?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The pharmaceutical research sector’s inability to develop new drugs has made it challenging to combat microbial resistance. Secondary metabolites from plant extracts have been identified as potential protective agents against multi-drug-resistant bacteria. This study aimed to analyze hydro-ethanol extracts’ antioxidant and antimicrobial activities from various parts of the Zahidi date (whole fruit, pit powder, and flesh). Phytochemical screening of phenolic and flavonoid compounds was performed using high-performance liquid chromatography (HPLC). Total phenolic and flavonoid compounds were extracted at their maximum levels, 71.89 mg/100 g and 843.06 mg/100 g, respectively, using EoH 60%. The whole fruit had the highest concentration of flavonoid quercetin (0.83 μg/g) and phenolic compoundsinapic acid (20.03 μg/g) at the same concentration. The findings from the broth micro-dilution method showed that the whole fruit had the strongest antimicrobial effects at 60% EoHagainst Salmonella typhi (30 mg/ml), Helicobacter pylori (27 mg/ml), Escherichia coli (22 mg/ml), and Staphylococcus aureus (44 mg/ml). Zahidi whole fruit extracts showed higher antioxidant potential at EoH 60% using 2, 2-diphenyl-1-picrylhydrazyl (DPPH) scavenging potential (78%) and ferric-reducing antioxidant power. Principal component analysis (PCA) revealed a significant negative correlation between antioxidant activity and the minimum inhibitory concentration (MIC). Evidence from this study highlights the considerable antioxidant and antimicrobial properties of Zahidi date fruit, suggesting its potential use in producing natural drugs and value-added foods to counteract contagious diseases.
Introduction
Date palm (Phoenix dactylifera L.) has been a staple throughout the Arabic region for over 6,000 years[Citation1]. Nearly 30% of the world’s date production comes from the Arab region, with Egypt, Saudi Arabia, and Iran among the leading producers.[Citation2] Globally, the area under date production in 2021 was around 1,301,979 ha, with an estimated date yield of 9,656,377 tons.[Citation3] However, due to poor quality, around 30% of date fruits are unsuitable for eating or use as animal feed.[Citation4] The fruit of the date palm, known as “dates,“has tremendous nutritional, economic, and cultural value. Date flesh (pericarp) comprises almost 85–90% of the total weight of the date fruit.[Citation5] Date production and trading meet dietary needs and generate significant economic profits in many areas.
Dates are well-known for their high nutritional content and ability to provide fast energy.[Citation6] They are rich in carbohydrates, fatty acids, proteins, amino acids, vitamins, carotenoids, dietary fiber, and essential minerals.[Citation7,Citation8] Dates’ natural sugar content ranges from 34% to 86%. Additionally, they are a good source of minerals such as magnesium (6.4 mg/g), copper (240 μg/g), potassium (7.1 mg/g), and selenium (310 μg/g).[Citation9] Furthermore, dates contain many vitamins, such as B2, B3, B6, and B9, which account for about 9% of the recommended daily requirement. The protein content in date fruit varies between 2.9 g and 4.6 g per 100 g, while its fat content ranges from 0.47 g to 2.61 g per 100 g.[Citation10,Citation11]
Besides their high nutritional value, date fruits have been used in ethnobotanical practices to treat various diseases, including liver infection, diarrhea, constipation, respiratory problems, and headaches.[Citation12,Citation13] Numerous research studies have revealed that date fruits possess strong anti-inflammatory,[Citation10] antioxidant,[Citation11–13] antihyperlipidemic,[Citation14] antimutagenic,[Citation15] anticancer,[Citation16] antiviral,[Citation17] antifungal,[Citation18] gastroprotective,[Citation19] hepatoprotective,[Citation20] nephroprotective,[Citation21] anti-hemolytic,[Citation22] gonadotrophic,[Citation23] neuropharmacological,[Citation24] and analgesic[Citation25] properties.
Date fruit contains phytochemical substances such as polyphenols, anthocyanins, carotenoids, tannins, procyanidins, sterols, flavonols, flavones, anthocyanidins, isoflavones, phenolic acids, and cinnamic acid derivatives, which contribute to its health benefits.[Citation26] Therefore, researchers have been interested in examining the phytochemicals in date fruit to discover their diverse pharmacological and nutritional effects.[Citation27,Citation28] Dates contain natural antioxidant compounds like chlorogenic acid, benzoic acid, and quercetin, which have strong radical-scavenging abilities. These compounds efficiently inhibit the production of free radical species and protect cells against oxidative damage.[Citation29]
The antimicrobial properties of date palms have been widely studied in many parts of the world. Leave extracts of date palm,[Citation30,Citation31] fruit,[Citation32–35] seeds,[Citation36] bark,[Citation37] and spathe[Citation38] were screened for their antimicrobial potential against a range of gram-negative and gram-positive bacterial species. These species include Enterococcus faecalis, Escherichia coli, Klebsiella pneumonia, Salmonella enteritidis, Pseudomonas aeruginosa, Listeria monocytogenes, Staphylococcus aureus, Bacillus subtilis, Pseudomonas aeruginosa, and Lactobacillus brevis.[Citation39–42] However, most studies have focused on the antibacterial activities or nutritional significance of specific parts of the date palm. Limited studies have been conducted on the antimicrobial potential properties of the entire date fruit,including the flesh and the pit, utilizing various solvent fractions.
Pakistan grow over 150 date varieties, the most popular of which are Zahidi, Dhakki, Hallawi, Khudravi, Begum Jangi, and Aseel. Pakistan is the seventh-highest producer of date palms and grows around 150 varieties.[Citation43] Zahidi, in particular, is one of Pakistan’s most widely exported date varieties. It yields semi-dry, medium-sized dates with a light brown color and a sweet taste. Pakistan is the seventh-highest producer of date palms.[Citation43] The study aimed to investigate the phytochemical profile, antioxidant potential, and antibacterial activity of various hydro-ethanol extracts of Zahidi date parts (whole fruit, pit, and flesh). We hypothesized that (i) different parts of the Zahidi date (whole fruit, pit, nd flesh) exhibit variations in their bioactive component composition; (ii) the antioxidant potential of Zahidi date extracts varies among the different parts; and (iii) Zahidi date extracts possess potent antimicrobial potential against drug-resistant microbes.
Materials and methods
Date material and extraction method
All chemicals and solvents were obtained from Sigma-Aldrich Co., Ltd. (St. Louis, France). Zahidi dates were purchased from the local market in Sargodha, Pakistan. The date fruits were collected during the Tamar stage, representing the fully ripe stage. Samples were randomly selected without preference for size, shape, color, or appearance.The date pits were manually separated from the flesh and washed with double-distilled water. The flesh was stored separately in plastic bags and refrigerated at 4°C. The pits were dried in the shade for four days and then ground to powder in a mechanical grinder for further analysis.
Polyphenol extraction and chemical characterization of Zahidi date extracts
The extraction of polyphenols from the whole fruit, pit, and flesh parts of theZahidi date was conducted separately using hydro-ethanol solvents with various concentrations, such as EoH 0%, EoH 20%, EoH 40%, EoH 60%, EoH 80%, and EoH 100%. Extracts from each date part were prepared using ethanol and water. The extraction involved mixing 150 g of each part in 500 mL of the extraction solvent and blending it using an electric blender. After mixing, the mixture was transferred into a conical flask and covered with aluminum foil to prevent solvent evaporation. Flasks were placed in a shaking incubator (Lab Line Instruments, Inc., 26 Melrose Park, IL, USA) for 6 hours at 35°C for continuous stirring. The resulting mixture was filtered using Whatman No. 1 filter paper and concentrated on a rotary evaporator (Model Heidolph, Germany) at 3000 rpm at 30°C until only one-fourth of the original volume remained. Afterward, all the extracts were preserved in plastic bottles and stored at −18°C for various phytochemical and radical scavenging assays.
Phytochemical analysis of date extracts
Total phenolic contents
TPC (total phenolic content) of date extracts was estimated using the Folin-Ciocalteu method with some modifications.[Citation44] A standard curve was constructed by diluting 3, 6, 10, 15, and 20 mg/ml of gallic acid in ethanol. 100 µl of each dilution was added to 500 µl of water, followed by 100 µl of Folin-Ciocalteu reagent.The mixture was allowed to stand for 50 minutes. Then, 1.5 ml of sodium carbonate (60 g/L) was added to the reaction mixture. The resulting solution was kept in the dark for 1 hour at 40°C, and absorbance was measured at 765 nm using a UV-visible spectrophotometer (Model T70, PG, USA). The exact process was repeated for the sample extracts.TPC contents were expressed as mg/L of GAE (gallic acid equivalents).The experiment was conducted in triplicate.
Total flavonoid contents
TFC (total flavonoid content) of date parts was evaluated using Park’s method.[Citation45] Quercetin was used as a standard to calculate the TFC of sample extracts, measured as quercetin equivalents. A standard curve of rutin was constructed by making various concentrations(0.1, 0.5, 1.0, 2.5, and 5 mg/ml) in ethanol.100 µl of each dilution was added to 500 µl of water, followed by 100 µl of 5% sodium nitrate.The mixture was left to stand for 5 minutes. Afterward,150 ml of a10% aluminum chloride solution was added to the reaction mixture and allowed to stand for another 5 minutes. Subsequently, 200 µl of NaOH solution was added.After30 minutes of incubation, absorbance was measured at 510 nm using a UV spectrophotometer. The same procedure was repeated with sample extracts.TFC was calculated using rutin equivalents (mgQE/g). All tests were performed in triplicate.
Quantitative phytochemical screening by HPLC
The flavonoids and phenolics in Zahidi date extracts were quantified using HPLC, as described by Ferreres.[Citation46] The following phenolic and flavonoid compounds were identified using standard solutions: quercetin, p-coumaric acid (all Sigma-Aldrich, Merck, Darmstadt, Germany), caffeic acid, benzoic acid, m-coumaric acid, vanillic acid, chlorogenic acid, sinapic acid, ferulic acid, and cinnamic acid (Acros Organics, Geel, Belgium). The solutions were prepared by mixing the standards in HPLC grade methanol (ultragradient grade; Carlo Erba, Milan, Italy) to formulate the stock solution of 100 mg/L, which was further used to make 50, 40, 30, 20 and 10 mg/L solutions for standard plots. In addition, a solution containing 30 mg/L of each phenolic compound was prepared in methanol. Two ferulic and p-coumaric acid solutions were prepared:50 mg/L each and the other with 70 mg/L of ferulic acid and 30 mg/L of p-coumaric acid. The vanillic and caffeic acid mixtures were made with the same concentrations. The absorbance of the sample (100 mg/L) of each phenolic and flavonoid compound was measured with a UV-Vis spectrophotometer (UV-2600; Shimadzu, Tokyo, Japan) at wavelengths between 180 and 480 nm to observe the wavelength for the HPLC-DAD measurements.
The HPLC analysis of the phenolic compounds and flavonoids was conducted using a Waters 2695 Alliance HPLC system (Waters Inc., Milford, CT, USA) equipped with a UV-Vis DAD. Samples were separated using a Waters Sunfire (C-18) column with 25 cm × 4.6 mm × 5 μm dimensions. Two solvents were utilized: solvent A (H2O: acetic acid, 94:6, pH = 2.27) and solvent B, 100% acetonitrile (mobile phase A, HPLC grade ≥ 99.9%; Honeywell Seelze, Germany), serving as the mobile phase for chromatographic separation. The complete runtime of the method was 60 minutes and the concentration gradient varies as follows; (a)initially 5% A and 95% B, (b) 15 min 35% A and 65% B, (c) 20 min 35% A and 65% B, (d) 30 min 40% A and 60% B, (e) 35 min 40% A and 60% B, (f) 40 min 50% A and 50% B, (g) 52 min 70% A and 30% B and (h) 60 min 5% A and 95% B. The separated phenolic compounds were analyzed using a UV-visible detector at a wavelength (λmax) of 280 nm. Phenolic compounds were identified by comparing their UV-visible spectra and retention times with standards. Quantification was performed through external calibration, and the results were expressed as mg/100 g DW (dry weight).
Antioxidants activity of date extracts
2, 2-diphenyl-1-picrylhydrazyl (DPPH)
DPPH radical scavenging assay of date extracts was performed following the method of Brand-Williams.[Citation47] A standard solution of 0.002% DPPH is prepared in ethanol, and the absorbance was measured at 515 nm. After that,100 µl of the sample was mixed thoroughly with 3 ml of DPPH solution.The mixture was incubated for 30 minutes at 37°C, and the antioxidant activity was again measured at 515 nm. The DPPH scavenging activity was calculated using the following formula:
The antioxidant activity of sample extracts was measured using a standard calibration curve of Ascorbic acid at 20, 40, 60, 80 and 100 µg/ml.
Ferric reducing antioxidant power assay (FRAP)
The reducing power capacity of date extracts was determined using the method outlined by Oyaizu.[Citation48] The stock solution comprised 3.1 g of 0.2 M sodium phosphate buffer solution (pH 6.6), a 10 mM TPTZ solution in 40 mM HCl, and a 20 mM FeCl3·6 H2O solution. The fresh solution was prepared by mixing 1 ml of buffer, 2.5 ml of TPTZ solution, and 1 ml of 1% K3Fe(CN)6. In test tubes, 3 ml of FRAP reagent was added, followed by 300 μl of standard sample or blank. After 5 minutes of incubation, absorbance was measured at 700 nm. A standard curve was created using ascorbic acid, and the results were expressed as μmol AS/100 g dw.
Minimum inhibitory concentration (MIC)
The in-vitro study was conducted based on the phenolic and flavonoid compounds that were found in higher quantities in the preliminary phytochemical analysis of date extracts. Specifically, extracts of zahidi whole fruit (EoH 60%), pit (EoH 100%), and flesh (EoH 0%) parts were chosen due to their rich phytochemical profiles and potent antioxidant activities. The concentration of specific compounds identified in all date extracts using HPLC served as the standard for the minimum inhibitory concentration (MIC) against the tested microorganisms.
The laboratory strain of S. typhi (ATCC 2680), obtained from the American Type Culture Collection (ATCC), was utilized for sensitivity testing against various date extracts. The bacterial strain was preserved on nutrient agar plates at 4°C and subcultured on freshly prepared agar plates 24 hours before the experiment. Salmonella-Shigella agar was employed to activate the Salmonella strain. The activation and regular maintenance of the strain were performed using agar nutrient media.
Clinical isolates of H. pylori were obtained from the gastric mucosa of patients diagnosed with gastritis, peptic ulcer disorder, or gastric cancer. Biopsies were inoculated on agar plates containing 7% sterile sheep blood, amphotericin B (250 mg), Campylobacter supplements, as well as trimethoprim (5 mg/L), vancomycin (10 mg/L), and polymyxin B (2500 units/L). The plates were incubated at 37°C for 3–5 days in a microaerophilic environment (10% carbon dioxide, 5% oxygen). Subsequently, bacterial isolates were subjected to testing for their antimicrobial potential.
The broth microdilution method was employed to determine the Minimum Inhibitory Concentration (MIC) of date extracts against S. typhi and H. pylori, utilizing 96-well plates.[Citation44] The date extracts were diluted in various aliquots of 50 µl, 25 µl, 12.5 µl, 6.25 µl, 3.12 µl, 1.56 µl, 0.78 µl, 0.39 µl, 0.19 µl, and 0.097 µl, and the final volume was attained by adding Muller Hinton Broth. The bacterial suspension was adjusted to 1.5 × 108 CFU, corresponding to McFarland turbidity.[Citation49]
In each well of a 96-well nutrient plate (Oxoid) (Corning Costar Ltd., USA), 50 µl of different extracts concentrations and 50 µl of bacterial suspension were added. The plate was incubated at 37°C for 18 to 24 hours. Ciprofloxacin (30 µg) was also included in the same steps as the standard antibiotic. The MIC was determined to be the lowest concentration in the sample, which resulted in complete inhibition (no visible colonies) of the tested bacteria. All experiments were conducted in triplicate.
Statistical analysis
The normality of all experimental data was tested using Kolmogorov’s test. The results were presented as mean ± SE. A general linear model was applied to compare the effect of extracts on TPC, TFC, DPPH, and FRAP assays, and on the minimum inhibitory concentration (MIC) of various microbes. Principal component analysis (PCA) and Pearson’s correlation were calculated to evaluate the relationship between antioxidants and MIC of different parts of zahidi. Data analysis was performed using Statistics 8 and Past 4.11.
Results
Total phenolic contents
The results revealed a significant difference (p < .01) in total phenolic content (TPC) among the whole fruit, pit, and flesh parts, as well as the solvent fractions of the Zahidi date (; ). TPC was measured usingFolinCiocalteu’s method, which was calculated by constructing a gallic acid standard curve. The whole fruit EoH 60% solvent fraction exhibited the highest TPC in mg GAE/100 g 66.5(p < .01), while the pit part showed a higher quantity of TPC at EoH 100% fraction. The flesh part had a higher concentration of TPC in the aqueous fraction, whereas TPC in the flesh part decreased (p < .01) with an increase in ethanol concentration.
Figure 1. (a) Total phenolic content; (b) total flavonoid content; (c) free radical scavenging activity (DPPH) and (d) ferric reducing power (FRAP) of hydro-ethanol extracts of Zahidi date whole fruit, pit, and flesh parts.
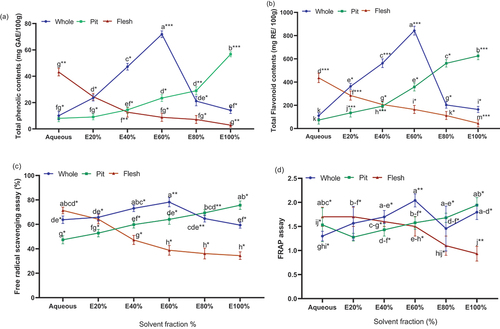
Table 1. General Linear Model (GLM) reveals zahidiextracts’effect on the TPC, TFC, DPPH and FRAP assay.
Total flavonoid contents
The results of the total flavonoid content (TFC) of the whole fruit, pit, and flesh parts, along with the solvent fractions of the zahidi date, are presented in and . The TFC was measured using an aluminum chloride colorimetric assay and determined by plotting arutin standard curve. Among the different date parts, the whole fruit had a significantly (p < .01) higher amount of TFC (mg RE/100 g 837)in the EoH 60% fraction, while the pit part contained a higher amount of TFC in the EoH 100% fraction. In the flesh part, the highest quantity of TFC was observed in the EoH 0% extract, and it was significantly lower (p < .01) in EoH 100% fractions.
2, 2-diphenyl-1-picrylhydrazyl (DPPH) free radical scavenging activity
The results of the DPPH scavenging activity are depicted in and . The significantly highest DPPH activity (79%)was recorded in the EoH 60% of zahidi date, whereas the lowest activity was observed in the different ethanol fractions of the zahidi date (p < .01). Among parts, whole fruit exhibited a higher amount of DPPH in EoH 60%, the pit part showed maximum DPPH concentration in EoH 100%, and the flesh part showed the highest level of DPPH in the EoH 0% ().
Ferric reducing antioxidant power (FRAP)
The results of the FRAP assay revealed that the whole fruit EoH 60% of Zahidi date exhibited the highest reducing activity compared to the pit EoH 100% and flesh EoH 0% extracts (; ; p < .01). Among the solvent fractions, theEoH 60% fraction of Zahidi date demonstrated the highest quantity of FRAP compared to other extracts (p < .01; ).
Antimicrobial activity of Zahidi extracts
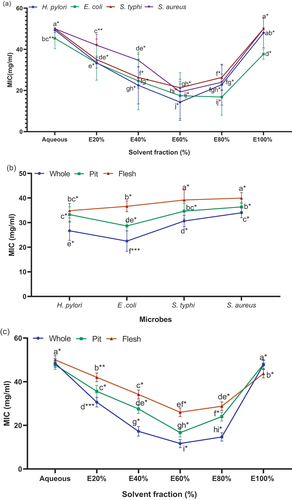
The minimum inhibitory concentration (MIC) results are depicted in . It was observed that S. aureus showed the highest MIC value, followed by S. typhi, E. coli, and H. pylori.The efficiency of the extracts decreased significantly when the ethanol concentrations increased above EoH 60% and decreased below EoH 60%.An increasing trend (p < .01) in extract antimicrobial efficiency was observed among the solvent fractions, particularly forEoH 60%.
Interaction between Zahidi parts and microbes
The results for the interaction between microbes and Zahididate parts on the MIC of the whole fruit, pit, and flesh extracts are presented in . Zahidi date extracts showed the highest MIC ofE. coli, while the lowest MIC (p < .01) was observed for S. aureus. Among different parts ofZahididates, whole fruit displayed a significantly higher MIC, followed by the pit and flesh parts (p < .01), against tested pathogens.
Interaction between Zahidi parts and solvent fractions
The results regarding the effect of different parts of Zahedi dates and solvent fractions on the minimum inhibitory concentration (MIC) of the whole fruit, pit, and flesh parts are presented in . Among various parts ofzahididates, whole fruit exhibited a significantly higher MIC against pathogens, while flesh parts showed significantly lower MIC (p < .01). In the case of solvent fractions, the EoH 60% fraction exhibited a significantly highest MIC value against the tested pathogens, while flesh EoH 100% showed the lowest MIC values (p < .01).
HPLC of polyphenolic compounds in Zahidi whole fruit, pit and flesh parts
HPLC was used to identify and quantify the phenolic and flavonoid compounds in zahidi’s whole fruit, pit, and flesh parts. The results showed that the maximum amounts of phenolics and flavonoids were present in the whole fruit, followed by the pit and flesh parts (). The main components identified in the phenolic and flavonoid analysis of whole fruit extracts were quercetin, benzoic acid, m-coumaric acid, sinapic acid, and p-coumaric acid. Quercetin, caffeic acid, benzoic acid, m-coumaric acid, and p-coumaric acid were the main components in pit extracts. In the flesh extracts, the prominent components were quercetin, benzoic acid, and m-coumaric acid. These findings indicate considerable variations in the phenolic and flavonoid compounds among different parts of zahidi’sextracts. Among the solvent fractions, the EoH 60% extract showed the highest amounts of quercetin (0.83 μg/g), benzoic acid (19.32 μg/g), m-coumaric acid (1.43 μg/g), sinapic acid (20.03 μg/g), and p-coumaric acid (0.86 μg/g). The EoH 100% extracts of the pit part had the highest concentrations of quercetin (0.76 μg/g), caffeic acid (2.25 μg/g), benzoic acid (12.9 μg/g), m-coumaric acid (0.86 μg/g), and p-coumaric acid (1.57 μg/g), as shown in . Furthermore, the interaction of solvent fraction and zahidi parts showed that the flesh parts had a higher quantity of phenolic and flavonoid compounds in EoH 0%, with quercetin (0.7 μg/g), benzoic acid (14.9 μg/g), and m-coumaric acid (4.23 μg/g) being the most prominent. In summary, the whole fruit had the highest amounts of phenolic and flavonoid compounds in EoH 60% extracts, the pit had the highest concentration in EoH 100% extracts, and the flesh part exhibited the highest quantities in EoH 0% extracts.
Table 2. HPLC analysis of phenolic compounds of Zahidi date whole fruit, pit and flesh.
Relationship among MIC, antioxidant activity, and various hydro-ethanolic extracts of Zahidi parts
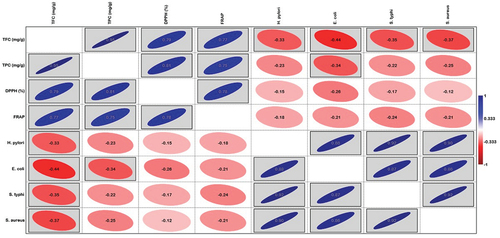
Principal Component Analysis (PCA) was applied to explore the correlation between MIC, antioxidant activity, and various hydro-ethanolic extracts of zahidi parts. The PCA bi-plot illustrates the relationship between microbes and antioxidant activity (). PC 1 (57.41%) and PC 2 (31.79%) account for 89.20% of the total variation. On PC 1, there is a clear separation between microbes such as H. pylori, E. coli, S. typhi, and S. aureus, as well as phytochemical compounds including flavonoids, phenolics, and antioxidant activities DPPH and FRAP. Additionally, all microbes demonstrated similar responses concerning antioxidant activity.
Figure 3. Principle component analysis (PCA) bi-plot showing the relationship between microbes, antioxidant activity and aqueous and ethanolic extracts of Zahidi date parts (b). (Symbols: Blue –Whole fruit, Red- Pit, Green-Flesh). (●EoH 0%, ■ EoH 20%, ▲EoH 40%, ♦EoH 60%, ×EoH 80%, *EoH 100%).
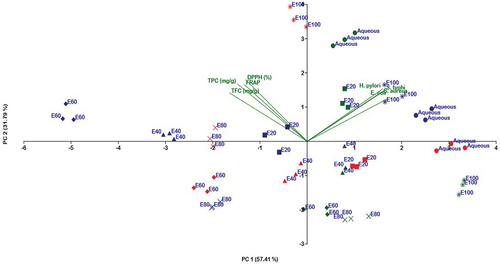
Various solvent fractions of whole fruit extracts were closely clustered with microbes, while most flesh extracts were positioned farther from phenolics, flavonoids, DPPH, and FRAP. Specifically, flesh extracts such as EoH 100%, EoH 80%, EoH 60%, and EoH 40% negatively correlation with the whole fruit extracts along PC2. On this axis, EoH 0% and EoH 20% of flesh, and EoH 100% of the pit were strongly correlated with each other and showed a positive correlation with the whole fruit extracts.
Pearson’s correlation coefficient also showed a significant correlation among phytochemical compounds, antioxidant potential, and MIC. The correlation analysis confirmed a significant positive correlation between phytochemicals and antioxidant activities and a significant negative correlation with bacterial MIC ().
Discussion
The Gallic acid equivalent was used to calculate total phenolic content due to its acidic stability and excellent sensitivity to the Folin-Ciocalteu reagent. In the present study, a higher concentration of TPC was found in the EoH 60% whole fruit extract of Zahidi’s date compared to the pit and flesh parts. This difference can be attributed to the composite nature of the whole fruit of zahidi’s date, which includes both hydrophobic phytochemicals from the pit part and hydrophilic phytochemicals from the flesh part.[Citation33] The hydrophobic phytochemicals are more soluble in organic solvents, whereas hydrophilic phytochemicals are more soluble in aqueous solvents.[Citation33] A recent study evaluated the TPC of four date cultivars by employing aqueous-organic solvent fractions and reported TPC values ranging from 32.24 to 35.84 mg CAE/100 g, consistent with the current study’s results.[Citation16] The combination of organic-aqueous solvent fractions makes an excellent medium for the maximum extraction of phytochemicals from plant materials because of its high ability to extract phenolics from protein matrix as they efficiently dissolve protein-phenolic complexes.[Citation17] The current findings regarding the pit part of zahidi’s date are consistent with previous research, which indicated that ethanol is the most effective solvent for extracting phenolics from date pits.[Citation18] Another study also reported higher levels of phenolics in the organic extracts of ajwa and zahidi pits.[Citation43] More recently, a study revealed the utilization of organic solvents to determine TPC (11.11 g GAE/100 g) in Egyptian date pits.[Citation35] This observation can be attributed to the significant presence of aromatic hydrophobic phytochemicals in the date pits, which are more soluble in organic solvents.[Citation33] Furthermore, the flesh part of zahidi dates displayed a higher TPC when dissolved in aqueous solvents due to high levels of aliphatic hydrophilic phytochemicals.[Citation33] These findings align with a previous study that reported higher TPC values (14.8 mg/g GAE) in date flesh when using aqueous solvents compared to organic solvents (10.31 mg/g GAE).[Citation35] Additionally, other studies have also demonstrated that aqueous solvents extract a greater quantity of phenolic compounds from date flesh compared to ethanol.[Citation50]
Flavonoids are well-known for their antioxidant and anti-inflammatory properties.[Citation51] In the present study, we observed the highest total flavonoid content (TFC) concentration in the whole fruit of the date when extracted using 60% ethanol.The stability was observed at an ethanol concentration of 60%, resulting in the maximum extraction of flavonoids. A researcher found that the flavonoid content in ajwa dates, as determined through ethanol extracts, ranged from 38.63 to 57.07 mg RE/100 g DW.[Citation52] However, the range of TFC observed in our study aligns with previous research, which demonstrated that the extraction of TFC from date seed extracts using various aqueous-organic solvent concentrations yielded a maximum value of 455.77 mg CEQ/100 g.[Citation53] In multiple studies, flavonoids have been reported to have a potent relationship with plant-based extracts’ antioxidant activity.[Citation54] Consistently, a previous study reported the highest TFC value in the ethanol extract of the date pit part from ten different date varieties using organic solvents.[Citation54] Similarly, another study showed that the zahidi pit had the highest TFC value (423.76 μg/mL RE) in pure organic extracts.[Citation43] Flavonoids and phenolic acids are natural antioxidants found in a wide variety of plant products.[Citation55] The quantity of phytochemicals found in different plants is greatly influenced by factors such as growing conditions, extraction methods, and the chemical structure of bioactive compounds.[Citation52]
In the current study, zahidi date extracts demonstrated the presence of quercetin in all parts, with the highest concentration observed in the whole fruit EoH 60% (0.83 ± 0.12 ppm), followed by the EoH 100% pit (0.76 ± 0.14 ppm) and EoH 0% flesh (0.7 ± 0.15 ppm) parts. In a previous study, a researcher utilized an aqueous-organic solvent combination to identify phenolic compounds and identified p-coumaric acid in the pits of four date cultivars.[Citation56] Phenolic compounds, including benzoic acid, caffeic acid, coumaric acid, and flavonoid compounds, such as quercetin, were documented in the pits of ten Saudi date varieties.[Citation57] Similarly, another study reported the presence of caffeic acid, benzoic acid, p-coumaric acid, and m-coumaric acid phenolic acids in pit extracts of four date palm cultivars, and these compounds were also identified in the pit extracts of the present study.[Citation58] Another study demonstrated the presence of phenolic compounds, including benzoic acid, p-coumaric acid, m-coumaric acid, and quercetin, in the flesh of twelve Saudi date cultivars using an aqueous solvent.[Citation59]
Reactive oxygen species (ROS) are unstable free radicals generated by normal metabolic processes or external factors that can cause harm to essential cellular components, including proteins, lipids, and DNA, leading to cancer, inflammation, and other degenerative diseases.[Citation60] The DPPH method is a very convenient and sensitive technique for evaluating the antioxidant potential of natural products.[Citation52] Among the different parts, the whole fruit EoH 60% extracts of zahidi displayed the highest antioxidant activity due to a high concentration of phytochemicals. Previous studies have identified that the elevated antioxidant activities observed in date extracts could be attributed to their reducing ability, as they contain antioxidant compounds capable of donating electrons, thereby reducing free radicals generated from lipid peroxidation.[Citation61] Various date palm varieties from Egypt, Morocco, Algeria, and Oman have also demonstrated strong antioxidant potential in both in vitro and in vivo studies.[Citation9,Citation14,Citation16,Citation35] A previous study reported high DPPH inhibition percentages in zahidi pit extracts using organic solvents.[Citation39,Citation43] In the current study, flesh EoH 0% extracts demonstrated the highest inhibition percentage, which aligns with previous studies that reported maximum antioxidant activity of Zahidi flesh using aqueous solvents.[Citation43,Citation55] It has been shown that the hydroxyl group of phenolic compounds is responsible for antioxidant activity, which donates protons to free radical species and scavenging them.[Citation52]
Furthermore, the scavenging capacity of phytochemicals in the extract depends not only on their concentrations but also on the nature and composition of the extraction solvent.[Citation61] The results revealed that the extracts with the highest reducing power exhibited significantly enhanced antioxidant activity in ethanol.[Citation62] Moreover, a previous study investigated the antioxidant activity (FRAP) of seeds of 14 date varieties, including zahidi, using various organic solvents and found higher antioxidant activity in zahidi seed organic extract than in other varieties.[Citation63] In another research, hydro-alcoholic extracts of the flesh from Kabkab and zahidi dates were studied, and it was found that zahidi flesh extracts exhibited higher antioxidant activity (FRAP) compared to Kabkab flesh extracts.[Citation64]
Hydro-ethanol extracts of Zahidi whole fruit, pit, and flesh parts exhibited the highest antibacterial activity against H. pylori, while the lowest activity was observed against S. aureus. The antibacterial results revealed that the whole fruit extract of zahidi date exhibited the lowest MIC value (p < .01), showing higher efficiency against H. pylori, E. coli, S. typhi, and S. aureus. Contrarily, the pit and flesh extracts demonstrated higher MIC values against these pathogenic bacteria. A lower MIC value suggests a higher level of effectiveness against pathogenic bacteria. The variations in MIC values observed against the tested pathogenic strains in the present study can be attributed to differences in extraction methods, size of the inoculum, duration of incubation, and the range of solvent concentrations used.[Citation40,Citation41] The lower antimicrobial potential of the extracts could be due to variations in the extraction solvent employed, the presence of unreleased or bound phenols in the extract matrices and the limited ability of the extracted compounds to diffuse into the antibacterial assay medium.[Citation42] Previous studies have shown that date fruit, epicarp, and seed extracts have effective antibacterial potential against both Gram-negative bacteria (E. coli) and Gram-positive bacteria (B. cereus).[Citation39] Similarly, a study found that the most resistant Gram-negative bacteria were S. dysenteriae, S. Typhi, and K. pneumonia.In contrast, the most resistant Gram-positive bacteria wereS. aureus against the antibacterial activity of date palm extracts.[Citation65]
Previous research has shown that hydro-ethanol is an excellent solvent for extracting the maximum amount of antibacterial chemicals from medicinal plants.[Citation66] The present study showed that S. aureus displayed high sensitivity to zahidi flesh and pit parts, followed by E. coli, H. pylori, and S. typhi. On the other hand, H. pylori demonstrated high sensitivity to the whole extract. S. aureus was inhibited at the lowest concentration of flesh extract, while the highest concentration of the whole extract inhibited its growth. In contrast, a high concentration of extracts was needed to inhibit the growth of S. typhi, possibly due to variations in their sensitivity to antibacterial agents. The absolute ethanol extracts of date pits also exhibited cellular destruction, as observed in various extracts with antimicrobial activity.[Citation67] The antibacterial effect of date pit EoH 100% extracts may be attributed to their ability to induce blebbing action on the bacteria cell walls, resulting in the leakage of cellular contents.[Citation68] A previous study investigating the antibacterial activity of organic date seed extracts against E. coli also demonstrated a similar trend attributed to bioactive components.[Citation40] In the current research, antimicrobial activity can be explained by the ability of phytochemicals to bind to extracellular proteins on the microbial cell wall, thereby inhibiting microbial growth and potentially causing oxidative damage.[Citation69]
The current finding was further supported by a study that investigating the antibacterial activity of phytochemical compounds extracted from various parts of the date fruit.[Citation70] The study revealed that these compounds demonstrated potent antibacterial activity against bacteria through mechanisms such as cell wall disruption and cell protoplasm deposition, ultimately resulting in bacterial death. When exposed to phytochemicals in date extracts for 24 hours, infectious bacteria such as E. coli exhibited DNA fragmentation, which was attributed to the presence of glycosides and flavonoids.[Citation71] The current research also observed that the maximum inhibition of date extracts was observed against the Gram-positive bacteria B. subtilis,a larger inhibition zone thanGram-negative pathogens. These findings are consistent with the previous study that reported higher antimicrobial activity of date seed extracts against Gram-positive S. aureusthan Gram-negative E. coli.[Citation72]
The current study suggests that zahidi date fruit may possess advantages over other conventional methods of antibacterial treatment. This is because date fruit contains beta-glucans, which have multiple health benefits, including boosting the immune system, increasing macrophage activity, and strengthening the body’s natural defenses against cell damage.Two forms of pectin from conventional sources exist: low methoxyl (LM) and high methoxyl (LM) pectin, which make non-uniform jams and jellies. Date pit powder is an excellent source of natural pectin, which has minimal variation and high stability, and could be used as a natural source of dietary fiber.
Conclusion
The levels of total phenols, flavonoids, antioxidant activity, and antimicrobial potential were assessed for the first time in hydro-ethanol extracts of zahidi’s whole fruit, pit, and flesh parts. The whole fruit, at a 60% EOH concentration, contained higher quantities of TPC and TFC and exhibited strong antioxidant activity. Furthermore, zahidi extracts contain highly efficient antibacterial bioactive components against multi-drug-resistant pathogenic bacteria, indicating their potential use in treating a variety of infectious disorders. Zahidi dates have been identified as possessing promising antioxidant properties, which may allow them to be used as food supplements to delay lipid oxidation and treat various ailments by scavenging free radicals. Further research is needed to establish the antioxidant and antimicrobial effectiveness of the compounds found in zahidi dates.
Author Contributions
Conceptualization, S.K., F.S. and M.A.; methodology, S.K. and F.S.; software, S.K and N.A.; validation, S.K, F.S. and M.A.; formal analysis, M.Z.K., W.K and S.Y.A.; investigation, S.K., and F.S; resources, F.S. and M.A. data curation, S.K., and F.S; writing – original draft preparation, S.K., and F.S.; writing – review and editing, S.K., F.S. and S. Y. A.; visualization, F.S., and M.A.; supervision, F.S. All authors have read and agreed to the published version of the manuscript.
Supplemental Material
Download MS Word (1.3 MB)Acknowledgments
The authors acknowledge support from the Institute of Food Science and Nutrition, University of Sargodha, Sargodha, for carrying out all research activities. This project was supported by Researchers Supporting Project Number [RSP2025R5] King Saud University, Riyadh, Saudi Arabia.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/10942912.2024.2365924
Additional information
Funding
References
- Ortiz-Uribe, N.; Salomón-Torres, R.; Krueger, R. Date Palm Status and Perspective in Mexico. Agriculture 2019, 9(3), 46. DOI: 10.3390/agriculture9030046.
- AbdulQadir, I. M.; Garba, I. D.; Eseigbe, E.; Omofonmwan, E. I. Nutritional Components of Date Palm and Its Production Status in Nigeria. IJARD 2011, 4(2), 83–89.
- FAO. FAO Statistical Database (FAOSTAT); Food and Agriculture Organization of United National. Roma, Italy, 2023; Available online: https://www.fao.org/faostat/es/#data/QCL. (accessed Mar 24, 2023).
- Fernández-López, J.; Viuda-Martos, M.; Sayas-Barberá, E.; Navarro-Rodríguez de Vera, C.; Pérez-Álvarez, J. A. Biological, Nutritive, Functional and Healthy Potential of Date Palm Fruit (Phoenix Dactylifera L.): Current Research and Future Prospects. Agronomy 2022, 12(4), 876. DOI: 10.3390/agronomy12040876.
- Siddiqi, S. A.; Rahman, S.; Khan, M. M.; Rafiq, S.; Inayat, A.; Khurram, M. S.; Jamil, F.; Jamil, F. Potential of Dates (Phoenix Dactylifera L.) As Natural Antioxidant Source and Functional Food for Healthy Diet. Sci. Total Environ. 2020, 748, 141234. DOI: 10.1016/j.scitotenv.2020.141234.
- Al-Farsi, M.; Lee, C. Nutritional and Functional Properties of Dates: A Review. Crit. Rev. Food Sci. Nutr. 2008, 48(10), 877–887. DOI: 10.1080/10408390701724264.
- Benkerrou, F.; Bachir Bey, M.; Amrane, M.; Louaileche, H. Ultrasonic-Assisted Extraction of Total Phenolic Contents from Phoenix Dactylifera and Evaluation of Antioxidant Activity: Statistical Optimization of Extraction Process Parameters. J. Food Meas. Charact. 2018, 12(3), 1910–1916. DOI: 10.1007/s11694-018-9805-5.
- Echegaray, N.; Pateiro, M.; Gullon, B.; Amarowicz, R.; Misihairabgwi, J. M.; Lorenzo, J. M. Phoenix Dactylifera Products in Human Health–A Review. Trends Food Sci. Technol. 2020, 105, 238–250. DOI: 10.1016/j.tifs.2020.09.017.
- Assirey, E. Nutritional Composition of Fruit of 10 Date Palm (Phoenix Dactylifera L.) Cultivars Grown in Saudi Arabia. J. Taibah Univ. Sci. 2015, 9(1), 75–79. DOI: 10.1016/j.jtusci.2014.07.002.
- Al-Shahib, W.; Marshall, R. J. The Fruit of the Date Palm: Its Possible Use as the Best Food for the Future? Int. J. Food Sci. Nutr. 2003, 54(4), 247–259. DOI: 10.1080/09637480120091982.
- Sheikh, B. Y.; Zihad, S. M. N. K.; Sifat, N.; Uddin, S. J.; Shilpi, J. A.; Hamdi, O. A. A.; Hossain, H.; Rouf, R.; Jahan, I. A. Comparative Study of Neuropharmacological, Analgesic Properties and Phenolic Profile of Ajwah, Safawy and Sukkari Cultivars of Date Palm (Phoenix dactylifera). Orient. Pharm Exp Med. 2016, 16(3), 175–183. DOI: 10.1007/s13596-016-0239-5.
- Younas, A.; Naqvi, S. A.; Khan, M. R.; Shabbir, M. A.; Jatoi, M. A.; Anwar, F.; Aadil, R. M.; Saari, N.; Aadil, R. M. Functional Food and Nutra-Pharmaceutical Perspectives of Date (Phoenix Dactylifera L.) Fruit. J. Food Biochem. 2020, 44(9), e13332. DOI: 10.1111/jfbc.13332.
- Mohamed, D. A.; Al-Okbi, S. Y. In vivo Evaluation of Antioxidant and Anti-Inflammatory Activity of Different Extracts of Date Fruits in Adjuvant Arthritis. Pol. J. Food Nutr. Sci. 2004, 13(54), 397–402.
- Mansouri, A.; Embarek, G.; Kokkalou, E.; Kefalas, P. Phenolic Profile and Antioxidant Activity of the Algerian Ripe Date Palm Fruit (Phoenix dactylifera). Food Chem. 2005, 89(3), 411–420. DOI: 10.1016/j.foodchem.2004.02.051.
- Vayalil, P. K. Antioxidant and Antimutagenic Properties of Aqueous Extract of Date Fruit (Phoenix Dactylifera L. Arecaceae). J. Agric. Food Chem. 2002, 50(3), 610–617. DOI: 10.1021/jf010716t.
- Al-Farsi, M.; Alasalvar, C.; Morris, A.; Baron, M.; Shahidi, F. Comparison of Antioxidant Activity, Anthocyanins, Carotenoids, and Phenolics of Three Native Fresh and Sun-Dried Date (Phoenix Dactylifera L.) Varieties Grown in Oman. J. Agric. Food. Chem. 2005, 53(19), 7592–7599. DOI: 10.1021/jf050579q.
- Lu, Y.; Yeap Foo, L. Antioxidant and Radical Scavenging Activities of Polyphenols from Apple Pomace. Food Chem. 2000, 68(1), 81–85. DOI: 10.1016/S0308-8146(99)00167-3.
- Abou-Elella, F.; Mourad, R. Anticancer and Anti-Oxidant Potentials of Ethanolic Extracts of Phoenix dactylifera, Musa Acuminata and Cucurbita Maxima. RJPBCS 2015, 6(1), 710–720.
- Jassim, S. A.; Naji, M. A. In vitro Evaluation of the Antiviral Activity of an Extract of Date Palm (Phoenix Dactylifera L.) Pits on a Pseudomonas Phage. Evid. Based Compl. Altern. Med. 2010, 7(1), 57–62. DOI: 10.1093/ecam/nem160.
- Shraideh, Z. A.; Abu-Elteen, K. H.; Sallal, A.-K. J. Ultrastructural Effects of Date Extract on Candida albicans. Mycopathologia 1998, 142(3), 119–123. DOI: 10.1023/A:1006901019786.
- Al-Qarawi, A.; Abdel-Rahman, H.; Ali, B. H.; Mousa, H. M.; El-Mougy, S. A. The Ameliorative Effect of Dates (Phoenix Dactylifera L.) on Ethanol-Induced Gastric Ulcer in Rats. J. Ethnopharmacol. 2005, 98(3), 313–317. DOI: 10.1016/j.jep.2005.01.023.
- Al-Qarawi, A. A., Mousa, H.M., Ali, B.H., Abdel-Rahman, H.; El-Mougy, S.A. Protective Effect of Extracts from Dates (Phoenix Dactylifera L.) on Carbon Tetrachloride-Induced Hepatotoxicity in Rats. Int. J. Appl. Res. Vet. Med. 2004, 2(3), 176–180.
- Sheikh, B. Y., Elsaed, W.M., Samman, A.H., Sheikh, B.Y.; Ladin, A.M.M.B. Ajwa Dates as a Protective Agent Against Liver Toxicity in Rat. Eur Sci J. 2013, 10, 358–368.
- Al-Qarawi, A.; Abdel-Rahman, H.; Mousa, H. M.; Ali, B. H.; El-Mougy, S. A. Nephroprotective Action of Phoenix dactylifera. in Gentamicin-Induced Nephrotoxicity. Pharm. Biol. 2008, 46(4), 227–230. DOI: 10.1080/13880200701739322.
- Abuharfeil, N. M.; Sukhon, S. E.; Msameh, Y.; Sallal, A.-K. J. Effect of Date Fruits, Phoenix Dactyliferia L. on the Hemolytic Activity of Streptolysin O. Pharm. Biol. 1999, 37(5), 335–339. DOI: 10.1076/phbi.37.5.335.6051.
- Puri, A.; Sahai, R.; Singh, K. L.; Saxena, R. P.; Tandon, J. S.; Saxena, K. C. Immunostimulant Activity of Dry Fruits and Plant Materials Used in Indian Traditional Medical System for Mothers After Child Birth and Invalids. J. Ethnopharmacol. 2000, 71(1–2), 89–92. DOI: 10.1016/S0378-8741(99)00181-6.
- Aljaloud, S.; Colleran, H. L.; Ibrahim, S. A. Nutritional Value of Date Fruits and Potential Use in Nutritional Bars for Athletes. Food Sci. Nutr. 2020, 11(6), 463. DOI: 10.4236/fns.2020.116034.
- El Abed, H.; Chakroun, M.; Fendri, I.; Makni, M.; Bouaziz, M.; Drira, N.; Mejdoub, H.; Khemakhem, B. Extraction Optimization and in vitro and in vivo Anti-Postprandial Hyperglycemia Effects of Inhibitor from Phoenix Dactylifera L. Parthenocarpic Fruit. Biomed. Pharmacother. 2017, 88, 835–843. DOI: 10.1016/j.biopha.2017.01.129.
- Hussain, M. I.; Farooq, M.; Syed, Q. A. Nutritional and Biological Characteristics of the Date Palm Fruit (Phoenix Dactylifera L.)–A Review. Food Biosci. 2020, 34, 100509. DOI: 10.1016/j.fbio.2019.100509.
- Demirpolat, A.; Akman, F.; Kazachenko, A. S. An Experimental and Theoretical Study on Essential Oil of Aethionema sancakense: Characterization, Molecular Properties and RDG Analysis. Molecules 2022, 27(18), 6129. DOI: 10.3390/molecules27186129.
- Perveen, K. Antibacterial Activity of Phoenix Dactylifera L. Leaf and Pit Extracts Against Selected Gram Negative and Gram Positive Pathogenic Bacteria. J. Med. Plant Res. 2012, 6(2), 296–300. DOI: 10.5897/JMPR11.1380.
- Maged, N. Q. A.; Abbas, N. A. Antibacterial Activity of Phoenix Dactylifera L. Leaf Extracts Against Several Isolates of Bacteria. Kufa Jou. Vete. Med. Sci 2013, 4(2), 45–50. DOI: 10.36326/kjvs/2013/v4i23969.
- Saleh, F. A.; Otaibi, M. M. Antibacterial Activity of Date Palm (Phoenix Dectylifera L.) Fruit at Different Ripening Stages. J. Food Process. Technol. 2011, 4(12). DOI: 10.4172/2157-7110.1000285.
- Ismail, I.; Altuwairki, D. Chemical Composition and Antimicrobial Efficacy of Date Palm Fruit of Saudi Arabia. World Appl. Sci. J. 2016, 34(2), 140–146. DOI: 10.5829/idosi.wasj.2016.34.2.1564.
- El Sohaimy, S. A.; Abdelwahab, A. E.; Brennan, C. S.; Aboul-Enein, A. M. Phenolic Content, Antioxidant and Antimicrobial Activities of Egyptian Date Palm (Phoenix Dactylifera L.) Fruits. Aust. J. Basic Appl. Sci. 2015, 9, 141–147.
- Samad, M. A.; Hashim, S. H.; Simarani, K.; Yaacob, J. S. Antibacterial Properties and Effects of Fruit Chilling and Extract Storage on Antioxidant Activity, Total Phenolic and Anthocyanin Content of Four Date Palm (Phoenix dactylifera) Cultivars. Molecules 2016, 21(4), 1–14. DOI: 10.3390/molecules21040419.
- Ammar, N. M.; Abou, L. T. Flavonoid Constituents and Antimicrobial Activity of Date (Phoenix Dactylifera L.) Seeds Growing in Egypt. Med Arom Plant. Sci Biotechnol. 2009, 3, 1–5.
- Al-Zoreky, N. S.; Al–Taher, A. Y. Antibacterial Activity of Spathe from Phoenix Dactylifera L. Against Some Food-Borne Pathogens. Ind. Crops Prod. 2015, 65, 241–246. DOI: 10.1016/j.indcrop.2014.12.014.
- Bentrad, N.; GAceb-Terrak, R.; Benmalek, Y.; Rahmania, F. Studies on Chemical Composition and Antimicrobial Activities of Bioactive Molecules from Date Palm (Phoenix Dactylifera L.) Pollens and Seeds. Afr. J. Tradit. Complement. Altern. Med. 2017, 14(3), 242–256. DOI: 10.21010/ajtcam.v14i3.26.
- Al-Zoreky, N. S.; Al, A. Y. Antibacterial Activity of Spathe from Phoenix Dactylifera L. Against Some Food-Borne Pathogens. Ind. Crop Prod. 2015, 65, 241–246. DOI: 10.1016/j.indcrop.2014.12.014.
- Nisar, M.; Qayum, M.; Shah, M. R.; Kaleem, W. A.; Ali, I. H. S. A. N.; Zia-Ul-Haq, M. Antimicrobial screening of Impatiens bicolor Royle. Pak. J. Bot. 2010, 42, 523–526.
- Al-Hassnawi, A. Evaluation of Antibacterial Activity of Aqueous and Methanolic Extracts of Pomegranate Peels (Punica Granatum Lin.) Against Some Bacteria. World J Pharm. Res. 2017, 2426–2436. DOI: 10.20959/wjpr20178-9193.
- Arshad, F. K.; Haroon, R.; Jelani, S.; Masood, H. B. A Relative in vitro Evaluation of Antioxidant Potential Profile of Extracts from Pits of Phoenix Dactylifera L. (Ajwa and Zahedi Dates). Int. J. Adv. Inf. Sci. Technol. 2015, 35(35), 28–37.
- Park, Y.; Jung, S.; Kang, S.; Heo, B.; Arancibia-Avila, P.; Toledo, F.; Drzewiecki, J.; Namiesnik, J.; Gorinstein, S. Antioxidants and Proteins in Ethylene-Treated Kiwifruits. Food Chem. 2008, 107(2), 640–648. DOI: 10.1016/j.foodchem.2007.08.070.
- Lim, Y.; Murtijaya, J. Antioxidant Properties of Phyllanthus Amarus Extracts As Affected by Different Drying Methods. LWT - Food Sci. Technol 2007, 40(9), 1664–1669. DOI: 10.1016/j.lwt.2006.12.013.
- Ferreres, F.; Grosso, C.; Gil-Izquierdo, A.; Valentão, P.; Azevedo, C.; Andrade, P. B. HPLC-DAD-ESI/MSn Analysis of Phenolic Compounds for Quality Control of Grindelia Robusta Nutt. and Bioactivities. J. Pharm. Biomed. Anal. 2014, 94, 163–172. DOI: 10.1016/j.jpba.2014.01.046.
- Brand-Williams, W.; Cuvelier, M.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT – Food Sci. Technol. 1995, 28(1), 25–30. DOI: 10.1016/S0023-6438(95)80008-5.
- Oyaizu, M. Studies on Products of Browning Reaction. Antioxidative Activities of Products of Browning Reaction Prepared from Glucosamine. Jpn. J. Nutr. 1986, 44(6), 307–315. DOI: 10.5264/eiyogakuzashi.44.307.
- Merveille, T. O.; Denis, Z.; Abdel, N. J.; Ursula, A. A.; Adie, A. F. A. M.; Smith, B. B.; Clement, A. N. J. Antimicrobial Activities of Some Medicinal Plants Against Multiresistants Microorganisms Causing Urogenital Tract Infections in Cameroon. JDMP 2017, 3(2), 33–41. DOI: 10.11648/j.jdmp.20170302.12.
- Zagoskina, N.; Nikolaeva, T.; Lapshin, P.; Zavarzin, A.; Zavarzina, A. Water-Soluble Phenolic Compounds in Lichens. Microbiol. 2013, 82(4), 445–452. DOI: 10.1134/S0026261713030132.
- Qadir, A.; Singh, S. P.; Akhtar, J.; Ali, A.; Arif, M. Phytochemical and GC-MS Analysis of Saudi Arabian Ajwa Variety of Date Seed Oil and Extracts Obtained by the Slow Pyrolysis Method. Orient. Pharm Exp Med. 2017, 17(1), 81–87. DOI: 10.1007/s13596-017-0257-y.
- Kadum, H.; Hamid, A. A.; Abas, F.; Ramli, N. S.; Mohammed, A. K. S.; Muhialdin, B. J.; Jaafar, A. H. Bioactive Compounds Responsible for Antioxidant Activity of Different Varieties of Date (Phoenix Dactylifera L.) Elucidated by 1H-NMR Based Metabolomics. Int. J. Food Prop. 2019, 22(1), 462–476. DOI: 10.1080/10942912.2019.1590396.
- Afifi, T.; Sareen, J.; Fortier, J.; Taillieu, T.; Turner, S.; Cheung, K.; Henriksen, C. Child Maltreatment and Eating Disorders Among Men and Women in Adulthood: Results from a Nationally Representative United States Sample. Int. J. Eat. Disord. 2017, 50(11), 1281–1296. DOI: 10.1002/eat.22783.
- Benmeddour, Z.; Mehinagic, E.; Meurlay, D.; Louaileche, H. Phenolic Composition and Antioxidant Capacities of ten Algerian Date (Phoenix Dactylifera L.) Cultivars: A Comparative Study. JFF. 2013, 5(1), 346–354. DOI: 10.1016/j.jff.2012.11.005.
- Bettaieb, I.; Ali Benabderrahim, M.; Rodríguez Arcos, R.; Jose Jiménez Araujo, A.; Elfalleh, W. Date Seeds (Phoenix Dactylifera): Antioxidant Potential and Profile of Free and Bound Polyphenols from Different Cultivars. Chem. & Biodiversity. 2023, 20(6), e202300179. DOI: 10.1002/cbdv.202300179.
- Al Juhaimi, F.; Özcan, M. M.; Adiamo, O. Q.; Alsawmahi, O. N.; Ghafoor, K.; Babiker, E. E. Effect of Date Varieties on Physico‐Chemical Properties, Fatty Acid Composition, Tocopherol Contents, and Phenolic Compounds of Some Date Seed and Oils. J. Food Process. Preserv. 2018, 42(4), e13584. DOI: 10.1111/jfpp.13584.
- El-Mergawi, R.; Al-Humaid, A. Searching for Natural Herbicides in Methanol Extracts of Eight Plant Species. Bull. Natl. Res. Cent. 2019, 43(1). DOI: 10.1186/s42269-019-0063-4.
- Ahmed, A.; Arshad, M.; Saeed, F.; Ahmed, R.; Chatha, S. Nutritional Probing and HPLC Profiling of Roasted Date Pit Powder. Pak. J. Nutr. 2016, 15(3), 229–237. DOI: 10.3923/pjn.2016.229.237.
- Hamad, I.; AbdElgawad, H.; Al Jaouni, S.; Zinta, G.; Asard, H.; Hassan, S.; Hegab, M.; Hagagy, N.; Selim, S. Metabolic Analysis of Various Date Palm Fruit (Phoenix Dactylifera L.) Cultivars from Saudi Arabia to Assess Their Nutritional Quality. Molecules 2015, 20(8), 13620–13641. DOI: 10.3390/molecules200813620.
- Kudryavtseva, A. V.; Krasnov, G. S.; Dmitriev, A. A.; Alekseev, B. Y.; Kardymon, O. L.; Sadritdinova, A. F.; Fedorova, M. S.; Pokrovsky, A. V.; Melnikova, N. V.; Kaprin, A. D. Mitochondrial Dysfunction and Oxidative Stress in Aging and Cancer. Oncotarget. 2016, 7(29), 44879–44905.
- Bourgou, S.; Ksouri, R.; Bellila, A.; Skandrani, I.; Falleh, H.; Marzouk, B. Phenolic Composition and Biological Activities of Tunisian Nigella Sativa L. Shoots and Roots. C.R. Biol. 2008, 331(1), 48–55. DOI: 10.1016/j.crvi.2007.11.001.
- Zineb, G.; Boukouada, M.; Djeridane, A.; Saidi, M.; Yousfi, M. Screening of Antioxidant Activity and Phenolic Compounds of Various Date Palm (Phoenix Dactylifera) Fruits from Algeria. Mediterr. J. Nutr. Metab. 2011, 5(2), 119–126. DOI: 10.3233/s12349-011-0082-7.
- Ardekani, M. R. S.; Khanavi, M.; Hajimahmoodi, M.; Jahangiri, M.; Hadjiakhoondi, A. Comparison of Antioxidant Activity and Total Phenol Contents of Some Date Seed Varieties from Iran. Iran. J. Pharm. Res. 2010, 9(2), 141.
- Biglari, F.; AlKarkhi, A.; Easa, A. Antioxidant Activity and Phenolic Content of Various Date Palm (Phoenix Dactylifera) Fruits from Iran. Food Chem. 2008, 107(4), 1636–1641. DOI: 10.1016/j.foodchem.2007.10.033.
- Ouahioune, L. A.; Bara, F.; Bariz, K.; Houali, K.; Djenane, D. Assessment of Antioxidant and Antibacterial Activity of Phoenix Dactylifera L. Seed Extracts: Perspective for the Development of New Foods. Nor. Afr. J. Food Nutr. Res. 2020, 4(8), 298–308. DOI: 10.51745/najfnr.4.8.298-308.
- Ali, H.; Uddin, S.; Jalal, S. Chemistry and Biological Activities of Berberis lyciumRoyle. J. Biolog. Active Product. Nat. 2015, 5(5), 295–312. DOI: 10.1080/22311866.2015.1073627.
- Zhang, H.; Rahman, S.; Li, W.; Fu, G.; Kaur, P. Characterization of a Novel Domain ‘GATE’ in the ABC Protein DrrA and Its Role in Drug Efflux by the DrrAB Complex. Biochem. Biophys. Res. Commun. 2015, 459(1), 148–153. DOI: 10.1016/j.bbrc.2015.02.086.
- Saritha, K.; Rajesh, A.; Manjulatha, K.; Setty, O.; Yenugu, S. Mechanism of Antibacterial Action of the Alcoholic Extracts of Hemidesmus Indicus (L.) R. Br. Ex Schult, Leucas Aspera (Wild.), Plumbago Zeylanica L. and Tridax Procumbens (L.) R. Br. Ex Schult. Front. Microbiol. 2015, 6, 6. DOI: 10.3389/fmicb.2015.00577.
- Taleb, H.; Maddocks, S.; Morris, R.; Kanekanian, A. The Antibacterial Activity of Date Syrup Polyphenols Against S. Aureus and E. Coli. Front. Microbiol. 2016, 7, 7. DOI: 10.3389/fmicb.2016.00198.
- Semaming, Y.; Pannengpetch, P.; Chattipakorn, S.; Chattipakorn, N. Pharmacological Properties of Protocatechuic Acid and Its Potential Roles as Complementary Medicine. Evid. Based Complement. Altern. Med. 2015, 2015, 1–11. DOI: 10.1155/2015/593902.
- Anandhi, D.; Srinivasan, P. T.; Kumar, G. P.; Jagatheesh, S. DNA Fragmentation Induced by the Glycosides and Flavonoids from C. Coriaria. Int. J. Curr. Microbiol. Appl. Sci. 2014, 3(12), 666–673.
- Barakat, A.; Hamed, A.; Bassuiny, R.; Abdel-Aty, A.; Mohamed, S. Date Palm and Saw Palmetto Seeds Functional Properties: Antioxidant, Anti-Inflammatory and Antimicrobial Activities. J. Food Meas. Charact. 2020, 14(2), 1064–1072. DOI: 10.1007/s11694-019-00356-5.