?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
This study examined the structural and physicochemical characteristics of starch isolated from seven yellow cassava genotypes. The structural properties of yellow cassava starch from these cultivars were elucidated by scanning electron microscopy, X-ray diffractometry and Fourier Transformed Infrared Spectroscopy (FTIR). Their water interaction properties, digestibility and viscoelastic behavior were also compared, and principal component analysis was used to establish factors associated with the variability in properties of the starch. All the starches were of the A-type diffraction pattern, with crystallinity ranging between 31 and 37%. Most of the granules exhibited spherical and oval shapes, some with a flat surface on one side. They had smooth surfaces and their sizes ranged from 4 µm for round granules to 23 µm for the major axis of oval-shaped granules. Significant differences (p < .05) were observed in amylose content, in-vitro digestibility, peak and breakdown viscosity of the starches, and these ranged between 13.6–18.1%, 11.4–18.5%, 354–520 BU and 233–366 BU, respectively. Significant differences were also recorded in the hydration and textural behavior of starches from these cassava cultivars. The differences observed in granular and physicochemical properties are likely to influence the performance of these cassava cultivars in food applications.
Introduction
Cassava is an important domestic and industrial crop in many parts of the world. Its food and industrial uses have been well documented. Starch is the main biomolecule in cassava, making up 75–85% of the dry matter of the edible root. Starch is composed of two polyglucans, amylopectin and amylose, which constitute 70–85% and 15–30% of its weight, respectively.[Citation1] While amylopectin is bulky, extensively branched and held together by α-1,6 and α-1,4 glycosidic bonds, amylose is largely linear and possesses much longer chains interconnected through α-1,4 glycosidic bonds. A small fraction of amylose, however, remains slightly branched.[Citation1] As indicated elsewhere,[Citation2] the semi-crystalline structure, granular properties, fine structure of amylopectin among other starch properties are crucial for determining the quality and end user experience of starchy foods.
To enrich the nutritional properties of cassava, elite yellow varieties with improved β-carotene levels, have been developed. While these are reported to have increased fat and reducing sugar content and longer storage ability, they generally have a low dry matter content.[Citation3,Citation4] Esuma et al.,,[Citation5] reported a mean dry matter content in some varieties to be lower than the levels (35%) in improved white varieties developed in Uganda. A study by Akinwale et al.[Citation6] showed that the deeper the yellow color in cassava, the lower its dry matter. A more recent study also reported a strong negative correlation between dry matter and carotenoid content in many yellow varieties.[Citation7] A similar relationship between dry matter or starch and carotenoid content has been reported in other crops such as sweetpotato[Citation8] and butternut squash.[Citation9] This reduction in dry matter ultimately affects the starch content since there is a genetic linkage between starch content and dry matter content of tuber crops such as cassava and sweetpotato.[Citation3]
Evidence suggests that the development of new variants of existing crops affect the functional behavior of the biomolecules such as starch, as reported in studies involving rice,[Citation10] maize, potato and sweetpotato.[Citation11] These differences include texture, appearance and cooking characteristics. In yellow cassava, differences in functionality of starches have manifested as vast differences in product behavior and quality. For instance, Vimala[Citation12] reported a diversity in the cooking quality of nearly 40 yellow cassava clones. To advance the fight against VAD using improved crop varieties, new lines of yellow cassava are under investigation. However, food application of these cultivars will be largely influenced by their starch properties. Studies on cassava starch have been widely reported, but many of these studies focused on the starch from white variants. Hence, it is important to extensively assess starch from newly developed yellow cassava genotypes to better understand their behavior, as this will guide targeted end utilization in both food and industrial applications, and also for further crop development. The aim of this study, therefore, was to examine the physicochemical functional and structural characteristics of starch from seven yellow cassava genotypes.
Materials and methods
Source of reagents and experimental materials
All the reagents used in the study were of analytical grade and were used as-is. Dimethyl Sulfoxide (DMSO) and Porcine pancreatic α-amylase were purchased from Sigma Chemical Co. (St. Louis, MO, USA). Seven yellow cassava cultivars were obtained from demonstration plots of the Council for Scientific and Industrial Research – Savannah Agricultural Research Institute. The cultivars, 11011797, 1082264, 1011412, 1083774, 1083594, 1090151, 1083461, were correspondingly designated as “S1,” “S2,” “S3,” “S4,” “S5,” “S6” and “S7” for easy referencing. After harvest, unblemished roots were washed and packaged into jute sacks and immediately transported to the laboratory for starch extraction.
Starch extraction
Yellow cassava starch was isolated following the method of Zhu et al.,[Citation11] with slight modifications. Fresh cassava roots were washed twice in potable water before manually peeling with a stainless-steel knife. Peeled tubers were washed thoroughly under running water, sliced and subsequently milled into a slurry in a laboratory blender (Waring E8420) using water to aid the process. The slurry was strained through a cheese cloth and the filtrate was left undisturbed for 4 h. The yellowish supernatant was discarded and the starch layer re-suspended in water and filtered through a 150 µm mesh. The starch was allowed to settle after standing for another 2 h, and the supernatant was discarded. Re-suspension was repeated once until a clear colorless supernatant was observed. Thereafter, the suspension was filtered (100 µm mesh) and allowed to settle overnight at 4°C. The clear supernatant was decanted and the wet starch dried in an air oven at 40°C for 6 h. The dried starch was milled into fine powder and sealed air-tight in high-density polyethylene (HDPE) bags for analyses.
Chemical composition of the yellow cassava starch
The starch was analyzed for protein, total fat, ash and starch content approved methods of the AOAC International.[Citation13]
Paste clarity and granular morphology
Starch paste clarity was determined on 1% starch suspension incubated in a boiling water bath for 30 min with continual shaking, according to Lawal,[Citation14] with slight modification. The suspension was cooled to room temperature (28°C) and its transmittance measured against a water blank at 650 nm on a double beam UV–VIS spectrophotometer (T80, PG Instruments, Leicestershire UK). Electronic micrographs of representative starch samples (pooled from replicates) were obtained using a Scanning Electron Microscope (SEM) (Phenom Desktop, Phenom-World, The Netherlands) after sputter-coating (108 Manual Sputter Coater, Ted Pella Inc. USA) with gold for 50 s. Images were obtained at an accelerating voltage of 15 kV and a magnification of 2500x.
Amylose determination
Amylose was determined following the method of Hoover and Ratnayake.[Citation15] Starch (20 mg) was dissolved in 8 mL of 90% DMSO in screw capped tubes and vigorously vortexed for 2 min. The tubes were heated in a water batch at 85°C for 15 min, with intermittent mixing, cooled to room temperature (28°C) and the content diluted with water to 25 mL in a volumetric flask. An aliquot (1 mL) of the dilute solution was mixed with 40 mL of water in a 50 mL volumetric flask. Five milliliters (5 mL) of I2/KI solution was then added before adjusting the final volume to 50 mL. The solution was vortexed, allowed to stand for 15 min for color development before measuring its absorbance at 600 nm.
Swelling and solubility indices
Starch swelling power and solubility index were determined as described elsewhere.[Citation16] Briefly, 150 mg of starch was mixed with 10 mL of distilled water and thoroughly vortexed for 30 s. The starch suspension was incubated in a water bath at 85°C for 30 min and later cooled to room temperature, centrifuged at 2000 × g for 35 min and decanted. The sediment was weighed directly, whereas the supernatant was dried to constant weight in a hot air oven. Swelling power (g/g) and solubility index (%) were calculated as follows;
In-vitro starch digestibility
Starch digestibility was estimated in vitro based on Zhang et al.,[Citation17] with slight modification. Starch (500 mg) was weighed into a previously weighed centrifuge tube, followed by 15 mL of phosphate buffer (0.15 M, pH 6.5), 30 mg CaCl2, 30 mg gelatin and 30 mg of pancreatin (Sigma Aldrich, St. Louis, USA), and incubated at 37°C. The tubes were shaken constantly to keep the starch in suspension for 6 h. Afterward, 5 mL of 1% H2SO4 was added to stop the reaction before centrifuging (5,000 × g) for 15 min, gently decanting and resuspending the sediment in 15 mL of 80% ethanol. This was followed by another round of centrifuging (5,000 × g for 5 min) before the supernatant was decanted. The pellet in the centrifuge tube was dried at 70°C to constant weight and starch digestibility calculated as follows;
X-ray diffraction
X-ray diffractograms of yellow cassava starch were obtained using a powder X-ray Diffractometer with a Cu anode (PANalytical Empyrean, Malvern Panalytical, UK).[Citation18] Crystallinity (%) was estimated as the ratio of area under the peaks to the total area under the curve using OriginPro 8.5 (OriginLab, Northampton USA).
FTIR spectroscopy
Fourier transformed infrared (FTIR) spectra were obtained by scanning powdered starch samples from 400 to 4000 cm−1 using a Spectrum II spectrometer (Perkin Elmer, UK) with a single bound diamond crystal Attenuated Total Reflectance (ATR) accessory. Starch samples were pressed onto the diamond probe and scanned at a resolution of 4 cm−1 .[Citation19] Spectral analysis was carried out on different samples and spectra representative of a single sample was reported.
Pasting properties
Pasting characteristics of cassava starch were quantitatively determined on an 8% suspension, using the Brabender Viscoamylograph E (Brabender Inc., Germany) with a 750 cmg cartridge. The starch suspension was heated from 50°C to 95°C at a rate of 1.5°C/min, held for 15 min at 95°C, cooled to 50°C at a rate of −1.5°C/min and held at 50°C for 15 min. Pasting indices including peak viscosity (highest viscosity obtained during the heating cycle), pasting temperature (temperature at which a significant change in viscosity occurred from the onset of heating), cool paste viscosity (viscosity of at the end of the cooling period), breakdown (difference between peak viscosity and trough viscosity) and setback viscosity (difference between peak viscosity and final viscosity) were derived from the profile using the Viscograph Software (Brabender Inc, Duisburg, Germany), as described by Akonor et al.[Citation16]
Gel texture analyses
For the purpose of gel texture analyses, a 10% cassava starch slurry was run in a Brabender viscoamylograph using the profile already described. The starch gel was allowed to cool to room temperature before evaluating its firmness and consistency by back extrusion. A portion of the gels were kept at 4°C overnight to set, and used for Texture Profile Analyses (TPA).[Citation11]
Back extrusion
The method described by Nasaruddin et al.[Citation20] was used for gel texture analyses by back extrusion. Back extrusion test was performed on 10% gel in a Perspex back extrusion rig, using a texture analyzer (TA-XT2 Plus, Stable Micro Systems, Surrey, UK) equipped with 5 kg load cell. The gel was filled into the Perspex rig to a height of 70 mm and compressed to a depth of 45 mm with a 40 mm plunger at a test speed of 1 mm/s. Gel firmness (the maximum compression force) and consistency (the area under the curve during the extrusion thrust) were reported in this test (Exponent Software, Stable Micro Systems, Godalming, UK).
Instrumental texture profile analysis (TPA)
TPA was performed on 10% starch gels from the pasting analysis using a texture analyzer (TA.XTplus, Stable Micro Systems, Surrey UK) with a 75 mm platen probe.[Citation16] A double bite compression cycle in which the probe was set to compress starch gels to about 50% of its height with a trigger force of 5 g, test speed of 1 mm/s per cycle, was used. Adhesiveness, springiness and cohesiveness of starch gels were also derived by the Texture Exponent software (Stable Micro System, Surrey UK) through integration of the area underneath the curve. All samples were tested in triplicates.
Experimental design and statistical analysis
A completely randomized design was used in this study, in which cultivar was considered as the principal factor. Analyses were run in triplicates and the data obtained were analyzed using single-factor ANOVA (Minitab 17.0.1) to compare means. The level of significance was set at p < .05. Principal component analyses (PCA) was performed on all relevant variables to examine variations observed in physicochemical, morphological and functional properties of the starches (XLSTAT, 2019.2.2, Addinsoft USA).
Results and discussions
Chemical composition of starch from yellow cassava
The chemical composition of starch isolated from the seven cultivars of yellow cassava is presented in . The results showed that, on the whole, very little amounts of other components were associated with the cassava starch extracted. The total starch content of samples ranged between 98 and 99%. While protein and fat were found in trace amounts (less than 0.5%), mineral levels ranged between 0.01 and 0.2%. The starches were without any significant amounts of fiber (0.01–0.2%), which may be from fibrous pith or cell wall materials encasing the starch granules.[Citation21] These results indicate a high purity of starch from the seven yellow cassava cultivars. Proteins, fat and minerals (such as phosphorus) are known to affect the functionality of starch.[Citation22] However, their presence in this study was trace and was therefore not expected to influence the functionality of the starches remarkably.
Table 1. Chemical composition of starch from seven yellow cassava cultivars (%db).
Optical and morphological characteristics of starch from yellow cassava
An important optical property of starches in food processing is the clarity of its paste when it is suspended in water and cooked. It is believed to occur because starch granules reflect light when swollen. This is particularly useful in the manufacture of jellies, fruit pastes and other products which require high transparency.[Citation23] The paste from Cultivar S4 was the most transparent, with a transmittance of nearly 56% (). This suggests that starch paste from Cultivar S4 contained lesser swollen granule remnants, and did not scatter as much light, but rather, allowed more light to pass through the paste. Paste from this cultivar may be useful in the production of fruit pie filling, which is usually transparent. On the other hand, Cultivar S5 was the opaquest, with less than 50% transmittance and would suit the manufacturing of salad dressing, a product which does not necessarily require high clarity. The presence of short chain amylose and amylopectin fractions, swollen granule remnants and the interaction of these materials in the paste,[Citation24] and the interactions between leached granules explain the differences observed in the clarity of starch pastes from these cassava cultivars. Albeit slight (r = −0.370, p = .413), starch amylose content had a negative influence on paste clarity. Paste clarity obtained in this study was higher (more transparent) than values reported in starches from other tuber crops such as sweetpotato (<40%), yam and taro (<10%) (Aprianita et al., 2009), tigernut (15–31%) .[Citation16]
Table 2. Optical and granular characteristics of starch from seven yellow cassava cultivars.
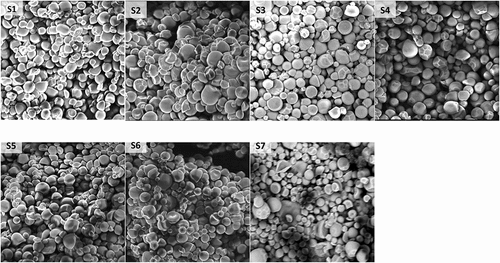
Electron microscopy revealed similarity in the morphological arrangement of starch granules among the various cultivars. Starch from the yellow cassava cultivars were composed of granules of varying sizes, ranging from 4.0 to 22.3 μm, with an overall mean of 10.2 μm. The seven cultivars were mainly composed of medium-sized granules (up to 22 μm), interspersed with small granules, measuring less than 10 μm. In these cultivars, an obvious bimodal distribution of granules was seen () between medium and small granules firmly clustered together to form a dense mass. Cultivars S2, S4 and S5 had granules of fairly uniform sizes, while Cultivar S4 was composed of small starch granules, measuring approx. 8.9 μm. Granule size of starch from these cultivars compares well with earlier studies on cassava starch in which sizes of 8–17 μm and 5–40 μm were reported by Rolland-Sabate et al.[Citation25] and Moorthy,[Citation26] respectively.
The starch granules had smooth surfaces, showing no evidence of pinholes or cracks as seen in , and were mainly spherical or oval, with other kettle drum and irregular shapes, typical of root and tuber starches, resembling the starch granules described by Moorthy.[Citation26] The micrographs also revealed truncated granular shapes similar to those observed independently by Mweta et al.,[Citation27] Ceballos et al.,[Citation28] and Vasconcelos et al.,[Citation29] in different cassava germplasms. However, no distinct shape(s) was observed to clearly distinguish between starches from the seven cultivars. The morphological features of these cultivars appear comparable to starch granules from other tuber crops such as sweetpotato.
Amylose and amylopectin are important components of starch which influences many starch properties and utilization potential. The yellow cassava starches exhibited differences in amylose content obtained by iodine binding, with levels ranging from 13.6% for Cultivar S4 to 18.1% for Cultivar S2 (). Whereas amylose primarily affects starch technological functionality including swelling, viscosity and product quality such as staling in bread, amylopectin influences gelatinization temperature[Citation18] and improves the sheen of products such as noodles.
Table 3. Physico-chemical properties of starch from seven yellow cassava cultivars.
These values were generally lower than the amylose content of 17–20% reported for cassava starch by Nuwamanya et al. .[Citation30] However, the amylose content of Cultivar S2 was comparable to 18.4% for starch from a wild cassava genotype[Citation31] and slightly higher than 17.3% in Torruco-Uco et al.[Citation32]
The ability of the starches to swell in excess water during heating together with the extent of their solubility varied widely among the seven cultivars. These indices depict the interaction of the constituent polymers with water. Swelling power was highest (33.8 g/g) for Cultivar S6 and lowest (27.3 g/g) for cultivar S5. Starch swelling is thought to be a property of amylopectin, with amylose acting as a diluent. Therefore, differences in starch swelling among these cultivars may be ascribed to variations in their amylose content and amylopectin chain length distribution.[Citation33] The extent of intermolecular bonding and differences in amylose content may also account for the varying swelling power among the starches. Albeit not a perfect fit, a pattern of high amylose – low swelling power (r = −0.703, p = .048) was observed in this study, which supports findings of earlier studies.[Citation11,Citation34] Water solubility index measures the extent of free amylose released from starch granules during heating in excess water, and varies from one botanical source to another. In this study, a range of 13 to 19% solubility was recorded, which is essentially comparable to 13% and 13.4–14.1%, respectively, reported by Gomand et al.,[Citation35] and Ceballos et al,[Citation28] but higher than values of waxy cassava starch (6.0%) obtained by Ceballos et al .[Citation28] Solubility index was strongly correlated (r = 0.836, p = .021) with amylose but negatively correlated (r = −0.886, p = .021) with crystallinity of the starch. ANOVA showed significant differences (p < .05) in swelling power and water solubility index among the seven cultivars studied, indicating differences in the level of interaction between starch chains within the amorphous and crystalline regions in granules of the various starches.
Starch digestibility affects the glycemic index of food and is influenced by the amylopectin structure, amylose content, granule size, granule structure.[Citation36] In this study, in vitro digestibility of raw cassava starch showed wide variation (11.4–18.49%) among the seven cultivars, with a mean digestibility of 15% (). Digestibility values obtained were low because uncooked native starch granules are densely packed, movement of their polymer chains is restricted, and the double helix conformation of their amylose is intact.[Citation37] The results were comparable to the digestibility of yam starch (16.55%) but lower than Taro starch (51.22%) and sweetpotato starch (98.95%) reported by Aprianita et al.[Citation38] The values were also lower than the digestibility values of different varieties of Chinese sweetpotato (29.5–41.2%),[Citation11] and 21.8% for uncooked native sweetpotato starch.[Citation39]
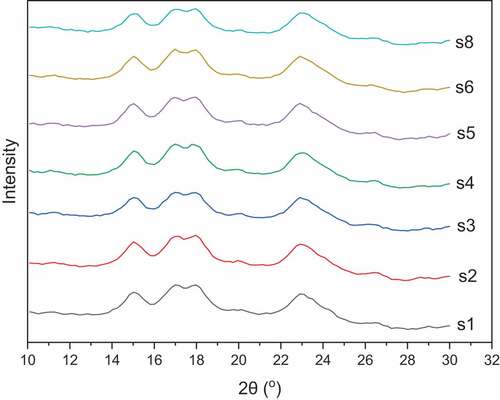
Wide angle X-ray Diffraction has been used severally to detect the crystalline structure of starch granules. Diffractograms of starches from the seven yellow cassava cultivars are presented in . The results showed that starches from these cassava varieties had similar diffraction patterns (A-type polymorph), but varying relative crystallinity. Without an exception, the starches exhibited strong diffraction peaks at of 15.0° and 22.9°, dual peaks occurring at 17.0° and 17.9° and a weak peak at 26.5°, an observation which is characteristic of A-type starch polymorphs. Starches, according to their x-ray diffraction pattern, exhibit three polymorphs, namely A-, B- and C-type. The A and B types are mainly found in cereal and tuber starches, respectively, while the C-type is a mixture of both A and B polymorphs and it is seen mainly in legume starches.[Citation40] That notwithstanding, many tuber starches including cassava starch also show A-type X-ray diffraction pattern, as observed in this study. The diffraction pattern observed in this study corroborates the findings of Gomand et al.,[Citation35] and Charoenkul et al.,[Citation41] who also reported strong diffraction peaks at 15°, 17°, 17.9° and 22.9°. However, these results of this study are inconsistent with Huang et al.[Citation42] and Rolland-Sabate et al.,[Citation43] who, both, found a C-type diffraction pattern; and Mbougueng et al.[Citation44] who reported a B-type polymorph for native cassava starch. The crystallinity pattern exhibited by starch is directly related to the chain length of its amylopectin. Generally, A-type starches are thought to have shorter chain length (<19.7) compared to starches of the B-type (≥21.6) and C-type (20.3–21.3) polymorphs forms, and digest faster because they contain less water molecules than the B- and C-types .[Citation45]
The crystalline structure of starch granules is associated with the packing of double helices of amylose and the structural water content. A-type starches contain less structural water compared to the other polymorphic forms. In this study, relative crystallinity ranged between 31 and 37% (), which is lower than 40–49% for wild type and amylose free cassava by Gomand et al.,[Citation31] and 42% reported by Dome et al.,[Citation46] but comparable to 30% reported by Ren,[Citation47] 37% by Kaewtatin and Tanrattanakul[Citation48] for cassava starch. Differences in relative crystallinity of the starches may be ascribed to differences in amylose content. In this study, a reasonable association existed between amylose content and crystallinity, in which sample with low amylose content had higher crystallinity. However, in agreement with Li et al.,[Citation49] this relationship was not linear. Other factors that influence starch crystallinity include amylopectin-chain length,[Citation50] amylose lipid complexation[Citation51] and starch granule size.[Citation52]
FTIR spectroscopy
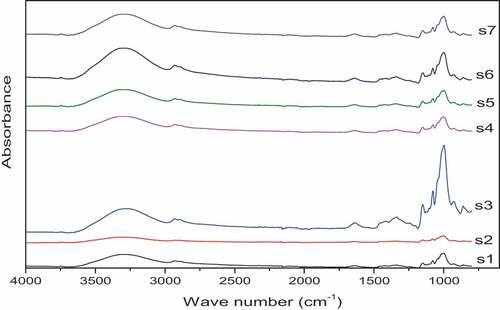
Infrared spectra of starch samples result from the vibrational modes of amylose and amylopectin, which are sensitive to changes in molecular structure, chain conformation and crystallinity.[Citation53] FTIR analysis of the yellow cassava starches showed slight differences in their transmittance intensity observed in the OH vibration region (3650–3000 cm−1) and the fingerprint region (below 1500 cm−1) (). For instance, whereas Cultivars S2 and S3 represented the extremes of signal intensities, Cultivars S4 and S5 closely resembled each other, showing intensities between the two extremes. This observation is ascribed to slight variations in amylose content, granular morphology and crystallinity as indicated by chemical analysis, SEM and XRD results. Whereas the sharp peak at 2932 cm−1 is characteristic of C – H stretching, the broad peak spanning from 3000 through 3500 cm−1 represents the complex vibrational stretching associated with the hydroxyl group which constitutes the gross structure of starch.[Citation54] Cultivar S6 had the highest peak due to OH stretching, whereas Cultivar S3 had the highest peak around the 995 region. In all cases, however, Cultivar S2 had the lowest peaks.
The shape of the infra-red spectra was similar among all the varieties, indicating that there were no conformational differences in the chemical groups of starch samples examined. The spectra in the region of 950 to 1075 is mainly characterized by three major modes with maximum absorbances at 1074, 1022 and 995 cm−1, arising out of C – O bond stretching. Bands at 1047 and 1022 cm−1 correspondingly depict the ordered and amorphous structures of starch, and the ratio of these two is used to quantify the degree of order.[Citation53] It ranged from 0.72 in sample S2 to 0.76 in sample S4. This implies that the S2 had a lower level of order compared to S4, as confirmed by the crystallinity results (). In this study, intensive signals were recorded around 995 (shift to 999, 1000) and 1074 cm−1. The peak around 1022 was unclear in all the samples, implying an appreciable level of crystallinity, as revealed by the X-ray diffraction analysis. This observation is similar to Sevenou et al.,[Citation55] who observed indistinct peaks at 1022 cm−1 in potato starch, which is also a tuber starch. Indeed, the 1022 band, which is strongly influenced by percentage amorphous fraction, increases with decreasing crystallinity and has been shown to be visible in freshly prepared hot starch gels.[Citation53]
Peaks observed around 1157 and 1105, which are attributed to C–O and C–C stretching, were also distinct as noted in other A-type starches. Peaks observed at 1409 and 1433 cm−1 depict C-H bending of CH2, while peaks at 1240, 1299 and 1333 represent O-H bending.[Citation56] The tightly bound water in starch is represented by the single peak that appeared around 1644 cm−1 .[Citation54]
Pasting and gel texture properties of yellow cassava starch
Starch from the seven yellow cassava cultivars showed similarities in their pasting profile, which was characterized by high peak and high breakdown viscosities. That notwithstanding, some of their pasting indices showed notable differences. Pasting time ranged between 11.1 and 13.5 min for Cultivars S7 and S2, respectively (), and pasting temperature ranged between 65.6 and 69.1°C with a mean of 66.9°C. Pasting temperature marks the commencement of the pasting process, which occurs after majority of the starch granules have gelatinized. At this point, there is a dramatic increase in viscosity,[Citation24] which primarily reflects the ease of swelling and cooking of starches. The pasting temperatures varied significantly (p < .05) among the cultivars, emphasizing the variability of molecular bonding forces among them. The high pasting temperature of Cultivar S2, for example, indicates its higher resistance to swelling compared to the other cultivars.
Table 4. Pasting properties of starch from seven yellow cassava cultivars.
Peak viscosity represents the highest viscosity attained during pasting and it is related to swelling power, which is, in turn, influenced by amylose content, granule size and other factors. Accordingly, Cultivar S6, which had the highest swelling power, recorded the highest peak viscosity of 510 BU. In this experiment, amylose content had a greater influence on the peak viscosity (r = −0.883, p = .008), since there was not much difference among the samples in respect of their granule size (r = −0.011, p = .981). Generally, starch from the cultivars with low amylose content exhibited high peak viscosities and low paste stability. This observation agrees with findings of previous studies by Zhu et al..[Citation11] These cultivars could be suitable in food processing applications such as puddings, in which high viscosity is required. The relatively higher amylose content in Cultivar S2 may have contributed to its lower peak viscosity. Peak viscosity values obtained were higher than the range (270.7–380.7 BU) reported by Afoakwa et al.,[Citation57] for six improved cassava varieties, but lower than values by Asare et al.,[Citation58] for native cassava starch.
Beyond the peak viscosity, viscosity of the starch paste reduced because of high temperature and continuous shearing. At this point, there is a gradual disruption of intermolecular bonds between starch chains. This thinning effect, known as breakdown viscosity, denotes the strength or stability of the gel formed. The stronger the intermolecular bonds, the lower the extent of disruption and the higher the paste stability. As indicated by their breakdown viscosity, cultivars S3 and S4 had a comparable gel strength. The remaining cultivars had significantly different gel-holding strengths. The low breakdown ratio of S4, for example, makes it suitable for use in food products such as noodles.
Setback is an important index in starch pastes, representing the phenomenal reassociation and partial re-crystallization of amylose and amylopectin molecules when starch pastes are cooled. Depending on the food application of interest, setback may or may not be desirable. Setback ratio, an index for predicting retrogradation tendency[Citation18] varied slightly among the starches but, Cultivars S1 and S7 were distinctly different (p < .05) from the rest, recording the lowest (1.38) and highest breakdown ratio (1.91), respectively. This indicates that “Cultivar S7” has a higher propensity to retrogradation compared to “Cultivar S1,” and may therefore be a comparatively poorer option for use in bakery products. In agreement with Zhu et al.,[Citation11] the results did not indicate an obvious association between amylose content and starch setback. This outcome was unexpected, since starch from these two cultivars did not have the lowest and highest amylose values among the lot. Perhaps, setback may have also been influenced by other starch properties such as amylopectin-chain length,[Citation59] which may interfere with the reassociation of amylose during cooling.
Gel texture properties have been correlated with starch granule size, with bigger granules forming softer, adhesive and cohesive gels compared to smaller starch granules.[Citation11] The yellow cassava starch exhibited differences in their gel texture properties. For instance, the firmest gel was obtained from Cultivar S2, while the most consistent, adhesive and cohesive gel was obtained from Cultivar S5 (). Starch gel from Cultivar S3 was the softest and the least consistent among the yellow cassava genotypes examined. Significant differences (p < .05) were recorded in the firmness of gels from the different cultivars, which ranged between 2.7 and 4.0 N. Gel firmness is caused by retrogradation, a phenomenon which primarily is a property of amylose,[Citation60] and this may explain why gel from cultivar S2 was the most firm. Also, Cultivar S7 which had the highest setback propensity, had a similar gel firmness as cultivar S2. Consistency impacts on mouthfeel and tongue propulsive forces required for swallowing liquid and semi-solid foods.[Citation61] The low consistency of gels from Cultivar S3 may have an undesirable impact on the mouthfeel of semisolid products made from this cultivar, even though they might be easier to swallow.
Table 5. Gel texture properties of starch from seven yellow cassava cultivars.
Adhesiveness of the gels ranged from 0.79 N for Cultivar 7 to 0.83 N for both Cultivars S1 and S6, with a mean of 0.84 N. ANOVA showed marginal differences in adhesiveness for the different starches apart from Cultivar S7 which was the least adhesive and significantly (p < .05) deviated from the mean. Springiness reflects the tendency of a deformed material to return to its initial state after the force of deformation is removed.[Citation62] Gels made from cultivar S5 recorded the highest springiness (0.76), and this was not surprising, as cultivar S5 had higher gel stability (the lowest breakdown viscosity). A plausible explanation would be the formation of a well-structured intermolecular network which makes the gel less prone to breakdown, hence increasing springiness. That notwithstanding, gel texture and strength may not be fully explained on the basis of the pasting properties alone. Even though a narrow range (0.13) of springiness was observed among the cultivars, variation among them were significant, and this may be due to differences in gel strength. Cohesiveness ranged from 519 to 839 and reflects the gel’s ability to withstand deformation.[Citation62] This is an important feature in solid starchy foods, and this is affected by the retrogradation of amylose. The gels showed significant differences (p < .05) in their cohesiveness, which may be attributed to differences in amylose content, extent of amylose leaching and its subsequent reassociation.[Citation63]
Principal component analysis
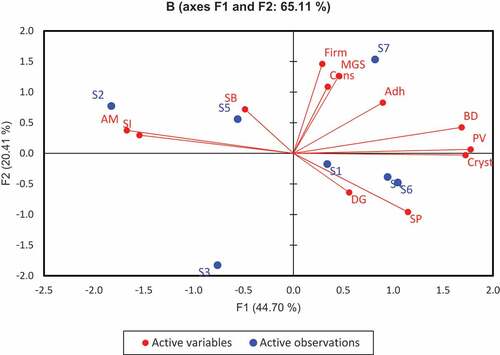
PCA was used to establish the relationship between starches from the seven cassava genotypes and also to determine the main factors associated with the variability in these starches. The first two principal components, F1 and F2, accounted for about 65.1% of the variability among starch samples (). Most of the variance was explained by the first principal component (F1), which was mainly associated with amylose, crystallinity, hydration properties (swelling power and water solubility index), peak viscosity and breakdown viscosity. The second principal component (F2), on the other hand, was characterized by texture properties of their gels (firmness and consistency) and mean starch granule size. The plot further reveals some association between the cassava genotypes. For instance, whereas Cultivar S4 and Cultivar S6 were closely related (by digestibility and swelling power) and had positive scores on F1, Cultivar S2 and S5 had a negative score on the same principal component axis, and were marked by their amylose, solubility and setback viscosity. Cultivar S7 was associated with high firmness and consistency on F2. Again, amylose content, swelling power and solubility index, crystallinity, peak viscosity and breakdown viscosity (similar among Cultivars S1, S2, S4, S5 and S6) loaded heavily on F1, whereas firmness, mean granule size and consistency were found on F2 (among Cultivars S3 and S7). The wide dispersion in observed in the PCA scores indicate that the differences in genotypes significantly affect the physicochemical characteristics of the starch, as reported by Li et al.[Citation64]
Figure 4. PCA biplot for physicochemical and functional properties of starch from seven yellow cassava cultivars.
AM – amylose, SI – water solubility index, SB – setback viscosity, BD – breakdown viscosity, PV – peak viscosity, Adh – adhesiveness, Cons – gel consistency, Firm – gel firmness MGS – mean granule size, Cryst – crystalinity, DG – digestibility, SP – swelling power.
Conclusion
There were considerable variations in physicochemical, structural, morphological and pasting properties among starches from the seven yellow cassava cultivars, making them suitable for use in processing diversity of food products. Amylose content of the starches and their digestibility, respectively, ranged from 13.6 to 18.1%, and 11.4 and 18.5%, while their morphological properties were marked by smooth surfaced spherical and oval-shaped granules, with granule size ranging from 4 to 22 µm. All the starches were of the A-type polymorph with no difference in their conformational structure, suggesting that the seven cultivars belong to a similar botanical complex. The diversity observed in the functionality of the starches provide useful information for assigning different cultivars to specific end-use, while ensuring a diversity of consumer preferences are met. Findings from this study could be beneficial for food and industrial utilization of yellow-fleshed cassava. For instance, the starches with high clarity may be useful as a thickener or coating in food and pharmaceutical applications while those with low peak viscosity may be used on infant foods. Also, those with high amylose and/or low digestibility be suitable for designing food for diabetics.
Acknowledgments
The technical assistance provided by the CSIR-Food Research Institute is duly acknowledged.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Bertoft, E. Understanding Starch Structure: Recent Progress. Agronomy. 2017, 7, 56. DOI: 10.3390/agronomy7030056.
- Zhang, Y.; Wang, Q.; Zhang, Y.; Wu, G.; Tan, L.; Zhang, Z. Effects of Moisture Content on Digestible Fragments and Molecular Structures of High Amylose Jackfruit Starch Prepared by Improved Extrusion Cooking Technology. Food Hydrocolloids. 2022, 133, 108023. DOI: 10.1016/j.foodhyd.2022.108023.
- Beyene, G.; Solomon, F. R.; Chauhan, R. D.; Gaitan-Solis, E.; Narayanan, N.; Gehan, J.; Siritunga, D.; Stevens, R. L.; Jifon, J.; Van Eck, J., et al. Provitamin A Biofortification of Cassava Enhances Shelf Life but Reduces Dry Matter Content of Storage Roots Due to Altered Carbon Partitioning into Starch. Plant Biotechnol. J. 2018, 16, 1186–1200. DOI: 10.1111/pbi.12862.
- Njoku, D. N.; Gracen, V. E.; Offei, S. K.; Asante, I. K.; Egesi, C. N.; Kulakow, P.; Ceballos, H. Parent-offspring Regression Analysis for Total Carotenoids and Some Agronomic Traits in Cassava. Euphytica. 2015, 206, 657–666. DOI: 10.1007/s10681-015-1482-4.
- Esuma, W.; Kawuki, R. S.; Herselman, L.; Labuschagne, M. T. Diallel Analysis of Provitamin A Carotenoid and Dry Matter Content in Cassava (Manihot Esculenta Crantz). Breed. Sci. 2016, 66, 627–635. DOI: 10.1270/jsbbs.15159.
- Akinwale, M. G.; Aladesanwa, R. D.; Akinyele, B. O.; Dixon, A. G. O.; Odiyi, A. C. Inheritance of ß-carotene in Cassava (Manihot Esculenta Crantz). Int. J. Genet. Mol. Biol. 2010, 2, 198–201.
- Olayide, P.; Large, A.; Stridh, L.; Rabbi, I.; Baldermann, S.; Stavolone, L.; Alexandersson, E. Gene Expression and Metabolite Profiling of Thirteen Nigerian Cassava Landraces to Elucidate Starch and Carotenoid Composition. Agronomy. 2020, 10, 424. DOI: 10.3390/agronomy10030424.
- Vimala, B.; Sreekanth, A.; Binu, H.; Wolfgang, G. Variability in 42 orange-fleshed Sweet Potato Hybrids for Tuber Yield and Carotene and Dry Matter Content. Gene Conserve. 2011, 40, 190–200.
- Ortiz, S.; Valdés, M. P.; Vallejo, F. A.; Baena, D. Genetic Correlations and Path Analysis in Butternut Squash Cucurbita Moschata Duch. Rev. Fac. Nal. Agr. Medellin. 2014, 68, 7399–7409. DOI: 10.15446/rfnam.v68n1.47827.
- Wang, L.; Xie, B.; Xiong, G.; Du, X.; Qiao, Y.; Liao, L. Study on the Granular Characteristics of Starches Separated from Chinese Rice Cultivars. Carbohydr. Polym. 2012, 87, 1038–1044. DOI: 10.1016/j.carbpol.2011.08.006.
- Zhu, F.; Yang, X.; Cai, Y.-Z.; Bertoft, E.; Corke, H. Physicochemical Properties of Sweetpotato Starch. Starch-Starke. 2011, 63, 249–259. DOI: 10.1002/star.201000134.
- Vimala, B.; Nambisan, B.; Thushara, R.; Unnikrishnan, M. Variability of Carotenoids in yellow-fleshed Cassava (Manihot Esculenta Crantz) Clones. Gene Conserve. 2009, 8(31).
- AOAC. Official Methods of Analysis. In Association of Official and Analytical Chemists, Washington DC, USA 17th. 2000
- Lawal, O. S. Composition, Physicochemical Properties and Retrogradation Characteristics of Native, Oxidised, Acetylated and acid-thinned New Cocoyam (Xanthosoma Sagittifolium) Starch. Food Chem. 2004, 87, 205–218. DOI: 10.1016/j.foodchem.2003.11.013.
- Hoover, R.; Ratnayake, W. S. Determination of Total Amylose Content of Starch. Curr. Protocols in Food Analytical Chemistry. 2001, 1, E2–3.
- Akonor, P. T.; Tortoe, C.; Oduro-Yeboah, C.; Saka, E. A.; Ewool, J. Physicochemical, Microstructural, and Rheological Characterization of Tigernut (Cyperus Esculentus) Starch. Int. J. Food Sci. 2019, 2019, 1–7. DOI: 10.1155/2019/3830651.
- Zhang, P.; Whistler, R. L.; BeMiller, J. N.; Hamaker, B. R. Banana Starch: Production, Physicochemical Properties, and Digestibility: A Review. Carbohydr. Polym. 2005, 59, 443–458. DOI: 10.1016/j.carbpol.2004.10.014.
- Gayin, J.; Bertoft, E.; Manful, J.; Yada, R. Y.; Abdel-Aal, E.-S. M. Molecular and Thermal Characterization of Starches Isolated from African Rice (Oryza Glaberrima). Starch/Starke. 2016, 68, 9–19. DOI: 10.1002/star.201500145.
- Warren, F. J.; Gidley, M. J.; Flanagan, B. M. Infrared Spectroscopy as a Tool to Characterise Starch Ordered structure—a Joint FTIR–ATR, NMR, XRD and DSC Study. Carbohydr. Polym. 2016, 139, 35–42. DOI: 10.1016/j.carbpol.2015.11.066.
- Nasaruddin, F.; Chin, N. L.; Yusof, Y. A. Effect of Processing on Instrumental Textural Properties of Traditional Dodol Using Back Extrusion. Int. J. Food Prop. 2012, 15(3), 495–506. DOI: 10.1080/10942912.2010.491932.
- Pallares A. P., Miranda, B. A., Truong, N. Q. A, Kyomugasho, C., Chigwedere, C. M., Hendrickx, M., Grauwet, T., et al., 2018. Process-induced cell wall permeability modulates the in vitro starch digestion kinetics of common bean cotyledon cells. Food and Function 9, 6544–6554.
- Delcour, J. A.; Hoseney, R. C. Principles of Cereal Science and Technology; AACC International: St. Paul, MN, 2010.
- Hazarika, B. J.; Sit, N. Effect of Dual Modification with Hydroxypropylation and cross-linking on Physicochemical Properties of Taro Starch. Carbohydr. Polym. 2016, 140, 269–278. DOI: 10.1016/j.carbpol.2015.12.055.
- Waterschoot, J.; Gomand, S. V.; Fierens, E.; Delcour, J. A. Production, Structure, Physicochemical and Functional Properties of Maize, Cassava, Wheat, Potato and Rice Starches. Starch/Stärke. 2015, 67, 14–29. DOI: 10.1002/star.201300238.
- Rolland-Sabaté, A.; Sánchez, T.; Buléon, A.; Colonna, P.; Jaillais, B.; Ceballos, H.; Dufour, D. Structural Characterization of Novel Cassava Starches with Low and high-amylose Contents in Comparison with Other Commercial Sources. Food Hydrocolloids. 2012, 27(1), 161–174. DOI: 10.1016/j.foodhyd.2011.07.008.
- Moorthy, S. N. Tropical Sources of Starch. In Starch in Food, Structure, Function and Application; Eliasson, A.-C., Ed.; Woodhead Publishing Ltd: Cambridge, 2004; pp 321–359.
- Mweta, D. E.; Labuschane, M. T.; Koen, E.; Benesi, I. R. M, Saka, J.D.K , et al. Some Properties of Starches from Cocoyam (Colocasia Esculenta) and Cassava (Manihot Esculenta Crantz.) Grown in Malawi. Afr. J. Food Sci. 2008, 2, 102–111.
- Ceballos, H.; Sanchez, T.; Morante, N.; Fregene, M.; Dufour, D.; Smith, A. M.; Denyer, K.; Perez, J. C.; Calle, F.; Mestres, C. Discovery of an amylose-free Starch Mutant in Cassava (Manihot Esculenta Crantz). J. Agric. Food Chem. 2007, 55, 7469–7476. DOI: 10.1021/jf070633y.
- Vasconcelos, L. M.; Brito, A. C.; Carmo, C. D.; Oliveira, E. J. Phenotypic Diversity of Starch Granules in Cassava Germplasm. Genet. Mol. Res. 2017, 16, 1–15. DOI: 10.4238/gmr16029276.
- Nuwamanya, E.; Baguma, Y.; Emmambux, N.; Rubaihayo, P. Crystalline and Pasting Properties of Cassava Starch are Influenced by Its Molecular Properties. Afr. J. Food Sci. 2010, 4, 8–15.
- Gomand, S. V.; Lamberts, L.; Visser, R. G. F.; Delcour, J. A. Physicochemical Properties of Potato and Cassava Starches and Their Mutants in Relation to Their Structural Properties. Food Hydrocolloids. 2010a, 24, 424–433. DOI: 10.1016/j.foodhyd.2009.11.009.
- Torruco-Uco, J. G.; Chel-Guerrero, L. A.; Betancur-Ancona, D. Isolation and Molecular Characterization of Makal (Xanthosoma Yucatanensis) Starch. Starch. 2006, 58, 300–307. DOI: 10.1002/star.200500451.
- Srichuwong, S.; Sunarti, T.; Mishima, T.; Isono, N.; Hisamatsu, M. Starches from Different Botanical Sources I: Contribution of Amylopectin Fine Structure to Thermal Properties and Enzyme Digestibility. Carbohydr. Polym. 2005, 60, 529–538. DOI: 10.1016/j.carbpol.2005.03.004.
- Kaur, A.; Singh, N.; Ezekiel, R.; Guraya, H. S. Physicochemical, Thermal and Pasting Properties of Starches Separated from Different Potato Cultivars Grown at Different Locations. Food Chem. 2007, 101, 643–651. DOI: 10.1016/j.foodchem.2006.01.054.
- Gomand, S. V.; Lamberts, L.; Derde, L. J.; Goesaert, H.; Vandeputte, G. E.; Goderis, B.; Visser, R. G. F.; Delcour, J. A. Structural Properties and Gelatinization Characteristics of Potato and Cassava Starches and Mutants Thereof. Food Hydrocolloids. 2010b, 24, 307–317. DOI: 10.1016/j.foodhyd.2009.10.008.
- Magallanes-Cruz, P. A. F.-S. P. C.; Bello-Perez, L. A. Starch Structure Influences Its Digestibility: A Review. J. Food Sci. 2017, 82, 2016–2023. DOI: 10.1111/1750-3841.13809.
- Bird, A. R.; Lopez-Rubio, A.; Shrestha, A. K.; Gidley, M. J. Resistant Starch in Vitro and in Vivo: Factors Determining Yield, Structure and Physiological Relevance; eds, Kasapis, S., Norton, I. T., Ubbink, J. B. Modern Biopolymer Sciences, Academic Press: London, 2009 449–512.
- Aprianita, A.; Purwandari, U.; Watson, B.; Vasiljevic, T. Physicochemical Properties of Flours and Starches from Selected Commercial Tubers Available in Australia. Int. Food Res. J. 2009, 16, 507–520.
- Huang, -T.-T.; Zhou, D.-N.; Jin, Z.-Y.; Xu, X.-M. N. D. C. H.-Q. Effect of Repeated heat-moisture Treatments on Digestibility, Physicochemical and Structural Properties of Sweetpotato Starch. Food Hydrocolloids. 2016, 54, 202–210. DOI: 10.1016/j.foodhyd.2015.10.002.
- Perez, S.; Bertoft, E. The Molecular Structures of Starch Components and Their Contribution to the Architecture of Starch Granules: A Comprehensive Review. Starch‐Stärke. 2010, 62, 389–420. DOI: 10.1002/star.201000013.
- Charoenkul, N.; Uttapap, D.; Pathipanawat, W.; Takeda, Y. Physicochemical Characteristics of Starches and Flours from Cassava Varieties Having Different Root Textures. LWT Food Sci. Technol. 2011, 44, 1774–1781. DOI: 10.1016/j.lwt.2011.03.009.
- Huang, Z. Q.; Lu, J. P.; Li, X. H.; Tong, Z. F. Effect of Mechanical Activation on physico-chemical Properties and Structure of Cassava Starch. Carbohydr. Polym. 2007, 68, 128‒135. DOI: 10.1016/j.carbpol.2006.07.017.
- Rolland-Sabate, A.; Sanchez, T.; Buleon, A.; Colonna, P.; Ceballos, H.; Zhao, S. S.; Dufour, D. Molecular and supra-molecular Structure of Waxy Starches Developed from Cassava (Manihot Esculenta Crantz). Carbohydr. Polym. 2013, 92, 1451–1462. DOI: 10.1016/j.carbpol.2012.10.048.
- Mbougueng, P. D.; Tenin, D.; Scher, J.; Tchiegang, C. Influence of Acetylation on Physicochemical, Functional and Thermal Properties of Potato and Cassava Starches. Influ. J. Food Eng. 2012, 108, 320–326. DOI: 10.1016/j.jfoodeng.2011.08.006.
- Htoon, A.; Shrestha, A. K.; Flanagan, B. M.; Lopez-Rubio, A.; Bird, A. R.; Gilbertd, E. P.; Gidley, M. J. Effects of Processing high-amylose Maize Starches under Controlled Conditions on Structural Organisation and Amylase Digestibility. Carbohydr. Polym. 2009, 75, 236–245. DOI: 10.1016/j.carbpol.2008.06.016.
- Dome, K.; Podgorbunskikh, E.; Bychkov, A.; Lomovsky, O. Changes in the Crystallinity Degree of Starch Having Different Types of Crystal Structure after Mechanical Pretreatment. Polymers. 2020, 12.
- Ren, S. Comparative Analysis of Some Physicochemical Properties of 19 Kinds of Native Starches. Starch/Stärke. 2017, 68, 1600367. DOI: 10.1002/star.201600367.
- Kaewtatin, K.; Tanrattanakul, V. Preparation of Cassava Starch Grafted with Polystyrene by Suspension Polymerization. Carbohydr. Polym. 2008, 73, 647–655. DOI: 10.1016/j.carbpol.2008.01.006.
- Li H.; Prakash, S.; Nicholson, T.; Fitzgerald, M.; Gilbert, R.; et al., 2016, The importance of amylose and amylopectin fine structure for textural properties of cooked rice grains. Food Chemistry. 196, 702–711.
- Shewry, P. R.; Underwood, C.; Wan, Y.; Lovegrove, A.; Bhandari, D.; Toole, G.; Mitchell, R. A. C. Storage Product Synthesis and Accumulation in Developing Grains of Wheat. J. Cereal Sci. 2009, 50, 106–112. DOI: 10.1016/j.jcs.2009.03.009.
- Moita, B. C.; Lourenco, D. S. C. A.; Bagulho, A. S.; Beirao-da-Costa, M. L. Effect of Wheat Puroindoline Alleles on Functional Properties of Starch. Eur. Food Res. Tech. 2008, 226, 1205–1212. DOI: 10.1007/s00217-007-0711-z.
- Ao, Z.; Jane, J. Characterization and Modeling of the A- and B- Granule Starches of Wheat, Triticale, and Barley. Carbohydr. Polym. 2007, 67, 46–55. DOI: 10.1016/j.carbpol.2006.04.013.
- Van Soest, J. J. G.; Tournois, H.; deWit, D.; Vliegenthart, J. F. G. Short-range Structure in (Partially) Crystalline Potato Starch Determined with Attenuated Total Reflectance Fourier-transform IR Spectroscopy. Carbohydr. Res. 1995, 279, 201–2014. DOI: 10.1016/0008-6215(95)00270-7.
- Fang, J. M.; Fowler, P. A.; Tomkinso, J.; Hill, C. A. S. The Preparation and Characterization of a Series of Chemically Modified Potato Starches. Carbohydr. Polym. 2002, 47, 245–252. DOI: 10.1016/S0144-8617(01)00187-4.
- Sevenou, O.; Hill, S. E.; Farhat, I. A.; Mitchell, J. R. Organisation of the External Region of the Starch Granule as Determined by Infrared Spectroscopy. Int. J. Biol. Macromol. 2002, 31, 79–85. DOI: 10.1016/S0141-8130(02)00067-3.
- Pozo, C.; Rodríguez-Llamazares, S.; Bouza, R.; Barral, L.; Castaño, J.; Müller, N.; Restrepo, I. Study of the Structural Order of Native Starch Granules Using Combined FTIR and XRD Analysis. J. Polym. Res. 2018, 25, 1–8. DOI: 10.1007/s10965-018-1651-y.
- Afoakwa, E. O.; Budu, A. S.; Asiedu, C.; Chiwona-Karltun, L.; Nyirenda, D. B. Viscoelastic Properties and Physico-Functional Characterization of Six High Yielding Cassava Mosaic Disease-Resistant Cassava (Manihot Esculenta Crantz) Genotypes. J. Nutr. Sci. 2012, 2, 129. DOI: 10.4172/2155-9600.1000129.
- Asare, P. A.; Galyuon, I.; Sarfo, J. K.; Tetteh, J. P. Functional and Pasting Properties of Cassava and Sweetpotato Starch Mixtures. Ghana J. Agric. Sci. 2014, 48, 77–85.
- Kong, X.; Bertoft, E.; Bao, J.; Corke, H. Molecular Structure of Amylopectin from Amaranth Starch and Its Effect on Physicochemical Properties. Int. J. Biol. Macromol. 2008, 43, 377–382. DOI: 10.1016/j.ijbiomac.2008.07.018.
- Sandhu, S. K.; Singh, N. Some Properties of Corn Starches II: Physicochemical, Gelatinization, Retrogradation, Pasting and Gel Texture Properties. Food Chem. 2007, 101, 1499–1507. DOI: 10.1016/j.foodchem.2006.01.060.
- Steele, C. M.; Alsanei, W. A.; Ayanikalath, S.; Barbon, C. E.; Chen, J.; Cichero, J. A.; … Wang, H. The Influence of Food Texture and Liquid Consistency Modification on Swallowing Physiology and Function: A Systematic Review. Dysphagia. 2015, 30, 2–26. DOI: 10.1007/s00455-014-9578-x.
- Bourne, M. Food Texture and Viscosity: Concept and Measurement, 2nd ed.; Massachusetts, USA: Academic Press. 2002, 416.
- Yan, W.; Yin, L.; Zhang, M.; Zhang, M.; Jia, X. Gelatinization, Retrogradation and Gel Properties of Wheat starch-wheat Bran Arabinoxylan Complexes. Gels. 2021, 7, 200. DOI: 10.3390/gels7040200.
- Li, B.; Zhang, Y.; Xu, F.; Khan, M. R.; Zhang, Y.; Huang, C.; … Liu, A. Supramolecular Structure of Artocarpus Heterophyllus Lam Seed Starch Prepared by Improved Extrusion Cooking Technology and Its Relationship with in Vitro Digestibility. Food Chem. 2021, 336, 127716. DOI: 10.1016/j.foodchem.2020.127716.