ABSTRACT
The microalga Spirulina platensis is recognized for its high nutritional value, particularly in terms of protein and bioactive compounds, and can be used to produce functional foods. The aim of this study was to develop a novel functional Ricotta cheese fortified with different amounts of spirulina powder (SRC) (0.25 , 0.5, 0.75, and 1.0) g/100 g cheese and to evaluate the effects on the cheese’s physicochemical, sensorial, and microbiological properties over a 21- day storage period at 5°C. Spirulina platensis had 70.4, 5.02, 6.77, 5.43, and 12.06 g/100 g (as drymatter) for protein, lipids, ash, fiber, and carbohydrate, respectively. It also had a high concentration of bioactive compounds, including 240.50 mg (as Gallic acid equivalents/100 g) of phenolic compounds and 30.79 mg/100 g of β-carotene. Ricotta cheese fortified with spirulina powder had a significant increase in protein, fat, ash, fibers, carbohydrates, and mineral contents. The total phenols, β-carotene, and 2,2-Diphenyl-1-picrylhydrazyl scavenging capacity of SRC also increased when compared with control. The textural properties of SRC cheese were improved, and it exhibited a more attractive color and desirable microstructure. The number of aerobic bacteria increased in SRC cheese, while no yeasts or mold were detected in all fresh cheese samples, but they appeared on the 14th day for the control sample and on the 21st day for the Ricotta cheese fortified with spirulina powder sample. Coliform bacterial cells were not observed in all fresh cheese or during storage. The addition of 0.75 g/100 g dry-solids SPP is found to be optimum in terms of color and other sensorial properties.
Introduction
Spirulina (Spirulina platensis) is a filamentous cyanobacteria that is phototrophic considered to be one of the most nutrient dense and comprehensive dietary sources available. It contains a wide array of essential nutrients, to making it a one of the most potent sources of macronutrients (proteins, minerals, fibers, and vitamins), and bioactive compounds (essential amino acids, monounsaturated and polyunsaturated fatty acids, polysaccharides, pigments, and enzymes), and their consumption might have positive effects on consumers’ health.[Citation1] The presence of the above-mentioned macronutrients and bioactive compounds in spirulina confers physiological and functional benefits, making it a promising ingredient for innovative and healthier food products to address malnutrition and provide other health benefits.
The Food and Drug Administration (FDA) and the National Sanitary Surveillance Agency (ANVISA) have both recognized spirulina as a “safe food” with no toxicological side effects, and have granted it GRAS (Generally Recognized as Safe) certification.[Citation2] Spirulina is used in a variety of food products, including juice smoothies, candy, food bars, baked goods, doughnuts, muffins, and pasta, as well as salad dressings, breakfast cereals, soups, and instant meals.[Citation3] In recent years, spirulina has also been incorporated into dairy products, including ice cream and cheese,[Citation4] fermented milk,[Citation5] yogurt,[Citation6] Kareish cheese,[Citation7] acidified Feta-type cheese,[Citation8] processed cheese,[Citation9] white brined cheese[Citation10] and ricotta cheese.[Citation11]
Whey is an important byproduct of the dairy industry and can be readily utilized in many food and pharmaceutical products due to its superior nutritional and functional properties. One of the most cost-effective methods of utilizing whey is to use it to produce Ricotta cheese. Fresh Ricotta is a white, soft, moist, unripened cheese that resembles cottage cheese curds in appearance.[Citation12] Scientists have attempted to improve the properties of Ricotta cheese by adding various ingredients such as skim milk powder, inulin, chestnut flour, and herbs including rosemary, thyme, and basil.[Citation13] To the best of our knowledge, there is currently very little information on the application of spirulina to enhance Ricotta cheese. Thus, aim of this study was to develop a novel functional Ricotta cheese that contains spirulina powder and evaluate its nutritional value and sensory quality by analyzing its physicochemical properties including, color, texture, microstructure, sensory and microbiological properties.
Material and methods
Materials
The study utilized cow milk and Ras cheese whey from the Department of Dairy Science at New Valley University (NVU) (New Valley, Egypt) to make Ricotta cheese. The cow milk and whey were analyzed for their physicochemical properties are presented in Table S1. Natural vinegar and table salt were purchased from a local market (New Valley, Egypt). Spirulina powder was obtained from the Soil Microbiology Department at the Water and Environment Research Institute (NVU, Egypt). All chemicals used were of analytical grade and were purchased from Sigma-Aldrich (St. Louis, Missouri, USA).
Preparation of S. platensis
The cultivation of S. platensis was performed using 100 mL of autoclaved sea water medium in 250 mL Erlenmeyer flasks in a growth room with a temperature of 28 ± 2°C and a light intensity of 55–60 μmol photon m−2 s−1 with a 16 h light and 8 h dark photoperiod. For large-scale production, raceway ponds in a semi-controlled greenhouse were utilized with an economical medium. The cultures were continuously circulated by paddle wheels at a flow rate of 20 cm/s, with a culture depth of 10 cm. Following 21–25 days of growth, the culture is harvested, filtered, washed, sun-dried, and stored at room temperature (25 ± 2°C) until use within a week.[Citation14] Powder form (mean diameter of approximately 0.3 mm) of the biomass obtained by grinding with a ball mill (Model 721, Moulinex, Egypt). The resulting powder is packaged in polypropylene bags and stored at chilled temperature (5°C) until further use (Figure S1).
Preparation of Ricotta cheese
Ricotta cheese samples were produced in the laboratory of the Dairy Science Department at the Faculty of Agriculture, New Valley University, Egypt, from April to May 2022. The production process involved the following steps : The cow milk is blended with whey of Ras cheese at a ratio of 10% (w/w), and the mixture is agitated for five minutes. The mixture is then heated to 85–90°C, acidified using natural vinegar to reach a pH of 5.4 for coagulation. The mixture was gently stirred until protein flakes were visible. Stirring was stopped once the curd became firm. The curd was collected in a plastic frame lined with fine cheesecloth over drainage table and allowed to achieve complete drainage in 24 h. The curd cheese is then seasoned with salt (0.5%) and Spirulina platensis powder (SPP) at levels of 0.25, 0.5, 0.75, and 1.0% (w/w) with mixing, respectively (Code SRC1, SRC2, SRC3 and SRC4) and control sample (CRC) produced without SPP addition. The resulting Ricotta cheese samples were pressing and divided into 100 g portions, packaged in plastic containers, and stored at 5°C for analysis. Analysis was carried out in fresh (one day) and on 7, 14, and 21 days of storage.
Fresh samples were collected to analyze variations in mineral content, antioxidant capacity, phenolic compounds, and vitamin concentrations. Both the fresh and stored samples were evaluated for their physiochemical characteristics, color attributes, textural characteristics, microbiological quality, and sensory properties.
Physicochemical analysis
The chemical composition of the SPP samples was analyzed for moisture, protein, lipid, fiber, ash, pH, and titratable acidity using the official methods of analysis (AOAC).[Citation15] The protein content was quantified by the macro-Kjeldahl method using the conversion factor of 6.25. Lipid content was determined using the Soxhlet extraction method with petroleum ether as the solvent. Ash content was determined by combusting the samples at 550°C. The total energy value of the samples was calculated from the proximate analysis, using the equation: Kcal/100 g = (protein % × 4) + (fat % × 9) + (carbohydrate % × 4). Carotenoids content was measured using a spectrophotometer (UVmin-1240, Shimadzu, Kyoto, Japan) at 450 nm according to Rodriguez-Amaya and Kimura.[Citation16] The total solids, protein, fat, ash, fiber, pH, and titratable acidity content of milk, whey, blended milk & whey and Ricotta cheese samples were determined following AOAC.[Citation15] Carbohydrates content was calculated by difference (total solids – (protein + fat + crude fiber + ash). The pH of the samples was measured using a laboratory digital pH meter (model Adwa 1030, Romania). The mineral content was quantified using a Perkin-Elmer atomic absorption spectrophotometer (model: GBC932AA) in accordance with AOAC[Citation15]
Amino acids profile
The amino acid content was determined by a high-performance amino acid analyzer, using the procedure outlined by AOAC.[Citation15] The recoverable amino acid amount was expressed as g/100 g of protein.
Total phenolic compounds and antioxidant activity
The total phenolic content was measured using the Folin-Ciocalteu assay, as described by Barros et al..[Citation17] A fine dried powder (1 g) was stirred with 50 mL of methanol at 25 C and 150 rpm for 1 h and filtered through Whatman No. 4 paper. The residue was then extracted with one additional 50-mL portion of methanol. The combined methanolic extracts were evaporated at 35 C under reduced pressure, redissolved in methanol at 20 mg/mL (stock solution), and stored at 4 C for further use. An aliquot of the extract solution was mixed with Folin-Ciocalteu reagent (5 mL, previously diluted with water 1:10 v/v) and sodium carbonate (75 g/L, 4 mL). The tubes were vortexed for 15 s and allowed to stand for 30 min. Absorbance was measured at 765 nm using a spectrophotometer (model 6505 UV/Vis, JENWAY, UK). Gallic acid was used as standard and the results were expressed as mg of gallic acid equivalents (GAE) per 100g.
The antioxidant capacity of the samples was determined using the DPPH (1, 1-diphenyl-2-picrylhydrazyl) radical scavenging activity method as described by Ravichandran et al..[Citation18] Specifically, 0.1 mL of extract was added to 3.9 mL of a DPPH solution (610–5 M) and the mixture was stirred and allowed to react for 30 minutes. The absorbance of the mixture was measured at 515 nm using the spectrophotometer, and the absorbance of the DPPH solution without extract was used as a control. The antioxidant activity was calculated using equation
DPPH radical scavenging activity (%) = [(Ac – As)/Ac] × 100
where, Ac and As represent the absorbance of the control and the sample at 515 nm, respectively.
Vitamins content
High-performance liquid chromatography (HPLC) analysis used according to Sami et al.,[Citation19] to determine the concentration of water-soluble vitamins (B1, B2, B3, B6, and B12) and fat-soluble vitamins (E and β-Carotene) in the samples. The analyses were conducted using an Agilent 1100 series HPLC system (Agilent Technologies, Santa Clara, CA, USA) equipped with a diode array detector. Separation of the compounds was achieved using an ODS C18 column (4.6 × 250 mm). The mobile phase consisted of a mixture of two phases: phase A comprised of 8% acetonitrile in water and phase B comprised of 22% of phase A in 0.1% phosphoric acid solution. The program started with 80% phase A and 20% phase B, with the percentage of phase A decreasing by 10% every 5 minutes and the percentage of phase B increasing by 10% for a total of 20 minutes. The flow rate and injection volume were 0.8 mL/min and 40 µL, respectively. The temperature of the column oven was maintained at 35°C. The samples and mobile phases were filtered through a 0.45 µm membrane filter (Millipore) prior to analysis. The presence of phenolic compounds was identified by comparing the retention times with those of commercial standards.
Color attributes
The color properties of the samples were evaluated by using Hunter Lab Color QUEST II Minolta CR-400 (Minolta Camera Co., Ltd., Osaka, Japan) with illuminate D63 according to Mensah.[Citation20] This method quantifies the lightness, or L* value, on a scale from 0 (black) to 100 (white), as well as the a* value, which ranged from − 100 (green) to + 100 (red), and the b* value, which ranged from −100 (blue) to + 100 (yellow). The values are the mean of three determinations.
Instrumental texture profile analysis
The textural properties of Ricotta cheese were evaluated according to the method described by Ateteallah et al.,[Citation21] using a Brookfield texture analyzer (Brookfield Engineering, CT3–4500, USA) under deformation force 25% of the sample. A sample of the cheese was placed in a container made of high-density polyethylene (50 mL capacity, 5.5 cm height, 4 cm internal diameter) to a height of 3.5 cm and the analysis was conducted at 20°C. Parameters measured were gumminess (hardness × cohesiveness), and chewiness (hardness× cohesiveness× springiness).
Scanning electron microscopy
Cheese samples were analyzed using a scanning electron microscope (SEM) according to Lobato‐Calleros et al.[Citation22] with minor modification. Cylindrical samples of 0.5 cm diameter and 0.5 cm height were fixed in a 2.5% buffered glutaraldehyde and 2% paraformaldehyde solution (0.1 M phosphate buffer, pH 7.4) for overnight at 4°C. The samples were then washed and dehydrated using increasing concentration of aqueous ethanol solutions (50%, 60%, 70%, 80%, 90%, and 100%, for 30 minutes each) and subsequently immersed in acetone for 1 hour. The specimens were then coated with gold-palladium membranes using a Jeol JSM-6510 L.V SEM at 30 kV and viewed at a magnification of 500× (50 μm).
Microbiological analyses
Microbial counts of the cheese samples were determined in a sterile environment according to Marshall.[Citation23] Ten g of cheese sample was homogenized using a sterile homogenizer with 90 mL of sterile physiological saline solution (0.85%) and serial dilutions were prepared in sterile 0.85% saline solution for bacterial analysis. The total aerobic bacteria (TAB) were enumerated using Plate Count Agar (Oxoid) and incubated at 37°C for 48 hours. Yeast and mold (YM) were quantified using Potato Dextrose Agar and incubated at 25°C for 5 days. Coliform count was enumerated using Violet Red Bile Agar and incubated at 37°C for 24 hours.
Sensory properties
Consumer acceptability of the Ricotta cheese samples was evaluated using according to Felfoul et al..[Citation24] A panel of 10 semi-trained evaluators (judges) made of laboratory staff and graduate students from the Department of Dairy Science at New Valley University, Egypt were used. A 9-point hedonic scale was used to assess the samples based on color, flavor, texture, and overall acceptability, with lower scores indicating lower levels of acceptability. A human ethical approval (No: 9–2022) was obtained from the human ethical committee at Faculty of Agriculture, New Valley University (Egypt) before conducting these tests.
Statistical analysis
Tests and analyses including each sample and each test parameter mentioned above were conducted in triplicate. The results are reported as the mean ± standard deviation. The collected data were statistically analyzed using the general linear model in IBM SPSS Statistics 25 software, and the Duncan’s multiple range test was applied to determine significance at a p-value of ≤ 0.05.
Results and discussion
Nutritional and chemical composition of spirulina powder
The chemical composition of spirulina powder is presented in . The moisture, protein, lipid, ash, carbohydrate, and fiber contents of this powder were 5.43, 70.40, 5.02, 6.77, 12.06, and 5.43%, respectively. These values are consistent with those of Ahsan et al.,[Citation25] who reported that their green spirulina specimen contained moisture in the range of 4–9%, 57–80% protein 2–6% fat, 3–11% ash, 15–25% carbohydrate, and 2–4% dietary fiber. The above data indicate that the protein content in spirulina surpasses that in dried soybeans (35%), peanuts (25%), or grains (8–10%). The protein content in our spirulina sample (70.40% on dry mass basis) was higher than that in meat, dried milk, eggs, and grains as well.[Citation26] According to other researchers, spirulina had a protein content of 65–71%, which was higher than that of soybean and more digestible since it lacked antinutrients including phytic acid and polyphenols, which are known to suppress proteolytic enzymes.[Citation27] Additionally, spirulina is rich in highly digestible (85–95%) natural fibers, which are conducive to digestive health. This ease of digestion is particularly important for individuals with intestinal malabsorption.[Citation28] The acidity and pH values of spirulina (0.19% and 6.82, respectively) are also consistent with those reported by Sami et al.[Citation19] The close to neutral pH of spirulina can help negate the impact of acidic foods and promote pH balance in the body. An acidic body pH has been linked to various modern diseases, including hypertension, cancer, diabetes, heart disease, gout, and rheumatism.[Citation29]
Table 1. Physiochemical properties, amino acid (g/100 g), minerals (mg/100 g), vitamins (mg/100 g), antioxidant indicators of spirulina (S. platensis) powder.
The data presented in indicate that Spirulina is a rich source of essential minerals, including iron, manganese, and zinc. It also contains calcium, phosphorus, and magnesium at levels comparable to those found in milk. Analysis of the amino acid composition revealed that leucine is the most prevalent essential amino acid (7.52%), followed by valine (6.34%). Among the nonessential amino acids, glutamic acid was the most abundant (13.44%) followed by aspartic acid (11.32%). These findings are consistent with previous research, including that of Bashir et al.[Citation30] who identified leucine as the most prevalent essential amino acid in spirulina samples, with valine, glutamic acid, and aspartic acid also found in substantial concentrations. As shown in , Spirulina is found to be a rich source of vitamins, including B1, B2, B3, B6, B12, and E, with concentrations of 2.77, 3.63, 13.69, 1.26, 0.23, and 10.50 (mg/100 g), respectively. Spirulina biomass also contained substantial amount of phenolic compounds, antioxidant activities as well as a notable β-carotene content of 240(mg GAE/100 g), 80.85%, and 30.79 (mg/100 g), respectively. A study conducted by the National Cancer Institute[Citation25] suggests that a daily intake of 6.0 mg of β-carotene, obtainable from 4.0 g of spirulina, may potentially be efficacious in reducing cancer risk.
Physicochemical properties of spirulina fortified cheese during the storage
The utilization of microalgae, including spirulina, for the fortification of food products has been shown to enhance various nutritional and compositional properties.[Citation31] The chemical composition of cheese samples fortified with microalgae (SRC) and control (CRC) was analyzed both fresh and during storage, as shown in Table S2. The incorporation of spirulina into SRC significantly increased the levels of total solids, protein, fat, ash, carbohydrate, fiber, and pH, but comparatively lower acidity when compared with those of the CRC samples. The levels of these parameters increased as the fortification level of spirulina increased (SRC1< SRC2< SRC3< SRC4). As expected, 1% fortification level (SRC4) resulted in the highest levels of all tested parameters except acidity, which was the lowest.
The results obtained from SRC cheese samples were consistent with previous findings reported by Tohamy et al.,[Citation9] who showed that the addition of spirulina powder to processed cheese led to an increase in nutritional content by increasing the total solids, fat, protein, ash, and fiber compared to control cheese. Our results also concur with those of Darwish,[Citation7] who found that incorporating spirulina in Kareish cheese at varying concentrations (0.5, 1, and 1.5% resulted in an increase in total solids, fat, protein, and ash content. The increased levels of these parameters in spirulina fortified cheese can be attributed to the higher concentrations of these parameters in spirulina.
The results from the analysis of SRC samples indicated that total solids, protein, fat, ash, carbohydrate, and fiber levels gradually increased during storage up to 21 days due to decreased moisture content. Additionally, the pH values of fresh SRC samples (as reported in ) were found to be higher than those of CRC samples, which may be attributed to the higher pH levels present in the spirulina powder used as a fortifying agent. These findings are consistent with previous studies, such as that of Golmakani et al.,[Citation8] who reported that the pH values of feta-style cheeses fortified with 0.5%, 1%, and 1.5% S. platensis were 4.81, 4.82, and 4.86, respectively, compared to the control sample with a pH of 4.6. However, as storage time increased while storing at 5°C, the pH values of samples decreased, accompanying an increase in acidity levels. This can be attributed to the conversion of residual lactose to lactic acid, as well as the production of free fatty acids and amino acids through lipolysis and proteolysis.[Citation32]
Table 2. Minerals*, total phenolic content and antioxidant capacity of fresh Ricotta cheese samples fortified with S. platensis powder.
Table 3. Physiochemical and Color parameters of Ricotta cheese fortified with S. platensis powder during storage at 5°C for 21 days.
Minerals, total phenolic content and antioxidant capacity of fresh Ricotta cheese
presents the changes in mineral content, total phenolic content, and antioxidant capacity of fresh Ricotta cheese samples. The results indicate that the incorporation of spirulina powder significantly impacted the mineral content of the cheeses when compared to the control samples (p ≤ .05). As the level of spirulina enrichment increased, a notable increase in mineral content was observed. These findings are also consistent with those reported by Tohamy et al.,[Citation9] who found that processed cheese fortified with spirulina powder increased the calcium, potassium, magnesium, iron, and zinc contents compared to those of the unfortified cheese.
Additionally, the Ricotta cheese fortified with 1% spirulina powder had significantly higher total phenolic content (7.03 ± 0.08 mg GAE/g) and antioxidant capacity (20.27 ± 0.80%) compared to that in control Ricotta cheese (5.11 ± 0.07 mg GAE/g and 15.25 ± 0.41%), respectively (). These findings are in line with the research published by Darwish,[Citation7] who reported high antioxidant and phenolic content in Kareish cheese fortified with spirulina. The high phenolic content and antioxidant capacity in the spirulina powder fortified Ricotta cheese can be attributed to the presence of beta-carotene, tocopherol, phenolic compounds, anthocyanins, and C-phycocyanin in the spirulina which are known to synergistically increase the antioxidant capacity.[Citation33]
Physical properties of Ricottacheese during the storage
Color parameters: Spirulina, a microalga, contains a number of pigments, including phenolic and flavonoid compounds, carotenoids, and chlorophyll, which can act as natural colorants. The color parameters of Ricotta cheese samples are presented in . Results indicate that control cheese samples (CRC) had a significantly higher L* value, a measure of lightness, than fortified (SRC) samples when they were fresh and after storage. Additionally, the L* values of the fortified samples decreased with increasing concentrations of spirulina. The highest L* value was observed in fresh CRC samples (91.23), while the lowest value was found in treatment in fortified (SRC4) samples at 1% Spirulina (76.84). These findings are consistent with those of Golmakani et al.,[Citation8] who reported a decrease in lightness with the addition of spirulina to acidified feta-type cheese.
The decrease in L* value was observed in all cheese samples containing spirulina during storage could be attributed to the increase in acidity and proteolysis that occurred during this period, as well as the solubilization of casein, which can result in a reduction in whiteness.[Citation34] The a* values, a measure of redness, of spirulina fortified samples were significantly lower than those of the control cheese samples at the start as well as at end of storage. The addition of spirulina powder resulted in a significant reduction in the a* values of fortified samples compared to the control. Furthermore, all spirulina powder fortified cheese samples were greener than the control ones, which may be due to the presence of the blue-green pigments chlorophyll and phycocyanin in spirulina.
Previous research has shown that incorporation of Aphanizomenon platensis powder in yogurt reduced a* values, indicating a shift in color from yellow to green.[Citation6] In our case, the yellowness (b* value) of the spirulina fortified cheese samples was higher than that of the control cheese when they were freshly prepared and also during storage. The yellowness value of the fortified cheese samples also increased with increasing concentrations of spirulina. At the end of the storage period, the highest color of yellowish green was observed, which could be attributed to the slow oxidation and slow degradation of microalgae pigments.[Citation5] Overall, the results from this study showed that the addition of spirulina to ricotta cheese samples reduced the lightness and redness values and increased the yellowness values. These findings are in agreement with previous research on the impact of microalgal pigments on cheese color.[Citation5,Citation7]
Textural profile analysis
The texture properties of Ricotta cheese cheese, such as hardness, cohesiveness, gumminess, springiness, and chewiness, are presented in . Hardness is an important factor that affects cheese texture. The hardness of SRC samples increased compared to CRC samples, both at the start and end of storage. This can be explained by the increase in dry matter and the decrease in water content in SRC cheeses. This pattern was also observed in previous studies, such as those conducted by Darwish,[Citation7] who reported that the inclusion of S. platensis in Kareish cheese resulted in increased firmness values. The protein and carbohydrate molecules in microalgae can play an important role in water absorption, which contributes to the development of cheese hardness.[Citation35]
Table 4. Texture profile analysis of Ricotta cheese fortified with S. platensis powder during storage at 5°C for 21 days.
The springiness of SRC samples increased with increasing levels of spirulina powder. Additionally, springiness increased in all cheese samples during storage until 21 days, compared to the fresh sample. The spirulina fortified cheese samples had lower cohesiveness, gumminess, and chewiness values right after preparation and also at the end of 21 days of storage than the control cheese sample, and this difference was reduced with the addition of SPP. During storage, these parameters increased in all cheese samples when compared to the fresh ones (). This increase can be attributed to the proteolysis of protein cheese during storage, which reduces the structural integrity of the protein matrix. There is a correlation between chewiness and cheese hardness; harder cheeses are more difficult to chew.[Citation36] Additionally, the samples that contained spirulina had a softer texture, making them easier to chew and disintegrate the cheese. Khemiri et al.[Citation11] noticed that microalgal biomass brought an overall positive structural effect when added to ricotta cheese.The application of microalgae to improve the textural qualities of cheese is therefore a promising area for the development of new food products.
Microstructure
Scanning electron microscopy (SEM) images of the FRC cheeses are presented in . The control cheese prepared without adding spirulina powder (CRC) appeared to have a compact protein matrix with a granular and rough appearance. This is an indication of the cheese’s loss of spongy texture and it becoming more compact. The reduction in interstitial spaces may be a cause or a result of the cheese’s lower fat content (Table S2). When spirulina powder was added to cheeses, the particles of the former likely interfered with the protein matrix and caused molecular rearrangement of proteins, resulting in strengthened linkages, as evidenced by the increased hardness of the cheese (). There were differences in microstructure among the SRC samples, with the matrix of the SRC1 and SRC2 cheeses displaying a diverse structure with a fine network. Casein micelles are more apparent in these samples compared to the control cheese. SRC3 and SRC4 showed a more compact and denser structure. Generally, SRC cheeses exhibited a higher degree of cross-linking and stronger links. The more compact matrix observed in these samples explains the increased hardness and deformability found in the texture profile of these cheese samples.
Figure 1. SEM micrographs of functional Ricotta cheese fortified with S. platensis powder as follows: CRC, control Ricotta cheese without Spirulina platensis powder; SRC1, Ricotta cheese with 0.25% Spirulina platensis powder; SRC2, Ricotta cheese with 0.5% Spirulina platensis powder; SRC3, Ricotta cheese with 0.75% Spirulina platensis powder; SRC4, Ricotta cheese with 1% Spirulina platensis powder.
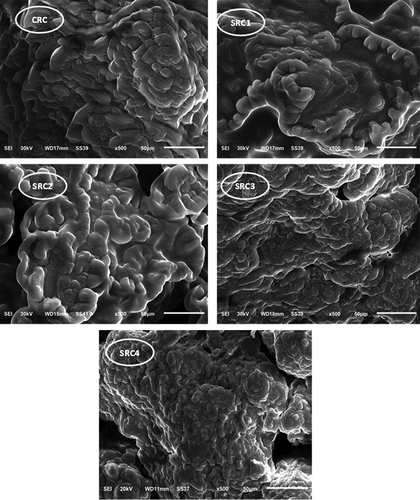
SEM also revealed that the protein matrices of cheese samples with increased levels of spirulina had larger aggregates compared to the control. As a result, casein clusters were thicker and more compact in the networks of treated cheeses SRC4 than in others, which may be attributed to the increased spirulina content. The addition of spirulina powder to cheese resulted in a smoother, more homogenous texture and structure due to its high fiber and protein content. It is known that the fibers in spirulina do not contain cellulose in their cell walls and are composed of soft mucopolysaccharides.[Citation28] These findings are consistent with those of Atallah et al.,[Citation37] who reported that the inclusion of spirulina in yogurt had a positive impact on its texture.
Microbial quality
Table S3 shows that the total viable bacterial count of cheese samples containing spirulina (SRC) was higher than that of control samples, whether they were fresh or stored. This increased growth and survival of microorganisms can be attributed to the abundance of nitrogenous compounds (free amino acids and peptone) present in spirulina biomass. Additionally, research has shown that spirulina has a significant buffering capacity due to its alkaline nature, which impedes acid production and promotes bacterial survival.[Citation38,Citation39]
Suna and Yilmaz-Ersan[Citation10] reported that during storage, white-brined cheese fortified with A. platensis had higher counts of total mesophilic aerobic bacteria compared to other samples. Yeast or mold counts were not detected in all fresh cheese samples, but they appeared at the 14th day for the CRC sample and on the 21st day for the SRC sample and increased until the end of storage. Notably, no coliform bacteria were discovered in any of the cheese samples. This is in agreement with Abdel-Moneim et al.[Citation40] who reported spirulina has an antifungal effect on Aspergillus niger, Aspergillus flavus, Aspergillus fumigatus, Candida tropicalis, Candida albicans, and Candida glabrata. It also possesses antimicrobial effect on B. cereus, B. subtilis, and E. coli.
Sensorial properties
Color, flavor, and texture are essential characteristics that determine the overall quality and consumer acceptability of a food product.[Citation41] The sensory evaluation data of fresh and stored Ricotta cheese samples is presented in . At the beginning of storage, the CRC samples had a sour taste. This sour taste declined in spirulina-fortified cheese samples (SRC). With regard to flavor (taste and smell), there was significant variations among SRC samples (p ≤ .05). The flavor was enhanced by increased levels of spirulina powder but, decreased when the SPP content was > 0.75%. This is likely due to the presence of earthy and musty odorous substances found in spirulina biomass. These results similar with that found by Khemiri et al.[Citation11] sample with 0.2% Chlorella spp. was the best. Concerning the color, found positively for samples with microalgal biomass (0.2 and 1% Chlorella spp.) and the 1.5% mixture was the least appreciated one as well as, had a very unpleasant fishy odor and aftertaste. Agustini et al.[Citation4] found that the addition of 1% spirulina to soft cheese was the best concentration. On the other hand, the same authors found panelists preferred ice cream without the addition of spirulina as it gave it a fishy or unpleasant odor and a dark green color. In this work, we found that that the addition of spirulina powder in cheese samples increased the green color. When the spirulina powder content was 1.0% the panelists perceived intense green color, and it received the lowest score in terms of acceptability. In contrast, the fresh sample with 0.5% SPP (SRC2) was more acceptable than the control and other treatments. This may be because the color of this sample was similar to that of Roquefort cheese. These results are in line with previous research (Jeon),[Citation42] which found that processed cheese containing 0.5% chlorella was preferred to the one with 1.0% chlorella or the one not containing it. Additionally, we found that the texture of the cheese samples fortified with spirulina powder was smoother and more homogeneous due to the presence of polysaccharides and dietary fibers. Over the course of the storage period, the sensory scores for all cheese samples gradually increased up to 14 days before declining at the end of the storage period.
Figure 2. Hedonic sensory evaluation of Ricotta cheese fortified with S. platensis powder during storage at 5°C for 21 day: (a) after 1 day, (b) after 7 days, (c) after 14 days, and (d) after 21 days. CRC, control Ricotta cheese without Spirulina platensis powder; SRC1, Ricotta cheese with 0.25% Spirulina platensis powder; SRC2, Ricotta cheese with 0.5% Spirulina platensis powder; SRC3, Ricotta cheese with 0.75% Spirulina platensis powder; SRC4, Ricotta cheese with 1% Spirulina platensis powder.
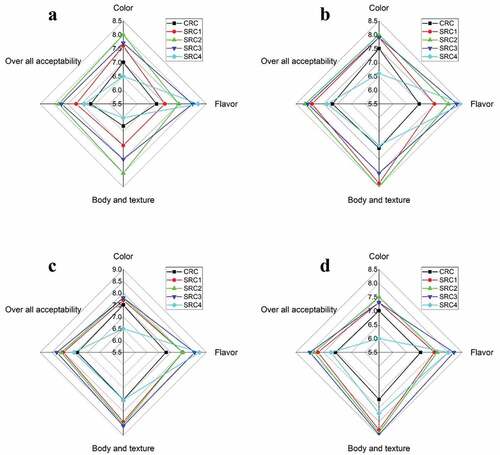
Fermentation can be an effective method to improve the sensory perception of food as it modifies the aromatic profile.[Citation43] This is likely due to the production of volatile compounds that can reduce the characteristic odor of spirulina. We found that the green color of spirulina-enriched Ricotta cheese was appreciated by the panelists and that acceptability, flavor, and texture all improved during storage. In general, all spirulina fortified cheese samples had good sensory scores, but the one containing 0.75% spirulina was the best performer at the end of the storage period.
Conclusion
Microalgae contain valuable macronutrients and other bioactive compounds that can be used as functional ingredients to create innovative and healthier food products. This research paper shows the feasibility of using microalgae (Spirulina platensis) to fortify and enhance the nutritional and quality properties of Ricotta cheese. The addition of spirulina powder to cheese samples led to a significant increase in nutritional content; make a texture smoother and more homogeneous texture, and better sensory properties than the unfortified cheese. Incorporation of 0.75% was found to be optimum from structure-function-sensory perspectives. In future research, spirulina algae will be used in more dairy industries, with a study of the vital and nutritional importance of the addition process, taking into account the quality properties of the resultant product.
Abbreviations
| AOAC | = | Association of official analytical chemists |
| APHA | = | American public health association |
| DPPH | = | 2,2-Diphenyl-1-picrylhydrazyl |
| A. platensis | = | Arthrospira platensis (Spirulina platensis) |
| SPP | = | Spirulina platensis powder |
| CFU | = | Colony forming unit |
| CRC | = | Cheese without Spirulina platensis (control) |
| SRC | = | Cheese fortified with Spirulina platensis |
| GAE | = | Gallic acid equivalents |
Author contribution
Hesham A. Ismail: Conceptualization, data curation, formal analysis, methodology, resources, software, supervision, writing – original draft. Talaat H. El-Sawah: Conceptualization, data curation, formal analysis, methodology, resources, software, supervision, writing – original draft. Mutamed Ayyash: Conceptualization, writing – review draft. Benu Adhikari: writing – review draft. Wael F. Elkot: Conceptualization, data curation, formal analysis, methodology, resources, software, supervision, writing – original draft.
Supplemental Material
Download ()Acknowledgments
The research presented in this work was financially supported by the Dairy Science and Technology Department at the Faculty of Agriculture & Natural Resources at Aswan University (Egypt), Dairy Science Department at the Faculty of Agriculture at New Valley University (Egypt), and United Arab Emirates University (UAEU). The authors would like to extend their gratitude to Professor Dr. Soha S.M. Mostafa from the Department of Microbiology at the Soils, Water, and Environment Research Institute (SWERI) at the Agricultural Research Center (ARC) in Egypt for providing the S. platensis powder used in this study.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
Data will be made available on reasonable request.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/10942912.2023.2238916
Additional information
Funding
References
- Matos, J.; Cardoso, C.; Bandarra, N. M.; Afonso, C. Microalgae as Healthy Ingredients for Functional Food: A Review. Food Fun. 2017, 8(8), 2672–2685. DOI: 10.1039/c7fo00409.
- Lucas, B. F.; Morais, M. G. D.; Santos, T. D.; Costa, J. A. V. Effect of Spirulina Addition on the Physicochemical and Structural Properties of Extruded Snacks. Food Sci. Tech. 2017, 37(spe), 16–23. DOI: 10.1590/1678-457x.06217.
- Simpore, J.; Kabore, F.; Zongo, F.; Dansou, D.; Bere, A.; Pignatelli, S.; Biondi, D. M.; Ruberto, G.; Musumeci, S. Nutrition Rehabilitation of Undernourished Children Utilizing Spiruline and Misola. Nutr. J. 2006, 5(1), 3. DOI: 10.1186/1475-2891-5-3.
- Agustini, T. W.; Maâ, W. F.; Widayat, W.; Suzery, M.; Hadiyanto, H.; Benjakul, S.; Application of S. Platensis on Ice Cream and Soft Cheese with Respect to Their Nutritional and Sensory Perspectives. J. Tek. 2016, 78 (4–2). DOI: 10.11113/jt.v78.8216.
- Mazinani, S.; Fadaei, V.; Khosravi‐Darani, K. Impact of Spirulina Platensis on Physicochemical Properties and Viability of Lactobacillus acidophilus of Probiotic UF Feta Cheese. J. Food Pro. Pre. 2016, 40(6), 1318–1324. DOI: 10.1111/jfpp.12717.
- Barkallah, M.; Dammak, M.; Louati, I.; Hentati, F.; Hadrich, B.; Mechichi, T.; Ayadi, M. A.; Fendri, I.; Attia, H.; Abdelkafi, S. Effect of S. Platensis Fortification on Physicochemical, Textural, Antioxidant and Sensory Properties of Yogurt During Fermentation and Storage. LWT. 2017, 84, 323–330. DOI: 10.1016/j.lwt.2017.05.071.
- Darwish, M. A. I. Physicochemical Properties, Bioactive Compounds and Antioxidant Activity of Kareish Cheese Fortified with S. Platensis. World J. Dairy Food Sci. 2017, 12(2), 71–78. DOI: 10.5829/idosi.wjdfs.2017.71.78.
- Golmakani, M. T.; Soleimanian-Zad, S.; Alavi, N.; Nazari, E.; Eskandari, M. H. Effect of Spirulina (Arthrospira Platensis) Powder on Probiotic Bacteriologically Acidified Feta-Type Cheese. J. App. Phy. 2019, 31(2), 1085–1094. DOI: 10.1007/s10811-018-1611-2.
- Tohamy, M.; Shaaban, H.; Ali, M.; Hasanain, A. Effect of S. Platensis as Nutrition Source on the Chemical, Rheological and Sensory Properties of Spreadable Processed Cheese. J. Bio. Sci. 19(1), 84–91. DOI: 10.3923/jbs.2019.84.91.
- Suna, G.; Yilmaz-Ersan, L. Utilization of Microalgae in Probiotic White Brined Cheese. Mljekarstvo: časopis za unaprjeđenje proizvodnje i prerade mlijeka. 2022, 72(2), 88–104. DOI: 10.15567/mljekarstvo.2022.0203.
- Khemiri, S.; Bouchech, I.; Berrejeb, N.; Mejri, M.; Smaali, I.; Nadia Khelifi, N. Effects of Growth Medium Variation on the Nutri-Functional Properties of Microalgae Used for the Enrichment of Ricotta. Food Tech. Bio. 2022, 60(1), 29–39. DOI: 10.17113/ftb.60.01.22.7105.
- Modler, H.; Emmons, D. The Use of Continuous Ricotta Processing to Reduce Ingredient Cost in ‘Further Processed’cheese Products. Int. Dairy. J. 2001, 11(4–7), 517–523. DOI: 10.1016/S0958-6946(01)00082-6.
- Niro, S.; Succi, M.; Cinquanta, L.; Fratianni, A.; Tremonte, P.; Sorrentino, E.; Panfili, G. Production of Functional Ricotta Cheese. Agro. Food Ind. Hi-Tech. 2013, 24, 56–59.
- Koli, D. K.; Rudra, S. G.; Bhowmik, A.; Pabbi, S. Nutritional Functional, Textural and Sensory Evaluation of Spirulina Enriched Green Pasta: A Potential Dietary and Health Supplement. Foods 2022, 11(7), 979. DOI: 10.3390/foods11070979.
- AOAC Official methods of analysis. Association of Official Analytical Chemists, 20th; Horwitz, W., Ed.; MAcademic Press, 2016.
- Rodriguez-Amaya, D. B.; Kimura, M. HarvestPlus Handbook for Carotenoid Analysis (Vol. 2); International Food Policy Research Institute (IFPRI) Washington, 2004.
- Barros, L.; Cabrita, L.; Boas, M. V.; Carvalho, A. M.; Ferreira, I. C. Chemical, Biochemical and Electrochemical Assays to Evaluate Phytochemicals and Antioxidant Activity of Wild Plants. Food Che. 2011, 127(4), 1600–1608. DOI: 10.1016/j.foodchem.2011.02.024.
- Ravichandran, K.; Saw, N. M. M. T.; Mohdaly, A. A.; Gabr, A. M.; Kastell, A.; Riedel, H.; Cai, Z.; Knorr, D.; Smetanska, I. Impact of Processing of Red Beet on Betalain Content and Antioxidant Activity. Food. Res. Int. 2013, 50(2), 670–675. DOI: 10.1016/j.foodres.2011.07.002.
- Sami, R.; Li, Y.; Qi, B.; Wang, S.; Zhang, Q.; Han, F.; Ma, Y.; Jing, J.; Jiang, L. HPLC Analysis of Water-Soluble Vitamins (B2, B3, B6, B12, and C) and Fat-Soluble Vitamins (E, K, D, A, and β-Carotene) of Okra (Abelmoschus esculentus). J. Chem. 2014, 2014, 1–6. DOI: 10.1155/2014/831357.
- Mensah, P. Fermentation the Key to Food Safety Assurance in Africa? Food Cont. 1997, 8, 271–278. DOI: 10.1016/S0956-7135(97)00020-0.
- Ateteallah, A. H.; Elkot, W. F.; Abd-Alla, A. E. Physicochemical, Antioxidant, Microstructure, Textural, and Organoleptic Characteristics of Soft Cheese Incorporated Corn Milk. J. Food Pro. Pre. 2022, 46, e16694.
- Lobato‐Calleros, C.; Ramírez‐Santiago, C.; Osorio‐Santiago, V.; Vernon‐Carter, E.; Hornelas‐Uribe, Y. Microstructure and Texture of Manchego Cheese‐Like Products Made with Canola Oil, Lipophilic and Hydrophilic Emulsifiers. J. Tex. Stu. 2002, 33(3), 165–182. DOI: 10.1111/j.1745-4603.2002.tb01343.x.
- Marshall, R. T. Standard Methods for the Examination of Dairy Products, 16th edn ed.; American Public Health Association: Washington DC, 1992.
- Felfoul, I.; Bornaz, S.; Baccouche, A.; Sahli, A.; Attia, H. Low-Fat Gouda Cheese Made from Bovine Milk-Olive Oil Emulsion: Physicochemical and Sensory Attributes. J. Food Sci. Tech. 2015, 52(10), 6749–6755. DOI: 10.1007/s13197-015-1736-0.
- Ahsan, M.; Habib, B.; Parvin, M.; Huntington, T. C.; Hasan, M. R. A Review on Culture, Production and Use of Spirulina as Food for Humans and Feeds for Domestic Animals. FAO Fisheri. Aquacult. Circul. 2008, No. 1034. https://www.fao.org/3/i0424e/i0424e00.pdf
- Ciferri, O. Spirulina, the Edible Microorganism. Mic. Rev. 1983, 47(4), 551–578. DOI: 10.1128/mr.47.4.551-578.1983.
- Salmeàn, G. G.; Castillo, L. F.; Cevallos, G. C. Nutritional and Toxicological Aspects of Spirulina (Arthrospira). Nut. Hos. 2015, 32(1), 34–40. DOI: 10.3305/nh.2015.32.1.9001.
- Soni, R. A.; Sudhakar, K.; Rana, R. Spirulina–From Growth to Nutritional Product: A Review. Tre. Food Sci. Tech. 2017, 69, 157–171. DOI: 10.1016/j.tifs.2017.09.010.
- Ismaiel, M. M. S.; El-Ayouty, Y. M.; Piercey-Normore, M. Role of pH on Antioxidants Production by Spirulina (Arthrospira) Platensis. Braz. J. Mic. 2016, 47(2), 298–304. DOI: 10.1016/j.bjm.2016.01.003.
- Bashir, S.; Sharif, M. K.; Butt, M. S.; Shahid, M. Functional Properties and Amino Acid Profile of S. Platensis Protein Isolates. Bio. Sci.-PJSIR. 2016, 59(1), 12–19. DOI: 10.52763/PJSIR.BIOL.SCI.59.1.2016.12.19.
- Figueira, F. D. S.; Crizel, T. D. M.; Silva, C. R.; Salas-Mellado, M. D. L. Pão sem glúten enriquecido com a microalga Spirulina platensis. Braz. J. Food Tech. 2011, 14(4), 308–316. DOI: 10.4260/BJFT2011140400037.
- Sulejmani, E. Effect of Vegetable Fat on the Texture, Colour and Sensory Properties of Macedonian White Brined Cheese. Mljekarstvo: časopis za unaprjeđenje proizvodnje i prerade mlijeka. 2021, 71(1), 25–34. DOI: 10.15567/mljekarstvo.2021.0103.
- Wang, Y.; Ocampo, M. F.; Rodriguez, B.; Chen, C. J.Resveratrol and Spirulina: Nutraceuticals That Potentially Improving Cardiovascular Disease. J. Car. Med. Cardiol. 2020, 7(2), 138–145. DOI: 10.17352/2455-2976.000129.
- Chudy, S.; Bilska, A.; Kowalski, R.; Teichert, J. Colour of Milk and Milk Products in CIE L* A* B* Space. Med. Weter. 2020, 76(2), 6327–2020. DOI: 10.21521/mw.6327.
- Raymundo, A.; Gouveia, L.; Batista, A.; Empis, J.; Sousa, I. Fat Mimetic Capacity of Chlorella Vulgaris Biomass in Oil-In-Water Food Emulsions Stabilized by Pea Protein. Food Res.Int. 2005, 38(8–9), 961–965. DOI: 10.1016/j.foodres.2005.02.016.
- Beal, P.; Mittal, G. Vibration and Compression Responses of Cheddar Cheese at Different Fat Content and Age. Milchwissenschaft. 2000, 55(3), 139–142.
- Atallah, A. A.; Morsy, O. M.; Gemiel, D. G. Characterization of Functional Low-Fat Yogurt Enriched with Whey Protein Concentrate, Ca-Caseinate and Spirulina. Int. J. Food Pro. 2020, 23(1), 1678–1691. DOI: 10.1080/10942912.2020.1823409.
- Akalin, A.; Unal, G.; Dalay, M. Influence of S. Platensis Biomass on Microbiological Viability in Traditional and Probiotic Yogurts During Refrigerated Storage. Ita. J. Food Sci. 2009, 21(3), 357–364.
- Molnár, N.; Gyenis, B.; Varga, L. Influence of a Powdered S. Platensis Biomass on Acid Production of Lactococci in Milk. Milchwissenschaft. 2005, 60(4), 380–382.
- Abdel-Moneim, A. E.; El-Saadony, M. T.; Shehata, A. M.; Saad, A. M.; Aldhumri, S. A.; Ouda, S. M.; Mesalam, N. M. Antioxidant and Antimicrobial Activities of Spirulina Platensis Extracts and Biogenic Selenium Nanoparticles Against Selected Pathogenic Bacteria and Fungi. Saud. J. Biolo. Sci. 2022, 29(2), 1197–1209. DOI: 10.1016/j.sjbs.2021.09.046.
- Ahsan, S.; Arefin, M. S.; Munshi, J. L.; Begum, M. N.; Maliha, M.; Rahman, S.; Bhowmik, A.; Kabir, M. S. In vitro Antibacterial Activity of S. Platensis Extracts Against Clinical Isolates of Salmonella enterica Serovars Typhi and Paratyphi (SUBP03). Stam. J. Micro. 2015, 5(1), 22–25. DOI: 10.3329/sjm.v5i1.26916.
- Jeon, J. K. Effect of Chlorella Addition on the Quality of Processed Cheese. J. Kor. Soc. Food Sci. Nut. 2006, 35(3), 373–377. DOI: 10.3746/jkfn.2006.35.3.373.
- Terpou, A.; Bosnea, L.; Mataragkas, M.; Markou, G. Influence of Incorporated Arthrospira (Spirulina) Platensis on the Growth of Microflora and Physicochemical Properties of Feta-Type Cheese as Functional Food. Mul. Dig. Pub. Ins. Pro. 2020, 70(1), 97. DOI: 10.3390/foods_2020-07659.
