ABSTRACT
The demand for marinated meat and natural antimicrobials has increased in the last decade. This study aimed to examine the antimicrobial and sensorial effects of natural citrox (0.2% v/w) and oregano essential oil (0.1% v/w) or their combination on marinated buffalo meat stored under vacuum at two storage conditions of 4°C and 12°C. In the control (without marinade), the population of all the spoilage microorganisms increased by 3.8–6.3 and 4.5–6.3 log cfu/g, at 4 and 12°C, respectively, during storage. On the last day of storage, at both 4 and 12°C, the spoilage microorganisms’ populations were lower for the marinated meat (without citrox and EO) by 1–2 log cfu/g (based on microorganism type) compared to its unmarinated counterpart. The combination of citrox and oregano essential oil (EO) resulted in decreases of 3.0–6.8 and 3.2–6.8 log cfu/g for mesophilic aerobic bacteria, Pseudomonas spp. lactic acid bacteria (LAB), Brochothrix thermosphacta, Enterobacteriaceae, and yeasts, at 4 and 12°C, respectively. The pH values of the treatments with citrox and oregano EO followed a steadily decreasing (P < .05) trend during storage. The recorded sensory data supported that meat marinated with those 2 natural antimicrobials led to a buffalo meat of acceptable quality. Marinade combined with citrox and oregano EO can provide a potential not previously studied solution for buffalo meat preservation.
Introduction
Water buffalo meat has emerged as a premium product, sought out by food connoisseurs worldwide. Buffalo meat products, either in a marinated form or as smoked sausages, are currently available in supermarkets and delicatessen shops and are becoming popular amongst consumers owing to their taste and flavor. Marination involves immersion of meat into a liquid mixture (marinade) that may contain ingredients (acid, herbs, spices, salt, organic acids, water, seasoning, sugar, wine, oil, aroma enhancers etc.) added to improve the quality and sensory attributes of the final product[Citation1].
Shelf-life of food products is usually extended using chemical preservatives. However, natural preservation techniques are the desired choice. Citrox (14WPlus, ProGarda) is a natural antimicrobial of plant origin, consisting of a citrus green extract, in addition to citric acid and a bioflavonoid. It complies with the British Standard European Norm BS EN 1276[Citation2,Citation2]: and British Standard European Norm BS EN 1275:2005.[Citation3] Citrox-ProGarda™ fruit & Vegetable Decontaminant (ref. 14WPlus) conforms to Organic Farming EU Regulation 2092/91.[Citation4] Citrox is currently not allowed to be used in food preparations, either as an ingredient or an additive. To the best of our knowledge, it can only be used as a washing (decontamination treatment) supplement for products, such as salads, cut vegetables, fruit, meat and fish products, etc. When applied at the correct dilutions, it gives reductions of 4–5 logs of Pseudomonas spp., Escherichia coli and Staphylococcus aureus (in accordance with British Standard European Norms.[Citation3] This product is recommended by the Australian Organic Council as a washing product for organic vegetables[Citation5] and its potential use for food preservation is under investigation. Recently, the effect of citrox (1% or 2%) alone or in combination with 1% chitosan was studied on the survival of Campylobacter jejuni in vacuum-packaged camel meat slices, stored at 4 or 10°C for 30 days.[Citation6] The results revealed that the shelf-life of camel meat increased by 30 days upon using 1% or 2% citrox, in combination with 1% chitosan as compared to using citrox alone. Meanwhile, the reductions in Campylobacter jejuni populations were up to 4.0 logs during the storage period at both 4 and 10°C. In the same study, it was reported that the quality of camel meat treated with citrox plus chitosan was better than that of the control meat.[Citation6] In another study, Citrox solution (1% and 2%) inhibited the growth of methicillin-resistant Staphylococcus aureus (MRSA) in vacuum packaged chicken fillets within the first three days of storage (1 log reduction).[Citation7] It was also reported that citrox on its own or combined with SO2 resulted in a decrease in the total aerobic plate count and coliforms, in “boerewors” a South African fresh sausage meat product. The action of citrox was reported to be comparable to that of SO2.[Citation8]
Other natural antimicrobials are essential oils (EO), known for their antioxidant, antimicrobial, and medicinal properties.[Citation9,Citation10] Their use has been suggested to increase the shelf-life of foods and improve sensorial and microbial quality.[Citation9,Citation11] Oregano EO showed promising results in several studies especially when combined with other antimicrobials. In one recent study[Citation12] Chitosan edible coating containing 0.15% oregano EO or 0.6% cinnamon EO prolonged the shelf-life by a week in roast duck slices. Marination with oregano EO (200 ppm) and tannic acid (10 ppm) combination on ground chicken breast and thigh meats showed positive sensory scores and antioxidant properties.[Citation13] In addition, the combination of Ethylenediaminetetraacetic acid (EDTA) (20 mM), air and modified atmosphere packaging (30%CO2/70%N2) and oregano EO (0.3 and 0.6% v/wt) achieved a shelf-life extension of 14–15 days in chicken liver samples.[Citation14]
To the best of our knowledge, data reported to date are from studies on effects of marinades mainly on chicken meat,[Citation15–17] pork[Citation18] and beef.[Citation19] Limited information is available on buffalo meat. This study was designed to investigate the effect of a commercially available marinade in combination with citrox and oregano EO on buffalo meat. The objectives of this study were: a) to examine the impact of different marinating treatments on both the microbiota and the shelf-life of buffalo meat when stored under vacuum conditions, b) to investigate the treatments efficiency at two storage temperatures: 4°C and 12°C, for a period of 22 and 11 days, respectively, and c) to evaluate consumer acceptability of marinated buffalo meat.
Materials and methods
Sample preparation
Fresh buffalo fillets (boneless cut or piece of meat) were obtained from the local supermarket in Greece and transferred to the laboratory under refrigeration temperatures within 30 min. They were subsequently cut into 10 g pieces (2 × 4 × 1 cm) with a sterilized knife and placed into polypropylene trays. In each of the 3 (separately conducted) experiments, approximately, 12.6 kg of buffalo meat was used (90 g; for microbiological, chemical and sensory analyses x 7 sampling days, x 5 treatments x 2 temperatures x 2 samples).
Citrus extract solution, oregano EO, and marinade preparation
For the marination of the samples, a commercially available marinade (a Ready-to-Use product (Bibasis Co., Ioannina, Greece)) was used. The composition of the commercially available marinade was (per 100 mL): a) solid mixture (1.7% w/v) of dextrose, black pepper, red pepper, all spice clove, oregano, and salt, b) a liquid marinade base (8.3% v/v) of dry red wine, olive oil, and sunflower oil, and c) water (90% v/v). Citrox (a commercial citrus extract) containing bioflavonoid powder (2%), anhydrous citric acid (18%), glycerin (5%), and water (demineralized; 75%) marketed under the trade name ProGarda 14WPlus, was purchased from POLYPAN GROUP S.A. (Athens, Greece). The extract complies with EU Regulation 2092/91 (Organic Farming EU Regulation).[Citation4] Pure oregano oil (Kokkinakis S.A., Athens, Greece) was used undiluted and extracted by hydrodistillation.
Addition of marinade, citrox or oregano EO to the samples and storage conditions
Marination of the buffalo samples was carried out in polypropylene trays at 4°C for 3 h by adding 1260 mL of marinade to 63 pieces of buffalo meat (10 g each). This volume was sufficient for the complete immersion of the samples into the marinades. Citrox (0.2%) or oregano EO (0.1%) (as secondary ingredients) were added to the main base marinade and mixed thoroughly for 3 min using a sterile spoon for homogenization. After that, buffalo meat samples were placed in vacuum pouches (control I, 9 pieces/pouch, Oxygen Transfer Rate 4.06 ml/package/day/atmosphere, LDPE/PA, VER PACK, Thessaloniki, Greece) with either marinade alone (control II) or with the added antimicrobials; Citrox (XC), oregano EO (XO) or their combination (XCO). A batch consisting of marinade but not the antimicrobials (X) and another batch devoid of antimicrobials and marinade (NX) were designated as controls. Previous studies indicated that concentrations of 0.1 and 0.2% of citrox and oregano EO, respectively, were sufficient to exert an antimicrobial effect.[Citation14] The samples placed in the vacuum pouches (one pouch/sampling day) were massaged by hand (from the outside of the vacuum pouch) to obtain a homogeneous distribution of the antimicrobials onto the marinated buffalo meat. Duplicate samples were subjected to microbiological, sensory and chemical analyses, while the experiment was conducted a total of three times (n = 2×3 = 6) using different batches of buffalo meat. The following sampling scheme was used: Days 0, 2, 4, 7, 10, 16 and 22 (storage temperature at 4°C) and 0, 1, 3, 5, 7, 9 and 11 (storage temperature at 12°C). These storage temperatures were chosen to simulate chill and mild abusive storage conditions in retail and home. All sampling and handling procedures were carried out using good hygienic practices (sterile utensils, laminar flow cabinet (class II safety), etc.) in order to reduce the risk of either direct contamination (through handling) or cross-contamination during sample preparation and packaging.
Microbiological and pH analyses
Buffalo meat samples (1 cube of meat, ca. 10 g) were aseptically transferred to 90 mL of 0.1% buffered peptone water (Merck, Darmstadt, Germany) in a sterile stomacher bag with an integrated filter (BagPage, Interscience, France) and homogenized in a stomacher (Seward BagMixer 400 West Sussex, United Kingdom) for 90 s at room temperature. For microbial enumeration, 1 or 0.1 ml samples of appropriate dilutions were poured or spread-plated on tryptone soy agar (Merck, Darmstadt, Germany) for mesophilic aerobic total viable counts, Pseudomonas agar base supplemented with cephalothin, fucidin, cetrimide (CFC) selective supplement (Merck, Darmstadt, Germany) for Pseudomonas spp., De-Man-Rogosa-Sharp medium (MRS) (Merck, Darmstadt, Germany) medium for lactic acid bacteria (LAB), Streptomycin Thallous Acetate agar (STAA) agar base (Merck, Darmstadt, Germany) for Brochothrix thermosphacta, Violet Red Bile Glucose (VRBG) (Merck, Darmstadt, Germany) for Enterobacteriaceae, and rose Bengal chloramphenicol (RBC) agar for yeasts (Merck, Darmstadt, Germany). The specifications and incubation conditions of these media have been previously described.[Citation20] The pH values of the samples were recorded using a pH meter (InterLab 427, Mettler-Toledo, GmbH, 8603, Schwerzenbach, Germany). To perform the pH analysis, a homogenate of buffalo meat (25 g) and distilled water (225 ml) was used.
Sensory analysis
Sensory evaluation was performed by seven in-house untrained laboratory staff members to simulate consumers’ preferences. The meat samples stored for 2 days at refrigeration temperatures was cooked in a microwave oven at 700 W for 10 min (non-marinated, marinated, and with added antimicrobials). Panelists evaluated the odor, taste, and tenderness (sensorial attributes) of the cooked buffalo meat presented in a random order in individual booths at room temperature. Between the evaluation of samples, a cracker and water was used to cleanse the palate. Scores were assigned based on a 3-point hedonic scale as follows: 3 (excellent), 2 (acceptable; a score of 2 was taken as the lower limit of acceptability), or < 2 (first off-odor, off-taste development) (Vergara, Gallego).[Citation21] Odor evaluation was performed according to the method described by Vergara & Gallego (2001) where samples were categorized as: 1 = not acceptable (strong off-odor); 2 = acceptable (slight off-odor) or 3 = very acceptable (no off-odor). The product was considered of unacceptable quality after the first off-odor or off-taste (supplementary file 1).[Citation22]
Statistical analysis
The effects of marination and addition of antimicrobials on the microbial populations, pH and sensory scores were analyzed using Statgraphics® plus version 5.1 (Statistical Graphics Corp., Rockville, MD, USA). For each of the different treatments, experiments were replicated twice (n = 2), and tests were run in triplicate for each (n = 2 × 3 = 6). Results are reported as mean values ± standard error of mean (SEM). Experimental data were subjected to two-way analysis of variance (ANOVA) with post-hoc analysis by Tukey HSD to detect the effect of treatments and their interactions on the tested nonpathogenic microorganisms during storage at 4 and 12°C. In addition, sensory evaluation data were analyzed by one-way ANOVA with post-hoc analysis (Tukey HSD) respectively. Statistical significance was defined as P < .05. Microbiological counts were converted to log cfu/g and subjected to analysis of variance.
Results
Effect of marinating treatments on buffalo meat microbiota
Mesophilic TVC, Pseudomonas spp., LAB, Brochothrix thermosphacta, Enterobacteriaceae, yeasts and molds were enumerated in the untreated (NX), marinated (X) and treated (XC, XO, XCO) samples () as these groups are likely to be present in the microbiota of buffalo meat. In the NX group, TVC, Pseudomonas spp., LAB, Enterobacteriaceae, Brochothrix thermosphacta increased by an average of 4–5 log cfu/g after storage for 22 days at 4°C, respectively (P < .05). In comparison, at 12°C, the microorganisms increased by similar numbers within a span of 11 days, respectively.
Figure 1. Population increase of Total viable counts (log10 CFU/g ± SEM) in different marinated essential oils samples after storage for 0, 2, 4, 7, 10, 16, and 22 days at 4°C. NX-Non marinated, X- Marinated, XO- Marinated +Oregano oil, XC- Marinated +Citrox, XCO- Marinated + Citrox+ Oregano oil.
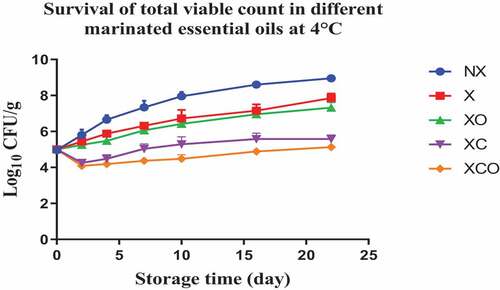
Figure 2. Population increase of Total viable count (log10 CFU/g ± SEM) in different marinated essential oils samples after storage for 0, 1, 3, 5, 7, 9, and 11 days at 12°C. NX-Non marinated, X- Marinated, XO- Marinated +Oregano oil, XC- Marinated +Citrox, XCO- Marinated + Citrox+ Oregano oil.
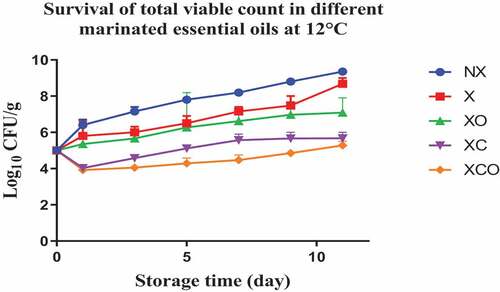
Figure 3. Population increase of pseudomonas (log10 CFU/g ± SEM) in different marinated essential oils samples after storage for 0, 2, 4, 7, 10, 16, and 22 days at 4°C. NX-Non marinated, X- Marinated, XO- Marinated +Oregano oil, XC- Marinated +Citrox, XCO- Marinated + Citrox+ Oregano oil.
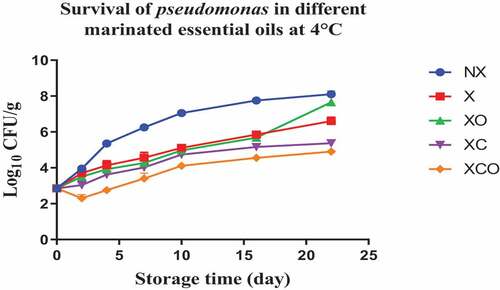
Figure 4. Population increase of pseudomonas (log10 CFU/g ± SEM) in different marinated essential oils samples after storage for 0, 1, 3, 5, 7, 9, and 11 days at 12°C. NX-Non marinated, X- Marinated, XO- Marinated +Oregano oil, XC- Marinated +Citrox, XCO- Marinated + Citrox+ Oregano oil.
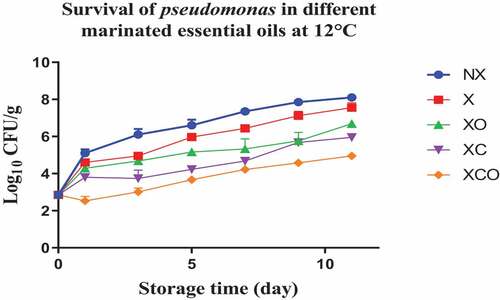
Figure 5. Population increase of Lactic acid bacteria (log10 CFU/g ± SEM) in different marinated essential oils samples after storage for 0, 2, 4, 7, 10, 16, and 22 days at 4°C. NX-Non marinated, X- Marinated, XO- Marinated +Oregano oil, XC- Marinated +Citrox, XCO- Marinated + Citrox+ Oregano oil.
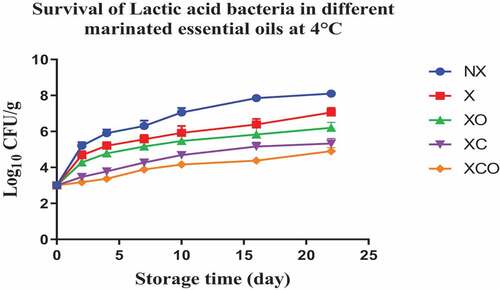
Figure 6. Population increase of Lactic acid bacteria (log10 CFU/g ± SEM) in different marinated essential oils samples after storage for 0, 1, 3, 5, 7, 9, and 11 days at 12°C. NX-Non marinated, X- Marinated, XO- Marinated +Oregano oil, XC- Marinated +Citrox, XCO- Marinated + Citrox+ Oregano oil.
Figure 7. Population increase of Enterobacteriaceae (log10 CFU/g ± SEM) in different marinated essential oils samples after storage for 0, 2, 4, 7, 10, 16, and 22 days at 4°C. NX-Non marinated, X- Marinated, XO- Marinated +Oregano oil, XC- Marinated +Citrox, XCO- Marinated + Citrox+ Oregano oil.
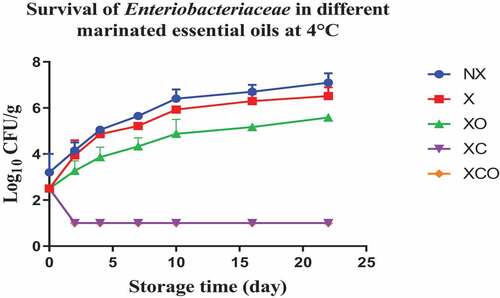
Figure 8. Population increase of Enterobacteriaceae (log10 CFU/g ± SEM) in different marinated essential oils samples after storage for 0, 1, 3, 5, 7, 9, and 11 days at 12°C. NX-Non marinated, X- Marinated, XO- Marinated +Oregano oil, XC- Marinated +Citrox, XCO- Marinated + Citrox+ Oregano oil.
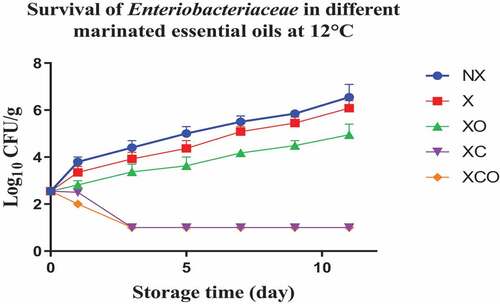
Figure 9. Population increase of yeast and molds (log10 CFU/g ± SEM) in different marinated essential oils samples after storage for 0, 2, 4, 7, 10, 16, and 22 days at 4°C. NX-Non marinated, X- Marinated, XO- Marinated +Oregano oil, XC- Marinated +Citrox, XCO- Marinated + Citrox+ Oregano oil.
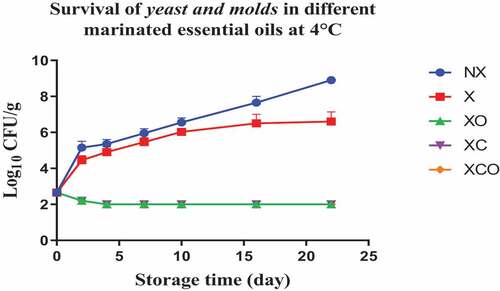
Figure 10. Population increase of yeast and molds (log10 CFU/g ± SEM) in different marinated essential oils samples after storage for 0, 1, 3, 5, 7, 9, and 11 days at 12°C. NX-Non marinated, X- Marinated, XO- Marinated +Oregano oil, XC- Marinated +Citrox, XCO- Marinated + Citrox+ Oregano oil.
Figure 11. Population increase of Brochothrix (log10 CFU/g ± SEM) in different marinated essential oils samples after storage for 0, 2, 4, 7, 10, 16, and 22 days at 4°C. NX-Non marinated, X- Marinated, XO- Marinated +Oregano oil, XC- Marinated +Citrox, XCO- Marinated + Citrox+ Oregano oil.
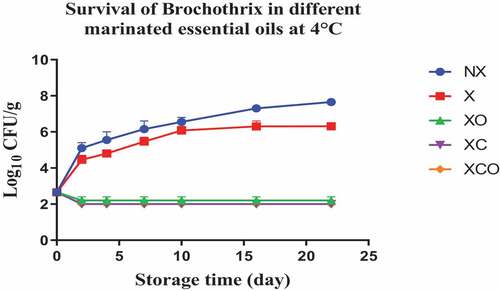
Figure 12. Population increase of Brochothrix (log10 CFU/g ± SEM) in different marinated essential oils samples after storage for 0, 1, 3, 5, 7, 9, and 11 days at 12°C. NX-Non marinated, X- Marinated, XO- Marinated +Oregano oil, XC- Marinated +Citrox, XCO- Marinated + Citrox+ Oregano oil.
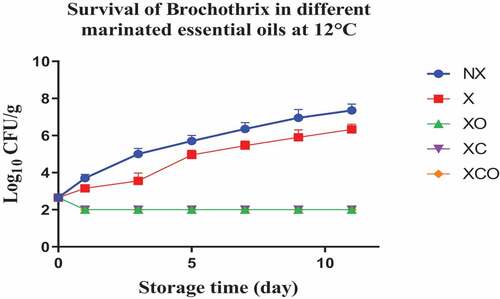
At 4°C, on day 22 (which marked the end of the storage period), the marinade (in comparison to NX) decreased TVC, Pseudomonas spp., LAB, Enterobacteriacea, yeasts and Brochothrix thermosphacta by 1.1, 1.5, 1.1, 0.6, 2.3 and 1.3 log cfu/g, respectively. In contrast, at 12°C, on day 11 (which marked the end of the storage period), the decrease was by 0.7, 0.6, 1.1, 0.5, 0.9 and 1.0 log cfu/g, respectively.
Oregano at 4°C, day 22 (in comparison to X) decreased TVC, LAB, Enterobacteriacea, yeasts and Brochothrix thermosphacta by 0.5, 0.9, 0.9, 4.6 and 4.1 log cfu/g, respectively. Meanwhile at 12°C, day 11, the decrease in TVC, Pseudomonas spp., LAB, Enterobacteriacea, yeasts and Brochothrix thermosphacta was by 1.6, 0.9, 0.5, 1.1, 3.3 and 4.3 log cfu/g, respectively.
Similarly, Citrox at 4°C, day 22, (in comparison to X) decreased TVC, Pseudomonas spp., LAB, Enterobacteriacea, yeasts and Brochothrix thermosphacta by 2.3, 1.2, 1.7, 5.5, 4.6 and 4.3 log cfu/g, respectively. Meanwhile at 12°C, day 11, the decrease was by 3.0, 1.6, 1.2, 5.1, 4.5 and 4.3 log cfu/g, respectively.
The overall mixture (marinade, citrox and EO) at 4°C, day 22, (in comparison to NX) decreased TVC, Pseudomonas spp., LAB, Enterobacteriacea, yeasts and Brochothrix thermosphacta by 3.8, 3.2, 3.2, 6.1, 6.9 and 5.7 log cfu/g, respectively. Meanwhile, at 12°C, day 11, the decrease was by 4.1, 3.2, 3.2, 5.6, 5.4 and 5.4 log cfu/g, respectively.
Effect of marinating treatments on pH changes in buffalo meat
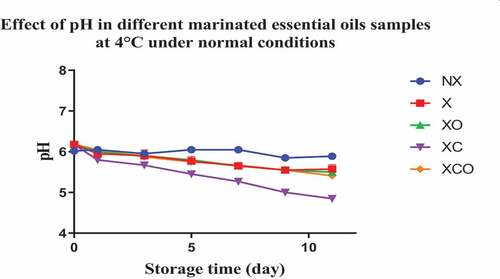
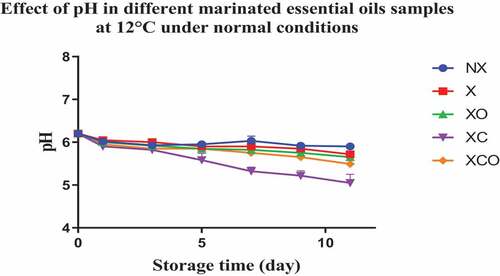
The initial pH of buffalo meat (day 0) with or without marination was around 6 for all trials (NX, X, XO, XC and XCO). During storage for 22 and 11 days at 4 and 12°C, respectively, the pH values of the NX samples remained almost unchanged (P > .05) (). In contrast, the pH values of the XO, XC, and XCO samples followed a steadily decreasing (P < .05) trend as the storage period increased. Among the treatments examined, the XC samples produced the lowest pH values (P < .05).
Effect of marinating treatments on sensory changes in buffalo meat
Overall, the treatments did not result in any significant change (P > .05) in taste and texture (). The average values for both parameters (or attributes) ranged from 2.7 to 3.0, respectively. Meanwhile, the color attribute increased significantly (P < .05) in all marinated samples as compared to the non-marinated ones. However, there was no difference in color between the treatments (XO, XC and XCO) and the marinated control (X). Amongst the treatment samples (XO, XC and XCO), the overall acceptability was the highest in the XC sample (2.85) followed by XCO (2.83) and then XO (2.78). However, the marinated control had the highest overall acceptability amongst all the treatments (2.98) (P < .05) ().
Table 1. Sensory evaluation results of cooked buffalo meat after 2 days of storage at refrigeration temperature (4°C) in the absence of marinade and antimicrobials (NX), in the presence of marinade (X), marinated + citrox (XC), marinated + oregano EO (XO) and marinated + citrox + oregano EO (XCO).
Discussion
The study was conducted at 4 and 12°C to mimic temperature abuse conditions which could occur due to constant opening/closing of refrigerator doors, during transport or due to a malfunctioning refrigerator. A very close resemblance in population growth was observed in bacterial growth especially in the control from day 2 to day 22 at 4°C, and from day 3 to day 11 at 12°C. This is understandable because 12°C is a comparatively more conducive environment for the microorganisms to thrive/proliferate as compared to refrigeration temperatures.
The initial microbial assessment of buffalo meat agreed with that reported previously[Citation16,Citation23] for chicken and pork meat. Data obtained in this study show that the highest decrease in microbial numbers was observed in XCO buffalo samples, which could be attributed to the combined (synergistic) effect of citrox and oregano EO. Citrox contains citric acid and bioflavonoids and oregano EO contains phenolic compounds (e.g., carvacrol), which are known to exhibit antimicrobial action.[Citation15] Marination alone, also caused reductions in the microbiota in buffalo meat, but this decrease was lower than that caused by the marination treatments with the added antimicrobials. The observed reduction could be attributed to the antimicrobial action of the constituents of the marinade. In another study, similar results have been observed with a winebased marinade containing thyme EO in beef filets.[Citation19] Antimicrobial activity was also reported action[Citation15] in chicken breast filets marinated with pomegranate, lemon juice, apple cider vinegar, and combinations of those stored at 4, 10, and 20°C.
Data obtained on Pseudomonas spp., LAB, and yeasts in NX and X buffalo samples support the hypothesis that these species are the main spoilage microorganisms. These species are usually psychrotrophic, a part of normal meat microbiota and become dominant toward the end of the shelf-life of the product (Karam et al.).[Citation24 Aerobic plate counts in meat products higher than 6 log cfu/g are often accompanied by the first signs of spoilage.[Citation23] In recent studies, LAB and TVC numbers have been used as indicators of spoilage in order to determine the shelf-life of marinated products.[Citation17] In our study, a Pseudomonas spp. count of 6 log cfu/g was considered to mark the end of shelf-life, as this group is typically associated with the spoilage of meat products.[Citation16,Citation17] Based on a Pseudomonas limit value of 6 log cfu/g, a microbiological shelf-life of 6, 16, and 18 days for NX, X, and XO was obtained, respectively. For both XC and XCO-treated buffalo samples, this limit value was never reached, even up to day 22 of storage (4°C). Vacuum packaging has been used widely in meat as a preservation method. The alteration of the surrounding gases in meat has been observed to change the phospholipids in the cell membrane bilayer in terms of saturation of bonds, their branching and overall structure in space.[Citation25] This in turn, has been observed to cell impact membrane permeability, acid resistance and oxygen consumption. As expected, at 12°C, all buffalo samples had shorter microbiological shelf-lives (compared to 4°C) of 2, 6, 8, and 11 days for NX, X, XO, and XC samples, respectively. XCO did not reach the end of shelf-life during all the storage period. At optimum higher temperatures, the bacterial cell wall, membrane potential, protein activity get altered to provide the bacteria with an optimum requirement for growth.[Citation26]
Currently, limited data is available in relation to marinated meat spoilage microbiota, including buffalo meat. The decrease in Pseudomonas spp. and LAB levels, observed at both 4°C and 12°C under XCO treatment, may be due to the synergistic effect of citrox and oregano EO. Additionally, the marinade itself may have also contributed to the inhibition of Pseudomonas spp. and LAB in buffalo meat, as decreases of 1–2 log units were observed. Similar decreases were also reported by Lytou et al.[Citation16,Citation17] for chicken under the effect of different marinating conditions and the influence of organic acid marinades. It is now well-established that organic acids act as membrane permeabilizers and pass the Gram-negative outer cell membrane, affecting the growth of bacterial species such as Pseudomonas.[Citation27] Nisiotou et al.[Citation28] previously reported significant reductions in Pseudomonas spp. by day 7 in beef filets treated with wine-based marinades. Lytou et al.[Citation17] also reported a reduction (5 logs) of Pseudomonas spp. in chicken breast filets, marinated in apple cider vinegar, pomegranate, and lemon juice (including their combinations), whereas Bolton et al.[Citation29] reported a reduction of up to 2.6 logs after treatment with 5% citric acid.
The results presented in this study indicate that the use of a combination of citrox and oregano EO leads to a significant reduction in LAB levels, regardless of the temperature. In another study however the acidic nature of lemon juice, apple cider vinegar, pomegranate juice, and their combinations did not result in a significant reduction in LAB counts in chicken breast filets.[Citation16] In vacuum packaged camel meat stored under refrigeration conditions, thymol (a component of oregano EO) decreased Enterobacteriaceae and Pseudomonas spp. populations by 1.2 and 1.4 log cfu/g, respectively.[Citation30] Thymol also decreased Enterobacteriaceae populations by 1.2 log cfu/g in the vacuum packaged camel meat stored at temperature abuse conditions (10°C).[Citation30] Oregano EO decreased LAB populations in vacuum packaged Tuscan sausages (stored under refrigeration conditions) to a level such that the shelf-life of the sausages increased by 8 and 14 days upon treatment at oregano EO 0.2 and 0.4% concentrations, respectively.[Citation31]
Interestingly, yeasts, Br. thermosphacta, and Enterobacteriaceae were significantly affected by the marinating treatments involving added antimicrobials. Significant reductions were obtained in buffalo samples under XCO, XC, and XO treatments, regardless of temperature. It is apparent that the use of citrox and oregano EO, either singly applied or combined, acted as an effective antimicrobial intervention. It was also reported that Br. thermosphacta was sensitive to the action of various antimicrobials applied in marinated chicken meat.[Citation16] The absence of an outer membrane perhaps, makes it even more sensitive to the antimicrobials’ action. In our study, the use of either citrus extract, alone or in combination with oregano EO, inhibited the growth of Enterobacteriaceae, B. thermosphacta, and yeasts in buffalo samples during storage at 4 or 12°C. Overall, a general trend of the treatments being more effective on TVC as compared to Pseudomonas spp. and LAB was observed. This could be because of the general different physical nature of the bacteria and their ability to develop resistance mechanisms to the antimicrobials (C.[Citation32] Interestingly, of the treatments in our study, citrox added to the marinated samples buffalo (XC) had a significant effect on pH, producing the lowest values at both temperatures, respectively. Such decrease in the pH values, may be attributed to the acidic nature of the citrox itself, and/or in combination with the marinade ingredients. However, this is a speculation and was not tested in our study.
In terms of mode of action, EOs, their active components and their phenolic compounds from oregano and citrus extracts exert their antimicrobial effects by attaching to bacterial cell membranes, penetrating them due to their lipophilic properties, and causing destabilization and degradation of the membrane layers. This can result in the inactivation of enzymes and disruption of the electron transport system.[Citation11,Citation33]
The no significant change (P > .05) in texture and color observed in the current study is similar to an experiment conducted previously on marinated camel meat where EO components namely carvacrol, cinnamaldehyde and thymol were used.[Citation34] The overall acceptability of marinated camel meat with 1% cinnamaldehyde (5.73) was significantly greater than the observation of the current study (2.85).[Citation34] However, with respect to taste the observations in this study are similar to a study conducted previously on fresh lamb meat marinated with oregano EO (0.1 and 0.3%). However, marinades, applied to meat products to enhance their sensory attributes, may negatively affect the microbiological shelf-life and could therefore mislead the determination of the “real” shelf-life. Marination ingredients may act as antimicrobial agents and increase the shelf life of meat.[Citation15] This could be exploited by meat retailers who might choose to marinate meat toward the end of shelf-life to enhance its flavor and appearance.[Citation16,Citation17] It is widely known that marinades contain several flavor enhancers that could mask the undesirable flavors of spoiled chicken meat. In this study, it is noteworthy that X samples recorded high populations of spoilage microorganisms (i.e., Pseudomonas spp. and/or Br. thermosphacta) toward the end of the storage period at both temperatures, and this indicates that marination may mask possible spoilage indicators, as also noted in the study of marinated pork steaks by Pichner et al..[Citation35]
Marinated meat products are a concern from a food safety perspective as it is possible that they are exposed to pathogenic cross-contamination from raw materials such as fresh meat, sauce, and other ingredients during processing and storage.[Citation36] Selecting a suitable spoilage indicator and threshold limit value to mark the end of the shelf-life of a marinated product is, therefore, crucial for the safety of consumers. Producers should consider any potential “masking” effect of marination, which may affect the safety of the product. They should be extremely cautious in assessment of the marinated product’s overall quality.
Conclusion
Citrox with oregano EO, acted synergistically with the marinade in terms of antimicrobial efficacy in buffalo meat. This is a cost-effective technique which would decrease spoilage of the product and enhance its safety. The extended shelf-life can offer potential benefits to meat processors and retail food service suppliers by allowing ample time for transport/export. However, retailers should keep the “masking effect” of the marinade into perspective. In addition, a more elaborate sensory analysis with a higher number of panelists would be highly recommended before scaling up for industrial applications in order to have a more representative sample of target consumers. Further studies are needed with different marinade ingredients and natural antimicrobial agents. It is necessary to carry out more studies on natural antimicrobials in poultry/chicken, their technological and organoleptic effects, their synergistic behavior and their functionality (bioavailability and bio accessibility studies). The potential use of natural citrox as a food additive should be further investigated as well.
Credit authorship contribution
Tareq Osaili, Layal Karam, Ioannis N. Savvaidis: Conceptualization; Tareq Osaili, Layal Karam, Ioannis N. Savvaidis: Data curation; Tareq Osaili, Layal Karam, Ali Atoui, Maria I. Tsiraki, Ioannis N. Savvaidis: Methodology; Tareq Osaili, Ioannis N. Savvaidis: Project administration; Tareq Osaili, Layal Karam, Ali Atoui, Maria I. Tsiraki, Ioannis N. Savvaidis: Writing – original draft; Tareq Osaili, Layal Karam, Ali Atoui, Maria I. Tsiraki, Ioannis N. Savvaidis: Writing – review & editing.
Acknowledgments
Open Access funding was provided by the Qatar National Library.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Yusop, S. M.; O’Sullivan, M. G.; Kerry, J. F.; Kerry, J. P. Effect of Marinating Time and Low PH on Marinade Performance and Sensory Acceptability of Poultry Meat. Meat Sci. 2010, 85(4), 657–663. DOI: 10.1016/j.meatsci.2010.03.020.
- British Standard European Norm BS EN 1276. Chemical Disinfectants and Antiseptics. Quantitative Suspension Test for the Evaluation of Bactericidal Activity of Chemical Disinfectants and Antiseptics Used in Food, Industrial, Domestic and Institutional Areas. Test method and requirements (phase 2, step 1). 2009.
- British Standard European Norm BS EN 1275. Chemical Disinfectants and Antiseptics. Quantitative Suspension Test for the Evaluation of Basic Fungicidal or Basic Yeasticidal Activity of Chemical Disinfectants and Antiseptics. Test method and requirements (phase 1). 2005.
- Commission Regulation (EC) No 394/2007. Council Regulation (EEC) No 2092/91 of 24 June 1991 on organic production of agricultural products and indications referring thereto on agricultural products and foodstuffs. 12 April 2007.
- Premier, R. Evaluation of Vegetable Washing Chemicals, VG09086; Horticulture Australia Ltd: Global F.S. Pty Ltd, 2012.
- Yehia, H. M.; Al-Masoud, A. H.; Elkhadragy, M. F.; Korany, S. M.; Nada, H. M. S.; Albaridi, N. A.; Alzahrani, A. A.; AL-Dagal, M. M. Improving the Quality and Safety of Fresh Camel Meat Contaminated with Campylobacter Jejuni Using Citrox, Chitosan, and Vacuum Packaging to Extend Shelf Life. Animals. 2021, 11(4), 1152. DOI: 10.3390/ani11041152.
- Yehia, H. M.; Fawzy Elkhadragy, M.; Abdullah Al-Megrin, W.; Hamad Al-Masoud, A. Citrox Improves the Quality and Shelf Life of Chicken Fillets Packed Under Vacuum and Protects Against Some Foodborne Pathogens. Animals. 2019, 9(12), 1062. DOI: 10.3390/ani9121062.
- Schalkwyk, V.; Charles, P. B.; Hugo, A.; Hugo, C. J.; Bothma, C. Evaluation of a Natural Preservative in a Boerewors Model System: Natural Preservative in Boerewors Model. J. Food Process. Preserv. 2013, 37(5), 824–834. DOI: 10.1111/j.1745-4549.2012.00706.x.
- Burt, S. Essential Oils: Their Antibacterial Properties and Potential Applications in Foods—A Review. Int. J. Food Microbiol. 2004, 94(3), 223–253. DOI: 10.1016/j.ijfoodmicro.2004.03.022.
- Yammine, J.; Gharsallaoui, A.; Fadel, A.; Mechmechani, S.; Karam, L.; Ismail, A.; Chihib, N. Enhanced Antimicrobial, Antibiofilm and Ecotoxic Activities of Nanoencapsulated Carvacrol and Thymol as Compared to Their Free Counterparts. Food Control. 2023, 143, 109317. DOI: 10.1016/j.foodcont.2022.109317.
- Yammine, J.; Chihib, N.; Gharsallaoui, A.; Dumas, E.; Ismail, A.; Karam, L. Essential Oils and Their Active Components Applied As: Free, Encapsulated and in Hurdle Technology to Fight Microbial Contaminations. A Review. Heliyon. 2022, 8(12), e12472. DOI: 10.1016/j.heliyon.2022.e12472.
- Chen, X.; Chen, W.; Xiao, L.; Mao, Y.; Luo, X.; Liu, G.; Zhu, L.; Zhang, Y. Effect of Chitosan Coating Incorporated with Oregano or Cinnamon Essential Oil on the Bacterial Diversity and Shelf Life of Roast Duck in Modified Atmosphere Packaging. Food Res. Int. 2021, 147(September), 110491. DOI: 10.1016/j.foodres.2021.110491.
- Al-Hijazeen, M.; Mendonca, A.; Lee, E. J.; Ahn, D. U. Effect of Oregano Oil and Tannic Acid Combinations on the Quality and Sensory Characteristics of Cooked Chicken Meat. Poultr. Sci. 2018, 97(2), 676–683. DOI: 10.3382/ps/pex285.
- Hasapidou, A.; Savvaidis, I. N. The Effects of Modified Atmosphere Packaging, EDTA and Oregano Oil on the Quality of Chicken Liver Meat. Food Res. Int. 2011, 44(9), 2751–2756. DOI: 10.1016/j.foodres.2011.06.011.
- Karam, L.; Roustom, R.; Abiad, M. G.; El-Obeid, T.; Savvaidis, I. N. Combined Effects of Thymol, Carvacrol and Packaging on the Shelf-Life of Marinated Chicken. Int. J. Food Microbiol. 2019, 291(February), 42–47. DOI: 10.1016/j.ijfoodmicro.2018.11.008.
- Lytou, A. E.; Panagou, E. Z.; Nychas, G.-J. E. Effect of Different Marinating Conditions on the Evolution of Spoilage Microbiota and Metabolomic Profile of Chicken Breast Fillets. Food Microbiol. 2017, 66(September), 141–149. DOI: 10.1016/j.fm.2017.04.013.
- Lytou, A. E.; Tzortzinis, K.; Skandamis, P. N.; Nychas, G.-J. E.; Panagou, E. Z. Investigating the Influence of Organic Acid Marinades, Storage Temperature and Time on the Survival/Inactivation Interface of Salmonella on Chicken Breast Fillets. Int. J. Food Microbiol. 2019, 299(June), 47–57. DOI: 10.1016/j.ijfoodmicro.2019.03.019.
- O’Neill, C.; Malco, M.; Cruz-Romero, C.; Duffy, G.; Joseph, P. K. Improving Marinade Absorption and Shelf Life of Vacuum Packed Marinated Pork Chops Through the Application of High Pressure Processing as a Hurdle. Food Pack. Shelf Life. 2019, 21(September), 100350. DOI: 10.1016/j.fpsl.2019.100350.
- Kargiotou, C.; Katsanidis, E.; Rhoades, J.; Kontominas, M.; Koutsoumanis, K. Efficacies of Soy Sauce and Wine Base Marinades for Controlling Spoilage of Raw Beef. Food Microbiol. 2011, 28(1), 158–163. DOI: 10.1016/j.fm.2010.09.013.
- Tsiraki, M. I.; Savvaidis, I. N. Citrus Extract or Natamycin Treatments on ‘Tzatziki’ – a Traditional Greek Salad. Food Chem. 2014, 142(January), 416–422. DOI: 10.1016/j.foodchem.2013.07.087.
- Vergara, H.; Gallego, L. Effects of Gas Composition in Modified Atmosphere Packaging on the Meat Quality of Spanish Manchega Lamb. J. Sci. Food Agric. 2001, 81, 1353–1357. DOI: 10.1002/jsfa.953.
- Mastromatteo, M.; Lucera, A.; Sinigaglia, M.; Rosaria Corbo, M. Combined Effects of Thymol, Carvacrol and Temperature on the Quality of Non Conventional Poultry Patties. Meat Sci. 2009, 83(2), 246–254. DOI: 10.1016/j.meatsci.2009.05.007.
- Holley, R. A.; Peirson, M. D.; Lam, J.; Bee Tan, K. Microbial Profiles of Commercial, Vacuum-Packaged, Fresh Pork of Normal or Short Storage Life. Int. J. Food Microbiol. 2004, 97(1), 53–62. DOI: 10.1016/j.ijfoodmicro.2004.03.029.
- Karam, L.; Chehab, R.; Osaili, T. M.; Savvaidis, I. N. Antimicrobial Effect of Thymol and Carvacrol Added to a Vinegar-Based Marinade for Controlling Spoilage of Marinated Beef (Shawarma) Stored in Air or Vacuum Packaging. Int. J. Food Microbiol. 2020, 332, 108769. DOI :10.1016/j.ijfoodmicro.2020.108769.
- Sandra, K.; Kienberger, H.; Kleigrewe, K.; Hilgarth, M.; Vogel, R. F. Effect of High Levels of CO2 and O2 on Membrane Fatty Acid Profile and Membrane Physiology of Meat Spoilage Bacteria. Eur. Food Res. Technol. 2021, 247(4), 999–1011. DOI: 10.1007/s00217-020-03681-y.
- Baatout, S.; De Boever, P.; Mergeay, M. Temperature-Induced Changes in Bacterial Physiology as Determined by Flow Cytometry. Ann. Microbiol. 2005, 55(January), 73–80.
- Lund, P.; Tramonti, A.; De Biase, D. Coping with Low PH: Molecular Strategies in Neutralophilic Bacteria. FEMS Microbiol. Rev. 2014, 38(6), 1091–1125. DOI: 10.1111/1574-6976.12076.
- Nisiotou, A.; Chorianopoulos, N. G.; Gounadaki, A.; Panagou, E. Z.; Nychas, G.-J. E. Effect of Wine-Based Marinades on the Behavior of Salmonella Typhimurium and Background Flora in Beef Fillets. Int. J. Food Microbiol. 2013, 164(2–3), 119–127. DOI: 10.1016/j.ijfoodmicro.2013.04.008.
- Bolton, D. J.; Meredith, H.; Walsh, D.; McDowell, D. A. The Effect of Chemical Treatments in Laboratory and Broiler Plant Studies on the Microbial Status and Shelf-Life of Poultry. Food Control. 2014, 36(1), 230–237. DOI: 10.1016/j.foodcont.2013.08.027.
- Osaili, T. M.; Hasan, F.; Al-Nabulsi, A. A.; Kumar Dhanasekaran, D.; Shaker Obaid, R.; Hashim, M. S.; Radwan, H. M.; Cheikh Ismail, L.; Hasan, H.; Faris, M. A.-I. E.; et al. Effect of Essential Oils and Vacuum Packaging on Spoilage-Causing Microorganisms of Marinated Camel Meat During Storage. Foods. 2021a, 10(12), 2980.
- Badia, V.; de Oliveira, M. S. R.; Polmann, G.; Milkievicz, T.; Cazonatto Galvão, A.; da Silva Robazza, W. Effect of the Addition of Antimicrobial Oregano (Origanum Vulgare) and Rosemary (Rosmarinus Officinalis) Essential Oils on Lactic Acid Bacteria Growth in Refrigerated Vacuum-Packed Tuscan Sausage. Braz. J. Microbiol. 2020, 51(1), 289–301. DOI: 10.1007/s42770-019-00146-7.
- Reygaert, C. W. An Overview of the Antimicrobial Resistance Mechanisms of Bacteria. AIMS Microbiol. 2018, 4(3), 482–501. DOI: 10.3934/microbiol.2018.3.482.
- Nannapaneni, R.; Muthaiyan, A.; Crandall, P. G.; Johnson, M. G.; O’Bryan, C. A.; Chalova, V. I.; Callaway, T. R.; Carroll, J. A.; Arthington, J. D.; Nisbet, D. J.; et al. Antimicrobial Activity of Commercial Citrus-Based Natural Extracts Against Escherichia coli O157: H7 Isolates and Mutant Strains. Foodborne Pathogens Dis. 2008, 5(5), 695–699.
- Osaili, T. M.; Hasan, F.; Dhanasekaran, D. K.; Obaid, R. S.; Al-Nabulsi, A. A.; Karam, L.; Savvaidis, I. N.; Olaimat, A. N.; Ayyash, M.; Al-Holy, M.; et al. Effect of Yogurt-Based Marinade Combined with Essential Oils on the Behavior of Listeria Monocytogenes, Escherichia coli O157: H7 and Salmonella Spp. in Camel Meat Chunks During Storage. Int. J. Food Microbiol. 2021b, 343, 109106. DOI: 10.1016/j.ijfoodmicro.2021.109106.
- Pichner, R.; Schönheit, C.; Kabisch, J.; Böhnlein, C.; Rabsch, W.; Beutin, L.; Gareis, M. Assessment of Microbiological Quality and Safety of Marinated Pork Products from German Retail During Shelf Life. Food Control. 2014, 46(December), 18–25. DOI: 10.1016/j.foodcont.2014.05.001.
- Choi, Y. M.; Bae, Y. Y.; Kim, K. H.; Kim, B. C.; Rhee, M. S. Effects of Supercritical Carbon Dioxide Treatment Against Generic Escherichia Coli, Listeria Monocytogenes, Salmonella Typhimurium, and E. Coli O157: H7 in Marinades and Marinated Pork. Meat Sci. 2009, 82(4), 419–424. DOI: 10.1016/j.meatsci.2009.02.016.