ABSTRACT
The aim of the current study was to explore the preservative effect of aqueous coriander extract and butylated hydroxytoluene (BHT) on the shelf life of refrigerated chicken patties for 9 days. For this purpose, BHT (100 ppm) and aqueous coriander extract (1%) was applied to improve the storage stability. The patties were evaluated for their physiochemical characterization including total phenolic content (TPC), thiobarbituric acid reactive substances (TBARS), peroxide value (PV), total carbonyl, metmyoglobin, and instrumental color, microbial analysis including (total plate count), and sensory attributes including (color and odor). The results showed that coriander extract has a significant (P < .05) effect in terms of delaying microbial growth, lipid and protein oxidation compared to BHT application. Likewise, higher phenolic contents were observed in patties treated with coriander extract. Conclusively, coriander extract did not impose any undesirable effect of physical attributes (instrumental color and color/odor) of cooked chicken patties. It is concluded that aqueous coriander extract is a plenteous source of various antioxidants and should be used as a functional food additive in meat products at 1% concentration without affecting the product’s quality attributes.
Introduction
In the last two decades, chicken has gained significant attention due to its unique taste and texture as compared to other meats. Poultry meat has a higher nutritional profile due to which it is highly recommended for different chicken-based products in the food industry.[Citation1] Furthermore, a natural balance and enriched nutritional profile categorized it into distinct food as compared to others like amino acids profile, high protein and lower fat content, and presence of various minerals and vitamins.[Citation2] A wide range of chicken-based products like burgers, sausages, and nuggets are much acceptable globally and commercially available. Fresh chicken flesh with higher water and nutritional content categorized it as highly perishable and jeopardizes its shelf stability.[Citation3]
Meat is more susceptible to oxidative damage owing to the presence of unsaturated lipids, metal catalysts, heme pigments, and other different oxidizing agents present in muscle tissues. Oxidation of biomolecules like proteins and lipids is considered one of the main reasons for chicken deterioration during the storage.[Citation4] Reactive oxygen species (ROS) such as singlet oxygen, superoxide anions (O2−), alkoxyl (RO−), peroxyl (RO2–), hydroxyl radicals, and reactive nitrogen species (RNS) including nitrogen dioxide radical NO2, nitric oxide radical (NO−) and peroxynitrite (ONOO−) are the main sources for oxidation.[Citation5–7] The products of lipid oxidation damage other molecules, particularly proteins that affect their solubility and functionality. Moreover, oxidized toxic products of lipids and proteins affect the sensory and nutritional attributes of chicken meat. The nature and extent of the reactions depend on the food ingredients and processing conditions.[Citation8–10]
In the food industry, antioxidants have gained more attention to inhibit oxidative reactions and are suitable for the preservation of different food products. Antioxidants can be broadly divided into two catagories: synthetic and natural antioxidants.[Citation11] Different synthetic antioxidants like butylated hydroxytoluene (BHT) and butylated hydroxyanisole (BHA) are commonly utilized in the food industry to improve the shelf life of chicken and chicken-based products; however, the potential toxicity and carcinogenic nature of synthetic antioxidants and consumer demand for chemical-free foods limit their use in food processing.[Citation12] To avoid the harmful effects of synthetic antioxidants, natural plant materials can serve as a better alternative to synthetic additives. Plant-based extracts with higher concentrations of phenolic compounds possess various potential activities such as antioxidant, anti-inflammatory, antimicrobial, and antimutagens.[Citation13–15]
Edible plants, particularly spices and herbs, are reliable sources of many powerful antioxidants such as ascorbic acid, carotenoids, alcohols (tocopherols, tocotrienols), and phenolic compounds (flavonoids, phenolic acid).[Citation16–18] Coriander (Coriandrum sativum L) is an important herb belonging to the Apiaceae family, mainly cultivated in the subcontinent and its leaves possess antioxidant, antibacterial, and anti-inflammatory activities.[Citation19] Coriander leaves are rich in volatile oils, including limonene, geraniol, and linalool, which have antibacterial, antifungal, and antioxidant properties.[Citation20,Citation21] Extraction of these bioactive compounds is a crucial step to utilize the potential activities of such plants. Conventional extraction techniques with organic solvents impose an adverse effect on the consumers and also on the environment. Furthermore, some residues of organic solvents remained in the extracts and after utilization in food processing makes it unpleasant. Green extraction techniques are known to eliminate the threat of toxic compounds and produce safe food.[Citation22] The aim of the current study is to investigate the effect of aqueous coriander extract supplementation in chicken patties and further characterize them with respect to their shelf stability. Keeping view of all the above discussion in this experiment, aqueous extraction was performed. As aqueous extraction has a lower quantity of flavoring and coloring compounds as compared to organic solvents. Moreover, aqueous extractions are economical, nontoxic, and environmentally friendly. However, this study aimed to elucidate the impact of aqueous coriander extract (green extraction) on the antioxidant profile, oxidative stability, microbial stability, and physiochemical attributes of patties made up of poultry meat.
Materials and methodology
Raw material
Raw materials including chicken and fresh coriander (Coriandrum sativum) were procured from the SB Departmental Store in Faisalabad, Pakistan. All the chemicals used in this experiment were of analytical grade and were procured from Sigma Aldrich and Merck.
Preparation of crude extract
Fresh coriander leaves were washed thoroughly and air-dried in the shade until they were completely dried and produced a chirping sound upon crushing. Dried leaves were then pulverized in the grinder (Philips HL7715) and macerated in water with a 1:10 overnight. Further, the solution was placed on a magnetic stirrer hot plate at 800 rpm for 48 h at room temperature (37°C) and the extract was concentrated through the application of a rotary evaporator (Rotavapor R210, Buchi, Postfach, Flawil, Switzerland) at 40°C temperature and 220 mbar at 35 rpm, and the extract was stored at refrigerated temperature (4°C) until further use.
Formation of chicken patties
Chicken patties (mixed mince) were prepared in triplicate by following the method of Elhadi et al.,[Citation23] with minor amendments in the formulation. Briefly, the mixture was prepared from the meat, and spices were designated into 50 g portions, then the extract was added as per the weight of the portion (v/w).
Total phenolic content
The level of TPC in aqueous coriander extract with various concentrations was evaluated by the previously described method by Singleton & Rossi.[Citation24] In brief, 0.5 ml sample was mixed with 2.5 ml folin reagent in 1:10 and preserved for 4 min. Two milliliters of sodium carbonate solution (75 g/L) was added and retained for 120 min at normal room temperature. The spectrophotometric absorbance was taken at 760 nm wavelength (Pharmaspec UV-1700, Shimadzu, Kyoto, Japan). In the patties, the concentration of TPC was taken by the method of Palmieri et al.[Citation21] In short, 25 ml of 70% acetone was used to mix with 5 g patties and then kept overnight at 4°C temperature. One ml of the extract was blended with distilled water. The above solution added sodium carbonate (1.25 ml, 20%) and 0.25 ml folin reagent. Absorbance was recorded at 725 nm.
Thiobarbituric acid reactive substances
Lipid peroxidation of patties was evaluated by measuring TBARS mg malondialdehyde (MDA) mg/kg by Cherian et al.,[Citation25] with minor changes. Chicken patties (3 g) were added to perchloric acid (PCA) (25 mL of 3.86%) and placed in a homogenizer (PCSIR, Lahore, Pakistan) for 20 s. After the filtration, filtrates (2 ml) were added to the TBA (2 ml, 20 mM). The solutions were stored at room temperature for 16 h then finally absorbance was taken at 531 nm using a spectrophotometer.
Peroxide value
The peroxide value of chicken patties was determined with the protocol described by Kinsella et al.,[Citation26] with minor modifications and results expressed as meq peroxide/kg. Briefly, chicken patties (5 g) were mixed with chloroform (30 ml) and anhydrous sodium sulfate (0.1 N) and centrifuged in a centrifuge machine (PCSIR, Lahore, Pakistan) at 10,000 rpm at 4°C temperature instead of filtration through a filter paper and the supernatant was collected. In the supernatant collected, glacial acetic acid (30 ml) and saturated potassium iodide (2 ml) solution was added and left for 2 min, with intermittent shaking. Afterward, distilled water (100 ml) and freshly prepared starch solution (1%, 2 ml) was added and titrated against 0.1 N sodium thiosulfate until a non-aqueous became colorless.
Total carbonyl
Protein oxidation was determined by a well-known method previously adopted by Salminen et al.[Citation27] Briefly, chicken patties (2 g) were mixed with NaCl (0.6 M, 25 ml) and contained a phosphate buffer, 4 aliquots of 0.2 mL were made. DNPH (1 ml, 0.2%) was dissolved in HCl (2 M) and filled into 2 aliquots while two were left blank. Incubated at room temperature and results were presented as nmol/mg protein.
Metmyoglobin (%)
Metmyoglobin percentage was determined by the method outlined by Chauhan et al.[Citation28] Briefly, 3 g of chicken patties sample were mixed with chilled phosphate buffer (30 ml, 0.04 M, pH 6.8). The sample was then homogenized with a homogenizer and kept for 60 min at 4°C. The sample was centrifuged in a refrigerated centrifuge machine at 8000 rpm at 4°C, and supernatant was collected and filtered through Whatman no.42 filter paper. Optical density was measured using a spectrophotometer (Pharmaspec UV-1700, Shimadzu, Kyoto, Japan) at 525, 572, and 700 nm, respectively.
Microbial analysis
In the microbial investigation, the total plate count was taken by applying the method already performed by Singh et al..[Citation29] Patties on respective storage days (0, 3, 6, and 9) were opened in the laminar flow (Behr, Germany) and pre-sterilized by ultra-violet (UV) radiation. Chicken patties (10 g) were mixed well with peptone water (90 ml sterile 0.1%) in a stomacher and dilutions were made. For the determination of total plate counts, plate count agar (Merck, Germany) was used, and plates were incubated in an incubator (PCSIR, Lahore, Pakistan) at 37 ± 2°C for 48 h. Moreover, the analysis was done by pour plate technique, and results were expressed as log CFU/g of the sample.
Sensory analysis
The sensory profile of the product was assessed by different trained and semi-trained judges. The panel of 20 judges consisted of different staff members and students including males and females of different age groups (20–40 years) by following the method illustrated by Trindade et al.[Citation30] The sensory evaluation performa was consisted of two parameters including color and odor and ranged from 1 to 9.
Statistical analysis
The obtained data were expressed as mean ± standard deviation (SD) using an average of three replications. Analysis of Variance (ANOVA) was applied for statistical analysis. Duncan’s multiple range test was adopted to evaluate the means differences. The data were treated with the statistical analysis package (SPSS Statistics 8.1 Window version, Chicago, USA). The level of significance was set at P < .05.
Results and discussion
Total phenolic content
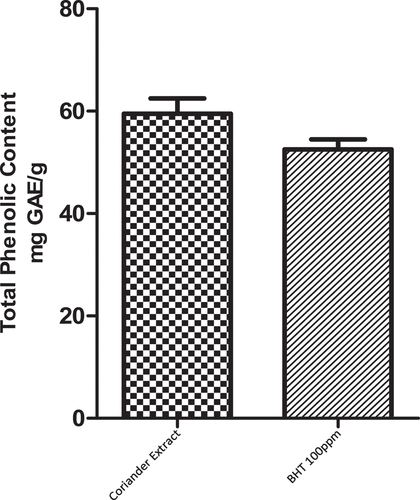
Polyphenols are a diverse class of plant bioactive compounds. These are famous for their strong antioxidant potential and free radical scavenging properties.[Citation31,Citation32] Therefore, the total phenolic content determination is important to elucidate the antioxidant potential of plant extracts. The total phenolic content of aqueous coriander extract and BHT is presented in . Results showed that 1% coriander (0.58 mg GAE/g) has a significantly (P < 0.05) higher level of TPC compared to BHT 100 ppm.
The mean values of the total phenolic content (TPC) of chicken patties formulated with aqueous coriander extract and BHT under refrigerated conditions are shown in . T1 had significantly (P < .05) higher values of TPC as compared to T2 and Control. In line with the previously reported data,[Citation33,Citation34] the current study revealed that the addition of coriander extract enhanced the TPC level of chicken patties. Similarly, Das et al.[Citation35] reported that the addition of lychee pericarp extracts significantly (P < .05) increased the total phenolic content of nuggets.
Table 1. Effect of Coriander extract and BHT on total phenolic of poultry meat patties.
Peroxide value
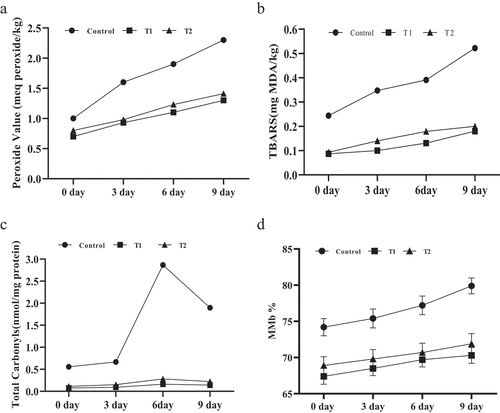
A peroxide value assay was done to determine the primary oxidation product formation. Data obtained from the assay are expressed in . The peroxide values on the first day of storage ranged from 0.7 to 1 meq peroxide/kg, and at the end of storage, they varied from 1.3 to 2.3 meq peroxide/kg, respectively. A regular upsurge was noticed in all samples throughout the storage interval. Initially, peroxide content was non-significant (P > .05); however, it became significant (P < .05) between control and treated samples. In other studies exploring the effect of plant extracts on peroxide values,[Citation36,Citation37] the current research found a positive effect of aqueous coriander extract as a natural preservative on the peroxide value of chicken patties in comparison to control and synthetic antioxidants.
Tbars
The effect of different treatments on TBARS values of cooked chicken patties is summarized in . The maximum amount of MDA/kg (0.24–0.52 mg) was measured in the control sample. The addition of antioxidants (natural/synthetic) significantly (P < .05) retarded the production of MDA. The lowest amount of MDA was recorded in the coriander 1% extract containing patties (0.08–0.18 mg), which is comparable with the synthetic antioxidant, BHT (0.09–0.2), and attributed to the strong antioxidant activity in preventing lipid oxidation. The results of the present study are in line with,[Citation38,Citation39] in which they noticed a significantly higher level of lipid oxidation in the control group when compared to the coriander and garlic-treated sausage samples.
The upsurge in the PV and TBARS is an indication of lipid peroxidation advancement. A fast increase in the PV and TBARS in the control sample might be due to the termination of the induction period. Moreover, chicken patties having antioxidants hold the induction period throughout the storage, thus implying that extracts can inhibit the formation of hydroperoxides which further take part in the formation of noxious oxidative compounds. This considerable difference in shelf-life between the treated and control samples revealed the strong antioxidant potential of aqueous coriander extract.
Lower PV might be due to the action of phenolics present in aqueous coriander extract, which slows down the formation of primary oxidation products. Progression in the TBARS is an indication of the overproduction of hydroperoxides, which leads to the formation of MDA. However, antioxidants retarded the lipid oxidation in treated samples, resulting in lower MDA. Hence, it can be concluded that coriander extraction possesses the primary oxidation retardation (hydroperoxide inhibition) capability.
Protein oxidation (carbonyl)
The total carbonyl values of patties made up of poultry meat are presented in . All the treated samples showed the same pattern except for the control. Due to the lack of antioxidants, the control sample exhibited significantly higher values (P < .05) of carbonyl content (0.55–1.89 nmol/mg). Cor1% containing sample efficiently inhibited the protein oxidation (0.075–0.14 nmol/mg) and/or equally effective as BHT (0.11–0.22 nmol/mg). A similar behavior was noted by Guedes-Oliveira et al.,[Citation40] in ground pork meat by using aqueous coriander extract, which retarded the formation of protein carbonyl formation (P < .05). Aldehydes formed during lipid oxidation and interact with proteins, resulting in the formation of protein carbonyls which can further cause degradation and fragmentation of proteins. In this study, the formation of protein carbonyls in the control sample was significantly (P < .05) higher (approx. six-folds) during the first 6 days of storage than in antioxidant-treated samples. A rapid decline was observed after 6 days of storage in the formation of protein carbonyls in all the samples, which is in accordance with the results presented by Batifoulier et al.[Citation41] and Khatun et al.[Citation42] Moreover, the rate of protein oxidation in the control sample was higher than the lipid oxidation indicating that the occurrence of protein oxidation is more rapid than lipid oxidation.
Metmyoglobin (%)
The amount of metmyoglobin is directly linked to the magnitude of protein oxidation in meat products. The heme proteins are mainly comprised of iron Fe+2 state and convert into Fe+3 via the process of autoxidation.[Citation43] The effect of antioxidants (Natural/Synthetic) on the formation of metmyoglobin is shown in . The maximum amount of metmyoglobin (74.2–79.9%) was measured in control after 9 days of storage, while in the treated samples T1 (67.4–70.3%) and T2 (68.9–71.9%), the presence of antioxidants restricted the development of MMb significantly (P < .05) which is attributed to the strong antioxidant activity. Singh et al.[Citation29] evaluated clove powder; ginger and garlic paste in chicken meat emulsion and found that all the treated samples significantly inhibited the formation of metmyoglobin as compared to the control. Such a non-significant (P > .05) difference between T1 and T2 indicates that aqueous coriander extract can be used as a substitute for synthetic antioxidants to prevent oxidation and discoloration.
Total plate count
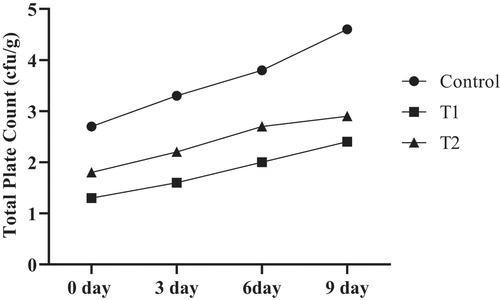
The total plate count of all the samples is shown in . T1 had the lowest amount of total plate count (1.3–2.4 CFU/g), while the highest value was observed in the control sample (2.7–4.6 CFU/g). In general, the plate count of all the samples increased with storage time. This implied that aqueous coriander extract (1%) played a pivotal role in the inhibition of microbes during storage and has strong antimicrobial activity. Nugboon and Intarapichet[Citation44] and Pateiro et al.[Citation45] also concluded that the Vietnamese coriander extract inhibited the growth of microbes in pork meatball samples, restricted the growth to <5 CFU/g, and retained a shelf life of up to 9 days at refrigerator temperature. The total plate count of all the treated samples was below the acceptable limit. The markedly antimicrobial activity might be due to the bioactive components present in the aqueous extract of coriander. Good hygiene and good manufacturing practices also play an important role in the safety of food.
Color stability
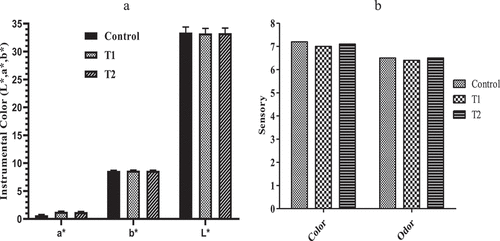
When evaluating meat, the consumer gives first attention to its color, which is a visual impression and is mainly affected by the presence of pigments, and is also influenced by tissue composition and meat texture. The overall mean values of L*, a*, and b* of all treatments during refrigerated storage are shown in . No significant difference (P > .05) was observed between the treated samples (T1 and T2); however, the overall mean of a* of control was significantly lower (P < .05). Despite this, treated samples in comparison to the control sample markedly retarded the metmyoglobin genesis; however, a significant reduction in a* values occurred in all the samples during storage intervals. During the 9 days of storage, L* values increased significantly (P < .05) and then remained constant and the values were not significantly different. From the data, the b* values of all the samples showed a non-significant difference (P > .05). A significant increase in the redness (a*) of chicken patties treated with pomegranate peel extract has been reported by Naveena et al.[Citation46] The present study claimed that the incorporation of natural extracts stabilizes the natural color of chicken patties and controls the degradation of redness. Metmyoglobin percentage is directly influenced by the color of muscle foods. Initially, the oxymyoglobin was produced from myoglobin which gives a slight pink color to the meat and further oxidation converts the oxymyoglobin into metmyoglobin which gives brownish color to the meat.[Citation47]
Sensory characteristics
Color and odor are the first preference of consumers in the acceptance and rejection of food. The results of the sensory evaluation of chicken patties are shown in . The incorporation of natural and synthetic antioxidants had no significant difference (P > .05). The presence of naturally occurring compounds in the coriander extract limited the formation of aldehydes and ketones which are mainly responsible for off-flavor and off-odor.[Citation48,Citation49] Despite the higher TBARS and PV values in the control sample, the sensory characteristics were not affected; it might be due to the sensory threshold. Hence, coriander extract did not impose any negative effect on the color and odor of cooked chicken patties.
Conclusion
Green solvent extraction is safe and environmentally friendly in comparison to solvent extraction. The aqueous coriander extract is found to be useful in extending the shelf-life of patties as well as a good alternative to synthetic antioxidants. The current research found that aqueous coriander extract did not harm any physical parameters of the product. Its activity was comparable to BHT as a preservative for meat products. According to the results, aqueous coriander extract reduced the formation of peroxides, TBARS, total carbonyls, and metmyoglobin formation and inhibited the growth of microorganisms in chicken patties stored for 9 days at 4°C.
Acknowledgments
The authors express their gratitude to Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2023R158), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Rebezov, M.; Neverova, O.; Nesterenko, A.; Kenijz, N.; Khayrullin, M.; Dolmatova, I. Bychkova, T. Study of the Nutritional and Biological Value of Meat Loaf from Chicken Meat and Chickpea Flour. Int. J. Mod. Agric. 2021, 10(2), 1972–1979.
- Pinto da Rosa, P.; Pio Ávila, B.; Damé Veber Angelo, I.; Garavaglia Chesini, R.; Albandes Fernandes, T.; da Silva Camacho, J., Gularte, M.; Roll, V. F. B.; Gularte, M. A. Impact of Different Chicken Meat Production Systems on consumers’ Purchase Perception. British Poul. Sci. 2021, 62(3), 387–395. DOI: 10.1080/00071668.2020.1857335.
- Candan, T.; Bağdatlı, A. Use of Natural Antioxidants in Poultry Meat. Celal Bayar University J. Sci. 2017, 13(2), 279–291.
- Domínguez, R.; Pateiro, M.; Gagaoua, M.; Barba, F. J.; Zhang, W.; Lorenzo, J. M. A Comprehensive Review on Lipid Oxidation in Meat and Meat Products. Antioxidants. 2019, 8(10), 429. DOI: 10.3390/antiox8100429.
- Contini, C.; Álvarez, R.; O’sullivan, M.; Dowling, D. P.; Gargan, S. Ó.; Monahan, F. J. Effect of an Active Packaging with Citrus Extract on Lipid Oxidation and Sensory Quality of Cooked Turkey Meat. Meat. Sci. 2014, 96(3), 1171–1176. DOI: 10.1016/j.meatsci.2013.11.007.
- Estévez, M.; Díaz-Velasco, S.; Martínez, R. Protein Carbonylation in Food and Nutrition: A Concise Update. Amino. Acids. 2021, 54(4), 1–15. DOI: 10.1007/s00726-021-03085-6.
- Qazi, M. A.; Molvi, K. I. Free Radicals and Their Management. Am. J. Pharm. Health Res. 2018, 6(4), 1–10. DOI: 10.46624/ajphr.2018.v6.i4.001.
- Heinonen, M.; Gürbüz, G.; Ertbjerg, P. Oxidation of Proteins. In Chemical Changes During Processing and Storage of Foods; Rodriguez-Amaya, D. B., Amaya-Farfan, J., Eds.; Elsevier, 2021; pp. 85–123.
- Levine, R. L. Carbonyl Modified Proteins in Cellular Regulation, Aging, and Disease. Free Radical Biol. Med. 2002, 32(9), 790–796. DOI: 10.1016/S0891-5849(02)00765-7.
- Rodriguez-Amaya, D. B.; Shahidi, F. Oxidation of Lipids. In Chemical Changes During Processing and Storage of Foods; Rodriguez-Amaya, D. B., Amaya-Farfan, J., Eds.; Elsevier, 2021; pp. 125–170.
- Lourenço, S. C.; Moldão-Martins, M.; Alves, V. D. Antioxidants of Natural Plant Origins: From Sources to Food Industry Applications. Molecules. 2019, 24(22), 4132. DOI: 10.3390/molecules24224132.
- Kumar, Y.; Yadav, D. N.; Ahmad, T.; Narsaiah, K. Recent Trends in the Use of Natural Antioxidants for Meat and Meat Products. Compr. Rev. Food Sci. Food Saf. 2015, 14(6), 796–812. DOI: 10.1111/1541-4337.12156.
- Heinonen, M. Antioxidant Activity and Antimicrobial Effect of Berry Phenolics–A Finnish Perspective. Mol. Nutr. Food Res. 2007, 51(6), 684–691. DOI: 10.1002/mnfr.200700006.
- Kähkönen, M. P.; Hopia, A. I.; Heinonen, M. Berry Phenolics and Their Antioxidant Activity. J. Agric. Food Chem. 2001, 49(8), 4076–4082. DOI: 10.1021/jf010152t.
- Vuorela, S.; Kreander, K.; Karonen, M.; Nieminen, R.; Hämäläinen, M.; Galkin, A.; PihlajaLaitinen, K.L.; Salminen, J. -P.; Moilanen, E.; Pihlaja, K., et al. Preclinical Evaluation of Rapeseed, Raspberry, and Pine Bark Phenolics for Health Related Effects. J. Agric. Food Chem. 2005, 53(15), 5922–5931. DOI: 10.1021/jf050554r.
- Huda-Faujan, N.; Noriham, A.; Norrakiah, A.; Babji, A. Antioxidant Activity of Plants Methanolic Extracts Containing Phenolic Compounds. Afr. J. Biotechnol. 2009, 8(3), 484–489.
- Nanasombat, S.; Teckchuen, N. Antimicrobial, Antioxidant and Anticancer Activities of Thai Local Vegetables. J. Med. Plants Res. 2009, 3(5), 443–449.
- Zheng, W.; Wang, S. Y. Antioxidant Activity and Phenolic Compounds in Selected Herbs. J. Agric. Food Chem. 2001, 49(11), 5165–5170. DOI: 10.1021/jf010697n.
- Kaiser, A.; Kammerer, D. R.; Carle, R. Impact of Blanching on Polyphenol Stability and Antioxidant Capacity of Innovative Coriander (Coriandrum Sativum L.) Pastes. Food Chem. 2013, 140(1–2), 332–339. DOI: 10.1016/j.foodchem.2013.02.077.
- Kačániová, M.; Galovičová, L.; Ivanišová, E.; Vukovic, N. L.; Štefániková, J.; Valková, V.; Borotová, E.P.; Žiarovská, J.; Terentjeva, M.; Felšöciová, S., et al. Antioxidant, Antimicrobial and Antibiofilm Activity of Coriander (Coriandrum Sativum L.) Essential Oil for Its Application in Foods. Foods. 2020, 9(3), 282. DOI: 10.3390/foods9030282.
- Palmieri, S.; Pellegrini, M.; Ricci, A.; Compagnone, D.; Lo Sterzo, C. Chemical Composition and Antioxidant Activity of Thyme, Hemp and Coriander Extracts: A Comparison Study of Maceration, Soxhlet, UAE and RSLDE Techniques. Foods. 2020, 9(9), 1221. DOI: 10.3390/foods9091221.
- Soquetta, M. B.; Terra, L. D. M.; Bastos, C. P. Green Technologies for the Extraction of Bioactive Compounds in Fruits and Vegetables. CyTA-J. Food. 2018, 16(1), 400–412. DOI: 10.1080/19476337.2017.1411978.
- Elhadi, D. A.; Elgasim, E. A.; Mohamed Ahmed, I. A. Microbial and Oxidation Characteristics of Refrigerated Chicken Patty Incorporated with Moringa (Moringa Oleifera) Leaf Powder. CyTA-J. Food. 2017, 15(2), 234–240. DOI: 10.1080/19476337.2016.1242157.
- Singleton, V. L.; Rossi, J. A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16(3), 144–158.
- Cherian, G.; Wolfe, F.; Sim, J. Dietary Oils with Added Tocopherols: Effects on Egg or Tissue Tocopherols, Fatty Acids, and Oxidative Stability. Poultr. Sci. 1996, 75(3), 423–431. DOI: 10.3382/ps.0750423.
- Kinsella, J.; Shimp, J.; Mai, J.; Weihrauch, J. Fatty Acid Content and Composition of Freshwater Finfish. J. Am. Oil Chem. Soc. 1977, 54(10), 424–429. DOI: 10.1007/BF02671025.
- Salminen, H.; Estévez, M.; Kivikari, R.; Heinonen, M. Inhibition of Protein and Lipid Oxidation by Rapeseed, Camelina and Soy Meal in Cooked Pork Meat Patties. Eur. Food Res. Technol. 2006, 223(4), 461–468. DOI: 10.1007/s00217-005-0225-5.
- Chauhan, P.; Pradhan, S. R.; Das, A.; Nanda, P. K.; Bandyopadhyay, S.; Das, A. K. Inhibition of Lipid and Protein Oxidation in Raw Ground Pork by Terminalia Arjuna Fruit Extract During Refrigerated Storage. Asian-Australas J. Anim. Sci. 2019, 32(2), 265. DOI: 10.5713/ajas.17.0882.
- Singh, P.; Sahoo, J.; Chatli, M. K.; Biswas, A. K. Shelf Life Evaluation of Raw Chicken Meat Emulsion Incorporated with Clove Powder, Ginger and Garlic Paste as Natural Preservatives at Refrigerated Storage (4±1 C). Int. Food Res. J. 2014, 21(4), 1363.
- Trindade, R.; Lima, A.; Andrade-Wartha, E.; Silva, E.; O, A.; Mancini-Filho, J.; Villavicencio, A. Consumer’s Evaluation of the Effects of Gamma Irradiation and Natural Antioxidants on General Acceptance of Frozen Beef Burger. Radiat. Phys. Chem. 2009, 78(4), 293–300. DOI: 10.1016/j.radphyschem.2008.12.003.
- Ge, X.; Jing, W.L.; Zhao, K.; Su, C.; Zhang, B.; Zhang, Q.; LiHan, L.; Yu, X.; Li, W. The Phenolic Compounds Profile, Quantitative Analysis and Antioxidant Activity of Four Naked Barley Grains with Different Color. Food Chem. 2021, 335, 127655. DOI: 10.1016/j.foodchem.2020.127655.
- Zhang, R.; Zeng, Q.; Deng, Y.; Zhang, M.; Wei, Z.; Zhang, Y.; Tang, X. Phenolic Profiles and Antioxidant Activity of Litchi Pulp of Different Cultivars Cultivated in Southern China. Food Chem. 2013, 136(3–4), 1169–1176. DOI: 10.1016/j.foodchem.2012.09.085.
- Šojić, B.; Pavlić, B.; Ikonić, P.; Tomović, V.; Ikonić, B.; Zeković, Z.; Kocić-Tanackov, M.S.; Jokanović, M.; Škaljac, S.; Ivić, M. Coriander Essential Oil as Natural Food Additive Improves Quality and Safety of Cooked Pork Sausages with Different Nitrite Levels. Meat Sci. 2019, 157, 107879. DOI: 10.1016/j.meatsci.2019.107879.
- Verma, A. K.; Rajkumar, V.; Banerjee, R.; Biswas, S.; Das, A. K. Guava (Psidium Guajava L.) Powder as an Antioxidant Dietary Fibre in Sheep Meat Nuggets. Asian-Australas J. Anim. Sci. 2013, 26(6), 886. DOI: 10.5713/ajas.2012.12671.
- Das, A. K.; Rajkumar, V.; Nanda, P. K.; Chauhan, P.; Pradhan, S. R.; Biswas, S. Antioxidant Efficacy of Litchi (Litchi Chinensis Sonn.) Pericarp Extract in Sheep Meat Nuggets. Antioxidants. 2016, 5(2), 16. DOI: 10.3390/antiox5020016.
- Marangoni, C.; Moura, N. F. D. Antioxidant Activity of Essential Oil from Coriandrum sativum L. in Italian Salami. Cienc. Technol. Aliment. 2011, 31(1), 124–128. DOI: 10.1590/S0101-20612011000100017.
- Rashidaie Abandansarie, S. S.; Ariaii, P.; Charmchian Langerodi, M. Effects of Encapsulated Rosemary Extract on Oxidative and Microbiological Stability of Beef Meat During Refrigerated Storage. Food Sci. Nutr. 2019, 7(12), 3969–3978. DOI: 10.1002/fsn3.1258.
- Bali, A.; Das, S. K.; Khan, A.; Patra, D.; Biswas, S.; Bhattacharyya, D. A Comparative Study on the Antioxidant and Antimicrobial Properties of Garlic and Coriander on Chicken Sausage. Int. J. Meat Sci. 2011, 1(2), 108–116. DOI: 10.3923/ijmeat.2011.108.116.
- Gazwi, H. S.; Mahmoud, M. E.; Toson, E. Analysis of the Phytochemicals of Coriandrum Sativum and Cichorium Intybus Aqueous Extracts and Their Biological Effects on Broiler Chickens. Sci. Rep. 2022, 12(1), 1–11. DOI: 10.1038/s41598-022-10329-2.
- Guedes-Oliveira, J.; Xue, S.; Setyabrata, D.; Kim, Y. Effect of Cilantro Extract (Coriandrum Sativum) Application on Color and Oxidative Stability of Ground Pork Under Different Packaging Conditions. Meat Muscle Biol. 2018, 2(2), 74. DOI: 10.22175/rmc2018.064.
- Batifoulier, F.; Mercier, Y.; Gatellier, P.; Renerre, M. Influence of Vitamin E on Lipid and Protein Oxidation Induced by H2O2-Activated MetMb in Microsomal Membranes from Turkey Muscle. Meat Sci. 2002, 61(4), 389–395. DOI: 10.1016/S0309-1740(01)00209-1.
- Khatun, M.; Hossain, M.; Ali, M. A.; Rahman, M.; Azad, M.; Hashem, M. Formulation of Value Added Chicken Nuggets Using Carrot and Ginger as a Source of Dietary Fiber and Natural Antioxidant: Value Addition of Chicken Nuggets. SAARC J. Agric. 2022, 20(1), 185–196. DOI: 10.3329/sja.v20i1.60611.
- Richards, M. P.; Dettmann, M. A. Comparative Analysis of Different Hemoglobins: Autoxidation, Reaction with Peroxide, and Lipid Oxidation. J. Agric. Food Chem. 2003, 51(13), 3886–3891. DOI: 10.1021/jf0212082.
- Nugboon, K.; Intarapichet, K. Antioxidant and Antibacterial Activities of Thai Culinary Herb and Spice Extracts, and Application in Pork Meatballs. Int. Food Res. J. 2015, 22(5), 1788–1800.
- Pateiro, M.; Munekata, P. E.; Sant’ana, A. S.; Domínguez, R.; Rodríguez-Lázaro, D.; Lorenzo, J. M. Application of Essential Oils as Antimicrobial Agents Against Spoilage and Pathogenic Microorganisms in Meat Products. Int. J. Food Microbiol. 2021, 337, 108966. DOI: 10.1016/j.ijfoodmicro.2020.108966.
- Naveena, B.; Sen, A.; Vaithiyanathan, S.; Babji, Y.; Kondaiah, N. Comparative Efficacy of Pomegranate Juice, Pomegranate Rind Powder Extract and BHT as Antioxidants in Cooked Chicken Patties. Meat Sci. 2008, 80(4), 1304–1308. DOI: 10.1016/j.meatsci.2008.06.005.
- Zhang, X.; Li, D.; Meng, Q.; He, C.; Ren, L. Effect of Mulberry Leaf Extracts on Color, Lipid Oxidation, Antioxidant Enzyme Activities and Oxidative Breakdown Products of Raw Ground Beef During Refrigerated Storage. J. Food Qual. 2016, 39(3), 159–170. DOI: 10.1111/jfq.12187.
- Tylewicz, U.; Inchingolo, R.; Rodriguez-Estrada, M. T. Food Aroma Compounds. In Nutraceutical and Functional Food Components; Galanakis, C. M., Ed.; Academic Press, 2022; pp. 363–409.
- Villalobos-Delgado, L. H.; Mateo, J.; Caro, I.; Ramos, M. Y. L.; Mendez, N. G.; Cansino, R. G.; Mondragón, E. G. G. Natural Antioxidants in Fresh and Processed Meat. In Sustainable Meat Production and Processing; Galanakis, C. M., Ed.; Academic Press, 2019; pp. 207–236.