 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Atemoya (Annona squamosa x A. cherimola hybrid) is a typical climacteric fruit that has a high respiration rate after harvest, short storage life, and is highly susceptible to fruit cracking and rot. Therefore, this study was intended to apply D-limonene nanoemulsion coating on fruit to prolong the storage life, reduce spoilage, and facilitate the expansion of the market. Green mature atemoya fruit coated with different concentrations of D-limonene nanoemulsion (0.25%, 0.5%, 1.0%, 1.5%, and control) was stored in a 14°C refrigerator for relevant analysis and investigation. The results showed that the use of 0.25% D-limonene nanoemulsion inhibited the browning on the fruit surface, and reduced physiological metabolisms. The browning phenomenon on the fruit surface increased as the D-limonene concentration ratio increases. Since the high concentration caused damage to the peel cells, 0.25% D-limonene was chosen as optimum for atemoya coating. The fruit of atemoya maintained green peel color (L × 28.6, a × 6.63, b × 12.3) after storage at 14°C for 21 days with 0.25% D-limonene nanoemulsion coating. At the same time, after 0.2% D-limonene nanoemulsion coating, the weight loss rate was about % less than that of the control group (0.25% treatment was 11.3%; control was 14.2%) and high pectin content (0.25% treatment was 16.9 mg AGA g−1AIS−1; control was 9.89 mg AGA g−1AIS−1). Coating of D-limonene nanoemulsion can reduce the physiological response of atemoya fruits after harvesting, prolong the storage time, and increase the feasibility of their export. From the experimental results, D-limonene showed the potential to be widely used in fruit preservation agents in the future.
KEYWORDS:
Introduction
Atemoya is an important tropical fruit, which is cultivated in the United States, Australia, Thailand, Israel, Egypt, and other countries [Citation1]. Atemoya (Annona squamosa x A. cherimola hybrids) is one of Taiwan’s most important export fruits because of its sweet and delicious taste. In Taiwan, the current cultivation area is about 2,700 hectares, and the main export countries include China and Hong Kong. On harvest, the atemoya fruit is fully matured, and the peel is bright green in color.[Citation2] It is a climacteric fruit with a high respiration rate and the peak respiration rate of atemoya fruit is about 200–250 mg CO2 when post-ripening at room temperature, while ethylene production peaks at approximately 100–300 μL kg−1 h−1.[Citation3,Citation4] The shelf-life of the fruit is very short about 3–6 days since the fruit continues to ripen after harvesting.[Citation5] Yamashita et al. (2002) reported that atemoya has a shelf life of about 13 days when stored at 15°C.[Citation6] Low-temperature refrigeration is an important measure to maintain fruit quality during long-term transportation. It helps to maintain the fruit quality and prolongs storage life.[Citation1,Citation7] However, the atemoya fruit is very sensitive to low temperatures. When the storage temperature is lower than 12°C, it is easy to cause cold damage to the fruit and fail to ripen or undergo abnormal ripening.[Citation8] Related research has been performed on postharvest preservation of atemoya, including cryogenic refrigeration, 1-methylcyclopropene (1-MCP), and modified atmosphere storage.[Citation6,Citation9–11]
“Green technology” can be stated that edible preservation technology that is a necessity of the present era. In comparison to traditional food preservation methods, these technologies help to prevent food spoilage besides preserving nutritional values. The bio-edible based coating is a more efficient strategy for improving the quality and safety of fresh produce than other techniques currently being utilized.[Citation12] Edible films and coatings can be used in fruits because they lower the exchange of moisture, gases, lipids, and volatiles between the food and the environment and by preventing surface contamination. Edible coatings are applied directly to the surface of fruits and vegetables by dipping, spraying, or panning, the latter of which involves combining the food and coating solution in a revolving basin before drying. This increases the efficiency of food packaging and lowers the need for petroleum-derived polymers, even though edible films and coatings are not expected to completely replace conventional packaging materials.[Citation13] The coating of fruit after harvest using edible films on the surface can reduce the loss of water content and gas exchange. It also maintains low oxygen and high carbon dioxide environment inside the fruit and delays the post-ripening and aging.[Citation14] Adding natural extracts or other antimicrobial substances to the coating material can inhibit the growth of microorganisms on the fruit surface and reduce fruit rot.[Citation15,Citation16]
Essential oils (EOs) have been shown to be efficient antibacterial agents. However, due to their volatility, their use for preserving fruit quality and delaying fungal rot is restricted. EOs must be encapsulated in a food-safe conveyance system in order to retain their biological pursuit, boost their effective usage rate, and reduce their effect on food organoleptic qualities.[Citation17] Because of the great number of constituents, EOs seem to have no specific cellular targets.[Citation18] As typical lipophiles, they pass through the cell wall and cytoplasmic membrane, disrupt the structure of their different layers of polysaccharides, fatty acids, and phospholipids, and permeabilize them. Cytotoxicity appears to include such membrane damage.[Citation19] EOs can coagulate the cytoplasm[Citation20] and damage lipids and proteins.[Citation21] Damage to the cell wall and membrane can lead to the leakage of macromolecules and to lysis.[Citation22] Limonene (4-I isopropenyl-1-methylcyclohexene) is a natural monoterpene found in citrus fruit EOs extracted from the peels (including citrus, lemon, and grapefruit) widely used as fragrances in cosmetics, detergents, and foods due to their lemon scent.[Citation23–25] In practical applications, D-limonene undergoes oxidative degradation and the hydrophobicity affects its activity. There are many methods to load D-limonene in different systems.[Citation26] Meanwhile, D-limonene is considered to be safe with low toxicity to humans without causing carcinogenic or nephrotoxic risks.[Citation24] Using D-limonene emulsion as a fruit coating can form a protective film on the fruit surface can prevent the direct contact of fruit skin to atmosphere and reduce microbial infection. It helps to slow down the respiration rate of the fruit and reduce the production of ethylene to maintain fruit quality.[Citation16] When D-limonene is loaded in the liposome coating, it increased the contact of D-limonene with microorganisms on the surface of blueberry and delays the release of D-limonene activity, which can have an antibacterial effect even at low concentrations.[Citation27] At present, there are insufficient materials available for the coating application of atemoya. In this study, the D-limonene nanoemulsion coating was used to maintain the quality and prolong the storage and transportation time of atemoya after harvesting. With this technology, it can strengthen the fresh-keeping mechanism of atemoya, expand new channels for export, and expand the overall market system.
Materials and methods
Preparation of D-limonene nanoemulsion coating
Referring to the method of Li et al. (2013) with a slight modification,[Citation28] the water phase was uniformly mixed with 1,2-propanediol, and the volume ratio was 2:1. The oil phase of different concentrations of D-limonene and 6% Tween 80 were evenly mixed, and the water phase was slowly added to the oil phase. The stirring rate was 800 rpm, the addition rate of the water phase was kept constant at 1.0 mL/min, and the stirring was continued for 6 minutes after the water phase was added. The mixture was allowed to form an O/W nanoemulsion, and added 1% sodium alginate to the emulsion, stirred evenly, and used it as a D-limonene nanoemulsion coating for further studies.
D-limonene nanoemulsion coating on atemoya
In a previous preliminary experiment, atemoya fruits were coated with 10% D-limonene essential oil, 6% Tween 80, 1% sodium alginate solution, and 33% propylene glycol solution, respectively, and then stored at room temperature to observe peel changes. Its purpose was to evaluate the effect of the main components of D-limonene nanoemulsion on fruit appearance. Atemoya produced in the spring of 2022, in Taitung, Taiwan, have been selected with similar color, shape, and maturity. The fruits have cleaned the surface, and using a clean plastic brush to different concentrations of D-limonene nanoparticles emulsion coating (0.25%, 0.5%, 1%, 1.5%, control) have been applied, and put it on a rack to remove the excess coating. The fruits were air-dried and stored at 14.0 ± 2.0°C for a 21 days storage test. The following analyses were performed on 0, 7, 14 and 21 days.
Appearance
At each measurement time point, the atemoya of each group was photographed and their appearance changes were recorded.
Color feature analysis
The atemoya samples were taken from three sampling points in the front, middle, and rear sections, respectively, and measured with a colorimeter (SA2000, Nippon Denshoku Industry Co., Ltd., Japan). The equipment was calibrated before analyzing the samples for L* (brightness), a* (red-green), b* (yellow-blue) values.
Physical and chemical analysis of atemoya
Weight loss
The weight of the sample was measured on the days of analysis using a digital balance (AP 224×, Shimadzu, Japan), and its weight loss percentage was calculated with the following formula.
where Wi = initial weight of the sample (g), Wf = weight of sample on days of analysis (g)
Firmness
Using a physical property analyzer (CR-500DX, Japan) the firmness of the samples has been measured during the days of analysis. After peeling the atemoya, three sampling points have been selected in the front, middle, and rear sections, and used a cylindrical probe with a diameter of 10 mm to measure the firmness of the atemoya pulp. The test conditions were 1 mm/sec and the penetration depth was 5 mm.
Determination of total pectin content
After peeling the atemoya, 95% ethanol was added to the pulp and homogenized it completely in a homogenizer (Simple blend10, Oster, USA). After standing for 2 hours, the mixture was filtered, rinsed with acetone several times, and vacuum dried at 30°C for 12 hours to prepare alcohol – insoluble solids (AIS).
Referring to the method of Hou et al. (2008) with a slight modification, 5 mg of AIS was taken and added 2 mL of pre-cooled concentrated sulfuric acid in an ice bath slowly.[Citation29] Later, 1 mL of deionized water was added and kept in an ice bath for 1 hour. Then, deionized water was added to 15 mL, filtered and diluted to 25 mL. From this 0.5 mL of sample solution was taken and 6 mL of 0.125 M borax solution was dissolved in concentrated sulfuric acid in an ice bath. Then, it was mixed well, heated in a boiling water bath for 5 minutes, immediately cooled in an ice bath. Later, 0.1 mL of 0.15% 3-phenylphenol solution dissolved in 0.5% NaOH was added at room temperature. Then it was mixed evenly and let stand for 5 minutes, measured the absorbance at 520 nm wavelength. Galacturonic acid solution (0–100 μg/mL) is the used as standard solution for color development and determination according to the same method. A standard curve for quantification was drawn, and the result is expressed as anhydrous galacturonic acid (AGA) content.
Total soluble solids
Atemoya (20 g) has been mixed with 80 mL of deionized water and homogenized completely using a homogenizer (Simple 30 Blend10, Oster, USA). The mixture was filtered using a filter paper (Advantec paper No. 1) and using a hand-held refractometer (Master-BX/S28M, Atago, Japan) total soluble solids of the filtrate have been measured.
Determination of titratable acidity
The filtrate from which the total soluble solids were determined was titrated to pH 8.3 with 0.1 N NaOH, and its titratable acidity was calculated by the following formula.
where F = 0.1 N NaOH, W = Atemoya sample weight (g). The results have been expressed as an equivalent percentage of malic acid, the main acid in the ripe atemoya.
Determination of total soluble sugars
Atemoya (0.2 g) has been mixed with 10 mL of 80% ethanol and kept in a water bath at 80°C for 30 minutes. The supernatant was diluted and mixed 400 μL of supernatant with 200 μL of 5% phenol and 1000 μL of concentrated sulfuric acid. The solution was mixed evenly, and let stand for a reaction for 20 minutes. After cooling, the absorbance was measured at a wavelength of 480 nm, and the glucose solution (0 ~ 100 μg/mL) was used as the standard solution for color development and measurement according to the same method, and a standard curve was drawn for quantification. The results were expressed as glucose (%) content.
Microbial analysis
Determination of total bacterial count
Atemoya epidermis (20 g from three sampling points in the front, middle, and back sections) have peeled and homogenized in 180 mL of sterilized PBS in a sterile bag using an iron stomacher. The test solution was diluted by serial dilution, and 100 μL of each dilution was plated on the Plate count agar (PCA). The inoculated Petri plates have been placed upside down in a 37°C incubator for 24 hours to count the number of colonies.
Yeast and mold count
Atemoya epidermis (20 g from three sampling points in the front, middle, and back sections) have peeled and homogenized in 180 mL of sterilized PBS in a sterile bag using an iron stomacher. The test solution was diluted by serial dilution, and 100 μL of each dilution was plated on Dichloran rose Bengalchloramphenicol agar (DBRC), and the number of colonies was counted after incubating at 25°C for 72 hours.
Statistics analysis
In each experiment, each group treated at least five repeated samples, and the test results were analyzed by one-way ANOVA with IBM SPSS 12.0 software. After the comparison, it was analyzed by Tukey method. p < .05 indicated that there was a significant difference. The differences of color, sugar degree, total pectin content, total soluble sugar, firmness, total bacterial count, and yeast and mold count of each group in different days were compared.
Results and discussion
Appearance of atemoya fruits
The low O2 and high CO2 concentration levels after harvesting inhibit ethylene biosynthesis, which can cause ripening genes to become active and cause changes in color, fragrance, and cell degeneration, which causes fruit softening. The efficiency of edible coatings depends on fruit respiration and coating permeability, modifies the environment in a manner similar to that of modified atmospheric packaging. Controlling the temperature is a crucial step since it can impact both the permeability and the rate of fruit respiration. Fruit respiration rates rise as temperature rises. Different substances, such as lipids, resins, polysaccharides, proteins, and synthetic polymers, can be used to make edible coatings. D-Limonene and emulsion coating combined with refrigeration technology can be a good method to prolong the shelf life of products.[Citation16,Citation30] shows the results of D-limonene nanoemulsion coating on the atemoya fruit and storage at room temperature for 3 days. In this study, the D-limonene concentration was initially set to a maximum of 10%, and the concentration addition test was carried out. It was found that when the 10% D-limonene nanoemulsion coating was applied to the atemoya and stored under room temperature conditions after 1 day, the browning reaction occurred on the surface of atemoya. With the increase in storage time, the browning of the peel became more and more serious. Other coating materials including sodium alginate, Tween 80 and propylene glycol, respectively, treated the fruit to remain normal without browning after 3 days of storage at room temperature. This result shows that 10% D-limonene is the main cause of browning and shrinkage on the surface of atemoya during storage. It may be that a high concentration of D-limonene will damage the epidermal cells of atemoya, which may cause damage. The enzyme is in contact with a large amount of air to produce a browning reaction. Surface browning, a symptom of phytotoxicity, was observed on all D-limonene treated apricots, and the severity of surface browning increased as the D-limonene concentration increased. Jain, (1985) and Mansour et al. (1986) pointed out that thymol is an essential oil from thyme (Thymus sp.) and has been used as a medicinal drug, food preserve, and beverage ingredient.[Citation31,Citation32] The use of thymol on fresh-market apricots may be limited since appearance is a critical component for fresh fruits, and thymol caused surface browning on apricots. Thymol fumigation on plums at a relatively low concentration, such as 2 or 4 mg/L, can greatly reduce postharvest decay without causing any phytotoxicity.[Citation33]
Table 1. The results of coating each material in the D-limonene nanoemulsion coating on atemoya and storing it for 3 days.
Alginates are brown algae-derived structural polysaccharides. Alginate has distinct colloidal properties that aid in emulsion stabilization and the preservation of fruit texture. Alginates are often used for a variety of purposes including protective coating for fresh fruits and vegetables.[Citation34] Alginate has certain beneficial characteristics, such as shrinkage reduction, moisture preservation, food odor, and color maintenance. Alginate is used to make strong edible coatings or films with low water resistance due to their hydrophilic aspect.[Citation35] Atemoya fruit will continue to mature after being harvested. The presence of polyphenol oxidase (PPO) further catalyzes the hydrolysis of phenolic compounds into o-quinone, which is then oxidized and polymerized into dark brown melanin (Melanin).[Citation36] Ramezanian et al. (2016) pointed out that treatment with appropriate essential oil concentration can delay the ripening of citrus fruit and make the peel turn slower.[Citation37] The Z. multiflora essential oil at concentration of 400 and 500 μl L−1 and T. vulgaris essential oil at concentration of 500 μl L−1 caused severe burns on the fruit peel surface that affected the fruit appearance.[Citation37] The atemoya coated with different concentrations of D-limonene nanoemulsion was stored for 21 days, and its appearance changes are as shown in . As shown, in the part of the control group, it can be seen that the atemoya began to turn black obviously on the 14th day of the storage period and then began to produce brown physiological spots, and the proportion was the highest on the 21st day, almost covering the entire atemoya. 0.5%, 1.0%, and 1.5% groups of atemoya started to have dark textures on the 7th day of the storage period, and the color became more obvious as time went on, and the phenomenon of shrinkage of atemoya was observed. The peel browning during storage was slightly lower in the 0.25% D-limonene nanoemulsion group than that of others. On the 21st day of the storage period, the degree of browning was lower than that of other groups of atemoya, and the unbrown green parts were still retained. During the entire storage period, only a small part of the surface of the atemoya produced a brown coloration. Delaying of browning and inhibition of brown physiological spots indicate the delay in the ripening effect of atemoya, which is beneficial to the quality of atemoya after harvesting and prolongs its shelf life.
Table 2. Effect on appearance changes of atemoya coated with different concentrations of D-Limonene nanoemulsion edible film and stored at 14.0 ± 2.0°C from 0 to 21 days.
Color characteristics of atemoya fruits
The color of fruit peel is an important factor that affects consumers’ purchase choices. It is closely related to the nutritional value and marketability of fruits and plays a key role in consumers’ preference and acceptance of fruit.[Citation38] The color properties of atemoya during storage were observed by measuring L* (brightness), a* (red-green), b* (yellow-blue) values.[Citation39] The results of the color characteristic change of atemoya fruits treated with different concentrations of D-limonene nanoemulsion coating for 21 days are given in . In the part of L* value, with the increase of storage time, each group showed a downward trend, which was due to the browning and brown spots on the surface of atemoya after overripe. The atemoya in the 0.25% group still maintained the highest value (28.61 ± 1.36) on the 21st day of the storage period, while the atemoya in the 1.0% and 1.5% groups showed a rapid decrease in L* value throughout the storage period. From the initial 45.11 ± 4.0, 45.17 ± 2.93 to 22.24 ± 1.50, 24.94 ± 1.60 on the 21st day of storage, because of the high concentration of D-limonene nanoemulsion coating, the surface color of atemoya has changed and produce browning reaction continuously. Ramezanian et al. (2016) used thyme essential oil for the peritoneal treatment of citrus peel.[Citation37] When the concentration of the essential oil exceeded 300–400 μl L −1, the peel would be phytotoxic and the L value of the peel decreased. In the part of the a* value, the values of atemoya have obviously changed. In contrast, the 1.0% group has the lowest trend at 21 days of storage, which is 4.13 ± 0.38, indicating that during the storage period the inner atemoya still retains a small part of its green color. In the part of b* value, the value of atemoya in each group generally decreased with the increase of time, because the color of atemoya changes from green to brown during the ripening process. Resulting in the decrease of b* value, and with the increase in storage time, each group showed a downward trend, and the 1.0% group had the most obvious decrease, from 20.54 ± 1.42 at the beginning to 7.18 ± 0.64 during the 21st day of storage. The phenomenon of peel browning caused by the aging of atemoya is unavoidable. The aging of the peel of the control group and the highest concentration of D-limonene nanoemulsion coating caused the peeling to be damaged and browning to accelerate. However, the use of a low concentration (0.25%) D-limonene nanoemulsion coating treatment can delay the occurrence of peel browning and maintain the bright green appearance of peel for a longer time. EOs at suitable concentrations maintain color quality probably due to the antioxidant properties that prevent the oxidation of pigments during storage.[Citation37]
Table 3. The effect of different concentrations of D-limonene nanoemulsion coating on the color properties of pineapple custard when stored at 14.0 ± 2.0°C for 0–21 days.
Data are expressed as mean ± standard deviation from triplicate determination (n = 5). Tukey’s test was performed, and the different letters within a column with the same storage time indicate significant differences at (p < .05) level.
Weight loss rate, texture, and pectin content of atemoya fruit
Post-harvest fruits and vegetables cause moisture and weight loss, thereby reducing the quality and value of fresh products.[Citation40] The weight loss of fruits during storage is related to physiological metabolism and tissue aging, water loss caused by evapotranspiration and energy consumption by respiration. Using thyme essential oil and chitosan a thin film applied to mango fruit reduced the evapotranspiration and respiration of the fruit, thereby reducing weight loss.[Citation34] The results of weight loss of atemoya treated with different concentrations of D-limonene nanoemulsion coating stored at 0–21 days are shown in . The weight loss rate of atemoya in each group showed an upward trend with storage time, and the increase in the 0.5% and 1.0% groups was more obvious on the 14th day. There was a significant difference (p < .05) between the control, 0.25%, and 1.5% groups, until the 21st day of the storage period. The weight loss increased to 14.44%, and 15.41%, which is more than 3% higher than the 0.25% group at the same time point. The D-limonene nanoemulsion coating treatment can significantly reduce the water loss of atemoya and prolong its shelf life, but when the D-limonene nanoemulsion concentration is too high (>0.5%), it will destroy the skin structure and accelerate water loss during storage. The weight loss rate increases with storage days. Ramezanian et al. (2016) used 300 μl L −1 Z. multiflora and 400 μl L −1 T. vulgaris essential oil treatment significantly reduced the weight loss rate of fruit.[Citation37] However, when Z. multiflora essential oil concentration exceeded 300 μl L −1 and T. vulgaris essential oil concentration exceeded 400 μl L −1the weight loss rate was increased. The use of high concentrations of EOs will damage the cytoplasmic membrane, increasing the rate of weight loss.[Citation37] Edible coating is a thin layer that acts as semipermeable membrane and operates as a barrier against gases, water leakage, hence, decreases the rate of respiration, enzymatic browning, and release of volatile compounds into the ambient environment.[Citation41]
Figure 1. (a) Effect on weight loss (a), firmness (b) and total pectin content (c) of atemoya coated with different concentrations of D-limonene nanoemulsion edible film and stored at 14 ± 2.0°C from 0 to 21 days. Data are expressed as mean deviation from triplicate determination (n=5). Tukey’s test was performed, and the different letters within a column with the same storage time indicate significant differences at (p < .05) level.
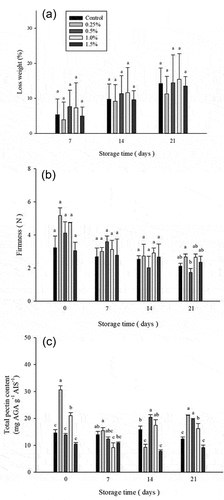
Atemoya is a climacteric fruit and will continue to mature after harvesting during the storage period. During the ripening period, the fruit firmness showed a decreasing trend. The decrease in firmness indicates the softening of the fruit, which is related to the dehydration and loss of the cell wall structure during the fruit ripening process.[Citation42] The firmness change of the atemoya treated with D-limonene nanoemulsion coating of different concentrations is shown in . The atemoya in the 0.5% group was rapidly ripened on the 14th day of the storage period, and the firmness dropped significantly to about 2 N. Although the 0.25% and 1.0% groups had a larger drop in the early stage, from the 7th day, the firmness showed a stable maintenance trend till 21st day of storage. The firmness of 0.25% and 1.0% still maintain higher values, which are 2.67 ± 0.18 N and 2.34 ± 0.67 N, respectively. The atemoya treated with the appropriate concentration of D-limonene nanoemulsion (0.25%, 1.0%), can effectively slow down the metabolic events, especially the softening and degradation rate of the pulp tissue during the post-harvest period. Firmness of fresh produce can be maintained with edible coating as it can protect the moisture loss and hold up the process of ripening.[Citation43] Papaya bars were coated with oxidized starch and chitosan, preserved at ambient temperature for 15 days and texture analysis was done. The results showed that papaya pulp coated with edible coating required more force to penetrate 5 mm of pulp. Firmness loss of 47.36% in coated samples and 92.02% was observed in uncoated control samples after 10 days of storage period.[Citation44]
Softening of the pulp during fruit ripening is associated with changes in cell wall structure,[Citation45] including increased solubility of cell wall pectin. The enzymes involved are cell wall hydrolases, polygalacturonase, pectin esterase, β-galacturon, lactosidases, and cellulases,[Citation46] which are affected. The content and properties of pectin will change by pectinases after harvesting in atemoya, in which protopectin will transform into pectin, and finally converted to pectic acid.[Citation47] According to Shah et al., the mango fruit was treated with thyme essential oil and chitosan, a film was formed on the peel to reduce the decomposition of pectic cellulose and maintain fruit firmness.[Citation48] Atemoya treated with different concentrations of D-limonene nanoemulsion coating and the change in the total pectin content of atemoya are shown in . On the 0th day, the total pectin content of the groups of 0.25% and 1.0% was higher, respectively, 30.6 mg/g and 21.03 mg/g. On the 14th day of storage, the 1.0% group pectin increased from 7.31 to 13.98 mg/g. While the 0.25% group slowly increased the pectin content and reached 16.99 mg/g until the 21st day of storage. This result indicates that the application of D-limonene nanoemulsion coating with an appropriate concentration can slow down the post-ripening phenomenon of atemoya.
Pulp quality of atemoya
The major pulp contents of atemoya fruit are glucose, fructose, and sucrose, which will increase with the increase in storage temperature and storage days. Sucrose is hydrolyzed into glucose and fructose during the ripening period.[Citation49] The changes in total soluble solids (TSS) of atemoya treated with D-limonene nanoemulsion during the storage period are shown in . The control, 0.5%, and the 1.0% group had a TSS content of 18.33 ± 1.02, 19.67 ± 1.25 and 17.5 ± 1.78°Brix, respectively, on the 14th day of storage. TSS concentrations increased in the 0.25% and 1.5% groups more slowly compared to other groups particularly to 16.33 ± 0.47 and 15.67 ± 0.47° Brix, respectively. The slower increase may have occurred due to a slowdown in fruit metabolism due to blocked gas exchange.[Citation50] A similar trend was found in a previous study using biopolymer coatings on fruits.[Citation51] However, in the control group and the 0.5% group on the 21st day of the storage period, TSS content showed a trend of slowing down and decreasing. It may be due to the over-ripening of atemoya resulting in glycolysis, resulting in a decrease in TSS content, while the 0.25% and 1.5% groups continued to increase, indicating that the fruit was still in the ripening stage.
Figure 2. Effect on total soluble solids (a), titratable acid (b) and total soluble sugar (c) of atemoya coated with different concentrations of D-limonene nanoemulsion edible film and stored at 14 ± 2.0°C from 0 to 21 days. Data are expressed as mean deviation from triplicate determination (n=5). Tukey’s test was performed, and the different letters within a column with the same storage time indicate significant differences at (p < .05) level.
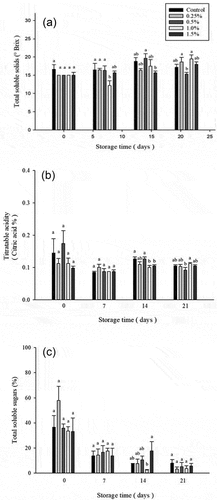
Malic acid and citric acid are the main organic acids in annona and soursop fruit species.[Citation8,Citation52] Citric acid is the main organic acid in atemoya fruit, while malic acid increases with post-ripening time.[Citation8] The titratable acid changes of atemoya treated with different concentrations of D-limonene nanoemulsion coating stored for 0–21 days as shown in . Changes in organic acids such as malic acid and citric acid showed that the use of an appropriate concentration of D-limonene nanoemulsion coating has no significant effect on the organic acids in atemoya, which is roughly consistent with the research results of Torres et al. (2010).[Citation53]
The change results of the total soluble sugars in atemoya coated with D-limonene nanoemulsion are shown in . The total soluble sugars are a good indicator of fruit ripening because the starch will be hydrolyzed into total soluble sugars during the ripening process.[Citation54] On the 14th day, the total soluble sugar (%) of all groups decreased with time, while the 1.5% group slowly increased to 17.72%, which may be due to the higher concentration of D-limonene nanoemulsion. The coating can block the contact between the atemoya and the air, slow down the respiration rate of the atemoya after ripening, and then reduce the hydrolysis of the starch.
Microbial content of atemoya
Atemoya fruit is rich in carbohydrates and sugars and is a source of some vitamins and minerals.[Citation55] Detection of total bacterial counts, yeast and mold counts is an important indicator for predicting the shelf life of fruits after processing.[Citation56] EOs are such antimicrobial compounds that have been in the top research areas of scientists these days. These EOs actually have a wide range of coverage and protection from microbial deterioration and post-harvest losses due to pathogen sensitiveness.[Citation57] The results of total plate count, yeast and mold count of atemoya treated with different concentrations of D-limonene nanoemulsion coating after storage for 0–21 days are shown in .
Figure 3. Effect on total plate count (a) and total yeast and mold count (b) of atemoya coated with different concentrations of D-limonene nanoemulsion edible film and stored at 14 ± 2.0°C from 0 to 21 days. Data are expressed as mean deviation from triplicate determination (n=5). Tukey’s test was performed, and the different letters within a column with the same storage time indicate significant differences at (p < .05) level.
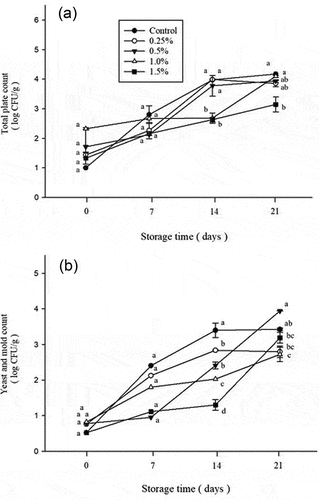
The total number of bacteria in each group of atemoya coated with D-limonene increased with time (). The increase in the control group was the most obvious, from the initial 1.03 Log (CFU/g) on the 21st day of storage to 4.17 Log (CFU/g). The groups treated with D-limonene nanoemulsion coating maintained good antibacterial activity in the early stage. On the 7th day of storage, the 0.25%, 0.5%, and 1.5% groups slowly increased from 1.43 Log (CFU/g), 1.71 Log (CFU/g), and 1.33 Log (CFU/g) to 2.26 Log (CFU/g), 2.12 Log (CFU/g) and 2.15 Log (CFU/g), respectively. On the 21st day of storage, the 1.5% group slowly increased from 1.33 Log (CFU/g) to 3.15 Log (CFU/g), which was suppressed by one more log value compared to the control group. The result indicating that D-limonene nanoemulsion coating can inhibit the growth of microorganisms in atemoya, and has a very high antibacterial activity at a concentration of 1.5%. The growth has a high inhibitory effect, but it will also cause dark brown lines on the surface of the atemoya, which will affect its appearance. Strawberries stored at 4°C for 14 days, treated with 50 μM, 50 mM D-limonene coating can effectively reduce fruit fungal decay, slow down respiration rate and maintain quality.[Citation15,Citation58] Treatment with D-limonene emulsion coating can maintain the fruit quality of strawberries stored at 4°C, reduce the bacterial load, and maintain the appearance of the fruit.[Citation16] Blueberries were stored at 4°C for 9 weeks and treated with 50 μM D-limonene liposome coating to inhibit spoilage and improve antibacterial ability.[Citation27] Essential oil components mainly destroy the cell membrane structure of pathogenic bacteria, leading to the depletion of substrates required for ATP production, and finally cell death.[Citation48] Randazzo et al. (2016) estimated the antimicrobial property of 8 EOs extricated from orange, lemon, and mandarin peel which he used to study with 76 bacterial strains of L. monocytogenes.[Citation59] The potential antibacterial impact of the EOs was found to show the maximum inhibition when added with methylcellulose or chitosan-based bio-decomposable films. The droplet size in nanoemulsions is a critical factor that influences their capacity to promote antibacterial essential oil characteristics.[Citation60]
The trend of yeast and mold counts during the storage period was similar to the results of the total bacteria counts (). The yeast and mold count increased to 3.42 Log (CFU/g) in control at the end of the storage period. While the 0.25%, 1.0%, and 1.5% groups respective counts were all lower than 3.20 Log (CFU/g), indicating that the antimicrobial activity of D-limonene nanoemulsion coating. In addition to good bacteriostatic ability against general bacteria, it also has a good inhibitory effect on fungi. Using the appropriate concentration of D-limonene nanoemulsion to coat the atemoya can maintain the quality of the fruits during storage, and prolong the shelf life.
Finally, to summarize this study, the green mature atemoya fruit after harvest rapidly ripened and senescence during storage, which resulted in a shortened shelf life. To this end, 0.25% D-limonene nanoemulsion was used for fruit coating to inhibit the browning of the peel during fruit storage, delay the color change of the peel and the fruit ripen, and at the same time inhibit the growth of food-borne microorganisms on the pulp. The D-limonene nanoemulsion coating effectively delays the ripening of atemoya fruit after harvest, thereby prolonging the shelf life and increasing the feasibility of its fruit export. Coating of D-limonene nanoemulsion has the potential to be widely used in fruit preservation in the future, which is worthy of further research and application.
Conclusion
Green mature atemoya fruit coated with varying concentrations of D-limonene nanoemulsion (0.25%, 0.5%, 1.0%, 1.5%, and control) was kept in a 14°C refrigerator. According to the study’s findings, 0.25% D-limonene nanoemulsion can successfully prevent fruit surface oxidation and delay physiological processes. As the ratio of D-limonene concentration increases, the browning phenomena on the fruit surface increases. D-limonene at a concentration of 0.25% was determined to be ideal for atemoya coating because a higher concentration harms the peel cells. When atemoya fruits are coated with D-limonene nanoemulsion, their physiological reaction is slow down after harvest, storage period is increased, and their export quality is improved.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Wongs-Aree, C.; Noichinda, S. Sugar apple (Annona squamosa L.) and atemoya (A. cherimola Mill. × A. squamosa L.). In Postharvest Biology and Technology of Tropical and Subtropical Fruits; Yahia, E. M.; Ed.; Woodhead Publishing, 2011; pp. 399–427e.
- Pinto, A.; Cordeiro, M. C. R.; De Andrade, S. R. M.; Ferreira, F. R.; Filgueiras, H. D. C.; Alves, R. E.; Kinpara, D. I. Annona species; International Centre for Underutilized Crops, University of Southampton: Southampton, 2005; p. 263.
- Brown, B.; Wong, L.; George, A.; Nissen, R. Comparative Studies on the Postharvest Physiology of Fruit from Different Species of Annona (Custard Apple). J. Hortic. Sci. 1988, 63, 521–528. DOI: 10.1080/14620316.1988.11515887.
- Kasiolarn, H. Postharvest Physiology of Sugar Apple (Annona Squamosa Linn.) and the Storage of Atemoya (Annona X“african Pride”) Fruit. M.S. Thesis, Kasetsart University, Bangkok, 1991.
- Pereira, M. C. T.; Braz, L. C.; Nietsche, S.; Da Mota, W. F. Determining the Harvesting Maturity of the Sugar Apple Fruits on Northern Minas Gerais. Acta Horticulturae. 2010, 864, 207–214.
- Yamashita, F.; Miglioranza, L.; Miranda, L.; Souza, C. Effects of Packaging and Temperature on Postharvest of Atemoya. Rev. Bras. Frutic. 2002, 24, 658–660. DOI: 10.1590/S0100-29452002000300021.
- Batten, D. J. Effect of Temperature on Ripening and Post-Harvest Life of Fruit of Atemoya (Annona Cherimola Mill.× A. Squamosa L.) Cv.‘african Pride’. Sci. Hortic. 1990, 45, 129–136. DOI: 10.1016/0304-4238(90)90075-P.
- WILLS, R. H.; Poi, A.; Greenfield, H.; Rigney, C. Postharvest Changes in Fruit Composition of Annona Atemoya During Ripening and Effects of Storage Temperature on Ripening. HortScience. 1984, 19, 96–97. DOI: 10.21273/HORTSCI.19.1.96.
- Aguiar, M. C. S.; Mizobutsi, G. P.; Sobral, R. R. S.; PINHEIRO, J.; Martins, J. C.; SANTOS, I.; ALVES, E.; Mizobutsi, E. H.; Aspiazú, I.; MOTA, W. Modified Atmosphere and Refrigeration in Postharvest Conservation of Atemoya Cv. Gefner. J. Exp. Agric. Int, London. 2019, 32, 1–12. DOI: 10.9734/jeai/2019/v32i430108.
- Lee, T. -C.; Hsieh, C. -H.; Chang, P. -T. Packaging Affects the Postharvest Quality of Atemoya Fruits (Annona Cherimola M.× Annona Squamosal L.). Net J. Agric. Sci, Sapele. 2016, 4, 58–62.
- Wijesinghe, G.; Kumara, G.; Kumara, J. Effect of 1-Methylcyclopropene on Shelf-Life and Postharvest Qualities of Four Annona (Annona Spp.) Species in Sri Lanka. J. Food and Agric. 2021, 14, 1. DOI: 10.4038/jfa.v14i1.5235.
- Armghan Khalid, M.; Niaz, B.; Saeed, F.; Afzaal, M.; Islam, F.; Hussain, M.; Mahwish, M. S.; Khalid, H.; Siddeeg, A.; Al-Farga, A. Edible Coatings for Enhancing Safety and Quality Attributes of Fresh Produce: A Comprehensive Review. Int. J. Food Prop. 2022, 25, 1817–1847. DOI: 10.1080/10942912.2022.2107005.
- Otoni, C. G.; Avena-bustillos, R. J.; Azeredo, H. M.; Lorevice, M. V.; Moura, M. R.; Mattoso, L. H.; McHugh, T. H. Recent Advances on Edible Films Based on Fruits and Vegetables—A Review. Compr. Rev. Food Sci. Food Saf. 2017, 16, 1151–1169. DOI: 10.1111/1541-4337.12281.
- Nawab, A.; Alam, F.; Hasnain, A. Mango Kernel Starch as a Novel Edible Coating for Enhancing Shelf-Life of Tomato (Solanum lycopersicum) Fruit. Int. J. Biol. Macromol. 2017, 103, 581–586. DOI: 10.1016/j.ijbiomac.2017.05.057.
- Dhital, R.; Mora, N. B.; Watson, D. G.; Kohli, P.; Choudhary, R. Efficacy of Limonene Nano Coatings on Post-Harvest Shelf Life of Strawberries. LWT. 2018, 97, 124–134. DOI: 10.1016/j.lwt.2018.06.038.
- Shao, P.; Zhang, H.; Niu, B.; Jiang, L. Antibacterial Activities of R-(+)-Limonene Emulsion Stabilized by Ulva Fasciata Polysaccharide for Fruit Preservation. Int. J. Biol. Macromol. 2018, 111, 1273–1280. DOI: 10.1016/j.ijbiomac.2018.01.126.
- Buranasuksombat, U.; Kwon, Y. J.; Turner, M.; Bhandari, B. Influence of Emulsion Droplet Size on Antimicrobial Properties. Food Sci. Biotechnol. 2011, 20(3), 793–800. DOI: 10.1007/s10068-011-0110-x.
- Carson, C. F.; Mee, B. J.; Riley, T. V. Mechanism of Action of Melaleuca Alternifolia (Tea Tree) Oil on Staphylococcus Aureus Determined by Time-Kill, Lysis, Leakage, and Salt Tolerance Assays and Electron Microscopy. Antimicrob. Agents Chemother. 2002, 46(6), 1914–1920. DOI: 10.1128/AAC.46.6.1914-1920.2002.
- Bakkali, F.; Verbeck, S. A.; Averbeck, D.; Idaomar, M. Biological Effects of Essential Oils–A Review. Food. Chem. Toxicol. 2008, 46, 446–475. DOI: 10.1016/j.fct.2007.09.106.
- Gustafson, J. E.; Liew, Y. C.; Chew, S.; Markham, J.; Bell, H. C.; Wyllie, S. G.; Warmington, J. R. Effects of Tea Tree Oil on Escherichia coli. Lett Appl. Microbiol. 1998, 26(3), 194–198. DOI: 10.1046/j.1472-765X.1998.00317.x.
- Burt, S. Essential Oils: Their Antibacterial Properties and Potential Applications in Foods–A Review. Int. J. Food Microbiol. 2004, 94(3), 223–253. DOI: 10.1016/j.ijfoodmicro.2004.03.022.
- Oussalah, M.; Caillet, S.; Saucier, L.; Lacroix, M. Antimicrobial Effects of Selected Plant Essential Oils on the Growth of a Pseudomonas putida Strain Isolated from Meat. Meat. Sci. Jun 2006, 73(2), 236–244. DOI: 10.1016/j.meatsci.2005.11.019.
- Rodríguez, A.; Peris, J. E.; Redondo, A.; Shimada, T.; Costell, E.; Carbonell, I.; Rojas, C.; Peña, L. Impact of D-Limonene Synthase Up-Or Down-Regulation on Sweet Orange Fruit and Juice Odor Perception. Food. Chem. 2017, 217, 139–150. DOI: 10.1016/j.foodchem.2016.08.076.
- Sun, J. D-Limonene: Safety and Clinical Applications. Altern. medi. rev. 2007, 12, 259.
- Zahi, M. R.; Liang, H.; Yuan, Q. Improving the Antimicrobial Activity of D-Limonene Using a Novel Organogel-Based Nanoemulsion. Food. Control. 2015, 50, 554–559. DOI: 10.1016/j.foodcont.2014.10.001.
- Ghasemi, S.; Jafari, S. M.; Assadpour, E.; Khomeiri, M. Nanoencapsulation of D-Limonene Within Nanocarriers Produced by Pectin-Whey Protein Complexes. Food. Hydrocoll. 2018, 77, 152–162. DOI: 10.1016/j.foodhyd.2017.09.030.
- Umagiliyage, A. L.; Becerra-Mora, N.; Kohli, P.; Fisher, D. J.; Choudhary, R. Antimicrobial Efficacy of Liposomes Containing D-Limonene and Its Effect on the Storage Life of Blueberries. Postharvest. Biol. Technol. 2017, 128, 130–137. DOI: 10.1016/j.postharvbio.2017.02.007.
- Li, Y.; Zhang, Z.; Yuan, Q.; Liang, H.; Vriesekoop, F. Process Optimization and Stability of D-Limonene Nanoemulsions Prepared by Catastrophic Phase Inversion Method. J. Food Eng. 2013, 119, 419–424. DOI: 10.1016/j.jfoodeng.2013.06.001.
- Hou, C. -Y.; Lin, Y. -S.; Wang, Y. T.; Jiang, C. -M.; Wu, M. -C. Effect of Storage Conditions on Methanol Content of Fruit and Vegetable Juices. J. Food Compost. Anal. 2008, 21, 410–415. DOI: 10.1016/j.jfca.2008.04.004.
- Joshi, P.; Becerra-Mora, N.; Vargas-Lizarazo, A. Y.; Kohli, P.; Fisher, D. J.; Choudhary, R. Use of Edible Alginate and Limonene-Liposome Coatings for Shelf-Life Improvement of Blackberries. Future. Foods. 2021, 4, 100091. DOI: 10.1016/j.fufo.2021.100091.
- Jain, S. K., Ed. Medicinal Plants, 5th ed.; National Book Trust: New Delhi, 1985.
- Mansour, F.; Ravid, U.; Putievsky, E. Studies of Essential Oils Isolated from 14 Species of Labiateae on the Carimine Spidermint Tetranychus Cinnabarinus. Phytoparasitica. 1986, 14, 137–142. DOI: 10.1007/BF02980899.
- Liu, W. T.; Chu, C. L.; Zhou, T. Thymol and Acetic Acid Vapors Reduce Postharvest Brown Rot of Apricots and Plums. HortScience. 2002, 37, 151–156. DOI: 10.21273/HORTSCI.37.1.151.
- Acevedo, C. A.; López, D. A.; Tapia, M. J.; Enrione, J.; Skurtys, O.; Pedreschi, F.; Osorio, F.; Creixell, W.; Osorio, F. Using RGB Image Processing for Designing an Alginate Edible Film. Food Bioproc. Tech. 2012, 5(5), 1511–1520. DOI: 10.1007/s11947-010-0453-y.
- Borchard, W.; Kenning, A.; Kapp, A.; Mayer, C. Phase Diagram of the System Sodium Alginate/Water: A Model for Biofilms. Int. J. Biol. Macromol. 2005, 35(5), 247–256. DOI: 10.1016/j.ijbiomac.2005.02.006.
- Martínez-Girón, J.; Arias-Rodas, A.; Hernández-Montaña, J.; Agudelo-Burbano, J.; Reina-Hernandez, B. Physicochemical, Microbiological and Sensory Characterization of Liquor Obtained from Atemoya Fruit (Annona Cherimola. × Annona squamosa). Scientia et technica. 2019, 24, 636–642. DOI: 10.22517/23447214.21501.
- Ramezanian, A.; Azadi, M.; Mostowfizadeh-Ghalamfarsa, R.; Saharkhiz, M. J. Effect of Zataria Multiflora Boiss and Thymus Vulgaris L. Essential Oils on Black Rot of ‘Washington Navel’orange Fruit. Postharvest. Biol. Technol. 2016, 112, 152–158. DOI: 10.1016/j.postharvbio.2015.10.011.
- Oms-Oliu, G.; Rojas-Graü, M. A.; González, L. A.; Varela, P.; Soliva-Fortuny, R.; Hernando, M. I. H.; Munuera, I. P.; Fiszman, S.; Martín-Belloso, O. Recent Approaches Using Chemical Treatments to Preserve Quality of Fresh-Cut Fruit: A Review. Postharvest. Biol. Technol. 2010, 57, 139–148. DOI: 10.1016/j.postharvbio.2010.04.001.
- Isas, A. S.; Celis, M. S. M.; Correa, J. R. P.; Fuentes, E.; Rodríguez, L.; Palomo, I.; Mozzi, F.; Van Nieuwenhove, C. Functional Fermented Cherimoya (Annona Cherimola Mill.) Juice Using Autochthonous Lactic Acid Bacteria. Food Res. Int. 2020, 138, 109729. DOI: 10.1016/j.foodres.2020.109729.
- Silva, G. M. C.; Silva, W. B.; Medeiros, D. B.; Salvador, A. R.; Cordeiro, M. H. M.; da Silva, N. M.; Santana, D. B.; Mizobutsi, G. P. The Chitosan Affects Severely the Carbon Metabolism in Mango (Mangifera Indica L. Cv. Palmer) Fruit During Storage. Food. Chem. 2017, 237, 372–378. DOI: 10.1016/j.foodchem.2017.05.123.
- Resende, N. S.; Gonçalves, G. A. S.; Reis, K. C.; Tonoli, G. H. D.; Boas, E. V. B. V. Chitosan/Cellulose Nanofibril Nanocomposite and Its Effect on Quality of Coated Strawberries. J. Food Qual. 2018, 2018, 1–13. DOI: 10.1155/2018/1727426.
- Deng, L. -Z.; Pan, Z.; Zhang, Q.; Liu, Z. -L.; Zhang, Y.; Meng, J. -S.; Gao, Z. -J.; Xiao, H. -W. Effects of Ripening Stage on Physicochemical Properties, Drying Kinetics, Pectin Polysaccharides Contents and Nanostructure of Apricots. Carbohydr. Polym. 2019, 222, 114980. DOI: 10.1016/j.carbpol.2019.114980.
- Han, C.; Zhao, Y.; Leonard, S. W.; Traber, M. G. Edible Coatings to Improve Storability and Enhance Nutritional Value of Fresh and Frozen Strawberries (Fragaria ×ananassa) and Raspberries (Rubus Ideaus). Postharvest. Biol. Technol. 2004, 33(1), 67–78. DOI: 10.1016/j.postharvbio.2004.01.008.
- Escamilla-García, M.; Rodríguez-Hernández, M. J.; Hernández-Hernández, H. M.; Delgado-Sánchez, L. F.; García-Almendárez, B. E.; Amaro-Reyes, A.; Regalado-González, C. Effect of an Edible Coating Based on Chitosan and Oxidized Starch on Shelf Life of Carica Papaya L., and Its Physicochemical and Antimicrobial Properties. Coatings. 2018, 8(9), 318. DOI: 10.3390/coatings8090318.
- Brummell, D. A. Cell Wall Disassembly in Ripening Fruit. Funct. Plant Biol. 2006, 33, 103–119. DOI: 10.1071/FP05234.
- Fischer, R. L.; Bennett, A. B. Role of Cell Wall Hydrolases in Fruit Ripening. Annu. Rev. Plant Biol. 1991, 42, 675–703. DOI: 10.1146/annurev.pp.42.060191.003331.
- Kojima, K.; Sakurai, N.; Kuraishi, S. Fruit Softening in Banana: Correlation Among Stress-relaxation Parameters, Cell Wall Components and Starch During Ripening. Physiol. Plant. 1994, 90, 772–778. DOI: 10.1111/j.1399-3054.1994.tb02536.x.
- Shah, S.; Hashmi, M. S.; Qazi, I. M.; Durrani, Y.; Sarkhosh, A.; Hussain, I.; Brecht, J. K. Pre-Storage Chitosan-Thyme Oil Coating Control Anthracnose in Mango Fruit. Sci. Hortic. 2021, 284, 110139. DOI: 10.1016/j.scienta.2021.110139.
- Durán-Soria, S.; Pott, D. M.; Osorio, S.; Vallarino, J. G. Sugar Signaling During Fruit Ripening. Front. Plant Sci. 2020, 11, 564917. DOI: 10.3389/fpls.2020.564917.
- Dave, R. K.; Ramana Rao, T.; Nandane, A. Improvement of Post-Harvest Quality of Pear Fruit with Optimized Composite Edible Coating Formulations. J. Food Sci. Technol. 2017, 54, 3917–3927. DOI: 10.1007/s13197-017-2850-y.
- Gutiérrez, M.; Lahoz, J.; Sola, M.; Pascual, L.; Vargas, A. Postharvest Changes in Total Soluble Solids and Tissue pH of Cherimoya Fruit Stored at Chilling and Non-Chilling Temperatures. J. Hortic. Sci. 1994, 69, 459–463. DOI: 10.1080/14620316.1994.11516475.
- Paull, R. Postharvest Variation in Composition of Soursop (Annona Muricata L.) Fruit in Relation to Respiration and Ethylene Production. J. Am. Soc. Hortic. Sci. 1982, 107, 582–585. DOI: 10.21273/JASHS.107.4.582.
- Torres, L. M. A. R.; Silva, M.; Guaglianoni, D. G.; Neves, V. A. Effects of Heat Treatment and Calcium on Postharvest Storage of Atemoya Fruits. Alimentos e Nutrição. Araraquara. 2010, 20, 359–368.
- Prabha, T.; Bhagyalakshmi, N. Carbohydrate Metabolism in Ripening Banana Fruit. Phyto. chemi. 1998, 48, 915–919. DOI: 10.1016/S0031-9422(97)00931-X.
- Padmanabhan, P.; Paliyath, G. Annonaceous Fruits. In Encyclopedia of Food and Health; Caballero, B., Finglas, P. Toldrá, F., Eds.; Academic Press: Oxford, 2016; pp. 169–173.
- Wiley, R. C. Preservation Methods for Minimally Processed Refrigerated Fruits and Vegetables, Minimally Processed Refrigerated Fruits and Vegetables; Springer: Boston, MA, 2017; pp. 187–237. DOI: 10.1007/978-1-4939-7018-6_6.
- González-aguilar, G. A.; Valenzuela-soto, E.; Lizardi-mendoza, J.; Goycoolea, F.; Martínez-téllez, M. A.; Villegas-ochoa, M. A.; Monroy-garcía, I. N.; Ayala-zavala, J. F. Effect of Chitosan Coating in Preventing Deterioration and Preserving the Quality of Fresh-cut Papaya ‘Maradol’. J. Sci. Food Agric. 2009, 89, 15–23. DOI: 10.1002/jsfa.3405.
- Dhital, R.; Joshi, P.; Becerra-Mora, N.; Umagiliyage, A.; Chai, T.; Kohli, P.; Choudhary, R. Integrity of Edible Nano-Coatings and Its Effects on Quality of Strawberries Subjected to Simulated In-Transit Vibrations. LWT. 2017, 80, 257–264. DOI: 10.1016/j.lwt.2017.02.033.
- Randazzo, W.; Jiménez-Belenguer, A.; Settanni, L.; Perdones, A.; Moschetti, M.; Palaz- Zolo, E.; Moschetti, G.; Vargas, M.; Germanà, M. A.; Moschetti, G. Antilisterial Effect of Citrus Essential Oils and Their Performance in Edible Film Formulations. Food. Control. 2016, 59, 750–758. DOI: 10.1016/j.foodcont.2015.06.057.
- Zhang, R.; Zhang, Z.; McClements, D. J. Nanoemulsions: An Emerging Platform for Increasing the Efficacy of Nutraceuticals in Foods. Colloids and Surfaces. B: Biointer 2020, 194, 111202. DOI: 10.1016/j.colsurfb.2020.111202.
