ABSTRACT
The Himalayan region supports a rich diversity of edible fruit species that can cope with global food and nutritional security by providing dietary diversity, genetic backup for commercial species and economic sustainability. Among these, Mahonia nepalensis DC. (Family Berberidaceae) is a medicinally important plant species of Himalaya with delicious edible berry fruits. Fruits of the species remain unexplored, however, their content of nutrients and phytochemicals and antioxidant potential changed with the ripening process. The current study assessed variations in nutritional, physicochemical, and phytochemical composition and antioxidant potential during the four ripening stages. A significant (p < .05) and sequential change in morphological (length, diameter, fresh weight, and volume), physicochemical (juice, juice pH, pomace, moisture, and juice pomace ratio), nutritive phytochemicals (riboflavin, lycopene, β-carotene, and total carotenoids), and antioxidant potential of the species were observed among the ripening stages. Total phenolic, flavonoid, and flavonol content exhibited an initial decrease followed by a consistent increase; however, tannin content decreased with the ripening process. Phenolic content identified in fruits exhibited order of concentration as chlorogenic acid > caffeic acid > syringic acid > ferulic acid > p-coumaric acid, and among these, chlorogenic acid decreased significantly (p < .05) with fruit ripening. Higher total phenolic, flavonoid, flavonol, and tannin contents and antioxidant activity in acidified methanolic extract than in methanolic extract indicated the presence of considerable content of glycosides as compared to aglycones in fruits. Presence of high nutritive phytochemicals, antioxidant potential, optimized extraction solvent, suitable harvesting stage in the species might be utilized as alternative dietary resources, potential nutraceutical and post-harvest management of Mahonia nepalensis berries.
Introduction
Berries are small-sized fruits derived from single or multiple ovaries and have three-layered pericarp embedded seed. These fruits are juicy and fleshy, oval to round shaped, soft and sweet to sour tasting, and have a variety of bright colors.[Citation1,Citation2] The berry fruits are gaining the attention of modern diets due to their high antioxidants, nutritional value, vitamins, and polyphenols, particularly anthocyanins.[Citation3] Antioxidant phytochemicals present in these fruits, such as anthocyanins, flavonoids, phenolic acids, ascorbic acids, and carotenes, are responsible for preventive effects related to aging and oxidative stress.[Citation4,Citation5] Recent epidemiological studies have indicated that increasing consumption of fruits has been associated with lowering the incidents of cardiovascular disease, gastrointestinal diseases, obesity, certain types of cancer, and diabetes due to their antioxidant and other beneficial properties.[Citation5–8] Meanwhile, the consumption of fruits and vegetables in developing countries, including India, is quite lower than recommended 400 g per day to obtain essential micronutrients.[Citation9] In such circumstances, wild native neglected small berry fruits can be a viable option for obtaining important minerals, vitamins, and nutrients with valuable health-beneficial effects in low-income groups in the developing world.[Citation10]
Indian Himalayan region (IHR), rich in wild edible diversity, harbors many locally popular underutilized nutritionally potent wild fruit species.[Citation11] Among these, Mahonia nepalensis DC. (syn: M. sikkimensis Takeda; Family Berberidaceae) is a Himalayan endemic evergreen medicinally important species with edible berry fruits.[Citation12] Generally, Mahonia genus comprises ca 60 species, mostly found as shrubs, or small trees praised for ornamental, dye production or medicinal value.[Citation13] High hybridization compatibility among species of Mahonia genus increases the quality of ornamental traits, reproductive capacity and vigor.[Citation14] Medicinally, Mahonia species are used in treating dysentery, jaundice, internal and external hemorrhage, acne vulgaris, and skin-related disorders, owing to the presence of alkaloids like, berberine, jatrorrhizine, and palmatine. Anthocyanin and antioxidant rich berry fruits of M. bealei and M. aquifolium are being extensively utilized by Europeans and Native Americans.[Citation15–19]
Besides the ornamental value, bark of M. nepalensis is used for the treatment of skin diseases like eczema and psoriasis, and for curing wounds.[Citation20] Dark colored mature ripe fruits are consumed in the hilly regions of India and Nepal.[Citation12] The bark of M. nepalensis exhibits significant antidiabetic,[Citation21] anti-inflammatory, antibacterial, and antifungal activities.[Citation20] Pharmacologically important alkaloids, berberine, protoberberines, bisbenzylisoquinolines, jattrrorzhine, O-methyl puljabine, isotetradine, homoaromaline, and so on, have been isolated from the stem bark of the species[Citation22,Citation23] and bisbenzyl-isoquinolines alkaloids (e.g., homoaromoline and isotetrandrine) from the roots.[Citation20] However, berry fruits of the species have not been investigated so far for their physicochemical, nutritional, phytochemical, and antioxidant potential. Additionally, berries from other Mahonia species, such as M. aquifolium, M. bealei and M. leschenaultii have been reported as a rich source of minerals, vitamins, anthocyanins, and antioxidants.[Citation16,Citation17] With the fruit development stages, phenolic acids, flavonoids, anthocyanins, and antioxidant activities of M. aquifolium berries changed significantly.[Citation17,Citation19] However, quantitative changes in nutritional and bioactive components in M. nepalensis during ripening stages may also be an important parameter for harvest management and quality control.
Generally, fruit ripening is a genetically controlled complex physiological and biochemical process, including change in chlorophyll level, development of color due to the biosynthesis of anthocyanin and other pigments, increase in sweetness due to accumulation and conversion of mono- and di-saccharides from starch, synthesis of aromatic compounds, decrease in the organic acid level, and degeneration of cell wall leading to fruit softening.[Citation24] This process is more important to understand in non-climacteric fruits, which progressed in the absence of ethylene through the involvement of ethylene-independent pathways and did not proceed after picking fruits from the plant.[Citation3,Citation24] Thus, determining the suitable harvesting stage of fruit is very important for harnessing optimum benefits in non-climacteric fruits of M. nepalensis.
M. nepalensis fruits are consumed only by the local dwellers of the hilly regions in the Indian Himalaya. However, the global market demand for berry fruit particularly, blackberry, blueberry, gooseberry, raspberry, strawberry, cranberry, and barberry is growing at a strident pace (CGAR 6.8%) and is continuously finding new agents as a novel food in the agri-food sector.[Citation2,Citation5] However, the knowledge gap on nutritional, phytochemical and antioxidant potential is restricting the scope for better utilization of the Mahonia berries. Keeping this in view, M. nepalensis was analyzed for morphological, physicochemical, nutritional, and phytochemical and antioxidant potential variability during ripening stages in the current study. The main objectives of the current study were to (i) analyze changes in the morphological, phytochemical, and antioxidant potential of the species with ripening, (ii) identify and quantify the phenolics present in juice and methanol (free phenolics) and acidified extracts (free and bounded phenolics), and (iii) assess the antioxidant potential of the species. To the best of our knowledge, this is the first study on morphological, physicochemical, nutritional, and phytochemical and antioxidant characterization of M. nepalensis. The findings of this study will be helpful in the management of nutritional security among communities of Himalayan using local bioresources and effective management of this important genetic resource in the future.
Materials and methods
Plant material collection
Fresh berry fruits of M. nepalensis were collected from the wild population of Pangthang area in Gangtok, Sikkim state of India (Latitude: 27° 21’ 48,” Longitude: 88° 34’ 04”; altitude: 2150 m asl). The ripening stages of berries were determined by the day after flowering, size, and change in color. Ripening stages were considered as small and green berries (S1, 30 days after flowering), maturing berries with an initial color change to light red color (S2, 45 days after flowering), fully grown and ripened berries with reddish-brown color (S3, 60 days after flowering), and ripened dark brown berries (S4, 75 days after flowering). Further, after the S4, over-ripened fruit lost its firmness and deterioration started. All the infected and damaged berries were removed, and only healthy berries were further processed for analysis. Immediately after collection, 10 berry fruits from each ripening stage were randomly selected for measuring morphological parameters. Meanwhile, physicochemical characteristics (juice content, pH of juice, pulp pomace, and moisture content) were assessed in triplicates from each ripening group of berries.
Reagents and chemicals
2,2-Diphenyl-2-picrylhydrazyl (DPPH), 2,2-azinobis-3-ethylbenzthiazoline-6-sulfonic acid (ABTS), 2,4,6-tri-2-pyridyl-1,3,5-triazin (TPTZ), ascorbic acid, gallic acid, quercetin, (+)-catechin, tannic acid, chlorogenic acid, caffeic acid, p-coumaric acid, syringic acid, ascorbic acid, riboflavin and β-carotene were procured from Sigma – Aldrich (Steinheim, Germany). While, ethanol, methanol, acetone, hydrochloric acid, glacial acetic acid, potassium permanganate, potassium persulfate, potassium chloride, potassium acetate, hydrogen peroxide, sodium sulfate, sodium carbonate, sodium acetate, Folin-Ciocalteu’s reagent, Folin – Dennis reagent, and ferric chloride were procured from Qualigens (Mumbai, India).
Morphological and physicochemical characterization
The width and length of 10 fresh berry fruits (each stage) were measured using a digital Vernier caliper with a sensitivity of 0.01 mm (Traceable Digital Caliper-6,” VWR International, Milano, Italy), and fresh weight was determined using a digital weighing machine with a sensitivity of 0.1 mg (Model CY510, Citizen). The volume of berry fruits was determined by the liquid displacement method. Berry fruits (50 g in three replicates) were squeezed to obtain the juice in mortar pistil and juice content was recovered by passing it from a cheesecloth. After the recovery of juice, pulp pomace was obtained and its content was determined using a digital weighing machine. The pH of the berry juice was measured using a digital pH meter (ECPH-70042S digital pH meter, Eutech Thermo Scientific, USA). The recovered juices were further stored at −4 ºC for analysis of phenolics and antioxidant activity. A total of 50 g berry fruits (in 3 replicates) were kept in a hot air oven at 65 ºC for drying till constant weight (4–5 days) and moisture content was calculated based on dry weight obtained.[Citation3]
Extraction and quantification of riboflavin content
Riboflavin was determined using the spectrophotometry method as described by Akwu and Josiah.[Citation25] Riboflavin was extracted by placing 10 g of the crushed edible part (pulp after removal of seed) in 100 ml of 50% ethanol solution and shaking for 1 h; the solution was filtered into a 100 ml flask. Thereafter, in 10 ml of the extract, 10 ml potassium permanganate (5%) and 10 ml hydrogen peroxide (30%) were added and allowed to stand over a hot water bath for 30 min. The total volume was made up to 50 ml by adding 20 ml of 40% sodium sulfate. Absorbance was measured at 510 nm using a UV–VIS spectrophotometer (UV-1800, Shimadzu, Kyoto, Japan). Quantifications were performed based on a standard curve of riboflavin prepared in 50% ethanol, and the results were expressed in mg/100 g fw.
Extraction and quantification of β-carotene
To measure the total carotenoid and β-carotene content, 10 g of fresh berries were homogenized in an ice bath with 5 mL acetone in a cold mortar pestle. Consequently, 1.0 g of anhydrous sodium sulfate (Na2SO4) was added to the achieved homogenization and was elutriated using a paper filter. Filtrated solution reached a volume of 10 mL with acetone and was centrifuged for 10 min at 5000 rpm. The upper phase was collected, and the absorbance of the solution was measured at 662, 645, 505, 453, 470, and 479 nm wavelengths using a UV–VIS spectrophotometer (UV-1800, Shimadzu, Kyoto, Japan).[Citation3] Acetone was used as a control and the carotenoids and β-carotene of each extract (in (mg/l)) were calculated using the following formulas.
Chlorophyll a (Ca) = 11.75 A662 - 2.350 A645
Chlorophyll b (Cb) = 18.61 A645 - 3.960 A662
Total carotenoids = 1000 A470 - 2.270 Ca - 81.4 Cb/227
β-carotene = 0.854 A479 - 0.312 A645 + 0.039 A663 - 0.005.
Lycopene = −0.0485 A662 + 0.204 A645 + 0.372 A505 – 0.0806 A453
Extraction and quantification of total monomeric anthocyanins
For the quantification of anthocyanins, 5 g of berry fruits were homogenized with 50 ml of 80% (v/v) acidified ethanol (95% ethanol + 5% 1.5 N HCl). After thorough mixing, the mixture were allowed to stand for overnight incubation at room temperature followed by filtering the extract. Extracts were stored at 4°C until analysis within 4 days.
The pH-differential method as described by Coklar and Akbulut was used for the determination of the content of total monomeric anthocyanin.[Citation16] The extracts were appropriately diluted in two buffers, 0.025 M potassium chloride pH 1.0 and 0.4 M sodium acetate pH 4.5. After 15 min of incubation at room temperature, absorbance was measured at 520 nm and 700 nm with a UV–VIS spectrophotometer (UV-1800, Shimadzu, Kyoto, Japan). The absorbance difference between the pH-1.0 and pH-4.5 samples was calculated:
A = (A520 nm - A700 nm) pH1.0 - (A520 nm - A700 nm) pH4.5
The monomeric anthocyanin pigment concentration was calculated using the following equation:
Monomeric anthocyanin pigment (mg/ml) = (A × MW × DF × 1000)/(ε × 1);
where MW = 449.2 and ε = 26,000, respectively, are molecular weight and molar absorptive of cyanidin-3-glucoside, which was used as a standard; DF is the dilution factor; l is the path length. The total monomeric anthocyanins were represented as mg/g fw.
Extraction preparation for quantification of phenolics and antioxidant activity
Fresh berry fruits (10 g) in each ripening stage were used to prepare extracts in three replicates to analyze phenolics (total phenolic, flavonoid, flavonol, and tannin contents) and antioxidant activity. After hand removal of seed, berry fruits were carefully homogenized using the grinder and kept in a flask, with 100 ml aqueous methanol (80% v/v) and acidified methanol (80% methanol with 1 N HCl in 4:1), separately, thereafter placed in the water bath at 60º C for 1 h. After cooling, the supernatant was kept for continuous shaking at room temperature for 15 h. The extracts were filtered, and the filtrate was centrifuged at 8,000 rpm for 10 min. The supernatant was stored at 4º C and used for quantification of phenolics and antioxidant activity within 2 days of extraction.
Determination of total phenolic, flavonoid, flavonol, and tannin content
The total phenolic content in the extracts was determined by Folin-Ciocalteu’s colorimetric method.[Citation26] Briefly, in 0.25 ml of diluted methanolic extract, 2.25 ml of distilled water and 0.25 ml Folin-Ciocalteu’s reagent was added and allowed to stand for reaction for up to 5 min. This mixture was neutralized by 2.50 ml of 7% sodium carbonate (w/v) and kept in the dark at room temperature for 60 min. The absorbance of the resulting blue color was measured at 765 nm using a UV–VIS spectrophotometer (UV-1800, Shimadzu, Kyoto, Japan)). Quantifications were done based on a standard curve of gallic acid prepared in 80% methanol (v/v), and results were expressed in mg gallic acid equivalent (GAE)/g of fresh weight (fw) of fruits.
The aluminum chloride colorimetric method was used to determine the total flavonoid content in the berry fruits.[Citation27] Briefly, 0.50 ml of methanolic extract of the sample was diluted with 1.50 ml of distilled water followed by adding 0.50 ml of 10% (w/v) aluminum chloride, 0.10 ml of 1 M potassium acetate, and 2.80 ml of distilled water. This mixture was incubated at room temperature (~20 ºC) for 30 min. The absorbance of the resulting reaction mixture was measured at 415 nm. Quantification of the flavonoid content was done based on a standard curve of quercetin prepared in 80% methanol. Total flavonol was estimated using the method of Dhyani et al.[Citation28] Briefly, in 2.0 ml of extract, 2.0 ml aluminum chloride solution (2% w/v) and 3.0 ml sodium acetate solution (5%) were added. The absorbance of the resulting solution was recorded at 440 nm after incubation (2.5 h) at room temperature (~20 ºC). Quantifications were performed based on a standard curve of catechin prepared in 80% (v/v) methanol. Results of total flavonoid and flavonol contents were expressed in mg quercetin equivalent (QE)/g fw.
The total tannin content was estimated following Badhani et al. with minor modifications.[Citation29] Briefly, 5 ml extract was added to 0.5 ml Folin – Dennis reagent (0.5 N) and 1 ml sodium carbonate solution (7%). The solution was diluted up to 10 ml with distilled water. After thorough mixing, the flasks were allowed to stand in a water bath at 25 ºC for 20 min. The absorbance of the resulting greenish-blue color was measured at 700 nm using a UV – vis spectrophotometer. The total tannin content was quantified at 700 nm based on a standard curve of tannic acid prepared in 80% (v/v) methanol. Results were expressed in mg tannic acid equivalent (TAE)/g fw.
Quantification of phenolic compounds
High performance liquid chromatography analysis was performed with a Shimadzu Prominence p-series binary HPLC system, equipped with including a CBM20A Lite controller, two LC20 AD BLK dual-plunger parallel flow pumps (L20105894240 & L20105894233), and an SPD-M40A PDA detector (Shimadzu (Shimadzu, Milan, Italy). Phenolic compounds were separated by using 4.6 × 250 mm, i.d.5 µm, Shim-Pack GWS C18 column. The Mobile phase used for the study included water, methanol, and acetic acid in the ratio of 80:20:1 and a flow rate of 0.8 ml/min in isocratic mode. Data were acquired using a PDA detector from 210 to 400 nm. The identification of phenolic compounds was done based on the retention time of the corresponding external standard. The UV–VIS spectra of the pure standard were used for plotting the standard calibration curve at different concentrations. The repeatability of the quantitative analysis was less than 3.0%. The mean value of content was calculated with ± standard deviation (SD). The results were expressed as mg/100 g fw of fruits.
Antioxidant activity by free radical – scavenging ability of DPPH cation (DPPH assay)
The traditional DPPH assay described by Coklar and Akbulut was used in this study with a few modifications.[Citation19] A cation solution of 0.1 mM DPPH prepared in 80% methanol (2.7 ml) was mixed with a 0.9 ml sample extract and kept in the dark at room temperature (~20 ºC) for 20 min. A reduction in the absorbance at 520 nm was recorded using a UV–VIS spectrophotometer (UV-1800, Shimadzu, Kyoto, Japan). Results were expressed in millimole (mM) ascorbic acid equivalent (AAE) per 100 g fresh weight (fw) of fruits.
Antioxidant activity by free radical – scavenging ability of ABTS cation (ABTS assay)
Total antioxidant activity was measured following the improved ABTS method described by Kim.[Citation30] ABTS salt (7.0 mM) and potassium persulfate (2.45 mM) were added for the production of ABTS cation (ABTS˙+) and kept in dark for 16 h at 23ºC. ABTS˙+ solution was diluted with distilled water until an absorbance of 0.700 ± 0.005 at 734 nm was obtained. Diluted ABTS˙+ solution (3.90 ml) was added to 0.10 ml of methanolic extract and allowed to stand for 6 min in the dark at room temperature (~20ºC). Absorbances were recorded at 734 nm using a UV–VIS spectrophotometer (UV-1800, Shimadzu, Kyoto, Japan) corresponding to a blank prepared with 80% (v/v) methanol. The standard curve of various concentrations of ascorbic acid was prepared in 80% v/v methanol for the equivalent quantification of antioxidant potential. Results were expressed in millimole (mM) ascorbic acid equivalent (AAE) per 100 g fresh weight (fw) of fruits.
Ferric reducing antioxidant power (FRAP) activity
Ferric reducing antioxidant power (FRAP) assay was performed following Gibson et al.[Citation31] FRAP reagent was prepared by adding 10 volumes of 300 mM acetate buffer (i.e., 3.1 g of sodium acetate and 16 ml glacial acetic acid/l), 1 volume of 10 mM 2,4,6-tri-2-pyridyl-1,3,5-triazine (TPTZ) in 40 mM HCl and 1 volume of 20 mM ferric chloride. The mixture was pre-warmed at 37°C and 3.0 ml of the mixture was added to 0.10 ml methanolic extract and kept at 37°C for 8 min. Absorbance was taken at 593 nm using a UV–VIS spectrophotometer. The blanks were prepared with ascorbic acid, and the results were expressed in millimole (mM) of ascorbic acid equivalent (AAE) per 100 g fresh weight (fw) of fruits.
Statistical analysis
Analysis of nutritive components, physicochemical parameters, phenolics, and antioxidant activity was carried out based on three replicates. Significant differences among mean values of ripening stages were tested using Duncan’s multiple-range test (P ≤ .05) using SPSS software Version 17.0 (SPSS Inc., Chicago, IL). An unpaired t-test was used to compare the mean values of the phenolics and antioxidant activity in the different solvent systems (methanolic and acidified methanolic).
Results and discussion
Morphological and physicochemical characterization
Morphological characteristics (fruit diameter, length, fresh weight, and volume) and physicochemical (juice %, juice pH, pomace %, moisture %, juice pomace ratio) characteristics of M. nepalensis have been presented in . Morphological parameters of berry fruits (diameter, length, fresh weight, and volume) exhibited a consistent significant increase (p < .05) with the progression of ripening. Fruit length increased up to 170% from S1 to S4 and reached up to 13.23 mm in the S4, and diameter increased by 215% from S1 to S4 and reached up to 8.66 mm in S4. Similarly, the fresh berry weight and volume increased by 10-times and 7.3-times and reached 0.06 g and 547.58 mm3 in S4, respectively. Among the physicochemical properties, moisture, and juice content were increased, and pomace content was decreased significantly (p < .05) along the ripening, thus juice pomace ratio increased consistently (p < .05) along the ripening. On the other hand, after a sharp decrease from S1 to S2, the juice pH increased from S2 to S4.
Table 1. Changes in morphological and physicochemical characters in fruits of M. nepalensis during different ripening stages.
Marakoglu et al. reported pomological properties as fruit thickness (7.05 mm), width (7.85 mm), length (9.10 mm), pulp mass ratio (88.21%), weight (0.28 g), geometric mean diameter (7.92 mm), volume (233 mm3), fruit density (1075 kg/m3) and bulk density (488.06 kg/m3) of M. aquifolium berries,[Citation32] and most of these parameters were found comparable or lower than M. nepalensis at the mature stage (S4) in the current study. However, among the different accessions of M. aquifolium grown in Ankara Turkey, a high genotypic variation was observed in morphological parameters (e.g., width, length, weight, number of seeds, seeds weight, and weight/seeds weight ratio; coefficient of variation between 15% and 43%). The average width and length were recorded as 8.4 and 10.2 mm,[Citation15] which were slightly lower than M. nepalensis recorded in the current study. Similarly, in a comparative study among different Berberis species of the family Berberidaceae in Ukraine, fruit weight was observed between 11.66 (in B. x declinata) to 39.66 g/100 fruits (B. amurensis),[Citation33] which were found comparable to M. nepalensis studied in the present work.
Generally, with fruit ripening, fruit volume increases by increasing the change in turgor pressure generated within cells by osmosis leading to fruit softening. Polysaccharides (e.g., pectin, cellulose, and hemicelluloses) present in cell wall of fruit tissues undergo solubilization, de-esterification, and depolymerization,[Citation24] which might be a possible reason for increased juice content and decreased pomace content during ripening. Also, a trend in pH with maturation represented by an initial sharp decrease followed by a consistent increase has been reported in blackberry fruits,[Citation34] cultivated strawberries,[Citation35] Himalayan wild strawberry,[Citation3] and lowbush blueberry,[Citation31] which might be due to reduction in content in organic acids after fruit development and utilized in the biosynthesis of sugars.[Citation31,Citation36]
Nutritive constituents
Various nutritive constituents such as riboflavin, lycopene, β-carotene, and total carotenoids, were quantified along the ripening stages using spectrophotometric methods (). Results revealed that the riboflavin content increased during ripening and reached up to 2.79 mg/100 g in S3, followed by a sharp decrease up to 1.08 mg/100 g fw in S4. Similarly, lycopene, β-carotene, and total carotenoids gradually increased with the ripening process and reached the maximum in S4 stage as 0.086 mg/100 g fw, 0.356 mg/100 g fw, and 3.351 mg/100 g fw, respectively. Among other berry fruits, β-carotene concentration has been recorded as 135.23 μg/100 g in blueberry, 8.22 μg/100 g in red raspberry, 44.27 μg/100 g in marionberry, and 240.52 μg/100 g oil in boysenberry seed oils.[Citation37] With the ripening, decreasing content of chlorophylls and increasing content of carotenoids have been identified as characteristic features of berry fruits of Berberidaceae. An increasing trend in carotenoid content has been recorded in fruits of Berberis vulgaris Linn, and B. chitria Lindl. with ripening; however, an opposite trend has been recorded in B. lycium Royle.[Citation38]
Figure 1. Change in nutritive constituents (in mg/100 g fw) such as, (a) riboflavin, (b) β-carotene, (c) total carotene and (d) lycopene content in fruits of M. nepalensis during different ripening stages. Ripening stage presented as S1 – Stage-1, S2 – Stage-2, S3 – Stage-3 and S4 – Stage-4.
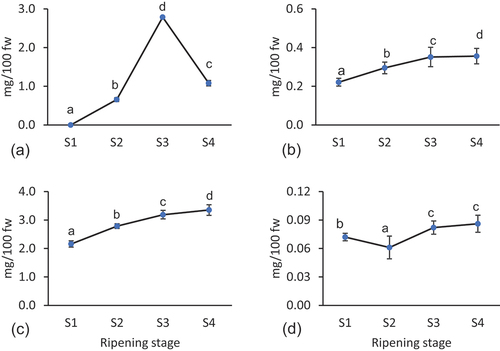
Spectrophotometric methods for carotenoids are a simple, inexpensive, rapid, and robust method that gives an estimate of different molecular classes; however, the results may be overestimated due to similar spectroscopic ehaviour of specific classes of other related molecules.[Citation39] These methods are still well accepted for general nutritional profiling of fruits and vegetables, and the results were found to be comparable to the results obtained through the HPLC method. The method has been used in the quantification of β-carotene, phenol, and anthocyanin content in several berries and other fruit species,[Citation3,Citation40] However, to avoid the overestimation of carotenoids, improved extraction, partial separation and saponification steps can be included, although this may lead to a loss of content of carotenoids.[Citation41]
Phenolic constituents
Phenolic constituents were analyzed in methanolic (mostly extracted aglycones), acidified methanolic (mostly extracted aglycones and glycosidic) extracts and in juice of M. nepalensis (). During the ripening, the total phenolic content exhibited a consistent increase in methanolic extract reached from 0.37 (S1) to 0.72 mg GAE/g fw (S4), while, in acidified methanolic extract, it exhibited an initial decrease in S2 followed by a consistent increase up to S4 and reached 8.84 mg/g GAE. However, the juice did not show any clear trend in phenolic content along the ripening and remained between 16.47 (S1) and 22.75 mg GAE/ml (S4). However, the total flavonoid content extracted from methanol initially decreased with ripening (0.84 mg QE/g in S1 and 0.71 mg QE/g in S2) and remained constant in S2, S3, and S4 stages. In contrast, it increased continuously from S1 (1.13 mg QE/g) to S4 (4.06 mg QE/g) in acidified methanolic extract; however, in juice, it decreased initially from 4.54 mg QE/g (S1) to 3.24 mg QE/g (S2) and then improved slightly in S4 as 3.40 mg QE/g. Also, the total flavonol content increased with ripening in methanolic extract, acidified methanolic extract, and in juice. The total tannin content increased during the S2 stage (4.14 mg TAE/g) and further decreased gradually up to S4 (3.61 mg TAE/g) in methanolic extract, while it decreased consistently from S1 (2.70 mg TAE/g) to S4 (2.06 mg TAE/g) in acidified methanolic. On contrary, it regularly increased in juice from 2.36 (S1) to 3.75 mg TAE/ml (S4) with ripening. Overall, acidified methanolic extract was found to be better for recovery of all types of phenolic constituents than methanolic extract.
Table 2. Changes in total phenolic, flavonoid, flavonol, and tannin content extracted in methanolic (free compounds) and acidified methanolic extracts (free and conjugated compounds) from fruits of M. nepalensis during different ripening stages.
Previously, a study on M. jaunsarensis revealed a comparable presence of total phenolics (1.00–1.39 mg GAE/g), tannins (1.01–1.11 mg TAE/g), flavonoids (1.07–1.22 mg QE/g), and flavonols (0.71–1.04 mg QE/g) in different solvent extractions.[Citation42] Similarly, the total phenolic content has been reported in fruits of cultivated M. aquifolium as 17.24 mg GAE/g dry weight.[Citation19] Similarly, the total phenolic contents in M. aquifolium berries were recorded from 1.30 mg (in chloroform extract) to 1049.40 mg GAE/100 g fw (in methanol extract) in another study.[Citation16] Also, in berries of M. leschenailtii found in the Western Ghats of India, total phenolic content was recorded as 65.8 mg/100 g GAE and total flavonoids as 95.5 mg/100 g QE, which was comparable to the current study.[Citation43] Among the Berberis species, total phenolic content was recorded as 8.99 mg GAE/g fresh weight in B. canadensis, 9.23 mg GAE/g fresh weight in B. amurensis, 10.52 mg GAE/g fresh weight in B. vulgaris, 12.43 mg GAE/g fresh weight in B. x declinata, and 13.63 mg GAE/g fresh weight in B. koreana, and total flavonoid between 1.02 and 2.10 mg rutin equivalent in these species.[Citation33] Thus, the fruits of M. nepalensis were found to be comparatively rich sources of polyphenolics.
Likewise, a decrease in total phenolic content with ripening has been reported in many berry fruits of Berberidaceae, such as, Berberis vulgaris Linn, B. chitria Lindl and B. lycium Royle,[Citation38] which was recorded as having a very sharp reduction in initial stages and become stable in lateral stages of ripening. However, in the fruits of Berberis buxifolia, an increase in total phenolic content has been reported after some initial decrease, while the total flavonoid content decreased consistently with ripening.[Citation44]
Results indicated that extraction of total phenolic, flavonoid, and flavonol content was significantly improved (p < .05) with acidified methanolic solvent as compared to aqueous methanolic solvent in all the ripening stages. It also indicated the presence of higher content of glycosides in the fruits of M. nepalensis and acid hydrolysis released the bounded phenolics during extraction.[Citation45] Acid hydrolysis improved extraction of total phenolic content with 12–23 times higher yield than methanol extraction, total flavonoids by 36%–481%, and total flavonol by 136%– 354% during different ripening stages. Along the ripening progression, the ratio of free total phenolic and bounded total phenolic content decreases with ripening stages. However, the ratio of methanol extractable (free) and acidified methanol extractable (free and bounded) total flavonoid and total flavonol contents were found to increase with the ripening stage. Significant improvement in the extraction of total phenolic (3%–10%) and flavonoid contents (60%–110%) with acid hydrolysis has been reported in Fragaria nubicola, and a similar change in the ratio of methanol and acidified methanol extractable total phenolic (3%–10%) and flavonoid contents was recorded with ripening.[Citation3] Similarly, improved extraction of total phenolic content with acid hydrolysis has been reported in strawberries,[Citation26] raspberries, blackcurrants, blackberries, cranberries, black chokeberries and blueberries.[Citation30]
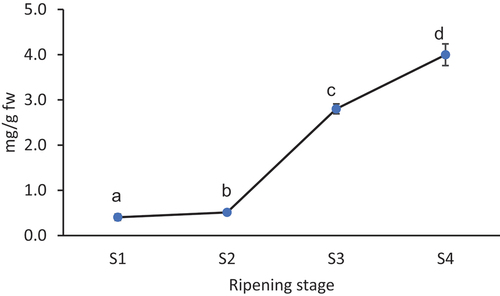
The total monomeric anthocyanin content significantly (p < .05) increased with fruit ripening stages and was recorded as 0.405 mg/g in S1, 0.509 mg/g in S2, 2.801 mg/g in S3, and 3.999 mg/g in S4 (). Therefore, S3 and S4 can be considered as very important stages for anthocyanin accumulation in berry fruits of M. nepalensis. Generally, berry fruits of Mahonia are considered a good source of anthocyanins, and it has been reported as 380.99 mg/100 g fw in ethanol extract of domesticated M. aquifolium fruits.[Citation17] However, it has been observed as 8.58 mg/100 g fw in M. leschenaultii fruits, which was comparatively less than M. nepalensis studied in the present work.[Citation43] Among the accessions of M. aquifolium grown in Ankara, Turkey, a relatively lower amount of total monomeric anthocyanin content was reported (between 5.28 and 36.10 and an average value of 14.10 mg/100 g) than the present results on M. nepalensis.[Citation15] Among the fruit ripening stages, a sharp increase in total anthocyanin content has also been reported in fruits of Berberis vulgaris Linn, B. chitria Lindl and B. lycium Royle.[Citation38]
Phenolic compositions
HPLC-DAD analysis was performed to identify and quantify the phenolic compounds in acidified methanolic extracts and results revealed that major phenolic compounds were chlorogenic acid, caffeic acid, syringic acid, p-coumaric acid, and ferulic acid in M. nepalensis fruits ( ; ). In the fruit commercial species M. aquifolium, chlorogenic acid was identified as a major phenolic compound followed by, ferulic acid, protocatechuic acid, gentisic acid, syringic acid, caffeic acid, and p-coumaric acid.[Citation17,Citation19,Citation46]
Figure 3. Chromatogram of methanolic extract of fruit at 254 nm. Peaks are presented as: 1, Chlorogenic acid; 2, Caffeic acid; 3, Syringic acid; 4, p-Coumaric acid; 5, Ferulic acid.
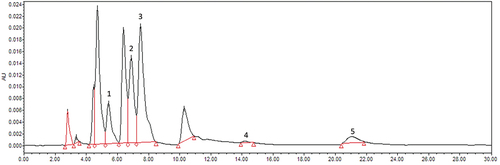
Table 3. Changes in phenolic composition in fruits of M. nepalensis during different ripening stages quantified by HPLC-DAD analysis.
Chlorogenic acid content decreased significantly from 15.33 mg/100 g in S1 to 9.64 mg/100 g in S4 along the ripening. In addition, caffeic acid (ranging from 0.43to 0.85 mg/100 g) drastically decreased in S2, followed by a significant (p < .05) increase with ripening. Syringic acid (0.22 to 0.41 mg/g), p-coumaric acid (0.09 to 0.21 mg/100 g), and ferulic acid (0.03 to 0.28mg/100 g) were also detected in trace quantity, but did not show any trend with fruit ripening. Previously, a decrease in the content of chlorogenic acid, gentisic acid, p-coumaric acid, and ferulic acid, and increases in the content of syringic acid and caffeic acid has been reported with the fruit ripening in M. aquifolium.[Citation19] Also, in a comparative study, higher amounts of chlorogenic acid and isoquercitrin, and lower amounts of ferulic acid and rutin have been reported in ripe fruit extract as compared to green fruit of M. aquifolium.[Citation17] Similarly, with fruit ripening, decreasing content of chlorogenic acid has been reported in many berry fruits, such as, Berberis asiatica, Myrica esculenta, Rubus ellipticus, Pyracantha crenulata, Morus alba,[Citation47] and blueberries.[Citation31] An increasing pattern of caffeic acid was also reported in Berberis asiatica,[Citation47] which partially followed the same pattern of M. nepalensis in the current study.
Biosynthesis of phenolic acids and flavonoids initiated from phenylalanine through the phenylpropanoid pathway, in which, phenylalanine ammonia lyase formed trans-cinnamic acid followed by hydroxylation of trans-cinnamic acid to generate p-coumaric acid in the presence of cinnamic acid 4-hydroxylase (a cytochrome P450 monooxygenase), and addition of a co-enzyme A to form p-coumaroyl-CoA by the 4-coumarate-CoA ligase.[Citation48] Here, p-coumaric acid can form caffeic acid and ferulic acid by p-coumarate 3-hydroxylase and 3-O-methyltransferase followed by the formation of other benzoic acid and cinnamic acid derivatives, including syringic acid.[Citation49] Meanwhile, chlorogenic acid is formed by p-coumaroyl-quinic acid and catalyzed by hydroxycinnamoyl-CoA shikimate/quinate hydroxycinnamoyl transferase, which is produced by p-coumaroyl-CoA in the presence of p-coumarate 3-hydroxylase. Caffeoyl-CoA is synthesized from chlorogenic acid or caffeoyl shikimic acid by hydroxycinnamoyl-CoA shikimate/quinate hydroxycinnamoyl transferase (HCT) and initiates the first step of flavonoid and anthocyanin biosynthesis through chalcone synthesis.[Citation50,Citation51] Caffeoyl-CoA also transforms to chlorogenic acid by the hydroxycinnamoyl CoA quinate hydroxycinnamoyl transferase. Thus, caffeic acid, caffeoyl-CoA, and ferulic acid exist in reversible transformations and exhibit a positive correlation with each other.[Citation52] With fruit ripening, anthocyanin biosynthesis accelerated and thus, a decreasing trend of chlorogenic acid and caffeic acid indicated its conversion to flavonoids and anthocyanins in fruits of M. nepalensis.
Antioxidant activity
The antioxidant activity of methanolic extract, acidified methanolic extracts, and juice of M. nepalensis berry fruit was assessed using three in vitro assays (). The ABTS assay revealed an increase in antioxidant activity across different ripening stages. It reached 7.98 to 11.12 mM AAE/100 g in methanolic extract, 11.35 to 12.73 mM AAE/100 g in acidified methanolic extract and 8.88–12.18 mM AAE/ml in juice. The DPPH assay revealed a decreasing pattern of antioxidant potential of acidified extract with fruit ripening; however, in juice and methanolic extracts the decrease in antioxidant activity was after S2, after the initial gain. The FRAP assay indicated a monotonic increase in the antioxidant activity with berry ripening in all the studied samples. Overall, antioxidant activity was recorded higher in acidified methanolic extract as compared to methanolic extract with ABTS assay.
Figure 4. Change in antioxidant activity in methanolic extract, acidified methanolic extract and fruit juice of M. nepalensis during different ripening stages using different in vitro assays namely, (a) ABTS assay, (b) DPPH assay and (c) FRAP assay. Ripening stage presented as S1 – Stage-1, S2 – Stage-2, S3 – Stage-3 and S4 – Stage-4. Different letters over the bar indicate statistically significant differences between the ripening stages (p < .05).
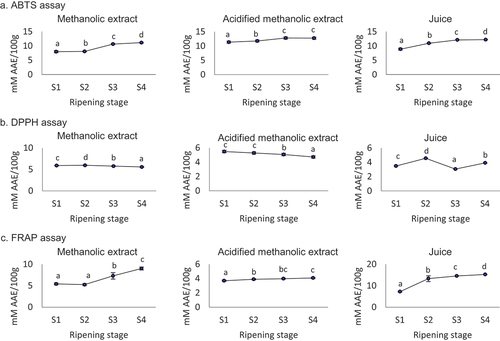
In an earlier study, antioxidant activity in fruits of M. jaunsarensis using the same in vitro assay method was found to be relatively lower than in the present study.[Citation42] In a previous study on M. aquifolium, the antioxidant activity was recorded as 35.26 mM by DPPH assay, 49.95 mM TE (Trolox equivalent)/kg by ABTS assay, and 136.34 mM TE/kg by FRAP mM TE/kg fresh weight in the methanolic extract.[16] Due to the antioxidant activity of M. aquifolium fruits, decreased serum nitric oxide, total oxidative status, 3-nitrothyrosine and TNF-alpha and increased total thiols were recorded as an indicator of anti-inflammatory activity in male albino Wistar rats.[Citation46] However, such variation among different studies might be due to different ripening stages, time of collection, post-harvest management, sample processing, and the genetic makeup of different genotypes.[Citation3,Citation29] Among the different solvent extraction systems, methanol was found best as compared to ethanol and distilled water in M. jaunsarensis,[Citation42] however, in this study, acidified methanol was found to be a better solvent for extraction than methanol due to its ability to extract glycosidic molecules also.[Citation3]
Major antioxidant molecules found in the fruits of M. nepalensis include phenolics, flavonoids, anthocyanins, and carotenoids. It has been reported that the antioxidant activity of anthocyanins is much higher than the activity of phenolic acids and flavonoids.[Citation16] Among the anthocyanins, it has been reported that cyanidin contains 3 times higher antioxidant activity than chlorogenic acid. Increasing anthocyanin with berry fruit ripening in M. nepalensis may lead to higher values of antioxidant activity with ABTS and FRAP assays. However, decreasing value of DPPH assay in lateral ripening stages might be due to compositional changes in non-lipophilic antioxidants,[Citation53] which is a more influencing factor for variable values of DPPH assay as compared to the other two assays.
Conclusion
Dark blue-blackish colored berry fruits of M. nepalensis were found to be a good source of nutritive phytochemicals, phenolic compounds, and antioxidants. The high nutritional and phytochemical value of the berries of the species indicated its potential application for nutraceutical development. Nutritive constituents and phytochemicals exhibit different accumulation and occurrence patterns during ripening depending on the particular group of molecules and thus, specific quality-related traits of fruits at each development stage can contribute in its best alternative uses in the future for raw consumption, production of juices of beverages, or for other industrial processing. Chlorogenic acid was found to be the major phenolic of the fruit and decreased with ripening, while other compounds and total content of phenolic, flavonoid, and flavonol increased as ripening proceeded. Higher concentrations of total phenolic, total flavonoid, total flavonol, tannin content, and antioxidant activity in methanolic and acidified methanolic extract indicated the presence of higher content of glycosides as compared to aglycones in fruits. Traditional spectrophotometric methods were used for nutrients and phytochemicals in the current study, and precise identification of these compounds remains a future area of research. Considering the nutritive constituents, phenolic composition, and antioxidant activity, fully ripened fruits could be recommended as the optimal harvesting stage for M. nepalensis. Thus, the current research allowed an opportunity to utilize this important edible bioresource and develop nutraceutical products from the species.
Authorship contribution statement
POB and SR conceptualized the idea and designed the experiments; POB, PA and AP collected the samples and conducted the experimentation; AP, PA, SR, and RJ analyzed the data and prepared figures and tables; AP, SR DK, RJ, and IDB wrote and edited the manuscript. All the authors read and approved the final version of the manuscript.
Ethical standards
There is no ethical standard related to the present review article.
Data archiving statement
This review manuscript does not use any data. There are no data uploaded to any database.
Acknowledgments
The authors would like to thank the Director of the Institute for providing facilities and encouragement. We also thank colleagues at the Sikkim Regional Centre for their support and help. Partial financial support received from the in-house program (project No-02) and the in-house program (project No-04) from the Institute and Himalayan Fellowship Program from the Mountain Division, GBPNIHE, is deeply acknowledged.
Disclosure statement
All the authors declared that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Funding
References
- Hickey, M.; King, C. The Cambridge Illustrated Glossary of Botanical Terms; Cambridge University Press: United Kingdom, 2000.
- Zhao, Y. Berry Fruit: Value-Added Products for Health Promotion, 1st ed.; CRC press: New York, 2007.
- Bhutia, P. O.; Kewlani, P.; Pandey, A.; Rawat, S.; Bhatt, I. D. Physico-Chemical Properties and Nutritional Composition of Fruits of the Wild Himalayan Strawberry (Fragaria Nubicola Lindle.) in Different Ripening Stages. J. Berry Res. 2021, 11(3), 481–496. DOI: 10.3233/JBR-210742.
- Bhatt, I. D.; Rawat, S.; Rawal, R. S. Antioxidants in Medicinal Plants. In Biotechnology for Medicinal Plants; Chandra, S. Lata, H. Varma, A., Eds.; Springer: Berlin, Heidelberg, 2013; pp. 295–326.
- Li, R.; Tao, M.; Xu, T.; Pan, S.; Xu, X.; Wu, T. Small Berries as Health-Promoting Ingredients: A Review on Anti-Aging Effects and Mechanisms in Caenorhabditis Elegans. Food. Funct. 2021, 13(2), 478–500. DOI: 10.1039/D1FO02184B.
- Hu, F. B. Plant-Based Foods and Prevention of Cardiovascular Disease: An Overview. Americ. J. Clin. Nutri. 2003, 78(3), 544S–551S. DOI: 10.1093/ajcn/78.3.544S.
- Riboli, E.; Norat; Norat, T. T. Epidemiologic Evidence of the Protective Effect of Fruit and Vegetables on Cancer Risk. Americ. J. Clin. Nutri. 2003, 78(3), 559S–569S. DOI: 10.1093/ajcn/78.3.559S.
- Aune, D.; Giovannucci, E.; Boffetta, P.; Fadnes, L. T.; Keum, N.; Norat, T.; Greenwood, D. C.; Riboli, E.; Vatten, L. J.; Tonstad, S. Fruit and Vegetable Intake and the Risk of Cardiovascular Disease, Total Cancer and All-Cause Mortality—A Systematic Review and Dose-Response Meta-Analysis of Prospective Studies. Int. J. Epidemiol. 2017, 46(3), 1029–1056. DOI: 10.1093/ije/dyw319.
- Minocha, S.; Thomas, T.; Kurpad, A. V. Are ‘Fruits and vegetables’ Intake Really What They Seem in India? Europ. J. Clin. Nutri. 2018, 72(4), 603–608. DOI: 10.1038/s41430-018-0094-1.
- Baldermann, S.; Blagojević, L.; Frede, K.; Klopsch, R.; Neugart, S.; Neumann, A.; Ngwene, B.; Norkeweit, J.; Schröter, D.; Schröter, A., et al. Are Neglected Plants the Food for the Future? Crit. Rev. Plant Sci. 2016, 35(2), 106–119.
- Samant, S. S.; Dhar, U. Diversity, Endemism and Economic Potential of Wild Edible Plants of Indian Himalaya. Int. J. Sust. Develop. World Ecol. 1997, 4(3), 179–191. DOI: 10.1080/13504509709469953.
- Singh, A. V.; Asha, H. Wild Edible Fruits of Arunachal Pradesh. Int. J. Innov. Res Sci. Engineer. Technol. 2017, 6, 12203–12209.
- He, J. M.; Mu, Q. The Medicinal Uses of the Genus Mahonia in Traditional Chinese Medicine: An Ethnopharmacological, Phytochemical and Pharmacological Review. J. Ethnopharmacol. 2015, 175, 668–683. DOI: 10.1016/j.jep.2015.09.013.
- Houtman, R. T.; Kraan, K. J.; Kromhout, H. Mahonia aquifoliumx wagneri en hybriden. Dendroflora. 2004, M. repens, M41, 42–69.
- Gunduz, K. Morphological and Phytochemical Properties of Mahonia aquifolium from Turkey. Pak. J. Agric. Sci. 2013, 50, 439–443.
- Coklar, H.; Akbulut, M. Anthocyanins and Phenolic Compounds of Mahonia aquifolium Berries and Their Contributions to Antioxidant Activity. J. Funct. Foods. 2017, 35, 166–174. DOI: 10.1016/j.jff.2017.05.037.
- Coklar, H.; Akbulut, M. Bioactive Compounds, Antioxidant Activity and Some Physicochemical Properties of the Seed and Seed-Oil of Mahonia aquifolium Berries. J. Food Measur. Character. 2019, 13(2), 269–1278. DOI: 10.1007/s11694-019-00042-6.
- Kakar, M. U.; Li, J.; Mehboob, M. Z.; Sami, R.; Benajiba, N.; Ahmed, A.; Nazir, A.; Deng, Y.; Li, B.; Dai, R. Purification, Characterization, and Determination of Biological Activities of Water-Soluble Polysaccharides from Mahonia Bealei. Sci. Rep. 2022, 12(1), 8150. DOI: 10.1038/s41598-022-11661-3.
- Coklar, H.; Akbulut, M. Changes in Phenolic Acids, Flavonoids, Anthocyanins, and Antioxidant Activities of Mahonia aquifolium Berries During Fruit Development and Elucidation of the Phenolic Biosynthetic Pathway. Hort. Environ. Biotech. 2021, 62(5), 785–794. DOI: 10.1007/s13580-021-00348-9.
- Mai, N. T.; Tuan, T. A.; Huong, H. T.; VanMinh, C.; Ban, N. K.; VanKiem, P. Bisbenzylisoquinoline Alkaloids from Mahonia Nepalensis. Vietnam J. Chem. 2009, 47(3), 368.
- Thusa, R.; Mulmi, S. Analysis of Phytoconstituents and Biological Activities of Different Parts of Mahonia Nepalensis and Berberis Aristata. Nepal J. Biotech. 2017, 5(1), 5–13. DOI: 10.3126/njb.v5i1.18864.
- Govindachari, T. R.; Pai, B. R.; Rajadurai, S.; Ramadas, R. U. Alkaloids of Mahonia Nepalensis D.C. Proceed. Indian Acad. Sci.-A. 1958, 47(1), 41–48. DOI: 10.1007/BF03052624.
- Mai, N. T.; Tuan, T. A.; Huong, H. T.; VanMinh, C.; Ban, N. K.; VanKiem, P. Secobisbenzylisoquinoline Alkaloid from Mahonia Nepalensis DC. Tạp. Chi. Khoa. Học. Va. Cong. Nghe. 2008, 46, 63–68.
- Prasanna, V.; Prabha, T. N.; Tharanathan, R. N. Fruit Ripening Phenomena–An Overview. Critic. Rev. Food Sci. Nutri. 2007, 47(1), 1–19. DOI: 10.1080/10408390600976841.
- Okwu, D.E.; Josiah, C. Evaluation of the chemical composition of two Nigerian medicinal plants.Afric. J. Biotech. 2006, 5(4), 357–361.
- Meyers, K. J.; Watkins, C. B.; Pritts, M. P.; Liu, R. H. Antioxidant and Antiproliferative Activities of Strawberries. J. Agric. Food. Chem. 2003, 51(23), 6887–6892. DOI: 10.1021/jf034506n.
- Bhatt, I. D.; Rawat, S.; Badhani, A.; Rawal, R. S. Nutraceutical Potential of Selected Wild Edible Fruits of the Indian Himalayan Region. Food. Chem. 2017, 215, 84–91. DOI: 10.1016/j.foodchem.2016.07.143.
- Dhyani, P.; Bahukhandi, A.; Rawat, S.; Bhatt, I. D.; Rawal, R. S. Diversity of Bioactive Compounds and Antioxidant Activity in Delicious Group of Apple in Western Himalaya. J. Food Sci. Technol. 2018, 55(7), 2587–2599. DOI: 10.1007/s13197-018-3179-x.
- Badhani, A.; Rawat, S.; Bhatt, I. D.; Rawal, R. S. Variation in Chemical Constituents and Antioxidant Activity in Yellow Himalayan (Rubus Ellipticus Smith) and Hill Raspberry (Rubus Niveus Thunb.). J. Food Biochem. 2015, 39(6), 663–672. DOI: 10.1111/jfbc.12172.
- Kim, J. S. Antioxidant Activities of Selected Berries and Their Free, Esterified, and Insoluble-Bound Phenolic Acid Contents. Prevent. Nutri. Food Sci. 2018, 23(1), 35–45. DOI: 10.3746/pnf.2018.23.1.35.
- Gibson, L.; Rupasinghe, H. V.; Forney, C. F.; Eaton, L. Characterization of Changes in Polyphenols, Antioxidant Capacity and Physico-Chemical Parameters During Lowbush Blueberry Fruit Ripening. Antioxidants. 2013, 2(4), 216–229. DOI: 10.3390/antiox2040216.
- Marakoglu, T.; Akbulut, M.; Calisir, S. Some Physicochemical Properties of Mahonia acquifolium Fruits. Asian J. Chem. 2010, 22, 1606–1614.
- Khromykh, N. O.; Lykholat, Y. V.; Kovalenko, I. M.; Kabar, A. M.; Didur, O. O.; Nedzvetska, M. I. Variability of the Antioxidant Properties of Berberis Fruits Depending on the Plant Species and Conditions of Habitat. Regulat. Mech. Biosyst. 2018, 9(1), 56–61. DOI: 10.15421/021807.
- Tosun, I.; Ustun, N. S.; Tekguler, B. Physical and Chemical Changes During Ripening of Blackberry Fruits. Sci. Agric. 2008, 65(1), 87–90. DOI: 10.1590/S0103-90162008000100012.
- Montero, T. M.; Mollá, E. M.; Esteban, R. M.; López-Andréu, F. J. Quality Attributes of Strawberry During Ripening. Sci. Hort. 1996, 65(4), 239–250. DOI: 10.1016/0304-4238(96)00892-8.
- Famiani, F.; Battistelli, A.; Moscatello, S.; Cruz-Castillo, J. G.; Walker, R. P. The Organic Acids That are Accumulated in the Flesh of Fruits: Occurrence, Metabolism and Factors Affecting Their Contents – a Review. Revista Chapingo Seri. Horticultura. 2015, 21(2), 97–128. DOI: 10.5154/r.rchsh.2015.01.004.
- Parry, J.; Su, L.; Luther, M.; Zhou, K.; Yurawecz, M. P.; Whittaker, P.; Yu, L. Fatty Acid Composition and Antioxidant Properties of Cold-Pressed Marionberry, Boysenberry, Red Raspberry and Blueberry Seed Oils. J. Agric. Food. Chem. 2005, 53(3), 566–573. DOI: 10.1021/jf048615t.
- Chandra, P.; Todaria, N. P. Maturation and Ripening of Three Berberis Species from Different Altitudes. Sci. Hort. 1983, 19(1–2), 91–95. DOI: 10.1016/0304-4238(83)90048-1.
- Biswas, A. K.; Sahoo, J.; Chatli, M. K. A Simple UV-Vis Spectrophotometric Method for Determination of β-Carotene Content in Raw Carrot, Sweet Potato and Supplemented Chicken Meat Nuggets. LWT-Food Sci. Technol. 2011, 44(8), 1809–1813. DOI: 10.1016/j.lwt.2011.03.017.
- Singh, A.; Singh, B. K.; Deka, B. C.; Sanwal, S. K.; Patel, R. K.; Verma, M. R. The Genetic Variability, Inheritance and Inter-Relationships of Ascorbic Acid, β-Carotene, Phenol and Anthocyanin Content in Strawberry (Fragaria × Ananassa Duch.). Sci. Horticult. 2011, 129(1), 86–90. DOI: 10.1016/j.scienta.2011.03.011.
- Biehler, E.; Mayer, F.; Hoffmann, L.; Krause, E.; Bohn, T. Comparison of 3 Spectrophotometric Methods for Carotenoid Determination in Frequently Consumed Fruits and Vegetables. J. Food Sci. 2010, 75(1), C55–61. DOI: 10.1111/j.1750-3841.2009.01417.x.
- Suyal, R.; Bahukhandi, A.; Rawal, R. S.; Upadhyay, S. Polyphenolics and Antioxidant Activity of Mahonia Jaunsarensis Ahrendt: A Narrow Endemic to West Himalaya. Natl. Acad. Sci. Lett. 2020, 43(6), 505–508. DOI: 10.1007/s40009-020-00916-0.
- Karuppusamy, S.; Muthuraja, G.; Rajasekaran, K. M. Antioxidant Activity of Selected Lesser Known Edible Fruits from Western Ghats of India. Indian J. Nat. Prod. Resour. 2011, 2, 174–178.
- Arena, M. E.; Zuleta, A.; Dyner, L.; Constenla, D.; Ceci, L.; Curvetto, N. Berberis Buxifolia Fruit Growth and Ripening: Evolution in Carbohydrate and Organic Acid Contents. Sci. Hort. 2013, 158, 52–58. DOI: 10.1016/j.scienta.2013.04.026.
- Pimpão, R. C.; Dew, T.; Oliveira, P. B.; Williamson, G.; Ferreira, R. B.; Santos, C. N. Analysis of Phenolic Compounds in Portuguese Wild and Commercial Berries After Multienzyme Hydrolysis. J. Agric. Food. Chem. 2013, 61(17), 4053–4062. DOI: 10.1021/jf305498j.
- Andreicut, A. D.; Pârvu, A. E.; Mot, A. C.; Pârvu, M.; Fischer, F. E.; Cătoi, A. F.; Feldrihan, V.; Cecan, M.; Irimie, A. Phytochemical Analysis of Anti-Inflammatory and Antioxidant Effects of Mahonia aquifolium Flower and Fruit Extracts. OXID. MED. CELL LONGEV. 2018, 2018, 2879793. DOI: 10.1155/2018/2879793.
- Belwal, T.; Pandey, A.; Bhatt, I. D.; Rawal, R. S.; Luo, Z. Trends of Polyphenolics and Anthocyanins Accumulation Along Ripening Stages of Wild Edible Fruits of Indian Himalayan Region. Sci. Rep. 2019, 9(1), 95894. DOI: 10.1038/s41598-019-42270-2.
- Liu, W.; Feng, Y.; Yu, S.; Fan, Z.; Li, X.; Li, J.; Yin, H. The Flavonoid Biosynthesis Network in Plants. Int. J. Mol. Sci. 2021, 22(23), 12824. DOI: 10.3390/ijms222312824.
- Marchiosi, R.; dos Santos, W. D.; Constantin, R. P.; de Lima, R. B.; Soares, A. R.; Finger-Teixeira, A.; Mota, T. R.; de Oliveira, D. M.; Foletto-Felipe, M. D. P.; Abrahão, J., et al. Biosynthesis and Metabolic Actions of Simple Phenolic Acids in Plants. Phytochem. Rev. 2020, 19(4), 865–906.
- Mahesh, V.; Million-Rousseau, R.; Ullmann, P.; Chabrillange, N.; Bustamante, J.; Mondolot, L.; Morant, M.; Noirot, M.; Hamon, S.; de Kochko, A., et al. Functional Characterization of Two P-Coumaroyl Ester 3′-Hydroxylase Genes from Coffee Tree: Evidence of a Candidate for Chlorogenic Acid Biosynthesis. Plant Mol. Biol. 2007, 64(1–2), 145–159.
- Chen, X.; Cai, W.; Xia, J.; Yu, H.; Wang, Q.; Pang, F.; Zhao, M. Metabolomic and Transcriptomic Analyses Reveal That Blue Light Promotes Chlorogenic Acid Synthesis in Strawberry. J. Agric. Food. Chem. 2020, 68(44), 12485–12492. DOI: 10.1021/acs.jafc.0c05020.
- Aseel, D. G.; Rashad, Y. M.; Hammad, S. M. Arbuscular Mycorrhizal Fungi Trigger Transcriptional Expression of Flavonoid and Chlorogenic Acid Biosynthetic Pathways Genes in Tomato Against Tomato Mosaic Virus. Sci. Rep. 2019, 9(1), 9692. DOI: 10.1038/s41598-019-46281-x.
- Rawat, S.; Jugran, A.; Giri, L.; Bhatt, I. D.; Rawal, R. S. Assessment of Antioxidant Properties in Fruits of Myrica esculenta: A Popular Wild Edible Species in Indian Himalayan Region. Evid.-Based Compl. Alternat. Med. 2011, 2011, 512787. DOI: 10.1093/ecam/neq055.