?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The Juglans regia root aqueous extract (JRRAE) phytochemical variability and the investigation of their antimicrobial, antibiofilm, insecticidal, and anti-leishmanial properties were studied. As a result, the JRRAE chemical profile revealed the presence of biomolecules using UV, HPLC, and FT-IR analysis. The investigations showed marked antibacterial activities, highlighted inhibited biofilm formation, induced lethality Leishmania amazonensis promastigotes (50%), and possess an insecticidal activity at 100 µg/ml. A molecular docking study was performed against S. aureus gyrase, S. aureus tyrosyl-tRNA synthetase, human peroxiredoxin 5, cyclooxygenase-2, and trypanothione reductase and confirmed that the activities are mainly due to coumarin, epicatechin, juglone, epicatechin, and epicatechin presence, respectively. The stability of these complexes is essentially related to the strong hydrogen bonds formed between the most active compounds and the binding site amino acids. The identified compounds were found to pass the absorption, distribution, metabolism, and excretion property as well as five Lipinski’s rules with good drug-likeness and pharmacokinetic behavior.
Introduction
The recourse to higher plants and their preparations to cure infectious diseases is an age-old practice. Recently, different traditional medicinal plant extracts have been scientifically evaluated.[Citation1,Citation2] It is widely recognized that plant therapeutic properties are due to their derivative bio-active compounds. Indeed, several studies have demonstrated that phenolic compounds are endowed with human health benefits.[Citation3,Citation4] Currently, the new plant application search is of great interest, especially when it comes to the creation of natural plant protection projects for sustainable agriculture. Indeed, “Green Pesticides” concept refers to all types of natural tools, focused on beneficial pest control that can reduce pest population and increase food production. They are harmless, eco-friendly, and more compatible with environmental components than synthetic pesticides.[Citation5] Walnut tree (Juglans regia) is the most widespread tree nut in the world of great economic interest. The plant is a well-known plant species used in folk medicine.[Citation2] In Tunisia, J. regia is known as the “Swak” tree and its bark is frequently used for teeth cleaning and for good oral hygiene maintenance.[Citation6] It has been recognized that various J. regia plant parts are medicinally useful for a wide array of diseases including diarrhea, sinusitis, stomach ache, arthritis, asthma, eczema, scrofula, skin disorders, diabetes mellitus, anorexia, and thyroid dysfunction.[Citation7] Antifungal, antibacterial, anthelmintic, antiparasitic, antioxidant, antidepressant, anti-inflammatory, anticancer, and antihypoxic activities of different J. regia extracts were reported. Biological properties of walnut tree are related to its chemical composition[Citation2]; however, the use of herbal medicines must be evidenced by toxicological data.[Citation7]
To the best of our knowledge, there is no available study regarding the J. regia root bark potency. In this context, the aim of the present study was to characterize and to identify the presence of bioactive molecules in J. regia root bark using UV, HPLC, and FT-IR analysis. The in vitro antimicrobial, anti-biofilm, and antileishmania properties as well as in vivo insecticidal activity were also investigated. A computational study has also been done to corroborate the in vitro results.
Material and methods
Standards and reagents
Folin-Ciocalteu reagent, 3,5-dinitrosalicylic acid (DNS) reagent, ethanol, methanol, and hexane were purchased from Sigma-Aldrich, PubChem, and Fluka. All other used chemicals were of analytical grade and purchased locally.
Microorganisms
In this study, 11 reference bacterial strains including Bacillus cereus (ATCC 14,579), Escherichia coli (ATCC 35,218), Enterococcus faecalis (ATCC 29,212), Salmonella enterica serovar Typhimurium (ATCC 1408), Staphylococcus aureus (ATCC25923), Staphylococcus epidermidis CIP (106510), Listeria monocytogenes (ATCC 19,115), Micrococcus luteus NCIMB (8166), Pseudomonas aeruginosa (ATCC 27,853), Vibrio alginolyticus (ATCC 33787), and Vibrio parahaemolyticus (ATCC 1780) were used.
Plant material and extraction procedure
J. regia fresh root bark was collected from different trees in the region of Fernana (North of Tunisia) (Latitude: 36 38’ 59.99“N, Longitude: 8 41’ 59.99” E) in March 2015. The species were identified and deposited at the herbarium of the Faculty of Sciences of Gafsa. Samples were dried at room temperature and separately ground into powder and then stored in a smoked bottle at 4°C. The plant extract was prepared by infusing 20 g of the powdered healthy and dried material in 200 mL distilled water for 48 h, and the obtained solution was filtered (Whatman no.1) and then lyophilized.[Citation8] The extraction yield (X0) was calculated as the percentage of mass of extract (mExtract) relative to total mass of raw material (RM), on wet basis (mRM), according to Equation:
Phytochemical screening
Qualitative analysis of secondary metabolites of the plant extract
The qualitative analysis of powdered extract was carried out with reference to phytochemical testing for the presence of various compounds by standard methods like alkaloids, glycosides, flavonoids, anthocyanins, tannins, phenolic compounds, coumarins, saponins, anthraquinone, quinones, proteins, reducing compounds, inulins, starch, resins, organic acids, carboxylic acid, oxalates, terpenoids, steroids, and sterols.[Citation9–11]
Phenolic compounds analysis
The total phenol content was estimated using the method described by Kassim et al. (2011).[Citation12] Briefly, 200 μl of aqueous extract was mixed with 1 ml Folin-Ciocalteu reagent. Then, after 5 min in the dark, 0.8 ml of 7% Na2CO3 was added. The absorbance was read at 760 nm after 30 min in darkness.
The total flavonoid content was estimated using the method of Kassim et al. (2011).[Citation12] To 500 μl of aqueous, 1500 μl of distilled water was added. At t = 0, 75 μl of 7% NaNO2 was added. After 5 min 75 μl of 10% AlCl3 was added and at 6 min 500 μl of NaOH (1 N) and 250 μl of distilled water were added. After incubation for 15 min at room temperature (23 ± 2°C), the absorbance was measured at 510 nm. Total phenolic and flavonoid compounds were calculated using a gallic acid and quercetin standard curve (concentration range: 50–200 μg/ml) and results were shown to be mg gallic acid or quercetin equivalents/g dry matter.
The flavonol levels were determined using the method of Almaraz-Abarca et al. (2007).[Citation13] Rutin calibration curve (100–750 µg/ml) was used, and results were shown to be mg rutin equivalent/100 g of dry matter. The anthocyanin quantification was performed using the pH differential method[Citation14] based on the anthocyanin color changes depending on pH. Each sample absorbance was measured at 520 nm and 700 nm. The anthocyanin concentration (mg/l) was calculated according to the following formula and expressed as Cyanidin-3-glucoside equivalents:
With: A is the absorbance = (A λ520 - A λ700) pH = 1 – (A λ520 - A λ700) pH = 4.5
Where MW is molecular weight of cyanidin 3-glucoside = 449.2 g/mol, DF is the dilution factor (DF = 10), Ε is the extinction coefficient of cyanidin 3-glucoside = 26 900 L mol−1 cm−1, d = 1 cm: length of the cell.
Biochemical composition: Total carbohydrates were obtained using the DNS method.[Citation15] Total protein and nitrogen content was determined using the Kjeldahl method.[Citation16]
UV-Vis absorption spectrum: The ultraviolet-visible absorption spectrum of J. regia root bark aqueous extract was recorded by a UV-Vis spectro-photometer (JENWAY/7315, United Kingdom) with the wavelength range of 200 to 800 nm at 25 °C.
Fourier-transform infrared spectroscopy (FT-IR) analysis: Sample was recorded by FTIR spectroscopy (VERTEX 70 FTIR). The sample was analyzed with an ATR A225 diamante using the middle infrared range (400–4000 cm−1). The obtained data were recorded with OPUS 7.2 software.
The quantitative and qualitative analyses of the plant extract were performed by a reverse-phase HPLC on a liquid chromatography (Shimadzu, Kyoto, Japan) equipped with a vacuum degasser (DGU-14A), a quaternary pump LC-10AT, a UV–Vis detector ((DAD) SPD-M20A), a manual injector (Rheodyne), and an LC-solution (CBM-20A) software for acquisition and processing of chromatogram. The separation was performed on a C18 column (Shimadzu CLC-ODS (M) Kyoto, Japan: 4.6 mm × 250 in length; 5 microns internal diameter) preceded by a guard column (Shimadzu G-ODSs (4), Kyoto, Japan: 4.6 mm × 12.5 mm). The injection volume was 20 µL. Organic acids are eluted with a gradient of phosphoric acid (0.1%) and methanol. During this analysis, the gradient increases linearly from 0 to 5% methanol for 10 min. After the next 10 min, the gradient will increase to 30% methanol, then up to 50% after the other 10 min. This state will last only 5 min and will end with the restoration of the initial situation following the decrease in the gradient from 50% to 10% of methanol. The last 5 min was used to recondition the column. The flow rate was 1.0 ml/min. The compound identification was done by comparing their retention times and the UV–Vis spectra to standard solutions (gallic acid, juglone, catechin, vanillic acid, epicatechin, and coumarin) used in the same conditions.
Antimicrobial activity assessment
MIC and MBC determination: Minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) were carried.[Citation10] Briefly, 0–512 µg/mL of J. regia plant extract diluted in Mueller-Hinton medium were added to a final total volume of 100 µL per well in 96-well microtiter plates. Each tested strain (100 µL), at a final concentration of 106 CFU/mL, was added to each well and incubated at 37 °C. The medium and chlorhexidine (CHX, 0.1% (w/v)) were used as the non-treated and positive controls, respectively. MIC was defined as the lowest concentration of the extract that completely inhibited growth in comparison with the non-treated control. MBC was defined as the lowest concentration that did not allow visible growth when 10 µL of the well contents were plated on agar and grown at 37 °C. All experiments were repeated thrice in duplicate.
Inhibition of the intestinal flora: The intestinal bacterial flora isolation from the fecal matter contained in the Wistar male rats large intestine, treated with 200 mg/kg of the extract, was performed. Three separate culture media named Hektoen,[Citation17] EMB,[Citation18] and Chapman[Citation19] were used.
Antibiofilm assay
The J. regia aqueous extract (100 µg/mL) effect on biofilm formation of each representative pathogenic bacterium was tested in ELISA plate (96-well).[Citation20] Briefly, two-fold plant extract serial dilutions were prepared with a final concentration of 100 µg/mL. A cell suspension of the tested strains was prepared as described in the MIC assay, and 100 µL (106 CFU/mL) were inoculated in each well of a 96-well plate. A 0.1% CHX, phosphate buffered saline (PBS) and extract free medium (Mueller-Hinton medium supplemented with 2% glucose (w/v)) were used as the positive, non-treated and blank controls, respectively. After incubation at 37 °C for 48 h, supernatants were discarded and washed 3 times with PBS and were dried at room temperature for 45 min. The cell adhesion rate was indirectly evaluated using the crystal violet staining.[Citation20]
Antileishmania activity assay
The plant extract, dissolved in RPMI medium (20, 40, 60, 80, and 100 mg/mL), was distributed into the tubes containing each 400,000 parasites. The procedure was performed in triplicate for each tested concentration. The used strain was obtained from a patient’s single lesion of cutaneous leishmaniasis and was identified as Leishmania amazonensis following its speciation using 12 isoenzymes.[Citation21] In the three control tubes, 1 mL of the complete RPMI medium was added. The Leishmania number was determined in each tube immediately using a hemocytometer. A parasite recount was performed in each tube after 48 h of incubation at 24–26°C in dark. Results were displayed as the concentration that inhibited parasite growth by 50% (IC50) compared with control tubes.[Citation22]
Insecticidal activity
Bioassay
The larvicidal activity of J. regia aqueous extract against neonatal S. littoralis larvae was assessed by dose-mortality bioassays.[Citation23] Mortality was recorded after 6 days, and the LC50 was calculated by Probit analysis using programs written in the R. language.[Citation24]
The histopathological effect of J. Regia extract in the midgut of S. littoralis: The histopathological effect of J. regia aqueous extract on S. littoralis midgut larvae was determined.[Citation25] The images were observed and photographed using a light microscope (Olympus Optical Co. LTD) operating at Olympus DP70 camera.
Docking studies
The organic extract contains some of the known bioactive compounds such as gallic acid (GA), juglone, catechin, vanillic acid (VA), coumarin, and epicatechin. The extract displays some potent antibacterial, antioxidant, anti-inflammatory, and anti-leishmania activities. To identify the most bioactive compounds for each biological activity, molecular docking studies have been performed. The bioactive compounds were docked into the active sites of S. aureus gyrase (PDB code 2 × CT) and S. aureus tyrosyl-tRNA synthetase (PDB code 1JIJ) for antibacterial activity,[Citation26,Citation27] human peroxiredoxin 5 (PDB code 1HD2) for antioxidant activity,[Citation28] cyclooxygenase-2 (PDB code 1C × 2) for anti-inflammatory activities,[Citation29] and trypanothione reductase (TR) from Leishmania infantum (PDB code 2JK6) for anti-leishmania.[Citation30] Molecular docking calculations were performed using the Autodock package.[Citation31] The re-docking of the original ligands into the binding sites of S. aureus gyrase (PDB code 2 × CT) and S. aureus tyrosyl-tRNA synthetase (PDB code 1JIJ), human peroxiredoxin 5 (PDB code 1HD2), cyclooxygenase-2 (PDB code 1C × 2) for anti-inflammatory activities, trypanothione reductase (TR) (PDB code 2JK6) is well reproduced with RMSD values less than 2.00Å. The stepwise molecular docking study is reported in our previous study.[Citation32]
ADME and molecular target predictions
Pharmacokinetics and druglikeness are crucial steps in drug design.[Citation33] The investigation of the identified bioactive molecules was performed through the freely available online Swiss ADME web tool.[Citation32,Citation34] Toxicity predictions were assessed using the pkCSM server (http://biosig.unimelb.edu.au/pkcsm/prediction). Molecular target predictions are important for finding phenotypical side effects or potential cross reactivity caused by the action of small biomolecules and were obtained by using the web tool (http://www.swisstargetprediction.ch/) and entering the smile formats of the desired drugs to obtain the targets. The prediction concerns the putative targets of the given molecule by utilizing 2D and 3D similarity index with known ligands.
Statistics
Statistical analysis was performed using GraphPad Prism Software (version GraphPad Prism 7.0, Northampton, MA, USA) using one-way analysis of variance (ANOVA) followed by the Tukey’s test. The differences were considered significant at p < .05.
Results and discussion
Extraction yield
The use of different polarity solvents is highly recommended to extract various phyto-phenolic compounds with high degree of accuracy. In addition, due to their diverse chemical properties influencing the solvent solubility, no particular solvent is suggested for the optimum recuperation of the bioantioxidants. In fact, the extract obtained from water exhibited an antioxidant better capacity with absence of toxicity.[Citation35] Results showed that the obtained yield of Juglans regia was 11.3%, which is higher than other plants from several families such as Zygophyllum album roots (6.10%).[Citation36] The fluctuations between values could be attributed to genetic variability of these plants, which affect the synthesis of bioactive metabolites.[Citation8]
Phytochemical qualitative analysis
Nature has been a huge source of medicinal agent for thousands of years, and an impressive number of modern drugs have been isolated from these sources.[Citation37] Plants have the ability to produce a large variety of secondary metabolites. In the present study, qualitative analysis of J. regia root bark aqueous extract shows the high content presence of flavonoids, tannins, anthraquinones, oxalates, phenols, proteins, inulins, and reducing sugar with lower levels of saponins, terpenoids, alkaloids, coumarins, resins, quinones, organic acids, starch, and anthocyanins (). These molecules are reported to have various physiological effects and medicinal properties. Notably, saponins are often used as intracellular histochemical dyes and as mild detergents.[Citation38] Terpenoids and flavonoids are used to treat inflammation, allergy, oxidative stress, and cancers. Further, the presence of glycosides enhances cardiac output and lowers heart diseases.[Citation11]
Table 1. Qualitative phytochemical tests of the JRRAE.
Phenolic compounds contents in J. regia extract
Phenolic compounds are the main predominant bioactive molecule in plants. In the current study, a preliminary screening was carried out () then dosages, using a spectrophotometer, were done for more detailed analyses (). The total phenolic compound content of the J. regia root bark aqueous extract was estimated to 668.38 ± 42.21 mg gallic acid equivalent (GAE) per 100 g of dry weight (DW), which is richer in polyphenols than the previously published content of an ethyl acetate extract 3483.3 mg GAE/100 g DW.[Citation6] The differences are attributed to many factors that could modify the root composition, including the climate conditions, origin, ripeness of the samples, and[Citation38] as well as extraction methods.[Citation6]
Table 2. Physicochemical characteristics of JRRAE.
The total flavonoid, flavonol, and anthocyanin contents were 267.75 ± 12.31 mg QE/100 g DW, 68 ± 3.23 mg of RE/100 g DW, and 29 ± 1.45 mg/L, respectively. The obtained results confirmed that J. regia is an important potential source of these bioantioxidants with values even greater than those reported for many medicinal species. Chromatographic analysis () revealed the presence of severals identified compounds, among them gallic acid, vanillic acid were the pre-dominant components. Flavonoids such as catechin and epicatechin have also been detected.
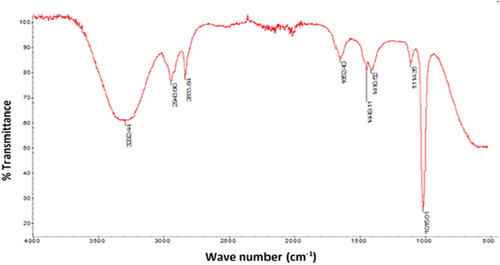
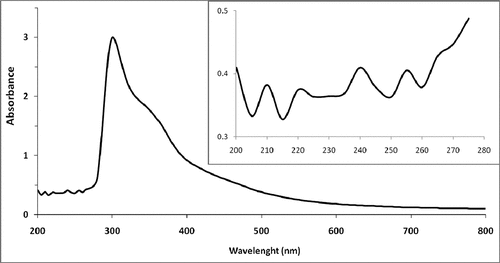
Figure 1b. UV-VIS spectra of JRRAE, HPLC chromatogram of JRRAE: (1) gallic acid, (2) juglone, (3) catechin, (4) vanillic acid, (5) epicatechin, (6) coumarin.IR spectrum of JRRAE.
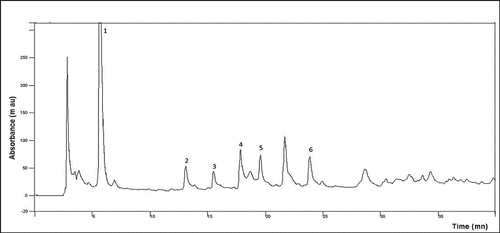
The obtained results are in agreement with the qualitative screening results as well as with literature data[Citation4,Citation7] which reported the clear occurrence of phenolic compounds in J. regia peculiarly phenolic acids and flavonoids. In the literature, there were no reports of individual phenolic compounds in J. regia water extract, but previous studies on other species suggested that phenolic compound composition can fluctuate between locations and solvent extract.[Citation39] It was worth noting that many peaks remained unidentified and their identification can offer numerous information about this species. The presence of all these beneficial and promising compounds in J. regia water extract would be of great importance to the pharmaceutical and food industries.
Sugar and protein content
To the best of our knowledge, this was the first reports about the phytochemical content (proteins and sugars) of J. regia root bark. Results in shows that the samples were rich with protein (ca. 31.39 ± 1.173 mg/mL) and contained important level of reducing sugars (17.986 ± 13.18 mg/mL).
The protein levels and carbohydrate content found here were lower than those reported from other species roots. In fact, the maturation stage, temperature, sun exposure, and climatic conditions played a major role in the plant protein content.[Citation40] Indeed, proteins, as an essential component of the diet of humans and other mammals, from unconventional plants should be explored and used as a raw material source for different population sectors.[Citation41] Furthermore, sugar content offer to J. regia an industrial importance. Recently, research has increased to investigate new sources of vegetable sugar from underexploited plants.[Citation2]
Spectral analysis of the aqueous extract of J. regia root bark
Ultraviolet absorption (UV) spectrum: As shown in , aqueous extract of J. regia root bark showed an absorption at 265 nm in the UV absorption spectra, suggesting the possible presence of a multitude of compounds such as phenolic compounds, mainly flavonoids (flavonols and their glycosyl derivatives), and at 500 nm that show the presence of anthocyanin, offering the natural red color.[Citation42] In accordance with data reported by Nour et al. (2014), a large absorbance peak at 500 nm was observed, which is a characteristic of naphthoquinones, namely juglone (5-hydroxy-1,4-naphthoquinone) which is unique to walnut.[Citation4] This latter shows a significant absorption in both the visible and UV domains with λmax = 420 nm[Citation43] and λmax = 255 nm,[Citation44] respectively. Also, a small peak observed in 280 nm and 375 nm is attributed to a trace of catechin and quercetin, respectively. This result was confirmed as previously reported.[Citation44]
Fourier infrared spectroscopy analysis (FT-IR)
The FTIR spectrum elucidates the major functional groups of polymers and determines their structural information. The FT-IR spectrum of the J. regia root bark aqueous extract is shown in and reveals a specific peak between 500 and 1500 cm−1 for phenolic and aromatic compounds. Peak at 1449 cm−1 indicates the presence of C = C bond of aromatic compound. The observed band at about 3300 cm−1 corresponds to O-H, as well as the H-bonded alcohols and phenols.[Citation12] Absorption of C-H band of CH2 and CH3 groups and O-H band of carboxylic acids were observed near 2943 cm−1. The absorption peaks at 1016, 1115, and 1410 cm−1 testify the C-O stretching of carboxylic acids, alcohols, and esters. Additionally, a peak at 1652 cm−1 revealed a characteristic band for the carbonyl group C=O.[Citation45] Another one at 2833 cm−1 has been observed due to the conjugation of C = C and C=O of carboxylic acid. These results are in agreement with the UV-Vis spectrum as well as with the HPLC spectrum.
The HPLC spectrum of the aqueous extract of J. regia root bark is shown in . Phytochemicals were separated and detected at 280 nm for catechin (+), epicatechin (-), tyrosol, and hydroxybenzoic acids,[Citation46] at 320 nm for p-coumaric, caffeic, caftaric, and ferulic acids, at 306 nm for trans-resveratrol, at 360 nm for myricetin, quercetin, and kaempferol, and at 420 nm for juglone [43]. Indeed, the chromatogram shows () the presence of phenolic compounds and their derivatives, namely gallic acid and vanillic acid. Flavonoids such as catechin and epicatechin have also been detected. The areas of the obtained peaks reveal a difference between the phytochemical amounts. This clearly demonstrates that phenols amounts depend on organ age, maturation state, and environment.[Citation44] Interestingly, juglone and coumarin were detected in the aqueous extract of J. regia root bark. The obtained results are in agreement with previous UV–visible spectrum and qualitative screening results as well as with literature data[Citation4,Citation7] which reported the occurrence of phenolic compounds in J. regia peculiarly phenolic acids and flavonoids. Nevertheless, other compounds have been detected, but we could not identify them for lack of standard solutions.
Antimicrobial activity assessment
Evaluation of MIC and MBC using the microdilution method
This study was conducted on 11 reference strains, and results are shown in . According to our findings, there is a series of fluctuations in MIC and MBC values varying with bacteria species. Staphylococcus aureus is the most sensitive strain toward the aqueous extract of J. regia root bark with MIC and MBC of 8 µg/mL and 16 µg/mL, respectively. In contrast, the Vibrio species are the most resistant with MICs and MBCs ranging from 256 to 512 µg/mL, respectively. This fluctuation can probably be linked to the unconformity of the cell wall and membrane structure and composition. Indeed, Gram-negative bacteria have an outer membrane that serves as a barrier for many molecules. This antimicrobial action of the bark extract may be more pronounced on Gram-positive than Gram-negative bacteria was also confirmed.[Citation47] Moreover, the presence of an efflux pump system has been established and can generate the resistance to natural compounds.[Citation1] Broadly, our results are in agreement with previous studies showing the potent antimicrobial effect of J. regia is mainly attributed to the presence of numerous flavonoids, which act essentially by enzymatic inhibition of DNA gyrase,[Citation11,Citation48] juglone,[Citation7,Citation43,Citation49] alkaloids,[Citation50] and saponins[Citation11] as bioactive antibacterial agents. Additionally, J. regia water extract showed an antibacterial activity similar to that of chlorhexidine.
Table 3. Antibacterial activity and effect of JRRAE on pathogenic bacterial adhesion evaluated by the crystal violet test.
Inhibition of the intestinal flora
Results of bacterial enumerations in intestinal flora are presented in . As expected, the extract showed an inhibitory effect against bacteria of the intestinal flora, proving once again the antibacterial activity of J. regia root bark water extract. This is in agreement with our previous MIC and MBC described results. Additionally, we noticed that inhibition of some bacteria by the extract has favored the development of others. Enterococci enumerated on Hektoen selective medium in the small intestine of control rats were 99 103 ± 0.816 bacteria/cm2, whereas they were totally absent in the treated rats. Moreover, the number of Enterobacteriaceae on EMB medium increased in the small intestine of control rats from 98 106 ± 3.949 bacteria/cm2 to 9,994.106 ± 8.256 bacteria/cm2 in treated rats. Similar observations were made for fecal matter in the large intestine. However, Staphylococci were absent in the small intestine of treated rats as well as controls. The aqueous extract causes the total disappearance of Staphylococci in the large intestine fecal matter of treated rats. The sensibility of Staphylococci was demonstrated again by Alkhawajah (1997).[Citation47] Another time, the antibacterial activity may be attributed to the presence of numerous molecules such as flavonoids, juglone,[Citation7,Citation43] alkaloids,[Citation50] and saponins[Citation11] as bioactive antibacterial agents.
Table 4. Bacterial count in the small intestine and feces in control and treated rats for 10 days by JRRAE at 200 mg/kg.
Antibiofilm activity assessment
Meanwhile, medicinal herbs have proven their valuable biological properties. Indeed, plant-derived compounds could influence biofilm formation by inhibiting peptidoglycan synthesis, destroying microbial membrane structures, and modulating quorum sensing.[Citation1] Antibiofilm activity of J. regia extract was carried out on 11 referenced strains, and the results are shown in . According to our findings, 100 µg/mL of extract induced a biofilm formation inhibition as well as an elimination of the preformed biofilm. Inhibition is evaluated by bacterial biomass decrease compared to control after crystal violet staining. The aqueous extract showed varying biofilm inhibition percentages ranging from 23% for Staphylococcus aureus ATCC 25,923 to 90% for Vibrio alginolyticus ATCC 33,787. Furthermore, the biofilm proportion varies considerably according to the environmental conditions[Citation1] and especially according to the physicochemical properties of the involved microorganisms. Indeed, suspension medium characteristics, such as pH, concentration, and temperature, are also considered as important factors that can influence the physicochemical properties of the substrate and bacterial surfaces[Citation1] and consequently microbial adhesion and biofilm formation. We can conclude from the given study that J. regia extract effectively penetrates into biofilms and causes lyses of test microorganisms. Previous reports confirmed the influence of J. regia on biofilm formation inhibition. In fact, J. regia extracts inhibit bacterial adhesion only by inhibition of glucan synthesis.[Citation48] Despite numerous reports that focused on the antimicrobial properties of plant extracts and essential oils, few studies have reported the antibiofilm activities of these compounds. Isolation and identification of effective compounds might be crucial to include them as alternatives in the control of biofilms, peculiarly in the medical field as well as in the food industries.[Citation20]
Antileishmania activity assay
The present study provides data on the effectiveness of the aqueous extract of J. regia root bark against Leishmania amazonensis. Results reveal that at 100 mg/mL, the extract has a potential effect on parasite viability and induced lethality to 50% of promastigotes. However, few studies were conducted on walnut leaf antileishmanial activity. To the best of our knowledge, this is the first report on the antileishmanial activities of J. regia root bark aqueous extract. Serakta et al. (2013) reported a significant reduction of the promastigote from Leishmania major in the presence of J. regia hydroalcoholic bark extract.[Citation51] Moreover, the ointment-based J. regia leaf extract induced a significant post-treatment decrease in the lesion size and parasite count of Leishmania major in infected animals, compared to control groups.[Citation52] These data are in agreement with our findings, which pave the way for further research in leishmaniasis curing.
It is important to mention that several factors such as drug toxicity, method of administration, and resistance of Leishmania spp to conventional remedies limit the leishmaniasis treatment.[Citation52] In fact, J. regia is frequently used for the management of parasitic diseases in central Italy and Morocco.[Citation51] Hence, this work proved that the aqueous extract of J. regia showed a promising anti-leishmanial activity; however, further investigations are needed to determine the mode of action of the bioactive compounds, which are behind the leishmanicidal activity.
Insecticidal activity
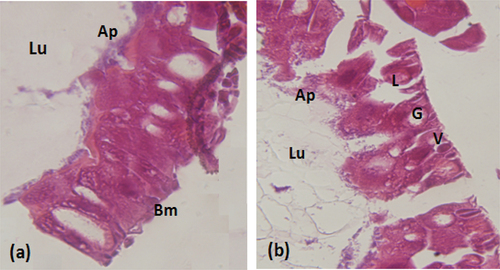
The bioassay of J. regia root bark aqueous extract was performed against the first instar larvae of S. littoralis. The obtained results showed that the plant extract exhibited insecticidal activity against S. Littoralis larvae with an LC50 of 130.99 µg/cm2. Larvae exposure to water, used as negative control, did not cause any mortality. In order to visualize the effect of the extract ingested by the larvae, histological sections were made in the midgut of the treated S. littoralis larvae. As shown in , the J. regia extract caused enlargement of epithelial cells, cytoplasmic vacuolization, appearance of vesicles at the apical part of the cells toward the midgut lumen and disruption of the basement membrane. In contrast, the midgut section of the control larvae showed usual structural organization with a well-developed brush border, a clear cytoplasm, and a normal adhesive basement membrane (). This result is a practical point of view, since S. littoralis is one of the most serious agricultural pests that can attack numerous economically important crops and cause considerable damage on vegetables throughout the year in Africa, Asia, and Europe.[Citation53] To our knowledge, only acaricidal activity of J. regia leaves extracts was reported.[Citation2] Consequently, we report for the first time the insecticidal activity of J. regia root bark aqueous extract showing its potential for use as a biocontrol agent against lepidopteran pests. Delaviz reported that the juglone from leaves, extracted from Juglans sp., is associated with the insecticidal activity.[Citation49]
Figure 2. Histopathological effect of the extract on the intestine of S.littoralislarvae (a): Normal appearance. (b): Histopathological aspect of the intestine of S.littoralistreated larvae. V:vesicles formation L:Lysis of epithelial cells. G:Large vacuolation of epithelial cells. Lu:lumen. Ap:apical membrane. Bm:basal membrane. (Magnification × 40).
Molecular docking
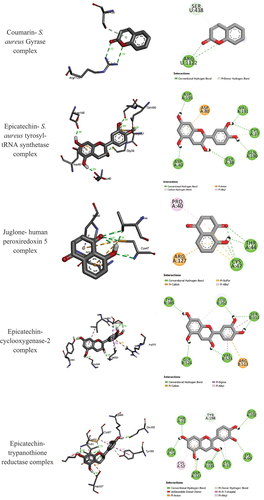
As mentioned in materials and methods, the organic extracts display potent antibacterial, antioxidant, anti-inflammatory, and anti-leishmania activities. The organic extract contains some bioactive compounds. In attempt to identify the most active compound for each biological activity, molecular docking study has been performed to determine the binding affinity of the bioactive compounds gallic acid (GA), juglone, catechin, vanillic acid (VA), coumarin, and epicatechin into the active sites of S. aureus gyrase (PDB code 2 × CT) and S. aureus tyrosyl-tRNA synthetase (PDB code 1JIJ), human peroxiredoxin 5 (PDB code 1HD2), cyclooxygenase-2 (PDB code 1C × 2) for anti-inflammatory activities, trypanothione reductase (TR) (PDB code 2JK6). gathers the calculated binding energies of stable complexes, ligand–receptor, number of conventional intermolecular hydrogen bonding established between the docked compounds and the active site residues of target enzymes.
Table 5. Docking binding energies, conventional hydrogen bonding, and the number of closest residues to the docked compounds into the active sites of S. aureus Gyrase (pdb 2 × CT), S. aureus tyrosyl-tRNA synthetase (pdb 1JIJ), human peroxiredoxin 5 (pdb 1HD2), cyclooxygenase-2 (pdb 1C × 2), and trypanothione reductase (pdb 2JK6).
All the bioactive compounds formed stable complexes into the binding sites of S. aureus Gyrase (pdb 2 × CT), S. aureus tyrosyl-tRNA synthetase (pdb 1JIJ), human peroxiredoxin 5 (pdb 1HD2), cyclooxygenase-2 (pdb 1C × 2), and trypanothione reductase (pdb 2JK6). The negative bending energies indicate that the inhibition is spontaneous and thermodynamically favorable. From the binding energies, one may easily deduce that the potency of the enzymatic inhibition of the bioactive compounds depends also on the target enzyme. For instance, the most stable ligand-receptor for S. aureus Gyrase (pdb 2 × CT), S. aureus tyrosyl-tRNA synthetase (pdb 1JIJ), human peroxiredoxin 5 (pdb 1HD2), cyclooxygenase-2 (pdb 1C × 2), and trypanothione reductase (pdb 2JK6) are obtained coumarin, epicatechin, juglone, epicatechin, and epicatechin with binding energies of −3.32, −7.74, −5.23, −7.97, and −8.43 kcal mol-1, respectively ( and ). Thus, one may conclude that the activity of the extract against S. aureus Gyrase (pdb 2 × CT), S. aureus tyrosyl-tRNA synthetase (pdb 1JIJ), human peroxiredoxin 5 (pdb 1HD2), cyclooxygenase-2 (pdb 1C × 2), and trypanothione reductase (pdb 2JK6) may be due to the presence of coumarin, epicatechin, juglone, epicatechin, and epicatechin, respectively. The stability of these complexes is mainly related to the strong hydrogen bonds formed between the most active compounds and the binding site amino acids of S. aureus Gyrase (pdb 2 × CT), S. aureus tyrosyl-tRNA synthetase (pdb 1JIJ), human peroxiredoxin 5 (pdb 1HD2), cyclooxygenase-2 (pdb 1C × 2), and trypanothione reductase (pdb 2JK6) ().
Pharmacokinetic and druglikeness properties
In order to explore the drug likeliness of the most promising compounds and reduce the risk of drug attrition in late stage, the ADME property profiling of the identified compounds was investigated using SwissADME software. As outlined in , all compounds satisfy the Lipinski’s rule of five, with TPSA values in the range 30.21–110.38 Å2 manifesting good oral absorption with good bioavailability score. The consensus Log Po/w values were found to be less than 1.82 indicating their good lipophilic character. Their pharmacokinetics show high GI absorption for all compounds with BBB permeation only for juglone and vanillic acid, P-gp substrate for coumarin, and CYP50 inhibitor only for coumarin (CYP1A2) and gallic Acid (CYP3A4).
Table 6. ADME properties of the identified compounds.
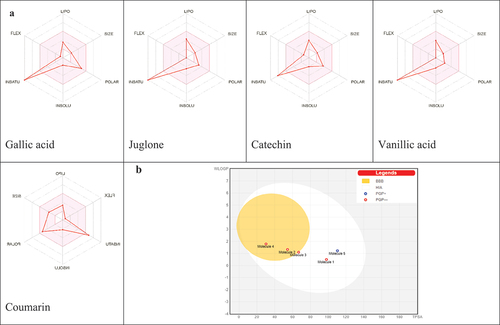
The BOILED-Egg graph for evaluation of passive gastrointestinal absorption and brain penetration and the bioavailability radar () to establish the drug likeness of compounds of have been also summarized. Results of the BOILED-Egg graph () clearly revealed that only coumarin appears with blue color in the white region meaning its high probability of passive absorption by the gastrointestinal tract and was exclusively a substrate for P-glycoprotein (PGP+), however those of gallic acid, juglone, catechin, and vanillic acid appeared in red color and therefore were P-glycoprotein (PGP-) with juglone and vanillic acid in the yolk region suggesting high probability to permeate through BBB to access CNS meanwhile catechin in the white region with high probability to be passively absorbed by the gastrointestinal tract. The druglikeness behavior was assessed by the bioavailability radar () for oral bioavailability prediction. The results of the druglikeness behavior revealed that all compounds are outside the pink area of the polygon only by one parameter suggesting their good oral bioavailability.
Figure 4. (A) Bioavailability radar of identified compounds based on physicochemical indices ideal for oral bioavailability. LIPO, Lipophilicity: −0.7 < XLOGP3 < þ 5; SIZE, Molecular size: 150 g/mol < mol. wt.<500 g/mol; POLAR, Polarity: 20 Å2 < TPSA<130 Å2; INSOLU, Insolubility: 0 < Log S (ESOL) < 6; INSATU, Insaturation: 0.25 < Fraction Csp3 < 1; FLEX, Flexibility: 0 < Number of rotatable bonds<9. The colored zone is the suitable physicochemical space for oral bioavailability. (B) Boiled-egg (B) model of compounds.
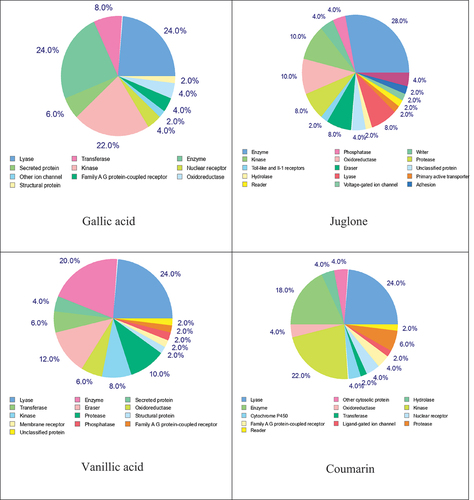
In order to estimate their targets and to and to rationalize their possible side-effects, the pie-chart () of the identified compounds has been predicted. The results of the target prediction analysis showed that the pie chart for gallic acid predicts a high level of 24% of lyase and enzyme and 22% of kinase. The pie chart for juglone predicts 28% of enzyme and 10% of kinase and oxidoreductase. Vanillic acid has attraction toward the specific binding site of lyase (28%) followed by enzyme (20%), eraser (12%), and protease (10%); however, coumarin may be binds preferentially to lyase (24%), kinase (22%), and enzyme (18%).
Conclusion
To the best of our knowledge, the results of the current work were the initial data on the characterization and biological properties of J. regia aqueous extract. Phytochemical characterization of J. regia aqueous extract by spectral analyses (UV, HPLC, and FTIR) was done, and several active phytochemicals have been found, such as flavonoids, hydrolysable tannins, reducing compounds, saponins, terpenoids, and starch. The J. regia aqueous extract exhibited a strong antibacterial inhibition activity toward several pathogen strains, a marked inhibition of biofilm formation and induction of lethality of Leishmania amazonensis promastigotes, for the first time. In addition, J. regia showed the potential to possess insecticidal activity mentioned for the first time. These actions are most likely mediated by their phytochemical constituents. Based on preliminary an in silico analysis, our promising findings supported very well the in vitro data and could become a basis for further studies at in vitro and in vivo levels in order to use these compounds as potential remedies to cure various diseases. Therefore, the outcomes obtained afford a new bioactive base for the development of J. regia as accessible therapeutic approach against oxidative stress-related diseases.
Acknowledgments
The authors thank the Faculty of Sciences of Gafsa for doing the work on their laboratories. Open Access funding provided by the Qatar National Library.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Kouidhi, B.; Al Qurashi, Y. M. A.; Chaieb, K. Drug Resistance of Bacterial Dental Biofilm and the Potential Use of Natural Compounds as Alternative for Prevention and Treatment. Microb. Pathog. 2015, 80, 39–49. DOI: 10.1016/j.micpath.2015.02.007.
- Shah, I. T.; Sharma, E.; Ahmad, G. Juglans regia Linn: A Phytopharmacological Review. World J. Pharm. Sci. 2014, 25, 364–373. ISSN (Print ISSN 2321-3310)
- Alminderej, F.; Bakari, S.; Almundarij, T. I.; Snoussi, M.; Aouadi, K.; Kadri, A. Antioxidant Activities of a New Chemotype of Piper cubeba L. Fruit Essential Oil (Methyleugenol/Eugenol): In Silico Molecular Docking and ADMET Studies. Plants. 2020, 9, 1534. DOI: 10.3390/plants9111534.
- Nour, V.; Trandafir, I.; Cosmulescu, S. HPLC Determination of Phenolic Acids, Flavonoids and Juglone in Walnut Leaves. J. Chromatog. Sci. 2014, 5, 883–890. DOI: 10.1093/chromsci/bms180.
- Koul, O.; Walia, S.; Dhaliwal, G. Essential Oils as Green Pesticides: Potential and Constraints. Biopestic. Int. 2008, 4, 63–84.
- Noumi, E.; Snoussi, M.; Trabelsi, N.; Hajlaoui, H.; Ksouri, R.; Valentin, E.; Bakhrouf, A. Antibacterial, Anticandidal and Antioxidant Activities of Salvadora Persica and Juglans Regia L Extracts. J. Med. Plants Res. 2011, 5, 4138–4146. DOI: 10.5897/JMPR.9000133.
- Panth, N.; Paudel, K. R.; Karki, R. Phytochemical Profile and Biological Activity of Juglans Regia. J. Integr. Med. 2016, 14, 359–373. DOI: 10.1016/S2095-4964(16)60274-1.
- Tlili, N.; Yahia, Y.; Feriani, A.; Labidi, A.; Ghazouani, L.; Nasri, N.; Khaldi, A.; Khaldi, A. Schinus terebinthifolius Vs Schinus molle: A Comparative Study of the Effect of Species and Location on the Phytochemical Content of Fruits. Ind. Crops Prod. 2018, 122, 559–565. DOI: 10.1016/j.indcrop.2018.05.080.
- Leke, L.; Abah-Onaji, R.; Ahmad, G.; Monday-Uchenna, O. Phytochemical Screening and Anti-Microbial Activity Studies of the Root Extract of Anthocleista djalonensis (Cabbage Tree). Inter. J. Chem. 2012, 4, 37–44. DOI: 10.5539/ijc.v4n4p37.
- Sharma, A. K.; Gangwar, M.; Kumar, D.; Nath, G.; Sudhir, A.; Sinha, K.; Tripathi, Y. P. Phytochemical Characterization, Antimicrobial Activity and Reducing Potential of Seed Oil, Latex, Machine Oil and Press Cake of Jatropha curcas. Avicenna. J. Plant. 2016, 6, 366–375.
- Sonam, M.; Pawan-Singh, R.; Pooja, S. Phytochemical Screening and TLC Profiling of Various Extracts of Reinwardtia indica. IJPPR. 2017, 9, 523–527. DOI: 10.25258/phyto.v9i4.8125.
- Kassim, M. J.; Hussin, M. H.; Ashmad, A.; Dahon, N. H.; Suan, T. K.; Hamdan, H. S. Determination of Total Phenol, Condensed Tannin and Flavonoid Contents and Antioxidant Activity of Uncaria gambir Extracts. Majalah. Farmasi. Indonesia. 2011, 22, 50–59.
- Almaraz-Abarca, N.; Campos, M. G.; Reyes, J. A. A.; Jiménez, N. N.; Corral, J. H.; Valdez, S. G. Antioxidant Activity of Polyphenolic Extract of Monofloral Honeybee-Collected Pollen from Mesquite (Prosopis juliflora Leguminosae). J. Food Comp. Analys. 2007, 20, 119–124. DOI: 10.1016/j.jfca.2006.08.001.
- Sava, C.; Sirbu, R.; Dumitrescu, C. Analyse qualitative et quantitative des anthocyanes dans des produits naturels. Sci. Study. Res. 2006, 7, 785–798.
- Miller, G. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 1959, 31, 426–428. DOI: 10.1021/ac60147a030.
- Mahmoudabadi, S. K.; Panahi, B.; Agharahimi, J.; Salajegheh, F. Determination of Compounds Existing in Fruits of Three Pistachio (Pistacia vera L.) Cultivars in Kerman Province. J. Biodivers. Environ. Sci. 2012, 6, 81–86.
- King, S.; Metzger, W. A New Plating Medium for the Isolation of Enteric Pathogens II. Comparison of Hektoen Enteric Agar with SS and EMB Agar. Appl. Microbiol. 1968, 16, 579–581. DOI: 10.1128/am.16.4.579-581.1968.
- Levine, M. Differentiation of B. Coli and B. Aerogenes on a Simplified Eosin-Methylene Blue Agar. J. Infect. Dis. 1918, 23, 43–47. DOI: 10.1086/infdis/23.1.43.
- Chapman, G. A Culture Medium for Detecting and Confirming Escherichia coli in ten Hours. Am. J. Public Health. 1951, 41, 1381. DOI: 10.2105/ajph.41.11_pt_1.1381.
- Ceylan, O.; Ugur, A.; Sarac, N.; Sahin, M. D. The Antimicrobial and Antibiofilm Activities of Mentha X Piperita L Essential Oil. J. Bio. Sci. Biotech. 2014, 2014, 23–27.
- Rahi, A.; Ali, M. A.; Al-Marjani, M. F. Characterization of Leishmania Species by Using Isozyme Analysis. IRJET. 2015, 6, 1063–1066. DOI: 10.4269/ajtmh.1980.29.199.
- Ebrahimisadr, P.; Ghaffarifar, F.; Hassan, Z. M. In-Vitro Evaluation of Antileishmanial Activity and Toxicity of Artemether with Focus on Its Apoptotic Effect. Iran. J. Pharm. Res. 2013, 12, 903–909.
- Abdelkefi-Mesrati, L.; Boukedi, H.; Dammak-Karray, M.; Sellami-Boudawara, T.; Jaoua, S.; Tounsi, S. Study of the Bacillus thuringiensis Vip3Aa16 Histopathological Effects and Determination of Its Putative Binding Proteins in the Midgut of Spodoptera littoralis. J. Invertebr. Pathol. 2011, 106, 250–254. DOI: 10.1016/j.jip.2010.10.002.
- Venables, W. N.; Smith, D. M., 2004. The R. Development Core Team. An Introduction to R. Version 1.9.1 http://wwwr-projectorg/
- Ben Khedher, S.; Boukedi, H.; Kilani, O.; Chaib, I.; Laarif, A.; Abdelkefi-Mesrati, L.; Tounsi, S. Bacillus amyloliquefaciens AG1 Biosurfactant: Putative Receptor Diversity and Histopathological Effects on Tuta Absoluta Midgut. J. Invertebr. Path. 2015, 132, 42–47. DOI: 10.1016/j.jip.2015.08.010.
- Bax, B. D.; Chan, P. F.; Eggleston, D. S.; Fosberry, A.; Gentry, D. R.; Gorrec, F.; Giordano, I.; Hann, M. M.; Hennessy, A.; Hibbs, M., et al. Type IIA Topoisomerase Inhibition by a New Class of Antibacterial Agents. Nature. 2010, 466, 935–940. DOI: 10.1038/nature09197.
- Qiu, X.; Janson, C. A.; Smith, W. W.; Green, S. M.; McDevitt, P.; Johanson, K.; Carter, P.; Hibbs, M.; Lewis, C.; Chalker, A., et al. Crystal Structure of Staphylococcus aureus tyrosyl‐tRNA Synthetase in Complex with a Class of Potent and Specific Inhibitors. Prot. Sci. 2001, 10, 2008–2016. DOI: 10.1110/ps.18001.
- Declercq, J. P.; Evrard, C.; Clippe, A.; Vander Stricht, D.; Bernard, A.; Knoops, B. Crystal Structure of Human Peroxiredoxin 5, a Novel Type of Mammalian Peroxiredoxin at 1.5 Å Resolution. J. Mol. Boil. 2001, 311, 751–759. DOI: 10.1006/jmbi.2001.4853.
- Kurumbail, R. G.; Stevens, A. M.; Gierse, J. K.; McDonald, J. J.; Stegeman, R. A.; Pak, J. Y.; Gildehaus, D.; Miyashiro, J. M.; Penning, T. D.; Seibert, K., et al. Structural Basis for Selective Inhibition of Cyclooxygenase-2 by Anti-Inflammatory Agents. Nature. 1996, 384, 644–648. DOI: 10.1038/384644a0.
- Baiocco, P.; Colotti, G.; Franceschini, S.; Ilari, A. Molecular Basis of Antimony Treatment in Leishmaniasis. J. Med. Chem. 2009, 52, 2603–2612. DOI: 10.1021/jm900185q.
- Morris, G. M.; Huey, R.; Lindstrom, W.; Sanner, M. F.; Belew, R. K.; Goodsell, D. S.; Olson, A. J. AutoDock4 and AutoDocktools4: Automated Docking with Selective Receptor Flexibility. J. Comput. Chem. 2009, 30, 2785–2791. DOI: 10.1002/jcc.21256.
- Othman, I. M.; Gad-Elkareem, M. A.; Aouadi, M.; Snoussi, K.; Kadri, A.; Kadri, A. Novel Fused Pyridine Derivatives Containing Pyrimidine Moiety as Prospective Tyrosyl-tRNA Synthetase Inhibitors: Design, Synthesis, Pharmacokinetics and Molecular Docking Studies. J. Mol. Struct. 2020, 1219, 128651. DOI: 10.1016/j.molstruc.2020.128651.
- Ghannay, S.; Bakari, S.; Msaddek, M.; Vidal, S.; Kadri, A.; Aouadi, K. Design, Synthesis, Molecular Properties and in vitro Antioxidant and Antibacterial Potential of Novel Enantiopure Isoxazolidine Derivatives. Arab J. Chem. 2020c, 13, 2121–2131. DOI: 10.1016/j.arabjc.2018.03.013.
- Kadri, A.; Aouadi, K. In Vitro Antimicrobial and α-Glucosidase Inhibitory Potential of Enantiopure Cycloalkylglycine Derivatives: Insights into Their In Silico Pharmacokinetic, Druglikeness, and Medicinal Chemistry Properties. J. App. Pharm. Sci. 2020, 10, 107–115. ISSN: 223-3354
- Ismail, B. B.; Pu, Y.; Guo, M.; Ma, X.; Liu, D. LC-MS/QTOF Identification of Phytochemicals and the Effects of Solvents on Phenolic Constituents and Antioxidant Activity of Baobab (Adansonia digitata) Fruit Pulp. Food. Chem. 2019, 277, 279–288. DOI: 10.1016/j.foodchem.2018.10.056.
- Feriani, A.; Tir, M.; Gómez-Caravaca, A. M.; Contreras, M.; Del, M.; Talhaoui, N.; Taamalli, A.; Allagui, M. S.; Mufti, A.; Tlili, N., et al. HPLC-DAD-ESI-QTOF-MS/MS Profiling of Zygophyllum album Roots Extract and Assessment of Its Cardioprotective Effect Against Deltamethrin-Induced Myocardial Injuries in Rat, by Suppression of Oxidative Stress-Related Inflammation and Apoptosis via NF-Κb Signaling Pathway. J. Ethnopharmacol. 2019, 247, 112266. DOI: 10.1016/j.jep.2019.112266.
- Krishnan, S. Traditional Herbal Medicines – A Review. IJRAR . 2018, 5, 611–614.
- Santhi, K.; Sengottuvel, R. Qualitative and Quantitative Phytochemical Analysis of Moringa concanensis Nimmo. Int. J. Curr. Microbiol. App. Sci. 2016, 5, 633–640. DOI: 10.20546/ijcmas.2016.501.064.
- Chirinos, R.; Pedreschi, R.; Rogez, H.; Larondelle, Y.; Campos, D. Phenolic Compound Contents and Antioxidant Activity in Plants with Nutritional and/or Medicinal Properties from the Peruvian Andean Region. Ind. Crops Prod. 2013, 47, 145–152–66900926–6690. ISSN DOI: 10.1016/j.indcrop.2013.02.025.
- Doukani, K.; Tabak, S. Profil Physicochimique Du Fruit “lendj” (Arbutus unedo L.). Revue. Nature et Technologie. 2015, 7, 51–64.
- Amjad, I.; Khalil, I. A.; Ateeq, N.; Sayyar Khan, M. Nutritional Quality of Important Food Legumes. Food. Chem. 2006, 97, 331–335. DOI: 10.1016/j.foodchem.2005.05.011.
- Kumar, S.; Pandey, A. K. Chemistry and Biological Activities of Flavonoids: An Overview. Sci. World J. 2013, 2013, 1–16. DOI: 10.1155/2013/162750.
- Sharma, N.; Ghosh, P.; Sharma, U.; Sood, S.; Arun, K. S.; Gulati, A. Microwave-Assisted Efficient Extraction and Stability of Juglone in Different Solvents from Juglans regia: Quantification of Six Phenolic Constituents by Validated RP-HPLC and Evaluation of Antimicrobial Activity. Anal. Letters. 2009, 42, 2592–2609. DOI: 10.1080/00032710903202055.
- Hadjmohammadi, M. R.; Kamel, K. Determination of Juglone (5-Hydroxy 1, 4-Naphthoquinone) in Pterocarya fraxinifolia by RP-HPLC. Iran. J. Chem. Eng. 2006, 4, 73–76. DOI: 10.30492/IJCCE.2006.8034.
- Kale, A.; Gaikwad, S. A.; Devare, S.; Nirmala, R. Spectrophotometric Validation and Standardization of a Bioactive Component from J. regia Stem Bark. Int. J. Pharm. Chem. Biol. Sci. 2012, 3, 133–136. ISSN: 2249-9504
- Burin, V. M.; Arcari, S. G.; Costa, L. L. F.; Bordignon-Luiz, M. T. Determination of Some Phenolic Compounds in Red Wine by RP-HPLC: Method Development and Validation. J. Chromatogr. Sci. 2011, 49, 647–651. DOI: 10.1093/chrsci/49.8.647.
- Alkhawajah, A. M. Studies on the Antimicrobial Activity of Juglans regia. Am. J. Chinese. Med. 1997, 25, 175–180. DOI: 10.1142/S0192415X97000202.
- Chaieb, K.; Kouidhi, B.; Ben Slama, R.; Fdhila, K.; Zmantar, T.; Bakhrouf, A. Cytotoxicity, Antibacterial, Antioxidant, and Antibiofilm Properties of Tunisian Juglans Regia Bark Extract. J. Herbs. Spices Med. Plants. 2013, 19, 168–179. DOI: 10.1080/10496475.2012.762818.
- Delaviz, H.; Mohammadi, J.; Ghalamfarsa, G.; Mohammadi, B.; Farhadi, N. A Review Study on Phytochemistry and Pharmacology Applications of Juglans Regia Plant. Pharmacogn. Rev. 2017, 11, 145–152. DOI: 10.4103/phrev.phrev_10_17.
- Kohelová, E.; Maříková, J.; Korábečný, J.; Hulcová, D.; Kučera, T.; Jun, D.; Cahlíková, L.; Jenčo, J.; Šafratová, M.; Hrabinová, M., et al. Alkaloids of Zephyranthes citrina (Amaryllidaceae) and Their Implication to Alzheimer’s Disease: Isolation, Structural Elucidation and Biological Activity. Bioorg. Chem. 2020, 107, 104567. DOI: 10.1016/j.bioorg.2020.104567.
- Serakta, M.; Djerrou, Z.; Mansour-Djaalab, H.; Kahlouche-Riachi, F.; Hamimed, S.; Trifa, W.; Belkhiri, A.; Edikra, N.; Pacha, H. Antileishmanial Activity of Some Plants Growing in Algeria: Juglans regia Lawsonia inermis and Salvia officinalis. African. J. Tradit. Complement. Altern. Med. 2013, 10, 427–430. DOI: 10.4314/ajtcam.v10i3.7.
- Chegeni, S. A.; Ezatpour, B.; Mohebali, M.; Mahmoudvand, H.; Zibaei, M.; Ebrahimzadeh, F.; Rashidipour, M.; Babaei, N.; Dokhaharani, S. C. Effect of Peel and Leaf Extract of Walnut (Juglans regia L.) on Cutaneous Leishmaniasis Caused by Leishmania Major in BALB/C Mice. J. Chem. Pharm. Sci. 2016, 9, 2490–2495.
- Pineda, S.; Schneider, M. S.; Smagghe, G.; Martínez, A.; Stal, P. D.; Vinuela, E.; Valle, J.; Budia, F. Lethal and Sublethal Effects of Methoxyfenozide and Spinosad on Spodoptera Littoralis (Lepidoptera: Noctuidae). J. Econ. Entomol. 2007, 100, 773–780. DOI: 10.1603/0022-0493(2007)100[773:LASEOM]2.0.CO;2.