ABSTRACT
Dairy waste management to reduce environmental pollution through its utilization in various food and non-food products is a major concern of the dairy industry. Whey and buttermilk are regarded as the major waste materials of the dairy industry. Therefore, the current review aimed to summarize the various extraction methods and potential application of buttermilk and cheese whey in production of different products, i.e., microencapsulation matrix, beverages, baked products, microorganisms’ cultivation, edible films, exopolysaccharides, organic acids, and essential fatty acids. These wasted components can be extracted from the dairy products by utilizing various extraction methods such as conventional homogenization, ultra-high-pressure homogenization, ultrafiltration, reverse osmosis, and nanofiltration. In microencapsulation technology, these have been used to encapsulate various essential oils, probiotics and several bioactive substances. Whey and buttermilk-based beverages have also been prepared to accentuate their application in the beverage industry. Whey and buttermilk-based microbial fermented products have a significant role in value-added food production. Edible coatings prepared from whey protein concentrate are more accessible, environmentally friendly, nontoxic, and low cost. In addition, several bioactive substances such as exopolysaccharides, bioactive peptides, essential fatty acids, and organic acids have been extracted from the whey and buttermilk. Moreover, these have the potential to improve the physicochemical, organoleptic, and rheological properties (i.e. water absorption, gelatinization temperature, dough development time) of baked goods in the bakery industry. In conclusion, utilization of dairy waste materials in various products can be proved beneficial in introducing various dairy-based healthy products, reduction of environmental pollution and economic growth of the country.
Introduction
The elevated demand for dairy products is the leading cause of the boom in the dairy sector globally. The sectors shift from custom to mechanized production and from smaller to larger-scale dairy-based products to approach this demand. Albeit, the rapid industrial development does not only result in massive production but it may also result in higher concentrations of toxic compounds in water or land reservoirs. Consequently, it enhances environmental pollution, which may lead to severe health disorders and diseases in human beings.[Citation1] Owing to the presence of higher amounts of organic compounds, the waste from the dairy industry is a tangible threat to the environment if it remains untreated. On the other hand, those waste materials contained higher amounts of nutrients, organic materials, inorganic materials, biological oxygen demand (BOD), chemical oxygen demand (COD), sterilizing agents, and a variety of alkaline and acids detergents.[Citation2]
Whey and buttermilk are considered among the top-ranked waste materials of the dairy industry. Whey is a pellucid liquid part of the milk with yellowish-green color obtained after milk coagulation and removal of casein during the production of casein and cheese. Whey color is linked to a substantial concentration of riboflavin (Vitamin B2). It imparts 80–90% to the milk volume and 50% of milk nutritional composition, i.e., lactose (approximately 70% depending on the whey acidity), minerals (12%), whey proteins (10%), fat, and vitamins. Calcium, sodium, magnesium, and potassium salts from most of these minerals (of more than 50% calcium salts, KCI, and NaCl), while other minerals such as copper and zinc are present in small concentrations.[Citation3] Buttermilk is a by-product of butter obtained either from the churning of cream (sweet buttermilk) or milk cream acidification (cultured buttermilk) and comprised of milk fat and milk proteins (whey and casein). Buttermilk nutritional composition and functional properties differ according to the source. So, the buttermilk obtained from whey protein has different properties than buttermilk obtained from sweet or sour cream. The lactose, ash, and protein concentrations are similar to skimmed milk.[Citation4] The buttermilk properties are dependent on polar lipid concentrations (in dry form) ranges from 1.2 to 2.1%.[Citation5] Buttermilk contains higher fat contents as compared to skimmed milk as well as sweet and sour cream. Buttermilk obtained from cheese whey is similar to the whey, while sweet or cultured buttermilk’s composition is similar to skimmed milk. The fundamental difference in various kinds of buttermilk is given in .
Table 1. Various types of buttermilk depending on its manufacturing technology.
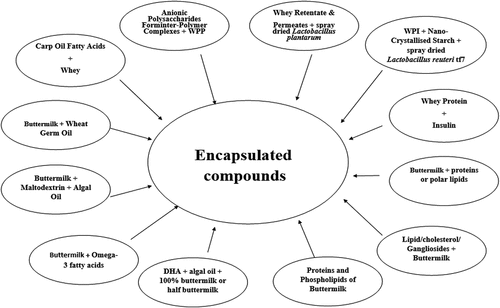
Whey retentate, permeates and every buttermilk part, like proteins and phospholipids, might be utilized exclusively as encapsulating materials for creating emulsions due to their inborn emulsifying properties.[Citation10] Whey proteins are utilized in formation of various products; whey in raw form, and buttermilk permeates are being utilized with anionic polysaccharides and other various proteins in manufacturing of inter-polymer complexes. The polysaccharides produced from whey proteins and buttermilk contain significant emulsifying, stabilizing, antioxidant and gelling attributes and are utilized as a feed for the growth of microbial culture in production of exopolysaccharides.[Citation11] Buttermilk constituents, i.e., proteins and phospholipids, might be utilized exclusively as encapsulating materials for creating emulsions due to their inborn emulsifying properties.[Citation12]
The new technology of bio-refinery has introduced to reduce the economic loss and maximize the utilization of waste of various industries (i.e., dairy sector) by producing the several value-added products simultaneously, i.e., bioplastics, biofuels, various chemicals and additives.[Citation13] Whey and buttermilk-based beverages have been prepared by various researchers from different parts of the world. These dairy based beverages can be categorized into whey protein-based beverages and fortified beverages (i.e., prebiotics, probiotics, fiber, vitamins and minerals fortified).[Citation14] Whey and buttermilk fermentation through utilization of various microorganism can be proved dual beneficial such as in reduction of environmental effects and enhancing the production of many values added products. The whey based fermented products can be developed by utilizing the various bacteria as probiotics such as Propionibacterium freudenreichii ssp. Globosum, commonly known as propionic acid bacteria (PAB), is responsible for the normal functioning of various microbes in human.[Citation15]
Scientists and technologists are continuously working to utilize the waste in production of various food and nonfood based commodities by using emerging technologies, i.e., biotechnology, genetic engineering, breeding techniques, and fermentation. Among these waste materials, buttermilk and cheese are gaining significant attention and numerous applications have been recognized for their utilization, i.e., bakery foods, dairy products, different beverages in bioactive substances utilization, microencapsulating materials, exopolysaccharides, fatty acids product, and several others. This review mainly focuses on utilization of whey and buttermilk in the production of nondairy items.
Different mechanisms for dairy waste products development
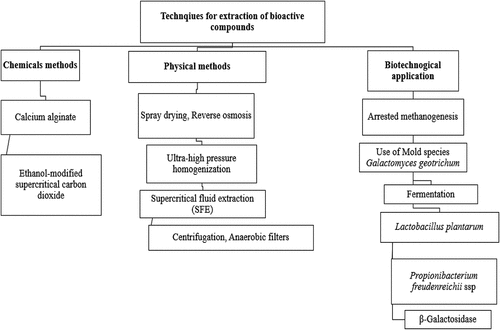
Dairy wastewater is generally treated with biological methods and physico-chemical.[Citation13] Whey is a pellucid liquid part of the milk with yellowish-green color obtained after milk coagulation and removal of casein during the production of casein and cheese. Likewise, buttermilk is produced during the manufacturing of whipping cream into margarine. Buttermilk proteins based encapsulation of Lactobacillus rhamnosus GG loaded with microbeads of calcium alginate was prepared by Hugues-Ayala et al.[Citation16] using an airbrush system. Anaerobic filters and up flow anaerobic sludge blanket reactors or related concepts were also used.[Citation17] Recently, a new strategy to produce concentrate of phospholipids from powder of buttermilk for the manufacturing of baked products was designed by using a new food-grade green technique depends on extraction with ethanol-modified supercritical carbon dioxide. The influence of extraction context, such as 50–60, 30 to 40 MPa pressure along with a 10, 15, or 20% ethanol concentration, the yield of total and phospholipid constituents was evaluated.[Citation18]
Conventional homogenization and ultra-high pressure homogenization were used to prepare the emulsions (oil-in-water) using 4–7% buttermilk, 10% chia, sunflower oil (50:50), and 30% maltodextrin at 30 MPa, and 200 MPa, respectively.[Citation19] Polymerase Chain Reaction-Denaturing Gradient Gel Electrophoresis (DGGE) technique was adopted for identification of microbiota.[Citation7] The production of EPS was also optimized with the help traditional method and response surface methodology.[Citation20] EPS can be separated by ultrafiltration techniques and centrifugations.[Citation21] Moreover, different techniques like reverse osmosis and nanofiltration can increase the production of dry whey from the waste of dairy industry.[Citation22] . is given for the different extraction methods of the dairy waste products, i.e., whey and butter milk.
Utilization of whey and buttermilk in development of microencapsulation matrix
Microencapsulation refers to the process of packaging the liquids, gaseous, or solid materials as an active ingredient with a continuous coating of film to form micrometer- to millimeter-sized capsules.[Citation23] The technique uses for the stability enhancement of various compounds with micro size ranges through the various encapsulation materials, i.e., different kinds of gums, casein particles, alginates and permeate of whey proteins. Whey proteins are utilized in formation of various products; whey in raw form and whey protein permeates are being utilized with anionic polysaccharides and other various proteins in manufacturing of inter-polymer complexes. The electrostatic interactions created through positive and negative charges on biopolymers are responsible for the complex formation. The main factors involved in the formation and strengthening of complexes are pH and ionic strength of biopolymers. One key benefit of this technique is that the chemical reagents and higher temperature is not required in complexes formation. These complexes enhance the nutritional and functional attributes of bioactive components that are being encapsulated.[Citation24] The utilization of whey protein permeates for encapsulation of carp oil fatty acids (COFAs) have capability (90.65%) to reduce the oxidation phenomenon.[Citation10]
Whey retentate and permeates are considered best among various microencapsulating material utilized for spray drying of Lactobacillus plantarum (ATCC-8014) due to their greater stability against higher temperatures in protection of bacterial cell. The 56-days storage study of microencapsulated particles at 20°C resulted cell counts more than log106 CFUg-1 through utilization of least numbers of probiotic bacteria in function food manufacturing.[Citation25] Microencapsulation of L. lactis R7 (sub-specie of Lactococcus lactis) was carried out with insulin and whey protein through spray dryer and stability of microencapsulated particles was measured against the various kind of adverse conditions. The higher encapsulation yield (94.61%) and cell viability (log1013 CFU/g) were observed from these synbiotic microcapsules and the cell viability reduced to >Log108 CFU/g after 6 months, when tested at various temperatures.[Citation26] Whey protein isolates (WPI) separately and in combination with nano-crystallized starch (NCS) were used for encapsulation of bile salt hydrolase producing bacteria (Lactobacillus reuteri TF-7) through spray dying and to increase their stability and survivability under poor environmental conditions. The survival rate of spherical microcapsules loaded in combined whey protein isolates and nano-crystallized starch is significantly greater than those loaded in whey protein isolates, when exposed to artificial gastrointestinal environment, pH and at various temperatures, i.e., 4, 25, and 35°C for long term storage.[Citation27]
Buttermilk is produced during the manufacturing of whipping cream into margarine. It involves water-soluble parts of the cream, i.e., lactose, milk proteins, minerals and the milk fat globule film (MFGM). Concurrently, fat globules are normally classified by the MFGM comprising of tri-layer which is manufactured by phospholipids and proteins and this tri-layer stabilizes fatty oils.[Citation28] Similarly, the MFGM is layer divider material for encapsulation of lipid. The cholesterol, gangliosides, proteins and polar lipids in the MFGM have nutritional activities, i.e., further developing cerebrum and intellectual framework advancement, disease prevention, developing insusceptibility, ensuring digestion and supporting sufficient development in sound newborn children.[Citation29] Every buttermilk constituent such as proteins and phospholipids, might be utilized exclusively as encapsulating materials for creating emulsions due to their inborn emulsifying properties.[Citation30] In this way, buttermilk could be a potential wall divider material for encapsulation of docosahexaenoic acid (DHA) and buttermilk has predominant properties as a wall material for encapsulation of algal oil. The algal oil embodied with 100% buttermilk or half buttermilk blended in with half maltodextrin had greater encapsulation proficiency, dispersion time, mass thickness and oxidation stability. The globule structures covered with phospholipids could emulate the normal milk fat globule layer. Many wall capsule materials utilized in the microencapsulation of material of food are accessible, including starches, for example, gum Arabic;[Citation31] maltodextrin;[Citation32] sucrose,[Citation33] sugars like lactose and mesquite gum;Citation34] and milk proteins, like whey protein and sodium caseinate.[Citation35]
Buttermilk has been investigated for its potential as an encapsulant for various essential oils such as omega-3 oils,[Citation30,Citation36] flaxseed oil,[Citation37] wheat germ oil,[Citation38] chia-seed oil,[Citation19] probiotics,[Citation16,Citation39] and curcuminoids[Citation40] in the recent years. Buttermilk alone or in combination with maltodextrin was used for encapsulation of algal oil. A total of 33% powdered buttermilk and 33% maltodextrin mixed with 33% algal oil resulted in efficient encapsulation, better protection from oxidation and improved storage stability of microencapsulated algal oil.[Citation41] Similarly, wheat-germ oil microencapsulated with buttermilk (1:2) along with 60% water showed significantly lower p-anisidine and peroxide values in comparison to non-encapsulated wheat-germ oil during the storage period of 24 hours at 60°C temperature. The stability of sensitive nutritional substances including carotenoids, phytosterols, and tocopherols in the encapsulated oil was also found to be higher than the non-encapsulated oil.[Citation16] Kumar et al.[Citation37] incorporated the flaxseed oil in the buttermilk and buttermilk concentrate based emulsion at 4 and 12% concentration, respectively. Buttermilk concentrate based emulsion showed lower anisidine value (45.09%) and peroxide value (66.08%) as compared to simple buttermilk with physical stability of emulsion was observed for the 15 days storage period. The improvement in the ultra-high pressure homogenization technology in comparison to conventional homogenization in producing omega-3 fatty acid rich emulsions was investigated.[Citation19] Conventional homogenization and ultra-high-pressure homogenization were used to prepare the emulsions (oil-in-water) using 4–7% buttermilk, 10% chia: sunflower oil (50:50), and 30% maltodextrin at 30 MPa, and 200 MPa, respectively. The findings of the study revealed the improved stability of buttermilk stabilized emulsion treated with ultra-high-pressure homogenization as compared to conventional method of homogenization particularly with regards to colloidal stability, particle size analysis, rheological characteristics and zeta-potential. The application of buttermilk as a carrier of curcuminoids was explained by Fu et al.[Citation42] using in vitro gastrointestinal digestion model. The bioaccessibility of curcuminoids (2% ethanol v/v) in the fasted conditions was in the range between 16 to 27.7% and 11 to 18.7% in buttermilk and with neat curcuminoids, respectively. While in the fed conditions, the values ranged from 37–69% with buttermilk and 45–79% with neat curcuminoids. The findings revealed that the presence of buttermilk as a carrier had significant influence on in vitro bioaccessibility of curcuminoids however the efficiency increased with increasing concentration of bile extract. Buttermilk proteins based encapsulation of Lactobacillus rhamnosus GG loaded with microbeads of calcium alginate were prepared using an airbrush system.[Citation16] The survivability of probiotics cells in buttermilk protein coated microbeads was significantly higher (8.91 CFU) than the non-coated microbeads (7.67 log CFU). The storage for 1 month resulted in 0.4 log and 0.8 log CFU/g in microbial cell count in the coated and uncoated microbeads, respectively. Similarly, the living cell count in buttermilk protein with Agave tequilana fructans after 14 days of storage at 20°C was 7.7 log CFU/ml. While the value was 3.0 log CFU/ml for alone buttermilk protein-based encapsulation. The studies mentioned here suggests that using buttermilk as a microencapsulating agent have significant potential in extending the potential use of several essential components as functional ingredients in several food formulations as well as in pharmaceutics. Whey and butter milk in various combinations are used to develop microencapsulated compounds as shown in .
Utilization of whey and buttermilk in beverages production
The diverting consumer behavior toward the healthy, nutritious and value-added products is a key factor of bringing boom in market of functional foods including dairy based beverage industry. These dairy based beverages can be categorized into whey protein-based beverages and fortified beverages (i.e., prebiotics, probiotics, fiber, vitamins and minerals fortified).[Citation43] As, the gut microbiota is directly interlinked to the diet and human health; its imbalance activity can be a good source of different metabolic disorders and dysbiosis in human body. The probiotic fortified beverages have significant potential to protect and reduce the various disorders linked with digestive system of human through improving the activity of gut microbiota.[Citation44] Due to this reason, the attention toward the probiotics incorporated whey-based functional beverages has increased globally.[Citation14] Whey provides the medium to the probiotics for their growth and feasibility which make it prospective source for probiotic-based whey beverages but these kinds of beverages are still limited.[Citation45] Whey in modified form or its separated membranes are beneficial medium for the manufacturing of functional nonalcoholic beverages. This has provided popularity to whey in beverage industry and market share of these beverages is increasing gradually.[Citation46] These ready to serve especially whey-based beverages are contributing the considerable share to the food market worldwide. Shortly, these kinds of beverages with high nutritional value are more convenient for the consumers of fast-moving world. In spite of high-quality proteins, various bioactive components, their derivatives and vitamins, i.e., riboflavin, cobalamin, and folic acid, are responsible for health of human.[Citation47]
Whey and buttermilk based beverages have been prepared by various researchers from different parts of the world. Meshram[Citation48] investigated the production of buttermilk-based orange, mango, and banana fruit beverages. The acceptable beverage composition was in the range between 10% (mango) to 35% (orange) while the buttermilk concentration of the beverages ranged between 62–83%. The buttermilk-based mango beverage was found to be more satisfactory among all the tested beverages. As buttermilk contains appreciable quantity of phospholipids, these can be utilized in the milkshakes. This aids in the creation of foamy structure at churning of buttermilk and helps to reduce the surface tension on liquid-air boundary.[Citation49] Mudgil et al.[Citation50] developed a novel buttermilk-based beverage using fortification of fiber. The findings of the investigation indicated that the buttermilk sample with 5% fiber content showed lowest phase separation and highest viscosity. While the samples with 4% fiber content showed the highest value in the sensory evaluation. Recently, a functional buttermilk fermented beverage manufacturing technique was developed and the impact of intake of beverage on the health was evaluated by Liutkevičius et al.[Citation51] It was concluded that a buttermilk beverage that is manufactured by buttermilk – skimmed and milk – milk protein concentrates of 0.3% reduced the high-density cholesterol and triacylglycerol level and also improve the syneresis, viscosity and flavor of beverage. Different kind of technological solutions have been investigated to enhance the manufacturing of foods based on buttermilk while the manufacturing of beverages seems to be most significantly acceptable from the technological as well as economic point of view. The application of buttermilk is assisted by the techniques of spray drying pasteurization, and concentration.[Citation52] The summarized recent studies conducted on the utilization of buttermilk and whey in beverages production are given in .
Table 2. Industrial utilization of buttermilk and whey in beverages productions.
Utilization of whey and buttermilk in production of baked products
The whey protein concentrates (WPC) and buttermilk have been used as additives, flavor and taste enhancers in the preparation of bakery products. Buttermilk increased the nutritional, rheological, and organoleptic properties of baked products.[Citation57] Buttermilk contains milk whey and casein protein constituents.[Citation40] Particularly in the US industries, buttermilk is commercially used for the baking industry; to prepare dry mixes 33% and 23% buttermilk is used for dairy products. Concurrently, buttermilk is used for improvement of flavor as well as texture of baked product. The water absorption capacity of the flour can be increased by increasing the quantity of buttermilk in the batter and dough as it increased the solubility of protein content, and hydrophobicity. In addition, buttermilk can modify the structure of dough thus allowing the more absorption of water because of hydrogen bonding. Increase water absorption limit of batter addresses consistency which is one of the engaging attributes in bread making. Furthermore, addition of buttermilk in the formulations of baked product improves the rheological characteristics (water absorption, gelatinization temperature, and dough development time), sensory quality, and physical properties of product such as volume, and weight. Therefore, the addition of 30% buttermilk is recommended to improve the nutritional as well as sensory attributes of baked products.[Citation58] Whey protein concentrate (WPC) as well as buttermilk powder were used in the dough unleavened and leavened flat-bread at around 0.4 and 8% concentration. The concentrate of whey protein and buttermilk powder, improves the characteristics of dough such as resistance value, dough stability along with resistance to extension. In addition, concentrate of buttermilk protein increase the protein content of flat bread up to 14%.[Citation59] By addition of 8% buttermilk powder, significantly increase in the content of mineral and ash, more yellowness on the crust surface of unleavened/leavened bread was observed. Buttermilk protein has significant influence on the functional properties e.g. foaming capacity, protein stability, and oil and water absorption capacity. These functional properties provide desirable water and fat stabilization, texture in the baked products. Furthermore, buttermilk improves the odor, flavor and taste of leavened and unleavened bread.[Citation60] In a study, Al-Jahani[Citation58] reported that the addition of buttermilk in the dough of wheat flour reduce the stability of dough. The reduction of dough stability was because of the fact that the constituents of buttermilk disrupted the gluten-starch network of wheat dough as the proteolytic activity increase resulting in the increase of gluten hydrolysis and reduction in the stability of protein network. Recently, a study investigated the optimal amount of milk, buttermilk and whey powder for compound milk chocolate formulation using mixture design. The effect of buttermilk as a substitute of milk and whey on the major physicochemical properties of compound milk chocolate was studied. The mixture optimization revealed that using 36.38% buttermilk, 35.6% milk powder, and 27.957% whey powder resulted in the production of optimum milk chocolate with the maximum acceptability without unacceptability in the quality parameters.[Citation61]
Recently, a new strategy to produce concentrate of phospholipids from powder of buttermilk for the manufacturing of baked products was designed by using a new food-grade green technique depends on extraction with ethanol-modified supercritical carbon dioxide. The influence of extraction context, such as 50–60, 30 to 40 MPa pressure along with a 10, 15, or 20% ethanol concentration, the yield of total and phospholipid constituents was evaluated. The finding showed that the concentration of ethanol had a significant impact on the extraction of total lipid or phospholipid constituents as compared to the pressure and temperature. Therefore, this extract of phospholipid can be supplemented in the manufacturing of functional foods as an emulsifier that improves texture of product.[Citation18] SA[Citation54] explained that phospholipids have better functional properties and improve sensorial characteristics of products. The unprocessed and fresh buttermilk was used in bread formulation and 50% supplementation improved dough rheology with significant volume, pleasant smell, taste and yellowish color of crumb with darker color of crust. Buttermilk also improved the concentration of protein, amino acids, and minerals. Currently, the use of various flavors in baked goods is increasing, particularly from natural origin that is paying worth attention toward the biotechnological techniques of flavor extraction with low cost, high efficiency rate along with simple process. Szudera-Kończal et al.[Citation53] evaluated the capability of the mold species Galac tomyces geotrichum to modify buttermilk and sour whey to produce complex mixtures of flavor with honey-rose pleasant aroma. Phenyl acetaldehyde content that give honey like flavor gave highest odor activity value while 2-phenylethanol (rose-like) from sour whey variant gave highest odor activity.
Innovations in technologies lead the change in food processing operations and proved more effective to manage production as well as waste. Different techniques like reverse osmosis, ultrafiltration and nanofiltration increased the production of dry whey from the waste of dairy industry. The supreme nutritional profile and functional properties of cheese whey (CW) acquired much more interest in food processing industries. CW is a chief mean of bioactive peptides and some of the amino acids, Immunoglobulins, Lactalbumin, Lactoferrin and α-Lactoglobulin. The main products obtained from dairy whey are hydrolyzed whey proteins (HWPs), whey protein concentrates (WPCs) and whey protein isolates (WPIs).[Citation22] These compounds are the byproducts of cheese manufacturing industry and are used in a broad range of food products. WPHs due to their high effectiveness over a range of pH promote cohesion, adhesion, and water binding capacity so, are used baking industry. This promotes emulsification and improves whipping also act as fat binding agent when polysaccharides are added. WPHs modify sensory properties, i.e., texture, color, and flavor.[Citation62] The addition of whey protein (15, 20, and 25% TS) to multigrain bread manufactured from flour of wheat, maize, sorghum and oat enhanced the firmness and stickiness of the product while gumminess was reduced. Whey in its concentrated form reduced the rate of proofing of the dough and this was recovered by the enhanced number of yeasts to the level of 6% and increased temperature, i.e., 45ºC during proofing.[Citation63] In Egyptian baladi bread water was replaced with sweet whey and results showed an improvement in the sensorial and physicochemical parameters of the bread. The significance increased with the increase in sweet cheese whey or milk permeate.[Citation64] In another study, Luz et al.[Citation65] developed bread supplemented with hydrolyzed goat milk whey to assess antifungal activity of the hydrolyzed whey. LC – ESI–TOF-MS was used for the characterization of the resulted peptides and found 27 peptides out of which lactoferrin, α-lactalbumin, κ-casein and β-lactoglobulin were associated with antifungal activity. The shelf life of the bread supplemented with hydrolyzed goat milk increased by 2 days with 1-log reduction in fungal growth and 85–100% production mycotoxin. Stoliar[Citation66] reported that lactose has the potential to enhance emulsification, flavor and crumb structure which can be isolated from cheese whey. The lactose can be isolated through the various pre-treatments of dairy effluents. The highest recovery of lactose (94.59%) was obtained from the combined heating and ultrasound pre-treatment.[Citation67]
Microorganisms’ cultivation from whey and butter milk
Whey produced as a by-product in dairy industry during the production of dairy based products, such as various kind of cheese and casein can cause the critical environmental issue. Its fermentation through utilization of various microorganism can be proved dual beneficial such as in reduction of environmental effects and enhancing the production of many values added products. The batch fermentation of whey permeates by Kluyveromyces lactis in different concentrated forms (normal, 1.5-folds and double) can produce the biomass and ethanol simultaneously with the higher activity of β-galactosidase. Therefore, it should be utilized in ethanol production instead of wasting it as by-product.[Citation68] The whey based fermented products can be developed by utilizing the various bacteria as probiotics such as Propionibacterium freudenreichii ssp. Globosum commonly known as propionic acid bacteria (PAB) is responsible for the normal functioning of various microbes in human. The various strain cultures of PAB growth rate are observed higher on whey medium with higher survival rates (minimum 108 CFU/cm3) making it more suitable for the production of fermented dairy products from whey proteins.[Citation15] Whey is also considered a good source for the production of microbial lactic acid. Permeates of whey proteins are excellent medium for lactic acid manufacturing because these permeates are formed after microfiltration which produce the purified and sterilized medium. These contains maximum amounts of lactose which can be extracted in maximum concentrations by utilizing the yeast extracts (0.2 g/L), eggs extracts (8 g/L) and peptone (5 g/L) to manufacture lactic acid on industrial scales.[Citation69] Whey derivatives, i.e., lactulose, are a desirable prebiotic for feeding specific probiotics. Moreover, the whey proteins are important as due to their nutritional value as well as their functions for probiotics in manufacturing of functional products, i.e., symbiotic.[Citation70]
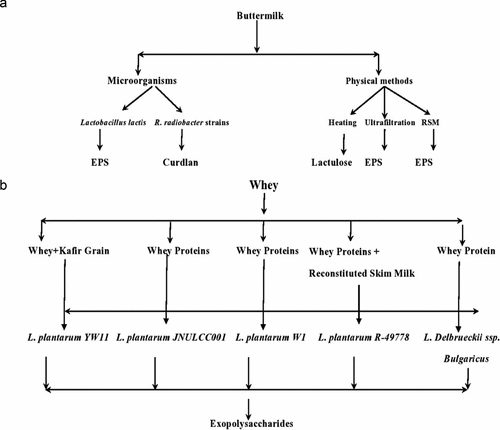
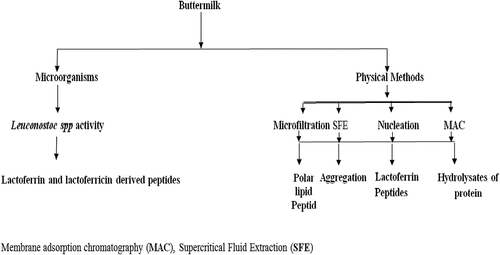
Lactic acid bacteria as well as yeasts were particularizing from fermented buttermilk. Polymerase Chain Reaction-Denaturing Gradient Gel Electrophoresis (DGGE) technique was adopted for identification of microbiota provided by Gebreselassie et al.[Citation7] The microbial composition was directed by strains of Lactococcus lactis ssp. lactis, Saccharomyces cerevisiae, and Lactobacillus plantarum. The existence of complex strains of LAB as well as yeasts in fermented buttermilk specified a prospective for production of various starter cultures to develop new dairy products. Similarly, the extraction of starter cultures Lc. lactis subsp. cremoris, Leu. citrovorum, as well as Leu. dextranicum from fermented buttermilk produce lactic acid that results tangy flavor.[Citation71] The utilization of buttermilk as a cultivation medium of probiotic lactobacilli has been proclaimed. Buttermilk has 106 to 107 CFU/ml of probiotics, i.e., Lactobacillus and Bifidobacterium.[Citation72] In another study, de Freitas Mascarello et al.[Citation52] extracted small concentration of yeast extract about 0.3% in the growth of Lactobacillus in buttermilk. Similarly, a probiotic Lb. acidophilus can be produced by Acidophilus buttermilk[Citation45] and enzymes, e.g. xanthine oxidase/dehydrogenase.[Citation73] Various products including organic acids and other products through microbial activities from whey and buttermilk are given in .
Table 3. Different products developed from buttermilk and cheese whey using microbial fermentation.
Table 4. Products developed from cheese whey using microbial fermentation.
Utilization of whey and buttermilk in edible films development
Food packaging material is hot point of discussion among the researchers for last two decades. Researchers and investigators are searching new techniques for manufacturing the safe, environment friendly and biodegradable packaging materials. The edible films are among the one of the solutions for the packaging material. Edible coating films formed by the whey proteins and lipids produce from the dairy industrial waste are getting intentions of researchers. Edible films are mostly being utilized in coating of perishable food commodities to hinder the nutritional deterioration and provide protection to organoleptic attributes of these commodities.[Citation103] Whey proteins obtained from the dairy industries as a by-product are considered sustainable raw material for edible coating formation. The whey proteins utilization has increased in formation of edible films because these are available in access amounts, friendly to environment, nontoxic, cheaper in cost, provide higher amounts nutrients and more suitable for the packaging material of food which protects from deterioration and damages.[Citation104] WPI and Whey protein concentrates (WPC) are two main types of whey proteins mostly utilized for development of edible coatings and films. The whey based edible coatings and films are dry and compacted polymer network along with 3-dimensional gel like structures. These are odorless, colorless, transparent and flexible with extraordinary hindering and mechanical attributes. They showed higher oxygen and vapor permeability with lower tensile strength in comparison to coatings and films form from other protein and polysaccharides. Furthermore, these films also play a key role as an active ingredient, i.e., antioxidants and antimicrobial, and act as probiotic in some foods with the additions of incredible properties to the packaging material desired by any packaging industry.[Citation105]
These edible films have capability to reduce the microbial growth and contamination rate, enhancing the shelf life of dairy based products produce by packed under modified atmospheric conditions, i.e., cheese, reduce moisture loss in frying products, i.e., doughnuts and French fries, before frying to reduce the oil absorption during frying. Moreover, these films prevent moisture absorption by baking products during long time storage, i.e., biscuits and breads, and help in maintaining hardness, freshness and chewiness of freshly cut food commodities, i.e., carrots, potatoes and apples.[Citation103] The psyllium seed gum (PSG) and WPI were utilized in various combinations to form edible films and their structural, physicochemical and mechanical attributes were evaluated. The combined films of WPI/PSG showed lower light transmitting and oxygen permeability properties and greater vapor permeability and contact angles of water in comparison to the films made up of simply either with PSG or WPI. The composites illustrated higher elongation attributes, declined degree and surface of cracks than single films. The increase in film whiteness and brightness index and decrease in differences of total colors was observed with the increase in PSG proportions. The PSG/WPI (1:1) composites showed the maximum elasticity, tensile potency, and maximum mechanical and physicochemical characteristics.[Citation106] In short, the whey-based edible coating and packaging films have changed the trends among packaging industries as these are environmental friendly along with more functions benefits. These edible films regardless of their edibility provide some other beneficial properties, i.e., provide hindrance against chemical and mechanical changes, are stable against microbial activity, maintain and improve the shelf life of products, have acceptable sensory attributes, are nontoxic in nature, are cheaper in production, and, more important, are compatible with products. Therefore, its utilization in packaging of various food industries, i.e., baking, meat, vegetables and fruits processing and dairy industry, has increased. Moreover, the prebiotics addition in this packaging material also improves the mechanical and physicochemical properties along with consumer acceptability.[Citation107]
Exopolysaccharides development from whey and buttermilk
Exopolysaccharides (EPS) are macromolecules present outer sides of cells excreted either in form of firmly bounded capsules or slime film loosely attached to inside the microorganisms. Exopolysaccharides play key role against osmotic stress, phagocytosis, phage attacks, desiccations, cell recognition, and toxic compounds. The polymers formed by these microbial capsules/film have prominent role in biotechnology and are extensively used in various fields including medicinal, nutraceutical and different food, sectors but the expensive raw material and increasing demand is a major issue in their production. The exploration and finding of cheaper, easily available and good quality raw material from the various by products such as dairy industrial waste including cheese whey proteins and a number other by products from food industries is a main focus of scientists of this era.[Citation108] After the progressive efforts, whey is suggested cheaper source for production of functional exopolysaccharides for their beneficial uses. Biotechnological methods are the good options to utilize cheese whey (dairy industrial by-product) in formation of polysaccharides.[Citation109] The polysaccharides produced from whey proteins contain significant emulsifying, stabilizing, antioxidant and gelling attributes and are utilized as a feed for the growth of microbial culture in production of exopolysaccharides.[Citation11]
The exopolysaccharides are produced through various methods; some of them are given below along with their functional importance (). The glucose and galactose containing homogeneous EPS polymers were prepared after purification through incorporation of whey (15%) and kafir grain (0.2 g/l/h) by Blandón et al.[Citation110] The hetero-exopolysaccharide was prepared from whey through fermentation by Lactobacillus plantarum bacterialstrain JNULCC001 and high activity of β-galactosidase. It contained various mono and disaccharides, i.e., glucose, galactose, arabinose, and mannose, and different functional groups such as amide, hydroxyl and carboxyl groups, providing heat stability. It contained various beneficial properties such as higher shear thinning and viscous behavior, stabilizing attributes in manufacturing of various nano-materials, i.e., selenium nanoparticles, and aids in bioremediation.[Citation111] The interaction between the whey proteins (WP) and EPS produced through the fermentation through Lactobacillus plantarum YW11 (EPS-YW11) was studied. The improvement in texture of complex compound (CC) formed with combination of WP and EPS-YW11 and increase in chewiness, gumminess, hardness, cohesiveness, resilience, and springiness was observed higher as compared to either single WP or EPS substances. The structure of CC was porous and branched which became more compact and stable on heating and showed improvement in functionality. Hence, this CC compound plays an important role in enhancement of various bioactivities, i.e., antibiofilm, cholesterol reducing and antioxidant properties, and texture improvement.[Citation106] The EPS were also produced by fermentation through various species, subspecies and strains of Lactobacillus including delbrueckii subsp. of bulgaricus, plantarum W1 and R-49778 from different sources, i.e., animal based milk whey proteins, reconstituted skim milk, and whey obtained from soy milk.[Citation112]
Buttermilk and cow milk are the sources of EPS and other secondary metabolites with the help of lactic acid bacteria such as Lactobacillus lactis. EPS from the buttermilk has been used as thickening agent in food industries.[Citation76] However, the data regarding the production of exopolysaccharides from the buttermilk is scarce. EPS has also role in yogurt production with the help of lactic acid bacteria as shown in . In addition to this, exopolysaccharides have antitumor, antioxidant, immunostimulatory as well as other therapeutic properties.[Citation113] Additionally, another exopolysacchride named as Curdlan which is produced by buttermilk using R. radiobacter strains has been shown to have good water holding properties as well as better thermal stability, so it is used as thickening and gelling agent. Moreover, they have excellent viscosity enhancing properties.[Citation77]
Lactobacillus lactis was used for the production of EPS by Almalki[Citation20] For this purpose, it was extracted from cultured milk. The production of EPS was optimized with the help traditional method and response surface methodology. L. lactis exhibit great capability to produced good quantity of exopolysaccharides as compared various other bacterial isolates. Furthermore, addition of 1% maltose increased the EPS production. The formed EPS exhibits antioxidant property due to which EPS and the Lactobacillus lactis can widely use in food industry. Oliveira et al.[Citation21] explained that evaporation of buttermilk leads to the crystallization of buttermilk. So, EPS can be separated by ultrafiltration techniques as well as separation of these crystals can be obtained by the help of centrifugation. In food and pharmaceutical industries, utilization of refined and edible lactose is common. Moreover, lactose is also added in different bakery and confectionary products in which it can improve viscosity and storage life of the food products. In addition to this, lactose can be used as carrier for taste and volatile aroma as well as it also acts as a sweetener in some products. The application of lactose also in the production of potato chips, salad dressing, sausages, etc. Kwak et al.[Citation114] explained that during heating lactose is converted into lactulose which act as growth promoter of bifidobacteria.
Bioactive peptides production through proteins and buttermilk utilization
Food industries are struggling continuously to produce the peptide based functional foods to improve the economy by utilizing the waste material of produce by various food industries and improve the health status of the people.[Citation115] Whey and buttermilk provide variety of peptides for the functional foods. Whey is considered as a major source of various bioactive compounds especially whey proteins have various health benefits. The whey proteins are being intensively studied for their health benefits. The biologically active peptides are formed from the various sequences of amino acids which are interlinked in primary proteins structures and release through proteolysis from these proteins.[Citation12] The Whey protein hydrolyzates (WPHs) formed through the enzymatic hydrolysis can improve various health related systems including cardiovascular, immune, GIT, cerebral, and nervous systems.[Citation115] These peptides have great potential to serve as anti-diabetic, antioxidant, antimicrobial and anti-hypertensive agents. The fermentation process is considered one of the best methods to produce the peptides from the whey proteins by utilizing gut microbiota.[Citation116,Citation117] These are being utilized in multiple fields for enhancement and protection of food quality to promote the health. These can be secluded from various proteins, besides their functionality and implementation in food development.[Citation118] The bioactive peptides can be secluded from whey proteins of artisan cheese by using Enterococcus faecalis. The bioactivities of these peptides can be measured by utilizing the silico approach. The WPHs depict that the dipeptidyl peptidase IV (DPP-IV) and Angiotensin-I-converting enzyme (ACE) are stringer inhibitors.[Citation119] The scotta, a by-product, produced during the manufacturing of ricotta (Italian cheese) are filtered with ultrafiltration membrane (500–4 kDa) to produce bioactive peptides.[Citation120]
The fermentation process is considered one of the best methods to produce the peptides from the buttermilk proteins by utilizing gut microbiota. In buttermilk, proteolytic bacteria such as lactic acid bacteria are present which can produce beneficial peptides with various functional properties. There is release of peptides having antimicrobial properties by heating the buttermilk.[Citation121] Antiviral, bactericidal, and fungicidal properties of the lactoferrin and lactoferricin derived peptides have been reported.[Citation122] Bioactive and functional characteristics as well as anti-cancer properties of bioactive components have been showed by the proteins and peptides present in buttermilk. Moreover, the free radical scavenging properties of the buttermilk peptides have been increased as antioxidant properties are improved by the peptides production. Lipopeptides and the milk fat globule molecule of the buttermilk act as biocatalyst as well as show attractive surface and biological properties.[Citation123] Additionally, peptides enriched buttermilk powder exposed to mice for 5–7 days resulting in the growth of Ig-A producing cells in the intestinal mucosa of the mice.[Citation124] Three potent lactic acid bacterial strains having bacteriocin producing potential were isolated from the homemade buttermilk. The produced bacteriocins were shown to have antibacterial activity against several grams +ive and gram – ive bacteria.[Citation125] Microfiltration (MF), CO2 and supercritical fluid extraction (SFE) was used in work of Olabi et al.[Citation126] to produce bioactive peptides. SFE with 30.9°C, 7.38 kPa is widely used as it showed low toxicity level, environmentally friendly and gaseous at low pressure and room temperature (). Furthermore, SFE is good separator of polar lipids and peptides. The content of buttermilk peptides as well as lactoferrin hydrolyzates was functionally extracted with membrane adsorption chromatography by Jean et al.[Citation127] As reported by Akalın[Citation128] racidin is an antimicrobial peptide linked with adhesion prevention as well as immune stimulation to support resistance against infections. Their findings gave valuable information to produce functional compounds from buttermilk in cationic extracts which can be utilized as a supplement in antibiotics in the feeding of poultry. Another study has been reported that most process of aggregation of protein based on nucleation. These type protein aggregates can be utilized in low-fat cheese as fat mimics or as modifiers of yogurt texture.[Citation52] In light of the dry weight, the primary parts of buttermilk got from sweet cream are protein 31.5–33.1%, fat 5.7–13.1%, and lactose (48.7–53.8%). The standard acidity level of buttermilk ought to be over 0.60% communicated as lactic acid, fermented by the biovariants of Lactococcus spp. and additionally Leuconostoc spp. Moreover, the base counts of specific microorganisms ought to be 107 CFU/g at the hour of sale. With its unique and rich composition, buttermilk can convey an assortment of practical constituents like stabilizers, emulsifiers, and nutrients coordinators for a various food items.[Citation5]
Utilization of whey proteins and buttermilk in organic acid production
The organic acids are extensively being utilized in agricultural, pharmaceuticals, foods, chemicals and detergents industries. The fermentation of whey is accomplished in two steps including carbohydrate conversion to lactic acid followed by its breakdown into hydrogen and various fatty acids, i.e., propionic acid, butyric acid, and acetic acid.[Citation80] Whey can be utilized in production of lactic acid as a culture medium of B. animalis (subsp. lactis BB12) and Lactobacillus acidophilus (LA5). However, the main factors affecting the lactic acid production includes bacterial type, incubation temperature, pH, and amounts of yeast extract used.[Citation129] \Arrested methanogenesis (AM) based simultaneous chemical digestion of brewery wastewater and cheese whey was used to produce the carboxylic acid and maximum production (78 g/L) was observed in 15 days batch processing at 7 pH and 40°C.[Citation130] Whey is a good source of succinic acid production along with Actinobacillus succinogenes cells available in free and immobilize form produced through alginate activity. The higher production of succinic acid can be obtained by utilizing 24.9 g/L whey along with 35 g/L lactose.[Citation131]
Through, combined digestion of sewage sludge and cheese whey can increase the production of volatile fatty acids and caproic acid production continuous reactor. The higher amounts of caproic acids were produced at 10-days hydraulic retention times (HRT) and 2 feeding intervals in one day which boost acidification rate (AR) up to 44%. The community dynamics of microbes in continuous reactor depicted that large number of microbes are involved in its production.[Citation132] The volatile fatty acids were produced from cheese whey by the anaerobic batch reactor. The process was optimized through HRT and solid retention times (SRT). The AR was increased from 0.73 to 0.83 and 0.79 to 0.87 by enhancing SRT till 15-days and reducing HRT till 1-day, accordingly.[Citation133] The galactonic acid and ethanol can be manufactured through the Gluconobacter oxydans and S. cerevisiae in two steps. Whey was degraded into galactose and glucose by using β-galactosidase. Then, galactose was converted into galactonic acid by G. oxydans based oxidation and glucose into ethanol by S. cerevisiae fermentation.[Citation24]
The concentration of different organic acids in the naturally fermented buttermilk was reported to be 52–1711.5 µg/g citric acid, 69.8–1297.4 µg/g succinic acid, 4.68–86.46 µg/g pyruvic acid, 5347.3–11557.0 µg/g lactic acid, 211.1–1393.7 µg/g acetic acid, 2.27–69 µg/galpha-ketoglutaric acid, 25.4–111.78 µg/g orotic acid, 2.94–25.54 µg/g uric acid, and 29.11–707.64 µg/g DL pyroglutamic acid, respectively.[Citation7] However, little literature is available on the commercial production and utilization of organic acids from the buttermilk. Nevertheless, the use of buttermilk in the production of yogurt has been investigated by various researchers. Buttermilk has been reported to enhance the titratable acidity, reduce the pH value, reduced gelation time, and hence reduced the fermentation period during yogurt production.[Citation134] Zhao et al.[Citation135] investigated the effect of different concentration of buttermilk on the sensory properties and composition of several volatile substances in the low-fat yogurt. Solid phase microextraction coupled with gas chromatography mass spectrometry (SPME-GC-MS) analysis revealed that the addition of buttermilk (1%) in the low-fat yogurt resulted in increase in some of the unique acids in the products. These acids include heptanoic acid, cyclohexane acetic acid, 2-oxopentanedioic acid, and phenylphosphonous acid.Production of bacteriocin-like-inhibitory substances by the lactic acid bacteria obtain from sour buttermilk was assessed by Iranmanesh et al.[Citation136] Bacterial species such as Lactobacillus paracasei, Lactobacillus pentosus, Pediococcus acidilactici, and Lactobacillus brevis were shown to generate proteinaceous inhibitory substances against several grams +ve and gram – ve bacteria including Listeria monocytogenes, Salmonella enteritidis, and Staphylococcus aureus.
Essential fatty acids production from buttermilk
Buttermilk and butter serum are rich source of sphingomyelin which is about 3.4–21 mg/g on the basis of dry matter but the amounts of bioactive ceramides are lower that is about 1:5% molecules in buttermilk and 1:10% molecules in butter serum. The lipids in the buttermilk are lower in cholesterol so it has common application in neonatal nutrition as well as in protection of brain, hepatic protection.[Citation137] There are several compounds such as glycerophospholipids and sphingolipids are common polar lipids which present in milk fat globule membrane.[Citation138] Moreover, the major phospholipids in buttermilk are phosphatidylethanolamine and sphingomyelin.[Citation139]
According to nutritional point of view sphingolipids are common bioactive components.[Citation140] There is difference in chain length, number of double bonds as well as in hydroxyl moieties in these sphingoid-based lipids.[Citation141] SM taken from milk performs biological functions in human body. SM is important for brain health as it is vital component of brain cells and cell membranes of different other vital organs.[Citation142] After 8 months of supplementation, the SM obtain from bovine milk was seen to be helpful in promotion of neurobehavioral development in premature babies.[Citation143] Butter serum contains 41% monounsaturated fatty acids as well as buttermilk contains 48.4% monounsaturated fatty acids. Among all unsaturated fatty acids, the amount of oleic acid is higher in buttermilk and butter serum.[Citation144] The amount of oleic acid is 35% of all polar lipids. Moreover, the amount of polyunsaturated fatty acids in buttermilk is 16.7% as well as butter serum contains 11.2% PUFA. In addition, the amount of essential fatty acids such as linolenic acid and alpha linolenic acid was higher in buttermilk when compared with butter serum. It was observed that the amount of DHA was higher in buttermilk. The comparison revealed that the amount of polar lipids is higher in butter milk as compared to butter serum. Furthermore, essential fatty acids such as linolenic acid and DHA can be added in the milk when these essential fatty acids are recovered from the milk of cows feeding on fresh grass.[Citation145]
Buttermilk significantly possesses the emulsifying properties because of its high protein content ratio.[Citation146] In previous literature it was showed that buttermilk contains higher content of phospholipids as compared to milk due to its high substance in milk fat globule molecules (MFGM) material, which is significantly affluent in phospholipids that establish around 33% of the MFGM. In contrast to milk, buttermilk exhibits 0.89 mg/g to 0.12 mg/g concentration of phospholipids.[Citation147] The high substance of phospholipids in buttermilk makes this dairy product a significantly important component in view of the emulsifying properties of phospholipids.[Citation148] Whey buttermilk exhibits higher degree of emulsification properties, while lower degree of foaming capacity in contrast to cultured and sweet buttermilk which might provide higher proportion of phospholipids (PLs) to protein. Roesch et al.[Citation149] evaluated the emulsions prepared along with buttermilk powder (BMP) and MFGM parts got by microfiltration of reconstituted buttermilk. The emulsions prepared with MFGM-advanced material were found to have a superior stability toward flocculation and creaming because of the more modest molecule size distribution. The strength of emulsion film firmly relied upon the sort of segregates delivered from buttermilk. Also, phospholipids have been displayed to have natural biological activity. PLs comprise 1–5% of the all-out lipid found in milk,[Citation150] Of which more than 60% are related with the MFGM that surrounds lipid droplets. Because of their particular amphiphilic nature along with close relationship with the MFGM, PLs assume a critical part in the emulsification of fat in milk.[Citation151]
Miscellaneous uses of whey proteins and buttermilk
The recovery of various valuable components from the dairy processing waste, i.e., lactose and proteins play a prominent role in lowering the environmental pollutions, COD and other concern issue. The lactose can be isolated through the various pre-treatments of dairy effluents. The highest recovery of lactose (94.59%) was obtained from the combined heating and ultrasound pre-treatment.[Citation67] The ultrafiltration in diafiltration mode was utilized to isolate the maximum amounts of proteins and lactose from retentate of whey and hollow fiber module was utilized for purification. Through this modern technology lactose and proteins were recovered with efficiency of 90% and 80% accordingly.[Citation152] The similar technique used for isolation of lactose and proteins enriched fractions. Different sizes of membranes, i.e., 3, 5, and 10kDa made up of recycled cellulose-based substance were used to evaluate the process efficiency and obtained 70–80%, 90–95%, and 100% lactose recovery rate from pure lactose solution. Moreover, 10kDa showed the similar results for wastewater sample.[Citation153] Albeit further researches are required including to improve and design refinery techniques, fermentation processes for whey conversion into multiple compounds, isolation of various components from cheese whey especially proteins and lactose to develop the value added products.[Citation154]
Plastic production and utilization have adverse effects on environment. It is need of this era to find out the environment friendly substitutes of plastic materials. These bioplastics as a substitute provide multi-benefits to the environment. Polyhydroxyalkanoates (PHAs) are ubiquitous group considered as prominent alternative for manufacturing plastic biologically and are biocompatible and biodegradable.[Citation85,Citation155] Albeit their production by carbon containing sources is limited due to higher costs. Therefore, the utilization of dairy based materials such as whey through the biotechnological processes is increasing in production of PHAs instead of carbon based other sources.[Citation109,Citation156] The combined biotechnological processes including fermentation and ultrafiltration are used to develop the PHAs. Its production efficiency can be tested by utilizing various methods and parameters, i.e., bioreactor systems, monitored and constant pH, temperature ranges and various stirring conditions. This pilot scale method can be utilized to manufacture the bioplastics on industrial scale to minimize the disposal issue of plastics.[Citation157] The continual management of wasted whey is now more familiarized to the food and biotechnological applications for the production of various valuable commodities and bioplastics manufacturing is one of them.[Citation154] Cheese whey is still considered a major polluting agent of the dairy sector.[Citation19]
In biotechnological field, the utilization of microbiological fermentation technology is playing a prominent role in production of various plastic materials, i.e., polyhydroxybutyrate (PHB). The waste water from the various sources can be utilized for production of PHB by fermenting them through Bacillus CYR1 (B-CYR1), B-CYR1-Cupriavidus sp. CY1, and their mixture. The COD and TOC (total oxygen carbon) analyzers are best equipment’s to validate the removal of carbon. The highest production rate was obtained from waste water of whey industry (151 mg/l) as compared to sewage treatment water.[Citation158] The life cycle assessment (LCA) of novel whey plastic developed by whey protein showed that 82.5 kg NOx and 115.3 kg CO2 emitted by consumption of 2.9KMJ during manufacturing of 1000 kg whey plastic which produce least toxicants to the marine and fresh water and has minimum global warming issue in comparison to other traditional plastic manifesting processes.[Citation159] Bio-refinery complexity index (BCI) is a main factor used to evaluate the economic and technological risk to design the biorefinery process using selected raw materials, manufacturing, extracting and purifying processes. The dairy industry BCI was 20, reflecting sustainable biorefinery and zero waste production suggesting biorefinery one of the best processes for dairy industry at both academic and industrial level.[Citation13]
Toxicological aspects of whey and buttermilk
The functions of various nutrients are carried out and maintain through the numbers of metabolic reactions. Protein digestion and development of bioactive peptides is carried out through same phenomenon. Hence, the exogenous peptides can disturb this phenomenon, which may be a main cause of health issues in body and must be mitigate to eliminate the safety issues.[Citation160] These exogenous peptides may stop at intestinal mucosal cells affecting the microfloral functionality in intestine.[Citation161] These kinds of various compounds are present in whey and butter milk which can cause the several health-related safety concerns on their consumptions. The 10 g/g BW of oligopeptides powder of milk with high protein content was an oral lethal dose for female and male mice. Similarly, they tracked down no unusual changes in the general status of health, histopathological assessment, biochemical pointers results, and other perception markers. Hussein et al.[Citation162] currently investigated the repeated-dose oral as well as acute toxicity of whey protein that was hydrolyzed by alcalase, which showed complications that are related with treatments while in the study of sub-acute dose, no toxicity modification was initiated. Subsequently, the mixed peptides specifically enhanced the symptoms in voluntary hypertensive rats. Different researchers have proved that whey protein enhanced the performance of athlete without any toxic impact as well as give significantly prime protein content for infant. Hence whey protein is supposed to be safe; there are some profound handling products that are produced from whey protein that might cause attention their before application in food products.[Citation163]
In view of basic ailments of health, unfriendly occasions were accounted for in 24 of 77 patients to whom whey protein from Clostridium difficile vaccinated cow-milk was given.[Citation164] However, no adverse effect on behavior, physical assessment, and substantial hematological as well as biochemical assessment. Lacterminis, a whey fragment in coorporating lactoferrin, and lactoperoxidase with bioactive peptides such as Lactermin, did not exhibit any kind of clinical adverse effect on body weight, organ weights, food intake, serum chemistry, hematology activity, observational behavior, ophthalmic effects, urinalysis or microscopic pathology.[Citation165] Hence the whey peptides are not toxic compound but usually people are deeply anxious about its allergy. β-lactoglobulin, is significantly precursor of causing allergy in infants. The whey protein can be fractionated into little peptides. Concurrently, a clinical examination has been done with a new ultrafiltrated hydrolysate of whey that are used in infant formula, which has sub-atomic weight under 8 kD and professed to be multiple times diminished in antigenicity.[Citation166] In vitro investigation propose that whey peptides under 3KD sub-atomic weight has extremely low antigenicity, and subsequently, exceptionally hydrolyzed whey protein has been recommended for baby equation antigenicity.[Citation167] Lactoferrin is an iron-restricting glycoprotein that is present in mammalian milk as well as exceptionally amassed in mammalian colostrums which assumes a basic part for maintain healthy immune system of infant.[Citation168] Acute as well as sub-chronic toxic study revealed no adverse harmful as well as mutagenic impact. Tamano et al.[Citation169] further completed ongoing toxicology investigations of cow-like lactoferrin, hydrolyzates of lactoferrin as well as the average peptide lactoferricin, along with no adverse impact was found. Hence, the lactoferrin along with its peptides can be utilized securely as dietary supplement for infant.
In buttermilk, four types of glycerophospholipids such as phosphatidylcholine, phosphatidylinositol, phosphatidylethanolamine, and phosphatidylserine are present.[Citation170] In these glycerophospholipids, Sphingomyelin is a significant sphingolipids of buttermilk.[Citation151] Hydroperoxyl and hydroxyl free radicals oxidize the membrane of phospholipids and directed toward lipid peroxidation process. In the membrane of phospholipids, polyunsaturated fatty acids are significantly present that are susceptible to the process of peroxidation. Similarly, free radical generate the generate the process of peroxidation of arachidonic acid as well as docosahexaenoic acid directed to catalyzed the metabolites of long chain polyunsaturated acids such as isoprostanes, neuroprostanes, and endoperoxides, that further exhibit toxicological and pharmacological action in various part of tissues.[Citation171] Concurrently, buttermilk is a dietetic product and prominent because of its high content of lecithin, fat and vitamin B but it is well tolerated by the people who are lactose intolerant. Hence buttermilk contains low quantity of protein but it exhibit good biological activity.[Citation172]
Conclusion
Sustainable development of dairy industry with respect to environmental safety and public health concerns is crucial to processors. Population growth and lack of land resources is the major challenge to waste management. Here are some approaches for dairy industrial waste management such as biotechnological applications, genetic engineering, breeding techniques, and fermentation. From dairy industry waste, by-products of high demand can be developed for further applications in bakery items, dairy products, different beverages, bioactive substances, biopolymers for microencapsulating materials, exopolysaccharides, short chain fatty acids, and several others. This review mainly focuses on utilization of whey and buttermilk for the production of valuable products which can be further utilized in different food processing operations.
Consent for publication
All the authors consent to the publication
Data availability
Additional data will be made available on request.
Acknowledgments
The authors are thankful to the faculty of Food Science and Nutrition, Bahauddin Zakariya University Multan, Department of Food Science, Government College University Faisalabad and Kampala International University for their support during the study.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Kyriakopoulou, K.; Keppler, J. K.; van der Goot, A. J.; Boom, R. M. Alternatives to Meat and Dairy. Annu. Rev. Food Sci. Technol. 2021, 12, 29–50. DOI: 10.1146/annurev-food-062520-101850.
- Ahmad, T.; Aadil, R. M.; Ahmed, H.; Ur Rahman, U.; Soares, B. C.; Souza Simone, L. Q.; Pimentel, T. C.; Scudino, H.; Guimarães, J. T.; Esmerino, E. A., et al. Treatment and Utilization of Dairy Industrial Waste: A Review. Trends Food Sci. Technol. 2019, 88, 361–372.
- Barukčić, I.; Lisak Jakopović, K.; Božanić, R. Valorisation of Whey and Buttermilk for Production of Functional Beverages–An Overview of Current Possibilities. Food Technol. Biotechnol. 2019, 57(4), 448–460. DOI: 10.17113/ftb.57.04.19.6460.
- Lambert, S.; Leconte, N.; Blot, M.; Rousseau, F.; Robert, B.; Camier, B.; Gassi, J. -Y.; Cauty, C.; Lopez, C.; Gésan-Guiziou, G. The Lipid Content and Microstructure of Industrial Whole Buttermilk and Butter Serum Affect the Efficiency of Skimming. Food. Res. Int. 2016, 83, 121–130. DOI: 10.1016/j.foodres.2016.03.002.
- Vanderghem, C.; Bodson, P.; Danthine, S.; Paquot, M.; Deroanne, C.; Blecker, C. Milk Fat Globule Membrane and Buttermilks: From Composition to Valorization. Biotechnol. Agron. Soc. Environ. 2010, 14(3), 485–500.
- Conway, V.; Couture, P.; Gauthier, S.; Pouliot, Y.; Lamarche, B. Effect of Buttermilk Consumption on Blood Pressure in Moderately Hypercholesterolemic Men and Women. Nutrition. 2014, 30(1), 116–119. DOI: 10.1016/j.nut.2013.07.021.
- Gebreselassie, N.; Abay, F.; Beyene, F. Biochemical and Molecular Identification and Characterization of Lactic Acid Bacteria and Yeasts Isolated from Ethiopian Naturally Fermented Buttermilk. J. Food Sci. Technol. 2016, 53(1), 184–196.
- Gebreselassie, N.; Abrahamsen, R. K.; Beyene, F.; Abay, F.; Narvhus, J. A. Chemical Composition of Naturally Fermented Buttermilk. Int. J Dairy Technol. 2016, 69(2), 200–208. DOI: 10.1111/1471-0307.12236.
- Skryplonek, K.; Dmytrów, I.; Mituniewicz-Ma\lek, A. The Use of Buttermilk as a Raw Material for Cheese Production. Int. J Dairy Technol. 2019, 72(4), 610–616. DOI: 10.1111/1471-0307.12614.
- Lehn, D. N.; Esquerdo, V. M.; Júnior, M. A. D.; Dall’agnol, W.; dos Santos, A. C. F.; de Souza, C. F. V.; de Almeida Pinto, L. A. Microencapsulation of Different Oils Rich in Unsaturated Fatty Acids Using Dairy Industry Waste. J. Clean. Prod. 2018, 196, 665–673. DOI: 10.1016/j.jclepro.2018.06.127.
- Ergene, E.; Ayşe, A. Effects of Cultural Conditions on Exopolysaccharide Production by Bacillus Sp. ZBP4. J. Agric. Sci. 2018, 24, 386–393. DOI: 10.15832/ankutbd.456666.
- Mann, B.; Athira, S.; Sharma, R.; Kumar, R.; Sarkar, P. Bioactive Peptides from Whey Proteins. In Whey Protein from Milk to Medicine; Deeth, H., and Bansal, N., Eds.; 2019; pp. 519–547. DOI: 10.1016/B978-0-12-812124-5.00015-1.
- Chandra, R.; Castillo-Zacarias, C.; Delgado, P.; Parra-Saldívar, R. A Biorefinery Approach for Dairy Wastewater Treatment and Product Recovery Towards Establishing a Biorefinery Complexity Index. J. Clean. Prod. 2018, 183, 1184–1196. DOI: 10.1016/j.jclepro.2018.02.124.
- Chizhayeva, A.; Oleinikova, Y.; Saubenova, M.; Sadanov, A.; Amangeldi, A.; Aitzhanova, A.; Alybaeva, A.; Yelubaeva, M. Impact of Probiotics and Their Metabolites in Enhancement the Functional Properties of Whey-Based Beverages. AIMS Agric. Food. 2020, 5(3), 521–542. DOI: 10.3934/agrfood.2020.3.521.
- Orlova, T. N.; Ott, E. F.; Musina, O. N. Perspective of Using Propionic Acid Bacteria to Produce Functional Foods Based on Milk Whey, in: AIP Conference Proceedings. AIP Publishing LLC. Ekaterinburg, Russia, 2021, 30006.
- Hugues-Ayala, A. M.; Sarabia-Sainz, J. A.; González-Rios, H.; Vázquez-Moreno, L.; Montfort, G.R. -C. Airbrush Encapsulation of Lactobacillus Rhamnosus GG in Dry Microbeads of Alginate Coated with Regular Buttermilk Proteins. LWT. 2020, 117, 108639. DOI: 10.1016/j.lwt.2019.108639.
- Faria, A.; Gonçalves, L.; Peixoto, J. M.; Peixoto, L.; Brito, A. G.; Martins, G. Resources Recovery in the Dairy Industry: Bioelectricity Production Using a Continuous Microbial Fuel Cell. J. Clean. Prod. 2017, 140, 971–976. DOI: 10.1016/j.jclepro.2016.04.027.
- Huang, Z.; Zheng, H.; Brennan, C. S.; Mohan, M. S.; Stipkovits, L.; Li, L.; Kulasiri, D. Production of Milk Phospholipid-Enriched Dairy Ingredients. Foods. 2020, 9(3), 263. DOI: 10.3390/foods9030263.
- Aghababaei, F.; Cano-Sarabia, M.; Trujillo, A. J.; Quevedo, J. M.; Ferragut, V. Buttermilk as Encapsulating Agent: Effect of Ultra-High-Pressure Homogenization on Chia Oil-In-Water Liquid Emulsion Formulations for Spray Drying. Foods. 2021, 10(5), 1059. DOI: 10.3390/foods9030263.
- Almalki, M. A. Exopolysaccharide Production by a New Lactobacillus Lactis Isolated from the Fermented Milk and Its Antioxidant Properties. J. King Saud Univ.-Sci. 2020, 32(2), 1272–1277. DOI: 10.1016/j.jksus.2019.11.002.
- Oliveira, D.; Fox, P.; O’Mahony, J. A. Byproducts from Dairy Processing. Byproducts Agricult Fisheries: Adding Value Food, Feed, Pharma, Fuels;. 2019, 57–106. DOI: 10.1002/9781119383956.ch4.
- Kumar, R.; Chauhan, S. K.; Shinde, G.; Subramanian, V.; Nadanasabapathi, S. Whey Proteins: A Potential Ingredient for Food Industry-A Review. Asian J. Dairy Food Res. 2018, 37, 283–290.
- Ozkan, G.; Franco, P.; De Marco, I.; Xiao, J.; Capanoglu, E. A Review of Microencapsulation Methods for Food Antioxidants: Principles, Advantages, Drawbacks and Applications. Food Chem. 2019, 272, 494–506. DOI: 10.1016/j.foodchem.2018.07.205.
- Zhou, X.; Hua, X.; Huang, L.; Xu, Y. Bio-Utilization of Cheese Manufacturing Wastes (Cheese Whey Powder) for Bioethanol and Specific Product (Galactonic Acid) Production via a Two-Step Bioprocess. Bioresources Technol. 2019, 272, 70–76. DOI: 10.1016/j.biortech.2018.10.001.
- Eckert, C.; Serpa, V. G.; dos Santos, A. C. F.; da Costa, S. M.; Dalpubel, V.; Lehn, D. N.; de Souza, C. F. V. Microencapsulation of Lactobacillus Plantarum ATCC 8014 Through Spray Drying and Using Dairy Whey as Wall Materials. LWT-Food Sci. Technol. 2017, 82, 176–183. DOI: 10.1016/j.lwt.2017.04.045.
- Rosolen, M. D.; Bordini, F. W.; de Oliveira, P. D.; Conceição, F. R.; Pohndorf, R. S.; Fiorentini, Â. M.; da Silva, W. P.; Pieniz, S. Symbiotic Microencapsulation of Lactococcus lactis Subsp. Lactis R7 Using Whey and Inulin by Spray Drying. LWT. 2019, 115, 108411. DOI: 10.1016/j.lwt.2019.108411.
- Puttarat, N.; Thangrongthong, S.; Kasemwong, K.; Kerdsup, P.; Taweechotipatr, M. Spray-Drying Microencapsulation Using Whey Protein Isolate and Nano-Crystalline Starch for Enhancing the Survivability and Stability of Lactobacillus Reuteri TF-7. Food Sci. Biotechnol. 2021, 30(2), 245–256.
- Livney, Y. D.; Ruimy, E.; Aiqian, M. Y.; Zhu, X.; Singh, H. A Milkfat Globule Membrane-Inspired Approach for Encapsulation of Emulsion Oil Droplets. Food Hydrocoll. 2017, 65, 121–129. DOI: 10.1016/j.foodhyd.2016.11.017.
- Lopez, C.; Cauty, C.; Guyomarc’h, F. Unraveling the Complexity of Milk Fat Globules to Tailor Bioinspired Emulsions Providing Health Benefits: The Key Role Played by the Biological Membrane. Eur. J. Lipid Sci. Technol. 2019, 121(1), 1800201. DOI: 10.1002/ejlt.201800201.
- Augustin, M. A.; Bhail, S.; Cheng, L. J.; Shen, Z.; Øiseth, S.; Sanguansri, L. Use of Whole Buttermilk for Microencapsulation of Omega-3 Oils. J. Funct. Foods. 2015, 19, 859–867. DOI: 10.1016/j.jff.2014.02.014.
- Tupuna, D. S.; Paese, K.; Guterres, S. S.; Jablonski, A.; Flôres, S. H.; de Oliveira Rios, A. Encapsulation Efficiency and Thermal Stability of Norbixin Microencapsulated by Spray-Drying Using Different Combinations of Wall Materials. Ind. Crops Prod. 2018, 111, 846–855. DOI: 10.1016/j.indcrop.2017.12.001.
- Tolun, A.; Altintas, Z.; Artik, N. Microencapsulation of Grape Polyphenols Using Maltodextrin and Gum Arabic as Two Alternative Coating Materials: Development and Characterization. J. Biotechnol. 2016, 239, 23–33. DOI: 10.1016/j.jbiotec.2016.10.001.
- Davidov-Pardo, G.; Arozarena, I.; Marín-Arroyo, M. R. Optimization of a Wall Material Formulation to Microencapsulate a Grape Seed Extracts Using a Mixture Design of Experiments. Food Bioprocess Technol. 2013, 6(4), 941–951.
- Kandansamy, K.; Somasundaram, P. D. Microencapsulation of Colors by Spray Drying-A Review. Int. J. Food Eng. 2012, 8, 3. DOI: 10.1515/1556-3758.2647.
- Hosseini, H.; Ghorbani, M.; Jafari, S. M.; Mahoonak, A. S. Encapsulation of EPA and DHA Concentrate from Kilka Fish Oil by Milk Proteins and Evaluation of Its Oxidative Stability. J. Food Sci. Technol. 2019, 56(1), 59–70.
- Zhang, M.; Luo, T.; Zhao, X.; Hao, X.; Yang, Z. Interaction of Exopolysaccharide Produced by Lactobacillus Plantarum YW11 with Whey Proteins and Functionalities of the Polymer Complex. J. Food Sci. 2020, 85(12), 4141–4151. DOI: 10.1111/1750-3841.15522.
- Kumar, R.; Sabikhi, L.; Rathod, G.; Chaudhary, N. Storage Studies of Flaxseed Oil Encapsulated by Buttermilk Solids. Food Bioprocess Technol. 2020, 13(8), 1392–1404.
- Aslan, K. S.; Karabulut, I.; Koc, T. B. Changes in Oxidative Stability and Phytochemical Contents of Microencapsulated Wheat Germ Oil During Accelerated Storage. Food Biosci. 2021, 44, 101415. DOI: 10.1016/j.fbio.2021.101415.
- Alvarado-Reveles, O.; Fernández-Michel, S.; Jiménez-Flores, R.; Cueto-Wong, C.; Vázquez-Moreno, L.; Montfort, G.R. -C. Survival and Goat Milk Acidifying Activity of Lactobacillus Rhamnosus GG Encapsulated with Agave Fructans in a Buttermilk Protein Matrix. Probiotics Antimicrob Proteins. 2019, 11, 1340–1347.
- Fu, S.; Shen, Z.; Ajlouni, S.; Ng, K.; Sanguansri, L.; Augustin, M. A. Interactions of Buttermilk with Curcuminoids. Food Chem. 2014, 149, 47–53. DOI: 10.1016/j.foodchem.2013.10.049.
- Zhang, X.; Zhao, Y.; Li, Y.; Zhu, L.; Fang, Z.; Shi, Q. Physicochemical, Mechanical and Structural Properties of Composite Edible Films Based on Whey Protein Isolate/Psyllium Seed Gum. Int. J. Biol. Macromol. 2020, 153, 892–901. DOI: 10.1016/j.ijbiomac.2020.03.018.
- Fu, S.; Augustin, M. A.; Shen, Z.; Ng, K.; Sanguansri, L.; Ajlouni, S. Bioaccessibility of Curcuminoids in Buttermilk in Simulated Gastrointestinal Digestion Models. Food Chem. 2015, 179, 52–59. DOI: 10.1016/j.foodchem.2015.01.126.
- Patel, K. S. Development of self-carbonated probiotic whey beverage. M. Tech PhD Thesis, Anand Agricultural University, Anand, Gujarat, 2012.
- Mudgil, D.: Barak, S. Dairy-Based Functional Beverages, In: Milk-Based Beverages. Elsevier, 2019; pp. 67–93. DOI: 10.1016/B978-0-12-815504-2.00003-7.
- Turkmen, N.; Akal, C.; Özer, B. Probiotic Dairy-Based Beverages: A Review. J. Funct. Foods. 2019, 53, 62–75. DOI: 10.1016/j.jff.2018.12.004.
- Akal, C.; Turkmen, N.; Özer, B. Technology of Dairy-Based Beverages, in Milk-Based Beverages. Elsevier. 2019, 331–372. DOI: 10.1016/B978-0-12-815504-2.00010-4.
- Joshi, J.; Gururani, P.; Vishnoi, S.; Srivastava, A. Whey Based Beverages: A Review. Octa J. Biosci. 2020, 300, 49–55.
- Meshram, B. D. Butter-Milk Based Fruit Juice Beverages. Asian J. Dairy Food Res. 2015, 34, 297–299.
- Deynichenko, G.; Yudina, T.; Rudochenko, O. Assessment of Quality Level of Buttermilk Milkshakes. Food Sci Food Technol. 2014, 4, 43–46. DOI: 10.15550/ASJ.2014.04.043.
- Mudgil, D.; Barak, S.; Darji, P. Development and Characterization of Functional Cultured Buttermilk Utilizing Aloe Vera Juice. Food Biosci. 2016, 15, 105–109. DOI: 10.1016/j.fbio.2016.06.001.
- Liutkevičius, A.; Speičienė, V.; Alenčikienė, G.; Mieželienė, A.; Narkevičius, R.; Kaminskas, A.; Abaravičius, J. A.; Vitkus, D.; Jablonskienė, V.; Sekmokienė, D. Fermented Buttermilk-Based Beverage: Impact on Young volunteers’ Health Parameters. Czech J. Food Sci. 2016, 34, 143–148. DOI: 10.17221/344/2015-CJFS.
- de Freitas Mascarello, A.; Pinto, G. I.; de Araújo, I. S.; Caragnato, L. K.; da Silva, A. L. L.; dos Santos, L. F. Technological and Biological Properties of Buttermilk: A Minireview, In: Whey-Biological Properties and Alternative Uses. IntechOpen. 2019. DOI: 10.5772/intechopen.80921.
- Szudera-Kończal, K.; Myszka, K.; Kubiak, P.; Majcher, M. A. Analysis of the Ability to Produce Pleasant Aromas on Sour Whey and Buttermilk By-Products by Mold Galactomyces Geotrichum: Identification of Key Odorants. Molecules. 2021, 26, 6239. DOI: 10.3390/molecules26206239.
- SA, S. S. New Ingredient in Bakery, Technological and Nutritional Effects of Buttermilk. 2019. DOI:10.2991/isils-19.2019.55.
- Shaikh, M. F. B.; Rathi, S. D. Utilisation of Buttermilk for the Preparation of Carbonated Fruit-Flavoured Beverages. Int. J Dairy Technol. 2009, 62, 564–570. DOI: 10.1111/j.1471-0307.2009.00527.x.
- Patel, B. K.; Patel, S. M.; Modi, Z. S.; Pinto, S. V. Shelf Life Studies of Buttermilk Supplemented with Moringa. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 552–567.
- Hassan, A. A.; El-Shazly, H. A.; Sakr, A. M.; Ragab, W. A. Influence of Substituting Water with Fermented Skim Milk, Acid Cheese Whey or Buttermilk on Dough Properties and Baking Quality of Pan Bread. World J. Dairy Food Sci. 2013, 8, 100–117.
- Al-Jahani, A. H. Effect of Buttermilk on the Physicochemical, Rheological, and Sensory Qualities of Pan and Pita Bread. Int. J. Food Sci. 2017, 2017, 1–8. DOI: 10.1155/2017/2054252.
- Ali, A. H. Current Knowledge of Buttermilk: Composition, Applications in the Food Industry, Nutritional and Beneficial Health Characteristics. Int. J Dairy Technol. 2019, 72, 169–182. DOI: 10.1111/1471-0307.12572.
- Madenci, A. B.; Bilgiçli, N. Effect of Whey Protein Concentrate and Buttermilk Powders on Rheological Properties of Dough and Bread Quality. J. Food Qual. 2014, 37, 117–124. DOI: 10.1111/jfq.12077.
- Rasouli Pirouzian, H.; Alakas, E.; Cayir, M.; Yakisik, E.; Toker, O. S.; Kaya, Ş.; Tanyeri, O. Buttermilk as Milk Powder and Whey Substitute in Compound Milk Chocolate: Comparative Study and Optimisation. Int. J Dairy Technol. 2021, 74, 246–257. DOI: 10.1111/1471-0307.12736.
- Jeewanthi, R. K. C.; Lee, N. -K.; Paik, H. -D. Improved Functional Characteristics of Whey Protein Hydrolysates in Food Industry. Korean J. Food Sci. Anim. Resour. 2015, 35, 350. DOI: 10.5851/kosfa.2015.35.3.350.
- Paul, S.; Kulkarni, S.; Rao, K. J. Effect of Indian Cottage Cheese (Paneer)-Whey on Rheological and Proofing Characteristics of Multigrain Bread Dough. J. Texture Stud. 2016, 47, 142–151. DOI: 10.1111/jtxs.12168.
- Gaber, A. M.; Satar, A. -E.; Abdeen, I. M. I. Utilization of Ras Cheese Sweet Whey, Acidic Cheese Whey and Permeate to Improve of Baladi Bread Properties. J. Food Dairy Sci. 2017, 8, 449–453.
- Luz, C.; Izzo, L.; Ritieni, A.; Mañes, J.; Meca, G. Antifungal and Antimycotoxigenic Activity of Hydrolyzed Goat Whey on Penicillium spp: An Application as Biopreservation Agent in Pita Bread. LWT. 2020, 118, 108717. DOI: 10.1016/j.lwt.2019.108717.
- Stoliar, M. US Whey Ingredients in Bakery Products. Applications Monograph Bakery; US dairy export council: USA, 2009; pp. 1–8.
- Khaire, R. A.; Gogate, P. R. Intensified Recovery of Lactose from Whey Using Thermal, Ultrasonic and Thermosonication Pretreatments. J. Food Eng. 2018, 237, 240–248. DOI: 10.1016/j.jfoodeng.2018.04.027.
- Sampaio, F. C.; de Faria, J. T.; da Silva, M. F.; de Souza Oliveira, R. P.; Converti, A. Cheese Whey Permeate Fermentation by Kluyveromyces lactis: A Combined Approach to Wastewater Treatment and Bioethanol Production. Environ. Technol. 2020, 41, 3210–3218. DOI: 10.1080/09593330.2019.1604813.
- Lech, M. Optimisation of Protein-Free Waste Whey Supplementation Used for the Industrial Microbiological Production of Lactic Acid. Biochem. Eng. J. 2020, 157, 107531. DOI: 10.1016/j.bej.2020.107531.
- Kareb, O.; Aïder, M. Whey and Its Derivatives for Probiotics, Prebiotics, Synbiotics, and Functional Foods: A Critical Review. Probiotics Antimicrob. Proteins. 2019, 11, 348–369.
- Kumar, R.; Kaur, M.; Garsa, A. K.; Shrivastava, B.; Reddy, V. P.; Tyagi, A. Natural and Cultured Buttermilk. Fermented Milk Dairy Prod. 2015, 2015, 203–225.
- Antunes, A. E.; Silva, E. R.; Van Dender, A. G.; Marasca, E. T.; Moreno, I.; Faria, E. V.; Padula, M.; Lerayer, A. L. Probiotic Buttermilk-Like Fermented Milk Product Development in a Semiindustrial Scale: Physicochemical, Microbiological and Sensory Acceptability. Int. J Dairy Technol. 2009, 62, 556–563. DOI: 10.1111/j.1471-0307.2009.00534.x.
- Fauquant, J.; Beaucher, E.; Sinet, C.; Robert, B.; Lopez, C. Combination of Homogenization and Cross-Flow Microfiltration to Remove Microorganisms from Industrial Buttermilks with an Efficient Permeation of Proteins and Lipids. Innov. Food Sci. Emerg. Technol. 2014, 21, 131–141. DOI: 10.1016/j.ifset.2013.10.004.
- Wang, K. Relationship between lactic acid bacteria, their lipolytic activity on milk phospholipids in buttermilk and potential health contribution. PhD Thesis, The Ohio State University, 2019.
- Parekh, S. L.; Balakrishnan, S.; Hati, S.; Aparnathi, K. D. Biofunctional Properties of Cultured Buttermilk Prepared by Incorporation of Fermented Paneer Whey. Int. J. Curr. Microbiol. App. Sci. 2017, 6, 933–945.
- Ilavenil, S.; Park, H. S.; Vijayakumar, M.; Valan Arasu, M.; Kim, D. H.; Ravikumar, S.; Choi, K. C. Probiotic Potential of Lactobacillus Strains with Antifungal Activity Isolated from Animal Manure. Sci. World J. 2015, 2015. DOI: 10.1155/2015/802570.
- Salah, R. B.; Jaouadi, B.; Bouaziz, A.; Chaari, K.; Blecker, C.; Derrouane, C.; Attia, H.; Besbes, S. Fermentation of Date Palm Juice by Curdlan Gum Production from Rhizobium Radiobacter ATCC 6466TM: Purification, Rheological and Physico-Chemical Characterization. LWT-Food Sci. Technol. 2011, 44, 1026–1034. DOI: 10.1016/j.lwt.2010.11.023.
- Ünver, Y. Utilization of Cheese Whey for Production of Azurin by Pseudomonas aeruginosa. Sak. Univ. J. Sci. 2021, 25, 601–609.
- Sharma, A.; Mukherjee, S.; Tadi, S. R. R.; Ramesh, A.; Sivaprakasam, S. Kinetics of Growth, Plantaricin and Lactic Acid Production in Whey Permeate Based Medium by Probiotic Lactobacillus Plantarum CRA52. LWT. 2021, 139, 110744. DOI: 10.1016/j.lwt.2020.110744.
- Asunis, F.; De Gioannis, G.; Francini, G.; Lombardi, L.; Muntoni, A.; Polettini, A.; Pomi, R.; Rossi, A.; Spiga, D. Environmental Life Cycle Assessment of Polyhydroxyalkanoates Production from Cheese Whey. Waste Manag. 2021, 132, 31–43. DOI: 10.1016/j.wasman.2021.07.010.
- Dinika, I.; Verma, D. K.; Balia, R.; Utama, G. L.; Patel, A. R. Potential of Cheese Whey Bioactive Proteins and Peptides in the Development of Antimicrobial Edible Film Composite: A Review of Recent Trends. Trends Food Sci. Technol. 2020, 103, 57–68. DOI: 10.1016/j.tifs.2020.06.017.
- Pandey, A.; Srivastava, S.; Rai, P.; Duke, M. Cheese Whey to Biohydrogen and Useful Organic Acids: A Non-Pathogenic Microbial Treatment by L. Acidophilus. Sci. Rep. 2019, 9, 1–9.
- Ricciardi, A.; Zotta, T.; Ianniello, R. G.; Boscaino, F.; Matera, A.; Parente, E. Effect of Respiratory Growth on the Metabolite Production and Stress Robustness of Lactobacillus Casei N87 Cultivated in Cheese Whey Permeate Medium. Front. Microbiol. 2019, 10, 851. DOI: 10.3389/fmicb.2019.00851.
- Cardoso, T.; Marques, C.; Dagostin, J. L. A.; Masson, M. L. Lactobionic Acid as a Potential Food Ingredient: Recent Studies and Applications. J. Food Sci. 2019, 84, 1672–1681. DOI: 10.1111/1750-3841.14686.
- Amaro, T. M.; Rosa, D.; Comi, G.; Iacumin, L. Prospects for the Use of Whey for Polyhydroxyalkanoate (PHA) Production. Front. Microbiol. 2019, 10, 992. DOI: 10.3389/fmicb.2019.00992.
- Luongo, V.; Policastro, G.; Ghimire, A.; Pirozzi, F.; Fabbricino, M. Repeated-Batch Fermentation of Cheese Whey for Semi-Continuous Lactic Acid Production Using Mixed Cultures at Uncontrolled pH. Sustainability. 2019, 11, 3330. DOI: 10.3390/su11123330.
- Chwialkowska, J.; Duber, A.; Zagrodnik, R.; Walkiewicz, F.; Lężyk, M.; Oleskowicz-Popiel, P. Caproic Acid Production from Acid Whey via Open Culture Fermentation–Evaluation of the Role of Electron Donors and Downstream Processing. Bioresources Technol. 2019, 279, 74–83. DOI: 10.1016/j.biortech.2019.01.086.
- Tagliazucchi, D.; Martini, S.; Solieri, L. Bioprospecting for Bioactive Peptide Production by Lactic Acid Bacteria Isolated from Fermented Dairy Food. Ferment. 2019, 5, 96. DOI: 10.3390/fermentation5040096.
- Longanesi, L.; Frascari, D.; Spagni, C.; DeWever, H.; Pinelli, D. Succinic Acid Production from Cheese Whey by Biofilms of Actinobacillus succinogenes: Packed Bed Bioreactor Tests. J. Chem. Technol. Biotechnol. 2018, 93, 246–256. DOI: 10.1002/jctb.5347.
- Park, J. -K.; Huh, C. -K.; Gim, D. -W.; Kim, Y. -J.; Kim, S. -H.; Kwon, Y. -K.; Bae, D.; Kim, Y. -D. Quality Characteristics of Whey Makgeolli Vinegar Produced Using Acetobacter Pomorum IWV-03. Korean J. Food Sci. Technol. 2018, 50, 61–68. DOI: 10.9721/KJFST.2018.50.1.61.
- Ren, Z. -Y.; Liu, G. -L.; Chi, Z.; Han, Y. -Z.; Hu, Z.; Chi, Z. -M. Overexpression of Both the Lactase Gene and Its Transcriptional Activator Gene Greatly Enhances Lactase Production by Kluyveromyces marxianus. Process Biochem. 2017, 61, 38–46. DOI: 10.1016/j.procbio.2017.06.001.
- Yadav, J. S. S.; Yan, S.; Ajila, C. M.; Bezawada, J.; Tyagi, R. D.; Surampalli, R. Y. Food-Grade Single-Cell Protein Production, Characterization and Ultrafiltration Recovery of Residual Fermented Whey Proteins from Whey. Food Bioprod. Process. 2016, 99, 156–165. DOI: 10.1016/j.fbp.2016.04.012.
- Lule, V. K.; Singh, R.; Pophaly, S. D.; Tomar, S. K. Production and Structural Characterisation of Dextran from an Indigenous Strain of Leuconostoc mesenteroides BA 08 in Whey. Int. J Dairy Technol. 2016, 69, 520–531. DOI: 10.1111/1471-0307.12271.
- Macwan, S. R.; Dabhi, B. K.; Parmar, S. C.; Aparnathi, K. D. Whey and Its Utilization. Int. J. Curr. Microbiol. Appl. Sci. 2016, 5, 134–155.
- Nath, A.; Verasztó, B.; Basak, S.; Koris, A.; Kovács, Z.; Vatai, G. Synthesis of Lactose-Derived Nutraceuticals from Dairy Waste Whey—A Review. Food Bioprocess Technol. 2016, 9, 16–48.
- Yadav, J. S. S.; Yan, S.; Pilli, S.; Kumar, L.; Tyagi, R. D.; Surampalli, R. Y. Cheese Whey: A Potential Resource to Transform into Bioprotein, Functional/Nutritional Proteins and Bioactive Peptides. Biotechnol. Adv. 2015, 33, 756–774. DOI: 10.1016/j.biotechadv.2015.07.002.
- Mollea, C.; Marmo, L.; Bosco, F. Valorisation of Cheese Whey, a By-Product from the Dairy Industry, In: Food Industry. IntechOpen. 2013. DOI: 10.5772/53159.
- Hsu, C. S. Integrated Rotating Fibrous-Bed Bioreactor-Ultrafiltration Process for Xanthan Gum Production from Whey Lactose. Dissertion, The Ohio State University and OhioLINK, 2011.
- Toyoda, T.; Ohtaguchi, K. Effect of Temperature on D-Arabitol Production from Lactose by Kluyveromyces lactis. J. Ind. Microbiol. Biotechnol. 2011, 38, 1179–1185. DOI: 10.1007/s10295-010-0893-4.
- Gupte, A. M.; Nair, J. S. β-Galactosidase Production and Ethanol Fermentation from Whey Using Kluyveromyces marxianus. NCIM. 2010, 3551 855–859.
- Fonseca, G. G.; Heinzle, E.; Wittmann, C.; Gombert, A. K. The Yeast Kluyveromyces marxianus and Its Biotechnological Potential. Appl. Microbiol. Biotechnol. 2008, 79, 339–354.
- Pham, P. L.; Dupont, I.; Roy, D.; Lapointe, G.; Cerning, J. Production of Exopolysaccharide by Lactobacillus Rhamnosus R and Analysis of Its Enzymatic Degradation During Prolonged Fermentation. Appl. Environ. Microbiol. 2000, 66, 2302–2310. DOI: 10.1128/AEM.66.6.2302-2310.2000.
- Di Pierro, P.; Mariniello, L.; Giosafatto, V. L.; Esposito, M.; Sabbah, M.; Porta, R. Dairy Whey Protein-Based Edible Films and Coatings for Food Preservation. Food Packag Preserv. Elsevier. 2018, 439–456. DOI: 10.1016/B978-0-12-811516-9.00013-0.
- Hammam, A. R. Technological, Applications, and Characteristics of Edible Films and Coatings: A Review. Sn. Appl. Sci. 2019, 1, 1–11.
- Kandasamy, S.; Yoo, J.; Yun, J.; Kang, H. -B.; Seol, K. -H.; Kim, H. -W.; Ham, J. -S. Application of Whey Protein-Based Edible Films and Coatings in Food Industries: An Updated Overview. Coatings. 2021, 11, 1056.
- Zhang, Y.; Pang, X.; Zhang, S.; Liu, L.; Ma, C.; Lu, J.; Lyu, J. Buttermilk as a Wall Material for Microencapsulation of Omega-3 Oils by Spray Drying. LWT. 2020, 127, 109320. DOI: 10.1016/j.lwt.2020.109320.
- Fernandes, L. M.: Guimarães, J. T.; Pimentel, T. C.; Esmerino, E. A.; Freitas, M. Q.; Carvalho, C. W. P.; Cruz, A. G.; Silva, M. C. Edible Whey Protein Films and Coatings Added with Prebiotic Ingredients. In Agri-Food Industry Strategies for Healthy Diets and Sustainability. Elsevier. 2020, 177–193. DOI: 10.1016/B978-0-12-817226-1.00007-2.
- Zikmanis, P.; Kolesovs, S.; Semjonovs, P. Production of Biodegradable Microbial Polymers from Whey. Bioresour. Bioprocess. 2020, 7, 1–15.
- Ahmad, T.; Aadil, R. M.; Ahmed, H.; Ur Rahman, U.; Soares, B. C.; Souza, S. L.; Pimentel, T. C.; Scudino, H.; Guimarães, J. T.; Esmerino, E. A. Treatment and Utilization of Dairy Industrial Waste: A Review. Trends Food Sci. Technol. 2019, 88, 361–372.
- Blandón, L. M.; Noseda, M. D.; Islan, G. A.; Castro, G. R.; de Melo Pereira, G. V.; Thomaz-Soccol, V.; Soccol, C. R. Optimization of Culture Conditions for Kefiran Production in Whey: The Structural and Biocidal Properties of the Resulting Polysaccharide. Bioact. Carbohydr. Diet. Fibre. 2018, 16, 14–21. DOI: 10.1016/j.bcdf.2018.02.001.
- Li, C.; Ding, J.; Chen, D.; Shi, Z.; Wang, L. Bioconversion of Cheese Whey into a Hetero-Exopolysaccharide via a One-Step Bioprocess and Its Applications. Biochem. Eng. J. 2020, 161, 107701. DOI: 10.1016/j.bej.2020.107701.
- Do, T. B. T.; Tran, B. K.; Tran, T. V. T.; Le, T. H.; Cnockaert, M.; Vandamme, P.; Nguyen, T. H. C.; Nguyen, C. C.; Hong, S. H.; Kim, S. Y., et al. Decoding the Capability of Lactobacillus Plantarum W1 Isolated from Soybean Whey in Producing an Exopolysaccharide. ACS Omega. 2020, 51, 33387–33394.
- Al-Dhabi, N. A.; Esmail, G. A.; Duraipandiyan, V.; Arasu, M. V. Chemical Profiling of Streptomyces Sp. Al-Dhabi-2 Recovered from an Extreme Environment in Saudi Arabia as a Novel Drug Source for Medical and Industrial Applications. Saudi J. Biol. Sci. 2019, 26, 758–766. DOI: 10.1016/j.sjbs.2019.03.009.
- Kwak, H. -S.; Lee, W. -J.; Lee, M. -R. Revisiting Lactose as an Enhancer of Calcium Absorption. Int. Dairy. J. 2012, 22, 147–151.
- Dullius, A.; Goettert, M. I.; de Souza, C. F. V. Whey Protein Hydrolysates as a Source of Bioactive Peptides for Functional Foods–Biotechnological Facilitation of Industrial Scale-Up. J. Funct. Foods. 2018, 42, 58–74. DOI: 10.1016/j.jff.2017.12.063.
- Mazorra-Manzano, M. A.; Robles-Porchas, G. R.; González-Velázquez, D. A.; Torres-Llanez, M. J.; Martínez-Porchas, M.; García-Sifuentes, C. O.; González-Córdova, A. F.; Vallejo-Córdoba, B. Cheese Whey Fermentation by Its Native Microbiota: Proteolysis and Bioactive Peptides Release with ACE-Inhibitory Activity. Fermentation. 2020, 6, 19. DOI: 10.3390/fermentation6010019.
- Mohanty, D. P.; Mohapatra, S.; Misra, S.; Sahu, P. S. Milk Derived Bioactive Peptides and Their Impact on Human Health–A Review. Saudi J. Biol. Sci. 2016, 23, 577–583. DOI: 10.1016/j.sjbs.2015.06.005.
- Martínez-Medina, G. A.; Carranza-Méndez, R.; Amaya-Chantaca, D. P.; Ilyna, A.; Gaviria-Acosta, E.; Hoyos-Concha, J. L.; Chávez-González, M. L.; Govea-Salas, M.; Prado-Barragán, L. A.; Aguilar-González, C. N. Bioactive Peptides from Food Industrial Wastes. In Bioactive Peptides; Onuh, J.; Selvamuthukumaran, M.; Pathak, Y. V.; Eds.; CRC Press: Boca Raton, 2021; pp. 169–203.
- Worsztynowicz, P.: Bia\las, W.; Grajek, W. Integrated Approach for Obtaining Bioactive Peptides from Whey Proteins Hydrolysed Using a New Proteolytic Lactic Acid Bacteria. Food Chem. 2020, 312, 126035. DOI: 10.1016/j.foodchem.2019.126035.
- Monari, S.; Ferri, M.; Russo, C.; Prandi, B.; Tedeschi, T.; Bellucci, P.; Zambrini, A. V.; Donati, E.; Tassoni, A.; Papadimitriou, K. Enzymatic Production of Bioactive Peptides from Scotta, an Exhausted By-Product of Ricotta Cheese Processing. PLoS One. 2019, 14, e0226834. DOI: 10.1371/journal.pone.0226834.
- Mills, S.; Ross, R. P.; Hill, C.; Fitzgerald, G. F.; Stanton, C. Milk Intelligence: Mining Milk for Bioactive Substances Associated with Human Health. Int. Dairy. J. 2011, 21, 377–401. DOI: 10.1016/j.idairyj.2010.12.011.
- van der Kraan, M. I.; Groenink, J.; Nazmi, K.; Veerman, E. C.; Bolscher, J. G.; Amerongen, A. V. N. Lactoferrampin: A Novel Antimicrobial Peptide in the N1-Domain of Bovine Lactoferrin. Peptides. 2004, 25, 177–183. DOI: 10.1016/j.peptides.2003.12.006.
- Chen, J.; Lindmark-Månsson, H.; Gorton, L.; Åkesson, B. Antioxidant Capacity of Bovine Milk as Assayed by Spectrophotometric and Amperometric Methods. Int. Dairy. J. 2003, 13, 927–935. DOI: 10.1016/S0958-6946(03)00139-0.
- Burns, P.; Molinari, F.; Beccaria, A.; Paez, R.; Meinardi, C.; Reinheimer, J.; Vinderola, G. Suitability of Buttermilk for Fermentation with Lactobacillus helveticus and Production of a Functional Peptide-Enriched Powder by Spray-Drying. J. Appl. Microbiol. 2010, 109, 1370–1378. DOI: 10.1111/j.1365-2672.2010.04761.x.
- Barman, S.; Ghosh, R.; Mandal, N. C. Production Optimization of Broad Spectrum Bacteriocin of Three Strains of Lactococcus lactis Isolated from Homemade Buttermilk. Ann. Agrar. Sci. 2018, 16, 286–296. DOI: 10.1016/j.aasci.2018.05.004.
- Olabi, A.; Jinjarak, S.; Jiménez-Flores, R.; Walker, J. H.; Daroub, H. Compositional and Sensory Differences of Products of Sweet-Cream and Whey Buttermilk Produced by Microfiltration, Diafiltration, and Supercritical CO2. J. Dairy. Sci. 2015, 98, 3590–3598. DOI: 10.3168/jds.2014-9141.
- Jean, C.; Boulianne, M.; Britten, M.; Robitaille, G. Antimicrobial Activity of Buttermilk and Lactoferrin Peptide Extracts on Poultry Pathogens. J. Dairy Res. 2016, 83, 497–504.
- Akalın, A. S. Dairy-Derived Antimicrobial Peptides: Action Mechanisms, Pharmaceutical Uses and Production Proposals. Trends Food Sci. Technol. 2014, 36, 79–95. DOI: 10.1016/j.tifs.2014.01.002.
- Amiri, S.; Rezaei Mokarram, R.; Sowti Khiabani, M.; Rezazade Bari, M.; Alizadeh Khaledabad, M. Production of Lactic Acid by Lactobacillus acidophilus LA5 and Bifidobacterium Lactis BB12 in Batch Fermentation of Cheese Whey and Milk Permeate. J Food Res. 2020, 30, 33–49.
- Wu, H.; Dalke, R.; Mai, J.; Holtzapple, M.; Urgun-Demirtas, M. Arrested Methanogenesis Digestion of High-Strength Cheese Whey and Brewery Wastewater with Carboxylic Acid Production. Bioresources Technol. 2021, 332, 125044. DOI: 10.1016/j.biortech.2021.125044.
- Uysal, U.; Hamamcı, H. Succinic Acid Production from Cheese Whey via Fermentation by Using Alginate Immobilized Actinobacillus Succinogenes. Bioresour. Technol. Rep. 2021, 16, 100829. DOI: 10.1016/j.biteb.2021.100829.
- Iglesias-Iglesias, R.; Portela-Grandío, A.; Treu, L.; Campanaro, S.; Kennes, C.; Veiga, M. C. Co-Digestion of Cheese Whey with Sewage Sludge for Caproic Acid Production: Role of Microbiome and Polyhydroxyalkanoates Potential Production. Bioresources Technol. 2021, 337, 125388. DOI: 10.1016/j.biortech.2021.125388.
- Lagoa-Costa, B.; Kennes, C.; Veiga, M. C. Cheese Whey Fermentation into Volatile Fatty Acids in an Anaerobic Sequencing Batch Reactor. Bioresources Technol. 2020, 308, 123226. DOI: 10.1016/j.biortech.2020.123226.
- Zhao, L.; Feng, R.; Mao, X. Addition of Buttermilk Powder Improved the Rheological and Storage Properties of Low-Fat Yogurt. Food Sci. Nutr. 2020, 8, 3061–3069. DOI: 10.1002/fsn3.1373.
- Zhao, L.; Feng, R.; Mao, X. Addition of Buttermilk Improves the Flavor and Volatile Compound Profiles of Low-Fat Yogurt. LWT. 2018, 98, 9–17. DOI: 10.1016/j.lwt.2018.08.029.
- Iranmanesh, M.; Ezzatpanah, H.; Mojgani, N.; Torshizi, M. Characterization and Kinetics of Growth of Bacteriocin Like Substance Produced by Lactic Acid Bacteria Isolated from Ewe Milk and Traditional Sour Buttermilk in Iran. J. Food Process. Technol. 2015, 6, 1–9.
- Bourlieu, C.; Cheillan, D.; Blot, M.; Daira, P.; Trauchessec, M.; Ruet, S.; Gassi, J. -Y.; Beaucher, E.; Robert, B.; Leconte, N., et al. Polar Lipid Composition of Bioactive Dairy Co-Products Buttermilk and Butterserum: Emphasis on Sphingolipid and Ceramide Isoforms. Food Chem. 2018, 240, 67–74. DOI: 10.1016/j.foodchem.2017.07.091.
- Lopez, C.; Madec, M. -N.; Jimenez-Flores, R. Lipid Rafts in the Bovine Milk Fat Globule Membrane Revealed by the Lateral Segregation of Phospholipids and Heterogeneous Distribution of Glycoproteins. Food Chem. 2010, 120, 22–33. DOI: 10.1016/j.foodchem.2009.09.065.
- Verardo, V.; Gómez-Caravaca, A. M.; Arráez-Román, D.; Hettinga, K. Recent Advances in Phospholipids from Colostrum, Milk and Dairy By-Products. Int. J. Mol. Sci. 2017, 18, 173. DOI: 10.3390/ijms18010173.
- Bourlieu, C.; Cheillan, D.; Blot, M.; Daira, P.; Trauchessec, M.; Ruet, S.; Gassi, J. -Y.; Beaucher, E.; Robert, B.; Leconte, N. Polar Lipid Composition of Bioactive Dairy Co-Products Buttermilk and Butterserum: Emphasis on Sphingolipid and Ceramide Isoforms. Food Chem. 2018, 240, 67–74. DOI: 10.1016/j.foodchem.2017.07.091.
- Zheng, W.; Kollmeyer, J.; Symolon, H.; Momin, A.; Munter, E.; Wang, E.; Kelly, S.; Allegood, J. C.; Liu, Y.; Peng, Q. Ceramides and Other Bioactive Sphingolipid Backbones in Health and Disease: Lipidomic Analysis, Metabolism and Roles in Membrane Structure, Dynamics, Signaling and Autophagy. Biochim. Biophys. Acta BBA-Biomembr. 2006, 1758, 1864–1884. DOI: 10.1016/j.bbamem.2006.08.009.
- Wehrmüller, K. Impact of Dietary Phospholipids on Human Health. ALP Sci. Switz. 2008, 12–15.
- Tanaka, K.; Hosozawa, M.; Kudo, N.; Yoshikawa, N.; Hisata, K.; Shoji, H.; Shinohara, K.; Shimizu, T. The Pilot Study: Sphingomyelin-Fortified Milk Has a Positive Association with the Neurobehavioural Development of Very Low Birth Weight Infants During Infancy, Randomized Control Trial. Brain Dev. 2013, 35, 45–52. DOI: 10.1016/j.braindev.2012.03.004.
- Huang, Z. Milk lipid complexation and interaction with food ingredients: Digestibility and absorption. Ph.D thesis, Lincoln University, 2019.
- Lopez, C.; Blot, M.; Briard-Bion, V.; Cirié, C.; Graulet, B. Butter Serums and Buttermilks as Sources of Bioactive Lipids from the Milk Fat Globule Membrane: Differences in Their Lipid Composition and Potentialities of Cow Diet to Increase N-3 PUFA. Food. Res. Int. 2017, 100, 864–872. DOI: 10.1016/j.foodres.2017.08.016.
- Phan, T. T. Q.; Asaduzzaman, M.; Le, T. T.; Fredrick, E.; Van der Meeren, P.; Dewettinck, K. Composition and Emulsifying Properties of a Milk Fat Globule Membrane Enriched Material. Int. Dairy. J. 2013, 29, 99–106. DOI: 10.1016/j.idairyj.2012.10.014.
- Sodini, I.; Morin, P.; Olabi, A.; Jiménez-Flores, R. Compositional and Functional Properties of Buttermilk: A Comparison Between Sweet, Sour, and Whey Buttermilk. J. Dairy. Sci. 2006, 89, 525–536. DOI: 10.3168/jds.S0022-0302(06)72115-4.
- Wong, P. Y. Y.; Kitts, D. D. A Comparison of the Buttermilk Solids Functional Properties to Nonfat Dried Milk, Soy Protein Isolate, Dried Egg White, and Egg Yolk Powders. J. Dairy. Sci. 2003, 86, 746–754. DOI: 10.3168/jds.S0022-0302(03)73655-8.
- Roesch, R. R.; Rincon, A.; Corredig, M. Emulsifying Properties of Fractions Prepared from Commercial Buttermilk by Microfiltration. J. Dairy. Sci. 2004, 87, 4080–4087. DOI: 10.3168/jds.S0022-0302(04)73550-X.
- Sánchez-Juanes, F.; Alonso, J. M.; Zancada, L.; Hueso, P. Distribution and Fatty Acid Content of Phospholipids from Bovine Milk and Bovine Milk Fat Globule Membranes. Int. Dairy. J. 2009, 19, 273–278. DOI: 10.1016/j.idairyj.2008.11.006.
- Contarini, G.; Povolo, M. Phospholipids in Milk Fat: Composition, Biological and Technological Significance, and Analytical Strategies. Int. J. Mol. Sci. 2013, 14, 2808–2831. DOI: 10.3390/ijms14022808.
- Das, B.; Sarkar, S.; Sarkar, A.; Bhattacharjee, S.; Bhattacharjee, C. Recovery of Whey Proteins and Lactose from Dairy Waste: A Step Towards Green Waste Management. Process Saf. Environ. Prot. 2016, 101, 27–33. DOI: 10.1016/j.psep.2015.05.006.
- Chen, Z.; Luo, J.; Wang, Y.; Cao, W.; Qi, B.; Wan, Y. A Novel Membrane-Based Integrated Process for Fractionation and Reclamation of Dairy Wastewater. Chem. Eng. J. 2017, 313, 1061–1070. DOI: 10.1016/j.cej.2016.10.134.
- Zandona, E.; Blažić, M.; Režek Jambrak, A. Whey Utilization: Sustainable Uses and Environmental Approach. Food Technol. Biotechnol. 2021, 59, 147–161.
- Khatami, K.; Perez-Zabaleta, M.; Owusu-Agyeman, I.; Cetecioglu, Z. Waste to Bioplastics: How Close are We to Sustainable Polyhydroxyalkanoates Production? Waste Manag. 2021, 119, 374–388. DOI: 10.1016/j.wasman.2020.10.008.
- Khattab, A. M.; Esmael, M. E.; Farrag, A. A.; Ibrahim, M. I. Structural Assessment of the Bioplastic (Poly-3-Hydroxybutyrate) Produced by Bacillus Flexus Azu-A2 Through Cheese Whey Valorization. Int. J. Biol. Macromol. 2021, 190, 319–332. DOI: 10.1016/j.ijbiomac.2021.08.090.
- Raho, S.; Carofiglio, V. E.; Montemurro, M.; Miceli, V.; Centrone, D.; Stufano, P.; Schioppa, M.; Pontonio, E.; Rizzello, C. G. Production of the Polyhydroxyalkanoate PHBV from Ricotta Cheese Exhausted Whey by Haloferax mediterranei Fermentation. Foods. 2020, 9, 1459. DOI: 10.3390/foods9101459.
- Reddy, M. V.; Mawatari, Y.; Onodera, R.; Nakamura, Y.; Yajima, Y.; Chang, Y. -C. Bacterial Conversion of Waste into Polyhydroxybutyrate (PHB): A New Approach of Bio-Circular Economy for Treating Waste and Energy Generation. Bioresour. Technol. Rep. 2019, 7, 100246. DOI: 10.1016/j.biteb.2019.100246.
- Chalermthai, B.; Giwa, A.; Schmidt, J. E.; Taher, H. Life Cycle Assessment of Bioplastic Production from Whey Protein Obtained from Dairy Residues. Bioresour. Technol. Rep. 2021, 15, 100695. DOI: 10.1016/j.biteb.2021.100695.
- Sato, K.; Iwai, K.; Aito-Inoue, M. Identification of Food-Derived Bioactive Peptides in Blood and Other Biological Samples. J. AOAC Int. 2008, 91, 995–1001. DOI: 10.1093/jaoac/91.4.995.
- Kan, J.; Cheng, J.; Xu, L.; Hood, M.; Zhong, D.; Cheng, M.; Liu, Y.; Chen, L.; Du, J. The Combination of Wheat Peptides and Fucoidan Protects Against Chronic Superficial Gastritis and Alters Gut Microbiota: A Double-Blinded, Placebo-Controlled Study. Eur. J. Nutr. 2020, 59, 1655–1666.
- Hussein, F. A.; Chay, S. Y.; Ghanisma, S. B. M.; Zarei, M.; Auwal, S. M.; Hamid, A. A.; Ibadullah, W. Z. W.; Saari, N. Toxicity Study and Blood Pressure–Lowering Efficacy of Whey Protein Concentrate Hydrolysate in Rat Models, Plus Peptide Characterization. J. Dairy. Sci. 2020, 103, 2053–2064. DOI: 10.3168/jds.2019-17462.
- Lam, F. -C.; Bukhsh, A.; Rehman, H.; Waqas, M. K.; Shahid, N.; Khaliel, A. M.; Elhanish, A.; Karoud, M.; Telb, A.; Khan, T. M. Efficacy and Safety of Whey Protein Supplements on Vital Sign and Physical Performance Among Athletes: A Network Meta-Analysis. Front Pharmacol. 2019, 10, 317.
- Young, K. W.; Munro, I. C.; Taylor, S. L.; Veldkamp, P.; van Dissel, J. T. The Safety of Whey Protein Concentrate Derived from the Milk of Cows Immunized Against Clostridium Difficile. Regul. Toxicol. Pharmacol. 2007, 47, 317–326. DOI: 10.1016/j.yrtph.2006.12.001.
- Dyer, A. R.; Burdock, G. A.; Carabin, I. G.; Haas, M. C.; Boyce, J.; Alsaker, R.; Read, L. C. In vitro and in vivo Safety Studies of a Proprietary Whey Extract. Food. Chem. Toxicol. 2008, 46, 1659–1665. DOI: 10.1016/j.fct.2007.12.029.
- Diao, X. An Update on Food Allergen Management and Global Labeling Regulations. Ph.D Thesis, University Of Minnesota, 2017. https://hdl.handle.net/11299/194287
- Knipping, K.; Simons, P. J.; Buelens-Sleumer, L. S.; Cox, L.; den Hartog, M.; de Jong, N.; Teshima, R.; Garssen, J.; Boon, L.; Knippels, L. M. Development of β-Lactoglobulin-Specific Chimeric Human IgEκ Monoclonal Antibodies for in vitro Safety Assessment of Whey Hydrolysates. PLoS One. 2014, 9, e106025. DOI: 10.1371/journal.pone.0106025.
- Bagwe, S.; Tharappel, L. J.; Kaur, G.; Buttar, H. S. Bovine Colostrum: An Emerging Nutraceutical. J. Complement. Integr. Med. 2015, 12, 175–185. DOI: 10.1515/jcim-2014-0039.
- Tamano, S.; Sekine, K.; Takase, M.; Yamauchi, K.; Iigo, M.; Tsuda, H. Lack of Chronic Oral Toxicity of Chemopreventive Bovine Lactoferrin in F344/DuCrj Rats. Asian Pac. J. Cancer Prev. 2008, 9, 313–316.
- Donato, P.; Cacciola, F.; Cichello, F.; Russo, M.; Dugo, P.; Mondello, L. Determination of Phospholipids in Milk Samples by Means of Hydrophilic Interaction Liquid Chromatography Coupled to Evaporative Light Scattering and Mass Spectrometry Detection. J. Chromatogr. 2011, A 1218, 6476–6482.
- Davies, S. S.; Guo, L. Lipid Peroxidation Generates Biologically Active Phospholipids Including Oxidatively N-Modified Phospholipids. Chem. Phys. Lipids. 2014, 181, 1–33.
- Augustyńska-Prejsnar, A.; Ormian, M.; Hanus, P.; Kluz, M.; Soko\lowicz, Z.; Rudy, M. Effects of Marinating Breast Muscles of Slaughter Pheasants with Acid Whey, Buttermilk, and Lemon Juice on Quality Parameters and Product Safety. J. Food Qual. 2019. DOI: 10.1155/2019/5313496.