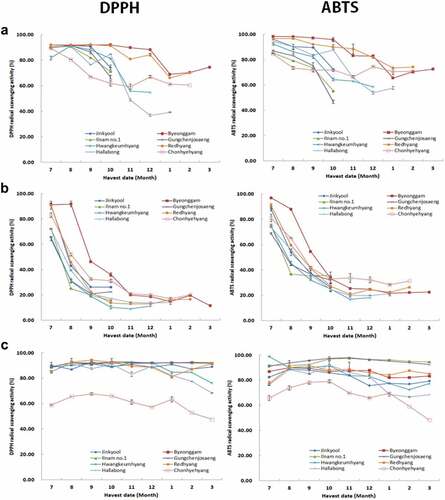
DPPH and ABTS radical scavenging activity of the citrus sample at the concentration of 1 mg/mL is depicted in . DPPH and ABTS radical scavenging activity is a common approach for evaluating antioxidant activities in a relatively short time compared with other methods.[Citation37] The ABTS assay is based on the generation of a blue/green ABTS that can be reduced by antioxidants, whereas the DPPH assay is based on the reduction of the purple DPPH to 1,1-diphenyl-2-picryl hydrazine.[Citation38] DPPH and ABTS methods depicted the same tendency, and most of them demonstrated high antioxidant activity in immature samples. On maturation, the antioxidant activity diminished, and the decrease was large in the order of flesh, peel, and leaves. The results of several reports[Citation33,Citation39] demonstrated that peel usually had high antioxidant activity, whereas flesh had lower activity. Ascorbic acid content is known to play a crucial role in the antioxidant effect. As the flesh matures, the ascorbic acid content is significantly lower than that of other parts and the antioxidant effect is greatly diminished.[Citation40] Recent reports state that immature citrus fruits have greater antioxidant activity than mature fruits.[Citation41] Kim et. al., (2009) reported that the antioxidant effect declines with maturity which is similar to our results. The antioxidant activity depicted a similar trend as total phenolic content. The present work can be beneficial in determining the suitable harvest time of citrus for use as materials. In the case of leaves, the change over time is small, thus it is expected to be competitive as a material
Acknowledgments
This study was supported by the Open Labs Program in partnership with Local Industrial Associates through the Commercialization Promotion Agency for R&D Outcomes of the Ministry of Science and ICT (NTIS1711139489).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Kim, D. S.; Lee, S.; Park, S. M.; Yun, S. H.; Gab, H. S.; Kim, S. S.; Kim, H. J. Comparative Metabolomics Analysis of Citrus Varieties. Foods. 2021, 10(11), 2826. DOI: 10.3390/foods10112826.
- Choi, S. Y.; Ko, H. C.; Ko, S. Y.; Hwang, J. H.; Park, J. G.; Kang, S. H.; Han, S. H.; Yun, S. H.; Kim, S. J. Correlation between Flavonoid Content and the NO Production Inhibitory Activity of Peel Extracts from Various Citrus Fruits. Biol. Pharm. Bull. 2007, 30(4), 772–778. DOI: 10.1248/bpb.30.772.
- Kim, Y. D.; Mahinda, S.; Koh, K. S.; Jeon, Y. J.; Kim, S. H. Reactive Oxygen Species Scavenging Activity of Jeju Native Citrus Peel during Maturation. J Korean Soc. Food Sci. Nutr. 2009, 38(4), 462–469. DOI: 10.3746/jkfn.2009.38.4.462.
- Ollitrault, P.; Navarro, L. Citrus. Fruit Breeding. 2011, 21, 623–662.
- Jin, Y. J.; Jang, M. G.; Kim, J. W.; Baek, S.; Ko, H. C.; Hur, S. P.; Kim, S. J. Anti-Obesity Effects of Polymethoxyflavone-Rich Fraction from Jinkyool (Citrus Sunki Hort. Ex Tanaka) Leaf on Obese Mice Induced by High-Fat Diet. Nutrients. 2022, 14(4), 865. DOI: 10.3390/nu14040865.
- Bermejo, A.; Llosa, M. J.; Cano, A. Analysis of Bioactive Compounds in Seven Citrus Cultivars. Food Sci. Tech. Int. 2011, 17(1), 0055–8. DOI: 10.1177/1082013210368556.
- Kawaii, S.; Tomono, Y.; Katase, E.; Ogawa, K.; Yano, M.; Koizumi, M.; Ito, C.; Furukawa, H. Quantitative Study of Flavonoids in Leaves of Citrus Plants. J. Agric. Food Chem. 2000, 48(9), 3865–3871. DOI: 10.1021/jf000100o.
- Shamloo, M. M.; Sharifani, M.; Daraei Garmakhany, A.; Seifi, E. Alternation of Secondary Metabolites and Quality Attributes in Valencia Orange Fruit (Citrus Sinensis) as Influenced by Storage Period and Edible Covers. J. Food Sci. Technol. 2015, 52(4), 1936–1947. DOI: 10.1007/s13197-013-1207-4.
- Kim, D. S.; Lim, S. B. Extraction of Flavanones from Immature Citrus Unshiu Pomace: Process Optimization and Antioxidant Evaluation. Sci. Rep. 2020, 10(1), 19950. DOI: 10.1038/s41598-020-76965-8.
- Mumivand, H.; Babalar, M.; Tabrizi, L.; Craker, L. E.; Shokrpour, M.; Hadian, J. Antioxidant Properties and Principal Phenolic Phytochemicals of Iranian Tarragon (Artemisia Dracunculus L.) Accessions. Hortic. Environ. Biotechnol. 2017, 58(4), 414–422. DOI: 10.1007/s13580-017-0121-5.
- Zhang, H. Y.; Yang, D. P.; Tang, G. Y. Multipotent Antioxidants: From Screening to Design. Drug Discov. Today. 2006, 11(15–16), 749–754. DOI: 10.1016/j.drudis.2006.06.007.
- Burda, S.; Oleszek, W. Antioxidant and Antiradical Activities of Flavonoids. J. Agric. Food Chem. 2001, 49(6), 2774–2779. DOI: 10.1021/jf001413m.
- Kris-Etherton, P. M.; Lefevre, M.; Beecher, G. R.; Gross, M. D.; Keen, C. L.; Etherton, T. D. Bioactive Compounds in Nutrition and health-research Methodologies for Establishing Biological Function: The Antioxidant and anti-inflammatory Effects of Flavonoids on Atherosclerosis. Ann. Rev. Nutr. 2004, 24(1), 511–538. DOI: 10.1146/annurev.nutr.23.011702.073237.
- Drewnowski, A.; Gomez-Carneros, C. Bitter Taste, Phytonutrients, and the Consumer: A Review. Am. J. Clin. Nutr. 2000, 72(6), 1424–1435. DOI: 10.1093/ajcn/72.6.1424.
- Yochum, L.; Kushi, L. H.; Meyer, K.; Folsom, A. R. Dietary Flavonoid Intake and Risk of Cardiovascular Disease in Postmenopausal Women. Am. J. Epidemiol. 1999, 149(10), 943–949. DOI: 10.1093/oxfordjournals.aje.a009738.
- Knekt, P.; Jarvinen, R.; Reunanen, A.; Maatela, J. Flavonoid Intake and Coronary Mortality in Finland: A Cohort Study. Brit. Med. J. 1996, 312(7029), 478–481. DOI: 10.1136/bmj.312.7029.478.
- Nichenametla, S. N.; Taruscio, T. G.; Barney, D. L.; Exon, J. H. A Review of the Effects and Mechanism of Polyphenolics in Cancer. Crit. Rev. Food Sci. 2006, 46, 161–183.
- Moon, Y. J.; Wang, X. D.; Morris, M. E. Dietary Flavonoids: Effects on Xenobiotic and Carcinogen Metabolism. Toxicol. Toxicology in Vitro. 2006, 20(2), 187–210. DOI: 10.1016/j.tiv.2005.06.048.
- Kris-Etherton, P. M.; Hecker, K. D.; Bonanome, A.; Coval, S. M.; Binkoski, A. E.; Hilpert, K. F.; Griel, A. E.; Etherton, T. D. Bioactive Compounds in Foods: Their Role in the Prevention of Cardiovascular Disease and Cancer. Am. J. Med The American J. Med. 2002, 113(9), 71–88. DOI: 10.1016/S0002-9343(01)00995-0.
- Asres, K.; Seyoum, A.; Veeresham, C.; Bucar, F.; Gibbons, S. Naturally Derived anti-HIV Agents. Phytother. ResPhytotherapy Res. 2005, 19(7), 557–581. DOI: 10.1002/ptr.1629.
- Cushnie, T. P. T.; Lamb, A. J. Antimicrobial Activity of Flavonoids. Int. J. Antimicrob. Agent Int J of Antimicrob Agents. 2005, 26(5), 343–356. DOI: 10.1016/j.ijantimicag.2005.09.002.
- Kim, H. P.; Son, K. H.; Chang, H. W.; Kang, S. S. Anti-inflammatory Plant Flavonoids and Cellular Action Mechanisms. J. Pharmacol. Sci. J. of Pharmacol. Sci. 2004, 96(3), 229–245. DOI: 10.1254/jphs.CRJ04003X.
- Kang, S. H.; Lee, Y. J.; Lee, C. H.; Kim, S. J.; Lee, D. H.; Lee, Y. K.; Park, D. B. Physiological Activities of Peel of Jeju-indigenous Citrus Sunki Hort. Tanaka. Korean J. Food Sci. Technol. 2005, 37(6), 983–988.
- Benavente-García, O.; Castillo, J.; Marín, F. R.; Ortuño, A.; Del Río, J. A. Uses and Properties of Citrus Flavonoids. J. Agric. Food Chem. 1997, 45, 4505–4515.
- Tijburg, L. B. M.; Mattern, T.; Folts, J. D.; Weisgerber, U. M.; Katan, M. B. Tea Flavonoids and Cardiovascular Diseases: A Review. Crit. Rev. Food Sci. 1997, 37, 771–785.
- Borrelli, F.; Izzo, A. A. The Plant Kingdom as a Source of anti-ulcer Remedies. Phytother. Res. 2000, 14, 581–591.
- Wightman, J. D. Red Berries and Their Health Benefits. Nutraceutical Beverages: Chemistry, Nutrition, and Health Effects (ACS Symp. Ser. 871),10. 2004, 123–132.
- Middleton, E.; Kandaswami, C. Effects of Flavonoids on Immune and Inflammatory Cell Functions. Biochem. Pharmacol. 1992, 43, 1167–1179.
- Javanmardi, J.; Stushnoff, C.; Locke, E.; Vivanco, J. M. Antioxidant Activity and Total Phenolic Content of Iranian Ocimum Accessions. Food Chem. 2003, 83, 547–550.
- Floegel, A.; Kim, D. O.; Chung, S. J.; Koo, S. I.; Chun, O. K. Comparison of ABTS/DPPH Assays to Measure Antioxidant Capacity in Popular Antioxidant-rich US Foods. J. Food Compost. Anal. 2011, 24, 1043–1048.
- Kim, Y. D.; Ko, W. J.; Koh, K. S.; Jeon, Y. J.; Kim, S. H. Composition of Flavonoids and Antioxidative Activity from Juice of Jeju Native Citrus Fruits during Maturation. Korean J. Nutr. 2009, 42(3), 278~290.
- Yamamoto, M.; Nishiguchi, N.; Shimada, A.; Matsumoto, R. Polymethoxylated Flavone Content of Major Cultivars and Local Accessions of Citrus Cultivated in Kagoshima, Japan. The Hortic J. 2019, 88(3), 320–328.
- Nogata, Y.; Sakamoto, K.; Shiratsuchi, H.; Ishii, T.; Yano, M.; Ohta, H. Flavonoid Composition of Fruit Tissues of Citrus Species. Biosci. Biotechnol. Biochem. 2006, 70(1), 178–192.
- Jeong, S. M.; Kim, S. Y.; Kim, D. R.; Jo, S. C.; Nam, K. C.; Ahn, D. U.; Lee, S. C. Effect of Heat Treatment on the Antioxidant Activity of Extracts from Citrus Peels. J. Agric. Food Chem. 2004, 52, 3389–3393.
- Yang, Y. T.; Kim, H. B.; Lee, S. H.; Park, Y. C. Composition Characteristics of Flavonoids in Citrus Juice. Hortic. Sci.Tech. 2019, 37(5), 651–662.
- Moon, S. H.; Assefa, A. D.; Ko, E. Y.; Park, S. W. Comparison of Flavonoid Contents and Antioxidant Activity of Yuzu (Citrus Junos Sieb. Ex Tanaka) Based on Harvest Time. Kor. J. Hort. Sci. Technol. 2015, 33(2), 283~291.
- Lim, H. K.; Yoo, E. S.; Moon, J. Y.; Jeon, Y. J.; Cho, S. K. Antioxidant Activity of Extracts from Dangyuja (Citrus Grandis Osbeck) Fruits Produced in Jeju Island. Food Sci. Biotechnol. 2006, 15(2), 312–316.
- Floegel, A.; Kim, D. O.; Chung, S. J.; Koo, S. I.; Chun, O. K. Comparison of ABTS/DPPH Assays to Measure Antioxidant Capacity in Popular antioxidant-rich US Foods. J. Food Compost. Anal. 2011, 24, 1043–1048.
- Ghasemi, K.; Ghasemi, Y.; Ebrahimzadeh, M. A. Antioxidant Activity, Phenol and Flavonoid Contents of 13 Citrus Species Peels and Tissues. Pak. J. Pharm. Sci. 2009, 22(3), 277–281.
- Xu, G.; Liu, D.; Chen, J.; Ye, X.; Ma, Y.; Shi, J. Juice Components and Antioxidant Capacity of Citrus Varieties Cultivated in China. Food Chem. 2008, 106(2), 545–551.
- Barreca, D.; Bellocco, E.; Caristi, C.; Leuzzi, U.; Gattuso, G. Flavonoid Composition and Antioxidant Activity of Juices from Chinotto (Citrus X Myrtifolia Raf.) Fruits at Different Ripening Stages. J. Agric. Food Chem. 2010, 58, 3031–3036.