 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Whereas herbs/spices serve as natural preservatives, and thermal processing makes animal meat products edible, combining them should complement each other. Additionally, the application of oven grilling to meat products continues to increase in popularity. However, there is a paucity of relevant published information specific to different marinated oven-grilled pork neck meat. Therefore, the quality attributes of different marinated oven-grilled pork neck meat were investigated, which involved chemical (pH, thiobarbituric acid reactive substance [TBARS], 2,2′-azinobis(3-ethylbenzothiaziline-6-sulfonate) [ABTS], 1,1-diphenyl-2-pierylhydrazy [DPPH], ferric reducing antioxidant power [FRAP]), physical (cooking weight loss, L*a*b* color, and textural cutting force), as well as organoleptic (sensory: flavor, appearance, tenderness, taste, and off-flavor; texture: hardness, chewiness, gumminess, graininess, and greasiness) aspects. In particular, the pork neck meat was procured from a porcine farm in Poland. Different marinated variants comprised constituent 0.5, 1, and 1.5% quantities of cranberry pomace (CP), grape pomace (GP), and Baikal skullcap (BS) that subsequently incorporated either African spice (AS) or industrial marinade/pickle (IM). Results showed decreases in ABTS, DPPH, FRAP, and TBARS in some marinated oven-grilled pork neck meat samples, alongside pH variations by difference that seemingly associated with increasing concentrations of either CP, BS, or GP, which might not always coincide with L*a*b* color trends as AS and IM were incorporated. Despite the many resemblances (p > .05), the sensory aspects fluctuated as textural chewiness, gumminess, and hardness increased in some samples, more evident when incorporating AS compared to IM. Overall, oven-grilling promises to moderate the range values of key quality attributes of different marinated pork neck meat samples in this study.
Introduction
Pork accounted for about 36% of global meat production as of 2013, which has placed this animal food product among the most widely consumed, as it has accounted for 110 million metric tonnes (mmt), and surpassed both beef (67 mmt) and chicken (104 mmt). China as of 2020 topped the global pork production, followed by European Union (EU), before United States[Citation1,Citation2] . In 2016, the pork production in the EU was recorded to reach 23.4 million tonnes, which translated to 45.9 kg per inhabitant.[Citation3] Indeed, the demand for pork meat gradually increases with global population, which has been poised to persist into the next few decades.[Citation1,Citation4] Specific to Poland, pork maintains a strong position in meat consumption, having recorded 21.8 million slaughtered pigs in 2016, which was largely driven by supply chain elements of procurement, processing, and distribution.[Citation3–5] Moreover, the various stages of pig production, from the breeding choices, through the on-site farm management/slaughter processes, to culinary aspects, remain very crucial to realize high-quality pork.[Citation6] Notable aspects of pork meat processing, largely three-fold, include slaughtering, meat cutting, and further processing. With respect to food service, the primal aspects of pork meat cutting/processing include leg, loin, belly, and shoulder.[Citation7] Moreover, when evaluating Poland’s domestic pork processing activity, Szymańska[Citation5] reported the following trends: meat products> cutting plants> slaughterhouses> meat mincing>mechanically separated meat.
Compared to beef, the pork carcass/meat is among USDA considered red meat that possesses ample protein, relatively high thiamin (vitamin B1), cholesterol, and saturated fat, with low myoglobin contents[Citation8–11] . There are some ante-/postmortem-associated biochemical/storage-related characteristics that contribute to influencing pork quality[Citation12,Citation13] . Like other meat products, moreover, the accelerated postmortem glycolysis that is triggered by lipid breakdown products post-slaughter typically brings about the process of quality deterioration.[Citation14] In addition to refrigerated storage that helps to curtail as well as manage both lipid breakdown and microbial proliferation, many pork consumers/stakeholders especially those of small-medium scale enterprises continually seek for enhanced/low-cost processing and shelf-life extension strategies, for example, the use of natural preservatives. Moreover, pork quality has been associated with such conditions as pale, soft, and exudative (PSE), which reflects both appearance and physical condition(s).[Citation15] More so, pork quality would equally depend on such factors as the effect(s) of diet and exercise (of the pig), changes associated with postmortem/rigor mortis, muscle structure and resultant water holding capacity, fiber type (of muscle) as well as processing yield of the overall (pork)meat.[Citation15]
In recent times, natural preservatives such as marinades are increasingly being pursued in many parts of the globe, especially their application to meat products, which has been largely focused to enhance various quality characteristics[Citation16–18] . The process at which meat muscle assimilates the marinade would depend on (meat) type, marination technique, as well as duration of the (marination) process.[Citation19] The most common marination process involves immersing the meat products in desired slurry/solution mix that can involve such components/ingredients like Baikal skullcap, cranberry pomace, herbs/spices, ginger, peanut, black/regular pepper, etc.,[Citation14,Citation16–18,Citation20–23] which would deliver such bioactive/health-promoting compounds as beneficial phenolic/phenols, flavonoids, polyphenols, etc.[Citation14,Citation23–25] Moreover, for animal meat products to become edible, there must be submitted to heat/thermal treatment, the latter which has helped to ensure decreased microbial proliferation and enhanced flavor/texture. Examples of thermal processing include aseptic processing, cook-chill, grilling/roasting, laser-based packaging, ohmic heating, etc[Citation26–28] . Despite the well-known difference between roasting (indirect heating method) and grilling (direct heating method), the physical characteristic outcomes post-application of both methods regards animal meat products might resemble.
There is increasing interest among researchers in grilling, which provides heat temperatures that are capable of delivering direct/radiant dry heat transferred by conduction. Additionally, a typical example increasingly employed across households globally is the oven-grill approach,[Citation28–30] which uses a facility that is widely available and commercially. This has made the application of oven grilling to animal meat products to increase in popularity.[Citation31–33] Despite this, there is a paucity of relevant published information specific to different marinated oven-grilled pork neck meat. Given that herbs/spices would capably serve as natural preservatives and considering the benefits thermal processing avails to meat products, it is a useful rationale to understand the effects oven-grill would have on a given marinated pork neck meat, especially from both consumer appeal and quality value standpoints, prior to storage considerations. To supplement existing information, therefore, this current work investigated the quality attributes of different marinated oven-grilled pork neck meat. In particular, the pork neck meat has been procured from a porcine farm in Poland.
Materials and methods
Schematic overview of experimental program
The schematic overview of the experimental program, which depicts the major stages, from the procurement of pork neck meat samples, preparation of marinade variants, through the oven-grilling activity, up to the various analytical measurements, is shown in . For emphasis, this work attempted to understand the effects oven-grilling would have on the quality attributes of different marinated pork neck meat. The marinades involved ground constituents of cranberry pomace, grape pomace, and Baikal skullcap, which subsequently incorporated African spice, and Industrial marinade/pickle. The quality attributes involved chemical (pH, thiobarbituric acid reactive substance [TBARS], 2,2′-azinobis(3-ethylbenzothiaziline-6-sulfonate) [ABTS], 1,1-diphenyl-2-pierylhydrazy [DPPH], ferric reducing antioxidant power [FRAP]), physical (cooking weight loss, L*a*b* color, and textural cutting force), as well as organoleptic (sensory = flavor, appearance, tenderness, taste and flavor; texture = hardness, chewiness, gumminess, graininess, and greasiness) aspects. The chemicals/reagents used were of analytical grade standard. All conducted laboratory procedures adhered to the standard guidelines set out by the Department of Functional Food Product Development, Wroclaw University of Environmental and Life Sciences, Poland.
Figure 1. The schematic overview of the experimental program, showing the key stages, from the procurement of pork neck meat samples, preparation of marinade variants, through oven-grilling activity, subsequently analytical measurements. ABTS = 2,2’-Azinobis-(3-ethylbenzthiazoline-6-sulfonate); DPPH = 1,1-diphenyl-2-pierylhydrazy (radical scavenging activity); FRAP = ferric reducing antioxidant power; UPWr = Uniwersytet Przyrodniczy we Wrocławiu (Wroclaw University of Environmental and Life Sciences-Poland).
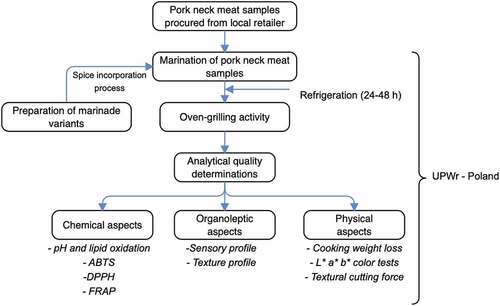
Procurement, and further preparation of pork neck meat samples
Freshly processed pork neck meat were supplied shortly after slaughter and packaging by a reputable local certified porcine retailer that supplies the Wroclaw’s Lower Silesia region. The dressed carcasses (~ 20 kg) placed in iced packed poly-boxes were received at the Department of Functional Food Products Development, Wroclaw University of Environmental and Life Sciences (Poland). Upon arrival, the pork neck samples were further prepared as described by Kim et al.,[Citation34] by cutting them into equivalent pieces of approximate thickness (9 × 9 × 3 cm), and subsequently placed in cold room refrigeration (~2°C), after which marination and subsequently oven-grilling were performed.
Preparation of marinades, and marination variants
The marinade preparation involved salt (1.6 g), ground cranberry pomace (CP), grape pomace (GP), and Baikal Skullcap (BS) at 0.5%, 1%, and 1.5% constituent quantities, which subsequently incorporated either African spice (AS) or Industrial marinade/pickle (IM) (4 g). The usage of CP, GP, and BS marinades, given their bioactive constituents, is to improve the nutritional status of the pork neck meat. The African spice product (Fresh and Tasty Kebab Powder) were from Fresh and Tasty Farms Ltd (Accra-North, Ghana) prepared according to the quality standards of Food and Drugs Authority (FDA) Ghana, with the label comprising ingredients peanut, ginger, as well as black/regular pepper. The use of this specific African spice product is believed to gain interest in barbecues across Poland. The industrial marinade/pickle (Marinate do mięs) product was from Regis(R) Food Technology (Regis sp. z o.o., Kraków-Poland) prepared according to the quality standards of International Organization for Standardization (ISO), British Retail Consortium (BRC), and International Food Standard (IFS), with the label comprising ingredients as thyme, oregano, rosemary, marjoram, and parsley. Also, this specific industrial marinade/pickle product is believed to have an established reputation in Poland and elsewhere in the EU.
The marination variants, which comprised increments of CP, GP, and BS concentrations that incorporated either AS or IM, were implemented as follows: 1) control (antioxidant additive % = 0.0); 2) control (antioxidant additive % = 0.5); 3) control (antioxidant additive % = 1.0); 4) control (antioxidant additive % = 1.5); 5) AS (antioxidant additive % = 0.0); 6) AS (antioxidant additive % = 0.5); 7) AS (antioxidant additive % = 1.0); 8) AS (antioxidant additive % = 1.5); 9) IM (antioxidant additive % = 0.0); 10) IM (antioxidant additive % = 0.5); 11) IM (antioxidant additive % = 1.0); 12) IM (antioxidant additive % = 1.5). As described by Sokołowicz et al.[Citation18] with some modifications, the immersion method was adapted to marinate the pork neck meat samples. We used plastic containers approved for contact with food to prepare the marinade using the 1:2 ratio to reflect the weight of meat (g) and marinade volume (mL). The pork meat samples were sufficiently dipped in the marinade variants for 24 h period. When immersion time was completed, marinated samples were allowed to drain (5 min), thereafter placed in folded foiled packages, and made ready for oven-grilling activity.
Oven-grilling procedure
The oven-grilling activity resembling the description given by Salmon, Knize, and Felton[Citation35] with some modifications was applied to the various marinated pork neck samples. The oven-grilling process was specifically conducted using a commercially available electric hot air convection (oven) type facility (CAMRY CR 6017, Serwis Centralny Camry, Warszawa, Poland). The oven-grilling operated with 2200 W power, and temperature set at 180°C. In the pre-heated oven, the pork neck meat samples, evenly spaced on grill rack, received heating of the set temperature that was evenly distributed from the bottom as well as top. During the cooking process, the oven-grill facility remained closed, and only opened on either the placement or removal of samples. Also, the internal temperature of the pork neck meat samples was checked routinely to ensure it roughly maintained at 75°C. The cooking time was constant (5 min), and was applied to all the marinated samples. When completed, the pork neck meat samples were allowed to cool (10 min) at ambient temperature, and thereafter refrigerated (4°C) during which analytical determinations were performed within 24 h period.
Determination of chemical aspects
The pH measurement slightly modified from Barido and Lee[Citation36] specifically taken before and after the oven-grilling activity. Roughly 5 g sample and 45 mL of distilled water were mixed using a homogenizer (PH91, SMT Chiba, Japan) at 10,000 rpm, for 1 min, thereafter tested using pH meter (HI 99163 Hanna Instrument Company, Vöhringen, Germany) that had been calibrated by buffer solutions (approximate pH 4.0, 7.0, and 9.0).
The thiobarbituric acid reactive substance (TBARS) measurement has been slightly modified from Luciano et al.[Citation37] and specifically determined before and after the oven-grilling process. With the help of stomacher, the pork neck meat samples (1.0 g) were homogenized with 10 mL of 10% trichloroacetic acid (TCA) for 1 min, thereafter centrifugation at 4000 × g (MPW-351 R refrigerated, MPW Med. instruments Warszawa, Poland), after which the emergent mix has been subject to filtration (Whatman #1 filter paper). Next, 2 mL of supernatant was transferred to 2 mL of 0.06 M thiobarbituric acid. The reaction mixture was submitted to water bath at 100°C for 40 min, then cooled under ice-water bath (~ 2 min). Calibration curve was prepared using 1,1,3,3-tetra-ethoxypropane TCA (standard solution). The samples were finally analyzed, reading the absorbance at 532 nm via UV-Vis Spectrophotometer (GENESYS™ 180, ThermoFisher Scientific Inc., Waltham, Massachusetts-USA). The TBARS values were reported as mg of malondialdehyde (MDA) per kg of meat sample.
The determination of 2,2′-azinobis(3-ethylbenzothiaziline-6-sulfonate) (ABTS+) radical scavenging activity has been slightly modified from Bai et al.[Citation38] The ABTS+ has been produced by mixing 7 mM of stock solution with 2.45 mM K2S2O8, thereafter incubated in darkness at 25°C for 12–16 h. From this, 990 μL of ABTS+ solution was mixed with 10 μL of meat tissue supernatant, thereafter incubated at ambient temperature (~ 25°C) for 6 min. The control comprised 990 μL of ABTS+ solution mixed with 10 μL EtOH 70%. The absorbance was spectrophotometrically measured at 734 nm. The ABTS+ radical scavenging activity values were reported as mM Trolox.
The determination of 1,1-diphenyl-2-pierylhydrazy (DPPH) radical scavenging activity has been slightly modified from Zhang et al.[Citation23] This involved aliquots (20 μL) from meat tissue supernatant vigorously mixed with 200 μL 0.3 mM of ethanolic DPPH radical solution by vortex for 1 min, and subsequently kept in the dark for 30 min under ambient temperature (25°C). The absorbance was recorded against a blank at 517 nm via UV-Vis Spectrophotometer (GENESYS™ 180, ThermoFisher Scientific Inc., Massachusetts-USA). The DPPH radical scavenging activity values were reported as mM Trolox.
The determination of ferric reducing antioxidant power (FRAP) has been slightly modified from Lengkidworraphiphat et al.[Citation39] Ethanol extracts of pork neck meat sample were prepared using 70% EtOH. The FRAP solution comprised 10 mM 2,4,6-tripyridyl-s-triazine (TPTZ), 20 mM ferric chloride, together with 300 mM sodium acetate buffer (pH 3.6), at a ratio of 1:1:10 (v:v:v), which were subsequently incubated for 30 min at 37°C. Control comprised 3 mL FRAP reagent mixed with 1 mL EtOH. The absorbance of resultant solution was recorded at 593 nm via UV-Vis Spectrophotometer (GENESYS™ 180, ThermoFisher Scientific Inc., Waltham, Massachusetts-USA). The FRAP values were reported as mM/dm3.
Determination of physical aspects
Cooking weight loss measurement has been slightly modified from Ali et al.[Citation40] Specifically, the samples had been weighed prior to and after oven-grilling, wherein the cooking weight loss depicted cooked sample (B) weight as a percentage of precooked sample (A) weight as shown by the equation below:
Color measurements has been slightly modified from Kopec et al.[Citation41], specifically conducted before and after oven-grilling by way of CIE L*a*b* scale (L* = darkness; a* = redness/greenness; and b* = yellowness/blueness) using a Minolta CR-40 reflection colorimeter (Konica Minolta Sensing Europe B.V., NL-3439 MR Nieuwegein-Netherlands). Three individual measurements had been performed on different areas on the pork neck meat surface, after which the readings were collected from the display results via the CIE L*a*b* colorimetric system at real-time.
Textural cutting force measurement has been slightly modified from Augustyńska-Prejsnar, Ormian, and Sokołowicz,[Citation42] which involved measuring the force required to cut a piece of pork neck meat. The cutting force (F-max) instrument was the Zwick/Roell machine (Zwick GmbH & Co. KG, Ulm, Germany), equipped with Warner-Bratzler V-blade knife, head speed of 100 mm/min and initial force of 0.2 N. The portions of pork neck meat samples to be cut had an estimated cross-sectional diameter of 100 mm2 and length of 50 mm.
Determination of organoleptic aspects
Organoleptic determinations of pork neck meat samples comprised sensorial analysis modified from Augustyńska-Prejsnar, Ormian, and Sokołowicz[Citation43], and textural profiling modified from Brambila, Bowker, and Zhuang.[Citation44] Sensory panelists comprised ten (10) staff and graduate students of the Department of Functional Food Products Development, Wrocław University of Environmental and Life Sciences (Poland), already familiar with the evaluation criteria to differentiate the levels of sensorial flavor, appearance, tenderness, taste, and off-flavor, as well as textural hardness, chewiness, gumminess, and graininess. The verbal consent taken prior to the sensory evaluation. Panelists’ participation was voluntary, and no name/gender was reported to ensure privacy. Panelists’ performed the organoleptic evaluation in well-ventilated room of neutral color, proper lighting, and distraction-free. For the organoleptic assessment, the evenly cut samples already cooled to 20°C ± 2°C were placed in coded white plastic plates. For the sensorial tests, each panelist used warm water to cleanse taste palates between samples, to ensure the previous evaluation did not affect the (taste of the) new one, consistent with Çakmakçı et al.[Citation45] The panelists reported the findings of coded samples based on 0–5 sensory scale (1 point being the lowest score and 5 points being the highest), and 0 to 15 texture scale (1 point being the lowest score and 15 points being the highest).[Citation46]
Statistical analysis
The data, independently generated from different samples and based on minimum of two determinations, were submitted to analysis of variance (ANOVA). The statistical significance was set at p < .05 (95% confidence level). Statistica 13.0 software (StatSoft GmbH, Hamburg Germany) was used to run the data.
Results and discussion
Changes in chemical aspects
Chemical aspects (specific to pH, ABTS, DPPH, FRAP, and TBARS) of different marinated oven-grilled pork neck meat were investigated (, and ). Across all samples, considering oven-grilling and antioxidant additives, the pH, TBARS, ABTS, DPPH, and FRAP values obtained varying ranges, from minimum (pH = ~5.22 at CP control pre-oven grill; TBARS = 4.27 ± 0.13 mg MDA/kg at AS + BS 0.5% pre-oven grill; ABTS = 1.80 ± 0.04 mM/Trolox at AS + BS 1.5%; DPPH = 0.21 ± 0.02 mM/Trolox at AS Control; FRAP = .07 ± 0.00 mM/dm3 at AS + BS 1% or Control + BS 1%) to maximum (pH = ~6.79 at BS Control pre-oven grill; TBARS = 22.09 ± 0.13 mg MDA/kg at IM + CP 1.5% after oven-grilling; ABTS = 2.29 ± 0.05 mM/Trolox at Control + GP 0.5%; DPPH = 0.75 ± 0.00 mM/Trolox at AS + BS 1.0%; FRAP = .14 ± 0.01 mM/dm3 at Control) values. The application of oven-grilling generally increased the pH, with a few exceptions, especially where either resemblances or decreases occurred. The pH range herein appears in contrast to data of Libera et al.[Citation47] for dry-cured pork neck (pH ranges of 5.42 and 5.76). Olsson and Pickova[Citation48] reported a well-fed and unstressed pig postmortem would have a pH typically fall from 7.2 to about 5.5, given by the biochemical and physical processes that help the conversion of (postmortem) muscle to meat. As shown in , there seems to be more variations of pH by difference associated with those of CP, before BS and then GP marinade concentrations. Nonetheless, Siroli et al.[Citation19] reported a reduced pH should favor the pork neck meat, which should provide it with a positive shelf potential, either to decrease the vulnerability to microbial proliferation, and/or facilitate the action of collagenases alongside other proteolytic enzymes associated with meat tenderization.
Figure 2. Changes in pH across the various marinated pork neck meat meat samples before and after oven-grilling. The different letters represent as follows: (a) = CP before oven-grill; (b) = CP after oven-grill; (c) = GP before oven-grill; (d) = GP after oven-grill; (e) = BS before oven-grill; (f) = BS after oven-grill; The number representations for different color shades are as follows: 1) control (antioxidant additive % = 0.0); 2) control (antioxidant additive % = 0.5); 3) control (antioxidant additive % = 1.0); 4) control (antioxidant additive % = 1.5); 5) AS (antioxidant additive % = 0.0); 6) AS (antioxidant additive % = 0.5); 7) AS (antioxidant additive % = 1.0); 8) AS (antioxidant additive % = 1.5); 9) IM (antioxidant additive % = 0.0); 10) IM (antioxidant additive % = 0.5); 11) IM (antioxidant additive % = 1.0); 12) IM (antioxidant additive % = 1.5). Cranberry pomace = CP; Grape pomace = GP; BS = Baikal Skullcap; African spice = AS; Industrial marinade/pickle = IM.
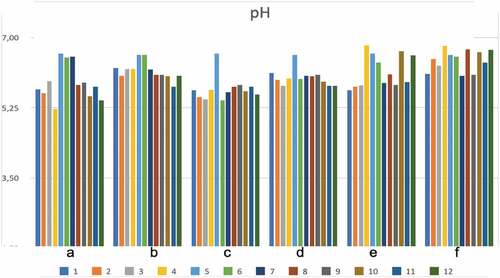
Figure 3. Variation of pH by difference across the various marinated oven-grilled pork neck meat samples compared to control. The number representations are as follows: 1) control (antioxidant additive % = 0.0); 2) control (antioxidant additive % = 0.5); 3) control (antioxidant additive % = 1.0); 4) control (antioxidant additive % = 1.5); 5) AS (antioxidant additive % = 0.0); 6) AS (antioxidant additive % = 0.5); 7) AS (antioxidant additive % = 1.0); 8) AS (antioxidant additive % = 1.5); 9) IM (antioxidant additive % = 0.0); 10) IM (antioxidant additive % = 0.5); 11) IM (antioxidant additive % = 1.0); 12) IM (antioxidant additive % = 1.5). African spice = AS; Industrial marinade/pickle = IM.
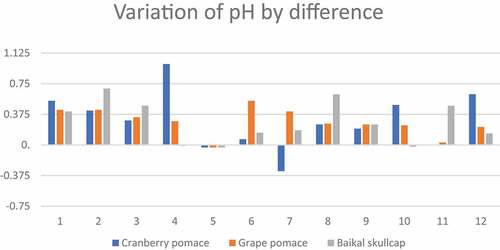
Figure 4. Changes in (a)ABTS (b) DPPH and (c)FRAP across the various marinated oven-grilled pork neck meat meat samples compared to control. Error bars show mean ± standard deviation (SD). ABTS = 2,2’-Azinobis-(3-ethylbenzthiazoline-6-sulfonate; DPPH = 1,1-diphenyl-2-pierylhydrazy (radical scavenging activity); FRAP = ferric reducing antioxidant power; Error bars shows mean values ± standard deviation (SD). African spice = AS; Industrial marinade/pickle = IM; CP = Cranberry pomace; GP = Grape pomace; BS = Baikal Skullcap.
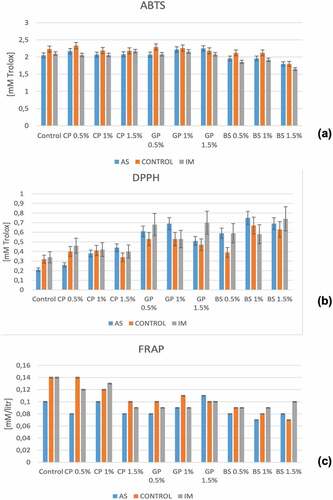
Table 1. Changes in thiobarbituric acid reactive substance (TBARS) across the various marinated oven-grilled pork neck meat samples compared to control.
As fluctuations seemingly persisted with ABTS and DPPH values especially when AS and IM were incorporated, the FRAP values would appear lower at AS alone compared to control, but not so for with IM alone. Despite this, ABTS, DPPH, and FRAP seemingly decrease with increasing CP, GP, and BS concentrations (). Biologically active ingredients present in marinades are believed to have the capacity to quench the DPPH+ radicals, which could depend on the muscle type[Citation49] that prevail in the pork neck meat of this current study. Hypothetically, to quench DPPH+ radical would entail slow (reaction) compounds that utilize more complex (reaction) mechanisms. Thus, the capacity of antioxidants to reduce/quench free radicals should help to extend the shelf-life of processed foods.[Citation49] Moreover, the proteins/peptides could affect some antioxidant action in meat muscle, which might facilitate the chelating capacity of oxidative metals to probably scavenge some free radicals.[Citation50]
The use of crushed seasonings/spices in meat processing could facilitate the release of polyphenols, which could become oxidized into electrophilic quinoae species. More so, the essence of using either CP, GP, or BS together with either AS or IM to make a herb mix herein, provides, not only adds flavor additives, but also, antioxidant and phenolic components to help regulate protein oxidation.[Citation51] Comparing , and , without AS and IM, the ABTS and FRAP values of oven-grilled pork neck meat seemingly decrease with increasing concentrations of either CP, GP, and slightly much less so at BS (particularly for FRAP). However, there are also some instances where pH and DPPH fluctuated with decreases and increases, like at CP concentrations before oven-grilling occurring alongside changes in TBARS, but not so for those of either GP or BS. Further, reveals that oven-grilling alone in some instances could significantly decrease (p < .05) the TBARS values as AS and IM were incorporated. Without AS and IM, however, the TBARS would significantly increase (p < .05) particularly with CP concentrations, but not so for GP and BS. It can be that the application of thermal processing (such as oven-grilling) disrupts the chemical structure especially the polysaccharides and other associated non-carbohydrate components of plant cell wall, which would allow for the onset of Maillard reaction.[Citation49]
Changes in physical aspects
Physical aspects (specific to L*a*b* color scales, cooking weight loss, and textural cutting force) of different marinated oven-grilled pork neck meat were investigated (, and ). Across all samples, considering oven-grilling and antioxidant additives, L*a*b* color scales, cooking weight loss, and textural cutting force values found various ranges, from minimum (L*color = 32.1 ± 1.9 at AS +BS 1.5% before oven-grill; a*color = 2.43 ± 0.4 at IM +GP 1.5% after oven-grill; b* color = 4.3 ± 0.9 at Control CP 1.5% before oven-grill; cooking weight loss = ~ 9.82% at AS + CP/BS; textural cutting force = 22.4 ± 6.7 N at GP + IM 1.5%) to maximum (L*color = 52.5 ± 1.6 at BS/GP + IM 0.0% after oven-grill; a*color = 13.2 ± 0.2 at Control BS 1.5% before oven-grill; b* color = 21.5 ± 3.1 at IM+ BS 0.5% after oven-grill; cooking weight loss = ~ 38.29% at AS+ GP 1.5%; textural cutting force = 127.0 ± 1.0 N at CP + IM 1.5%) values. Results showed oven-grilling seemingly produced varying decreasing and increasing L*a*b* color values at some instances across the different marinated pork neck meat samples. Whereas the L* values would largely increase (p < .05) with few exceptions of slight decrease at BS control, the a* values would largely decrease (p < .05) with few exceptions at some CP, and GP samples. Moreover, increases in CP, GP, and BS concentrations may not always occur with L*a*b* color values. Besides b* color to influence the top layer/surface color of pork meat,[Citation52] marinades that possess coloring compounds may equally influence some (pork) color attributes.[Citation19] Any decrease in a* value may not necessarily depict an enhanced antioxidant effect, which would help to stabilize the color.[Citation47] At slaughter, as muscle glycogen increases the resistance to stress-induced (glycogen) depletion, and coincides with obvious pH decreases, there would be an inevitable influence on the meat structure with high reflectance (paler color).[Citation48]
Figure 5. Changes in L*a*b* color of (a) cranberry pomace, (b) grape pomace, and (c) Baikal skullcap, across the various marinated oven-grilled pork neck meat samples. The number representations for different color shades are as follows: 1) control (antioxidant additive % = 0.0); 2) control (antioxidant additive % = 0.5); 3) control (antioxidant additive % = 1.0); 4) control (antioxidant additive % = 1.5); 5) AS (antioxidant additive % = 0.0); 6) AS (antioxidant additive % = 0.5); 7) AS (antioxidant additive % = 1.0); 8) AS (antioxidant additive % = 1.5); 9) IM (antioxidant additive % = 0.0); 10) IM (antioxidant additive % = 0.5); 11) IM (antioxidant additive % = 1.0); 12) IM (antioxidant additive % = 1.5). African spice = AS; Industrial marinade/pickle = IM.

Figure 6. Changes in cooking weight loss (%) across the various marinated oven-grilled pork neck meat samples. The number representations for different color shades are as follows: 1) control (antioxidant additive % = 0.0); 2) control (antioxidant additive % = 0.5); 3) control (antioxidant additive % = 1.0); 4) control (antioxidant additive % = 1.5); 5) AS (antioxidant additive % = 0.0); 6) AS (antioxidant additive % = 0.5); 7) AS (antioxidant additive % = 1.0); 8) AS (antioxidant additive % = 1.5); 9) IM (antioxidant additive % = 0.0); 10) IM (antioxidant additive % = 0.5); 11) IM (antioxidant additive % = 1.0); 12) IM (antioxidant additive % = 1.5). African spice = AS; Industrial marinade/pickle = IM.
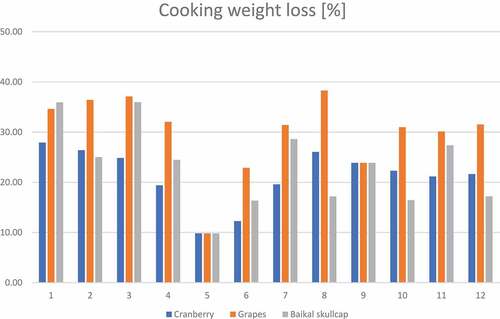
Table 2. Changes in textural cutting force across the various marinated grilled pork neck meat samples compared to control.
Prior to and even after thermal processing, some moisture could still be held in the muscle tissues of the pork meat.[Citation53] Particularly at the beginning of refrigerated storage, Siroli et al.[Citation19] reported the marination process could reduce the cooking weight loss of pork meat. In this current work, the cooking weight loss fluctuated increasingly with decreases and increases at various instances, despite (increased) CP, GP, and BS concentrations and incorporating AS and IM. Moreover, the occurrence of cooking/drip loss would likely render the meat muscle (such as in pork neck) less acceptable, which could affect (product) color, weight, etc.[Citation53,Citation54] Interestingly, across the marinated oven-grilled pork neck meat, incorporating 0.5% CP seemingly increased the textural cutting force (p < .05), but would decrease when incorporating 0.5% GP, as well as 0.5% BS concentrations. Believed to negatively relate to muscle tenderness, any increase in cutting force would reflect the cracking phenomena potentially commencing within the muscle fibers.[Citation54] For emphasis, the muscle tissue comprises connective aspect that involve myofibrillar proteins, which contribute to build up the meat tenderness.[Citation55]
Changes in organoleptic aspects
The palatability of pork meat largely depends on the condition of the product being tested by the sensory panel. It also depends on the training the sensory panelists undertake, as well as the structure of the (sensory)test.[Citation56] More so, a high score of sensory attributes for fatty taste, followed by meaty, and burnt taste as the least may reflect the direct heating nature of oven grill, which tends to mimic roasting.[Citation52]
In this current work, the organoleptic aspects of various marinated oven-grilled pork neck meat were tested, specific to sensory appearance, flavor, taste, tenderness, and off-flavor, as well as textural chewiness, graininess, greasiness, gumminess, and hardness, respectively, shown in . Across samples and considering oven-grilling and antioxidant additives, there were various range values in sensory appearance (from 3.00 ± 0.84 to 4.63 ± 0.92), flavor (from 3.33 ± 0.52 to 4.50 ± 0.93), taste (from 2.83 ± 0.82 to 4.56 ± 0.52), tenderness (from 2.63 ± 0.46 to 4.63 ± 0.92), with the exception of off-flavor (from 4.44 ± 0.79 to 5.00 ± 1.00), as well as textural chewiness (from 3.00 ± 1.41 to 6.33 ± 2.14), graininess (from 2.00 ± 1.28 to 3.88 ± 1.73), greasiness (from 2.67 ± 1.21 to 4.86 ± 3.72), gumminess (from 3.00 ± 2.48 to 6.00 ± 1.87), and hardness (from 3.13 ± 0.35 to 6.33 ± 0.41). Despite the many resemblances (p > .05), the sensory attributes showed fluctuations with increasing CP, GP, and BS concentrations, and as AS and IM were incorporated. Only the flavor seemingly decreases especially when incorporating AS. With respect to textural profile, in some marinated oven-grilled pork neck samples, the concentrations of CP and GP increase with hardness, and to some extent chewiness and gumminess, whereas in some others, the textural chewiness, gumminess, and hardness would increase especially when incorporating AS, slightly above those of IM.
Table 3. Sensory profile by way of flavour, appearance, tenderness, taste and off-flavour across the various marinated grilled pork neck meat samples compared to control.
Table 4. Textural profile by way of hardness, chewiness, gumminess, graininess, and greasiness across the various marinated grilled pork neck meat samples compared to control.
The many data resemblances as well as fluctuations make establishing a specific trend across CP, GP, and BS concentrations of this current study especially for the organoleptic sensorial attributes seemingly quite challenging. Despite this, sensory evaluation remains among the most popular approach to evaluate the freshness of animal meat products as it provides useful information about product quality.[Citation57] Connecting meat tenderness, for instance, with sensory often appear challenging because the sensation associated with consumption requires the understanding of various intricate stages, from initial ease to masticate, ease of grinding during chewing to achieve particles, to the mouthfeel of residue accumulated post-mastication.[Citation55] Moreover, the combination of instrumental texture with sensory tenderness acceptability would corroborate shear force value, which may concur with unacceptable meat toughness.[Citation58] The application of marinades, despite its influence on color of meat, would not negate the panelists’ sensory evaluation.[Citation19] Applicable to pork neck meat of this current work, the preservative potential of marinades would be better evidenced by refrigerated storage, which for instance is often demonstrated by differences in flavor, juiciness, and tenderness.[Citation59]
Conclusion
Various range values occurred across the quality attributes of the marinated oven-grilled pork neck meat samples. Decreases in ABTS and FRAP, with variations of pH by difference that seemingly associated with increasing concentrations of either CP, BS, and GP, would not always coincide with L*a*b* color trends. To establish a specific organoleptic sensory and texture trend across CP, GP, and BS proved challenging. Overall, the oven-grilling process promises to moderate the range values of key quality attributes of the different marinated pork neck meat of this current study. Considering the results, the direction of future work should be to evaluate the microbiological quality of the different marinated oven-grilled pork neck meat, in order to deduce the potential microbial entities that could be of interest. When such information is established, it would then be useful to submit this various marinated oven-grilled pork neck meat to different refrigerated storage/packaging conditions. This will help establish the preservative efficacy of marinades/marination variants as well as oven-grilling treatment.
Authorship contribution
Conceptualization, CORO and MK; Data curation, HS, CORO, SJ, KL, and MK; Formal analysis, HS, CORO, SJ, KL, and MK; Funding acquisition, CORO, SJ, KL, and MK; Investigation, CORO, HS, SJ, KL; Methodology, CORO, HS, SJ, KL, MK; Project administration, MK; Software, HS, SJ, KL; Supervision, MK and RPFG; Validation, HS, SJ, KL, RPFG; Visualization, HS, SJ, KL, RPFG; Writing – original draft, CORO and MK; Writing – review & editing, CORO and RPFG. All authors reviewed and approved the final manuscript.
Acknowledgments
Authors CORO, KL, SJ, and MK acknowledge financial support from Wroclaw University of Environmental and Life Sciences, Poland. Author RPFG acknowledges financial support from Polytechnic Institute of Viseu, Portugal.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
The datasets generated during and/or analyzed can be made available upon reasonable request from the corresponding authors.
Additional information
Funding
References
- McGlone, J. J.;. The Future of Pork Production in the World: Towards Sustainable, Welfare-Positive Systems. Animals. 2013, 3(2), 401–415. DOI: 10.3390/ani3020401.
- FAO’s Animal Production and Health Division: Meat & Meat Products. https://www.fao.org/agriculture/animal-production-and-health/en (Accessed 04 September 2022)
- Eurostat. (2017). Pork production up in the EU. Products Eurostat News, Published 19‑09‑2017. https://ec.europa.eu/eurostat/web/products-eurostat-news/-/DDN-20170919-1 (Accessed 28 December 2022)
- Cook, R. (2022). Ranking Of Countries That Produce The Most Pork (USDA), Beef2Live. https://beef2live.com/story-ranking-countries-produce-pork-usda-508-213044 Accessed 04 September 2022
- Szymańska, E. J.;. Functioning of the Supply Chain of Pork in Poland. J. Agribus. Rural Dev. 2015. DOI: 10.17306/JARD.2015.59.
- Florowski, T.; Pisula, A.; Słowński, M.; Orzechowska, B. Processing Suitability of Pork from Different Breeds Reared in Poland. Acta Scientiarum Polonorum Technologia Alimentaria. 2006, 5(2), 55–64.
- BC Cook Articulation Committee. (2015). Meat Cutting and Processing in the Food Service Industry. Victoria, B.C.: BCcampus. https://opentextbc.ca/meatcutting
- Arnarson, A. (2015). Pork 101: Nutrition Facts and Health Effects, In: Authority Nutrition: An Evidence-Based Approach, https://web.archive.org/web/20150408004138/http://authoritynutrition.com/foods/pork (Accessed 05 September 2022)
- Calorie-Count. (2022). Calories in Pork, Fresh, Loin, Tenderloin: Separable Lean Only, Cooked, Roasted. https://web.archive.org/web/20070906122726/http://www.calorie-count.com/calories/item/10061.html (Accessed 04 September 2022)
- National Institutes for Health (NIH). (2022). Thiamin- Fact Sheets for Professionals, National Institutes for Health -office of Dietary Supplements, US Department of Health, and Human Services. https://ods.od.nih.gov/factsheets/Thiamin-HealthProfessional/#h3 (Accessed September 4, 2022)
- Whitbread, D. (2022). Top 10 Foods Highest in Thiamin (Vitamin B1), Powered by USDA Nutrition Data, https://myfooddata.com/articles/thiamin-b1-foods.php (Accessed 04 September 2022)
- Toldrá, F.; Flores, M. The Use of Muscle Enzymes as Predictors of Pork Meat Quality. Food Chem. 2000, 69(4), 387–395. DOI: 10.1016/S0308-8146(00)00052-2.
- Moya, V. J.; Flores, M.; Aristoy, M. C.; Toldrá, F. Pork Meat Quality Affects Peptide and Amino Acid Profiles during the Ageing Process. Meat Sci. 2001, 58(2), 197–206. DOI: 10.1016/S0309-1740(00)00152-2.
- Martini, S.; Cattivelli, A.; Conte, A.; Tagliazucchi, D. Black, Green, and Pink Pepper Affect Differently Lipid Oxidation during Cooking and in Vitro Digestion of Meat. Food Chem. 2021, 350, 129246. DOI: 10.1016/j.foodchem.2021.129246.
- Cassens, R. G.;. Historical Perspectives and Current Aspects of Pork Meat Quality in the USA. Food Chem. 2000, 69(4), 357–363. DOI: 10.1016/S0308-8146(00)00048-0.
- Cheok, C. Y.; Chin, N. L.; Yusof, Y. A.; Mustapa Kamal, S. M.; Sazili, A. Q. Effect of Marinating Temperatures on Physical Changes of Traditionally Marinated Beef Satay. J. Food Process. Preserv. 2011, 35(4), 474–482. DOI: 10.1111/j.1745-4549.2010.00490.x.
- Istrati, D.; Simion Ciuciu, A. M.; Vizireanu, C.; Ionescu, A.; Carballo, J. Impact of Spices and Wine‐Based Marinades on Tenderness, Fragmentation of Myofibrillar Proteins and Color Stability in Bovine Biceps Femoris Muscle. J. Texture Stud. 2015, 46(6), 455–466. DOI: 10.1111/jtxs.12144.
- Sokołowicz, Z.; Augustyńska-Prejsnar, A.; Krawczyk, J.; Kačániová, M.; Kluz, M.; Hanus, P.; Topczewska, J. Technological and Sensory Quality and Microbiological Safety of RIR Chicken Breast Meat Marinated with Fermented Milk Products. Animals. 2021, 11(11), 3282. DOI: 10.3390/ani11113282.
- Siroli, L.; Baldi, G.; Soglia, F.; Bukvicki, D.; Patrignani, F.; Petracci, M.; Lanciotti, R. Use of Essential Oils to Increase the Safety and the Quality of Marinated Pork Loin. Foods. 2020, 9(8), 987. DOI: 10.3390/foods9080987.
- Zhang, H.; Wu, J.; Guo, X. Effects of Antimicrobial and Antioxidant Activities of Spice Extracts on Raw Chicken Meat Quality. Food Sci. Human Wellness. 2016, 5(1), 39–48. DOI: 10.1016/j.fshw.2015.11.003.
- Shahidi, F.; Hossain, A. Bioactives in Spices, and Spice Oleoresins: Phytochemicals and Their Beneficial Effects in Food Preservation and Health Promotion. J. Food Bioactives. 2018, 3, 8–75. DOI: 10.31665/JFB.2018.3149.
- Jalali, M.; Mahmoodi, M.; Moosavian, S. P.; Jalali, R.; Ferns, G.; Mosallanezhad, A.; … Mosallanezhad, Z. The Effects of Ginger Supplementation on Markers of Inflammatory and Oxidative Stress: A Systematic Review and Meta‐analysis of Clinical Trials. Phytotherapy Res. 2020, 34(8), 1723–1733. DOI: 10.1002/ptr.6638.
- Zhang, Y.; Henning, S. M.; Lee, R. P.; Huang, J.; Zerlin, A.; Li, Z.; Heber, D.; Turmeric and Black Pepper Spices Decrease Lipid Peroxidation in Meat Patties during Cooking. Int. J. Food Sci. Nutr. 2015, 663, 260–265. DOI:10.3109/09637486.2014.1000837.
- Amber, K.; Badawy, N. A.; El-Sayd, A. E. N. A.; Morsy, W. A.; Hassan, A. M.; Dawood, M. A. Ginger Root Powder Enhanced the Growth Productivity, Digestibility, and Antioxidative Capacity to Cope with the Impacts of Heat Stress in Rabbits. J. Thermal Biol. 2021, 100, 103075. DOI: 10.1016/j.jtherbio.2021.103075.
- Awuchi, C. G.; Okpala, C. O. R. Natural Nutraceuticals, Especially Functional Foods, Their Major Bioactive Components, Formulation, and Health Benefits for Disease prevention-An Overview. J. Food Bioact. 2022, 19, 97–123. DOI: 10.31665/JFB.2022.18317.
- Richardson, P.; Ed. Improving the Thermal Processing of Foods; Woodhead Publishing Limited: Cambridge, England, 2004; pp 520.
- Viegas, O.; Amaro, L. F.; Ferreira, I. M.; Pinho, O. Inhibitory Effect of antioxidant-rich Marinades on the Formation of Heterocyclic Aromatic Amines in pan-fried Beef. J. Agric. Food Chem. 2012, 60(24), 6235–6240. DOI: 10.1021/jf302227b.
- Okpala, C. O. R.; Juchniewicz, S.; Leicht, K.; Korzeniowska, M.; Guiné, R. P. Antioxidant, Organoleptic and Physicochemical Changes in Different Marinated Oven-Grilled Chicken Breast Meat. Foods. 2022, 11(24), 3951. DOI: 10.3390/foods11243951.
- Schröder, M. J. A.;. Food Quality and Consumer Value: Delivering Food that Satisfies. Archived from the original on 11 June 2016.; Springer: Berlin, 2003; pp 150.
- Ježek, F.; Kameník, J.; Macharáčková, B.; Bogdanovičová, K.; Bednář, J. Cooking of Meat: Effect on Texture, Cooking Loss and Microbiological quality–a Review. Acta Vet. Brno. 2020, 88(4), 487–496. DOI: 10.2754/avb201988040487.
- Farhadian, A.; Jinap, S.; Abas, F.; Sakar, Z. I. Determination of Polycyclic Aromatic Hydrocarbons in Grilled Meat. Food Control. 2010, 21(5), 606–610. DOI: 10.1016/j.foodcont.2009.09.002.
- Kerth, C. R.; Blair‐Kerth, L. K.; Jones, W. R. Warner‐Bratzler Shear Force Repeatability in Beef Longissimus Steaks Cooked with a Convection Oven, Broiler, or Clam‐shell Grill. J. Food Sci. 2003, 68(2), 668–669. DOI: 10.1111/j.1365-2621.2003.tb05729.x.
- Khan, M. I.; Min, J. S.; Lee, S. O.; Yim, D. G.; Seol, K. H.; Lee, M.; Jo, C. Cooking, Storage, and Reheating Effect on the Formation of Cholesterol Oxidation Products in Processed Meat Products. Lipids Health Dis. 2015, 14(1), 1–9. DOI: 10.1186/s12944-015-0091-5.
- Kim, E. J.; Lee, S.; Park, D. H.; Kim, H.; Choi, M. J. Physicochemical Properties of Pork Neck and Chicken Leg Meat under Various Freezing Temperatures in a Deep Freezer. Food Sci. Anim. Resour. 2020, 40(3), 444. DOI: 10.5851/kosfa.2020.e24.
- Salmon, C. P.; Knize, M. G.; Felton, J. S. Effects of Marinating on Heterocyclic Amine Carcinogen Formation in Grilled Chicken. Food Chem. Toxicol. 1997, 35(5), 433–441. DOI: 10.1016/S0278-6915(97)00020-3.
- Barido, F. H.; Lee, S. K. Effect of Detoxified Rhus Verniciflua Extract on Oxidative Stability and Quality Improvement of Raw Chicken Breast during Cold Storage. J. Anim. Sci. Technol. 2022, 64(2), 380. DOI: 10.5187/jast.2022.e20.
- Luciano, G.; Moloney, A. P.; Priolo, A.; Rohrle, F. T.; Vasta, V.; Biondi, L.; Monahan, F. J. Vitamin E and Polyunsaturated Fatty Acids in Bovine Muscle and the Oxidative Stability of Beef from Cattle Receiving Grass or concentrate-based Rations. J. Anim. Sci. 2011, 89, 3759–3768. DOI: 10.2527/jas.2010-3795.
- Bai, W. K.; Zhang, F. J.; He, T. J.; Su, P. W.; Ying, X. Z.; Zhang, L. L.; Wang, T. Dietary Probiotic Bacillus Subtilis Strain Fmbj Increases Antioxidant Capacity and Oxidative Stability of Chicken Breast Meat during Storage. PLoS One. 2016, 11(12), e0167339. DOI: 10.1371/journal.pone.0167339.
- Lengkidworraphiphat, P.; Wongpoomchai, R.; Taya, S.; Jaturasitha, S. Effect of Genotypes on Macronutrients and Antioxidant Capacity of Chicken Breast Meat. Asian-australas. J. Anim. Sci. 2020, 33(11), 1817. DOI: 10.5713/ajas.19.0736.
- Ali, M. D.; Kang, G. H.; Yang, H. S.; Jeong, J. Y.; Hwang, Y. H.; Park, G. B.; Joo, S. T. A Comparison of Meat Characteristics between Duck and Chicken Breast. Asian-australas. J. Anim. Sci. 2007, 20(6), 1002–1006. DOI: 10.5713/ajas.2007.1002.
- Kopec, W.; Jamroz, D.; Wiliczkiewicz, A.; Biazik, E.; Pudlo, A.; Korzeniowska, M.; … Skiba, T. Antioxidative Characteristics of Chicken Breast Meat and Blood after Diet Supplementation with Carnosine, L-histidine, and β-alanine. Antioxidants. 2020, 9(11), 1093. DOI: 10.3390/antiox9111093.
- Augustyńska-Prejsnar, A.; Ormian, M.; Sokołowicz, Z.; The Influence of Frozen Storage Duration and Thawing Methods on the Meat Quality of Broiler Chickens. Apar Badaw Dydakt 2017, 22.
- Augustyńska-Prejsnar, A.; Ormian, M.; Sokołowicz, Z. Physicochemical and Sensory Properties of Broiler Chicken Breast Meat Stored Frozen and Thawed Using Various Methods. J. Food Qual. 2018, 2018.
- Brambila, G. S.; Bowker, B. C.; Zhuang, H. Comparison of Sensory Texture Attributes of Broiler Breast Fillets with Different Degrees of White Striping. Poultr. Sci. 2016, 95(10), 2472–2476. DOI: 10.3382/ps/pew165.
- Çakmakçı, S.; Topdaş, E. F.; Kalın, P.; Han, H.; Şekerci, P.; Köse, L. P.; Gülçin, İ. Antioxidant Capacity and Functionality of Oleaster (Elaeagnus angustifolia L.) Flour and Crust in a New Kind of Fruity Ice Cream. Int. J. Food Sci. Technol. 2015, 50(2), 472–481. DOI: 10.1111/ijfs.12637.
- Civille, G. V.; Thomas Carr, B. Sensory Evaluation Techniques, 5th ed. CRC Press: Florida-USA, 2015.
- Libera, J.; Kononiuk, A.; Kęska, P.; Wójciak, K. Use of Grape Seed Extract as a Natural Antioxidant Additive in dry-cured Pork Neck Technology. Biotechnol. Food Sci. 2018, 82, 2.
- Olsson, V.; Pickova, J. The Influence of Production Systems on Meat Quality, with Emphasis on Pork. AMBIO: J. Hum. Environ. 2005, 34(4), 338–343. DOI: 10.1579/0044-7447-34.4.338.
- Moroney, N. C.; O’Grady, M. N.; Lordan, S.; Stanton, C.; Kerry, J. P. Seaweed Polysaccharides (Laminarin and Fucoidan) as Functional Ingredients in Pork Meat: An Evaluation of anti-oxidative Potential, Thermal Stability and Bioaccessibility. Mar. Drugs. 2015, 13(4), 2447–2464. DOI: 10.3390/md13042447.
- Brychcy, E.; Król, Ż.; Kulig, D.; Jarmoluk, A. The Effect of Carrageenan and Gelatine Hydrosols Incorporated with Acidic Electrolysed Water on Surface Microbiota and Quality Changes on Pork Meat. Int. J. Food Sci. Technol. 2016, 51(7), 1618–1629. DOI: 10.1111/ijfs.13132.
- Xiong, Y. L.;. Muscle Protein Oxidation and Functionality: A Global View of a once-neglected Phenomenon. Meat Muscle Biol. 2022, 5(3), 14349. DOI: 10.22175/mmb.14349.
- Biller, E.; Kleniewska, M. The Composition of Volatile Compounds, Parameters of Colour and Sensory Quality Attributes of Roasted Pork Neck Shoulder Derived from Polish Meat. Polish J. Appl. Sci.2017, 2(2), 59–65.
- Kerr, W. L.; Wang, X.; Choi, S. G. Physical and Sensory Characteristics of Low‐fat Italian Sausage Prepared with Hydrated Oat. J. Food Qual. 2005, 28(1), 62–77. DOI: 10.1111/j.1745-4557.2005.00010.x.
- Xia, X.; Kong, B.; Liu, J.; Diao, X.; Liu, Q. Influence of Different Thawing Methods on Physicochemical Changes and Protein Oxidation of Porcine Longissimus Muscle. LWT Food Sci. Technol. 2012, 46(1), 280–286. DOI: 10.1016/j.lwt.2011.09.018.
- Migdał, W.; Różycki, M.; Mucha, A.; Tyra, M.; Natonek-Wiśniewska, M.; Walczycka, M.; Krępa-Stefanik, K. Meat Texture Profile and Cutting Strength Analyses of Pork Depending on Breed and Age. Ann. Anim. Sci. 2020, 20(2), 677–692. DOI: 10.2478/aoas-2019-0085.
- Miller, R.; Prusa, K. Sensory Evaluation of Pork; National Pork Board. Pork Quality. American Meat Science Association, USA: Pork, 1998; pp 1–20.
- Smaoui, S.; Hlima, H. B.; Ghorbel, R. The Effect of Sodium Lactate and Lactic Acid Combinations on the Microbial, Sensory, and Chemical Attributes of Marinated Chicken Thigh. Poult. Sci. 2012, 91(6), 1473–1481. DOI: 10.3382/ps.2011-01641.
- Schilling, M. W.; Schilling, J. K.; Claus, J. R.; Marriott, N. G.; Duncan, S. E.; Wang, H. Instrumental Texture Assessment and Consumer Acceptability of Cooked Broiler Breasts Evaluated Using a Geometrically uniform-shaped Sample. J. Muscle Foods. 2003, 14, 11–23. DOI: 10.1111/j.1745-4573.2003.tb00342.x.
- Kim, Y. J.; Jin, S. K.; Park, W. Y.; Kim, B. W.; Joo, S. T.; Yang, H. S. The Effect of Garlic or Onion Marinade on the Lipid Oxidation and Meat Quality of Pork during Cold Storage. J. Food Qual. 2010, 33, 171–185. DOI: 10.1111/j.1745-4557.2010.00333.x.
