ABSTRACT
This research aimed to verify how the germination method affects the pasting, thermal and structural properties of haricot bean flours to expand their potential for application in various food products. The pasting properties of flours showed considerable variation due to variety, germination, and interaction between variety and germination. Onset, peak and end of gelatinization temperature and enthalpy of haricot bean flours ranged with germination from 65.4 to 67.9, 70.6 to 73.3, 77.0 to 79.4 C, and 3.5 to 4.9 J/g, respectively. The Crystallinity index of 632 G flour was higher than that of 119 G and 125 G flour. The data revealed that the soaked and germinated haricot bean flour showed major diffraction peak values of the 2θ at 18.25°,18.40°,18.25°, 17.68°, 18.74°, and 18.34°, respectively for 119S, 119 G, 632S, 632 G,125S, and 125 G. FT-IR spectroscopy of four samples did not show any variations in spectra, but a change in the intensity of bands was observed. The SEM images reveal that starch granules were surrounded by a protein matrix.
Introduction
Legumes have been considered the most significant food source, especially for low-income groups of people in developing countries,[Citation1] containing a relatively high amount of proteins.[Citation2] Dry beans are the dicotyledonous seed of plants belonging to the Leguminosae family, which are widely cultivated worldwide due to their global adaptability and drought tolerance.[Citation3] Among the commonly consumed dry beans, haricot beans (Phaseolus vulgaris L.) occupy a significant place in human nutrition in the East and Great Lakes regions of Africa.[Citation4] However, their role appears to be limited because of several factors including the presence of anti-nutritional compounds in the seeds which may have adverse effects on human nutrition, low protein, and starch digestibility,[Citation5] and poor mineral bioavailability.[Citation6] Transforming the beans into flour aids in improving the nutritional requirements of consumers, especially in developing countries.[Citation7] The germination processes are easy and cost-effective treatments given to legumes to modify macronutrient structure,[Citation8] improve nutritional, functional, and biological properties, as well as removing antinutritional factors such as tannin, phytic acid, oxalate, and enzyme inhibitors.[Citation9,Citation10] The consumption of grain sprouts has become common among people interested in improving and keeping their health by altering dietary habits. Due to the health benefits of germination, different studies have reported on the incorporation of germinated seeds into various food manufacturing ingredients, such as pasta,[Citation11] crackers,[Citation12] beverages,[Citation13] muffins,[Citation14] and yogurt.[Citation15] Depending on the food products, the addition of legumes in a germinated form has different effects.[Citation16] Eventhough the compositional changes of haricot beans during germination have been investigated to a certain degree, the research performed on changes in the thermal and structural properties of flour during the germination of haricot beans is limited. It is based on this background that this work was undertaken to investigate the effect of germination on pasting, thermal, morphological, FTIR, and XRD properties of flours from three varieties of haricot bean seeds.
Material and methods
Sample preparation
Haricot bean (Phaseolus Vulgaris L.) varieties (SER 119, SAB 632, and SER 125) were collected from Awash Melkasa Agricultural Center based on the facts that they have shown to have a high production percentage, disease tolerance, short period to ripe and easy to adopt in Ethiopia.[Citation17] The soaked and germinated haricot bean flour was prepared according to previously done methods.[Citation10] The resulting haricot bean flours were placed in plastic bags, sealed, and stored at 4°C in a desiccator until needed for further analysis.
Pasting properties
The pasting properties of the flours were measured using a Rapid Visco Analyzer (Perten RVA 4800, PerkinElmer, Sweden). An aqueous dispersion of flour on a 14% moisture basis (12.28%, w/w; 28.5 g total weight) was equilibrated at 50°C for 1 min, heated at the rate of 12.2°C/min to 95°C, held for 2.5 min, cooled to 50°C at the rate of 11.8°C/min and again held at 50°C for 2 min. A constant paddle rotational speed (160 rpm) was used throughout the entire analysis, except for rapid stirring at 960 rpm for the first 10 s to disperse the sample.[Citation18]
Thermal properties
The thermal properties of flours were analyzed using DSC (SKZ1052B, Hunan, China) equipped with a thermal analysis data station. A 10 mg sample was weighed into a 40 µl capacity aluminum pan and 20 µl distilled water was added with the help of a Hamilton micro syringe. Pans were hermetically sealed and allowed to stand for 1 h at room temperature before heating in DSC. The DSC was calibrated using indium, and an empty aluminum pan was used as a reference. Sample pans were heated at a rate of 10°C/min from 20 to 180°C, and thermal parameters viz. onset (To), peak (Tp), conclusion (Tc) temperature, and enthalpy (DH) were calculated from the DSC curves.[Citation18]
Fourier transform infrared analysis
The spectra of the samples were recorded using Fourier transform infrared (FTIR) spectrometer system (Nicolet is50 ABX, Thermofisher Scientific, German) coupled to an attenuated total reflection (ATR) accessory. Analyses were carried out at room temperature, and spectra were acquired in the range of 4000–500 cm−1 at a resolution of 4 cm−1.[Citation19]
Xrd
Samples were loaded into the XRD sample holder, and XRD pattern was obtained using an X-ray diffractometer (XRD-7000, Shangai Drawel scientific instrument co., Ltd., China) equipped with a divergence slit, operating at 40 kV and 40 mA at a scan speed of 1o/min. The relative crystallinity (RC) was calculated using the areas of the crystalline and amorphous regions on the X-ray diffractogram.[Citation20]
Scanning electron microscopy
The flour was placed on an adhesive tape attached to a circular aluminum specimen stub and then coated vertically with gold palladium. The images of samples were captured at an accelerator potential of 10 kV using a scanning electron microscope (JCM-6000 plus, Jeol Ltd., Korea).[Citation18]
Statistical analysis
All analyses were conducted in triplicates observations except FTIR and SEM analysis, and data obtained were subjected to analysis of variance (ANOVA) using software package SAS version 9.0 (SAS Institute, Inc., Cary, North Carolina, USA). Tukey’s HSD test at the significance level of 5% (P < .05) was used to determine significant differences among means.
Results and discussion
Pasting properties
The pasting properties of the haricot bean four samples are presented in . Significant differences were observed in pasting characteristics of flours due to variety, processing method and interaction between them. While the non-germinated 119S flour had the highest peak viscosity value (1832 ± 187 cp) but flour from germinated SER125 variety had the lowest peak viscosity value (564 ± 36cp).
Table 1. Effects of interaction between particle size and pretreatment on pasting properties of pumpkin flour.
Peak viscosity is indicative of the strength of the paste, which is made from gelatinization during processing in food applications. It also reflects the extent of granule swelling.[Citation21] As revealed in , peak and breakdown viscosities decreased with the germination. The decrease in peak viscosity with germination may be attributed to protein and starch degradation or debranching to simpler units by the enzymes activated during the germination,[Citation22] similar to Enyinnaya, Adewuyi, & Oneh[Citation23] and Obalolu & Cole[Citation24] report on malted maize and blends of cowpea and soybean flour, respectively. The high breakdown viscosity is indicative of the low ability of the flour to withstand heating and shear stress during cooking.[Citation25] Flour from germinated SAB 632 seeds (190.0 ± 0.8 cp) had higher breakdown viscosity than 119 G (42. ± 6) and 125 G (42 ± 6) flour samples. High breakdown viscosity for 632 G flour reflects its less resistance to the shear, and the starch granules have more susceptibility to disintegration than starch granules from other flours under study. Breakdown viscosity value measures the ease with which the swollen granules can be disintegrated.[Citation26] Trough viscosity values ranged from 522 ± 42to 1401 ± 166 cp, with the highest values recorded by 119S and the lowest by 125 G flour sample. As reported by Enyinnaya et al.[Citation23] and Liu et al.,[Citation27] the decrease in trough viscosity with germination may be attributed to starch degradation, which caused a decrease in viscosity value. Trough viscosity is the minimum viscosity value in the constant temperature phase of the RVA profile and measures the ability of the paste to withstand breakdown during cooling. There is no significant difference (P < .05) in the final viscosity among flour samples except for the 119S flour sample with the highest value (3516 ± 692), and also final viscosity decreases with germination. The observed low values of final viscosity for the germinated flours might be due to the attendant breakdown of starches, which is associated with the increase in amylase activity during seed germination of the legumes.[Citation28] Final viscosity indicates the ability of a material to form a viscous paste after cooking and cooling.[Citation29] The soaked bean (control) flour had significantly (P ≤ .05) the highest setback viscosity with the highest values attained by the 119S flour sample (2114 ± 525cp) and the SER119 germinated flour had the least setback of 444 ± 96cp. The lower setback values of germinated bean flour indicates its lowest rate of retrogradation and this will be useful in the prolonged shelf life of the flour.[Citation30] In line with this finding Acevedo et al.[Citation31] reported a decrease in setback viscosity of pigeon pea, Dolichos bean, and jack bean flours after germination. The highest setback viscosity of 119 G flour indicates its higher tendency to retrograde than other flours. According to Wani, Sogi, & Gill,[Citation32] retrogradation results from hydrogen bonding between starch molecules that have hydroxyl and hydrogen acceptor sites. shows a significant difference (p < .05) among flour pasting temperatures. The pasting temperature ranged from 75–82.3°C, the lowest for 125 G and the highest for 632S flour samples. Pasting temperatures of all three flours decreased upon germination. This might be due to the enzymatic degradation of cell wall-wrapped protein and starch, in pulse seeds facilitating starch and protein to contact and absorb water, which increases the heat transfer rate.[Citation8] The reduction in pasting temperature during germination was in agreement with the decrease in Tc of gelatinization. Low pasting temperature translates to low energy cost and other component stability.[Citation28] According to this finding, pasting temperature results are lower than Felker et al.[Citation33] for the raw navy, black, and pinto bean flours (between 80 and 83°C) and higher than 72°C for chickpea flour. Peak time ranged from 6.8 to 7.20 min, with the non-germinated SER 119 variety having the highest peak time (7.20 min) while germinated SER 125 variety had the lowest peak time (6.8 min). The peak time indicates the minimum temperature required to cook the flour. Peak time values reported in this study are higher than the peak time (5.13 to 5.80 min) reported by Adebowale et al.,[Citation29] implies that it needs extended times for forming pastes and hence longer processing times. Generally, the activated amylases and proteases during germination might cause a decline in the pasting viscosity of flours, due to the degradation of starch chains and disruption of disulfide linkages, respectively[Citation22,Citation34] Especially the protein degradation and loss of structural integrity in flours increased the mechanical breakability of the swollen starch granules, which caused a substantial decline in pasting viscosity.[Citation22]
Thermal properties
Values are mean ± SD. Means that sharing the same superscript letters in columns are not significantly different from each other (Tukey’s HSD test, p < .05). *** Significant effect at p < .05, NS, Not significant. 119S, SER 119 variety Soaked; 119 G,SER 119 variety germinated; 632S, SAB 632 variety soaked; 632 G,SAB 632 variety germinated; 125S, SER125 variety soaked;125 G= SER125 variety germinated;To, onset temperature; Tp, peak temperature; Tc, conclusion temperature; DH, gelatinization enthalpy.
The thermal properties of haricot bean flour were significantly affected by the varieties and germination as well as by the interaction between varieties and germination (p < .05) . But the difference between the temperatures at the beginning and end of gelatinization is not too large, signifying a low variability of the thermal properties of flour molecules. The thermal properties indicated that the hydrogen bond of starch in germinated flour could be broken down at a temperature lower than that of control flours (). To, Tp, Tc and ΔHgel for haricot bean flours ranged between 61 ± 1–66 ± 2, 67. ± 2–71.7 ± 0.2, 78 ± 2–80 ± 2 oC, and 4.9 ± 0.3–7.8 ± 0.4 J/g, respectively. The value of onset temperature of 632S flour (66 ± 2°C) was significantly higher than that of the other flours. An insignificant change was observed in Tp and Tc of flour with germination. The germinated haricot bean flour samples showed low thermal properties than native flour. The decrease in thermal properties of flour during germination might be due to decreased fat content, which covers the starch granules and thus become easily assessable for swelling.[Citation35] In addition, the reduction in Tc may be linked with the degradation of interactions between starch and protein/lipid during germination.[Citation22] The differences in thermal properties between different cultivars may be attributed mainly to the difference in starch properties, crystallinity, and protein content.[Citation36] Similar results of a decrease in thermal properties of chickpea and brown rice flour due to germination were reported by Xu et al.[Citation37] and Wu et al.,[Citation35] respectively.
Table 2. Effects of interaction between variety and processing method on thermal properties of haricot bean flour.
Gelatinization enthalpy (ΔH) can be used to predict the energy required to break down the intermolecular hydrogen bonds of starch granules.[Citation38] Flour from soaked SER 119 seeds showed a higher ΔH value than flour from other cultivars. The ΔH of germinated flours decreases, suggesting the reduction of energy required to convert the chemical composition of bean flour from an ordered to a disordered form; also, it indicates that reduction in the number of double helical,[Citation39] which may be attributed to the degradation and consumption of starch during sprouting. According to Xu et al.,[Citation8] during germination, the partial hydrolysis of starch by the activated enzymes diminishes the intermolecular hydrogen bonds of starch, making them easily detached on heating. This result suggests that the gelatinization enthalpy values obtained were higher than those reported on lentil flours (3.35 to 3.70 J/g) by M. Xu et al.,[Citation8] and lower than chickpea flour (15.2 J g−1) by J. Huang et al.[Citation40]
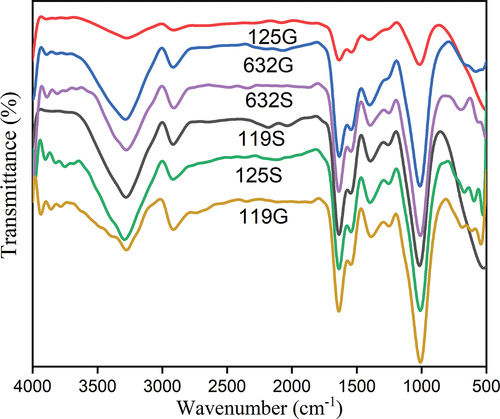
Fourier transform infrared spectroscopy (FT-IR)
The soaked (control) and germinated haricot bean flour samples were analyzed by FT-IR spectroscopy to study the change in functional groups upon the interaction between variety and germination. The FT-IR spectroscopy of haricot bean flour samples did not show any variations in spectra, but a change in the intensity of bands was observed (). The spectra obtained for all samples were similarly based on the form but differed in the intensity of the major peaks as presented in . All tested flour samples showed high absorption spectra at the wavenumbers 3278.6, 2912.3, 1635.5, 1391.4, 1252.6, and 1008.3 cm−1. The bands observed in the region 3278 cm−1 in the spectra of the flour can be assigned to the stretching vibration of the – OH groups caused by the formation of hydrogen bonds in the[Citation41] starches and starch – proteins interaction and also from various compound like alcohol, phenols, and carboxylic acid.[Citation42] In the literature, it was highlighted that the spectral range between 3000 and 2800 cm−1 corresponding to lipid compound, due to vibration produced by carbonyl group of triglyceride (C-H)[Citation16] The signal at 2,912 cm − 1 in a sample confirmed the development of more C─H bonds, possibly from unsaturated C-bonds. The spectral range between 1700 and 1500 cm − 1 corresponds to the protein content of the samples. The lengthening at 1635 cm−1 indicated the presence of C=O stretching, which may be related to the amide I region[Citation16,Citation43] The peaks at 1420 –1380 cm−1 are assigned to C-H deformation vibrations. The signals in the region between 1200–900 cm−1 are ascribed to C―O and C―C stretching vibrations of the polysaccharide molecules.[Citation44,Citation45]
X-ray diffraction (XRD)
XRD has been widely used to reveal the characteristics of the crystalline structure of starch granules.[Citation46] As exhibited in , the crystallinity index, diffraction peaks, peak intensity, and D-Spacing of all six flour samples ranged from 44–59.1%, 17.68–18.49°, 370.43 ± 1.01–537.6 ± 1.0, and 27.4 ± 0.8–28.6 ± 0. 5 nm, respectively. Both soaked and germinated haricot bean flours showed insignificant changes in diffraction peaks, suggesting no change in the type of crystalline structure due to variety and germination, as well as the interaction between them. It can be seen that the crystallinity index for the 125 G flour sample was higher (59.1 ± 0.9) than that of other flours. The variation of the crystallinity index of flour implies the differences in the extent of starch damage. According to Li et al.,[Citation47] during germination, the starch is hydrolyzed to provide energy, while the amorphous regions of the starch granules are hydrolyzed first, and then the crystalline regions are hydrolyzed so that the overall value in the calculation of the ratio decreased and the result of crystallinity increased. reveals that the soaked haricot bean flour showed strong intensities as compared to the germinated flour, with the 119S flour being the highest and 632 G the lowest.
Figure 2. XRD patterns of haricot beans flours: 119S, SER 119 variety Soaked; 119G,SER 119 variety germinated; 632S, SAB 632 variety soaked; 632G,SAB 632 variety germinated; 125S, SER125 variety soaked;125G= SER125 variety germinated.
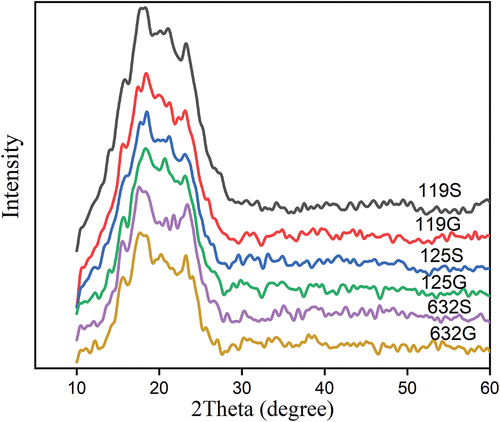
Table 3. Effects of interaction between variety and processing method on XRD parameters of haricot bean flour.
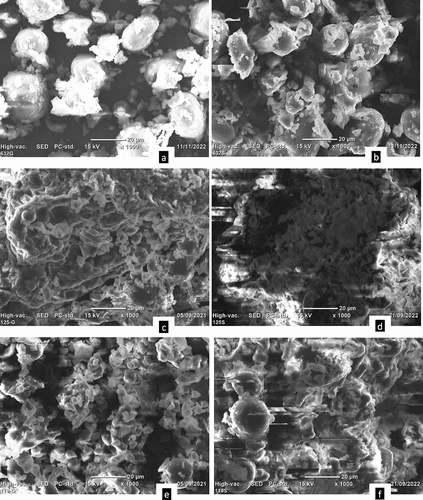
Scanning electron microscopy
Microstructures of the soaked and germinated haricot bean flours were examined by scanning electron microscopy () and the images reveal that some residual proteins deposited or fragments of protein matrix on the granule surfaces, which confirms that starch granules were surrounded by a protein matrix.[Citation3] In addition, it may be included mineral and fiber components, as reported by other workers.[Citation18] There were visible changes observed between the micrographs of the soaked and germinated flour samples of all varieties (). This indicates that the germination could affect the shape of haricot bean flour granules. A similar finding on the structural change of sprouted onion powder and sprouted sorghum was observed by Majid, Dar, & Nanda[Citation48] and Yan et al.,[Citation49] respectively. The compositional changes during germination account for microstructural changes. This morphological change has an important influence on the flowability behavior of the powders
Conclusion
The study showed a significant effect of variety and germination on the pasting, thermal, XRD, FTIR, and SEM properties of haricot bean flour. The germination process decreases flour’s thermal and pasting properties, which could enhance its better utilization in the food industry due to the loosening structure caused by increased amylase activity. So the flour from germinated haricot bean seeds can provide a potential application for producing foods that require low viscosity compared to control flour. It was found that there were significant differences between the onset temperature and gelatinization enthalpy of the flours, with 632S flour showing the highest To and Tp, but 125 G flour exhibited the lowest ΔHgel. Germinated SER 125 and SAB 632 flour samples had high crystallinity index than other flours. This study may promote haricot bean flour utilization in the food industry.
Abbrevaition
| 119 G | = | Germinated SER 119 variety flour |
| 119S | = | Soaked SER 119 variety flour |
| 125 G | = | SER125 variety germinated flour |
| 125S | = | Soaked SER125 variety flour |
| 632 G | = | Germinated SAB 632 variety flour |
| 632S | = | Soaked SAB 632 variety flour |
| DH | = | Gelatinization Enthalpy |
| Tc | = | Conclusion Temperature |
| To | = | Onset Temperature |
| Tp | = | Peak Temperature |
Acknowledgments
The first author would like to acknowledge Wolkite University Food Process Engineering Department for providing us laboratory facilities.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Tharanathan, R. N.; Mahadevamma, S. Grain legumes - A boon to human nutrition. Trends Food Sci. Technol. 2003, 14(12), 507–518.
- De, M.; Tavares, J. A.; Carvalho, D.; Jaeger, L. M.; de M Vieira, A. C.; de Castro, I. M. Scanning Electron Microscopy and Crystallinity of Starches Granules from Cowpea, Black and Carioca Beans in Raw and Cooked Forms. Food Sci. Technol. 2019, 39, 718–724. DOI: 10.1590/fst.30718.
- Hollman, M.; Zhang, Y. Physicochemical Properties and Rheological Behavior of Fl Ours and Starches from Four Bean Varieties for Gluten-Free Pasta Formulation. J. Agric. Food Res. 2019, 1(149), 100001. DOI: 10.1016/j.jafr.2019.100001.
- Shimelis, E. A.; Ã, S. K. R. Proximate Composition and Physico-Chemical Properties of Improved Dry Bean (Phaseolus Vulgaris L .) Varieties Grown in Ethiopia. LWTFood Sci. Technol 2005, 38, 331–338.
- Negi, A.; Boora, P.; Khetarpaul, N. Starch and Protein Digestibility of Newly Released Moth Bean Cultivars: Effect of Soaking, Dehulling, Germination and Pressure Cooking. Nahrung. Food. 2001, 45(4), 251–254. DOI: 10.1002/1521-3803(20010801)45:4<251:AID-FOOD251>3.0.CO;2-V.
- Kamchan, A.; Puwastien, P.; Sirichakwal, P. P.; Kongkachuichai, R. In vitro Calcium Bioavailability of Vegetables, Legumes and Seeds. J. Food Compos. Anal. 2004, 17(3–4), 311–320. DOI: 10.1016/j.jfca.2004.03.002.
- Njintang, Y. N.; Mbofung, C. M. F.; Waldron, K. W. In vitro Protein Digestibility and Physicochemical Properties of Dry Red Bean (Phaseolus Vulgaris) Flour: Effect of Processing and Incorporation of Soybean and Cowpea Flour. J. Agric. Food. Chem. 2001, 49(5), 2465–2471. DOI: 10.1021/jf0011992.
- Minwei Xu, Z. J.; Simsek, J.; Rao, S.; Chen, B.; Rao, J.; Chen, B. E Ff Ect of Germination on the Chemical Composition, Thermal, Pasting, and Moisture Sorption Properties of Fl Ours from Chickpea, Lentil, and Yellow Pea. Food. Chem. 2019, 295, 579–587. DOI: 10.1016/j.foodchem.2019.05.167.
- Sajad Ahmad Sofi, J. S.; Muzaffar, K.; Ahmad, S.; Dar, M. B. N. Effect of Germination Time on Physico-Chemical, Functional, Pasting, Rheology and Electrophoretic Characteristics of Chickpea Flour. J. Food Meas. Charact. 2020, 14, 2380–2392.
- Wodajo, D.; Emire, S. A. Haricot Beans (Phaseolus Vulgaris L .) Flour: Effect of Varieties and Processing Methods to Favor the Utilization of Underconsumed Common Beans. Int. J. Food. Prop. 2022, 25(1), 1186–1202. DOI: 10.1080/10942912.2022.2074029.
- Torres, A.; Frias, J.; Granito, M.; Vidal-Valverde, C. Germinated Cajanus Cajan Seeds as Ingredients in Pasta Products: Chemical, Biological and Sensory Evaluation. Food. Chem. 2007, 101(1), 202–211. DOI: 10.1016/j.foodchem.2006.01.018.
- Polat, H.; Dursun Capar, T.; Inanir, C.; Ekici, L.; Yalcin, H. Formulation of Functional Crackers Enriched with Germinated Lentil Extract: AResponse Surface Methodology Box-Behnken Design. LWT - Food Sci. Technol. 2020, 123, 109065.
- Chavan Mayuri, Y. G.; Harmalkar, R.; Waghmare, M.; Waghmare, R. Development of Non-Dairy Fermented Probiotic Drink Based on Germinated and Ungerminated Cereals and Legume. LWT. 2018, 91, 339–344. DOI: 10.1016/j.lwt.2018.01.070.
- Kaczmarska, K. T.; Chandra-Hioe, M. V.; Frank, D.; Arcot, J. Enhancing Wheat Muffin Aroma Through Addition of Germinated and Fermented Australian Sweet Lupin (Lupinus Angustifolius L.) and Soybean (Glycine Max L.) Flour. LWT. 2018, 96, 205–214. DOI: 10.1016/j.lwt.2018.05.034.
- Park, K. B.; Oh, S. H. Production of Yogurt with Enhanced Levels of Gamma-Aminobutyric Acid and Valuable Nutrients Using Lactic Acid Bacteria and Germinated Soybean Extract. Bioresources. Technol. 2007, 98(8), 1675–1679. DOI: 10.1016/j.biortech.2006.06.006.
- Denisa Atudorei, S. S.; Gabriela, G.; Codină, G. G. Impact of Germination on the Microstructural and Physicochemical Properties of Different Legume Types. Plants. 2021, 10, 592. DOI: 10.3390/plants10030592.
- Wodajo, D.; Admassu, S.; Dereje, B. Geometric Characteristics and Mass-Volume-Area Properties of Haricot Beans (Phaseolus Vulgaris L.): Effect of Variety. Int. J. Food. Prop. 2021, 24(1), 885–894. DOI: 10.1080/10942912.2021.1937210.
- Ahmed, I.; Singh, D.; Abas, A.; Singh, B. Physico-Chemical and Functional Properties of Fl Ours from Indian Kidney Bean (Phaseolus Vulgaris L .) Cultivars. LWT - Food Sci. Technol. 2013, 53(1), 278–284. DOI: 10.1016/j.lwt.2013.02.006.
- Wani, I.; Andrabi, S.; Sogi, D.; Hassan, I. Comparative Study of Physicochemical and Functional Properties of Flours from Kidney Bean (Phaseolus Vulgaris L.) and Green Gram (Vigna Radiata L.) Cultivars Grown in Indian Temperate Climate. Legum. Sci. 2019, 11.
- Chinma, C. E.; Abu, J. O.; Asikwe, B. N.; Sunday, T.; Adebo, O. A. Effect of Germination on the Physicochemical, Nutritional, Functional, Thermal Properties and in vitro Digestibility of Bambara Groundnut Flours. LWT - Food Sci. Technol. 2021, 140, 110749. DOI: 10.1016/j.lwt.2020.110749.
- Liang, X.; King, J. M. Pasting and Crystalline Property Differences of Commercial and Isolated Rice Starch with Added Amino Acids. J. Food Sci. 2003, 68(3), 832–838. DOI: 10.1111/j.1365-2621.2003.tb08251.x.
- Li, C.; Jeong, D.; Lee, J. H.; Chung, H. J. Influence of Germination on Physicochemical Properties of Flours from Brown Rice, Oat, Sorghum, and Millet. Food Sci. Biotechnol. 2020, 29(9), 1223–1231. DOI: 10.1007/s10068-020-00770-2.
- Enyinnaya, C.; Adewuyi, O.; Oneh, J. Effect of Germination on the Chemical, Functional and Pasting Properties of Flour from Brown and Yellow Varieties of Tigernut (Cyperus Esculentus). Food. Res. Int. 2009, 42(8), 1004–1009. DOI: 10.1016/j.foodres.2009.04.024.
- Obalolu, V. A.; Cole, A. H. Functional Property of Complementary Blends of Soybean and Cowpea with Malted or Unmalted Maize. Food. Chem. 2000, 70(2), 147–153. DOI: 10.1016/S0308-8146(99)00248-4.
- Adebowale, A. A.; Sanni, L.; Awonorin, S. Effect of Texture Modifiers on the Physicochemical and Sensory Properties of Direct Fufu. Food Sci. Technl Intl. 2005, 11(5), 373–382. DOI: 10.1177/1082013205058531.
- Kaur, M.; Singh, N. Studies on Functional, Thermal and Pasting Properties of Flours from Different Chickpea Cultivars. Food. Chem. 2005, 91(3), 403–411. DOI: 10.1016/j.foodchem.2004.06.015.
- Liu, Y.; Su, C.; Saleh, A. S. M.; Wu, H.; Zhao, K.; Zhang, G.; Jiang, H.; Yan, W.; Li, W. Effect of Germination Duration on Structural and Physicochemical Properties of Mung Bean Starch. Int. J. Biol. Macromol. 2020, 154, 706–713. DOI: 10.1016/j.ijbiomac.2020.03.146.
- Clifford, O.; Chika, O.; Jude, I.; Tochi, E. Use of Seed Sprouting in Modification of Food Nutrients and Pasting Profile of Tropical Legume Flours. Niger. Food J. 2014, 32(1), 117–125. DOI: 10.1016/S0189-7241(15)30104-1.
- Adebowale, A. A.; Sanni, S. A.; Oladapo, F. O. Chemical, Functional and Sensory Properties of Instant Yam–Bread Fruit Flour. Niger. Food J. 2008, 26(1), 2–12. DOI: 10.4314/nifoj.v26i1.47417.
- Lucretia, P. C. O.; Peace, I. B. Effect of Germination and Pre-Gelatinization on the Proximate Composition and Pasting Properties of Maize Flour a Base Ingredient for Cereal-Based Infant Complementary Food. Int. J. Biotechnol. Food Sci. 2019, 7(3), 30–37.
- Acevedo, B. A.; Thompson, C. M. B.; González Foutel, N. S.; Chaves, M. G.; Avanza, M. V. Effect of Different Treatments on the Microstructure and Functional and Pasting Properties of Pigeon Pea (Cajanus Cajan L.), Dolichos Bean (Dolichos Lablab L.) and Jack Bean (Canavalia Ensiformis) Flours from the North-East Argentina. Int. J. Food Sci. Technol. 2017, 52(1), 222–230. DOI: 10.1111/ijfs.13271.
- Wani, I. A.; Sogi, D. S.; Gill, B. S. Physicochemical and Functional Properties of Flours from Three Black Gram (Phaseolus Mungo L.) Cultivars. Int. J. Food Sci. Technol. 2013, 48(4), 771–777. DOI: 10.1111/ijfs.12025.
- Felker, F. C.; Kenar, J. A.; Byars, J. A.; Singh, M.; Liu, S. X. Comparison of Properties of Raw Pulse Flours with Those of Jet-Cooked, Drum-Dried Flours. LWT. 2018, 96(June), 648–656. DOI: 10.1016/j.lwt.2018.06.022.
- Li, C.; Oh, S. G.; Lee, D. H.; Baik, H. W.; Chung, H. J. Effect of Germination on the Structures and Physicochemical Properties of Starches from Brown Rice, Oat, Sorghum, and Millet. Int. J. Biol. Macromol. 2017, 105, 931–939. DOI: 10.1016/j.ijbiomac.2017.07.123.
- Wu, F.; Chen, H.; Yang, N.; Wang, J.; Duan, X.; Jin, Z.; Xu, X. Effect of Germination Time on Physicochemical Properties of Brown Rice Flour and Starch from Different Rice Cultivars. J. Cereal Sci. 2013, 58(2), 263–271. DOI: 10.1016/j.jcs.2013.06.008.
- Aparecida Correia Bento, J.; Bassinello, P. Z.; Carvalho, R. N.; Souza Neto, M. A. D.; Caliari, M.; Soares Júnior, M. S. Functional and Pasting Properties of Colorful Bean (Phaseolus Vulgaris L) Flours: Influence of the Cooking Method. J. Food Process Preserv. 2021, 45(e15899), 1–14. DOI: 10.1111/jfpp.15899.
- Xu, J.; Zhang, H.; Guo, X.; Qian, H. The Impact of Germination on the Characteristics of Brown Rice Flour and Starch. J. Sci. Food Agric. 2012, 92(2), 380–387. DOI: 10.1002/jsfa.4588.
- Hoover, R.; Hughes, T.; Chung, H. J.; Liu, Q. Composition, Molecular Structure, Properties, and Modification of Pulse Starches: A Review. Food. Res. Int. 2010, 43(2), 399–413.
- Huang, T. T.; Zhou, D. N.; Jin, Z. Y.; Xu, X. M.; Chen, H. Q. Effect of Repeated Heat-Moisture Treatments on Digestibility, Physicochemical and Structural Properties of Sweet Potato Starch. Food .Hydrocoll. 2016, 54, 202–210.
- Huang, J.; Schols, H. A.; van Soest, J. J. G.; Jin, Z.; Sulmann, E.; Voragen, A. G. J. Physicochemical Properties and Amylopectin Chain Profiles of Cowpea, Chickpea and Yellow Pea Starches. Food. Chem. 2007, 101(4), 1338–1345.
- Pelissari, F. M.; Andrade-Mahecha, M. M.; Do a Sobral, P. J.; Menegalli, F. C. Comparative Study on the Properties of Flour and Starch Films of Plantain Bananas (Musa Paradisiaca). Food. Hydrocoll. 2013, 30(2), 681–690.
- Kumar, Y.; Sharanagat, V. S.; Singh, L.; Mani, S. Effect of Germination and Roasting on the Proximate Composition, Total Phenolics, and Functional Properties of Black Chickpea (Cicer Arietinum). Legum. Sci. 2020, 2(1), 1–7.
- Tarahi, M.; Shahidi, F.; Hedayati, S. Physicochemical, Pasting, and Thermal Properties of Native Corn Starch–Mung Bean Protein Isolate Composites. Gels. 2022, 8(11), 693.
- Kamarudin, F.; Gan, C. Y. Molecular Structure, Chemical Properties and Biological Activities of Pinto Bean Pod Polysaccharide. Int. J. Biol. Macromol. 2016, 88, 280–287.
- Martínez-Castaño, M.; Díaz, D. P. M.; Contreras-Calderón, J.; Cabrera, C. G. Physicochemical Properties of Bean Pod (Phaseolus Vulgaris) Flour and Its Potential as a Raw Material for the Food Industry. Rev. Fac. Nac. Agron. Medellin. 2020, 73(2), 9179–9187.
- Kaptso, G. K. Characterization of Morphology and Structural and Thermal Properties of Legume Flours: Cowpea (Vigna Unguiculata L. Walp) and Bambara Groundnut (Vigna Subterranea L. Verdc .) Varieties. Int. J. Food Eng. 2015, 12(2), 139–152.
- Li, C. Y.; Li, C.; Lu, Z. X.; Li, W. H.; Cao, L. P. Morphological Changes of Starch Granules During Grain Filling and Seed Germination in Wheat. Starch/staerke. 2012, 64(2), 166–170.
- Majid, I.; Dar, B. N.; Nanda, V. Rheological, Thermal, Micro Structural and Functional Properties of Freeze Dried Onion Powders as Affected by Sprouting. Food. Biosci. 2018, 22(January), 105–112.
- Yan, S. Properties of Field-Sprouted Sorghum and Its Performance in Ethanol Production. J. Cereal Sci. 2010, 51(3), 374–380.