ABSTRACT
Starch nanoparticles resist digestion in the upper intestine but not in the colon. They are proven to exert prebiotic effects on the human body. Starch-based nanoparticles have generated a great interest in the food and agriculture sector due to their biocompatibility, wide array of natural sources and ease of modification. In the current study, two starch sources (rice and water chestnut) were used to synthesize nanoparticles for the encapsulation of probiotic bacteria. Obtained nanoparticles were characterized for their morphological, molecular, and structural attributes using Fourier Transform Infrared Spectroscopy (FTIR), scanning electron microscopy (SEM), X-ray diffraction (XRD), and zeta sizer. Furthermore, free and nano-encapsulated probiotics were subject to simulated gastrointestinal digestion conditions. The average size of rice and water chestnut starch-based nano-capsules ranged from 309 to 427 nm. The encapsulation efficiency was recorded for rice and water chestnut starch as 89% and 95%, respectively. SEM micrographs exhibited entrapment of probiotics in wall materials. The surface of the capsules showed tiny, smooth surface polygonal granules. XRD images showed loss of crystallinity structure following encapsulation. Higher probiotic viability was recorded under simulated gastrointestinal conditions for nano-encapsulated probiotics compared to free probiotics. Conclusively, water chestnut and rice-based starch nanoparticles showed overall the best results regarding the viability of probiotics under stressed conditions.
Introduction
In recent years, encapsulation of probiotics has a significant amount of attention not only because of their ability to promote gut health but also because of their potential ability to prevent or treat a variety of conditions such as cardiovascular disorders and hypercholesterolemia[Citation1]. Probiotics are living microorganisms, and they need to be able to survive while traveling through the harsh environment of the stomach. Additionally, an adequate number of probiotics need to reach the large intestine for them to colonies and proliferate, which will allow them to maintain their beneficial effects on the host.[Citation2]
In recent years, there has been a growing interest in nano-encapsulation technology, which has shown itself to be more useful for improving bioaccessibility and ensuring the delivery of bioactive substances to specific locations.[Citation3] Starch is often regarded as the most accessible and inexpensive natural polymer, and it possesses a significant amount of potential for use in the manufacture of starch-based nanoparticles for use in a variety of applications within the pharmaceutical and food industries. Encapsulating probiotic bacteria in starch has been shown to improve the bacteria’s ability to live in symbiotic ice cream, which has been used as a food model, as well as in vitro conditions.[Citation4]
Resistant starch (RS) is a form of dietary fiber that can produce short-chain fatty acids (SCFAs), including acetate, propionate, and butyrate, which can support metabolic and colonic health. Resistant starch is resistant to enzymatic breakdown in the small intestine and can instead reach the colon.[Citation5] Various researchers have tried to encapsulate probiotics using native starch molecules[Citation6]; nevertheless, the efficiency of starch-based nanoparticles in encapsulating live probiotic microorganisms still needs to be explored. In this investigation, we made use of a non-traditional supply of rice starch and water chestnut starch.
Bioactive delivery systems have been a crucial technology area spanning from food and agricultural applications to nutraceutical advances due to the growing concern of protecting and improving the physiological effects of the bioactive substances in the gastrointestinal tract. Due to their exceptional durability, Pickering emulsions stabilized by solid particles have recently attracted increasing interest. Due to their advantageous qualities, including biocompatibility, nontoxicity, renewability, and low cost, starch nanoparticles (SNPs) have emerged as one of the promising solid stabilizers for use in Pickering emulsions.[Citation7]
Although the nanomaterials have demonstrated promising results for the transport of bioactive substances, it is not known whether or not they are suited for the encapsulation of live probiotic cells.[Citation8] The key objective of the present study is to evaluate the effect of starch-based nanoparticles encapsulation on the viability and stability of probiotics under adverse conditions. Moreover, water chestnut starch-based nanoparticles encapsulation was compared in terms of different attributes. The survival of free and encapsulated probiotics was also studied under in vitro simulated gastrointestinal conditions.
Materials and methods
The present study was designed to explore the effect of starch-based nano-encapsulating wall materials on the viability and stability of probiotics, which was carried out at the Food Safety and Biotechnology Laboratory, Department of Food Sciences, Government College (GC) University Faisalabad.
Procurement of materials
Water chestnuts and rice were purchased from a local market in Faisalabad. All the chemicals were acquired from the Food Safety and Biotechnology Laboratory, Department of Food Science. The chemicals used in the present study were of analytical grade. The freeze-dried culture of Lactobacillus rhamnosus (GG) was sourced by Culturelle.
Extraction of starch
The samples of water chestnut were peeled and cut into small portions as previously reported by Zehra et al.[Citation9] The small pieces were ground in a domestic blender, and starch was extracted by alkali method. Briefly, the flour was soaked in 0.2% NaOH solution for 24 h. The sample was homogenized (FSH 2A) and secerned through a muslin cloth. The mixture was centrifuged (Heraeus megafuge 8 R) with rpm 4000 for 10 min. Two layers were formed. The vicious layer is washed away, and the precipitate is put into petri dishes. For 4 h at 60°C, isolated starch was dried in a hot air oven. The rice starch was extracted according to the method of Ashwar et al.[Citation10] The rice grains were steeped in distilled water for 4 h, washed, and then soaked for 1 h in a 0.2% NaOH solution. The grains were combined with an alkaline solution for 5 min. Screens were used to filter the rice slurry. The raw starch crystallized after being allowed to settle in the starch suspension. The filtrate was decanted, and the remaining sediment was washed with 0.2% NaOH to remove the protein until the yellow layer dissipated. The remaining starch was then neutralized with 1 N HCl and washed with distilled water then centrifuged it for 12 min at 10,000 rpm. At room temperature, purified rice starch was dried and stored in a desiccator until used.
Preparation of starch nanoparticles
Starch nanoparticles of water chestnut were prepared as described by Ahmad et al.[Citation8] Briefly, 15 g of water chestnut and rice starch were pretreated in 0.1N NaOH solution. The solution mixture was kept in a shaking incubator (Thermostable IS-20 R) for 3 days at 60°C. After 3 days, suspension was centrifuged (Heraeus megafuge 8 R) at 4000 rpm for 10 min. After centrifugation, the suspensions were washed with distilled water to neutralize the solution. The washing process was repeated three times in acetone water, and the precipitated starch was discharged for solvent replacement. Contrary to this, starch nanoparticles of rice were prepared by alkali freezing treatment following the method of Xiao et al.[Citation11] Fifteen grams of rice starch was dissolved in the 0.1N NaOH solution. The solution mixture was kept in a shaking incubator (Thermostable IS-20 R) for 3 days at 60°C. After 3 days, the suspension was centrifuged at 4000 rpm for 10 min. After centrifugation, distilled water was used to wash the suspensions and neutralize the solution. By washing three times in acetone, the precipitated starch was discharged for solvent replacement.
Activation of bacterial culture
The procedure described by Afzaal et al.[Citation12] was followed to activate freeze-dried cell cultures. Briefly, cells of Lactobacillus rhamnosus were inoculated in 100 mL of MRS broth. The bacterial culture was incubated at 37°C for 24 h. Following 5 min of centrifugation at 4°C and 4500 rpm, cells were collected. After that, cells were twice washed with a sterile solution of peptone (0.1%). Bacterial culture was magnetically stirred to minimize bacterial sedimentation rate.
Nano-encapsulation of Lactobacillus rhamnosus
Encapsulation of probiotic Lactobacillus rhamnosus was done by the dispersion method as described by Islam et al.[Citation13] Bacterial suspension was added in starch nanoparticle dispersion solutions followed by continuous magnetic stirring. Four samples of nano-encapsulated Lactobacillus rhamnosus were formed by using water chestnut (1% and 2%) and rice starch (1% and 2%). All samples were formed as F1 probiotic encapsulated with 1% (starch nanoparticles of water chestnut), F2 probiotics encapsulated with 2% (starch nanoparticles of water chestnut), F3 probiotic encapsulated with 1% (starch nanoparticles of rice) and F4 probiotic encapsulated with 2% (starch nanoparticles of rice). Results indicate that the size of encapsulated L. Rhamnoses was decreased while the encapsulation efficiency of capsules was increased.
Characterization of probiotic loaded nanoparticles
Determination of particle size
The size of probiotic encapsulated starch nanoparticles was evaluated by scanning electron microscopy (SEM) conducted at the Physics Department, Government College University, Faisalabad.
Determination of zeta potential
Zeta potential of all samples was calculated utilizing the technique outlined by Ni et al.[Citation14] The samples with various treatments were suspended in 0.1 mM KCL for zeta potential measurements, the pH was set to 6, and the samples were given the night to equilibrate before testing.
PDI (Poly Dispersity Index)
Polydispersity (PDI) was determined to estimate the average uniformity of solution with the help of Zeta potential analyzer that was used to obtain the PDI.
Encapsulation efficiency
The encapsulation efficiency of the probiotic encapsulated starch nanoparticles was calculated by following the method as described by Afzaal et al.[Citation15] The percentage encapsulation efficiency (% EE) was calculated by using the formula as shown.
A0 is the total number of free cells before encapsulation, and A is the number of live entrapped cells released after encapsulation.
Scanning electron microscopy (SEM)
The surface morphologies of the probiotic encapsulated starch nanoparticles were determined by SEM (Emcraft cubeseries, South Korea). High-quality images were captured at a voltage of 8 kV and a current of 1.203 mA. SEM was performed in the Physics Department, nanotechnology lab at GC University Faisalabad.
X-ray powder diffraction
The probiotic encapsulated starch nanoparticles (PENs) were analyzed for XRD analysis (Siemens (D 5005 Germany) to check the crystalline nature of encapsulated starch nanoparticles. XRD analyses were performed at the High TEC laboratory at GC University, Faisalabad.
Fourier transform infrared (FTIR) spectroscopy
FTIR spectra of the encapsulated starch nanoparticle molecules were measured using FTIR spectrophotometer (FTIR was performed at NTU Faisalabad). The encapsulated probiotic was mixed well with potassium bromide in the range of 1%. The finely powdered KBr was used to get optimum results because it absorbs more humidity from the air. The pellets were scanned in the range of 4000 to 650 cm−1 and images of all samples were collected.
In vitro studies
Preparation of gastric stock solution Survival of free and encapsulated L. Rhamnosus was examined by protocol of Amira et al.[Citation16] with slight modifications. Briefly, the stimulated gastric juice was prepared using 0.3% (w/v) pepsin, 0.5% (w/v) NaCl, 0.22% (w/v) NaHCO3, and 0.22% (w/v) CaCl2 the stimulated gastric juice was acidified with HCL (0.1 M) to pH 2.5. Non-encapsulated and encapsulated probiotic cells of water chestnut starch and rice starch nanoparticles were added in the test tubes and were incubated at 37°C. The viability of free and encapsulated cells was analyzed at 0, 30, 60, 90, and 120 min.
Preparation of intestinal stock solution
By following the method as described by Amira et al.[Citation16] stimulated intestinal stock solution was prepared to assess the viability under stimulated intestinal conditions. With 0.1% (w/v) pancreas, 0.0128% (w/v) NaCl, 0.023% (w/v) KCL, 0.64% (w/v) NAHCO3, and 0.5% (w/v) bile salts, stimulated intestinal juice was made, and the pH was adjusted to 7.5. The viability and stability were also determined under simulated intestinal circumstances. Test tubes containing the solutions were filled with free and encapsulated cells, and results were recorded after 0, 30, 60, 90, and 120 min. Concentrations of chemicals for the stock solution have been shown in .
Table 1. Preparation of Gastric and intestinal stock solution.
Thermal resistance
The thermal resistance of free and encapsulated probiotic starch nanoparticles was assayed according to the method suggested by Ahmad et al.[Citation8] One ml of free suspension and one-gram probiotic encapsulated starch nanoparticles were put into test tubes containing 10 mL of sterile distilled water and provided to different heat treatments in the water bath at 50°C, 60°C, 70°C, and 80°C for 15 min.
Statistical analysis
To assess the variable-dependent numbers of live probiotics, each experiment was performed three times with cells determined in the viability assays according to the probiotic (with SLM (probiotic chocolate), free cells (without encapsulation), and controls (no added probiotic). The analysis of variance with α = 0.05 and average differences was calculated with the help of statistical software.
Results and discussions
Particle size
Encapsulated probiotics with starch nanoparticles were investigated to determine the particle size. The average particle size of probiotics encapsulated with starch nanoparticles is shown in . The average particle size diameter of F1 (probiotic encapsulated with 1% water chestnut) and F2 (probiotic encapsulated with 2% water chestnut) have sizes 322.67 ± 3.06 nm and 427.0 ± 6.25 nm. Likewise, the concentration of F3 (probiotic encapsulated with 1% rice starch) and F4 (probiotic encapsulated with 2% rice starch) having particle size diameters 257.0 ± 6.56 nm and 217.67 ± 7.51 nm.
Table 2. Descriptive analysis of nano-encapsulated particle size.
Ahmed et al.[Citation8] concluded that the encapsulated starch nanoparticles have different particle sizes due to amylopectin in starch molecules. Therefore, particle size may be reduced by ultra-sonication process because this technique brake down the amylopectin structures. These current findings are in line with Ahmed et al.[Citation8] where they prepared water chestnut starch particles encapsulated with catechin and these particles were 559 nm in size. Similarly, Xiao et al.[Citation11] developed rice starch nanoparticles having the particle size of 100 nm and 200 nm.
Zeta potential
The zeta potential of prepared encapsulated probiotics (L. Rhamnosus) with different concentrations of water chestnut and rice starch nanoparticles significantly (p < .05) is shown in . As the anionic nature of starch nanoparticles, the results have indicated that all zeta potentials are negatively charged. The F2 2% concentration of water chestnut starch nanoparticles showed a significantly high zeta potential value was −18 ± 0.03 mV while F4 (2% concentration of rice starch nanoparticles) has a minimum zeta potential value was −14 ± 0.04 mV.
Table 3. The effect of Zeta potential.
Ahmed et al.[Citation8] developed the starch nanoparticles of water chestnut encapsulated probiotic Pediococcus acidolactici having zeta potential −20 mV. This study showed that starch nanoparticles of water chestnut encapsulated probiotics have great potential for the stability of probiotics. According to Chen et al.[Citation17] starch nanoparticles synthesized from maize encapsulated with rutin showed a zeta potential of −20.8 mV. Due to high zeta potential starch nanoparticles have great stability.
Polydispersity index
The homogeneity of particle size distribution is assessed by the polydispersity index (PDI) value. The numerical value of PDI ranges from 0.0 to 1.0. PDI values range from 0.1 to 0.5 for accurate measurements and high-quality colloidal suspensions to values around 0.7 for low-quality samples, with a wide size distribution that may contain aggregates or large particles. PDI is an effective tool to evaluate the value of particle size distribution. Acevedo-Guevara et al.[Citation18] reported that banana starch based used for nanoencapsulation of curcumin showed 0.25 PDI value. Likewise, Chang et al.[Citation19] developed starch nanoparticles by ultrasonication tehnique and reported value of PDI between 0.25 to 0.35 in treatments suggested that starch nanoparticles were more uniform in size. The particle size distribution and PDI are highly important physical attributes of nanoparticles. These values can affect the bulk properties, product efficiency stability, and viability of the end product. The results of encapsulated probiotic starch nanoparticles have been shown in .
Table 4. Polydispersity Index.
The mean results from all treatments of encapsulated probiotic starch nanoparticles illustrated that the maximum reading for PDI 0.73 was detected in treatment F2 (encapsulated probiotic with 2% water chestnut starch nanoparticles, while the minimum value was detected in treatment F4 (encapsulated probiotic with 2% rice starch nanoparticles). The difference in PDI values indicates the difference in the structure of both rice and water chestnut starch.
Encapsulation efficiency
The encapsulation efficiency determines whether a wall material can keep the tiny bioactive molecules inside its hollow structure. The initial counts of cells before encapsulation were 8.54, 8.63, 8.24, and 9.43 (Log CFU/g). After encapsulation, the final counts of cells were 8.047, 8.25, 7.38, and 8.538, respectively. The encapsulation efficiency of encapsulated probiotic starch nanoparticles is shown in . The encapsulation of both water chestnut and rice starch nanoparticles encapsulated with probiotics was found to be 94.2%, 95.22%, 89.11%, and 91.2%, respectively. The ability of small particles to create a better film around the core materials results in improved encapsulation efficacy due to smaller particle size diameter. Panichikka et al.[Citation20] developed rice starch nanoparticles encapsulated with encapsulated Bacillus licheniformis where the encapsulation efficiency was 96.66%. Panichikkal et al.[Citation21] developed zinc oxide nanoparticles encapsulated with probiotics. In this study, the encapsulation efficiency of all types of beads was found to be more than 90%.
Table 5. Encapsulation Efficiency.
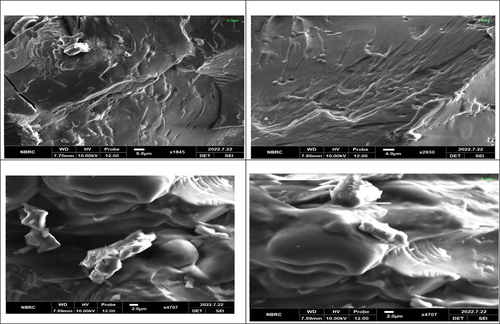
Scanning electron microscopy
Scanning Electron Microscopy was used to observed the morphological characteristics of nanoparticles.
SEM analysis, also known as scanning electron microscopy (SEM), is an effective analytical technique that may be used on a variety of materials at high magnifications and provide high-resolution images. SEM analysis. The results are shown in . According to the results, the encapsulated probiotic starch nanoparticles have smooth or porous surfaces and are oval or circular. Samples showed tiny, smooth-surfaced polygonal granules, although some also had dents or hollows at one end. By following the previous literature Atraki & Azizkhani,[Citation4] the preparation of nano-encapsulated probiotics with corn starch described the SEM images that showed round shapes and symmetry in their structure. Ashwar et al.[Citation10] developed rice starch nanoparticles and showed the SEM images that showed the regular and round-shaped patterns. The biological origin, biochemistry of the amylose and amylopectin of starch molecules, and probiotic physiology may be responsible for the variation in granule form.
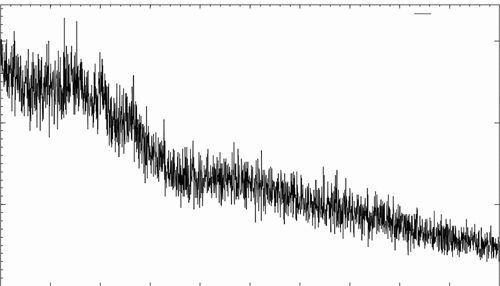
Crystallography analysis by XRD
A special technique for determining the crystallinity of various substances is X-ray diffractometric (XRD). Any size nanoparticles are characterized by XRD, and conclusions about how crystal structure and cell characteristics change as a function of nanoparticle size and shape are drawn from the observed changes in placements of diffraction peaks shown in . It is a unique method to understand the crystalline structure formation and chemical and physical behavior of samples. By using X-Ray Diffraction (XRD) analysis, the crystal structures of produced encapsulated probiotic starch nanoparticles have been identified. Xiao et al.[Citation11] developed rice starch nanoparticles and characterized by XRD noted the results that all starch nanoparticles contain crystalline regions. Similarly, Das & Sit[Citation22] developed taro starch nanoparticles which also showed crystalline nature. Moreover, the result of the current study is aligned with together. The XRD analysis of prepared nano-encapsulated probiotics (L. Rhamnosus) with 2% concentrations of water chestnut starch nanoparticles is shown in the figure. The water chestnut starch nanoparticles encapsulated with probiotics showed distinctive peaks that showed disclosed the crystalline structure. The difference in peak intensity is due to the interactions between different groups of polysaccharides through OH bonding.
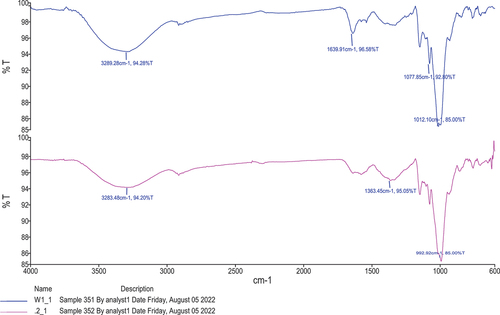
Fourier transform infrared spectroscopy (FTIR)
The FTIR spectra were used to determine the interactions between probiotic L. rhamnosus and starch nanoparticles. The infrared spectrum of transmission or absorption of material was obtained by using the FTIR method. FTIR spectrum is like a chemical fingerprint; it can be used to characterize both inorganic and organic chemicals and also identify and verify known and unknown samples. The specific molecular groups differ depending on the infrared absorption frequency range of 600–4000 cm−1. The FTIR analysis of prepared F2 nano-encapsulated probiotic (L. rhamnosus) starch nanoparticles is shown in . The findings showed that probiotics encapsulated in water chestnut starch nanoparticles have varying transmittance percentages on various intensities. The highest peak 3289.8 cm−1 was recorded for F2 at 94.28% transmittance and the lowest peak showed 1012.10 cm−1 at 85% transmittance. The average absorption peak between 3290 and 3246 cm−1 in the FTIR spectra, which is attributable to the -OH stretching, was visible, and its breadth revealed how much inter- and intramolecular hydrogen was formed. The O-H stretching peaks in starch nanoparticles encapsulated probiotic changed to a higher wavelength. In the previous study by Ahmad et al.[Citation8] the starch nanoparticles of water chestnut, lotus stem, and horse chestnut were prepared for characterization of starch nanoparticles. The results revealed that the structural analysis of all the starches with their nanoparticles had hydrogen bonds and electrostatic force interaction due to the main reason for starch nanoparticles. Remanan & Zhu[Citation23] also developed maize starch nanoparticles with the encapsulation of rutin results of FTIR were obtained concerning nanoparticles of starch at different concentrations, although the results of the current study are the same with and align with references.
In vitro studies
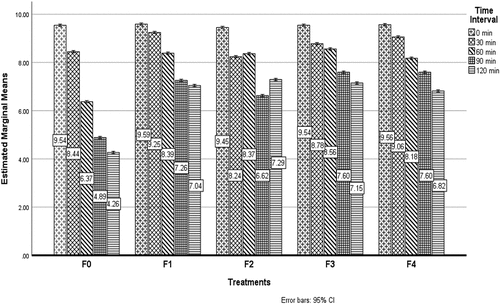
Simulated gastric fluid (SGF)
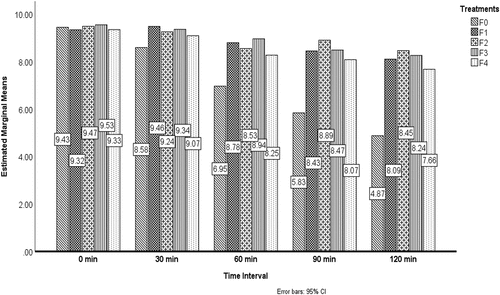
Bacteria must enter the host’s digestive tract and colonize there for the good of the host. For bacteria to tolerate the unfavorable environment of the GIT, the wall material is of utmost importance for this reason. The results of the effect of probiotics under stimulated gastric conditions from the replicates of treatments are shown in . From the results it has been illustrated that Fo (unencapsulated/free probiotic) has the lowest survival rate 4.87 CFU/ml at the storage time of 120 min and the maximum value was detected in F2 (encapsulated pro`biotic water chestnut starch nanoparticles at 2%) was 8.87 at 90 min. The results revealed that all treatments’ survival rate increased as the concentration of starch nanoparticles enhanced. The survivability of probiotic cells under SGF conditions was observed by using various wall materials for nano-encapsulation, according to Atraki and Azizkhani.[Citation4] L. rhamnus, acidophilus. The findings showed that nano-encapsulation of probiotics increases the viability of cells throughout time. The result of this study align with Ahmed et al.[Citation8] The viability effect of nano-encapsulated probiotics is shown in .
Simulated intestinal fluid (SIF)
Bacteria must enter the host’s digestive tract and colonize there for the good of the host. For bacteria to tolerate the unfavorable environment of the GIT, the wall material is of utmost importance for this purpose. The vitality of the bacteria was assessed when the free and nano-encapsulated probiotic cells were individually subjected to intestinal conditions. In simulated intestinal fluids, nano-encapsulation produced enhanced protection. The data regarding the analysis of the effect of stimulated intestinal fluid are shown in . While had shown the release behavior of encapsulated probiotics under stimulated intestinal conditions (SIF). According to Atraki & Azizkhani,[Citation4] indicated that the viability of probiotic bacteria in stimulated intestinal conditions was observed and indicated that insulted nano-encapsulation.
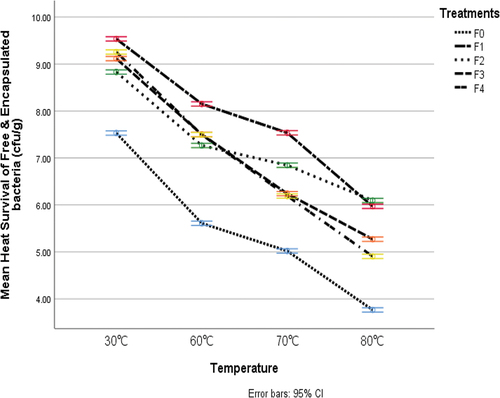
Thermal resistance
The impact of probiotic encapsulation of L. rhamnosus in rice and water chestnut starch nanoparticles on the viability of cells under various heat treatments are shown in . The data demonstrated the major differences between starch nanoparticles in terms of their capacity to protect cells from various heat treatments. The results from all treatments of probiotics illustrated that the maximum reading of heat tolerance was 9.51 was detected in treatment F1 (probiotic encapsulated with 1% starch nanoparticles), while the minimum value of heat tolerance of 3.76 was detected in F0 (unencapsulated probiotic). The findings show that unencapsulated probiotics did not provide cells with protection at high temperatures. The probiotic that was encapsulated in starch nanoparticles, in contrast, offered cells better defense against all time-temperature combinations. The finding of this research is in line with the findings of Razavi et al.[Citation24] this study noted that heat tolerance with encapsulated a better barrier at elevated temperatures.
Conclusion and recommendations
The results of the study exhibited that starch-based wall materials are effective and prolong the viability and stability of L. rhamnosus under hostile conditions and in model food. Water chestnut starch nanoparticles showed the best results in all aspects compared to rice starch nanoparticles. Water chestnut starch-based nano-encapsulation improved bioaccessibility and cell permeability. Furthermore, water chestnut starch-based encapsulation does not affect the sensory attributes of functional food products (chocolate milk). Starch-based nanoparticles are effective in the delivery of sensitive ingredients. The adaptation of starch-based nano-encapsulation in the food industry could be a way forward for the development of functional food for community health promotion. It could be encapsulated in starch nanoparticles as a wall material, which increased the viability and stability of probiotics. According to the study’s findings, using sources of starch-based nanoparticles, nano-encapsulation may be an effective way to get around this restriction. When included in fortified functional foods, the nanoparticles can protect the probiotic during the process since they have improved. Among the two wall materials, it was observed that water chestnut starch had the highest encapsulation efficiency and produced lower particle size. Nano-encapsulation using starch-based nanoparticles protected the probiotic against harsh gastric conditions and helped in retaining its bioactive properties during the in vitro digestion process.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
For this type of study, formal consent is not required.
Credit authorship contribution statement
Muhammad Noman, Muhammad Afzaal, and Farhan Saeed proposed this idea and conducted this research. Aftab Ahmad, Ali Imran, Aasma Asghar, Yasir Abbas Shah, Huda Ateeq, Noor Akram, Mohammad Rizwan Khan, and José M. Lorenzo prepared the manuscript and also aided in conducting this research and in preparing figures and tables.
Consent to participate
Corresponding and all the coauthors are willing to participate in this manuscript.
Acknowledgments
The authors would like to thank the Researchers Supporting Project number (RSP2023R138), King Saud University, Riyadh, Saudi Arabia
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Even though adequate data has been given in the form of tables and figures, all authors declare that if more data is required, the data will be provided on a request basis.
Additional information
Funding
References
- Stenman, L. K.; Burcelin, R.; Lahtinen, S. Establishing a Causal Link Between Gut Microbes, Body Weight Gain and Glucose Metabolism in Humans–Towards Treatment with Probiotics. Benefic. Microbes. 2016, 7(1), 11–22. DOI: 10.3920/BM2015.0069.
- Gani, A.; Shah, A.; Ahmad, M.; Ashwar, B. A.; Masoodi, F. A. β-D-Glucan as an Enteric Delivery Vehicle for Probiotics. Int. J. Biol. Macromol. 2018, 106, 864–869. DOI: 10.1016/j.ijbiomac.2017.08.093.
- Karim, A.; Rehman, A.; Feng, J.; Noreen, A.; Assadpour, E.; Kharazmi, M. S.; Lianfu, Z.; Jafari, S. M. Alginate-Based Nanocarriers for the Delivery and Controlled-Release of Bioactive Compounds. Adv. Coll. Interf. Sci. 2022, 307, 102744. DOI: 10.1016/j.cis.2022.102744.
- Atraki, R.; Azizkhani, M. Survival of Probiotic Bacteria Nanoencapsulated within Biopolymers in a Simulated Gastrointestinal Model. Innovative Food Sci. Emerging Technol. 2021, 72, 102750. DOI: 10.1016/j.ifset.2021.102750.
- DeMartino, P.; Cockburn, D. W. Resistant Starch: Impact on the Gut Microbiome and Health. Curr. Opin. Biotechnol. 2020, 61, 66–71. DOI: 10.1016/j.copbio.2019.10.008.
- Ashwar, B. A.; Gani, A.; Gani, A.; Shah, A.; Masoodi, F. A. Production of RS4 from Rice Starch and Its Utilization as an Encapsulating Agent for Targeted Delivery of Probiotics. Food Chem. 2018, 239, 287–294. DOI: 10.1016/j.foodchem.2017.06.110.
- Hu, X.; Miao, M. Starch-Based Nanoparticles for Fabrication of Nutraceutical Delivery System. In Polysaccharide Nanoparticles; Elsevier, 2022; pp. 341–375.
- Ahmad, M.; Mudgil, P.; Gani, A.; Hamed, F.; Masoodi, F. A.; Maqsood, S. Nano-Encapsulation of Catechin in Starch Nanoparticles: Characterization, Release Behavior and Bioactivity Retention During Simulated in-Vitro Digestion. Food Chem. 2019, 270, 95–104. DOI: 10.1016/j.foodchem.2018.07.024.
- Zehra, K.; Nawab, A.; Alam, F.; Hadi, A.; Raza, M. Development of Novel Biodegradable Water Chestnut Starch/PVA Composite Film. Evaluation of Plasticizer Effect Over Physical, Barrier, and Mechanical Properties. J. Food Process. Preserv. 2022, 46(3), e16334. DOI: 10.1111/jfpp.16334.
- Ashwar, B. A.; Shah, A.; Gani, A.; Rather, S. A.; Wani, S. M.; Wani, I. A.; Masoodi, A.; Gani, A. Effect of Gamma Irradiation on the Physicochemical Properties of Alkali-Extracted Rice Starch. Radiat. Phys. Chem. 2014, 99, 37–44. DOI: 10.1016/j.radphyschem.2014.02.002.
- Xiao, H.; Yang, F.; Lin, Q.; Zhang, Q.; Zhang, L.; Sun, S.; Han, W.; Liu, G.-Q. Preparation and Characterization of Broken-Rice Starch Nanoparticles with Different Sizes. Int. J. Biol. Macromol. 2020, 160, 437–445. DOI: 10.1016/j.ijbiomac.2020.05.182.
- Afzaal, M.; Khan, A. U.; Saeed, F.; Arshad, M. S.; Khan, M. A.; Saeed, M.; Maan, A. A.; Khan, M. K.; Ismail, Z.; Ahmed, A. Survival and Stability of Free and Encapsulated Probiotic Bacteria Under Simulated Gastrointestinal Conditions and in Ice Cream. Food Sci. Nutr. 2020, 8(3), 1649–1656. DOI: 10.1002/fsn3.1451.
- Islam, F.; Noman, M.; Afzaal, M.; Saeed, F.; Shabana, A.; Zubair, M. W.; Awuchi, C. G.; Hussain, M.; Ateeq, H.; Awuchi, C. G. Synthesis and Food Applications of Resistant Starch-Based Nanoparticles. J. Nanomater. 2022, 2022, 1–10. DOI: 10.1155/2022/8729258.
- Ni, F.; Luo, X.; Zhao, Z.; Yuan, J.; Song, Y.; Liu, C.; Gu, Q. Enhancing Viability of Lactobacillus Plantarum Encapsulated by Alginate-Gelatin Hydrogel Beads During Gastrointestinal Digestion, Storage and in the Mimic Beverage Systems. Int. J. Biol. Macromol. 2022, 224, 94–104. DOI: 10.1016/j.ijbiomac.2022.10.106.
- Afzaal, M.; Saeed, F.; Ateeq, H.; Akhtar, M. N.; Imran, A.; Ahmed, A.; Awuchi, C. G. Probiotics Encapsulated Gastroprotective Cross‐Linked Microgels: Enhanced Viability Under Stressed Conditions with Dried Apple Carrier; Food Science & Nutrition, 2022.
- Amira, S.; Alioua, Z.; Harchouche, K. Gonadal Development and Fecundity of Bogue Boops boops (Linnaeus, 1758) (Sparidae) on the Central Algerian Coast. Turk. J. Zool. 2019, 43(1), 12–29. DOI: 10.3906/zoo-1805-44.
- Chen, Y. Y.; Liu, K.; Zha, X. Q.; Li, Q. M.; Pan, L. H.; Luo, J. P. Encapsulation of Luteolin Using Oxidized Lotus Root Starch Nanoparticles Prepared by Anti-Solvent Precipitation. Carbohydr. Polym. 2021, 273, 118552. DOI: 10.1016/j.carbpol.2021.118552.
- Acevedo-Guevara, L.; Nieto-Suaza, L.; Sanchez, L. T.; Pinzon, M. I.; Villa, C. C. Development of Native and Modified Banana Starch Nanoparticles as Vehicles for Curcumin. Int. J. Biol. Macromol. 2018, 111, 498–504. DOI: 10.1016/j.ijbiomac.2018.01.063.
- Chang, Y.; Yan, X.; Wang, Q.; Ren, L.; Tong, J.; Zhou, J. High Efficiency and Low Cost Preparation of Size Controlled Starch Nanoparticles Through Ultrasonic Treatment and Precipitation. Food Chem. 2017, 227, 369–375. DOI: 10.1016/j.foodchem.2017.01.111.
- Panichikkal, J.; Puthiyattil, N.; Raveendran, A.; Nair, R. A.; Krishnankutty, R. E. Application of Encapsulated Bacillus licheniformis Supplemented with Chitosan Nanoparticles and Rice Starch for the Control of Sclerotium Rolfsii in Capsicum Annuum (L.) Seedlings. Curr. Microbiol. 2021, 78(3), 911–919. DOI: 10.1007/s00284-021-02361-8.
- Panichikkal, J.; Prathap, G.; Nair, R. A.; Krishnankutty, R. E. Evaluation of Plant Probiotic Performance of Pseudomonas sp. Encapsulated in Alginate Supplemented with Salicylic Acid and Zinc Oxide Nanoparticles. Int. J. Biol. Macromol. 2021, 166, 138–143. DOI: 10.1016/j.ijbiomac.2020.10.110.
- Das, A.; Sit, N. Modification of Taro Starch and Starch Nanoparticles by Various Physical Methods and Their Characterization. Starch‐Stärke. 2021, 73(5–6), 2000227. DOI: 10.1002/star.202000227.
- Remanan, M. K.; Zhu, F. Encapsulation of Rutin Using Quinoa and Maize Starch Nanoparticles. Food Chem. 2021, 353, 128534. DOI: 10.1016/j.foodchem.2020.128534.
- Razavi, S.; Janfaza, S.; Tasnim, N.; Gibson, D. L.; Hoorfar, M. Nanomaterial-Based Encapsulation for Controlled Gastrointestinal Delivery of Viable Probiotic Bacteria. Nanoscale Adv. 2021, 3(10), 2699–2709. DOI: 10.1039/D0NA00952K.