ABSTRACT
Demand for almond is in rise, due to its desired flavor and beneficial health-related properties. Roasting is the most common practice in almond processing, improving aroma and texture, as well as nutritional properties. “Shefa,” a new Israeli almond variety, was recently introduced; however, no information is available regarding its phytochemical composition and response to roasting. The aim of the current work was to characterize its health-related composition, in addition to describe its response to moderate roasting conditions, including temperature and duration. For this, “Shefa” almonds were roasted at 100°C, 125°C, 150°C, and 175°C for 15 and 25 min. and compared to raw unroasted kernels. Health and nutritional quality parameters included antioxidant capacity (DPPH, FRAP, and ABTS; spectrophotometric), total polyphenol, tocopherol and phytosterol content (spectrophotometric), and fatty acid profiling (GC-MS). Our results demonstrate that “Shefa” variety phytochemical composition resembles that of other Mediterranean almond varieties, e.g. Italian and Turkish are high in tocopherols and phytosterols, with average polyphenol content. Its antioxidant activity was 19.11, 25.56, and 22.18 mg TE/100 g DW for DPPH, FRAP, and ABTS, respectively. In addition, under the roasting conditions evaluated in the current work, “Shefa” almonds presented an improved health-related composition, with levels of antioxidant capacity, polyphenols, tocopherols, and phytosterols increasing with temperature and duration. “Shefa” response to roasting resembled that of other almond varieties evaluated under comparable roasting conditions. To conclude, the newly introduced Israeli almond “Shefa” has a healthy and nutritionally beneficial phytochemical composition, which improves with moderate roasting. Further investigation is needed to profile its polyphenol and tocopherol composition, to provide additional valuable insights into its phytochemical composition.
Introduction
Almond holds significant global importance as a leading nut tree crop in terms of commercial production[Citation1] with over 3.6 million metric tons in 2022.[Citation2] C-1: References should be number format California is the primary almond-producing region, contributing to more than half of the worldwide output, followed by Australia, Spain, Turkey, Morocco, China, and Iran.[Citation2,Citation3] Israel’s annual almond production comprises 11,000 tins, introducing new varieties like “Kochva,” “Gilad,” and “Shefa” .[Citation4] However, only little research is available concerning the nutritional quality and chemical composition of these newer almond varieties.
Almonds’ nutritional benefits significantly contribute to their commercial value and increasing consumer demand. These nuts boast a diverse array of nutritionally advantageous phytochemicals, e.g., polyphenols, encompassing phenolic acids, flavonoids, condensed tannins, lignans, as well as tocopherols (α-, β-, γ-, and δ).[Citation5,Citation6] Additionally, almonds are a rich source of phytosterols alongside desirable unsaturated fatty acids like oleic acid (C18:1), linoleic acid (C18:2), and linolenic acid (C18:3), an essential omega-3 fatty acid.[Citation7] Furthermore, almond kernels and skin exhibit remarkable antioxidant bioactivity.[Citation8–11] This array of phytochemicals and bioactivities makes almonds a functional food, providing various health benefits.
Most commercial almonds undergo roasting, to enhance their sensory appeal and overall palatability.[Citation12–14] Dry heat is commonly used for the roasting process, contributing to the development of the desired flavor and texture. Dry roasting is typically carried out at temperatures ranging from 130°C to 150°C, with the duration adjusted accordingly.[Citation15] Roasting temperature and duration play a significant role in determining the final roasting outcomes.[Citation16,Citation17]
Roasting processes significantly impact the physical structure and chemical composition of almonds.[Citation18] These alterations encompass the specialized metabolite profile, fatty acid composition, antioxidant activity, and aroma volatile profile.[Citation15,Citation17] Recent studies have indicated that roasting may lead to a decrease in vitamin E content due to oxidation reactions.[Citation19] Additionally, roasting has been found to negatively affect almond’s thiamine and carotenoid contents.[Citation20] Contrarily, some studies have observed an increase in general antioxidant activity, likely attributed to the rise in Maillard reaction products.[Citation17] These authors also reported a gradual increase in phenolic components with higher roasting temperatures and longer durations. However, a study evaluating the roasting effect on almond skin demonstrated that roasted almonds exhibited a 26% lower total phenol content (TPC) and a 34% lower Ferric ion reducing antioxidant power (FRAP) antioxidant activity compared to raw almonds.[Citation21] Similar effects have been reported for other tree nuts, e.g., walnuts, hazelnuts, macadamia, and pine nuts.[Citation19]
The primary objective of the current study was twofold. Firstly, we aimed to investigate the phytochemical composition of Israeli “Shefa” almonds, as no existing data on this specific variety is available. Additionally, we sought to examine the impact of various roasting conditions (including temperature and duration) on the health-related properties of “Shefa” almonds.
Materials and methods
Plant material
Commercial samples of “Shefa” almond variety used in the current study were provided by Mr. Moshe Levi roasting and packing house, Bikat Arad, Israel. A representative 5 kg sample of raw Israeli-grown “Shefa” almonds was provided on January 2021, of fresh almonds harvested on August 2020. All reagents used in this present study were of analytical grade, purchased from Sigma-Aldrich (Rehovot, Israel).
Roasting
Almond kernels were roasted in an oven at 100°C, 125°C, 150°C, and 175°C for 15 and 25 min, and then ground by a coffee grinder. Almond powders were kept at 4°C pending analysis.
DPPH antioxidant activity
DPPH analysis was performed as previously published.[Citation17] Briefly, 0.9 mL of ethyl acetate was added to 200 mg of almond powder, placed in a thermoshaker for 10 min at 25°C, followed by 5-min centrifugation at 17 KG. 100 μL of the supernatant is then transferred to a 96-well plate, 100 μL of DPPH solution (0.18 mg/mL in 80% ethanol) is added and plate is read at 517 nm in triplicates. Quantification was done using a 6-point calibration curve (0–0.1 mg/mL) of trolox and presented as mg trolox equivalent (TE)/g.
FRAP antioxidant activity
FRAP analysis was performed as published before.[Citation21] 1 mL of 80% ethanol was added to 200 mg almond powder and placed in a thermoshaker for 10 min at 25°C, followed by 5-min centrifugation at 17 KG. 30 μL of the supernatant is then transferred to a 96-well plate, followed by 200 μL of FRAP solution (10 mL of 0.3 M acetate buffer (pH = 3.6), 1 mL of TPTZ solution (10 mM in 40 mM of HCl in DH2O) and 1 mL of FeCl3•6 H2O (20 mM in water)) and read at 593 nm in triplicates. Quantification was done using a 6-point calibration curve (0–0.1 mg/mL) of trolox and presented as mg trolox equivalent (TE)/g.
ABTS antioxidant activity
ABTS analysis was done for hydrophobic and hydrophilic bioactivity from the same sample as previously published,[Citation22] with slight modifications. For hydrophobic analysis, 900 μL hexane were added to 200 mg almond powder. Samples were then placed in a thermoshaker for 10 min at 25°C, followed by 6-min centrifugation at 17 KG. 20 μL of the supernatant was then transferred to a 96-well plate, followed by 200 μL ABTS•+ (in 85% IPA) and read at 750 nm in triplicates, and quantification was done using a 6-point calibration curve of α tocopherol (0–0.1 mg/mL). For the hydrophilic antioxidant bioactivity analysis for the same sample, the pellet from the hydrophobic analysis was first dried by speed-vac to dryness. Then, 450 μL of acetone and 450 μL of acetate buffer (pH = 4.3) were added, and samples were then placed in a thermoshaker for 10 min at 25°C, followed by 6-min centrifugation at 17 KG. 10 μL of the supernatant were then transferred to a 96-well plate, followed by 200 μL ABTS•+ (in 75% ethanol) and read at 745 nm in triplicates, and quantification was done using a 6-point calibration curve of trolox (0–0.1 mg/mL) and presented as mg trolox equivalent (TE)/g.
Total polyphenol content (TPC)
Polyphenols content was determined using the Folin-Ciocalteu spectrophotometric method as published before.[Citation17] Briefly, 1 mL of 80% ethanol solution was added to 200 mg almond powder. Samples were then placed in a thermoshaker for 10 min 25°C and centrifuged for 5 min at 17 KG. 30 μL of the supernatant was transferred to a 96-well plate, followed by 100 μL FC solution (10% in DH2O) and 100 μL sodium carbonate solution (2% in DH2O), and after 90 min read at 765 nm in triplicates. Quantification was based on a 6-point gallic acid calibration curve (0–03 mg/mL) and presented as mg gallic acid equivalents (GAE) per g.
Total tocopherol content
Total tocopherol content was measured as published before.[Citation23] Briefly, 1 mL of isopropyl alcohol (IPA) was added to 100 mg almond powder and placed in a thermoshaker for 10 min at 25°C, followed by 10-min centrifugation at 17 KG. To 750 μL of the supernatant 200 μL of FeCl3 solution (0.2% (w/v) in IPA) and 200 μL of 2.2 dipyridyl solution (0.2% (w/v) in ethanol:IPA 1:3) were added. After thermoshaking for 20 minutes at 25°C, 200 μL were transferred to a 96 well plate, and read at of 515 nm in triplicates. Quantification was based on a 6-point a-tocopherol calibration curve (0–0.1 mg/mL).
Total phytosterol content
Total phytosterol analysis was performed as published before.[Citation23] Briefly, 1 mL ethyl acetate was added to 200 mg of almond powder and placed in a thermoshaker for 20 min at 30°C, followed by 5-min centrifugation at 17 KG. 100 μL of the supernatant was transferred to a 96-well plate, 100 μL of LB reagent (1 mL H2SO4 in 10 mL cold acetic anhydride) was added, and the plate was read at 675 nm in triplicates. Quantification was done using a 6-point calibration curve (0–2 mg/mL) of β-sitosterol.
Fatty acid profile
Fatty acids were profiled as published before.[Citation23] Briefly, 1.5 mL hexane (HPLC grade) was added to 300 mg almond powder, vortexed and centrifuged for 5 min at 17 KG. 1 mL of the supernatant was transferred to a 2 mL vial, followed by 200 μL methanolic KOH (2 M). 200 μL of C17:0 (1 mg/mL in hexane) was added as internal standard (IS). Analysis was done on a 7890 GC instrument coupled to a 5890 MS (Agilent Technologies, Santa Clara, CA, USA). and a DB-23 capillary column (60 m long, 0.25 mm diameter, 0.25 μm film thickness; J&W Scientific, Folsom, CA USA). Helium was employed as the carrier gas (1 mL/min). The detector temperature was set at 250°C. The oven temperature was held at 175°C for 3 min, then increased at 4°C min−1 to 225°C and then 10°C min−1 to 240°C and held for 6 min until the end of the 23 min run. The injection volume was 1 µL at 20:1 split ratio. Fatty acids were identified by comparing retention times with standard compounds (FAME Mix C8–C24, Supelco, Bellefonte, PA, USA). The relative composition of fatty acids in the oil was determined as percentage of total fatty acids.
Statistical analysis
Data were analyzed using Analysis of Variance (ANOVA) in JMP version 16.0 (SAS Inst. Inc., Cary, NC., USA). All analyses were done in triplicates and results expressed as mean ± standard error. Significance level used for all statistical analyses was p < .05, and post-hoc test used was a t-test, with no FDR correction.
Results
In the current work we evaluated the health-related composition of “Shefa,” a new Israeli almond variety,[Citation4] as well as studied the effect of roasting conditions, including temperature and duration, on its nutritional quality parameters.
‘Shefa’ almond variety chemical composition
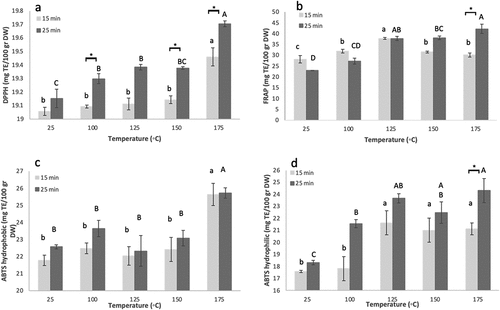
Almond kernel antioxidant bioactivity was evaluated through three methods: DPPH, FRAP, and ABTS (hydrophobic and hydrophilic). The DPPH bioactivity of untreated kernels was 19.11 ± 0.05 mg TE/100 g DW (), and the FRAP bioactivity of “Shefa” was 25.56 ± 0.91 mg TE/100 g DW (). In addition, raw nuts presented 22.18 ± 0.21 and 17.95 ± 0.14 mg TE/100 g DW for ABTS hydrophobic and hydrophilic bioactivity, respectively.
Figure 1. Effect of roasting temperature and duration on new Israeli “Shefa” almond antioxidant capacity. a. DPPH; b. FRAP; c. Hydrophobic ABTS; d. Hydrophilic ABTS. Presented are means+standard error of 3 replicates (n=3).
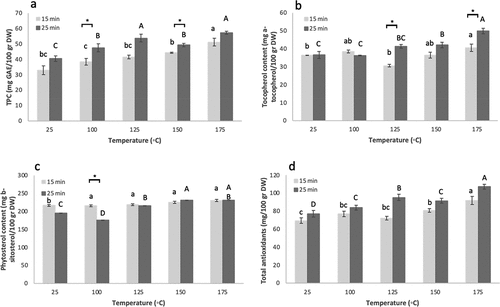
Furthermore, we measured three other quality parameters of almond kernels, namely TPC, total tocopherol contents, and total phytosterol contents. The average TPC of raw “Shefa” almond was 36.73 ± 2.37 mg GAE/100 g. Tocopherol contents averaged 36.49 ± 1.06 mg a-tocopherol equivalent/100 g for raw nuts, and phytosterol contents was 205.92 ± 5.61 mg b-sitosterol equivalent/100 g for raw nuts.
The fatty acid (FA) profile of raw “Shefa” almonds includes C18:1 (9, Z-) as the main FA, accounting for 73.96 ± 0.41%, followed by C18:2 (9,12, Z-, Z-) consisting of 17.17 ± 0.43%, and C16:0, (6.99 ± 0.02%), and C18:0 (1.22 ± 0.06%) () (). Other FA are lower than 0.5%: C16:1 (7,Z-) (0.51 ± 0.02), C14:0 (0.23 ± 0.01%), and C20:1 (0.12 ± 0%), with some found at trace levels (<0.05%, C12:0, C16:1 (9,Z-), C16:1 (11,Z-), C18:3 (9,12,15, Z-, Z-, Z-), and C20:0). Monounsaturated FA (MUFA) and polyunsaturated FA (PUFA) made up 74.64 ± 0.41% and 17.71 ± 043% of total FA, respectively, and their ratio was 4.35. Saturated FA (SFA) and unsaturated FA (UFA) made up 8.24 ± 0.06% and 91.85 ± 0.2% of total FA, respectively.
Figure 2. GC-MS chromatogram of fatty acid methyl ester (FAME) analysis, profiling raw “Shefa” almond fatty acids.
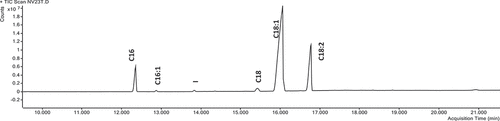
Table 1. Fatty acid profile of Israeli “Shefa” variety under various roasting conditions (GC-MS). Presented are means+standard error of 3 replicates (n = 3).
Effect of roasting conditions on ‘Shefa’ quality parameters
DPPH values of roasted almonds ranged between 19.05 and 19.73 mg TE/100 g DW (), while FRAP values ranged 25.54–44.95 mg TE/100 g DW (). ABTS antioxidant capacity of roasted kernels ranged 20.99–16.86 and 15.83–26.67 mg TE/100 g DW for hydrophobic and hydrophilic bioactivity, respectively ().
As for the other quality parameters of roasted almonds, TPC of roasted kernels ranged 34.38–58.58 mg GAE/100 g, tocopherol contents ranged 30.60 to 52.92 mg a-tocopherol equivalent/100 g, and phytosterol contents was 175.22–239.96 mg b-sitosterol equivalent/100 g for roasted nuts.
Fatty acid profile of roasted almonds was mostly similar to that of raw nuts, with oleic acid (73.07–75.61%), linoleic acid (15.32–17.44%), palmitic acid (6.34–8.84%), and stearic acid (1.07–1.32%) as main FA, followed by C16:1 (7,Z-) (0.45–0.58%), tetradecanoic acid (0.22–0.27%) and eicosanoic acid (0.10–0.13%) (). MUFA and PUFA in roasted almonds ranged 73.82–76.30% and 15.37–17.48%, respectively, and their ration was 4.23–4.98. SFA and UFA ranged 7.93–8.47% and 91.29–92.09%, respectively.
Roasting temperature significantly affected antioxidant activity of “Shefa” almonds: DPPH values were generally unchanged at 15- min for all temperatures, with an increased at 175°C (). For 25-min roasting, values increased with increasing temperature, with highest activity at 175°C (). FRAP values for 15-min roasting increased until 125°C and then decreased, while in 25-min values increased with increasing temperature, peaking at 175°C (). Hydrophobic ABTS antioxidant activity values followed the trend of DPPH, remaining unchanged up until 150°C with an increase at 175°C, for both 15- and 25-min roasting (). Hydrophilic ABTS antioxidant bioactivity increased with temperature, with highest values at 125°C and higher, for both durations ().
Roasting temperature also affected other almond health-related parameters. TPC remain unchanged with increasing temperature for 15-min roasting, then showing an increase at 175°C, while for 25-min an increase was observed with temperature, with highest values recorded at 125°C and 175°C (). Tocopherol content was generally constant in all temperatures during 15-min roasting, while for 25-min roasting an increase with temperature was observed (). Total phytosterol content increased between control kernels and 100°C and remained unchanged with increasing temperatures above 100°C for 15-min roasting, while for 25-min the trend was of an increase with temperature and highest levels at 150°C and 175°C (). We added up the two almond main antioxidants, polyphenols and tocopherols, to ‘total antioxidant contents” (in contrast to antioxidant bioactivity measured by DPPH, FRAP, and ABTS, presented at ). This parameter follows the same trend as both TPC and total tocopherol content, remaining generally unchanged with increasing temperature for 15-min roasting, with a peak at 175°C, and for 25-min increasing with temperature with highest value at 175°C ().
Figure 3. Effect of roasting temperature and duration on new Israeli “Shefa” almond health-related properties. a. TPC; b. total tocopherol contents; c. total phytosterol content; d. total antioxidants. Presented are means+standard error of 3 replicates (n=3).
FA profile was mostly stable for all fatty acids for 15-min roasting, with the exception of the main FAs: oleic acid was stable with increasing temperature, with highest percentage at 175°C, while a completing trend was observed for linoleic acid, which was stable with increasing temperatures, and decreased at 175°C (). Concomitantly, MUFA content showed the same trend described for oleic acid, PUFA followed linoleic acid trend, and their ratio (MUFA/PUFA) presented the same trend as oleic acid. For UFA the only significant difference was higher percentage at 150°C compared to 175°C, and vice versa for UFA.
For 25-min roasting, significant changes were observed for most FAs-Palmitic acid was stable up until 125°C, showed the highest percentage at 150°C, and decreased at 175°C, and so did C16:1 (7, Z-) (). Oleic acid was unchanged 25°C- 125°C, and the only significant difference was higher percentage at 175°C compared to 150°C. Eicoseaic acid was fluctuating, with no specific trend to be identified. As expected, MUFA was following the same trend as oleic acid (the main unsaturated FA), while SFA that of palmitic acid (the main saturated FA).
Roasting duration was found significant in only comparisons. For antioxidant activity analysis, these were 100°C, 150°C and 175°C for DPPH, 175°C for FRAP and hydrophilic ABTS (). For beneficial compounds the significant differences between the two duration groups were observed at 100°C and 150°C for TPC, 125°C for tocopherols, and 100°C for phytosterol contents (). No significant effect of roasting time was found for FA profile ().
Discussion
The objective of the current work was to characterize the health-related properties of the newly introduced Israeli “Shefa” almond variety while also investigating the influence of roasting temperature and duration on its beneficial compounds. Our findings indicate that “Shefa” almonds exhibit health-related properties similar to those found in many other varieties, mainly Mediterranean, with roasting under the conditions studied in this work positively impacting these properties.
‘Shefa’ phytochemical composition
Concerning the health-related composition of “Shefa,” it appears to align well with other almond varieties worldwide. Its DPPH bioactivity is comparable to the Turkish Akbadem/Mugla variety[Citation24] and the Greek variety,[Citation25] exhibiting values of 26 and 67.5 mg TE/100 g kernels, respectively. Similarly, the FRAP bioactivity of “Shefa” is akin to that of Turkish varieties (20 mg TE/100 g),[Citation24] and certain Californian varieties (92–223 mg TE/100 g).[Citation9] While information regarding the hydrophobic and hydrophilic ABTS bioactivity of almonds is limited, available reports confirm that “Shefa” displays a high ABTS antioxidant bioactivity, generally comparable to some Turkish varieties (91 mg TE/100 g) [Citation24] and surpassing that of Portuguese varieties, ranging from 0.156 to 1.16 mg TE/100 g.[Citation26]
In food systems, antioxidants also play an important role in preservation, as well as maintaining oxidative stability of the product.[Citation27] Specifically in oil and oil-seeds, antioxidants are important for product shelf-life, slowing down oxidative chain reactions by quenching free radicals, hence delaying deterioration in quality.[Citation28] For example, in olive oil, polyphenols impart oxidative stability and prolong shelf-life, while in almond high levels of tocopherols were correlated with longer shelf-life of the kernels. Thus, inevitably, healthier oil is more oxidative stable, and vice versa.[Citation29] Concomitantly, various methods have been developed for the analysis of food antioxidant activity, e.g., DPPH, ABTS, and FRAP, measuring specific mechanisms of anti-oxidant capacity.[Citation30]
TPC of almonds exhibits significant variation, and TPC values for “Shefa” align with published data for certain Italian varieties (39–1103 mg GAE/100 g)[Citation31] and California-grown varieties (58–159 mg GAE/100 g).[Citation9] Polyphenols stand out as one of almond’s most health-related bioactive compounds and have been associated with several desirable effects, including beneficial antioxidant, anti-inflammatory, cardiovascular, metabolic, cognitive and anti-cancer effects.[Citation5,Citation32] Consuming polyphenols as part of a healthy diet has been linked to various health benefits, partly attributed to their impact on gut microbiota,[Citation33] making them a recommended component of a healthy lifestyle.
The tocopherol contents of almond varieties exhibit significant variation, with recorded values ranging from 21.8 to 77.7 mg a-tocopherol/100 g for Italian varieties[Citation34], 21.9 to 31.0 mg a-tocopherol/100 g for California-grown varieties,[Citation3] and 8.5 to 19 mg a-tocopherol/100 g for Spanish varieties.[Citation35] “Shefa” almonds show tocopherol levels comparable to those found in some of the Italian varieties.
Vitamin E comprises two subgroups, tocopherols and tocotrienols, each containing four homologs: α-, β-, γ-, and δ, characterized by the numbers and positions of their methyl groups.[Citation36] α- tocopherol is the most biologically active form, preferentially utilized by the human body compared to other forms, although generally tocotrienols are considered more biologically active than tocopherols.[Citation37] α-Tocopherol’s ability to quench free radicals and break lipid peroxidation chain reactions stems mainly from scavenging reactive oxygen species (ROS), particularly peroxyl radicals, and formation of resonance-stabilized tocopheroxyl radicals (α-T•).[Citation38] As the most abundant lipophilic antioxidants in cells, the presence of tocopherols contributes to the health-related properties of almonds, including cardiovascular, anti-oxidant and anti-inflammatory, as well as osteal, ocular, nephrological and neurological benefits.[Citation39] Among tocopherols α- is the predominant form in almond kernels, followed by b-, g-, and d-tocopherol.[Citation35] Almond kernels contain 240–440 μg/g of α-tocopherol playing a crucial role in maintaining the oxidative stability of almond oil and kernels during storage.[Citation40]
The phytosterol contents in Italian almonds have been reported as 112.6–277.7 mg/100 g,[Citation41] while Moroccan varieties exhibited levels of 108–162 mg/100 g.[Citation42] Notably, the “Shefa” almond variety shows a relatively high phytosterol content, comparable to that of the Italian varieties. Plant sterols, also known as phytosterols, are bioactive components renowned for their ability to promote heart health and reduce blood cholesterol levels.[Citation43] Among the phytosterols found in almonds, β-sitosterol is the most abundant, comprising approximately 95% of the total sterols in kernel, followed by campesterol and stigmasterol.[Citation44] The substantial presence of phytosterols, especially β-sitosterol, in “Shefa” almonds contributes to their potential health benefits, particularly in supporting cardiovascular health and managing blood cholesterol levels.
The fatty acid (FA) profile of almonds is typically consistent, with oleic acid being the predominant FA, making up about 60–75% of the total FA content, followed by linoleic (12–25%), palmitic (4.5–7%), and stearic acid (1–3%).[Citation7] While there is some variability in FA composition among different almond cultivars, it is not as pronounced as in other crops, e.g., olive. From a health and nutritional standpoint, a high percentage of unsaturated FA (both mono (MUFA) and poly unsaturated FAs (PUFA) is desirable, together with low levels of saturated FAs, as these proportions have been correlated with positive health-related effects. The presence of the omega-3 essential FA linolenic acid (<1% of composition) is also advantageous, as it offers neurological benefits. Nevertheless, unsaturated FAs present challenges during kernel postharvest storage and processing, as they are prone to oxidation, leading to higher susceptibility to oil and kernel oxidation and reduced oxidative stability, negatively affecting nutritional, health, and sensory quality.[Citation45]
Specifically in almonds, the oleic/linoleic (O/L) ratio holds significant importance as a quality parameter, as a higher O/L ratio is associated with improved oxidative stability, thereby preserving kernel’s overall quality.[Citation46]
Effect of roasting on ‘Shefa’ almond health-related composition
Roasting plays a crucial role in the almond industry, as it imparts desirable sensory characteristics to the kernels, enhancing their color, texture, and aroma. Moreover, roasting has been reported to positively impact the nutritional value of almonds by increasing the bioavailability of phenolic compounds,[Citation47] and decreasing the contents of antinutrients.[Citation48] However, the high temperatures involved in the roasting process might also lead to phytonutrients degradation and deteriorated nutritional quality. Roasting conditions are thus of utmost importance in determining the overall outcome of the process, and their optimization is essential in achieving the desired beneficial effects while maintaining kernel’s quality and avoiding harmful effects.
It’s essential to note that despite its favorable effects, almond roasting can also lead to the formation of harmful substances. Notably, furans, classified as possibly carcinogenic to humans (Group 2B) by the International Agency for Research on Cancer and acrylamide (Group 2A) are among the harmful compounds that may form during the roasting process.[Citation19,Citation49,Citation50] Recently, novel roasting methods have been suggested as alternatives to oven roasting, aiming to minimize the harmful effects, including microwave, infrared, and radio-frequency roasting.[Citation51–53]
Roasting effect on the health-related phytochemical composition and antioxidant capacity has been reported in California almond skins treated at 146°C for 14 min resulting in a significant 34% decrease in FRAP compared to raw kernels.[Citation21] Another study reported that DPPH, ABTS+, and FRAP levels increased with higher roasting temperatures (150 or 180ºC) and longer durations (5, 10, or 20 min).[Citation17] A third work found that roasting almonds at 190°C for 10 min led to reduced ABTS•+ and DPPH• levels, but an increase was observed at 170°C, making 170°C the recommended roasting temperature by the authors.[Citation54] Moreover, a separate investigation showed that roasting almond skin at 145°C significantly enhanced its antioxidant activity.[Citation10]
Our study aligns with these findings, recording an increase in the antioxidant activity of almond kernels with higher roasting temperatures (ranging from 125 to 175ºC) and longer durations (15 and 25 min), as assessed by DPPH, ABTS, and FRAP parameters, also supporting current knowledge regarding the increase in almond antioxidant capacity upon roasting.
In the work by Bolling and colleagues, almond skin TPC levels were negatively affected by roasting, resulting in a 26% decrease.[Citation21] However, a more detailed investigation in kernels by Lin et al. revealed that various phenolic components, such as flavonoids, condensed tannins, and phenolic acids increased with roasting temperature and duration (150°C or 180°C, 5, 10, or 20 min).[Citation17] Additionally, another work reported a significant enhancement in the polyphenol content of almond skin after roasting.[Citation10] Our current data are in-line with these findings, observing increased TPC in roasted kernels. It should also be noted that the observed effects, although significant, are sometimes small in magnitude. Nevertheless, such differences might be meaningful to the consumer, as a difference of 18 mg GAE/100 g upon roasting represents a 55% increase in polyphenol consumption. Given a recommended daily intake of 1 ounce almonds,[Citation55] such difference is equivalent to 180 mg/month, the amount in 1 liter of olive oil from some varieties.
Regarding FAs, Lin and colleagues’ roasting treatment led to increased levels of unsaturated fatty acids, including linoleic, oleic, and elaidic acids, as well as saturated fatty acids (palmitic and stearic acids) in almond kernels.[Citation17] In contrast, a different study found that roasting almond kernels at varying temperatures (ranging from 123ºC/25 min to 185.5ºC/25 min) did not significantly alter FA composition.[Citation19] Lipan et al., on the other hand, observed a reduction in polyunsaturated fatty acids at 190°C, whereas no such change was observed at 170°C.[Citation54] Our results for unsaturated FAs align with previous reports, showing enhanced levels of oleic acid, a slight decrease in linoleic acid, a decrease in palmitic acid, and no change in stearic acid levels upon roasting. The variation in outcomes between studies could be attributed to an array of factors, as discussed below.
Roasting almonds at temperatures above 150°C resulted in a significant reduction in tocopherol levels,[Citation19] also observed in another study, with almonds roasted at 140ºC/25 min to 160ºC/15 min.[Citation20] Tocopherols are relatively heat-sensitive, prone to degradation when exposed to high temperatures, which explains their decrease during the roasting process.[Citation38] Interestingly, our data reveals a slight increase in tocopherol contents, not in agreement with previous findings, and possible causes for observed discrepancy might include genetic as well as environmental factors, as discussed further.
Information is severely lacking regarding the effect of roasting on almond phytosterol contents. Works in other oil seeds, e.g., cocoa and peanut, showed that phytosterols are decreasing with increased roasting temperature, same as for tocopherols.[Citation56] However, an increase was observed in walnuts roasted at 140°C, 160°C, and 180°C for 5 min, 10 min, and 15 min,[Citation57] and no effect was observed in cashew nuts.[Citation58] Our data presents an induction of phytosterol levels, as expected by some of those previous works on other nuts.
The increase in the levels of beneficial compounds observed during nut roasting has been extensively discussed. Several hypotheses have been proposed, including the degradation of polymerized polyphenols, hydrolysis of glycosylated flavonoids, and the decomposition of aglycones.[Citation59] However, the most widely accepted hypothesis is related to the possible role of newly generated Maillard reaction products, produced during thermal treatment. It has been suggested that these compounds, e.g., melanoidins, typically possess a reductone-type structure,[Citation60] thereby possessing antioxidative activity, contributing to the enhancement of the antioxidant attributes in processed foods.[Citation17,Citation21]
To conclude, these data suggest that the phytochemical composition of the new Israeli “Shefa” almond resembles that of other Mediterranean almond varieties. Furthermore, antioxidant capacity and levels of beneficial compounds in whole “Shefa” almonds may increase upon roasting under moderate temperature up to 175°C for short duration of up to 25 min, same as previously observed for other almond varieties under comparable conditions. These findings highlight the significance of the roasting process in enhancing the overall nutritional value and health benefits of almonds, also emphasizing the delicate balance in achieving desirable nutritional quality, flavor, and texture through roasting while preserving essential nutrients. Further investigation is warranted to shed light over the mechanism underlying roasting impact on health and nutritional beneficial content in almonds and to develop and optimize effective roasting strategies that improve the nutritional value of this popular nut. Moreover, further exploration is needed to profile polyphenols, tocopherols, and tocotrienols composition in “Shefa” and other new Israeli varieties, to provide valuable insights into their potential health benefits.
Research highlights
The phytochemical profile of a newly bred Israeli almond variety ‘Shefa” is characterized.
Varity’s response to roasting temperature and duration was also evaluated.
Mild roasting conditions were found to increase almond’s health-related composition.
“Shefa” profile of beneficial properties resembles that of other Mediterranean almond varieties.
CRediT Taxonomy
ZT- Conceptualization, formal analysis, funding acquisition, methodology, supervision, visualization, writing – original draft, writing – review & editing; NV- investigation, writing – original draft; IG- investigation, data curation, methodology, project administration, resources, validation; SM- methodology, validation, RHB- investigation; DH- Conceptualization, funding acquisition, writing – review & editing.
Acknowledgments
The authors are thankful to the Israeli Almond Board for the partial funding for this work. We also want to thank Mrs. Rachel Buchris from “Ort Aryeh-Meir” high school for her valuable support and contribution to this project. Many thanks to Mr. Moshe Levi for the kernels, and Prof. Arnon Dag for his helpful comments.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Kodad, O.; Estopañán, G.; Juan, T.; Alonso, J. M.; Espiau, M. T.; Socias i Company, R. Oil Content, Fatty Acid Composition and Tocopherol Concentration in the Spanish Almond Genebank Collection. Sci. Hortic. 2014, 177, 99–107. DOI: 10.1016/j.scienta.2014.07.045.
- FAO. FAO Stats; 2022. https://www.fao.org/faostat/en/#home.
- Yada, S.; Huang, G.; Lapsley, K. Natural Variability in the Nutrient Composition of California-Grown Almonds. J. Food Compost. Anal. 2013, 30(2), 80–85. DOI: 10.1016/j.jfca.2013.01.008.
- Holland, D.; Bar-Ya’akov, I.; Hatib, K.; Albert, T.; Mani, Y.; Spiegel-Roy, P. ’Shefa’ Almond. HortScience 2006, 41(6), 1502–1503. DOI: 10.21273/HORTSCI.41.6.1502.
- Bolling, B. W. Almond Polyphenols: Methods of Analysis, Contribution to Food Quality, and Health Promotion. Compr. Rev. Food Sci. Food Saf. 2017, 16(3), 346–368. DOI: 10.1111/1541-4337.12260.
- Roncero, J. M.; Álvarez-Ortí, M.; Pardo-Giménez, A.; Rabadán, A.; Pardo, J. E. Review About Non-Lipid Components and Minor Fat-Soluble Bioactive Compounds of Almond Kernel. Foods 2020, 9(11), 1646. DOI: 10.3390/foods9111646.
- Ouzir, M.; Bernoussi, S. E.; Tabyaoui, M.; Taghzouti, K. Almond Oil: A Comprehensive Review of Chemical Composition, Extraction Methods, Preservation Conditions, Potential Health Benefits, and Safety. Comprehensive Reviews in Food Science and Food Safety. Compr. Rev. Food Sci. Food Saf. 2021, 20(4), 3344–3387. DOI: 10.1111/1541-4337.12752.
- Bolling, B. W.; Chen, C.-Y. O.; McKay, D. L.; Blumberg, J. B. Tree Nut Phytochemicals: Composition, Antioxidant Capacity, Bioactivity, Impact Factors. A Systematic Review of Almonds, Brazils, Cashews, Hazelnuts, Macadamias, Pecans, Pine Nuts, Pistachios and Walnuts. Nutr. res. rev. 2011, 24(2), 244–275. DOI: 10.1017/S095442241100014X.
- Bolling, B. W.; Dolnikowski, G.; Blumberg, J. B.; Chen, C.-Y. O. Polyphenol Content and Antioxidant Activity of California Almonds Depend on Cultivar and Harvest Year. Food Chem. 2010, 122(3), 819–825. DOI: 10.1016/j.foodchem.2010.03.068.
- Garrido, I.; Monagas, M.; Gómez‐Cordovés, C.; Bartolomé, B. Polyphenols and Antioxidant Properties of Almond Skins: Influence of Industrial Processing. J Food Sci 2008, 73(2), C106–C115. DOI: 10.1111/j.1750-3841.2007.00637.x.
- Yang, J.; Liu, R. H.; Halim, L. Antioxidant and Antiproliferative Activities of Common Edible Nut Seeds. LWT Food Sci. Technol. 2009, 42(1), 1–8. DOI: 10.1016/j.lwt.2008.07.007.
- Oliveira, I.; Meyer, A. S.; Afonso, S.; Vilela, A.; Goufo, P.; Trindade, H.; Gonçalves, B. Bioactive Compounds, Antioxidant Activities, Fatty Acids, and Sensorial Characteristics of Almond (Prunus Dulcis) After Roasting and Blanching. In Proceedings of the 1st International Electronic Conference on Agronomy, 3–17 May 2021; MDPI: Basel, Switzerland, 2021. DOI: 10.3390/IECAG2021-09908.
- Franklin, L. M.; King, E. S.; Chapman, D.; Byrnes, N.; Huang, G.; Mitchell, A. E. Flavor and Acceptance of Roasted California Almonds During Accelerated Storage. J. Agric. Food Chem. 2018, 66(5), 1222–1232. DOI: 10.1021/acs.jafc.7b05295.
- Franklin, L. M.; Chapman, D. M.; King, E. S.; Mau, M.; Huang, G.; Mitchell, A. E. Chemical and Sensory Characterization of Oxidative Changes in Roasted Almonds Undergoing Accelerated Shelf Life. J. Agric. Food Chem. 2017, 65(12), 2549–2563. DOI: 10.1021/acs.jafc.6b05357.
- Xiao, L.; Lee, J.; Zhang, G.; Ebeler, S. E.; Wickramasinghe, N.; Seiber, J.; Mitchell, A. E. HS-SPME GC/MS Characterization of Volatiles in Raw and Dry-Roasted Almonds (Prunus Dulcis). Food Chem. 2014, 151, 31–39. DOI: 10.1016/j.foodchem.2013.11.052.
- Erten, E. S.; Cadwallader, K. R. Identification of Predominant Aroma Components of Raw, Dry Roasted and Oil Roasted Almonds. Food Chem. 2017, 217, 244–253. DOI: 10.1016/j.foodchem.2016.08.091.
- Lin, J.-T.; Liu, S.-C.; Hu, C.-C.; Shyu, Y.-S.; Hsu, C.-Y.; Yang, D.-J. Effects of Roasting Temperature and Duration on Fatty Acid Composition, Phenolic Composition, Maillard Reaction Degree and Antioxidant Attribute of Almond (Prunus Dulcis) Kernel. Food Chem. 2016, 190, 520–528. DOI: 10.1016/j.foodchem.2015.06.004.
- Perren, R.; Escher, F. E. Impact of roasting on nut quality. In Improving the safety and quality of nuts, Woodhead Publishing, 2013; pp. 173–197.
- Schlörmann, W.; Birringer, M.; Böhm, V.; Löber, K.; Jahreis, G.; Lorkowski, S.; Müller, A. K.; Schöne, F.; Glei, M. Influence of Roasting Conditions on Health-Related Compounds in Different Nuts. Food Chem. 2015, 180, 77–85. DOI: 10.1016/j.foodchem.2015.02.017.
- Stuetz, W.; Schlörmann, W.; Glei, M. B-Vitamins, Carotenoids and α-/γ-Tocopherol in Raw and Roasted Nuts. Food Chem. 2017, 221, 222–227. DOI: 10.1016/j.foodchem.2016.10.065.
- Bolling, B. W.; Blumberg, J. B.; Chen, C.-Y. O. The Influence of Roasting, Pasteurisation, and Storage on the Polyphenol Content and Antioxidant Capacity of California Almond Skins. Food Chem. 2010, 123(4), 1040–1047. DOI: 10.1016/j.foodchem.2010.05.058.
- Vinokur, Y.; Rodov, V. Method for Determining Total (Hydrophilic and Lipophilic) Radical-Scavenging Activity in the Same Sample of Fresh Produce. I International Symposium on Natural Preservatives in Food Systems 709, 2005.
- Tietel, Z.; Dag, A.; Yermiyahu, U.; Zipori, I.; Beiersdorf, I.; Krispin, S.; Ben‐Gal, A. Irrigation‐Induced Salinity Affects Olive Oil Quality and Health‐Promoting Properties. J. Sci. Food Agric. 2019, 99(3), 1180–1189. DOI: 10.1002/jsfa.9287.
- Kamiloglu, S.; Pasli, A. A.; Ozcelik, B.; Capanoglu, E. Evaluating the in vitro Bioaccessibility of Phenolics and Antioxidant Activity During Consumption of Dried Fruits with Nuts. LWT Food Sci. Technol. 2014, 56(2), 284–289. DOI: 10.1016/j.lwt.2013.11.040.
- Kalogeropoulos, N.; Chiou, A.; Ioannou, M. S.; Karathanos, V. T. Nutritional Evaluation and Health Promoting Activities of Nuts and Seeds Cultivated in Greece. Int. J. Food Sci. Nutr. 2013, 64(6), 757–767. DOI: 10.3109/09637486.2013.793298.
- Oliveira, I.; Meyer, A. S.; Afonso, S.; Sequeira, A.; Vilela, A.; Goufo, P.; Trindade, H.; Gonçalves, B. Effects of Different Processing Treatments on Almond (Prunus Dulcis) Bioactive Compounds, Antioxidant Activities, Fatty Acids, and Sensorial Characteristics. Plants 2020, 9(11), 1627. DOI: 10.3390/plants9111627.
- Carocho, M.; Morales, P.; Ferreira, I. C. Antioxidants: Reviewing the Chemistry, Food Applications, Legislation and Role As Preservatives. Trends Food Sci. Technol. 2018, 71, 107–120. DOI: 10.1016/j.tifs.2017.11.008.
- Sharma, S.; Cheng, S.-F.; Bhattacharya, B.; Chakkaravarthi, S. Efficacy of Free and Encapsulated Natural Antioxidants in Oxidative Stability of Edible Oil: Special Emphasis on Nanoemulsion-Based Encapsulation. Trends Food Sci. Technol. 2019, 91, 305–318. DOI: 10.1016/j.tifs.2019.07.030.
- Lanza, B.; Ninfali, P. Antioxidants in Extra Virgin Olive Oil and Table Olives: Connections Between Agriculture and Processing for Health Choices. Antioxidants. 2020, 9(1), 41. DOI: 10.3390/antiox9010041.
- Shahidi, F.; Zhong, Y. Measurement of Antioxidant Activity. J. Funct. Foods. 2015, 18, 757–781. DOI: 10.1016/j.jff.2015.01.047.
- Summo, C.; Palasciano, M.; De Angelis, D.; Paradiso, V. M.; Caponio, F.; Pasqualone, A. Evaluation of the Chemical and Nutritional Characteristics of Almonds (Prunus Dulcis (Mill). D.A. Webb) As Influenced by Harvest Time and Cultivar. J. Sci. Food Agric. 2018, 98(15), 5647–5655. DOI: 10.1002/jsfa.9110.
- Özcan, M. M. A Review on Some Properties of Almond: ımpact of Processing, Fatty Acids, Polyphenols, Nutrients, Bioactive Properties, and Health Aspects. J. Food Sci. Technol. 2023, 60(5), 1493–1504. DOI: 10.1007/s13197-022-05398-0.
- Zheng, B.; He, Y.; Zhang, P.; Huo, Y.-X.; Yin, Y. Polyphenol Utilization Proteins in the Human Gut Microbiome. Appl. Environ. Microbiol. 2022, 88(3): e01851–21. DOI: 10.1128/aem.01851-21.
- De Giorgio, D.; Leo, L.; Zacheo, G.; Lamascese, N. Evaluation of 52 Almond (Prunus Amygdalus Batsch) Cultivars from the Apulia Region in Southern Italy. J. Horticultural Sci. Biotechnol. 2007, 82(4), 541–546. DOI: 10.1080/14620316.2007.11512271.
- Kodad, O.; Socias i Company, R.; Alonso, J. M. Genotypic and Environmental Effects on Tocopherol Content in Almond. Antioxidants 2018, 7(1), 6. DOI: 10.3390/antiox7010006.
- Tietel, Z.; Hammann, S.; Meckelmann, S. W.; Ziv, C.; Pauling, J. K.; Wölk, M.; Würf, V.; Alves, E.; Neves, B.; Domingues, M. R., et al. An Overview of Food Lipids Toward Food Lipidomics. Comprehensive Reviews in Food Science and Food Safety. Compr. Rev. Food Sci. Food Saf. 2023, 22(6), 4302–4354.
- Peh, H. Y.; Tan, W. D.; Liao, W.; Wong, W. F. Vitamin E Therapy Beyond Cancer: Tocopherol versus Tocotrienol. Pharmacology & Therapeutics. 2016, 162, 152–169. DOI: 10.1016/j.pharmthera.2015.12.003.
- Sabliov, C. M.; Fronczek, C.; Astete, C. E.; Khachaturyan, M.; Khachatryan, L.; Leonardi, C. Effects of Temperature and UV Light on Degradation of α-Tocopherol in Free and Dissolved Form. J. Am. Oil Chem. Soc. 2009, 86(9), 895–902. DOI: 10.1007/s11746-009-1411-6.
- Azzi, A. Many Tocopherols, One Vitamin E. Mol Aspects Med. 2018, 61, 92–103. DOI: 10.1016/j.mam.2017.06.004.
- Gouta, H.; Laaribi, I.; Ksia, E.; Juan, T.; Estopañan, G.; Martínez-Gómez, P. Physical Properties, Biochemical and Antioxidant Contents of New Promising Tunisian Almond Genotypes: Traits Stability, Quality Aspects and Post-Harvest Attributes. J. Food Compost. Anal. 2021, 98, 103840. DOI: 10.1016/j.jfca.2021.103840.
- Kodad, O.; Fernandez-Cuesta, A.; Velasco, L.; Estopañán, G.; El Baji, M.; Martínez-García, P. J.; Martínez-Gómez, P.; I Company, R. S. Genotype and Environment Effects on Phytosterol and Tocopherol Contents in Almond Kernel Oil. Seeds 2022, 1(4), 260–270. DOI: 10.3390/seeds1040022.
- Kodad, O.; Fernández-Cuesta, Á.; Karima, B.; Velasco, L.; Ercişli, S. Natural Variability in Phytosterols in Almond (Prunus Amygdalus) Trees Growing Under a Southern Mediterranean Climate. J. Horticultural Sci. Biotechnol. 2015, 90(5), 543–549. DOI: 10.1080/14620316.2015.11668712.
- Moreau, R. A.; Nyström, L.; Whitaker, B. D.; Winkler-Moser, J. K.; Baer, D. J.; Gebauer, S. K.; Hicks, K. B. Phytosterols and Their Derivatives: Structural Diversity, Distribution, Metabolism, Analysis, and Health-Promoting Uses. Prog. Lipid Res. 2018, 70, 35–61. DOI: 10.1016/j.plipres.2018.04.001.
- Maestri, D.; Martínez, M.; Bodoira, R.; Rossi, Y.; Oviedo, A.; Pierantozzi, P.; Torres, M. Variability in Almond Oil Chemical Traits from Traditional Cultivars and Native Genetic Resources from Argentina. Food Chem. 2015, 170, 55–61. DOI: 10.1016/j.foodchem.2014.08.073.
- Prescha, A.; Grajzer, M.; Dedyk, M.; Grajeta, H. The Antioxidant Activity and Oxidative Stability of Cold‐Pressed Oils. J. Am. Oil Chem. Soc. 2014, 91(8), 1291–1301. DOI: 10.1007/s11746-014-2479-1.
- Kodad, O.; Estopañán, G.; Juan, T.; Socias i Company, R. Tocopherol Concentration in Almond Oil from Moroccan Seedlings: Geographical Origin and Post-Harvest Implications. J. Food Compost. Anal. 2014, 33(2), 161–165. DOI: 10.1016/j.jfca.2013.12.010.
- Li, M.; Lu, P.; Wu, H.; de Souza, T. S. P.; Suleria, H. A. R. In vitro Digestion and Colonic Fermentation of Phenolic Compounds and Their Bioaccessibility from Raw and Roasted Nut Kernels. Food Funct. 2023, 14(6), 2727–2739. DOI: 10.1039/D2FO03392E.
- Lu, P.; Wu, H.; Gu, J.; Nawaz, M. A.; Ma, X.; Suleria, H. A. Impact of Processing on Bioaccessibility of Phytochemicals in Nuts. Food Rev. Int. 2023, 39(8), 5968–5985.
- Cha, C.-Y.; Lee, K.-G. Effect of Roasting Conditions on the Formation and Kinetics of Furan in Various Nuts. Food Chem. 2020, 331, 127338. DOI: 10.1016/j.foodchem.2020.127338.
- Nematollahi, A.; Kamankesh, M.; Hosseini, H.; Hadian, Z.; Ghasemi, J.; Mohammadi, A. Investigation and Determination of Acrylamide in 24 Types of Roasted Nuts and Seeds Using Microextraction Method Coupled with Gas Chromatography–Mass Spectrometry: Central Composite Design. J. Food Meas. Charact. 2020, 14(3), 1249–1260. DOI: 10.1007/s11694-020-00373-9.
- Bagheri, H.; Abbaszadeh, S. Application of Infrared Heating for Roasting Nuts. J. Food Qual. 2020, 2020, 1–10. DOI: 10.1155/2020/8866369.
- Lipan, L.; , M.; , L.; , C.L.; ,S.; , H.; , V.A.; , C.; , C.B. Nutrition Quality Parameters of Almonds As Affected by Deficit Irrigation Strategies. Molecules 2019, 24(14), 2646. DOI: 10.3390/molecules24142646.
- Milczarek, R. R.; Avena-Bustillos, R. J.; Peretto, G.; McHugh, T. H. Optimization of Microwave Roasting of Almond (P Runus Dulcis). J. Food Process. Preserv. 2014, 38(3), 912–923. DOI: 10.1111/jfpp.12046.
- Lipan, L.; Cano-Lamadrid, M.; Vázquez-Araújo, L.; Sendra, E.; Hernández, F.; Corell, M.; Moriana, A.; Carbonell-Barrachina, Á. A. How Does Water Stress and Roasting Temperature Affect the Physicochemical Parameters of Almonds? LWT 2021, 150, 112073. DOI: 10.1016/j.lwt.2021.112073.
- Board, C. A. Snack for Life. 2023. https://www.almonds.com/why-almonds/snacking#:~:text=1%20ounce%20of%20almonds%2C%20or,the%20Dietary%20Guidelines%20for%20Americans (accessed Jul 2023).
- Oracz, J.; Nebesny, E.; Żyżelewicz, D. Effect of Roasting Conditions on the Fat, Tocopherol, and Phytosterol Content and Antioxidant Capacity of the Lipid Fraction from Cocoa Beans of Different Theobroma Cacao L. Cultivars. Eur. J. Lipid Sci. Technol. 2014, 116(8), 1002–1014. DOI: 10.1002/ejlt.201300474.
- Gao, P.; Cao, Y.; Liu, R.; Jin, Q.; Wang, X. Phytochemical Content, Minor‐Constituent Compositions, and Antioxidant Capacity of Screw‐Pressed Walnut Oil Obtained from Roasted Kernels. Eur. J. Lipid Sci. Technol. 2019, 121(1), 1800292. DOI: 10.1002/ejlt.201800292.
- Massimo, L.; Laura, D. E.; Ginevra, L.-B. Phytosterols and Phytosterol Oxides in Bronte’s Pistachio (Pistacia Vera L.) and in Processed Pistachio Products. Eur. Food Res. Technol. 2020, 246(2), 307–314. DOI: 10.1007/s00217-019-03343-8.
- Chang, S. K.; Alasalvar, C.; Bolling, B. W.; Shahidi, F. Nuts and Their Co-Products: The Impact of Processing (Roasting) on Phenolics, Bioavailability, and Health Benefits–A Comprehensive Review. J. Funct. Foods 2016, 26, 88–122. DOI: 10.1016/j.jff.2016.06.029.
- Taş, N. G.; Gökmen, V. Phenolic Compounds in Natural and Roasted Nuts and Their Skins: A Brief Review. Curr. Opin. Food Sci. 2017, 14, 103–109. DOI: 10.1016/j.cofs.2017.03.001.
