Abstract
The objectives of this work were to determine the gelatinization temperature of jackfruit starch and to compare the effect of pH on the viscosity and density of different botanical sources of starch pastes. The study was conducted according to a three × four factorial design with three repetitions, being three levels for the starch paste (jackfruit seed, corn and cassava) and four levels for pH (3.4; 4.4; 5.4 and 7.0). The results showed a gelatinization temperature (GT) of jackfruit seed starch ranging from 75 to 80 °C, superior to GT range of common starch sources. Density of all starch pastes increased with pH. The effect of the kind of starch was not significant (P > 0.05). Viscosity of corn and cassava starch pastes varied with pH according to quadratic models. The viscosity of seed starch paste from jackfruit did not significantly vary with pH, indicating acid resistance.
El objectivo de este trabajo fue determinar la temperatura de gelatinización del almidón de la semilla de jaca y comparar el efecto del pH sobre la viscosidad y densidad de diferentes fuentes botánicas de pastas de almidón. El estudio se realizó siguiendo un diseño factorial de tres por cuatro con tres repeticiones, habiendo tres niveles para la pasta de almidón (semilla de jaca, maíz y yuca) y cuatro niveles para el pH (3,4; 4,4; 5,4 y 7,0). Los resultados mostraron una temperatura de gelatinización para el almidón de la semilla de jaca en el intervalo de 75 a 80 °C, superior al intervalo GT de otras fuentes comunes de almidón. La densidad de todas las pastas de almidón se incrementó con el pH. El efecto de la clase del almidón no fue significativo (P > 0,05). La viscosidad de la pasta del almidón de maíz y yuca variaron con el pH siguiendo modelos cuadraticos. La viscosidad de la pasta del almidón de jaca no varió significativamente con el pH, indicando resistencia ácida.
Palabras clave:
Introduction
Starch is the main reserve of substance in superior plants. It supplies 70–80% of the calories consumed by people. It is a renewable, biodegradable and non-toxic raw material, being largely used in food industries as an improvement agent of functional properties such as solubility, viscosity, force of gelatinization or adhesion in food products. The relationship between structural characteristics and thermal properties of starches and those of starch paste has received considerable attention (BeMiller, Citation1997; Leonel and Cereda, Citation2002; Liu, Selomulyo, & Zhou, Citation2008; Van der Burgt et al., Citation2000).
The starch world industry is at a decisive moment in its growth and development. It is questionable whether new chemical or derivative reactants will be approved for utilization in food products. In existing starches, the permitted levels of chemical modification treatments will remain static. These restrictions are based on consumer protection, workplace safety, environmental protection and decrease of production costs (BeMiller, Citation1997). Therefore, the food industry is interested in identifying species that produce native starches with adequate physicochemical characteristics once processed, in order to replace the chemically modified starches currently used as stabilizers and thickeners (Kim, Wisenborn, Orr, & Grant, Citation1995). These starches must be resistant to the natural acidity of various food products and to the heating processes to which they are submitted.
Starch granules are insoluble in cold water. However, after heating, there is an irreversible swelling of the granules which produces a viscous paste (Murphy, Citation2000). This phenomenon is known as starch gelatinization and it depends on factors such as the source of the starch and its concentration and temperature during the heating process (Morikawa and Nishinari, Citation2000). Gelatinization is defined as the collapse or rupture of the molecular order inside the starch granule, causing irreversible changes in its properties, such as the increase of the granule's size, crystals fusion, loss of birefringence and solvency. This occurs above a certain range of temperature; larger granules gel first and the smaller ones later (Thomas and Atwell, Citation1999).
Measurements of viscosity and density can be utilized as indirect measurements of the starch's resistance to acidity. Reduction of pH catalyzed by an increase of temperature causes a break in the molecule with consequent attrition reduction, molecular weight and an increase of the hydration degree of the molecules.
The objectives of this research were to determine the gelatinization point of the starch extracted from jackfruit seeds and to compare the pH effect on the density and viscosity of starch pastes obtained from jackfruit seed, corn and cassava.
Material and methods
Materials
Commercial starches of corn (Maizena®, Unilever Brasil, Pernambuco) and cassava (Juparanã®, Juparanã Industry, Espírito Santo) were obtained at the local market. Starch from jackfruit seed was extracted from fruits of the soft variety at the Process Engineering Laboratory of the Southwest Bahia State University (UESB), in the city of Itapetinga, Bahia.
Starch was extracted according to Singh and Singh (Citation2001), substituting sodium metabisulfite solution with distilled water. All chemical reagents were of analytical grade.
Determination of gelatinization temperature (GT) range
GT range was determined according to a method proposed by Souza and Andrade (Citation2000), who consider the rupture of 70% of the granules as the gelatinization point. The temperature range around this value is then the GT range.
A plate heater was used to maintain the dispersion of 50 g L−1 of jackfruit seed starch at the desired temperature (between 40 °C and 90 °C at 5 °C intervals). Treated samples were diluted ten times and analyzed in an optical microscope (Model BX50, Olympus, Tokyo). This determination was conducted three times, each one in triplicate.
Density determination
Density was determined using solutions of 100 g L−1 of cassava, corn and jackfruit seed corn starches in a citric acid–sodium phosphate buffer (0.1 M) with pH values of 3.4, 4.4, 5.4 and 7.0. The solutions were heated until 80 °C, until gelatinization of the samples. Then, the samples were cooled down to 25 °C and density was measured adopting the picnometric method, utilizing a 10-mL picnometer (Coimbra et al., Citation2006). The analyses were conducted three times, each one in triplicate.
Apparent viscosity determination
Apparent viscosity was determined using the same solutions used for density determination. Solutions of 100 g L−1 of cassava, corn and jackfruit seed corn starches in citric acid–sodium phosphate buffer (0.1 M) with pH values of 3.4, 4.4, 5.4 and 7.0 were prepared and heated up to 80 °C, until gelatinization of the samples.
As soon as gelatinized pastes were obtained, they were cooled down to 25 °C. Viscosity was determined using a digital rheometer (model DV-II plus, Brookfield) using a LV-3 spindle and 80 rpm of rotation. The analyses were conducted three times, each one in triplicate.
Statistical analysis
In order to study the influence of both pH and the kind of starch on the density and apparent viscosity of starch pastes, an experiment was set up in a 3 × 4 factorial design, entirely casual. Three kinds of starch (jackfruit seed, corn and cassava) were used under four levels of pH (3.4, 4.4, 5.4 and 7.0) and three repetitions were conducted, each one in triplicate.
Experimental data were submitted to Analysis of Variance (ANOVA), using the Fisher test (P < 0.05) and linear regression analysis, adopting as choice criteria for best models the significance parameters (Student test with P < 0.05), residual analysis, determination coefficient (r 2) and standard errors.
Results and discussion
GT range
The increase of temperature resulted in an increased swelling of jackfruit starch granules. An excessive swelling occurred at temperatures from 75 to 80 °C, the GT range, with the granules losing their molecular organization. Therefore, this interval was determined as the gelatinization point. The phenomenon described can be observed in , displaying a loss of molecular structure in the granules of 40–86%, respectively.
Figure 1. Optical micrographs of jackfruit seed starch granules at gelatinization temperature range ((a) treated at 75 °C, average diameter of 9.2 μm; (b) treated at 80 °C, average diameter of 14.2 μm).
Figura 1. Microfotografía de granulos de almidón de semilla de jaca en el intervalo de temperature de gelatinización ((a) tratado a 75 °C, diámetro medio de 9,2 μm; (b) tratado a 80 °C, diámetro medio de 14,2 μm).
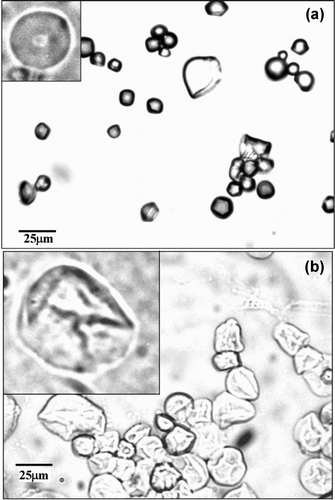
The GT range of starch extracted from the jackfruit seeds is considered high if compared to the values determined by microscopic technique of other starch sources such as normal corn, which has a GT ranging from 70 to 75 °C (Ratnayake and Jackson, Citation2007; Souza and Andrade, Citation2000), rice and cassava (from 55 to 60 °C), potato (from 65 to 70 °C) and wheat, with a GT ranging from 70 to 75 °C (Ratnayake and Jackson, Citation2007).
This elevated GT, associated with the presence of strong connections inside granules which resist breaking during the heating period (Murphy, Citation2000), indicates the presence of greater proportions of long ramified chains of amilopectin in starch extracted from jackfruit seeds.
The elevated GT range has advantages that can be used in the food industry. Because of the high gelatinization point, higher temperatures can be applied to drying starch compared to traditional starches, accelerating its acquisition. In some products, such as biscuits and sausages, a higher level of gelatinization is not desirable during cooking. The use of jackfruit starch can be used to optimize this characteristic.
Effect of pH and kind of starch on density
Density is a parameter which can be utilized as a measure of starch hydrolysis; consequently it is an efficient parameter to evaluate starch resistance in processes at low pH levels.
presents the result of Variance Analysis (ANOVA) for density measurements. There was a significant difference (P < 0.05) in density values at different levels of pH, a non-significant difference (P > 0.05) for different kinds of starch, and the pH-starch interaction. Therefore, a unique model needs to be adjusted, with density values at each pH level obtained from the average of all kinds of starch under test.
Table 1. ANOVA test of effect of pH and starches on density and apparent viscosity.
Tabla 1. Prueba ANOVA del efecto del pH y almidón sobre la densidad y viscosidad aparente.
Density values for pH 3.4, 4.4, 5.4 and 7.0 were equal to 1017.95 kg m−3, 1019.15 kg m−3, 1020.13 kg m−3 and 1023.88 kg m−3, respectively. shows the experimental values and fitted model.
Figure 2. Density of starch pastes in function of pH levels.
Figura 2. Densidad de pastas de almidón en función del pH.
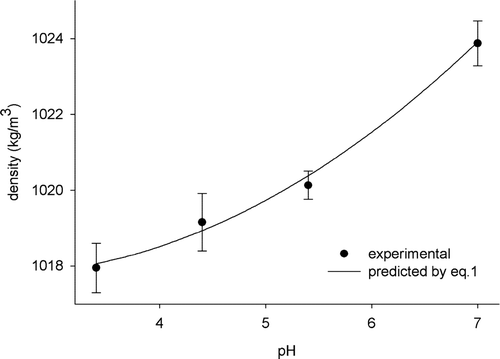
The results show that density increased as pH increased. Through regression analysis, it was verified that the relationship between pH and density could be approached by a quadratic model, EquationEquation (1), as shown below. The equation parameters were significant (P < 0.05), residual analysis was satisfactory and the determination coefficient (r
2) was 0.99.
The behavior described in EquationEquation (1) and observed in is associated with the degree of hydration of the starch molecule. The pH is inversely proportional to the hydration degree of the starch. pH reduction causes more starch acid hydrolysis, which becomes more hydrated (Murphy, Citation2000). The increase in hydration degree is related to the increase in the granule volume and total solution volume and, consequently, to density reduction.
Effect of pH and kind of starch on apparent viscosity
presents the results from ANOVA regarding the analysis of viscosity as a function of pH and different kinds of starches. The significant effect of pH, the kind of starch and the interaction between them were verified.
Each kind of starch presented a different behavior for apparent viscosity within the pH range. The jackfruit seed starch paste did not present variation of apparent viscosity under different pH values (P > 0.05), remaining constant at 7.15 mPa s.
Thus, cassava starch paste and corn starch paste present alterations in their apparent viscosity under the pH range. The behavior of each one was approached to fit the quadratic model. For both, the parameters were significant (P < 0.05), residual analyses were satisfactory and high determination coefficients were obtained. Fitted equations are shown in Equations (Equation2) and (Equation3
), respectively.
shows a maximum viscosity value for corn and cassava starch pastes between pH 5 and 6. The first derivative of obtained models shows pH values of exactly 5.18 for a maximum viscosity of corn starch paste and 5.66 for cassava starch paste. For these values, apparent viscosity values are 143.7 mPas and 170.5 mPas to corn and cassava starches pastes, respectively.
Figure 3. Apparent viscosity of the studied starch pastes.
Figura 3. Viscosidad aparente de las pastas de almidón estudiada.
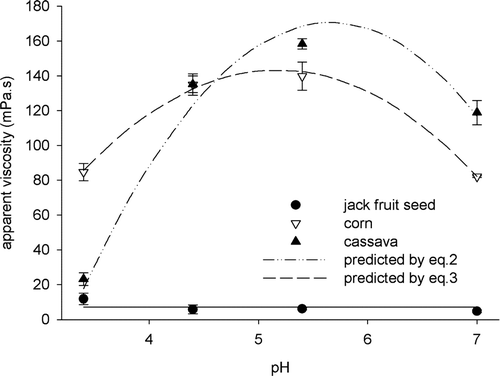
Furthermore, the observed behavior shows that jackfruit seed starch paste was resistant to acidity for the range of pH under study, while other pastes were greatly influenced by the pH values, leading to starch hydrolysis.
Saartrat, Puttanlek, Rungsardthong, and Uttapap (Citation2005) investigated the effect of an acid environment on the stability of canna starches and observed that peak viscosity decreased as pH changed from 2.5 to 7.0. The authors reported that acidic treatment affected the pasting properties of canna starches by partial hydrolysis of the glycosidic linkages of starch molecules in the granules.
With corn and cassava pastes, the initial change of pH from 7.0 to near 5.0 caused partial hydrolysis of starch granules, as large molecules that originally were inside granules hydrated, increasing viscosity. Reducing pH to values less than 5.0 caused an intense starch hydrolysis, with a large number of small molecules that did not contribute much to viscosity.
Despite the low apparent viscosity value of jackfruit seed starch paste, its acidity resistance could be used to formulate acid products, such as salad sauces and fermented dairy drinks, where once modified starches were generally employed. In order to amplify the use of starch in food industries, the applications of the starch under examination need further research.
Conclusion
Jackfruit seed starch presents an elevated GT range, from 75 to 80 °C, compared to common starches, such as corn and cassava. The density behavior of starch paste from corn, cassava and jackfruit seeds did not present significant differences (P > 0.05). Their values increased as pH increased. Jackfruit starch was resistant to acid environments of the pH range under study. It did not show alterations in apparent viscosity values, becoming a new alternative for acid products formulation.
Notes
Research conducted at Process Engineering Laboratory, Southwest Bahia State University – UESB.
References
- BeMiller , J. N. 1997 . Starch modification: challenges and prospects . Starch/Stärke , 49 : 127 – 131 .
- Coimbra , J. S.R. , Gabas , A. L. , Minim , L. A. , Garcia Rojas , E. E. , Telis , V. R.N. and Telis-Romero , J. T. 2006 . Density, heat capacity and thermal conductivity of liquid egg products . Journal of Food Engineering , 74 : 186 – 190 .
- Kim , Y. S. , Wisenborn , D. P. , Orr , P. H. and Grant , L. A. 1995 . Screening potato starch for novel properties using differential scanning calorimetry . Journal of Food Science , 60 : 1060 – 1065 .
- Leonel , M. and Cereda , M. P. 2002 . Caracterização físico-química de algumas tuberosas amiláceas . Ciência e Tecnologia de Alimentos , 22 : 65 – 69 .
- Liu , Y. , Selomulyo , V. O. and Zhou , W. 2008 . Effect of high pressure on some physicochemical properties of several native starches . Journal of Food Engineering , 88 : 126 – 136 .
- Morikawa , K. and Nishinari , K. 2000 . Effects of concentration dependence of retrogradation behavior of dispersions for native and chemically modified potato starch . Food Hydrocolloids , 14 : 395 – 401 .
- Murphy , P. 2000 . “ Starch (chapter 3) ” . In Handbook of hydrocolloids , Edited by: Phillips , G. O. and Williams , P. A. 41 – 65 . Boca Raton, , Florida : CRC Press .
- Ratnayake , W. S. and Jackson , D. S. 2007 . A new insight into the gelatinization process of native starches . Carbohydrate Polymers , 67 : 511 – 529 .
- Saartrat , S. , Puttanlek , C. , Rungsardthong , V. and Uttapap , D. 2005 . Paste and gel properties of low-substituted acetylated canna starches . Carbohydrate Polymers , 61 : 211 – 221 .
- Singh , J. and Singh , N. 2001 . Studies on the morphological, thermal and rheological properties of starch separated from some Indian potato cultivars . Food Chemistry , 75 : 67 – 77 .
- Souza , R. C.R. and Andrade , C. T. 2000 . Investigação dos processos de gelatinização e extrusão de amido de milho . Polímeros , 10 : 24 – 30 .
- Thomas , D. J. and Atwell , W. A. 1999 . Starches: Practical guides for the food industry , 94 Saint Paul, , Minnesota : Eagan Press .
- Van der Burgt , Y. E.M. , Bergsma , J. , Bleeker , I. P. , Mijland , P. J.H.C. , Kamerling , J. P. and Vliegenhart , J. F.G. 2000 . Structural studies on methylated starch granules . Starch/Stärke , 52 : 40 – 43 .