ABSTRACT
Non-thermal processing has been developed to increase the quality and storage life of fruit juices in response to consumer desire. Ozonation is a recently developed method that generates volatile oxygen atoms that can eliminate a broad spectrum of microbes and extend the shelf stability of food products. The present research was intended to investigate the ozone processing on the physicochemical characteristics, microbiological quality, sensory attributes, and antioxidant potential of Kinnow juice. In this study, Kinnow juice was processed to 150 mg/h of ozone gas at a constant concentration for 5, 10, and 15 min. The sample was then taken in a plastic container and stored at 35°C ± 2 for three months. The results revealed that ozone processing had a significant (p˂0.05) effect on the total phenolics (TP) contents, total flavonoids (TF) contents, total soluble solids (TSS), acidity, total antioxidant activity (TAA) and DDPH radical scavenging activity of Kinnow juice. In addition, the ozonation application also significantly (p˂0.05) decreased the population of the microbes when the ozone time was increased from 5–15 min. Among the treatments, it was observed that T3 had the highest TSS, acidity, TF contents, TAA, and DDPH radical scavenging activity values and the lowest mold and plate counts. Regarding sensory qualities, T0+ showed the highest sensory score during storage. Thus, it may be concluded from the findings of our study that ozone application improved the quality of Kinnow juice by increasing TSS, phytochemicals and decreasing the microbial population, thereby prolonging their shelf life.
Introduction
The nutritional benefits of minerals and vitamins of fruit juices are widely recognized. The fruit juices are also substantial sources of bioactive compounds such as vitamin C, carotenoids, and phenolics.[Citation1] The Rutaceae family includes sweet lime, Kinnow, grapefruit, tangerines, lemon, oranges, bitter oranges and lime.[Citation2] Citrus fruits are distinguished by their exceptional flavor, nutritional value, and phytochemical variety.[Citation3] Citrus fruits are among the most frequently produced worldwide, with an annual harvest area of 10.07 million hectares and a yield of 158.49 million metric tonnes.[Citation4] Among the citrus fruits, Kinnow (Citrus reticulata Blanco) (also known as mandarin), a hybrid of King and willow leaf, is a popular fruit in Pakistan. With an estimated yield of 370,000 tonnes and a trade value of 222 million dollars, Pakistan is the 6th largest mandarin producer worldwide.[Citation5] Pakistan contributes 6.5% of the world’s mandarin production, with 60% Kinnow and 5% other mandarin varieties.[Citation6] Kinnow postharvest losses range between 25 to 30%. These losses can be minimized by converting high-value-added products such as marmalade, jam, squash, juice blends, jelly, and RTS[Citation7,Citation8]
Kinnow juice is very delicious, reviving, and nutrient-rich liquid. It has been reported to have antioxidant, anti-microbial, and anti-blood clotting properties. It decreases the probability of developing a heart condition.[Citation9,Citation10] It comprises 88.9%water, 10.1% carbohydrates, 0.5% protein, 0.2% fiber, 0.2% fat. In addition, it also contains 1 mg sodium, 18 mg calcium, 8 mg magnesium, 14 mg phosphorus and 178 mg potassium out of 100 g.[Citation10,Citation11] Kinnow juice also contains abundant quantities of primary metabolites (amino acids, vitamins (provitamin-A, folate, and ascorbic acid)), and secondary metabolites such as flavones, flavonoids, limonoids, phenolics, and carotenoids.[Citation12,Citation13]
Consumers demand natural, healthy, and safe products. The nutritional and qualitative qualities were altered through a controlled process. Heat treatment destroys pathogenic bacteria, inactivates pectin methyl esterase, and reduces the juice’s flavor and nutritional value.[Citation14] Chemical preservatives are utilized to increase the shelf life of the foodstuff but contribute to many side effects in the human body, including allergy, asthma, GI tract, cancer, and respiratory disorder. Due to the drawbacks mentioned earlier of thermal procedures and chemical preservatives, many innovative non-thermal ways have been implemented to increase the shelf life of processed food items. Non-thermal technologies, including HPP, ozone, ultrasonic, and plus-electric field, are used. As a result, these methods can lessen heat damage to the nutrients and quality of food.[Citation15–17]
Ozone is produced from three oxygen atoms and has no flavor or color. Owing to its great oxidizing power and bactericidal and oxidant properties, it can remove microorganisms such as bacteria, protozoa, and viruses from food products, extending their shelf life. Compared to other thermal processing, ozonation is a quick, efficient, inexpensive, and accessible technology that can be utilized as an alternative. It is a green biochemical method that needs GRAS approval. As an antibacterial, food is preserved with ozone approved by the FDA.[Citation14,Citation18] Ozonation has antibacterial properties, like releasing free oxygen atoms that can kill many microbes and make food last longer. It has been implemented in industrial sectors like orange juice, tomato juice, apple juice, and apple cider. It has not impacted fruit juices’ acidity, NEB, or pH.[Citation15,Citation19] Glowacz et al.[Citation20] reported that ozone can be efficient in the reduction of microbial load of the product without affecting their textural, visual, and even nutritional quality. It was also proven that ozone can preserve fruits and vegetables by providing antimicrobial activity as well as increased contents of antioxidant (vitamin C and phenolics) activities.[Citation21] The analysis of the study indicated that the exposure to ozone treatments above 2.4 mg/L for higher durations significantly reduced the microbial load and showed better retention of other quality parameters like ascorbic acid, firmness, color and overall acceptability. In another study, ozone treatment of sugarcane juice was found to be effective in the inactivation of enzymes and microbial destruction, thereby leading to enhanced anti-browning and antimicrobial properties.[Citation22] To date, no published literature was found regarding influence of ozone on the quality of Kinnow juice at ambient temperature. Hence, the current study was aimed to investigate the impact of ozone treatment on microbial load, bioactive contents, and physicochemical properties of Kinnow juice at room temperature (35°C ± 2) for three months.
Materials and methods
Collection of fruit
Kinnow fruits were collected from a local farm and sorted to remove defective and infected fruit. The fruits were then rinsed with tap water to eliminate dirt and dust.
Chemical reagents
All analytical chemicals were acquired from Sigma-Aldrich (Gillingham, UK) and are readily available on the local market.
Extraction of Kinnow juice
The fruits were peeled before extracting the juice with an electrical juice extraction extractor (Model. No. KJ 1003 operating at 450W, frequency 60 Hz, and voltage 45 V). The clear juice was obtained by filtering the juice through a No. 18 filter sieve. Finally, the Kinnow juice was placed in a plastic container at ambient temperature (35°C ± 2) for three months.
Ozone processing of Kinnow juice
Ozone was done by subjecting 250 mL of Kinnow juice in a beaker to ozone gas at a fixed concentration of 150 g/ha via a pipe linked to a diffuser (Model No. TR-YCA, operating at 15 W of power, 50 Hz of frequency, and 220 V of voltage) that made bubbles and spread the gas evenly (). The juice was exposed to ozone for 5 to 15 min. The samples were taken in sterilized bottles and stored at room temperature (35°C ± 2) for three months.
Chemical preservative
KMS (0.1%) was added to treatment T0+ to compare the juice to the control and other treatments. The treatment plan is detailed in .
Table 1. Treatment plan of the present study.
Physicochemical analyses
Total soluble solids were determined using a digital refractometer (Model AVl41984, ERMA, India). According to method 981.12 of the AOAC[Citation23] the pH of Kinnow juice was determined using a pH meter (CPH-102, Contech, India). The acidity of the samples was measured using the standard titration method in line with method 942.15 of the AOAC.[Citation23] The viscosity of Kinnow juice was determined using a ROTAVISC revolving viscometer with a No. L2 spindle head. 100 mL of each juice sample was taken in a beaker. Subsequently, the spindle was adjusted to a rotational speed of 1000 revolutions per min at a temperature of 28°C ± 1, following which the viscometer interpretation was recorded.[Citation24]
Total phenolic (TP) contents
Total phenolic contents were measured using slightly modifying the Folin – Ciocalteu reagent technique.[Citation25] .5 mL prepared sample was taken in different test tubes. 0.5 mL Folin solution (10%) was placed in the test tube and left for 6 minmin. Then, 1 mL of Na2CO3 (20%) was taken to a test tube and stayed in the incubator at 30°C for 1 hour. Using a spectrophotometer (Du-8800d, UV-VIS double beam), the absorbance was measured at 760 nm. The findings were stated as mg equivalent gallic acid (GAE) per 100 mL of juice.
Total flavonoid (TF) contents
According to Iqbal et al.,[Citation14] the total flavonoids (TF) of Kinnow juice were calculated. 250 µL sample was taken in different test tubes by preparing 1 mL diluted juice. Each sample made three replicates. Then, t 75 µL sodium nitrite was added and wait left for 6 min. 150 µL aluminum chloride was taken in the above mixture and left for 5 min. Then, 500 µL NaOH was added, and further 9 mL distilled water was also added. The absorbance at 510 nm was then measured using a spectrophotometer (Du-8800d, UV-VIS double beam, Diepoldsau, Switzerland). The results were expressed in µg catechin equivalent (CE) per 100 mL of juice.
Antioxidant activity (TAA)
Kinnow juice samples were examined using the Prieto et al.[Citation26] method to determine the antioxidant activity. The results were represented as µg ascorbic acid equivalent/100 mL juice and were calibrated using ascorbic acid as the standard. The evaluations were carried out in triplicate.
DDPH free radical scavenging activity
Kinnow juice samples were tested for DPPH free radical scavenging capacity using the prior method developed by Yi et al.,[Citation27] with slight changes. The following calculation was used to calculate the change in absorbance:
(A0 - A1/A0)×100= DPPH radical scavenging activity
A0indicates the absorbance of the control, and A1indicatesthe absorbance of the extracts. Using Trolox as a standard, the identical procedure was used to assess DPPH radical scavenging activity, and the results were stated as µM equivalent of Trolox/mL juice.
Total plate count (TPC)
TPC determination was performed by FDA’s standard method outlined in the Bacteriological Analytical Manual.[Citation28] Using a sterilized pipette, 1 mL of the sample was transferred to a test tube, and 9 mL of saline solution was diluted. After adding diluents and shaking at a low speed for one to two min, the sample became completely clouded. 9 mL of saline solution was used to follow the 10-fold serial dilution protocol. Each dilution was carefully stirred. In the petri dish, 12–15 ml of PCA (Plate Count Agar) was poured, sample dilutions were combined with media, and the media was solidified. The petri dishes were flipped over and incubated at 32°C for 48 hours. The total bacterial populations in the sample were calculated, multiplied by the reciprocal, and stated as log colony-forming units (CFU) per mL of juice.
Mold count (MC)
MC determination was performed by FDA’s standard method outlined in the Bacteriological Analytical Manual.[Citation28] One milliliter of juice was taken in a test tube. A solution exhibiting turbidity was obtained after 9 mL of saline solution and subsequent agitation for one to two min. Then, 1 mL of the 10 mL dilution was added to the 9 mL dilution previously prepared. All dilutions were thoroughly blended. Subsequently, 12–15 ml of PDA (Potato dextrose agar) was poured into a petri dish, the diluted sample was added, and the mixture was solidified. All petri dishes were incubated between 20 to 25°C. After 48 hours of incubation, the MC in each plate was measured and reported as log CFU/mL of juice. Each analysis was conducted in triplicate.
Sensory analyses
The purpose of assessing the product using the human senses is through sensory evaluation. Kinnow juice was evaluated in a sensory assessment laboratory for sensory attributes like texture, color, flavor, appearance, and overall acceptability at ambient temperature by a panel of semi-trained judges on a 9-point scale. The Hedonic Scale was described in detail in Land and Shepherd.[Citation29]
Statistical analysis
The result was evaluated using statistics and the two-variance analysis method. Mean differences were assessed using Tukey's test with a 5% significance level.[Citation30]
Results and discussion
Impact of ozone treatments on TSS, pH, acidity, and viscosity
The ozone processing resulted in highly significant alterations in the TSS of Kinnow juice, but three months of storage led to a substantial reduction (). It was observed that the total soluble contents (TSS) were varied between treatments. The highest TSS were detected in T3 (14.80°B), and the lowest values were in T0- (13.77°B) at 0 day. Over time, TSS reduced from 14.80 to 4.10°B. After three months, the highest value of TSS in T0+ (13.10°B) and the lowest in T0- (4.10°B).The present findings of the study on TSS are consistent with prior research that stated similar patterns. Bulu and Koyuncu[Citation31] and Aday et al.[Citation32] also found the decreasing trends of TSS in ozonated pomegranate and strawberry fruits throughout the storage time, correlating with current results. The TSS is decreased by the breakdown of sugar into water, CO2, and ethyl alcohol. The raspberry samples undergoing ozone treatment (60 and 120 minmin) exhibited a higher soluble solid concentration than the control group. It may be attributed to a rise in the predominant carbohydrates, i.e. glucose and fructose, as a consequence of the results of ozone.[Citation33]
Table 2. Effect of ozone treatments (T0-= Control juice, T0+ = Juice with KMS preservative, T1 = 5 min ozone treatment, T2 = 10 min ozone treatment, T3 = 15 min ozone treatment)on physicochemical characteristics of Kinnow juice during storage (35°C ± 2).
The findings revealed that the treatments and storage time significantly impacted the pH of Kinnow juice (). The pH was varied among the treatments. The pH of treated samples was increased during three months of storage. The highest pH value was noticed in T0- (3.81) and the lowest in T3 (3.60) at 0 day. Over time, pH increased from 3.60 to 3.99. After three months, the highest value of pH in T0- (3.99) and the lowest in T3 (3.81) were observed. Concurrent to the current findings, Alencar et al.[Citation34] and Barboni et al.[Citation35] demonstrated a rising tendency of pH in ozone-treated banana and kiwi fruit through storage. The same patterns were noticed across treatments (Fundo et al., 2018).The pH of both filtered and unfiltered ozone-processed watermelon juice decreased substantially (p˂0.05) over the duration of 5 to 25 min. The decrease in pH may be a result of the increase in malic acid in unfiltered and filtered ozone-processed watermelon juice after ozonation.[Citation36] The rise in pH may be related to the inverse correlation between pH and acidity. The increase in pH may be the consequence of the conversion of acids to sugars or the chemical interaction between the organic components of the juice as a result of temperature and enzyme action.[Citation37]
The findings revealed that the treatments and storage time significantly impacted the acidity of Kinnow juice (). The acidity varied among the treatments. The acidity of treated samples was decreased during three months of storage. The highest acidity value was noticed in T3 (0.173%), and the lowest acidity value in T0- (0.126%) at 0 day. Over time, acidity decreased from 0.173 to 0.081%. After three months, the highest acidity value was in T3 (0.109%) and the lowest in T0- (0.081%). Ozone-treated watermelon and peach juices showed similar trends throughout treatments and storage durations.[Citation24,Citation36] During storage, the acidity of blends of Kinnow juice decreased. The invertase enzyme may be responsible for the degradation of acids into salt and sugar, hence decreasing acidity.[Citation38]
The findings revealed that the treatments and storage time significantly impacted the viscosity of Kinnow juice (). The viscosity was varied among the treatments. The viscosity of treated samples was reduced during three months of storage. The highest viscosity value was noticed in T0+ (0.574 m.Pas), and the lowest was in T3 (0.464 m.Pas) at 0 day. Over time, viscosity decreased from 0.574 to 0.143 m.Pas. After three months, the highest viscosity value was in T0+ (0.218 m.Pas) and the lowest in T3 (0.143 m.Pas). The findings are consistent with previous research, indicating a reduction in the viscosity of ozone-processed apple juice (1 to 4.8% w/w ozone) over a 12-min exposure period[Citation39] Jaramillo-Sánchez et al.[Citation24] found reductions in apparent viscosity of ozonized juices. Ozone exposure has been shown to reduce the molecular weight of dietary polysaccharides like pectins due to their potent oxidizing activity, which lowers viscosity.[Citation40]
Impact of ozone treatment on total plate counts (TPC) and mold counts (MC)
The findings revealed that the treatments and storage time significantly impacted the TPC of Kinnow juice (). The TPC were varied among the treatments. The TPC of treated samples were increased during three months of storage. The highest TPC value was noticed in T0- (1.75 CFU/mL) and the minimum TPC value in T3 (1.59 CFU/mL) at 0 day. After three months, the highest value of TPC was in T0- (2.05 CFU/mL) and the lowest in T3 (1.90 CFU/mL). The reduction of microbial populations in ozone-processed fresh-cut papaya fruits was noticed.[Citation41] The ozone technique reduced the microbial population in soy milk by 0.19, 0.35, and 0.57 Logs at 1, 3, and 5 min of treatment.[Citation42]
Figure 2. Effect of ozone treatments on total plate counts (TPC) (CFU/mL, Means ±SD) and mold counts (MC) (CFU/mL, Means ±SD) of Kinnow juice during storage at 35°C ± 2.
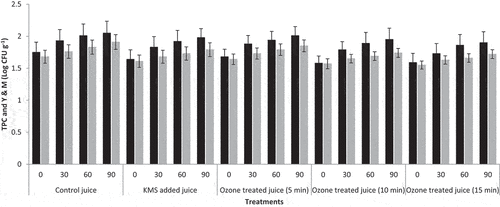
The findings revealed that the treatments and storage time significantly impacted the MC of Kinnow juice (). The MC was varied among the treatments. The MC of treated samples were increased during three months of storage. The highest MC value was perceived in T0- (1.55 CFU/mL) and the minimum MC value in T3 (1.68 CFU/mL) at 0 day. After three months, the highest value of MC was in T0- (1.72 CFU/mL) and the lowest in T3 (1.91CFU/mL). The study conducted by Panigrahi et al.[Citation22] stated that an increase in ozone processing time resulted in a significant decrease in MC in sugar cane juice. The mold and yeast count in filtered and non-filtered ozonated watermelon juice was reduced after 25 min. The findings demonstrated that the processing duration of ozone and the concentration of solids in watermelon juice affected the efficacy of ozone against yeast and mold.[Citation36]
Sensory analyses
The findings about sensory characteristics are presented in . After three months of storage, the sensory evaluation score indicated that treatment T0+ was highly acceptable, as determined by the sensory panel. The highest color score was observed in T0+ (7.65), and the lowest was in T3 (7.00) at 0 day (). During storage, the treatments exhibited a gradual decrease in color. During storage, the color score of Kinnow juice ranged from 7.65 to 2.75. The change in color might be due to changes in volatile compounds. Bhardwaj and Nandal[Citation38] found that the color of Kinnow juice blend was significantly decreased during storage time at ambient and refrigerated temperatures with related present findings. Rajashri et al.[Citation43] reported similar trends. The reduction of color may be attributed to the breakdown of pigment and copolymerization.
Figure 3. Effect of ozone treatments (T0-= Control juice, T0+ = Juice with KMS preservative, T1 = 5 min ozone treatment, T2 = 10 min ozone treatment, T3 = 15 min ozone treatment) on sensory scores (Means ±SD, color (a), taste (b), texture (c), of Kinnow juice during storage at 35°C ± 2.
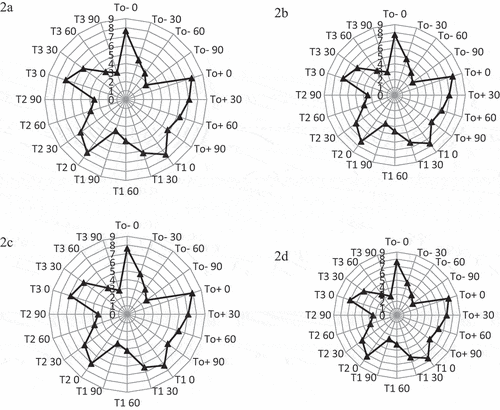
The findings revealed that the treatments and storage time significantly impacted the taste of Kinnow juice (). The highest taste score was observed in T0+ (7.75), and the lowest was in T3 (6.90) at 0 day. During storage, the treatments exhibited a gradual decrease in taste. During storage, the taste score of Kinnow juice ranged from 7.75 to 2.80. The loss of flavor in juice may be the result of considerable fermentation. Panigrahi et al.[Citation22] found a slight change in the taste of ozone-processed sugar cane juice compared to the control, but non-significant results were observed. The taste of star fruit and sweet orange juices blended cordial was reduced with time.[Citation44] The taste may be reduced due to the breakdown of ascorbic acid. Similar trends were observed in Kinnow banana RTS during storage at ambient and refrigerated temperatures. The taste of Kinnow banana RTS was reduced during storage.[Citation45]
The findings revealed that the treatments and storage time significantly impacted the texture of Kinnow juice (). The highest texture score was observed in T0+ (7.90), and the lowest was in T3 (6.85) at 0 day. During storage, the treatments exhibited a gradual decrease in texture. During storage, the texture score of Kinnow juice ranged from 7.90 to 2.75. Similar trends were shown in Kinnow banana RTS during storage at ambient and refrigerated temperatures. The texture of Kinnow banana RTS was reduced after three months.[Citation45]
The findings revealed that the treatments and storage time significantly impacted the overall acceptance of Kinnow juice (). The highest overall acceptance score was observed in T0+ (7.75), and the lowest was in T3 (7.0) at 0 day. During storage, the treatments exhibited a gradual decrease in overall acceptance. The overall acceptability of star fruit and sweet orange juice blend fruit cordial was reduced with time.[Citation44] The reduction of overall acceptance might be attributed to the loss of appearance, uniformity, and flavor component of the juice. Similar trends were found in Kinnow banana RTS during storage at ambient and refrigerated temperatures. The overall acceptability of Kinnow banana RTS was reduced during storage by Kumar and Singh.[Citation45] The reduction of overall acceptability of aloe juice complemented Kinnow nectar was also found by Shubhra et al.[Citation46]
Impact of ozone treatment on phytochemicals (TP contents, TF contents), TAA and DPPH radical scavenging activity
The findings revealed that the treatments and storage time significantly impacted the TP content of Kinnow juice (). The TP contents were varied among the treatments. The TP contents of treated samples were reduced during three months of storage. The highest TP contents were noticed in T2 (489 mg GAE/mL), and the lowest were in T0- (450.33 mg GAE/mL) at 0 day. After three months, the highest values of TP contents were in T2 (337 mg GAE/mL) and the lowest in T0- (301 mg GAE/mL). A similar trend was observed by Shah et al..[Citation15] Alothman et al.[Citation47] found that the total phenols increased substantially when pineapple and banana were exposed to ozone for up to 20 min. Ozone exposure may have altered the cell wall, which may have allowed some related phenolic chemicals to be released from the cell wall, leading to a rise in phenolic contents. Numerous free radicals are produced alongside the auto-decomposition of ozone. Various chemical reactions may also significantly contribute to the rise of these phenolic compounds. These reactions may involve a direct interface between ozone and the target molecule or its intermediate radicals. Shah et al.[Citation48] observed that the total phenol of ozone – processed pummelo juice decreased during storage. It might be caused by the phenolic components performing their protective functions by scavenging ozone-generated free radicals, decreasing the polyphenol concentration of pummelo juice.
Figure 4. Effect of ozone treatments on phytochemicals (total phenolics (black bars, mg GAE/mL, Means ±SD) and flavonoids (gray bars, mg CE/mL, Means ±SD)), total antioxidant capacity (TAA, (white bars, µg AAE/mL, Means ±SD)) and DPPH radical scavenging activity (line, µM AAE/mL, Means ±SD)) of Kinnow juice during storage at 35°C ± 2.
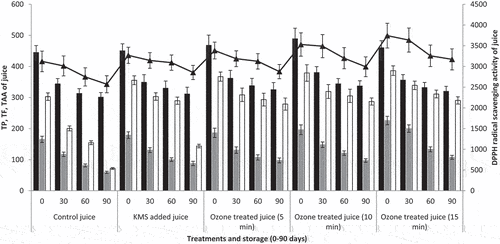
The findings revealed that the treatments and storage time significantly impacted the TF content of Kinnow juice (). The TF contents varied among the treatments. The TF contents of treated samples were reduced during three months of storage. The highest TF contents were observed in T3 (225.93 mg CE/mL), and the lowest were in T0- (165.43 mg CE/mL) at 0 day. After three months, the highest value of TF contents was in T3 (107.4 mg CE/mL) and the lowest in T0- (59.26 mg CE/mL). During storage, the TF concentration in ozone-processed pineapple and tender coconut water were decreased.[Citation43,Citation47] The TF contents may be caused by the hyperresponsiveness of ozone, which prevents numerous enzymes responsible for the oxidation of TF content, such as polyphenol oxidase and peroxidase.
The findings revealed that the treatments and storage time significantly impacted the TAA of Kinnow juice (). The TAA varied among the treatments. The TAA of treated samples was reduced during three months of storage. The highest TAA value was noticed in T3 (386.42 µg AAE/mL) and the minimum TAA value in T0- (302.81 µg AAE/mL) at 0 day. The antioxidant capacity of freshly sliced kiwifruit was initially improved by ozone processing for 10 min, which may be associated with the proliferation of antioxidants.[Citation49] Ozone processing raised AA, TP, and TF contents, thereby increasing TAA. Ozone lowered polyphenol oxidases, which caused enzymatic browning and increased TAA. After three months, the highest value of TAA was in T3 (290.63 µg AAE/mL) and the lowest in T0- (71.71 µg AAE/mL). After 10 to 20 min of exposure to ozone, the formation of lipoxygenase may contribute to an increase in TAA.[Citation41,Citation47] Al It was also observed that during the storage period, the TAA of ozone-processed raspberries decreased.[Citation50]
The findings revealed that the treatments and storage time significantly impacted the DDPH radical scavenging activity of Kinnow juice (). The DDPH radical scavenging activity was varied among the treatments. The DDPH radical scavenging activity of treated samples was reduced during three months of storage. The highest DDPH activity value was noticed in T3 (3744.2 µM AAE/mL) and the minimum DDPH activity value in T0- (3122.1 µM AAE/mL) at 0 day. Over time, DDPH activity reduced from 3744.2 to 2570.6 µM Trolox/mL. After three months, the highest value of DDPH activity was in T3 (3171.2 µM AAE/mL) and the lowest in T0- (2570.6 µM AAE/mL). Increased DPPH activity after ozone exposure may have produced antioxidant enzymes like superoxide dismutase and phenylalanine ammonia-lyase, which remove harmful reactive oxygen species.[Citation51]
Conclusion
The results of this study indicated that ozone processing resulted in Kinnow juice of superior quality, exhibiting exceptional stability in terms of both bioactive components and physicochemical attributes throughout the storage period. The ozone processing moderately increased the total phenols, flavonoids, acidity, DDPH activity, total soluble solid contents, and antioxidant activity of Kinnow juice. T3 was the most effective treatment in increasing the bioactive components, physicochemical characteristics, and antioxidant capacity while lowering microbial loads within the permitted range.
Thus, ozone processing was a simple, cost-effective alternative to existing techniques for preserving product quality parameters and prolonging their shelf life.
Author contributions
A.I, M.N and A.A performed the methods and investigation. T.M.Q, W.K and F.M conceptualization, funding acquisition and writing of original draft. S.R, I.A.M.A and M.Z.K helped in writing of this manuscript. While A.R, A.N and M.T.N helped in software, MZK and W.K supported in analysis and supervision of research work, I. A. M. A. validation, visualization, funding acquisition, and writing – review and editing. All authors have read and agreed to the published version of the manuscript.
Acknowledgments
The authors are highly obliged to the University of Sargodha (UOS), and IT Department, Higher Education Commission (HEC, Islamabad) for access to journals, books and valuable database.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Obasi, B. C.; Whong, C. M. Z.; Ameh, J. B. Nutritional and Sensory Qualities of Commercially and Laboratory Prepared Orange Juice. Afr. J. Food Sci. 2017, 11(7), 189–199. DOI: 10.5897/ajfs2015.1371.
- Purewal, S. S.; Sandhu, K. S. Nutritional Profile and Health Benefits of Kinnow: An Updated Review. Int. J. Fruit Sci. 2020, 20, S1385–S1405. DOI: 10.1080/15538362.2020.1792390.
- Suri, S.; Singh, A.; Nema, P. K. Current Applications of Citrus Fruit Processing Waste: A Scientific Outlook. Appli. Food Res. 2022, 2(1), 100050. DOI: 10.1016/j.afres.2022.100050.
- FAOSTAT. Food and Agriculture Organization of the United Nations 2022. http://www.fao.org/faostat/en/#data (accessed March 21, 2022).
- Nawaz, R.; Abbasi, N. A.; Hafiz, I. A.; Khalid, A.; Ahmad, T. Economic Analysis of Citrus (Kinnow Mandarin) During On-Year and Off-Year in the Punjab Province, Pak. J. Of Horti. 2018, 4, 05. DOI: 10.4172/2376-0354.1000250.
- Altaf, S.; Khan, M. M.; Jaskani, M. J.; Khan, I. A.; Usman, M.; Sadia, B.; Khan, A. I. Morphogenetic Characterization of Seeded and Seedless Varieties of Kinnow Mandarin (‘Citrus reticulata’Blanco). Aust. J. Crop Sci. 2014, 8(11), 1542–1549.
- Ilame, S. A.; Singh, S. V. Physico-Chemical Properties of Ultrafiltered Kinnow (Mandarin) Fruit Juice. J. Food Sci. Tech. 2018, 55(6), 2189–2196. DOI: 10.1007/s13197-018-3136-8.
- Hashimi, S. M.; Ayub, M.; Durrani, Y. Quality Evaluation of Whole Mandarin (Citrus Reticulata) Juice During Storage. J. Int. Acad. Res. Multidiscip. 2021, 11(4), 1–8.
- Singh, S. K. Characterization of Kinnow Mandarin Fruit Juice Stored Under Incubator. Ann. Bio. 2018, 34(2), 126–129.
- Musara, C.; Aladejana, E. B.; Mudyiwa, S. M. Review of the Nutritional Composition, Medicinal, Phytochemical and Pharmacological Properties of Citrus Reticulata Blanco (Rutaceae). F1000research. 2020, 9(1387), 1387. DOI: 10.12688/f1000research.27208.1.
- USDA. 2019. USDA National Nutrient Database for Standard Reference. United States Department of Agriculture. http://www.ars.usda.gov/services/ (Data accessed Dec 15 2022).
- Barros, H. R. D. M.; Ferreira, T. A. P. D. C.; Genovese, M. I. Antioxidant Capacity and Mineral Content of Pulp and Peel from Commercial Cultivars of Citrus from Brazil. Food Chem. 2012, 134(4), 1892–1898. DOI: 10.1016/j.foodchem.2012.03.090.
- Codoñer-Franch, P.; Valls-Bellés, V. Citrus as Functional Foods. Curr. Top. Nutraceutical Res. 2010, 8, 173–184.
- Iqbal, A.; Nadeem, M.; Ainee, A.; Ameer, K.; Ather Nadeem, M.; Sultan, M.; Malik, F.; Siddeeg, A. The Impact of Ozonation on the Physicochemical Properties, Antioxidant Potential and Shelf Life of Kinnow (Citrus Reticulata Blanco) Juice. Int. J. Food. Prop. 2022, 25(1), 2551–2560. DOI: 10.1080/10942912.2022.2148165.
- Shah, N. N. A. K.; Sulaiman, A.; Sidek, N. S. M.; Supian, N. A. M. Quality Assessment of Ozone-Treated Citrus Fruit Juices. Int. Food Res. J. 2019, 26(5), 1405–1415.
- Malik, F.; Nadeem, M.; Ainee, A.; Kanwal, R.; Sultan, M.; Iqbal, A.; Mahmoud, S. F.; Alshehry, G. A.; Al-Jumayi, H. A.; Hassan, E., et al. Quality Evaluation of Lemon Cordial Stored at Different Times with Microwave Heating (Pasteurization). Nat. Prod. Res. 2022, 4, 1–12. DOI: 10.1080/14786419.2022.2092864.
- Nadeem, M.; Ranjha, M. M. A. N.; Ameer, K.; Ainee, A.; Yasmin, Z.; Javaria, S.; Teferra, T. F. Effect of Sonication on the Functional Properties of Different Citrus Fruit Juices. Int. J. Fruit Sci. 2022, 22(1), 568–580. DOI: 10.1080/15538362.2022.2079584.
- Porto, E.; Alves Filho, E. G.; Silva, L. M. A.; Fonteles, T. V.; Do Nascimento, R. B. R.; Fernandes, F. A. N.; de Brito, E. S.; Rodrigues, S. Ozone and Plasma Processing Effect on Green Coconut Water. Food. Res. Int. 2020, 131, 109000. DOI: 10.1016/j.foodres.2020.109000.
- Fundo, J. F.; Miller, F. A.; Tremarin, A.; Garcia, E.; Brandão, T. R. S.; Silva, C. L. M. Quality Assessment of Cantaloupe Melon Juice Under Ozone Processing. Innov. Food Sci. Emerg. Technol. 2018, 47, 461–466. DOI: 10.1016/j.ifset.2018.04.016.
- Glowacz, M.; Colgan, R.; Rees, D. The Use of Ozone to Extend the Shelf-Life and Maintain Quality of Fresh Produce. J. Sci. Food Agric. 2014, 95(4), 662–671. DOI: 10.1002/jsfa.6776.
- Tzortzakis, N.; Chrysargyris, A. Postharvest Ozone Application for the Preservation of Fruits and Vegetables. Food Rev. Int. 2016, 33(3), 270–315. DOI: 10.1080/87559129.2016.1175015.
- Panigrahi, C.; Mishra, H. N.; De, S. Effect of Ozonation Parameters on Nutritional and Microbiological Quality of Sugarcane Juice. J. Food Process. Eng. 2020, 43(11), e13542. DOI: 10.1111/jfpe.13542.
- AOAC. Official Methods of Analysis of AOAC International. Association Of The Official Analytical Chemists, Gaithersburg 20th. 2016, 210–219.
- Jaramillo-Sánchez, G. M.; Garcia Loredo, A. B.; Gómez, P. L.; Alzamora, S. M. Ozone Processing of Peach Juice: Impact on Physicochemical Parameters, Color, and Viscosity. Ozone Sci. Eng. 2018, 40(4), 305–312. DOI: 10.1080/01919512.2017.1417111.
- Navida, M.; Nadeem, M.; Qureshi, T. M.; Pashameah, R. A.; Malik, F.; Iqbal, A.; Sultan, M.; Abo-Dief, H. M.; Alanazi, A. K. The Synergistic Effects of Sonication and Microwave Processing on the Physicochemical Properties and Phytochemicals of Watermelon (Citrullus Lanatus) Juice. Agriculture. 2022, 12(9), 1434. DOI: 10.3390/agriculture12091434.
- Prieto, P.; Pineda, M.; Aguilar, M. Spectrophotometric Quantitation of Antioxidant Capacity Through the Formation of a Phosphomolybdenum Complex: Specific Application to the Determination of Vitamin E. Anal. Biochem. 1999, 269(2), 337–341. DOI: 10.1006/abio.1999.4019.
- Yi, Z.; Yu, Y.; Liang, Y.; Zeng, B. In vitro Antioxidant and Antimicrobial Activities of the Extract of Pericarpium Citri Reticulatae of a New Citrus Cultivar and Its Main Flavonoids. LWT - Food Sci. & Tech. 2008, 41(4), 597–603. DOI: 10.1016/j.lwt.2007.04.008.
- FDA. FDA Center for Food Safety and Applied Nutrition. In U.S. Food and Drug Administration Bacteriological Analytical Manual; 8th ed.; U.S. 2001; Vol. 2.
- Land, D. G.; Shepherd, R. Scaling and Ranking Methods. In In Sensory Analysis of Foods, 2nd ed.; Elsevier Appl. Sci. 1988; pp. 155–185.
- Steel, R. G. D.; Torrie, J. H.; Dicky, D. A. Principles and Procedures of Statistics: A Biometrical Approaches, 3rd ed., McGraw Hill Book Co. Inc: Singapore, 1997; pp. 204–227.
- Buluc, O.; Koyuncu, M. A. Effects of Intermittent Ozone Treatment on Postharvest Quality and Storage Life of Pomegranate. Ozone Sci. Eng. 2020, 43(5), 1–9. DOI: 10.1080/01919512.2020.1816449.
- Aday, M. S.; Büyükcan, M. B.; Temizkan, R.; Caner, C. Role of Ozone Concentrations and Exposure Times in Extending Shelf Life of Strawberry. Ozone Sci. Eng. 2014, 36(1), 43–56. DOI: 10.1080/01919512.2013.833851.
- Onopiuk, A.; Półtorak, A.; Moczkowska, M.; Szpicer, A.; Wierzbicka, A. The Impact of Ozone on Health-Promoting, Microbiological, and Colour Properties ofRubus IdeausRaspberries. CyTa – J. Food. 2017, 15(4), 563–573. DOI: https://doi.org/10.1080/19476337.2017.1317669.
- Alencar, E. R.; Faroni, L. R.; Pinto, M. S.; da Costa, A. R.; Carvalho, A. F. Effectiveness of Ozone on Postharvest Conservation of Pear (Pyrus Communis L.). J. Food Process. Technol. 2014, 5(4). DOI: 10.4172/2157-7110.1000317.
- Barboni, T.; Cannac, M.; Chiaramonti, N. Effect of Cold Storage and Ozone Treatment on Physicochemical Parameters, Soluble Sugars and Organic Acids in Actinidia Deliciosa. Food Chem. 2010, 121(4), 946–951. DOI: 10.1016/j.foodchem.2010.01.024.
- Lee, B. J.; Ting, A. S. Y.; Thoo, Y. Y. Impact of Ozone Treatment on the Physico-Chemical Properties, Bioactive Compounds, Pectin Methylesterase Activity and Microbiological Properties of Watermelon Juice. J. Food Sci. & Tech. 2021, 59(3), 979–989. DOI: 10.1007/s13197-021-05102-8.
- Islam, M. A.; Ahmad, I.; Ahmed, S.; Sarker, A. Biochemical Composition and Shelf Life Study of Mixed Fruit Juice from Orange & Pineapple. J. Environ. Sci. Nat. Res. 2014, 7(1), 227–232. DOI: 10.3329/jesnr.v7i1.22175.
- Bhardwaj, R. L.; Nandal, U. Effect of Storage Temperature on Physico-Chemical and Sensory Evaluation of Kinnow Mandarin Juice Blends. J. Food Proc. & Tech. 2014, 05(8), 05. DOI: 10.4172/2157-7110.1000361.
- Torres, B.; Tiwari, B. K.; Patras, A.; Wijngaard, H. H.; Brunton, N.; Cullen, P. J.; O’Donnell, C. P. Effect of Ozone Processing on the Colour, Rheological Properties and Phenolic Content of Apple Juice. Food Chem. 2011, 124, 721–726. DOI: 10.1016/j.foodchem.2010.06.050.
- Mapalagama, O. V.; Amunugoda, P. A.; Ranaweera, K. K. D. S. Decontamination of Pineapple (Ananas Comosus) Juice Using Ozone as a Non-Thermal Sterilization Method. Future Food: J. Food Agric. Soc. 2021, 9(5), 15–26.
- Yeoh, W. K.; Ali, A.; Forney, C. F. Effects of Ozone on Major Antioxidants and Microbial Populations of Fresh-Cut Papaya. Postharvest. Biol. Technol. 2014, 89, 56–58. DOI: 10.1016/j.postharvbio.2013.11.006.
- Anam, C.; Perwitasari, A. A.; Jannah, H. N.; Yaqin, N.; Yulianto, E.; Sasmita, E.; Restiwijaya, M.; Kinandana, A. W.; Aryanto, F.; Nur, M. Effect of Ozone Treatment on Microbiological and Physicochemical Properties of Soymilk Beverage. IOP Conference Series: Earth and Environmental Science, 2020, 443, 012100. DOI: 10.1088/1755-1315/443/1/012100.
- Rajashri, K.; Roopa, B. S.; Negi, P. S.; Rastogi, N. K. Effect of Ozone and Ultrasound Treatments on Polyphenol Content, Browning Enzyme Activities, and Shelf Life of Tender Coconut Water. J. Food Process Preserv. 2020, 44(3), e14363. DOI: 10.1111/jfpp.14363.
- Kesavanath, J.; Premakumar, K.; Inthujaa, Y. Nutritional and Sensory Characteristics of Star Fruit and Sweet Orange Juices Blend Fruit Cordial. Int. J. Multi. Stud. 2015, 2(2), 42. DOI: 10.4038/ijms.v2i2.72.
- Kumar, V.; Singh, S. K. Quality and Evaluation of Banana and Kinnow Fruit Based RTS Beverage as Influenced by Blending Ratio and Their Storage Study at Different Conditions. Int. Agric. Eng. J. 2013, 6(2), 409–414.
- Shubhra, B.; Swati, K.; Singh, R. P.; Savita, S. Studies on Aloe Juice Supplemented Kinnow Nectar. Res. J. Agric. For. Sci. 2014, 2(8) , 14–20.
- Alothman, M.; Kaur, B.; Fazilah, A.; Bhat, R.; Karim, A. A. Ozone-Induced Changes of Antioxidant Capacity of Fresh-Cut Tropical Fruits. Innov. Food Sci. Emerg. Technol. 2010, 11(4), 666–671. DOI: 10.1016/j.ifset.2010.08.008.
- Shah, N. N. A. K.; Supian, N. A. M.; Hussein, N. A. Disinfectant of Pummelo (Citrus Grandis L. Osbeck) Fruit Juice Using Gaseous Ozone. J. Food Sci. Technol. 2018, 56(1), 262–272. DOI: 10.1007/s13197-018-3486-2.
- Wang, Y.; Li, Y.; Yang, S.; Wu, Z.; Shen, Y. Gaseous Ozone Treatment Prolongs the Shelf-Life of Fresh-Cut Kiwifruit by Maintaining Its Ascorbic Acid Content. LWT. 2022, 172, 114196. DOI: 10.1016/j.lwt.2022.114196.
- Piechowiak, T.; Antos, P.; Kosowski, P.; Skrobacz, K.; Józefczyk, R.; Balawejder, M. Impact of Ozonation Process on the Microbiological and Antioxidant Status of Raspberry (Rubus Ideaeus L.) Fruit During Storage at Room Temperature. Agri. Food Sci. 2019, 28(1), 35–44. DOI: 10.23986/afsci.70291.
- Supian, N. A. M.; Shah, N. N. A. K.; Shamsudin, R.; Sulaiman, A. Effects of Aqueous Ozone Treatment on the Nutritional Attributes of Mango (Mangifera Indica L.) Fruit Juice. Int. Food Res. J. 2022, 29(5), 1005–1019.