ABSTRACT
In this study, the change of Msalais quality and antioxidant capacity during storage were investigated. The experiments were carried out over a period of 9 months, and analyses were performed monthly. The results showed that the pH, total sugar, and alcohol content of Msalais during the storage period exhibited a decreasing trend, while the total acid content increased. The antioxidant capacity indicated that the contents of total phenols and flavonoids and the scavenging ability of Msalais for DPPH and ABTS decreased markedly with storage time, and stabilizing after 9 months of storage. Furthermore, a total of 27 aroma components, including alcohols, aldehydes, ketones, esters and acids, were detected. After 9 months of storage, the content of most aroma components in Msalais had changed. Among them, the values of the characteristic aroma ingredient phenylethyl alcohol decreased during storage. However, the contents of diisobutyl phthalate, hexadecanoic acid ethyl ester, and octadecanoic acid ethyl ester increased substantially with the following 9 months of storage time. Overall, during the storage process, alcohols, aldehydes and ketones continued to decrease, while esters and acids continued to increase; it was determined that within the 9 month storage period, the longer the storage time, the greater the difference. The obtained findings provide reference data for the optimization of Msalais storage.
Introduction
Msalais is a traditional wine mainly produced by Uygur ethnic people in the A′wati region in Xinjiang, China.[Citation1] The raw materials used to produce Msalais are local grapes, which are a kind of Vitis vinifera Hetianhong grape,[Citation2] fermented with boiled grape juice for approximately two months.[Citation3] Due to the unique brewing process of Msalais, it is a unique wine with variations in grape variety, technique, and characteristics compared to other wines.[Citation4] The quality of Msalais reflects a texture of stiffness, high nutritional value, and a delicious, rich and mellow taste.[Citation5] Msalais is rich in flavonoids, total phenols and other nutrients, endowing it with anticancer, anti-inflammatory, free radical scavenging, anti-platelet agglutination and other effects. In addition, Msalais is rich in essential amino acids and organic acids, which are beneficial to the human body. As a form of intangible national cultural heritage, Msalais has strong regional and ethnic characteristics. Due to its unique flavor and nutritional value, this wine has attracted much attention and has gradually gained recognition from a wider range of consumers.[Citation6] With the vigorous development of China’s wine industry, the production of Msalais has ushered in development opportunities.
In recent years, Msalais has received increasing attention, but research regarding this wine is currently limited. Most of the current research has focused on the screening of Saccharomyces cerevisiae[Citation7–9] and the identification of volatile components during fermentation,[Citation10,Citation11] and some studies have focused on security control. Zhu et al.[Citation12] analyzed the Msalais-fermenting microbes and aromatic compounds formed during natural Msalais fermentation by using high-throughput sequencing and gas chromatography‒mass spectrometry, respectively. Hou et al.[Citation13] identified phenolic profiles and antioxidant characteristics of winemaking using chromatographic analysis. Wang et al.[Citation14] performed transcriptomics sequencing of the liver and kidney of rats to evaluate the toxicity of ethyl carbamate in Msalais; however, there is almost no research on aging or storage after fermentation. As is well known, the final flavor of aged wine can be affected by a wide variety of factors, including the original type (yeast variety, production method), storage temperature, aging time, storage container (glass bottle or wooden, etc.) and oxygen concentration.[Citation15,Citation16] Avizcuri’s research[Citation17] showed that wine stored at 25°C for 6 months under controlled and varying oxygen levels resulted in a range of total phenolic contents. In addition, wine aging development is complex and varies among different wines. Curko, N. et al.[Citation18] showed that red wine was partially oxidized during aging and storage, which contributed to color stabilization and aroma improvement. However, due to the low phenolic content of white wine, aging has little impact on its quality.[Citation19] Additionally, during storage, aroma components are modified due to various reactions, including hydrolysis, oxidation, and esterification.[Citation20,Citation21] Ethanol itself is relatively stable during wine aging, but it can participate in reactions with other wine components. For instance, ethanol can react with acetaldehyde to form ethyl acetate, a volatile compound contributing to wine aroma.[Citation22] However, excessive alcohol content in wine can contribute to poor wine quality. High alcohol levels can disrupt wine balance, leading to a perception of heat or burn on the palate. With different factors, such as storage time and temperature, the degree of these reactions will also vary[Citation23–25]; this means that wine does not remain chemically stationary during storage, and if the optimal storage conditions are not maintained, the quality of the wine and even its consumption can be affected. Therefore, the aging process of wine and its suitable management are crucial to preserve the varietal characteristics and to obtain wines with the desired quality.
The aim of this work was to study the change of physicochemical properties, antioxidant capacity, and aroma components in Msalais throughout 9 months of storage and to provide reference data and support for quality control during the storage process.
Materials and methods
Sake materials
The simulations took place in the laboratory using the processing technology of DaoLang Msalais Co., Ltd., Awati, China. The steps were as follows.[Citation13] The preparation of Msalais samples began with obtaining grape juice by squeezing fresh grapes through a screw press. The extracted juice was then transferred to a steam-boiling tank to boil for 12 h-14 h to achieve a sugar content of 22–26 °Brix before being cooled in cold water to obtain condensed juice. Grape juice was then pumped into a stainless steel tank for fermentation under conditions of fixation for 45 days and the final alcohol content was controlled at 12%-14%. Finally, aged, and stored for 9–12 months in bottles at 10°C in a dark place.
Reagents and instruments
Ethanol, n-hexane, ethylacetate, cyclohexanone, anhydrous disodium hydrogen phosphate and anhydrous sodium dihydrogen phosphate were purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). ABTS, DPPH, Trolox, trichloroacetic acid, FeCl3, and potassium ferricyanide were all purchased from Macklin Biochemical Co., Ltd. (Shanghai, China). Rutin, gallic acid and Folin – Ciocalteu’s phenol reagent were all purchased from Laibao Technology Co., Ltd. (Beijing, China).
Sample processing
The Msalais sample was stored in a glass bottle at room temperature. According to the periodic measurement, samples were taken every month for a total of 9 months. Three parallel experiments were performed for each sample.
Analysis
pH: A Leici PHB-4 handheld pH meter was used for measurement.
Total sugar and total acid: Total sugar and total acid were analyzed according to the official method of the national standards, China.[Citation26]
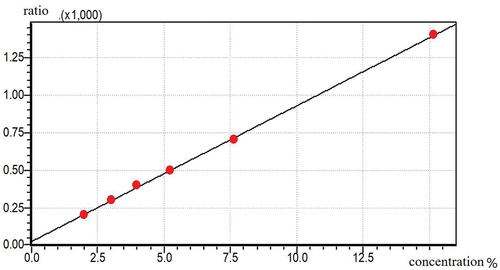
Alcohol content: The alcohol content was measured by gas chromatography.[Citation27] The standard curve is shown below (). The standard curve equation for measuring alcohol was Y = 90.65X + 24.36, R2 = 0.995.
Total phenolic content
The Folin – Ciocalteu colorimetric method of Liu et al.[Citation28] was performed to determine the total phenolic content. Then, 0.2 mL of Msalais was added to 10 mL of distilled water. Then, 0.3 mL of 2 N Folin-Ciocalteu reagent was added and shaken well. After the reaction was carried out for 5 min, 1 mL 15% Na2CO3 was added. The sample reacted for 120 min at room temperature and in the dark, and the absorbance was measured at 746 nm. All experiments were repeated in triplicate. A standard solution of 0–0.5 mg/mL gallic acid was prepared, and a standard curve was prepared for calculating the total phenolic content in Msalais. The standard curve regression equation was obtained: y = 0.8577×+0.0052,R2 = 0.9991.
Total flavonoid content
The total flavonoid content was determined according to the method described by Liu et al. .[Citation28] Then, 1 mL of Msalais was added to 4 mL of 80% ethanol, and 0.5 mL 10% (w/w) NaNO2 solution was added and kept at room temperature for 6 min. Next, 0.5 mL 10% (w/w) Al(NO3)3 solution was added, and 4 mL 4% NaOH solution was added after 10 min-20 min. After mixing, the absorbance was measured at 510 nm. All experiments were repeated in triplicate. A standard solution of 0–0.16 mg/mL rutin was prepared, and a standard curve was prepared for calculating the total flavonoid content in Msalais. The standard curve regression equation was obtained: y = 0.2534×-0.01,R2 = 0.9985
Antioxidant capacity
1,1-Diphenyl-2-picrylhydrazyl (DPPH) radical scavenging activity assay: DPPH radical scavenging activity was determined as described by Shopska et al.[Citation29] Briefly, the Msalais samples were centrifuged at 4000 r/min for 5 min for use. One hundred microliters of wine was added to 3.9 mL of 12.5% DPPH solution in methanol; the mixture was allowed to react for 20 min in the dark, and then the absorbance at 517 nm was determined. All experiments were repeated in triplicate. A standard solution of 0–1000 μmol/L Trolox was prepared, and a standard curve was prepared for calculating the DPPH radical scavenging activity assay in Msalais. The standard curve regression equation was obtained:y = −0.0005×+0.6904,R2 = 0.9947.
ABTS radical scavenging activity assay: The ABTS assay was based on the method of Anna Floegel (2011)[Citation30] with slight modifications. Briefly, the Msalais samples were centrifuged at 4000 r/min for 5 min and diluted 5 times. Then, 5 mL and 88 µL of 7 × 10−3 mol/L ABTS solution and 0.14 mol/L potassium persulfate solution, respectively, were added, and the mixture was placed in the dark for 12 h-16 h. The mixture was diluted with methanol until the absorbance was 0.70 ± 0.02 at 734 nm. Next, 0.1 mL of Msalais was added to 3.9 mL of ABTS solution for 8 min in the dark, and the absorbance value was measured at 734 nm. All experiments were repeated in triplicate. A standard solution of 0–1000 μmol/L Trolox was prepared, and a standard curve was prepared for calculating the ABTS radical scavenging activity assay in Msalais. The standard curve regression equation was obtained:y = −0.0007×+0.6771, R2 = 0.9981.
Aroma component analysis
The extraction method of aroma compounds was optimized for Zhang Yu’s[Citation31] extraction method. Five Msalais samples, containing 10 μL of 20 μg/mL cyclohexanone as an internal standard and ddd 1 g of sodium chloride, were added to a 10 mL glass vial and mixed at 60°C for 30 min. The extraction time was 30 min at the same temperature. Next, the gas was analyzed for 3 min.
GC-MS(Agilent Company, USA) analysis conditions: VF-WAXms column (60 m × 0.25 mm, 0.25 µm film; Agilent), the initial temperature was 60°C, then it rose to 110°C at a heating rate of 5 ℃/min, and then rose to 215°C at a heating rate of 3 ℃/min for 3 min; the temperature was raised to 240°C at a rate of 5 ℃/min and maintained for 10 min. The carrier gas was helium, with a flow rate of 1.2 mL/min, a sample inlet temperature of 250°C, and a detector temperature of 240°C.
Mass spectrometry conditions: EI ionization source, electron energy of 70 eV, and filament flow rate of 0.20 mA. The detector voltage was 350 V. The scanning range was 50 amu-550 amu, the ion source temperature was 230°C, and the transmission line temperature was 270°C. Qualitative and quantitative analysis of aroma components shall be carried out in accordance with Chen’s method.[Citation27]
Statistical analysis
All data are the mean values of three parallel experiments and are expressed as the mean ± standard deviation. Significant differences among different groups were compared by one-way analysis of variance (ANOVA) with SPSS 18.0 software. Differences were considered significant at the level of p < .05.
Results and discussion
Changes in physicochemical parameters during storage of Msalais
The physicochemical parameters are the most important parameters applied to monitor the storage condition of Msalais.[Citation32] The changes in total sugar, total acid, pH, alcohol, and alcohol content are typically used to determine the flavor and quality of Msalais.[Citation33] The physicochemical properties of Msalais at different storage stages are displayed in .
Table 1. Chemical indices of Msalais at different storage times.
In the storage of Msalais, the level of total acid constantly increased, reaching 6.42 g/L ±0.02 g/L after 9 months of storage. However, the pH significantly decreased (p < .05) (0–6 months) and then gradually decreased and remained stable (6–9 months). The possible reasons for these differences in pH and total acid could be the degree of secondary fermentation or the kind of microbes involved during the course of secondary fermentation during storage.[Citation34] Perhaps due to the action of lactic acid bacteria, they would continue to convert sugars and other compounds into acid, and some alcohol was oxidized to acetaldehyde, which was unstable and quickly oxidized back to acetic acid, which increases acidity. Additionally, the total sugar was significantly decreased (p < .05) (0 month–9 month), possibly because some sugars are mainly consumed by microorganisms for secondary fermentation, which was helpful to elevate the acid content.[Citation16] The alcohol content gradually decreased from 14.40 ± 0.10 to 13.05 ± 0.05 (0 month–7 month), followed by almost remaining constant (p > .05). This result was basically in agreement with the results of a previous study.[Citation35] The monitoring of the above physicochemical parameters provides important guidance for the quality of Msalais during storage.
Changes in total phenols, flavonoids and antioxidant activity during storage of Msalais Polyphenols and total flavonoids are the main antioxidant components in Msalais,[Citation36] which could reflect the antioxidant capacity of Msalais.[Citation37,Citation38] The results showed that the content of total phenols and flavonoids showed a slow downward trend during storage (). The total phenol content decreased from 0.575 mg/mL to 0.418 mg/mL (0–9 months). In addition, the total flavonoid content decreased from 0.734 mg/mL to 0.569 mg/mL; after 9 months of storage, it basically stabilized. The reduction in total phenols and flavonoids has been observed in other red wines and is most likely caused by the formation of pigment substances during storage or partial dissolution of oxygen, leading to the oxidation and reduction of total phenols and flavonoids,[Citation39–41] Others, phenolic compounds can bind or precipitate with nonvolatile compounds such as proteins and other phenolic compounds, reducing their content in liquids.[Citation42]
Figure 2. Antioxidant substances in Msalais during storage, with corresponding changes, DPPH radical scavenging activity, and ABTS radical scavenging activity.
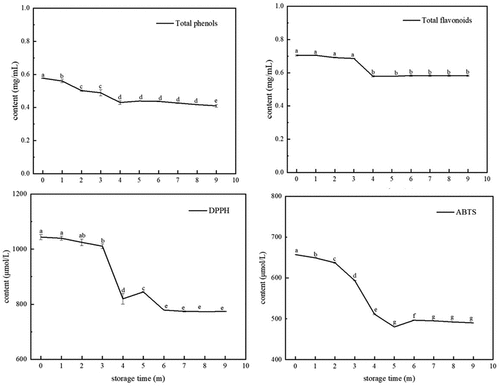
DPPH and ABTS are common methods to evaluate the antioxidant capacity of wine. The radical scavenging abilities are shown in . The results indicated that with the prolongation of storage time, the scavenging ability of Msalais on DPPH and ABTS free radicals showed a decreasing trend; this is positively correlated with the trend of changes in total phenols and flavonoids.[Citation30] However, shows that the antioxidant capacity detected by the DPPH method was higher than that detected by the ABTS method. This phenomenon is caused by various reaction mechanisms,[Citation43,Citation44] which could be explained by different kinds or contents of free radicals in Msalais leading to different test results for the two antioxidants.
Changes in aroma composition during storage of Msalais
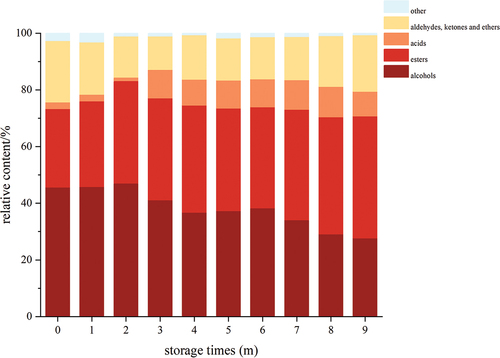
The changes in the aroma composition of Msalais during storage are shown in . In the early stage of storage (month 0), only 22 aroma components were detected in Msalais, with a total content of 253.41 µg/L of aroma components. Among them, the content of phenylethyl alcohol was the highest. During the 9-month storage period, its contents in phenylethyl alcohol varied between 60.96 µg/L and 98.71 µg/L with a higher level of content. Overall, the values of phenylethyl alcohol decreased during storage; this was similar to N. Moreira’s[Citation45] research results. Moreover, studies have shown that phenylethyl alcohol contributes significantly to the wine bouquet, imparting fruity and flowery flavors.[Citation46] After 9 months of storage, the content of most aroma components in Msalais had changed. The contents of diisobutyl phthalate, hexadecanoic acid ethyl ester, and octadecanoic acid ethyl ester increased substantially with the following 9 months of storage time. These changes could occur in Msalais due to esterification of the acid and alcohol components.[Citation47] At the later stage of storage (9 months), 25 aroma components were detected in Msalais, with a total content of 314.43 µg/L of aroma components; among them, the contents of diisobutyl phthalate and hexadecanoic acid ethyl ester were higher, and their contents in the late storage period (9 months) were 1.5 times and 3.6 times, respectively, those in the early storage period (0 months). These constituted the main aroma components of Msalais, and these results were consistent with the research by Chen et al. [Citation27]
Table 2. Analysis of aroma components in Msalais with different storage times.
The aroma components detected in Msalais include alcohols, aldehydes, ketones, esters and acids. The content of alcohols and esters was relatively high, accounting for a relatively low proportion of aldehydes, ketones and acids; this indicates that alcohols and esters were the main aroma components in Msalais (). In the early stage of storage (0 months), the content of alcohols in Msalais was the highest, accounting for 46.65% of the total aroma components. With the extension of storage time, the content of alcohols showed a trend of first increasing and then decreasing. However, the percentage of alcohols in the total aroma components continued to decrease. In the later stage of storage (9 months), the percentage of alcohols in the total aroma components decreased to 27.71%. The content of esters was second only to alcohols in the early stage of storage (0 months), accounting for 27.66% of the total aroma components. With the extension of storage time, the content of esters continued to rise. In the 9th month of storage, the percentage of esters in the total aroma components increased to 43.01%. Aldehydes and ketones decreased with increasing storage time. As expected, the results showed that storage time had a great impact on the aroma compounds of Msalais, similar to the research results of N. Moreira et al.[Citation45]
The selected aroma data were all standardized, and as the storage time was prolonged, the differences in aroma component structure in Msalais increased (). In the early stage of storage (0 months), the aroma component structure in Msalais was most similar to the aroma components stored for 1 month; its components include 1-propanol, oleic acid, ethane, 2-chloro-1,1-dimethoxy-, 3-furanmethanol, 5-hydroxymethylfurf, ethyl hydrogen succinate, ethanone 1-(3-thienyl)-, 1-butanol 3-methyl-, thiophene tetrahydro-2-methyl-, phenylethyl alcohol, hexadecanoic acid ethyl ester, ethyl oleate, 2,3-butanediol, [R-(R*,R*)]-, decanoic acid ethyl ester, and other components. After 9 months of storage, the composition structure of aroma components showed a significant difference compared to 0 months, but was more similar to that stored for 8 months. Similar components include pentadecanoic acid, octanoic acid, dodecanoic acid ethyl ester, 9-hexadecenoic acid, tetradecanoic acid, hexanedioic acid bis(2-ethylhexyl) ester, linoleic acid ethyl ester, n-hexadecanoic acid, octanoic acid ethyl ester, methyl vinyl ketone, n-decanoic acid, (E)-9-octadecenoic acid ethyl ester, furfural, and other components. In summary, during the storage process, alcohols, aldehydes and ketones continued to decrease, while esters and acids continued to increase; the longer the storage time, the greater the difference. This result indicated that storage time had an impact on the aroma components of Msalais.
Conclusion
This work presents an investigation of the changes in the physicochemical properties, antioxidant capacity, and aroma components of Msalais during storage. The results indicated that the pH, total sugar, and alcohol content of Msalais during the storage period showed a decreasing trend, while the total acid content increased. The content of total phenols and flavonoids and the scavenging ability of Msalais on DPPH and ABTS decreased markedly with storage time; after 9 months of storage, it basically stabilizes. A significant difference in aroma components between storage in month 0 and storage in month 9 was observed. Overall, during the storage process, alcohols, aldehydes and ketones continued to decrease, while esters and acids continued to increase. These results provide reference data and support for the development and optimization of the storage process of Msalais.
Acknowledgments
The work described in this article was supported by the Tarim University Presidents’ Fund (TDZKSS202218) and research on the Development of New Products and Comprehensive Utilization Technology of by-products for Msalais (2022B02024-3). We thank Elsevier for its linguistic assistance during the preparation of this manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Zhu, L.; Wang, L.; Yang, W.; Guo, D. Physicochemical Data Mining of Msalais, a Traditional Local Wine in Southern Xinjiang of China. Int. J. Food. Prop. 2016, 19(11), 2385–2395. DOI: 10.1080/10942912.2015.1033549.
- Guo, D. L.; Zhang, J. Y.; Liu, C. H. Genetic Diversity in Some Grape Varieties Revealed by ScoT Analyses. Mol. Biol. Rep. 2012, 39(5), 5307–5313. DOI: 10.1007/s11033-011-1329-6.
- Zhu, L.-X.; Zhang, M.-M.; Shi, Y.; Duan, C.-Q. Evolution of the Aromatic Profile of Traditional Msalais Wine During Industrial Production. Int. J. Food. Prop. 2019, 22(1), 911–924. DOI: 10.1080/10942912.2019.1612428.
- Zhu, L.-X.; Wang, H.; Zhang, M.-M.; Shi, Y.; Xiang, X.-F.; Lan, Y.-B.; Zhang, R.-L. Aromatic and Chemical Differences Between Msalais Wines Produced at Traditional Craft Workshops and Modern Plants. J. Food Compost. Anal. 2023, 116, 1–12. DOI: 10.1016/j.jfca.2022.105029.
- Yuanqing, Z. Design of an Annual Production Capacity of 500 Tons of Moussaires Wine Factory [D]; Tarim University: Alar, 2021.
- Xiaojie, H.; Study on Brewing Technology of Low Foaming Moussales [D]; Tarim University: Alar, Xinjiang; 2021.
- Zhu, L.-X.; Wang, G.-Q.; Xue, J.-L.; Gou, D.-Q.; Duan, C.-Q. Direct Stamp of Technology or Origin on the Genotypic and Phenotypic Variation of Indigenous Saccharomyces cerevisiae Population in a Natural Model of Boiled Grape Juice Fermentation into Traditional Msalais Wine in China. FEMS Yeast Res. 2017, 17, 108. DOI: 10.1093/femsyr/fow108.
- Jiabin, Z.; Guanqun, W.; Tongguo, C.; Quan, M.; Lixia, Z. Laboratory-Scale Development of Combined Starter for Msalais Production. China Brew 2017, 36, 115–120.
- Zhu, L.; Xue, J. Modern Technology Homogenizes Enological Traits of Indigenous Saccharomyces cerevisiae Strains Associated with Msalais, a Traditional Wine in China. World J. Microbiol. Biotechnol. 2017, 33(3), 63. DOI: 10.1007/s11274-017-2227-4.
- Zhu, L.-X.; Zhang, M.-M.; Liu, Z.; Shi, Y.; Duan, C.-Q. Levels of Furaneol in Msalais Wines: A Comprehensive Overview of Multiple Stages and Pathways of Its Formation During Msalais Winemaking. Molecules. 2019, 24(17), 3104. DOI: 10.3390/molecules24173104.
- Zhu, L.-X.; Zhang, M.-M.; Xiang, X.-F.; Lan, Y.-B.; Shi, Y.; Duan, C.-Q.; Zhang, R.-L. Aromatic Characterization of Traditional Chinese Wine Msalais by Partial Least-Square Regression Analysis Based on Sensory Quantitative Descriptive and Odor Active Values, Aroma Extract Dilution Analysis, and Aroma Recombination and Omission Tests. Food Chem. 2021, 361, 129781. DOI: 10.1016/j.foodchem.2021.129781.
- Zhu, L.-X.; Hui, W.; Han, P.-J.; Lan, Y.-B. Identification of Dominant Functional Microbes That Contribute to the Characteristic Aroma of Msalais, Traditional Wine Fermented from Boiled Local Grape Juice in China. Food Chem. 2023, 100778, 2590–1575. DOI: 10.1016/j.fochx.2023.100778.
- Hou, X.; Chen, S.; Pu, Y.; Wang, T.; Xu, H.; Li, H.; Ma, P.; Hou, X. Effect of Winemaking on Phenolic Compounds and Antioxidant Activities of Msalais Wine. Molecules. 2023, 28(3), 1250. DOI: 10.3390/molecules28031250.
- Wang, W.; Han, Z.; Guo, D.; Xiang, Y. Renal Transcriptomics Reveals the Carcinogenic Mechanism of Ethyl Carbamate in Musalais. OncoTargets Ther. 2021, 14, 1401–1416. DOI: 10.2147/OTT.S282125.
- Boerzhijin, S.; Isogai, A.; Mukai, N. Impact of Storage Conditions on the Volatile Aroma Compounds of Aged Sake. J. Food Compost. Anal. 2023, 121(105351), 0889–1575. DOI: 10.1016/j.jfca.2023.105351.
- Romina Castellanos, E.; Jofre, V. P.; Fanzone, M. L.; Assof, M. V.; Catania, A. A.; Mariela Diaz-Sambueza, A.; Heredia, F. J.; Mercado, L. A. Effect of Different Closure Types and Storage Temperatures on the Color and Sensory Characteristics Development of Argentinian Torrontes Riojano White Wines Aged in Bottles. Food Control. 2021, 130, 0956–7135. DOI: 10.1016/j.foodcont.2021.108343.
- Avizcuri, J. M.; Sáenz-Navajas, M. P.; Echávarri, J. F.; Ferreira, V.; Fernández-Zurbano, P. Evaluation of the Impact of Initial Red Wine Composition on Changes in Color and Anthocyanin Content During Bottle Storage. Food Chem. 2016, 213, 123–134. DOI: 10.1016/j.foodchem.2016.06.050.
- Curko, N.; Ganić, K. K.; Tomašević, M.; Gracin, L.; Jourdes, M.; Teissedre, P. L. Effect of Enological Treatments on Phenolic and Sensory Characteristics of Red Wine During Aging: Micro-Oxygenation, Sulfur Dioxide, Iron with Copper and Gelatin Fining. Food Chem. 2021, 339, 339. DOI: 10.1016/j.foodchem.2020.127848.
- Ortega-Heras, M.; González-Sanjosé, M. L.; González-Huerta, C. Consideration of the Influence of Aging Process, Type of Wine and Oenological Classic Parameters on the Levels of Wood Volatile Compounds Present in Red Wines. Food Chem. 2007, 103(4), 1434–1448. DOI: 10.1016/j.foodchem.2006.10.060.
- Coetzee, C.; Oxidation Treatments Affecting Sauvignon Blanc Wine Sensory and Chemical Composition [D]; Stellenbosch University: Stellenbosch, Western Cape; 2014.
- Coetzee, C.; Van Wyngaard, E.; Suklje, K.; Silva Ferreira, A. C.; Du Toit, W. J. Chemical and Sensory Study on the Evolution of Aromatic and Nonaromatic Compounds During the Progressive Oxidative Storage of a Sauvignon Blanc Wine. J. Agric. Food Chem. 2016, 64(42), 7979–7993. DOI: 10.1021/acs.jafc.6b02174.
- Styger, G.; Prior, B.; Bauer, F. F.; Wine Flavor and Aroma. J. Ind. Microbiol. Biotechnol. 2011, 38(9), 1145. DOI: 10.1007/s10295-011-1018-4.
- Cejudo-Bastante, M. J.; Hermosín‐Gutiérrez, I.; Pérez‐Coello, M. S. Accelerated Aging Against Conventional Storage: Effects on the Volatile Composition of Chardonnay White Wines. J. Food Sci. 2013, 78(4), 507–513. DOI: 10.1111/1750-3841.12077.
- Hopfer, H.; Ebeler, S. E.; Heymann, H. The Combined Effects of Storage Temperature and Packaging Type on the Sensory and Chemical Properties of Chardonnay. J. Agric. Food Chem. 2012, 60(43), 10743–10754. DOI: 10.1021/jf302910f.
- Echave, J.; Barral, M.; Fraga-Corral, M.; Prieto, M. A.; Simal-Gandara, J. Bottle Aging and Storage of Wines: A Review. Molecules 2021, 26(3), 713. DOI: 10.3390/molecules26030713.
- General Administration of Quality Supervision, Inspection and Quarantine of the China, Standardization Administration of China. GB/T 15038-2006. Bibliographic Description Rules for Bibliographic References [S]; China Light Industry Press: Beijing, 2006.
- Shenghuizi, C.; Study on Physicochemical Quality and Characteristic Flavor of Musalais in Xinjiang [D]; Tarim University: Alar, Xinjiang; 2022.
- Liu, W.; Ji, R.; Aimaier, A.; Sun, J.; Pu, X.; Shi, X. Weidong Cheng, Bin Wang, Adjustment of Impact Phenolic Compounds, Antioxidant Activity and Aroma Profile in Cabernet Sauvignon Wine by Mixed Fermentation of Pichia kudriavzevii and Saccharomyces cerevisiae. Food Chem. 2023, 18, 2590–1575. DOI: 10.1016/j.fochx.2023.100685.
- Shopska, V.; Kostova, R. D.; Zarcheva, M. D.; Teneva, D.; Denev, P.; Kostov, G. Comparative Study on Phenolic Content and Antioxidant Activity of Different Malt Types. Antioxidants. 2021, 10(7), 1124. DOI: 10.3390/antiox10071124.
- Floegel, A.; Kim, D.-O.; Chung, S.-J.; Koo, S. I.; Chun, O. K. Comparison of ABTS/DPPH Assays to Measure Antioxidant Capacity in Popular Antioxidant-Rich US Foods. J. Food Compost. Anal. 2011, 24(7), 1043–1048. DOI: 10.1016/j.jfca.2011.01.008.
- Yu, Z.; Electronic Nose and GC-MS Techniques Were Used to Study the Changes of Aroma Substances in Musalais Q8 Wine [D]; Tarim University: Alar, Xinjiang; 2017.
- Tyl, C.; Sadler, G. PH and Titratable Acidity. In Food Analysis. Food Science Text Series, Nielsen, S., Ed. Springer: Cham, 2017; pp. 389–406.
- Coelho, E. M.; da Silva Padilha, C. V.; Miskinis, G. A.; de Sá, A. G. B.; Pereira, G. E.; de AzevêAzevêDo, L. C.; dos Santos Lima, M. Simultaneous Analysis of Sugars and Organic Acids in Wine and Grape Juices by HPLC: Method Validation and Characterization of Products from Northeast Brazil. J. Food Compos. Anal. 2018, 66, 160–167. DOI: 10.1016/j.jfca.2017.12.017.
- Nemo, R.; Bacha, K. Microbial, Physicochemical and Proximate Analysis of Selected Ethiopian Traditional Fermented Beverages. LWT - Food Sci. Technol. 2020, 131, 1–7. DOI: 10.1016/j.lwt.2020.109713.
- Huang, Z.-R.; Guo, W.-L.; Zhou, W.-B.; Li, L.; Xu, J.-X.; Hong, J.-L.; Lv, X.-C.; Zeng, F.; Bai, W.-D.; Liu, B. Microbial Communities and Volatile Metabolites in Different Traditional Fermentation Starters Used for Hong Qu Glutinous Rice Wine. Food Res. Int. 2019, 121, 593–603. DOI: 10.1016/j.foodres.2018.12.024.
- Generalić Mekinić, I. G.; Skračić, Z.; Kokeza, A.; Soldo, B.; Ljubenkov, I.; Banović, M.; Šimat, V.; Skroza, D. Effect of Enzyme-Assisted Vinification on Wine Phenolics, Colour Components, and Antioxidant Capacity. Pol. J. Food Nutr. Sci. 2020, 70(2), 113–123. DOI: 10.31883/pjfns/115461.
- Danila. The Antioxidant Capacity of Red Wine in Relationship with Its Polyphenolic Constituents. Food Chem. 2008, 111, 45–49. DOI: 10.1016/j.foodchem.2008.03.037.
- Angeles, M. A.; Dominguez, C. Determination of Antioxidant Power of Red and White Wines by a New Electrochemical Method and Its Correlation with Polyphenolic Content. J. Agric. Food Chem. 2002, 50(11), 3112–3115. DOI: 10.1021/jf0116101.
- Liqiong, C.; Dynamic Changes of Main Physical and Chemical Indexes and Antioxidant Activity of Wine During Q10 Storage [D]; Lanzhou University of Technology: Lanzhou, Gansu; 2016.
- Mulero, J.; Pardo, F.; Zafrilla, P. Effect of Principal Polyphenolic Components in Relation to Antioxidant Activity in Conventional and Organic Red Wines During Storage.European. Food Res. Technol. 2009, 229(5), 807–812. DOI: 10.1007/s00217-009-1117-x.
- Pati, S.; Crupi, P.; Savastano, M.; Benucci, I.; Esti, M. Evolution of Phenolic and Volatile Compounds During Bottle Storage of a White Wine without Added Sulfite. J. Sci. Food Agric. 2019, 100(2), 775–784. DOI: 10.1002/jsfa.10084.
- Arnous, A.; Makris, D. P.; Kefalas, P. Effect of Principal Polyphenolic Components in Relation to Antioxidant Characteristics of Aged Red Wines. J. Agric. Food. Chem. 2001, 49(12), 5736–5742. DOI: 10.1021/jf010827s.
- Zhang, H.; Yang, Y.-F.; Zhou, Z.-Q. Phenolic and Flavonoid Contents of Mandarin (Citrus Reticulata Blanco) Fruit Tissues and Their Antioxidant Capacity as Evaluated by DPPH and ABTS Methods. J. Integr. Agric. 2018, 17(1), 256–263. DOI: 10.1016/S2095-3119(17)61664-2.
- Zhang, H.; Xi, W. P.; Yang, Y. F.; Zhou, X. Y.; Liu, X.; Yin, S. S.; Zhang, J. M.; Zhou, Z. Q. An On-Line HPLC-FRSD System for Rapid Evaluation of the Total Antioxidant Capacity of Citrus Fruits. Food Chem. 2015, 172, 622–629. DOI: 10.1016/j.foodchem.2014.09.121.
- Moreira, N.; Lopes, P.; Ferreira, H.; Cabral, M.; de Pinho, P. G. Influence of Packaging and Aging on the Red Wine Volatile Composition and Sensory Attributes. Food Pack. Shelf Life 2016, 8, 14–23. DOI: 10.1016/j.fpsl.2016.02.005.
- Rapp, A.; Mandery, H. Wine Aroma. Experientia. 1986, 42(8), 873–884. DOI: 10.1007/BF01941764.
- Ramey, D. D.; Ough, C. S. Volatile Esters Hydrolysis or Formation During Storage of Model Solutions and Wines. J. Agric. Food Chem. 1980, 28(5), 928–934. DOI: 10.1021/jf60231a021.