ABSTRACT
The demand for probiotic-based functional food is increasing globally owing to its health-endorsing attributes. There are various driving forces behind probiotic therapy. However, Intestinal dysbiosis in humans is the prime driving force behind this increasing trend in the consumption of probiotic-based functional food. Probiotics have numerous health potentials, however, their target delivery and stability is a great challenge for food manufacturer. Microencapsulation with various types of coating materials is trending for the target and stable delivery of potential probiotics. There are various encapsulation techniques with pros and cons. The type of probiotic bacteria, encapsulation methods, and coating materials are considered crucial factors to prolong the viability of probiotics under hostile conditions. The current review addresses the opportunities, challenges, and future trends surrounding matrix materials used in probiotic encapsulation. The review also describes the current studies and their findings on the various types of encapsulation materials. This comprehensive review could be a way forward in the selection of efficient and effective wall material for the target delivery of sensitive ingredients.
Introduction
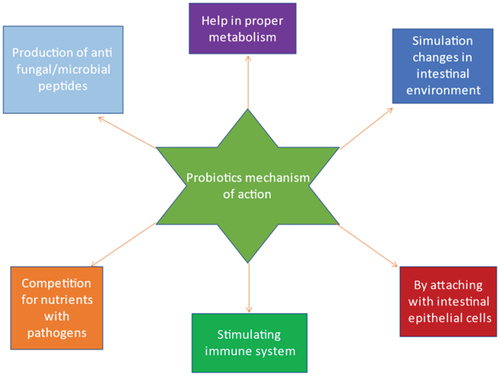
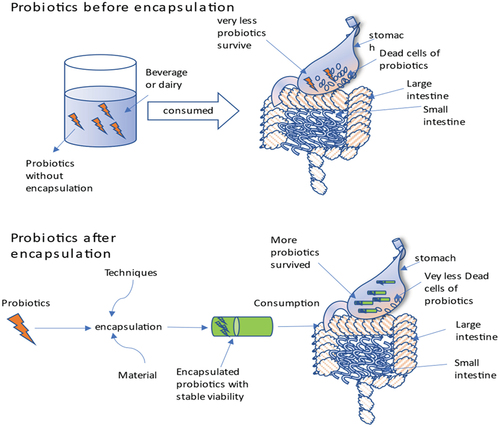
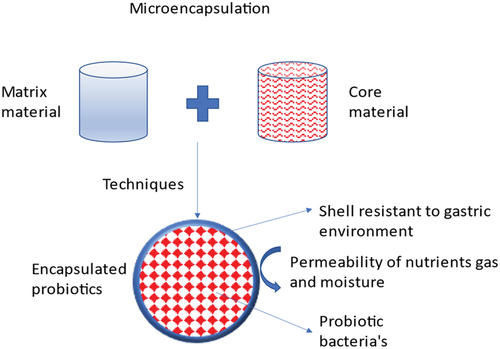
A fast-growing trend is the use of probiotic bacteria in the production of pharmaceuticals and nutritional supplements. As per the definition by the Food and Agriculture Organization (WHO) and World Health Organization of the United Nations (FAO), probiotics are “living bacteria that, when ingested in suitable proportions, confer health advantages to the host” and revised by international Scientific association for probiotics and prebiotics (ISAPP) in 2013 with minor grammatical changes as Live microorganisms that, when administered in adequate amounts, confer a health benefit on the host[Citation1,Citation2]. C-2: References should be in number format. There are many different ways to describe probiotic bacteria, and depending on how they function, they can benefit people’s health.[Citation3] It has been demonstrated that probiotics have several advantageous impacts on the human body, including an anti-pathogenic effect, immune system boosting effects, and antagonistic actions against dangerous bodies. The bacterial culture works by encouraging the development of probiotics that are beneficial to humans in the gut and by strengthening the immune system’s ability to defend against potentially dangerous microbes.[Citation4,Citation5] Several processes have been proposed to explain how these microbes benefit the host’s health are shown in .[Citation6,Citation7] These characteristics enable a significant number of these helpful microbes survive to colonizing in the GIT effectively.[Citation8,Citation9] To boost the body’s health, the amount of live probiotic bacteria in the product at the moment of consumption should be approximately 107 CFU/mL (colony-forming units).[Citation10,Citation11] Lactobacillus and Bifidobacterium are two important microorganisms found in the human colon that work together to help the immune system enhance and repair inflammatory ailments.[Citation12,Citation13] Analysts predict that the probiotics business will be worth more than 3 billion US dollars worldwide in 2024.[Citation14–16] As a result, their effectiveness is closely related to both their quantity and to both the viability during preservation and product shelf-life.[Citation17,Citation18] A comparison of the viability of probiotics before and after encapsulation is shown in . In recent decades, microencapsulation has become a well-liked method for enhancing probiotic bacteria’s survivability.[Citation19] The process of encasing microparticles or droplets of solid or liquid material in a coating or embedding them in a heterogeneous or homogeneous polymeric matrix film to produce tiny capsules with a range of useful properties is referred to as microencapsulation.[Citation20,Citation21] Probiotics, flavors, enzymes, perfumes, antimicrobials, antioxidants, edible pigments, nucleic acids, lipids, minerals, and other items are commonly carried through microencapsulation with edible coatings.[Citation22,Citation23] The consumption of microcapsules with probiotics as edible film or coating material in food materials provides health benefits to the host.[Citation24] The process of microencapsulation is shown in . The encapsulating material used for probiotics capsule formation is classed as macro, micro and nano-capsules with range (>5000 μm), (0.2 to 5000 μm), and (<0.2 μm) respectively based on their size.[Citation25] In simulated gastrointestinal fluid (SGIF) models, microencapsulation has been shown to minimize probiotic cell damage and increase survival.[Citation19] Encapsulating material protects the probiotics from environmental stress which includes: gas exchange, free radicals production rate, moisture, solute migration, etc. Additionally, it provides defense against harmful environmental factors including heat and UV radiation. Microencapsulation of probiotic bacteria has been researched utilizing a range of technological methods based on chemical and physical principles. Successful microencapsulation technologies include spray freezing, spray drying, layer-by-layer drying, spray chilling, extrusion, fluidized bed drying, electro-spraying, and other physicochemical processes likewise coacervation and emulsification. Additionally, the majority of the coating materials utilized to microencapsulate probiotics today consist mostly of lipids, proteins, and polysaccharides. These organically existing polymers, or chemically altered versions of them, are frequently employed alone or in mixes to make structural coatings.[Citation26] The compatibility of each element the kind of microbe, the microencapsulation technique, and the coating material is crucial for a successful probiotic microencapsulation. The final characteristics of the microcapsule can be greatly altered by small variations in the coating or shell composition.[Citation27] The purpose of this article summarized the encapsulation material used so far for probiotic microencapsulation. This article initially provides an overview of current microencapsulation techniques before going on to cover the frequently utilized coating substances, including proteins, lipids and polysaccharides.
Probiotic microencapsulation techniques
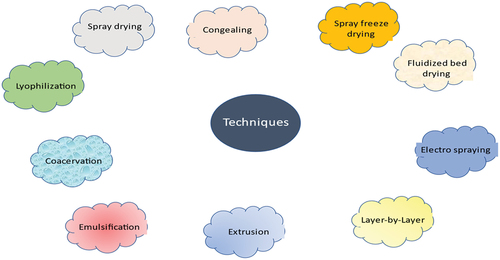
The probiotic microbe, the process of encapsulation, and the coating admixtures all have a big role in determining the chemical and/or physical features of the edible microcapsule. Over the past few decades, new and inventive microencapsulation techniques have been developed, resulting in the production of a wide variety of useful probiotic microcapsules. It is crucial to note that the food industry is inclined to favor less expensive procedures. Therefore, from an industrial standpoint, the first consideration in selecting a manufacturing process is its cost; it must be both affordable and effective without sacrificing morals or the quality of the end product. Probiotic bacteria were microencapsulated using a variety of techniques, including several chemical and physical principles. Different techniques used for microencapsulation are shown in . Spray drying,[Citation28,Citation29] spray chilling[Citation30,Citation31] (also known as congealing or spray cooling), spray freeze drying,[Citation32,Citation33] lyophilization,[Citation34,Citation35] electro spraying,[Citation36,Citation37] layer-by-layer,[Citation38,Citation39] fluidized bed drying,[Citation40,Citation41] extrusion,[Citation42,Citation43] and its modified variant, the vibrating nozzle[Citation44,Citation45] are all effective techniques for microencapsulating probiotics. The physicochemical processes of emulsification[Citation11,Citation46] and coacervation[Citation47,Citation48] are also significant and often employed. Even though in various applications, a consistent particle size distribution may be preferable, these various microencapsulation techniques result in microcapsules with a wide variety of particle sizes.
The viability and stability of Probiotics in the gastrointestinal tract have an inverse relation with the size of encapsulated probiotics produced. The greater the size, the lesser the chance of encapsulated probiotics surviving in the gut.
As shown in . The purpose of the different techniques listed below is to cover live microorganisms in a protective covering (). These approaches, however, operate on distinct fundamental tenets. There are numerous similarities between the spray chilling and spray drying procedures for microencapsulating probiotics. Both processes atomize the core material to spread it into a chamber that fosters the coating’s solidification. The created microcapsules are then isolated from the damp air by a filtration system or cyclone so that they may be collected as powder. The primary distinction between the two approaches is the temperature of the container used to harden the coating[Citation68]Spray chilling hardens the hot, molten combination of core-coating ingredients by atomizing it in an environment chilled below the melting temperature of the coating substance. To hasten the evaporation of the solvent in which the coating material was dissolved, warm air is employed during spray drying.[Citation68,Citation69]
Table 1. Researches reported on use of different coating materials with different probiotics and techniques.
Other names for freeze-drying include lyophilization and cryodesiccation. In the freeze-drying method, firstly frozen, the solvent medium is then sublimed at low pressure. It involves spraying a liquid containing the shell material and core components; the spray droplet is frozen. These droplets are lyophilized and produce dry elements; they are no longer frozen.[Citation68,Citation70]
Basic methods for creating hydrocolloid microcapsules include extrusion, which is also known as the droplet method and emulsification, which is often known as the two-phase system method. The extrusion process involves a hydrocolloid solution that contains viable cells. The suspension is then driven through a tiny opening, such as a syringe prickle, allowing the resultant droplets to fall freely into the solution for hardening.
The droplet scheme is a traditional, affordable, and widely used method for producing microcapsules. But despite the low budget of manufacturing, the major drawback of this method is that the coating on the microcapsules solidifies slowly, making it difficult to scale up the technique.[Citation71] The vibrating nozzle technique is an advancement in the extrusion process.[Citation72] An extruded jet is subjected to a vibrating frequency with a specific amplitude in this procedure, which causes the laminar jet to fragment into droplets of a given size.[Citation73] Droplet size is determined by jet diameter, extruded fluid velocity, viscosity, surface tension, and disturbance frequency. The vibrating technique has been employed in investigations focusing on probiotic microencapsulation throughout the last few decades.[Citation74,Citation75]
The emulsification process is another low-cost approach for the microencapsulation of probiotics that, unlike extrusion, can be readily scaled up. A “solution-like” comprised of minute droplets is generated by combining bacteria with coated polymer in oil and aqueous part.[Citation65] The agitation rate of the liquid determines the size of the microcapsule in this procedure. Emulsifying chemicals (such as polyoxyethylene sorbitan) reduce the surface tension between the two immiscible liquids, resulting in improved homogenization and the ability to prepare smaller capsules.[Citation76]
Microencapsulation via coacervation consists mostly of three processes carried out under continuous agitation. In the first stage, three immiscible chemical segments are formed: the core medium, the assembly liquid and the coating substance. The next stage is a coating deposition phase in which the base substance is disseminated in the coating solution. The last phase is outside layer solidification, which utilizes heated, cross-connect, or desolation processes to make the immiscible coating material rigid. Furthermore, the coacervation procedure can be classified as simple or complicated. This method is sensitive to heat-sensitive material. When a particularly hydrophilic substance is added to a colloid solution, two phases emerge, whereas complicated coacervation tries to manipulate the acidic/basic character of extra colloid systems to encourage the formation of microcapsules.[Citation77]
Several approaches were discussed earlier, and there are two techniques electrospraying and the second is fluidized bed drying, the use of probiotics microencapsulation. The electro-spraying method is based on the electrohydrodynamic concept utilized for microencapsulation. Electro-spraying is another term for electro-hydrodynamic atomization. Typically, a tube through which liquid core material is sprinkled toward an ionized collector where the circular drops are accumulated is supplied with a high-voltage electrical field. This strategy was incorporated with other microencapsulation methods to increase the process’ efficacy. Probiotic encapsulation has been effectively accomplished via the electro-spray extrusion method.[Citation36,Citation78]
The chemical electrostatic interaction of positively and negatively charged substances underpins the layer-by-layer technique (LbL).[Citation79] To create microcapsules using LbL, components with opposing charges are electrostatically attracted, where they self-assemble into layers. Using this method, multi-layered capsules may be constructed by successively introducing charged substrates to the surface of the core material. The procedure can be carried out again to produce the necessary number of coating layers. The fluidized bed drying process is an efficient method for microencapsulation. In this method, the gaseous phase is mixed with small particles due to electrostatic forces coating material to form a layer.[Citation40,Citation41]
Edible coating materials
The barrier that encapsulates the core medium and shields it from the environment is the coating material. It goes by other names, including matrix material, carrier material, exterior phase, wall, shell, or membrane. The coating substance can also be arranged in more than two coating layers that include the core substance. The creation of ingestible probiotic microcapsules has faced significant obstacles in the past. In this context, during the past 20 years, several researchers have focused on bioactive compounds for coating materials.[Citation24,Citation80]
Being chemically unreactive with the core material, being capable of sealing and confining the core material within the capsule, having the potential to offer safety against adverse circumstances, and being sustainable and affordable are all important qualities in a coating material. Since it is not possible to concurrently enhance the coating qualities, there is currently no perfect coating that serves all objectives. Finding a balance between desirable qualities, such as resistance to the impacts of pressure, moisture, gas exchange (O2/CO2), acidity, and/or heat aggression, is thus necessary to generate acceptable coatings for microcapsules as a probiotic delivery method. However, the interaction between the microencapsulation technique and the probiotic bacteria should also be considered for an appropriate selection of the exterior phase. The natural or synthetic polymers used are determined by the core components and the desired properties of microcapsules.[Citation81,Citation82] Figure 5 illustrates several coating materials. Proteins (such as soy protein, zein, gelatine, and collagen), polysaccharides (starch, chitosan, alginate and like cellulose derivatives), and lipids are the main components of edible coating materials made of bio-polymers (such as fats and waxes).[Citation26]
In the development of edible covering for microencapsulation, the qualities of edible film-forming ingredients must be taken into account. Probiotics need water and CO2, and O2. Conveniently, materials that developed edible films have selective permeability (such as water vapor and gases), allowing for controlled gas exchange and bacterial growth. It has been demonstrated that mixing various edible film-forming components can advance or alter the final edible film’s physical characteristics. However, it’s crucial to take into account the various testing circumstances, such as the temperature and relative humidity, when comparing these figures (RH). For instance, the permeability is significantly impacted by RH; even little changes in RH during testing can cause considerable variations in the permeability.[Citation83]
When RH is low, polysaccharide and protein-based films frequently have excellent gas barrier properties, but since they are hydrophilic, they typically have extremely poor water barriers. Gas permeability increases noticeably as RH increases. In comparison to most protein-based films, the majority of pure polysaccharide-based films usually have subordinate WVP values. Moreover, WVP values increased with high RH in protein-based films; this material is sensitive to moisture contents. Second, lipid-based films are hydrophobic and crystalline forms. Therefore, they have low WVP values and good gas fence qualities. In general, the permeability of the film decreases as the degree of lipid crystalline increases. The lipid crystalline structure is disrupted due to branch structure, decreased C-C double bonds or the length of the carbon chains, which lowers oxygen permeability.[Citation83]
The features of each component may be combined due to the formation of composites, as was already explained. To lower the gas permeability and WVP values of polysaccharide or protein films, lipids can be added. As a result, the permeability to gases and water at high RH decreases when lipid chemicals are added to the films.[Citation83,Citation84]
Furthermore, coating materials should have desirable commercial food attributes such as being cost-effective, meeting standards for quality and safety, being chemically inert with the base materials and having rheological properties (elastic, viscous) that are efficient for encapsulation.[Citation82,Citation85] Encapsulating material has more qualities throughout encapsulation processing, including the capacity to stick and sustain the interior material within its structure throughout processing, diffuse or emulsify the active ingredient and stabilize the resulting emulsion, favor desolventization, this provides the best safety from environmental factors with the encapsulated material throughout the process. The most popular coating materials utilized recently for probiotic microencapsulation are described in the sections that follow. Usually, polysaccharides, lipids and proteins are used for the encapsulation of probiotics. The duration of probiotics has been increased throughout simulated gastrointestinal conditions and processing.
Proteins
Despite being great materials for microencapsulating probiotics, proteins are commonly coupled with another coating mediator (). Until now, few works have employed proteins as the sole wall material.[Citation32,Citation37,Citation61,Citation100] Many proteins are commonly utilized as matrix martial because they possess properties that serve as an important deterrent against oxygen and carbon dioxide permeability. Its distinct amino acid chain permits a variety of intra- and intermolecular relations, as well as interactions with other elements used to create the edible matrix.[Citation24,Citation26]
Table 2. Protein, carbohydrates and lipid based material for encapsulation of probiotics.
Depending on their origin, the proteins utilized as shell material for probiotic microcapsules can be categorized as either vegetable or animal proteins. Casein, gelatine, whey protein isolate (WPI), whey protein concentrate (WPC), caseinates and egg white are a few examples of proteins derived from animal sources. In contrast, examples of proteins from vegetable sources include those found in soy, wheat, pea, and maize (zein). The optimal proteins for developing or enhancing coatings in line with certain microencapsulation techniques are emphasized in particular. For instance, gelatine is a big, fibrillar protein produced by partially hydrolyzing collagen.[Citation102] Gelatine is a protein for use as a coating mediator in the production of capsules since it is one of the earliest and most useful dietary ingredients.[Citation37] Gelatine may also be utilized as a wall material in a variety of encapsulation techniques, including spray drying, spray chilling, complex coacervation, and lyophilization, owing to its properties and capacity to react with a broad range of polysaccharides.[Citation86,Citation103] Gelatin A and gelatin B are used in nanoparticle formation. Type B gelation has negatively charged at neutral pH in the isoelectric point where the negatively charged particles have long shelf life in blood circulation, on the other hand, type A gelation has positively charged at neutral pH and short shelf life in blood circulation.[Citation104]
Another benefit of using gelatine protein as an encapsulation material because it has a higher oxygen resistance than globular proteins due to its linear structure.
However, there are some disadvantages to using gelatine for probiotic microencapsulation, including variable purity, which makes the precise molecular weight within preparatory work unknown, or the fact that it frequently needs to be combined with previous substances to attain meticulous characteristics like gel strength, thickness, or glueyness. Moreover, owing to its animal source, it cannot be used in the production of capsulation material that here to the kosher or vegan trends (Meng & Cloutie, 2014; Thies 2007).[Citation105,Citation106]
Whey proteins, soy proteins, egg white and soy proteins are spherical proteins used in probiotic encapsulation (albumen). Because of their excellent emulsifying and gelling characteristics, these proteins are regarded to be the premium resources for microencapsulation via the coacervation process (Xiao et al., 2014; Thies 2007; Weingbreck et al., 2003).[Citation87,Citation88,Citation106] But, there hasn’t been much research employing whey proteins to create probiotic coatings through coacervation.[Citation60,Citation88,Citation107] Over coacervation utilizing globular proteins as coating materials, other methods have been favored. By electro-spraying and fluidized bed drying, for instance, Lactobacillus acidophilus was preserved using a covering made of stearic acid and egg albumen. Similar to this, L. plantarum was microencapsulated by extrusion using a mixture of alginate and soy protein isolate, while L. acidophilus was microencapsulated by spray drying utilizing maltodextrin and soy extract as carrier material.[Citation29,Citation108]
Soy proteins were gained popularity in recent times as a component of good bacterial coatings. Soy protein isolate is one of the accessible sources of good quality proteins and a dependable substitute for vegans and those with a milk allergy.[Citation109] Soy protein has emulsification and gelation properties therefore it can be used as a encapsulating material.[Citation110,Citation111] Both natural and processed milk proteins are available for use as carrier material. Whey proteins WPI and WPC), Caseins and products with both whey proteins and casein have been used in numerous recent studies on the microencapsulation of probiotics. Bovine milk has a protein content of ~3.5%, primarily made up of casein and whey proteins. Casein is a notable protein, accounting for over 79–80% of the whole protein in milk. Whey proteins are thus described just like any protein that stays in solution after the casein in milk has been eliminated.[Citation112,Citation113] Caseins primarily consist of αS1-, αS2-, β-, and κ-casein types, but whey proteins contain immunoglobulins, serum albumins, and α-lactalbumin (α-LA)β-lactoglobulin(β-LG).
Genetic variation in caseins and whey proteins gives them innate characteristics, including varying isoelectric points, molecular weight, hydrophobicity, and other characteristics.[Citation114] Caseins are insoluble at a pH of 4.6, whereas whey proteins are. Therefore, the primary method for extracting casein from dairy products is isoelectric precipitation. Casein precipitates are cleaned and dried during the curdling process, which separates them from whey proteins. Caseinate is produced when the water-soluble acid casein derivatives react with alkaline solutions.[Citation115] The most popular casein variant utilized as a coating material is sodium caseinate (SC), which has outstanding surface-active qualities comparable to caseins and a higher confrontation to temperature denaturation. In fact, in the spray drying method, caseinates are preferred over whey protein for drying milk (Augustin & Oliver, 2014). A commercial enzyme called transglutaminase (TGase) is also well-recognized for improving the sensory appeal and texture of dairy products. To form intra- and intermolecular isopeptide linkages between proteins, this enzyme crosslinks glutamine and lysine residues.[Citation116] To successfully microencapsulate heat-sensitive, live probiotics, TGase can promote the gelation of caseinates under moderate circumstances (Heidebach & Kulozik, 2010; Heidebach & Kulozik, 2009).[Citation117,Citation118] Commercially, whey proteins are offered isolates (WPI) and concentrate (WPC), with corresponding protein contents of 35%–85% and >95%. Their methods of separation and purification, as well as their compositions, differ. WPC is created via ultrafiltration or diafiltration, centrifugation, and spray drying. In contrast, additional processes, including ion exchange chromatography, are used to produce WPI (Fox 2008). These variations in developed WPCs were defined by their little cholesterol and fat content, as well as their elevated stage of lactose and overall fats, whereas WPIs have a significant amount of protein and a lower content of lipids and lactose (Morr & Ha, 1993).[Citation119]
Bovine milk provides the most whey protein composition (50–60%) in the form ofβ-lactoglobulin(β-LG) protein. As a result, the 162 amino acid residues of this ubiquitous globular protein have been thoroughly examined. The excellent thermogelification qualities of the β-LG dictate the gelation characteristics of the WPC.[Citation120]
α-Lactalbumin (α-LA) (calcium metalloprotein) accounts for around 20% of all whey proteins. The capacity to gel and gel-like characteristics (such as potency, stiffness, or viscoelasticity) was shown to enhance when β-LG and α-LA were coupled in studies, showing a synergistic impact of these proteins on gelation actions (Rojas et al., 1997).[Citation121] This protein can bind to other proteins and is thermally stable due to the calcium atom’s capacity to encourage the configuration of intermolecular ionic associations.[Citation122,Citation123] Several types of whey proteins have recently been studied as wall materials for probiotic microencapsulation.[Citation35,Citation37,Citation48,Citation97,Citation97,Citation100] Consider sweet whey (SW) as a last example of a product that combines both whey proteins and casein. It is a high protein-content condensed, the dried by-product of the cheese-making process. The primary proteins include α-LA,β-LG, αS1-casein, lactoferrin (a tiny phosphoglucose protein), bovine serum albumin (BSA), fatty acid binding proteins (FABP) and immunoglobulins light chain and G heavy (IgG-LC and IgG-HC respectively)[Citation110,Citation124] Spray drying was used to successfully microencapsulate Bifidobacterium lactis utilizing sweet whey as the coating medium.[Citation125]
Polysaccharides
In recent advances, polysaccharides are used as an encapsulating material that has excellent properties to bear the harsh environment. The most common use of polysaccharides is chitosan, alginate, pectin, carrageenan, gum Arabic etc.[Citation126] The monosaccharide building blocks containing hydroxyl groups are what make up the biopolymers known as polysaccharides or poly glycans. Through intra- and intermolecular hydrogen interactions, these groups can communicate with water or other molecules. However, the nature of the poly glycans monomers and substituent groups also has an impact on how they behave, allowing for a wide range of molecular and functional variations. Unmodified (natural polysaccharides) or modified (synthesized or semi-natural polysaccharides) substituent groups are both possible. Anionic, cationic, nonionic, and amphoteric are the five classifications for polysaccharides that are frequently utilized as matrix materials for probiotic encapsulation, regardless of their place of origin or composition.[Citation127]
Anionic Polyglycans Anionic poly glycans often exhibit a negative pH response when their pKa value is exceeded and a neutral pH response when it is much below. Natural anionic polysaccharides like pectin, alginate, xanthan, carrageenan, gum Arabic and Gellan gum are primarily used in probiotic microencapsulation (Table-2); however, synthetic anionic polysaccharides like chitin liquid also known as carboxymethyl chitin (CMCH), and cellulose gum, also known as sodium carboxymethyl cellulose (CMC), are also frequently used. Ionic species in the environment have the potential to change the electrical charge of carbohydrates. In interactions with multivalent or monovalent ions like Ca2+ or Na+, the negatively ionic groups on the polymeric sequence may alter the overall ionic properties. Anionic polysaccharides gel when they interact with groups on the polymer chain that have opposing charges. Alginates and pectins, for instance, can gel when divalent metal ions like Ca2+ are present.[Citation128]
Alginate is a substance that is frequently utilized as a coating material in the extrusion and spray-drying processes used to create microcapsules using ionic gelation. Pectin, in contrast to alginate, demonstrated comparable abilities but is thought to be more tolerant to acids and the digestive environment.[Citation60] Unbranched heteropolysaccharide, known as alginate, is obtained from the cell wall of brown algae (Laminaria spp.). It contains ß-D-mannuronic acid and α-L-guluronic acid combined by ß-1,4 glycosidic bonds. Pectin, on the contrary, is derived from some fruits or peels of fruits (such as citrus peels and apple pomace), and by calcium gelification, it makes a coating structure. The main constituents of this structure were linked with D-galacturonic acid and highly branching segments containing additional disaccharides galactose, xylose and arabinose (Kwiecien & Kwiecien, 2018). In place of pectin, alginate is now the wall substance that probiotic bacteria are most typically microencapsulated (Coghetto et al., 2016; Lee et al., 2019; Gaudreau et al., 2016; Pitigraisorn et al., 2016; Nualkaekul et al., 2013).[Citation36,Citation40,Citation43,Citation89,Citation129]
Carrageenans are made of various sulfated polysaccharide combinations and are derived from red seaweeds (Rhodophyta). Three different forms of commercially available carrageenans that change in their chemical arrangement and characteristics may be produced by red seaweed.[Citation130] Importantly, only the κ-and ί- (mono-sulfated) and (bisulfated) respectively carrageenan categories have anhydrous crossing that permits gelation when K+ and Ca2+ are present, among other cations. On the contrary, the(trisulfated)λ-carrageenan variety prevents gelation because it cannot create hydrogen bridges.[Citation131] Because of this, carrageenans show a vast range of gel properties depending on the category (κ-, ί-, and λ-).[Citation90,Citation91] All forms of carrageenan dissolve in hot water. In contrast to ί-carrageenan, which is soluble in frosty water and creates spongy, flexible gels, κ-carrageenan forms a hard, brittle gel that melts when heated at a low temperature. Notwithstanding this, ί-carrageenan’s potential use in probiotic microencapsulation has not drawn much attention.[Citation130] Additionally, by mixing κ-carrageenan with other wall materials such as calcium alginate, vegetable oils, and other gums, its gel properties have been improved (for example, Gellan, xanthan and locust bean gums). These mixes had used to successfully microencapsulate probiotics by emulsification.[Citation91,Citation92,Citation132]
Other anionic polysaccharides utilized in probiotic microencapsulation include gellan gum, xanthan gum, and gum Arabic. The bacterial extracellular polyglycansgellan gums and xanthan are generated by Sphingomonas elodea and Xanthomonas campestris, respectively. Gum Arabic (gum acacia) belongs to the Acacia family (Burnside 2014). Several plants of the Xanthomonadaceae family generate xanthan gum (XG). However, a plant-associated bacterium, Xanthomonas campestris, is being used in industrial production. The pentasaccharide repeating units that makeup XG’s chemical structure has one glucuronic acid fragment squished between two mannose molecules and are associated with every other glucose unit on the linear cellulose backbone (β-(1–4)-linked glucose).[Citation133] The configuration of XG also holds O-acetyl and pyruvyl end products in varying concentrations based on the microbial species and the fermentation circumstances. The acetyl and pyruvyl residues are important because they protonate at pH values greater than 4.5, which gives XG a polyanionic charge signature. The relations of the XG groups with the pyruvyl/acetyl end products go ahead to intermolecular crosslinking, which encourages modifications in the ruin order of the XG structure. These poly glycan characteristics mostly depend on the environment, including pH, the kind of ionic strength and electrolyte.[Citation134] Recent studies have shown that the existence of pyruvate or acetyl on the exterior mannoses affects the constancy of the helical (ordered) conformation of xanthan gum.[Citation135,Citation136]
The presence of pyruvate and acetyl groups is also a crucial consideration for a useful purpose. For instance, larger quantities of pyruvyl substitutions encourage a gel-like behavior, whereas lesser levels impart low viscosity.[Citation134,Citation136] The pyruvyl and acetyl residues in the XG are essential for setting up the conditions for complexation with divalent cations like Mg2+ or Ca2+ (Bergmann et al., 20018). For the microencapsulation of probiotics, XG has shown temperature stability and encapsulation properties that simulate gastrointestinal conditions.[Citation66,Citation136,Citation137,Citation137] XG has been efficiently paired with various wall materials and improved the properties of encapsulation.[Citation53]
Microencapsulation of Lactobacillus plantarum LAB12 with XG and alginate increased the probiotic viability in gastric juice and bile salts. Therefore, L. plantarum LAB12 showed more protection from high temperatures and low pH by chitosan coating complex XG alginate (Fareez et al., 2017; Fareez et al., 2015).[Citation66,Citation138] Most studies reported that XG-chitosan and XG-chitosan-XG conjunction showed increased L. acidophilus viability in microcapsules, and this was added to milk-based drinks. Furthermore, the XG-gellan gum combination increased Bifidobacterium lactis survival in wall material kept in sodium phosphate buffer (pH 6–6.8).[Citation53]
An anionic bacterial polysaccharide called gellan gum (GG) has a straight chain sequence of tetrasaccharides and contains two by-products of β-D-glucose. Differentiating characteristics, such as their gelation behavior, are conferred by variations in the acyl group concentration of GG. GG is now offered in two forms: one is deacylated, and the second is acylated, which was marketed under the names Kelcogel and Gelrite, respectively.[Citation67]
Each GG variety has distinctive qualities. The low acyl GG cools to stiff and rigid hydrogels at 40°C, but soft and flexible hydrogels are made by acylated GG at 65°C.[Citation67] The deacylated form of GG was effectively used as an encapsulation for bacteria. When XG-GG was used in combination,[Citation53] the probiotic viability in bile salt environments and simulated gastric fluid was increased by microencapsulating L. casei with a sodium caseinate-GG mixture as a wall material.[Citation61]
Gum Arabic’s major components are L-arabinose, D-galactose, and L-rhamnose. GA has a complicated chemical structure composed of 1–3-linked β-D-galactopyranosyl linkage, α-L-rhamnopyranose, linkages can be found in both the form of chain and branch.[Citation139,Citation140] Unexpectedly, GA is covalently connected to a protein moiety that contains a lot of proline, serine, and hydroxyproline amino acid residues. When compared to other exudate gums, GA has a very low viscosity and is water soluble (up to 50% w/v). These traits are connected to its light molecular weight and highly branching structure. On the other hand, the polysaccharide’s surface activity, foaming capabilities, and emulsifying qualities are all brought about by the protein moiety of GA.[Citation141,Citation142]
In this case, the miracle fruit (Synsepalum dulcificum) leaf, seed, or pulp extracts were combined with isolated whey protein (WPI), gelatine-GA, and other materials as shell materials for the encapsulation of bacteria. When evaluated to unencapsulated cells, these coatings effectively increased the lifespan of bacterial cells during storage, processing, and in vitro simulated gastrointestinal conditions.[Citation143]
Carboxymethyl chitin (CMCH) and carboxymethyl cellulose (CMC) are semi-synthetic anionic polysaccharides that are modified anionic polysaccharides. CMCH and CMC are derivatives of chitin and cellulose, respectively. Surprisingly, one of the most naturally occurring polysaccharides is cellulose which is followed by chitin on the planet. Cellulose has β-1,4-linked D-glucose, but the structure of chitin has β-1,4-linked units of an amino sugar (N-acetyl-glucosamine).[Citation143,Citation144] CMCH and CMC are both widely utilized in the food sector, particularly as coating materials in probiotic microencapsulation. CMC is a cellulose derivative that is water soluble. The partial substitute of anhydrous glucose’s hydroxyl group with the carboxymethyl group was made by alkali and chloroacetic acid.
Research that employed chitosan and CMC as wall materials for L. acidophilus microencapsulation discovered that these materials might increase probiotic viability throughout simulated gastrointestinal transit.[Citation38] In a different investigation, mixtures ofκ-carrageenan and CMC were utilized as wall resources for the encapsulation of L. plantarum. These mixtures demonstrated appropriate for the formation of microcapsules for the oral administration of good bacteria.[Citation92]
CMCH, commonly referred to as chitin liquid, is an anionic polysaccharide that dissolves in water (). Chitin’s hydroxyl groups are changed to carboxymethyl groups to create CMCH. Numerous different applications, including antibacterial, culinary, cosmetic, and medication delivery systems, have made extensive use of CMCH.[Citation145] Concerning its possible matrix material for beneficial bacteria encapsulation, however, nothing has been done. In recent work, Bifidobacterium was microencapsulated using CMCH and sodium alginate as a shell material. Free cells were less likely to survive under simulated in vitro gastrointestinal circumstances than microencapsulated probiotics did, which indicates an effective way to create microcapsules as a probiotic delivery strategy.[Citation2]
Cationic Polysaccharides: Chitosan is a cationic polysaccharide. The primary component of chitosan is (1,4)-linked 2-amino-2-deoxy-D-glucan, which is produced when chitin is partially deacetylated (). Insect exoskeletons and other bacterial parasites also contain chitosan naturally, but in too tiny an amount for commercial use.[Citation128] Despite having a wide antibacterial range, chitosan has been combined with other encapsulating agents to create probiotic microcapsules.[Citation22,Citation58] Several probiotic microbes were protected by chitosan when combined with substances such as starch, alginate, xanthan gum and whey protein isolate in simulated in vitro gastrointestinal settings. This may be a useful method for providing probiotic advantages to consumers.[Citation54,Citation146–148]
The encapsulation of Bacillus coagulants utilizing chitosan and alginates shell materials is a superb example. According to Anselmo et al.[Citation94] these probiotic microcapsules let microorganisms survive despite simulated gastrointestinal conditions. Additionally, they showed that, as compared to free cells, microencapsulated probiotics stick more strongly to the mucosal area of fresh pig colon tissues and to the EpiIntestinal system, an isolated intestinal model that replicates physiological gut architecture. Additionally, they evaluated in vivo probiotic survival using a mouse model, and they discovered that microencapsulated probiotics had a considerable survival advantage over free cells.[Citation94]
Other artificial cationic polysaccharides with aesthetic uses have also been discussed in the past, including cationic hydroxyethyl cellulose, cationic guar, and cationic hydroxypropyl guar. None of them has yet been identified as a matrix material for probiotic microencapsulation because it has potential cationic materials.
Nonionic Polysaccharides: These polymers, known as nonionic polysaccharides, lack a prescribed charge. But, nearby environmental factors could have an impact on their charge properties, altering their typical solution behavior. Cellulose ethers (such as hydroxypropyl-cellulose and hydroxypropyl methyl) are adapted as encapsulating material.[Citation91,Citation127] A soft, tasteless, white powder is starch. It is generated by farms and mostly comprises two types of D-glucose polysaccharides one is linear amylase and the other is helical amylose. Whereas amylose is nearly entirely composed of linear molecules with α-(1,4)-linked D-glucose units that form a helical shape.[Citation91] The starch’s intrinsic properties are determined by the amylose/amylopectin content, which varies depending on the source. Sometimes referred to as resistant starch (amylase maize starch), it is a common example of starch that has a high amylose concentration and is renowned for producing strong and stretchy films; this is most likely owing to the predominate amylose structure and its crystallization (RS).[Citation145,Citation149]
Starch films are ideally suited as a shell material for encapsulation since they conveniently possess several features. Toxic-free, tasteless, colorless, odorless, and selectively permeable to moisture, carbon dioxide, and oxygen, as well as flavor and lipid contents, are all characteristics of starch films.[Citation149] A transformed form of starch called octenyl-succinate starch is estimated in this respect because of a protective substance for the encapsulation of probiotics (Bifidobacterium). E1450 (Octenyl-succinate starch) is a food additive that was chosen because of its special appropriateness for the spray drying procedure used for microencapsulation. When introduced to dry food preparations or in acidic environments, this microencapsulation procedure did not increase probiotic viability compared to free cells. However, the encapsulation process employing the spray drying technique and E1450 as the shell material was effectively improved to produce microcapsules consisting of viable Bifidobacterium probiotic cells. A possible prebiotic ingredient for the microencapsulation of several probiotics was also mentioned in relation to starch. In other investigations, RS was mixed with various coating substances, including alginate, chitosan, sodium caseinate, or hydrolyzed or separated whey protein, to microencapsulate probiotics.[Citation95,Citation116,Citation121,Citation146]
In its official definition, maltodextrin is described as “purified, nutritious mixes of saccharide polymers formed by partial hydrolysis of edible starch.” Any starch may be used to make maltodextrins when D-glucose units are linked together in strands of different lengths. The two variables that differ across maltodextrins and ultimately dictate their qualities are the degree of polymerization (DP) and dextrose equivalent (DE) (BeMiller 2019).[Citation96] Conveniently, some of these characteristics, such as film formation, high solubility, easy digestion, moisture control, ease of spray-drying, and capacity to create gels, are appropriate for microencapsulation. Maltodextrin has a good record of working with other edible ingredients to enhance the probiotic coatings’ drying capabilities during microencapsulation. For instance, the probiotic’s survival and viability were greatly increased when Lactobacillus paracasei was encapsulated by spray freeze-drying and coated with trehalose and maltodextrin.[Citation90] Subsequent research employing soy extract and maltodextrin as protective material and spray-drying L. acidophilus produced comparable results.[Citation29]
A microbial enzyme Cyclodextrin glycosyltransferase (CGTase), degrades the starch during exercise and oligosaccharide cyclization creates cyclic oligosaccharides termed cyclodextrins (CDs) (Van et al., 2000). Now, the most studied cyclic oligosaccharides are β-CD and its variants. Numerous advantages of β-CD have been shown, including its metabolism and safety, the elimination of cholesterol from numerous foods (such as dairy and egg products), and the avoidance of a spike in triacylglycerols and plasma cholesterol, among other advantages.[Citation34] Furthermore, cross-linked β-CD microcapsules are widely delivered drugs with controlled liberate.[Citation150,Citation151] However, there aren’t many pieces of research that have been done using β-CD as a wall material for probiotic encapsulation. Lactobacillus acidophilus, Saccharomyces boulardii, and Bifidum have all recently been characterized as being microencapsulated using gum Arabic and β-CD as shell materials. In comparison to free cells, probiotics microencapsulated with β-CD generally have thermal resistance and improved viability in SGI circumstances. On the other hand, described the utilization of β-CD in conjunction with xanthan gum, alginate, and chitosan as matrix materials for the microencapsulation of L. plantarum LAB12. Additionally, they showed that microcapsules enclosing probiotics and β-CD had the potential to decrease cholesterol.
Another organic, nonionic polysaccharide is guaran, popularly known as guar gum (GUG). Cyanaposis tetragonolobus cluster bean plant seeds made it. In alginate-starch microcapsules, Salaria et al.[Citation152] disclosed microencapsulating L. acidophilus LA1 with fructo oligosaccharide (FOS) or partially-hydrolyzed GUG as co-encapsulating material. One more study used XG or GUG as the wall material to microencapsulate with a mixed culture of probiotics (L. rhamnosus,L. acidophilus,and B. longum). To create a beneficial probiotic snack, microcapsules were then successfully added to cream cookies.[Citation98] In order to add probiotics to milk chocolate beverages, lactobacillus strain microcapsules were made using alginate and GUG as shell agents (.[Citation42] Both studies indicated that adding probiotic microcapsules did not change the final goods’ flavor or taste, in addition to improving microbial vitality throughout product storage. Recently reported that GUG was used for the encapsulation of Yeast S. cerevisiae. Probiotic microcapsules were later employed as an additive for fish feed, in contrast to earlier investigations. It’s interesting to note that administering microencapsulated probiotics to fish hosts also increased growth rates, and feed conversion ratios, and boosted immune responses.[Citation34]
Other nonionic cellulose ethers can be used as coating components for probiotic microencapsulation in addition to carboxymethyl cellulose (CMC). nonionic cellulose ethers models include Hydroxyethyl cellulose (HEC), Methylcellulose (MC), hydroxypropyl methylcellulose (HPMC), hydroxypropyl cellulose (HPC), and microcrystalline cellulose(MCC). Pop et al. (2015) reported the co-encapsulating material, in this alginate combined with several nonionic polysaccharides. The goal of the study was to create microcapsules with acceptable physical/biochemical qualities capable of withstanding probiotic viability and practical for scaling up by exploring the encapsulation of B. lactis 300B. New co-encapsulating wall material includes 2 kinds of starches pullulan and dextrin, Sodium-carboxymethyl cellulose (Na-CMC), hydroxypropyl methylcellulose (HPMC), microcrystalline cellulose (MCC) are used for encapsulated wall material. They discovered that the composition of the encapsulating substance has a significant impact on the probiotic’s lifespan. The two admixtures that offered the probiotic the greatest protection throughout the encapsulation procedure and after 15 days of storage were alginate-pullulan and alginate-HPMC. However, B. lactis had microencapsulated utilizing nonionic polysaccharides as wall resources and a layer-by-layer procedure. The exterior layer was composed of a mixture of HPC and poloxamer, with HPMC serving as the inner layer. A triblock copolymer of poly (ethylene oxide), poly (propylene oxide), andpoly (ethylene oxide) makes up the nonionic surfactant poloxamer (PEO-PPO-PEO). The fast microscopic changes takes place in its structure depending on the lower critical solution temperature (LCST). The polymer is soluble below the LCST but becomes insoluble when the temperature rises above the LCST. During the reconstitution of powdered infant formula (PIF), the probiotic survival was assessed after the addition of the microencapsulated probiotic. Because the coating of the microcapsules may produce an insoluble gel that shelters encapsulated cells. Moreover, the probiotic organisms are revealed when the covering gel breaks upon cooling at 40°C.[Citation41]
Amphoteric Polysaccharides: Polymers known as amphoteric polysaccharides have anionic and cationic charges carried along the same chain. Typically, natural polysaccharides are used as the building blocks in their production. The cosmetic industry includes carboxymethyl chitosan (CMCS), N-[(2”-Hydroxy-2,”3’−dicarboxy) ethyl] chitosan, sulfated chitosan, and modified amphoteric starches.[Citation127] Chitosan’s amino and hydroxyl groups are swapped out for carboxymethyl groups to create CMCS. In contrast to chitosan, CMCS has advantageous qualities such as high moisture absorption, enhanced water solubility, non-toxicity, high viscosity, biocompatibility, and a good capacity to produce hydrogels and films.[Citation59,Citation99] Only CMCS has been mentioned as a matrix material for probiotic encapsulation up until this point. Using the extrusion process, Lactobacillus casei ATCC 393 was microencapsulated. The medium used were chitosan, alginate, and CMCS. L. casei survived longer with microcapsules during cold-air-flow drying and in simulated gastric environments.[Citation153]
Lipids
A diverse category of chemicals known as lipids include waxes, phospholipids, fatty acids, and fats. Due to their low polarity and ability to largely inhibit moisture transport, lipids can be employed as edible matrix materials. However, the produced coatings are more brittle due to their hydrophobic character. Lipids are combined with polysaccharides or proteins and made protective wall material for encapsulation.[Citation154] For instance, the introduction of lipids to the composition enhances its resistivity to water vapor, whereas polysaccharides or proteins contribute structural cohesiveness, durability and integrity as well as selective permeability to gases (O2 or CO2).[Citation155] Fats make up the majority of the lipids used in probiotic microencapsulation. Edible fats can come from either plants or animals, depending on their source. For instance, animal-sourced fats like fish oil, butter, or pork oil. Sunflower, maize, or olive oils are examples of plant fats, on the other hand. Mono-, di-, or triglycerides, which are made up of fatty acids and glycerol, are the most common forms of fat. As a result, the fatty acid makeup greatly influences their characteristics. The amount of carbon and hydrogen atoms that make up a molecule’s carboxylate hydrophilic head and a hydrophobic tail, which together make up fatty acids, determines the molecular mass of the group. The molecular mass of the fatty acid affects the melting point; a higher molecular weight results in a greater melting point. Compared to unsaturated fatty acids, saturated fatty acids contain greater melting points. At its melting point thermal solidification is caused, the key factor employed for microencapsulation is the melting point of lipids. Probiotics have frequently been microencapsulated using vegetable lipids as co-encapsulating materials by emulsification[Citation91,Citation100]or spray drying.[Citation31] Silva et al., however, reported microencapsulating probiotics using either gelatin-gum Arabic or vegetable oil as the only coating medium in a recent investigation. In comparison to unbound cells, this method of microencapsulation effectively preserved probiotic cells during simulated gastric circumstances and stress conditions (such as temperature, pH, sucrose and sodium chloride).[Citation30] Despite the stated success, there is still room for improvement in the microencapsulated probiotic’s storage viability.
Lipid substances called waxes are frequently employed in the food sector. Candelilla wax, Beeswax, and Carnauba wax are a few examples of organically occurring waxes. The chemical molecules that make up natural waxes are a complex combination of long alcohol chains, long alkyl chains, long fatty acid chains, aldehydes, ketones, and fatty acid esters. Beeswax is a natural wax, which honeybees produce and utilize to make their combs. It has an excellent solubility, melting point (MP) of 61–65°C than other waxes or oils. At ordinary temperatures, beeswax is a flexible substance; yet, at low temperatures, it becomes brittle. Furthermore, carnauba waxes have a melting point of 82.5–86°C. Its structure is made up of saturated fatty acid esters and saturated long-chain alcohols (C24–C32). Among all natural waxes, this wax has the specific gravity and greatest melting point. On the other hand, oxidized polyethylene wax and paraffin wax are two synthetic waxes that are frequently utilized in the food sector.
Both of these items are made from petroleum and may be dissolved into water and form pellets, powder and flakes-like shapes. Hydrocarbon fractions make up paraffin wax (MP ~ 50°C) and its general formula is CnH2n + 2 and carbon has a range of C18–C32. Food additive E914 is another name for oxidized polyethylene wax (MP 97–115°C).[Citation2,Citation101]
Mandal et al.[Citation95] explained that probiotic microcapsules made with alginate and resistant starch can have an exterior coating of stearic acid, beeswax, or poly-L-lysine. The probiotic cells of Lactobacillus casei that were encapsulated in microcapsules coated with stearic acid and beeswax demonstrated an increased rate of survival under simulated gastric conditions. Particularly, the stearic acid covering indicated a complete release of probiotics that had been encapsulated in a simulated colonic pH solution and offered higher protection.
Similar research by Rao et al.[Citation152] explained that encapsulation of probiotic made with cellulose-acetate-phthalate using beeswax or stearic acid (CAP) are used. In contrast, they discovered that Bifidobacterium pseudolongum survived the longest in microcapsules coated with beeswax following consecutive incubation in simulated stomach juice and intestinal juice.
A wide group of lipids known as phospholipids, which may combine to produce emulsions, micelles, and liposomes, are often employed in the food business. These phosphorus-containing lipids are crucial for the metabolic and structural functions of living cells. Simple lipids are less complicated than phospholipids (fats and waxes). Phospholipids include substances like phosphatidylethanolamine (cephalin), phosphatidic acid (phosphatidate), phosphatidylcholine (lecithin), and phosphatidylserine, (PS) for instance.
The hydrophobic properties and neutral charge of the fatty acid tails are provided. These properties give phospholipids an amphipathic character; It plays a crucial role in the synthesis of cellular membranes.[Citation156] Phospholipids, which are the main building blocks of liposomes in this regard, collect in water to create a distinctive bilayer as a result of the interaction between the hydrophilic water environment and hydrophobic fatty acid chains. Phospholipids are the main building blocks of liposomes. Such interactions encourage the creation of liposomes, which are closed, sealed vesicles.[Citation152] Another microencapsulation method used in the food business is liposome entrapment. Liposomes have mostly been used as delivery vehicles for bioactive substances, including medications, vitamins, enzymes, and other substances. Although liposomes have shown significant promise for the regulated release and encapsulation of nutritious components, their employment in meals has not yet been completely explored.[Citation157] For instance, liposome entrapment of probiotics has not been documented yet. This may be because of the high budget for the ingredients and the procedure (Sarao & Arora, 2017).[Citation57] The liposome entrapment methodology is used for microencapsulation. Research has to be done on the effectiveness of probiotic distribution in the intestinal environment, as well as the liposomes’ resilience to gastric and intestinal acids. The greatest drawback of the fat as an encapsulating material includes the use of solvent and recovery of solubility of the actives in the solvents that can make it highly expensive. Moreover, large size dispersion of capsules and temperature-sensitive fats and oils are volatilization and oxidation takes place[Citation158]
Conclusion
Microencapsulation is an effective method to increase the efficacy of ingesting good (probiotic) bacteria among the several methods utilized for the preservation of probiotics. Probiotic bacteria have the best rate of survival when they are produced, stored, and delivered into the consumer’s gastrointestinal tract thanks to microencapsulation. The interaction between the encapsulating procedure, the kind of probiotic bacterium, and the coating materials must be taken into account while designing probiotic microcapsules. Biopolymers are used for the microencapsulation of probiotics. The most researched capsulation materials are made of polysaccharides. The polysaccharides most often utilized with almost all documented microencapsulation techniques are alginate and starches. The majority of documented encapsulation methods spray freezing techniques use various whey protein forms as the protein-based material. There are still several protein-based products that may be used, including gelatine, egg albumin, and vegetable proteins. They are readily accessible and inexpensive materials. However, there hasn’t been much research done on using lipids as shell materials for probiotic encapsulation. The majority of reports only cover the use of vegetable fats as co-encapsulating agents when using spray drying and emulsification techniques. Wax and phospholipids are lipid-based encapsulating materials that can still be researched as pure or mixed matrix materials because of their relative availability and permeability features. Despite recent developments in this area, new methods for microencapsulating probiotics provide a range of potential research areas. For instance, the encapsulation method of liposome entrapment was widely utilized as a wall material for probiotic encapsulation bioactive substances; however, probiotics have not yet been reported to be microencapsulated using this method, which opens a field of research prospects to be studied in the future. However, research must be done to uncover innovative pairings of current coating materials with microencapsulation techniques, or the invention/discovery of new edible film-forming materials, for the field of probiotic microencapsulation to expand in the future. Despite extensive research into a variety of methods, coatings, and probiotic microorganisms, very few studies have concentrated on in vivo evaluations of the survivability and biological characteristics of probiotic microencapsulated. As a result, whether or if the survival effects of microencapsulated probiotics transfer into human or animal models is an important question that must be answered. C-3: Remove references from the conclusion section
Acknowledgments
This work was supported by the China Agriculture Research System (CARS-48) and the Anhui Agriculture Research System (AARS-08).
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
Even though adequate data has been given in the form of tables and figures, however, all authors declare that if more data required then the data will be provided on request basis.
Additional information
Funding
References
- Abbas, M. S.; Saeed, F.; Afzaal, M.; Jianfeng, L.; Hussain, M.; Ikram, A.; Jabeen, A. Recent Trends in Encapsulation of Probiotics in Dairy and Beverage: A Review. J. Food Process. Preserv. 2022, 46, e16689. DOI: 10.1111/jfpp.16689.
- Pech-Canul, A. D. L. C.; Ortega, D.; García-Triana, A.; González-Silva, N.; Solis-Oviedo, R. L. A Brief Review of Edible Coating Materials for the Microencapsulation of Probiotics. Coatings. 2020, 10(3), 197. DOI: 10.3390/coatings10030197.
- Riaz, Q. U. A.; Masud, T. Recent Trends and Applications of Encapsulating Materials for Probiotic Stability. Crit. Rev. Food Sci. Nutr. 2013, 53(3), 231–244. DOI: 10.1080/10408398.2010.524953.
- Tripathi, M. K.; Giri, S. K. Probiotic Functional Foods: Survival of Probiotics During Processing and Storage. J. Funct. Foods. 2014, 9, 225–241. DOI: 10.1016/j.jff.2014.04.030.
- Corcoran, B. M.; Stanton, C.; Fitzgerald, G. F.; Ross, R. Survival of Probiotic Lactobacilli in Acidic Environments is Enhanced in the Presence of Metabolizable Sugars. Appl. Environ. Microbiol. 2005, 71(6), 3060–3067. DOI: 10.1128/AEM.71.6.3060-3067.2005.
- Sanders, M. E.; Benson, A.; Lebeer, S.; Merenstein, D. J.; Klaenhammer, T. R. Shared Mechanisms Among Probiotic Taxa: Implications for General Probiotic Claims. Curr. Opin. Biotechnol. 2018, 49, 207–216. DOI: 10.1016/j.copbio.2017.09.007.
- Lebeer, S.; Bron, P. A.; Marco, M. L.; Van Pijkeren, J. P.; Motherway, M. O. C.; Hill, C.; Pot, B.; Roos, S.; Klaenhammer, T. Identification of Probiotic Effector Molecules: Present State and Future Perspectives. Curr. Opin. Biotechnol. 2018, 49, 217–223. DOI: 10.1016/j.copbio.2017.10.007.
- Anadón, A.; Ares, I.; Martínez-Larrañaga, M. R.; Martínez, M. A. Probiotics: Safety and Toxicity Considerations. In Nutraceuticals; Academic Press: 2021; pp. 1081–1105. DOI:10.1016/B978-0-12-821038-3.00065-3.
- Sanders, M. E.; Akkermans, L. M.; Haller, D.; Hammerman, C.; Heimbach, J. T.; Hörmannsperger, G.; Huys, G. Safety Assessment of Probiotics for Human Use. Gut Microbes. 2010, 1(3), 164–185. DOI: 10.4161/gmic.1.3.12127.
- Pop, O. L.; Dulf, F. V.; Cuibus, L.; Castro-Giráldez, M.; Fito, P. J.; Vodnar, D. C.; Coman, C.; Socaciu, C., & Suharoschi, R. Characterization of a Sea Buckthorn Extract and Its Effect on Free and Encapsulated Lactobacillus Casei. Int. J. Mol. Sci. 2017, 18(12), 2513. DOI: 10.3390/ijms18122513.
- Ding, W. K.; Shah, N. P. An Improved Method of Microencapsulation of Probiotic Bacteria for Their Stability in Acidic and Bile Conditions During Storage. J. Food Sci. 2009, 74(2), M53–61. DOI: 10.1111/j.1750-3841.2008.01030.x.
- Liao, N.; Pang, B.; Jin, H.; Xu, X.; Yan, L.; Li, H.; Shao, D., & Shi, J. Potential of Lactic Acid Bacteria Derived Polysaccharides for the Delivery and Controlled Release of Oral Probiotics. J. Controlled Release. 2020, 323, 110–124. DOI: 10.1016/j.jconrel.2020.04.022.
- Ragavan, M. L.; Das, N. Optimization of Exopolysaccharide Production by Probiotic Yeast Lipomyces starkeyi VIT-MN03 Using Response Surface Methodology and Its Applications. Ann. Microbiol. 2019, 69(5), 515–530. DOI: 10.1007/s13213-019-1440-9.
- Lee, N. K.; Paik, H. D. Prophylactic Effects of Probiotics on Respiratory Viruses Including COVID-19: A Review. Food Sci. Biotechnol. 2021, 30(6), 773–781. DOI: 10.1007/s10068-021-00913-z.
- Dimitrellou, D.; Kandylis, P.; Petrović, T.; Dimitrijević-Branković, S.; Lević, S.; Nedović, V.; Kourkoutas, Y. Survival of Spray Dried Microencapsulated Lactobacillus Casei ATCC 393 in Simulated Gastrointestinal Conditions and Fermented Milk. LWT Food Sci. Technol. 2016, 71, 169–174. DOI: 10.1016/j.lwt.2016.03.007.
- Arena, M. P.; Caggianiello, G.; Russo, P.; Albenzio, M.; Massa, S.; Fiocco, D.; Capozzi, V., & Spano, G. Functional Starters for Functional Yogurt. Foods. 2015, 4(4), 15–33. DOI: 10.3390/foods4010015.
- Li, X. Y.; Chen, X. G.; Cha, D. S.; Park, H. J.; Liu, C. S. Microencapsulation of a Probiotic Bacteria with Alginate–Gelatin and Its Properties. J. Microencapsulation. 2009, 26(4), 315–324. DOI: 10.1080/02652040802328685.
- Kim, S. J.; Cho, S. Y.; Kim, S. H.; Song, O. J.; Shin, I. S.; Cha, D. S.; Park, H. J. Effect of Microencapsulation on Viability and Other Characteristics in Lactobacillus acidophilus ATCC 43121. LWT Food Sci. Technol. 2008, 41(3), 493–500. DOI: 10.1016/j.lwt.2007.03.025.
- Shori, A. B. Microencapsulation Improved Probiotics Survival During Gastric Transit. HAYATI Journal Of Biosciences. 2017, 24(1), 1–5. DOI: 10.1016/j.hjb.2016.12.008.
- Yang, W.; Wang, L.; Ban, Z.; Yan, J.; Lu, H.; Zhang, X.; Wu, Q.; Aghdam, M. S.; Luo, Z.; Li, L. Efficient Microencapsulation of Syringa Essential Oil; the Valuable Potential on Quality Maintenance and Storage Behavior of Peach. Food Hydrocoll. 2019, 95, 177–185. DOI: 10.1016/j.foodhyd.2019.04.033.
- Gharsallaoui, A.; Roudaut, G.; Chambin, O.; Voilley, A.; Saurel, R. Applications of Spray-Drying in Microencapsulation of Food Ingredients: An Overview. Food Res. Int. 2007, 40(9), 1107–1121. DOI: 10.1016/j.foodres.2007.07.004.
- Călinoiu, L. F.; Eugenia Ştefănescu, B.; Delia Pop, I.; Muntean, L.; Cristian Vodnar, D. Chitosan Coating Applications in Probiotic Microencapsulation. Coatings. 2019, 9(3), 194. DOI: 10.3390/coatings9030194.
- Monnard, P. A.; Oberholzer, T.; Luisi, P. Entrapment of Nucleic Acids in Liposomes. Biochim. Biophys. Acta Biomembr. 1997, 1329(1), 39–50. DOI: 10.1016/S0005-2736(97)00066-7.
- Pavli, F.; Tassou, C.; Nychas, G. J. E.; Chorianopoulos, N. Probiotic Incorporation in Edible Films and Coatings: Bioactive Solution for Functional Foods. Int. J. Mol. Sci. 2018, 19(1), 150. DOI: 10.3390/ijms19010150.
- Ayoub, A.; Sood, M.; Singh, J.; Bandral, J. D.; Gupta, N.; Bhat, A. Microencapsulation and Its Applications in Food Industry. J. Pharmacogn. Phytochem. 2019, 8(3), 32–37.
- Quirós-Sauceda, A. E.; Ayala-Zavala, J. F.; Olivas, G. I.; González-Aguilar, G. A. Edible Coatings as Encapsulating Matrices for Bioactive Compounds: A Review. J. Food Sci. Technol. 2014, 51(9), 1674–1685. DOI: 10.1007/s13197-013-1246-x.
- Janjarasskul, T.; Krochta, J. M. Edible Packaging Materials. Ann. Rev. Food Sci. Technol. 2010, 1(1), 415–448. DOI: 10.1146/annurev.food.080708.100836.
- Fazilah, N. F.; Hamidon, N. H.; Ariff, A. B.; Khayat, M. E.; Wasoh, H.; Halim, M. Microencapsulation of Lactococcus lactis Gh1 with Gum Arabic and Synsepalum Dulcificum via Spray Drying for Potential Inclusion in Functional Yogurt. Molecules. 2019, 24(7), 1422. DOI: 10.3390/molecules24071422.
- Acordi Menezes, L. A.; Matias de Almeida, C. A.; Mattarugo, N. M. D. S.; Ferri, E. A. V.; Bittencourt, P. R. S.; Colla, E.; Drunkler, D. A. Soy Extract and Maltodextrin as Microencapsulating Agents for Lactobacillus acidophilus: A Model Approach. J. Microencapsulation. 2018, 35(7–8), 705–719. DOI: 10.1080/02652048.2019.1579264.
- Silva, M. P.; Tulini, F. L.; Matos-Jr, F. E.; Oliveira, M. G.; Thomazini, M.; Fávaro-Trindade, C. S. Application of Spray Chilling and Electrostatic Interaction to Produce Lipid Microparticles Loaded with Probiotics as an Alternative to Improve Resistance Under Stress Conditions. Food Hydrocoll. 2018, 83, 109–117. DOI: 10.1016/j.foodhyd.2018.05.001.
- Arslan-Tontul, S.; Erbas, M. Single and Double Layered Microencapsulation of Probiotics by Spray Drying and Spray Chilling. LWT Food Sci. Technol. 2017, 81, 160–169. DOI: 10.1016/j.lwt.2017.03.060.
- Dolly, P.; Anishaparvin, A.; Joseph, G. S.; Anandharamakrishnan, C. Microencapsulation of Lactobacillus Plantarum (Mtcc 5422) by Spray-Freeze-Drying Method and Evaluation of Survival in Simulated Gastrointestinal Conditions. J. Microencapsulation. 2011, 28(6), 568–574. DOI: 10.3109/02652048.2011.599435.
- Semyonov, D.; Ramon, O.; Kaplun, Z.; Levin-Brener, L.; Gurevich, N.; Shimoni, E. Microencapsulation of Lactobacillus Paracasei by Spray Freeze Drying. Food Res. Int. 2010, 43(1), 193–202. DOI: 10.1016/j.foodres.2009.09.028.
- Boonanuntanasarn, S.; Ditthab, K.; Jangprai, A.; Nakharuthai, C. Effects of Microencapsulated Saccharomyces cerevisiae on Growth, Hematological Indices, Blood Chemical, and Immune Parameters and Intestinal Morphology in Striped Catfish, Pangasianodon Hypophthalmus. Probiotics Antimicrob. Proteins. 2019, 11(2), 427–437. DOI: 10.1007/s12602-018-9404-0.
- Rajam, R.; Kumar, S. B.; Prabhasankar, P.; Anandharamakrishnan, C. Microencapsulation of Lactobacillus Plantarum MTCC 5422 in Fructooligosaccharide and Whey Protein Wall Systems and Its Impact on Noodle Quality. J. Food Sci. Technol. 2015, 52(7), 4029–4041. DOI: 10.1007/s13197-014-1506-4.
- Coghetto, C. C.; Brinques, G. B.; Siqueira, N. M.; Pletsch, J.; Soares, R. M. D.; Ayub, M. A. Z. Electrospraying Microencapsulation of Lactobacillus Plantarum Enhances Cell Viability Under Refrigeration Storage and Simulated Gastric and Intestinal Fluids. J. Funct. Foods. 2016, 24, 316–326. DOI: 10.1016/j.jff.2016.03.036.
- Gomez-Mascaraque, L. G.; Morfin, R. C.; Pérez-Masiá, R.; Sanchez, G.; Lopez-Rubio, A. Optimization of Electrospraying Conditions for the Microencapsulation of Probiotics and Evaluation of Their Resistance During Storage and in-Vitro Digestion. LWT Food Sci. Technol. 2016, 69, 438–446. DOI: 10.1016/j.lwt.2016.01.071.
- Priya, A. J.; Vijayalakshmi, S. P.; Raichur, A. M. Enhanced Survival of Probiotic Lactobacillus acidophilus by Encapsulation with Nanostructured Polyelectrolyte Layers Through Layer-By-Layer Approach. J. Agric. Food Chem. 2011, 59(21), 11838–11845. DOI: 10.1021/jf203378s.
- McHugh, D. J. Production and Utilization of Products from Commercial Seaweeds; FAO: Rome, Italy, 1987.
- Pitigraisorn, P.; Srichaisupakit, K.; Wongpadungkiat, N.; Wongsasulak, S. Encapsulation of Lactobacillus acidophilus in Moist-Heat-Resistant Multilayered Microcapsules. J. Food Eng. 2017, 192, 11–18. DOI: 10.1016/j.jfoodeng.2016.07.022.
- Penhasi, A. Microencapsulation of Probiotic Bacteria Using Thermo-Sensitive Sol-Gel Polymers for Powdered Infant Formula. J. Microencapsulation. 2015, 32(4), 372–380. DOI: 10.3109/02652048.2015.1028497.
- Deshpande, H.; Kharat, V.; Katke, S.; Jadhav, V. B. Studies on Process Standardization and Sensory Evaluation of Probiotic Chocolate. Int. J. Curr. Microbiol. Appl. Sci. 2019, 8, 1527–1534.
- Lee, S.; Kirkland, R.; Grunewald, Z. I.; Sun, Q.; Wicker, L.; de La Serre, C. B. Beneficial Effects of Non-Encapsulated or Encapsulated Probiotic Supplementation on Microbiota Composition, Intestinal Barrier Functions, Inflammatory Profiles, and Glucose Tolerance in High Fat Fed Rats. Nutrients. 2019, 11(9), 1975. DOI: 10.3390/nu11091975.
- De Prisco, A.; Maresca, D.; Ongeng, D.; Mauriello, G. Microencapsulation by Vibrating Technology of the Probiotic Strain Lactobacillus Reuteri DSM 17938 to Enhance Its Survival in Foods and in Gastrointestinal Environment. LWT Food Sci. Technol. 2015, 61(2), 452–462. DOI: 10.1016/j.lwt.2014.12.011.
- Pop, O. L.; Brandau, T.; Schwinn, J.; Vodnar, D. C.; Socaciu, C. The Influence of Different Polymers on Viability of Bifidobacterium Lactis 300b During Encapsulation, Freeze-Drying and Storage. J. Food Sci. Technol. 2015, 52(7), 4146–4155. DOI: 10.1007/s13197-014-1441-4.
- Setijawati, D.; Nursyam, H.; Salis, H. Carrageenan: The Difference Between PNG and KCL Gel Precipitation Method as Lactobacillus acidophilus Encapsulation Material. IOP Conference Series: Earth And Environmental Science. April 2018, 137(1), 012073. IOP Publishing. DOI: 10.1088/1755-1315/137/1/012073.
- Silva, T. M. D.; Barin, J. S.; Lopes, E. J.; Cichoski, A. J.; Flores, E. M. D. M.; Silva, C. D. B. D.; Menezes, C. R. D. Development, Characterization and Viability Study of Probiotic Microcapsules Produced by Complex Coacervation Followed by Freeze-Drying. Ciência Rural. 2019, 7, 49.
- Bosnea, L. A.; Moschakis, T.; Nigam, P. S.; Biliaderis, C. G. Growth Adaptation of Probiotics in Biopolymer-Based Coacervate Structures to Enhance Cell Viability. LWT. 2017, 77, 282–289. DOI: 10.1016/j.lwt.2016.11.056.
- Martins, E.; Poncelet, D.; Rodrigues, R. C.; Renard, D. Oil Encapsulation Techniques Using Alginate as Encapsulating Agent: Applications and Drawbacks. J. Microencapsulation. 2017, 34(8), 754–771. DOI: 10.1080/02652048.2017.1403495.
- Motalebi Moghanjougi, Z.; Rezazadeh Bari, M.; Alizadeh Khaledabad, M.; Amiri, S.; Almasi, H. Microencapsulation of Lactobacillus acidophilus LA‐5 and Bifidobacterium Animalis BB‐12 in Pectin and Sodium Alginate: A Comparative Study on Viability, Stability, and Structure. Food Science & Nutrition. 2021, 9(9), 5103–5111. DOI: 10.1002/fsn3.2470.
- Holkem, A. T.; Raddatz, G. C.; Barin, J. S.; Flores, É. M. M.; Muller, E. I.; Codevilla, C. F.; Jacob-Lopes, E.; Ferreira Grosso, C. R.; de Menezes, C. R. Production of Microcapsules Containing Bifidobacterium BB-12 by Emulsification/Internal Gelation. LWT Food Sci. Technol. 2017, 76, 216–221. DOI: 10.1016/j.lwt.2016.07.013.
- Özer, B.; Uzun, Y. S.; Kirmaci, H. A. Effect of Microencapsulation on Viability of Lactobacillus acidophilus LA‐5 and Bifidobacterium Bifidum BB‐12 During Kasar Cheese Ripening. Int. J. Dairy Technol. 2008, 61(3), 237–244. DOI: 10.1111/j.1471-0307.2008.00408.x.
- McMaster, L. D.; Kokott, S. A. Micro-Encapsulation of Bifidobacterium Lactis for Incorporation into Soft Foods. World J. Microbiol. Biotechnol. 2005, 21(5), 723–728. DOI: 10.1007/s11274-004-4798-0.
- Chávarri, M.; Marañón, I.; Ares, R.; Ibáñez, F. C.; Marzo, F.; Del Carmen Villarán, M. Microencapsulation of a Probiotic and Prebiotic in Alginate-Chitosan Capsules Improves Survival in Simulated Gastro-Intestinal Conditions. Int. J. Food Microbiol. 2010, 142(1–2), 185–189. DOI: 10.1016/j.ijfoodmicro.2010.06.022.
- Yeung, T. W.; Üçok, E. F.; Tiani, K. A.; McClements, D. J.; Sela, D. A. Microencapsulation in Alginate and Chitosan Microgels to Enhance Viability of Bifidobacterium longum for Oral Delivery. Front. Microbiol. 2016, 7, 494. DOI: 10.3389/fmicb.2016.00494.
- O’riordan, K.; Andrews, D.; Buckle, K.; Conway, P. Evaluation of Microencapsulation of a Bifidobacterium Strain with Starch as an Approach to Prolonging Viability During Storage. J. Appl. Microbiol. 2001, 91(6), 1059–1066. DOI: 10.1046/j.1365-2672.2001.01472.x.
- Sarao, L. K.; Arora, M. Probiotics, Prebiotics, and Microencapsulation: A Review. Crit. Rev. Food Sci. Nutr. 2017, 57(2), 344–371. DOI: 10.1080/10408398.2014.887055.
- Sahariah, P.; Másson, M. Antimicrobial Chitosan and Chitosan Derivatives: A Review of the Structure–Activity Relationship. Biomacromolecules. 2017, 18(11), 3846–3868. DOI: 10.1021/acs.biomac.7b01058.
- Zhao, L.; Zhu, B.; Jia, Y.; Hou, W.; Su, C. Preparation of Biocompatible Carboxymethyl Chitosan Nanoparticles for Delivery of Antibiotic Drug. Biomed Res. Int. 2013, 2013, 1–7. DOI: 10.1155/2013/236469.
- Ribeiro, M. C. E.; Chaves, K. S.; Gebara, C.; Infante, F. N.; Grosso, C. R.; Gigante, M. L. Effect of Microencapsulation of Lactobacillus acidophilus LA-5 on Physicochemical, sensory and Microbiological Characteristics of Stirred Probiotic Yoghurt. Food Res. Int. 2014, 66, 424–431. DOI: 10.1016/j.foodres.2014.10.019.
- Nag, A.; Han, K. S.; Singh, H. Microencapsulation of Probiotic Bacteria Using Ph-Induced Gelation of Sodium Caseinate and Gellan Gum. Int. Dairy J. 2011, 21(4), 247–253. DOI: 10.1007/s11694-018-9835-z.
- Mokhtari, S.; Jafari, S. M.; Khomeiri, M.; Maghsoudlou, Y.; Ghorbani, M. The Cell Wall Compound of Saccharomyces cerevisiae as a Novel Wall Material for Encapsulation of Probiotics. Food Res. Int. 2017, 96, 19–26. DOI: 10.1016/j.foodres.2017.03.014.
- Gbassi, G. K.; Vandamme, T.; Ennahar, S.; Marchioni, E. Microencapsulation of Lactobacillus Plantarum Spp in an Alginate Matrix Coated with Whey Proteins. Int. J. Food Microbiol. 2009, 129(1), 103–105. DOI: 10.1016/j.ijfoodmicro.2008.11.012.
- Doherty, S. B.; Gee, V. L.; Ross, R. P.; Stanton, C.; Fitzgerald, G. F.; Brodkorb, A. Development and Characterisation of Whey Protein Micro-Beads as Potential Matrices for Probiotic Protection. Food Hydrocoll. 2011, 25(6), 1604–1617. DOI: 10.1016/j.foodhyd.2010.12.012.
- Krasaekoopt, W.; Bhandari, B.; Deeth, H. Evaluation of Encapsulation Techniques of Probiotics for Yoghurt. Int. Dairy J. 2003, 13(1), 3–13. DOI: 10.1016/S0958-6946(02)00155-3.
- Fareez, I. M.; Lim, S. M.; Lim, F. T.; Mishra, R. K.; Ramasamy, K. Microencapsulation of Lactobacillus Sp. Using Chitosan‐alginate‐xanthan Gum‐β‐cyclodextrin and Characterization of Its Cholesterol Reducing Potential and Resistance Against pH, Temperature and Storage. J. Food Process Eng. 2017, 40(3), e12458. DOI: 10.1111/jfpe.12458.
- Zia, K. M.; Tabasum, S.; Khan, M. F.; Akram, N.; Akhter, N.; Noreen, A.; Zuber, M. Recent Trends on Gellan Gum Blends with Natural and Synthetic Polymers: A Review. Int. J. Biol. Macromol. 2018, 109, 1068–1087. DOI: 10.1016/j.ijbiomac.2017.11.099.
- Fang, Z.; Bhandari, B. Spray Drying, Freeze Drying and Related Processes for Food Ingredient and Nutraceutical Encapsulation. In Encapsulation Technologies and Delivery Systems for Food Ingredients and Nutraceuticals; Woodhead Publishing: 2012; pp. 73–109. DOI:10.1533/9780857095909.2.73.
- Oxley, J. D. Spray Cooling and Spray Chilling for Food Ingredient and Nutraceuticalencapsulation. In Encapsulation Technologies and Delivery Systems for Food Ingredients and Nutraceuticals; Woodhead Publishing: 2012; pp. 110–130. DOI:10.1533/9780857095909.2.110.
- Ali, M. E.; Lamprecht, A. Spray Freeze Drying as an Alternative Technique for Lyophilization of Polymeric and Lipid-Based Nanoparticles. Int. J. Pharmaceutics. 2017, 516(1–2), 170–177. DOI: 10.1016/j.ijpharm.2016.11.023.
- Mortazavian, A.; Razavi, S. H.; Ehsani, M. R.; Sohrabvandi, S. Principles and Methods of Microencapsulation of Probiotic Microorganisms. Iran. J. Biotechnol. 2007, 5(1), 1–18.
- Whelehan, M.; Marison, I. W. Microencapsulation Using Vibrating Technology. J. Microencapsulation. 2011, 28(8), 669–688. DOI: 10.3109/02652048.2011.586068.
- Rayleigh, L. On the Capillary Phenomena of Jets. Proc. R. Soc. London. 1879, 29(196–199), 71–97.
- Olivares, A.; Silva, P.; Altamirano, C. Microencapsulation of Probiotics By efficient Vibration Technology. J. Microencapsulation. 2017, 34(7), 667–674. DOI: 10.1080/02652048.2017.1390005.
- Shi, L. E.; Li, Z. H.; Li, D. T.; Xu, M.; Chen, H. Y.; Zhang, Z. L.; Tang, Z. X. Encapsulation of Probiotic Lactobacillus Bulgaricus in Alginate–Milk Microspheres and Evaluation of the Survival in Simulated Gastrointestinal Conditions. J. Food Eng. 2013, 117(1), 99–104. DOI: 10.1016/j.jfoodeng.2013.02.012.
- Garti, N.; Aserin, A. Micelles and Microemulsions as Food Ingredient and Nutraceutical Delivery Systems. In Encapsulation Technologies and Delivery Systems for Food Ingredients and Nutraceuticals; Woodhead Publishing: 2012; pp. 211–251. DOI:10.1533/9780857095909.3.211.
- Singh, H.; Thompson, A.; Liu, W.; Corredig, M. Liposomes as Food Ingredients and Nutraceutical Delivery Systems. In Encapsulation Technologies and Delivery Systems for Food Ingredients and Nutraceuticals; Woodhead Publishing: 2012; pp. 287–318. DOI:10.1533/9780857095909.3.287.
- Jaworek, A. Electrohydrodynamic Microencapsulation Technology. In Encapsulations; Academic Press: 2016; pp. 1–45. DOI:10.1016/B978-0-12-804307-3.00001-6.
- Yan, J.; Luo, Z.; Ban, Z.; Lu, H.; Li, D.; Yang, D.; Aghdam, M. S.; Li, L. The Effect of the Layer- By-Layer (LBL) Edible Coating on Strawberry Quality and Metabolites During Storage. Postharvest. Biol. Technol. 2019, 147, 29–38. DOI: 10.1016/j.postharvbio.2018.09.002.
- Ramos, P. E.; Cerqueira, M. A.; Teixeira, J. A.; Vicente, A. A. Physiological Protection of Probiotic Microcapsules by Coatings. Crit. Rev. Food Sci. Nutr. 2018, 58(11), 1864–1877. DOI: 10.1080/10408398.2017.1289148.
- Jeyakumari, A.; Zynudheen, A. A.; Parvathy, U. Microencapsulation of Bioactive Food Ingredients and Controlled Release-A Review. 2016.
- Poshadri, A.; Aparna, K. Microencapsulation Technology: A Review. Journal Of Research ANGRAU. 2010, 38(1), 86–102.
- Tzia, C.; Tasios, L.; Spiliotaki, T.; Chranioti, C.; Giannou, V. 16 Edible Coatings and Films to Preserve Quality of Fresh Fruits and Vegetables. Food Preservation. 2016, 531.
- Lacroix, M. Mechanical and Permeability Properties of Edible Films and Coatings for Food and Pharmaceutical Applications. In Edible Films and Coatings for Food Applications; Springer: New York, NY, 2009; pp. 347–366. DOI: 10.1007/978-0-387-92824-1_13.
- Desai, K. G. H.; Jin Park, H. Recent Developments in Microencapsulation of Food Ingredients. Drying Technol. 2005, 23(7), 1361–1394. DOI: 10.1081/DRT-200063478.
- de Almeida Paula, D.; Martins, E. M. F.; de Almeida Costa, N.; de Oliveira, P. M.; de Oliveira, E. B.; Ramos, A. M. Use of Gelatin and Gum Arabic for Microencapsulation of Probiotic Cells from Lactobacillus Plantarum by a Dual Process Combining Double Emulsification Followed by Complex Coacervation. Int. J. Biol. Macromol. 2019, 133, 722–731. DOI: 10.1016/j.ijbiomac.2019.04.110.
- Xiao, J. X.; Huang, G. Q.; Wang, S. Q.; Sun, Y. T. Microencapsulation of Capsanthin by Soybean Protein Isolate‐chitosan Coacervation and Microcapsule Stability Evaluation. J. Appl. Polym. Sci. 2014, 131(1). DOI: 10.1002/app.39671.
- Weinbreck, F.; De Vries, R.; Schrooyen, P.; De Kruif, C. G. Complex Coacervation of Whey Proteins and Gum Arabic. Biomacromolecules. 2003, 4(2), 293–303. DOI: 10.1021/bm025667n.
- Nualkaekul, S.; Cook, M. T.; Khutoryanskiy, V. V.; Charalampopoulos, D. Influence of Encapsulation and Coating Materials on the Survival of Lactobacillus Plantarum and Bifidobacterium longum in Fruit Juices. Food Res. Int. 2013, 53(1), 304–311. DOI: 10.1016/j.foodres.2013.04.019.
- Tavassoli-Kafrani, E.; Shekarchizadeh, H.; Masoudpour-Behabadi, M. Development of Edible Films and Coatings from Alginates and Carrageenans. Carbohydratepolymers. 2016, 137, 360–374. DOI: 10.1016/j.carbpol.2015.10.074.
- Wandrey, C.; Bartkowiak, A.; Harding, S. E. Materials for Encapsulation. In Encapsulation Technologies for Active Food Ingredients and Food Processing; Springer: New York, NY, 2010; pp. 31–100. DOI: 10.1007/978-1-4419-1008-0_3.
- Dafe, A.; Etemadi, H.; Zarredar, H.; Mahdavinia, G. R. Development of Novel Carboxymethyl Cellulose/k-Carrageenan Blends as an Enteric Delivery Vehicle for Probiotic Bacteria. Int. J. Biol. Macromol. 2017, 97, 299–307. DOI: 10.1016/j.ijbiomac.2017.01.016.
- Yucel Falco, C.; Amadei, F.; Dhayal, S. K.; Cárdenas, M.; Tanaka, M.; Risbo, J. Hybrid Coating of Alginate Microbeads Based on Protein‐biopolymer Multilayers for Encapsulation of Probiotics. Biotechnol. Prog. 2019, 35(3), e2806. DOI: 10.1002/btpr.2806.
- Anselmo, A. C.; McHugh, K. J.; Webster, J.; Langer, R.; Jaklenec, A. Layer‐by‐layer Encapsulation of Probiotics for Delivery to the Microbiome. Adv.Mate. 2016, 28(43), 9486–9490. DOI: 10.1002/adma.201603270.
- Mandal, S.; Hati, S.; Puniya, A. K.; Khamrui, K.; Singh, K. Enhancement of survival of alginate‐encapsulated Lactobacillus casei NCDC 298. J. Sci. Food Agric. 2014, 94(10), 1994–2001. DOI: 10.1002/jsfa.6514.
- BeMiller, J. N. Starches: Conversions, modifications, and uses. Carbohydrate chemistry for food scientists. 2019, 191–221.
- Ying, D.; Sanguansri, L.; Weerakkody, R.; Bull, M.; Singh, T. K.; Augustin, M. A. Effect of encapsulant matrix on stability of microencapsulated probiotics. J. Funct. Foods. 2016, 25, 447–458. DOI: 10.1016/j.jff.2016.06.020.
- Muzzafar, A.; Sharma, V. Microencapsulation of probiotics for incorporation in cream biscuits. J. Food Meas. Charact. 2018, 12(3), 2193–2201. DOI: 10.1007/s11694-018-9835-z.
- Tzaneva, D.; Simitchiev, A.; Petkova, N.; Nenov, V.; Stoyanova, A.; Denev, P. Synthesis of carboxymethyl chitosan and its rheological behaviour in pharmaceutical and cosmetic emulsions. J. Appl. Pharm. Sci. 2017, 7(10), 070–078. DOI: 10.7324/JAPS.2017.71010.
- Zou, Q.; Zhao, J.; Liu, X.; Tian, F.; Zhang, H. P.; Zhang, H.; Chen, W. Microencapsulation of Bifidobacterium bifidum F‐35 in reinforced alginate microspheres prepared by emulsification/internal gelation. International journal of food science & technology. 2011, 46(8), 1672–1678. DOI: 10.1111/j.1365-2621.2011.02685.x.
- Baldwin, E. A.; Hagenmaier, R.; Bai, J., Eds. Edible coatings and films to improve food quality; CRC press, 2011.
- Karim, A. A.; Bhat, R. Gelatin alternatives for the food industry: recent developments, challenges and prospects. Trends in food science & technology. 2008, 19(12), 644–656. DOI: 10.1016/j.tifs.2008.08.001.
- Flores-Belmont, I. A.; Palou, E.; López-Malo, A.; Jiménez-Munguía, M. T. Simple and double microencapsulation of Lactobacillus acidophilus with chitosan using spray drying. Int. J. Food Stud. 2015, 4(2).
- Aramwit, P.; Jaichawa, N.; Ratanavaraporn, J.; Srichana, T. A comparative study of type A and type B gelatin nanoparticles as the controlled release carriers for different model compounds. Mater. Express. 2015, 5(3), 241–248. DOI: 10.1166/mex.2015.1233.
- Meng, Y.; Cloutier, S. Gelatin and other proteins for microencapsulation. In Microencapsulation in the food industry; Academic Press: 2014; pp. 227–239. DOI:10.1016/B978-0-12-821683-5.00021-2.
- Thies, C. Microencapsulation of flavors by complex coacervation. Encapsulation and controlled release technologies in food systems. 2007, 149–170. DOI: 10.1002/9780470277881.ch7.
- Holkem, A. T.; Favaro-Trindade, C. S. Potential of solid lipid microparticles covered by the protein-polysaccharide complex for protection of probiotics and proanthocyanidin-rich cinnamon extract. Food Res. Int. 2020, 136, 109520. DOI: 10.1016/j.foodres.2020.109520.
- Praepanitchai, O. A.; Noomhorm, A.; Anal, A. K. Survival and behavior of encapsulated probiotics (Lactobacillus plantarum) in calcium-alginate-soy protein isolate based hydrogel beads in different processing conditions (pH and temperature) and in pasteurized mango juice. Biomed Res. Int. 2019, 2019, 1–8. DOI: 10.1155/2019/9768152.
- Rizzo, G.; Baroni, L. Soy, soy foods and their role in vegetarian diets. Nutrients. 2018, 10(1), 43. DOI: 10.3390/nu10010043.
- Wang, X.; Luo, K.; Liu, S.; Adhikari, B.; Chen, J. Improvement of gelation properties of soy protein isolate emulsion induced by calcium cooperated with magnesium. J. Food Eng. 2019, 244, 32–39. DOI: 10.1016/j.jfoodeng.2018.09.025.
- Wang, X.; Zeng, M.; Qin, F.; Adhikari, B.; He, Z.; Chen, J. Enhanced CaSO4 induced gelation properties of soy protein isolate emulsion by pre-aggregation. Food Chem. 2018, 242, 459–465. DOI: 10.1016/j.foodchem.2017.09.044.
- Augustin, M. A.; Oliver, C. M. Use of milk proteins for encapsulation of food ingredients. Microencapsulation in the food industry. 2014, 211–226. DOI: 10.1016/B978-0-12-404568-2.00019-4.
- Mulvihill, D.; Donovan, M. Whey proteins and their thermal denaturation-a review. Irish Journal of Food Science and Technology. 1987, 11(1), 43–75.
- Visker, M. H. P. W.; Heck, J. M.; Van Valenberg, H. J.; Van Arendonk, J. A.; Bovenhuis, H. Short communication: A new bovine milk-protein variant: α-Lactalbumin variant. DJ Dairy Sci. 2012, 95(4), 2165–2169. DOI: 10.3168/jds.2011-4794.
- Sarode, A. R.; Sawale, P. D.; Khedkar, C. D.; Kalyankar, S. D.; Pawshe, R. D. Casein and caseinate: methods of manufacture. 2016.
- DeJong, G. A. H.; Koppelman, S. J. Transglutaminase catalyzed reactions: impact on food applications. J. Food Sci. 2002, 67(8), 2798–2806. DOI: 10.1111/j.1365-2621.2002.tb08819.x.
- Heidebach, T.; Först, P.; Kulozik, U. Transglutaminase-induced caseinate gelation for the microencapsulation of probiotic cells. Int. Dairy J. 2009, 19(2), 77–84. DOI: 10.1016/j.idairyj.2008.08.003.
- Heidebach, T.; Först, P.; Kulozik, U. Influence of casein-based microencapsulation on freeze-drying and storage of probiotic cells. J. Food Eng. 2010, 98(3), 309–316. DOI: 10.1016/j.jfoodeng.2010.01.003.
- Morr, C. V.; Ha, E. Y. W. Whey protein concentrates and isolates: processing and functional properties. Critical Reviews in Food Science & Nutrition. 1993, 33(6), 431–476. DOI: 10.1080/10408399309527643.
- Fox, P. F. Milk: an overview. Milk proteins. 2008, 1–54. DOI: 10.1016/B978-0-12-374039-7.00001-5.
- Rojas, S. A.; Goff, H. D.; Senaratne, V.; Dalgleish, D. G.; Flores, A. Gelation of commercial fractions of β-lactoglobulin and α-lactalbumin. Int. Dairy J. 1997, 7(1), 79–85. DOI: 10.1016/S0958-6946(96)00045-3.
- Santos, M. B.; da Costa, N. R.; Garcia‐rojas, E. E. Interpolymeric complexes formed between whey proteins and biopolymers: Delivery systems of bioactive ingredients. Compr. Rev. Food Sci. Food Saf. 2018, 17(3), 792–805. DOI: 10.1111/1541-4337.12350.
- Layman, D. K.; Lönnerdal, B.; Fernstrom, J. D. Applications for α-lactalbumin in human nutrition. Nutr. Rev. 2018, 76(6), 444–460. DOI: 10.1093/nutrit/nuy004.
- Larsen, L. B.; Wedholm-Pallas, A.; Lindmark-Månsson, H.; Andrén, A. Different proteomic profiles of sweet whey and rennet casein obtained after preparation from raw versus heat-treated skimmed milk. Dairy Science & Technology. 2010, 90(6), 641–656. DOI: 10.1051/dst/2010024.
- Pinto, S. S.; Verruck, S.; Vieira, C. R.; Prudêncio, E. S.; Amante, E. R.; Amboni, R. D. Influence of microencapsulation with sweet whey and prebiotics on the survival of Bifidobacterium-BB-12 under simulated gastrointestinal conditions and heat treatments. LWT Food Sci. Technol. 2015, 64(2), 1004–1009. DOI: 10.1016/j.lwt.2015.07.020.
- Liu, H.; Xie, M.; Nie, S. Recent trends and applications of polysaccharides for microencapsulation of probiotics. Food Front. 2020, 1(1), 45–59. DOI: 10.1002/fft2.11.
- Gruber, J. V. Polysaccharide-based polymers in cosmetics. Cosmetic Science and Technology Series. 1999, 325–390.
- Matalanis, A.; Jones, O. G.; McClements, D. J. Structured biopolymer-based delivery systems for encapsulation, protection, and release of lipophilic compounds. Food Hydrocoll. 2011, 25(8), 1865–1880. DOI: 10.1016/j.foodhyd.2011.04.014.
- Gaudreau, H.; Champagne, C. P.; Remondetto, G. E.; Gomaa, A.; Subirade, M. Co-encapsulation of Lactobacillus helveticus cells and green tea extract: Influence on cell survival in simulated gastrointestinal conditions. J. Funct. Foods. 2016, 26, 451–459. DOI: 10.1016/j.jff.2016.08.002.
- Chakraborty, S. Carrageenan for encapsulation and immobilization of flavor, fragrance, probiotics, and enzymes: A review. J. Carbohydr. Chem. 2017, 36(1), 1–19. DOI: 10.1080/07328303.2017.1347668.
- Yuguchi, Y.; Thuy, T. T. T.; Urakawa, H.; Kajiwara, K. Structural characteristics of carrageenan gels: temperature and concentration dependence. Food Hydrocoll. 2002, 16(6), 515–522. DOI: 10.1016/S0268-005X(01)00131-X.
- Özer, B.; Kirmaci, H. A.; Şenel, E.; Atamer, M.; Hayaloğlu, A. Improving the viability of Bifidobacterium bifidum BB-12 and Lactobacillus acidophilus LA-5 in white brined cheese by microencapsulation. Int. Dairy J. 2009, 19(1), 22–29. DOI: 10.1016/j.idairyj.2008.07.001.
- Jansson, P. E.; Kenne, L.; Lindberg, B. Structure of the extracellular polysaccharide from Xanthomonas campestris. Carbohydr. Res. 1975, 45(1), 275–282. DOI: 10.1016/S0008-6215(00)85885-1.
- Petri, D. F. Xanthan gum: A versatile biopolymer for biomedical and technological applications. J. Appl. Polym. Sci. 2015, 132(23). DOI: 10.1002/app.42035.
- Kool, M. M.; Gruppen, H.; Sworn, G.; Schols, H. A. The influence of the six constituent xanthan repeating units on the order–disorder transition of xanthan. Carbohydr. Polym. 2014, 104, 94–100. DOI: 10.1016/j.carbpol.2013.12.073.
- Wu, M.; Qu, J.; Shen, Y.; Dai, X.; Wei, W.; Shi, Z.; Li, G.; Ma, T. Gel properties of xanthan containing a single repeating unit with saturated pyruvate produced by an engineered Xanthomonas campestris CGMCC 15155. Food Hydrocoll. 2019, 87, 747–757. DOI: 10.1016/j.foodhyd.2018.09.002.
- Valero-Cases, E.; Frutos, M. J. Effect of different types of encapsulation on the survival of Lactobacillus plantarum during storage with inulin and in vitro digestion. LWT. Food Sci. Technol. 2015, 64(2), 824–828. DOI: 10.1016/j.lwt.2015.06.049.
- Fareez, I. M.; Lim, S. M.; Mishra, R. K.; Ramasamy, K. Chitosan coated alginate–xanthan gum bead enhanced pH and thermotolerance of Lactobacillus plantarum LAB12. Int. J. Biol. Macromol. 2015, 72, 1419–1428. DOI: 10.1016/j.ijbiomac.2014.10.054.
- Karlton-Senaye, B. D.; Ibrahim, S. A. Impact of gums on the growth of probiotics. Agro food Ind. Hi Tech. 2013, 24(4), 10–14.
- Nussinovitch, A. Hydrocolloids in Flavor Encapsulation. Water-Soluble Polymer Applications in Foods; Nussinovitch, A. Ed, 2003; pp. 31–113.
- Burnside, E. Hydrocolloids and gums as encapsulating agents. In Microencapsulation in the food industry; Academic Press, 2014; pp. 241–252.
- Cui, S. W. Food carbohydrates: chemistry, physical properties, and applications; CRC press, 2005.
- Elieh-Ali-Komi, D.; Hamblin, M. R. Chitin and chitosan: production and application of versatile biomedical nanomaterials. Int. J. Adv. Res. 2016, 4(3), 411.
- Wallick, D. Cellulose polymers in microencapsulation of food additives. In Microencapsulation in the Food Industry; Academic Press: 2014; pp. 181–193. DOI:10.1016/B978-0-12-404568-2.00017-0.
- Liu, H.; Yang, Q.; Zhang, L.; Zhuo, R.; Jiang, X. Synthesis of carboxymethyl chitin in aqueous solution and its thermo-and pH-sensitive behaviors. Carbohydr. Polym. 2016, 137, 600–607. DOI: 10.1016/j.carbpol.2015.11.025.
- de Araújo Etchepare, M.; Raddatz, G. C.; de Moraes Flores, É. M.; Zepka, L. Q.; Jacob-Lopes, E.; Barin, J. S.; Ferreira Grosso, C. R. & de Menezes, C. R. Effect of resistant starch and chitosan on survival of Lactobacillus acidophilus microencapsulated with sodium alginate. LWT Food Sci. Technol. 2016, 65, 511–517. DOI: 10.1016/j.lwt.2015.08.039.
- Trabelsi, I.; Bejar, W.; Ayadi, D.; Chouayekh, H.; Kammoun, R.; Bejar, S.; Salah, R. B. Encapsulation in alginate and alginate coated-chitosan improved the survival of newly probiotic in oxgall and gastric juice. Int. J. Biol. Macromol. 2013, 61, 36–42. DOI: 10.1016/j.ijbiomac.2013.06.035.
- Nualkaekul, S.; Lenton, D.; Cook, M. T.; Khutoryanskiy, V. V.; Charalampopoulos, D. Chitosan coated alginate beads for the survival of microencapsulated Lactobacillus plantarum in pomegranate juice. Carbohydr. Polym. 2012, 90(3), 1281–1287. DOI: 10.1016/j.carbpol.2012.06.073.
- Shah, U.; Naqash, F.; Gani, A.; Masoodi, F. A. Art and science behind modified starch edible films and coatings: a review. Compr. Rev. Food Sci. Food Saf. 2016, 15(3), 568–580. DOI: 10.1111/1541-4337.12197.
- Gidwani, B.; Vyas, A. A comprehensive review on cyclodextrin-based carriers for delivery of chemotherapeutic cytotoxic anticancer drugs. Biomed Res. Int. 2015, 2015, 1–15. DOI: 10.1155/2015/198268.
- Pariot, N.; Edwards-Levy, F.; Andry, M. C.; Lévy, M. C. Cross-linked β-cyclodextrin microcapsules: preparation and properties. Int. J. Pharmaceutics. 2000, 211(1–2), 19–27. DOI: 10.1016/S0378-5173(00)00576-7.
- Rao, A. V.; Shiwnarain, N.; Maharaj, I. Survival of microencapsulated Bifidobacterium pseudolongum in simulated gastric and intestinal juices. Can. Inst. Food Sci. Technol. J. 1989, 22(4), 345–349. DOI: 10.1016/S0315-5463(89)70426-0.
- Li, X. Y.; Chen, X. G.; Sun, Z. W.; Park, H. J.; Cha, D. S. Preparation of alginate/chitosan/carboxymethyl chitosan complex microcapsules and application in Lactobacillus casei ATCC 393. Carbohydr. Polym. 2011, 83(4), 1479–1485. DOI: 10.1016/j.carbpol.2010.09.053.
- Shit, S. C.; Shah, P. M. Edible polymers: challenges and opportunities. J. Polym. 2014, 2014, 1–13. DOI: 10.1155/2014/427259.
- Vargas, M.; Pastor, C.; Chiralt, A.; McClements, D. J.; Gonzalez-Martinez, C. Recent advances in edible coatings for fresh and minimally processed fruits. Crit. Rev. Food Sci. Nutr. 2008, 48(6), 496–511. DOI: 10.1080/10408390701537344.
- Jackson, L. S.; Lee, K. Microencapsulation and the food industry. Lebensm Wiss Technol. 1991, 24(4), 289–297.
- Singh, M. N.; Hemant, K. S. Y.; Ram, M.; Shivakumar, H. G. Microencapsulation:A promising technique for controlled drug delivery. Res. Pharm. Sci. 2010, 5(2), 65. DOI: 10.1016/j.carbpol.2015.10.074.
- Salaria, A.; Thompkinson, D. K.; Kumar, M. H.; Sabikhi, L. Prebiotics in the microencapsulating matrix enhance the viability of probiotic Lactobacillus acidophilus LA1. International Journal of Fermented Foods. 2013, 2(1), 33–45.