?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Phenolic compounds are considered to be an important autotoxic substances. The interactions between them and soil microbes are increasingly becoming a focus of the allelopathic research studies. In the present study, we have evaluated the effect of 4-hydroxybenzoic acid on soil microbial community structure and functional diversity. Bulk soil without grapevine cuttings (Group 1) and rhizosphere soil with Beta (Vitis riparia × Vitis labrusca) grapevine cuttings (Group 2) were used as research subjects. The bacterial (16s rRNA) and fungal internal transcribed spacer regions community structures were obtained using a denaturing gradient gel electrophoresis and substrate utilization patterns were determined using Biolog EcoPlates. In this research, the results showed that the 4-hydroxybenzoic acid caused a shift in the soil bacterial and fungal community structures and changed the soil microbial functional diversity. The results also showed that the diversity of the microbial community structure and function differed between the two designated groups. The application of 4-hydroxybenzoic acid (1 and 2 mg/g) to the soil resulted in a greater decrease in the average diversity of the bacterial community structure in Group 2, compared to Group 1. Moreover, the average diversity of the fungal community structure in Group 2 became higher than that in Group 1. However, the bacterial and fungal community structural diversities increased with the application of 0.5 mg/g of 4-hydroxybenzoic acid. Thus, we concluded that both soil microbes and root exudates might be the recipients of 4-hydroxybenzoic acid.
Introduction
The development of sustainable agriculture is crucial because of land restrictions and environmental considerations. However, the replant problem is a serious constraint on the development of sustainable agriculture. The deterioration of soil physical and chemical properties, soil-borne diseases and autotoxicity are considered as the main reasons for this problem.[Citation1] Out of these three reasons, autotoxicity is considered the most serious problem.[Citation2,Citation3] Autotoxicity is a form of intraspecific allelopathy, where plants release toxic chemicals into the environment to inhibit the growth of their own species.[Citation4]
Numerous studies have focused on the effect of allelochemicals in the soil environment. Although phytotoxicity of allelopathic substances has been observed in vitro, these substances are not present in soils at high enough concentrations or for long enough periods of time to account for the observed inhibition phenomenon.[Citation5] Inderjit et al. [Citation6] reported that the phytotoxic effects of catechin may be exerted through microbes in some soils. In addition, Cantor et al. [Citation7] found that low allelochemical concentrations, detected in garlic mustard-invaded forest soils, inhibited fungal growth and arbuscular mycorrhizal fungi spore germination. Further, Zhou and Wu [Citation8] discovered that phenolic acid could inhibit cucumber (Cucumis sativus L.) seedling growth and change the soil microbial community. Numerous studies have focused on the shift in the microbial community in rhizosphere soil with the replant problem.[Citation1,Citation9–11] Huang et al. [Citation12] found that autotoxins influence soil microbes, leading to an increased or decreased degree of soil sickness.
Grapevine (Vitis vinifera L.) is a very important fruit tree. However, it has suffered from a serious replant problem. This phenomenon can be traced to the last century.[Citation13,Citation14] Studies have focused on how to overcome or ease the grapevine replant problem and results have shown that soil fumigation is an effective solution.[Citation15–17] These studies support the hypothesis that biotic factors play a key role in the grapevine replant problem.[Citation18] However, factors that account for changes in biotic factors after the grapevine replanting have not been studied yet. Brinker and Creasy [Citation19] found a negative effect of old grapevine root exudates and this phenomenon was subsequently interpreted as autotoxicity. However, the toxic substances were not determined. In our preliminary study, 4-hydroxybenzoic acid and salicylic acid were first detected in grapevine root exudates, which had significant autotoxicity effects on tissue culture plantlets and pot cuttings of grapevine.[Citation20] However, studies on how the allelochemicals influence the soil microbial community have not been conducted.
In this study, we hypothesized that the microbial community structural and functional diversities differed between the control (soil, untreated with 4-hydroxybenzoic acid) and soil, treated with 4-hydroxybenzoic acid. In addition, the microbial responses to the 4-hydroxybenzoic acid in the soil, in which the grapevine cuttings were planted, differed from those in the soil without grapevine cuttings. The objectives of the present work were to examine the effects of different concentrations of artificially applied 4-hydroxybenzoic acid on the soil microbial community structure and function, and to determine the role of root exudates on the autotoxicity effects of phenolic compounds.
Materials and methods
Field experiments
This experiment was conducted in the vineyard of Shenyang Agricultural University, Liaoning province, China (41.8°N, 123.4°E). The field experiment was divided into two groups (Group 1 did not contain grapevine; Group 2 did contain grapevine) that were subjected to rain-shelter conditions (28 °C during the day/18 °C during the night, relative humidity 60%–80%, 16 h light/8 h dark). Beta cuttings (V. riparia × V. labrusca) were used. The same soil, treatment concentrations and sampling times (6, 12, 18, 24 and 30 d after treatment) were used for both groups. The soil was a mixture of leisure soil (grapevine was not previously planted in the soil), peat moss and sand (7:2:0.5 V/V/V), containing 14.47 mg/kg available N, 96.54 mg/kg available P, 265.5 mg/kg available K, 8.48 g/kg total P, 4.86 g/kg total K, 1.06 g/kg total N, with a pH of 6.70. Each pot (20 cm diameter, 15 cm height) contained 2.4 kg of dry culture medium. The pots were filled with soil in May and water was added daily to maintain 60% soil water holding capacity. The height and diameter of the stems were measured when all cuttings reached a height of 5 cm. We selected uniform cuttings and randomly divided them into four subgroups. Different concentrations of 4-hydroxybenzoic acid (0.5 mg/g, 1.0 mg/g, 2.0 mg/g dry soil) were applied to the pots. To adjust the solution pH to 7.0, 1 mol/L NaOH was used. The control was treated with distilled water (0.0 mg/g 4-hydroxybenzoic acid). These concentrations of 4-hydroxybenzoic acid exerted different effects on grapevine cuttings based on our preliminary experiment.[Citation21]
Plant growth parameters and soil sampling
The cuttings were separated into aboveground (leaves and stem) and belowground (roots) components. The fresh weight of these components was measured on three replicates after the soil sampling was completed. At each sampling period (6, 12, 18, 24 and 30 d after treatment), three pots were randomly selected for the collection of soil samples. The growth parameters of all remaining cuttings were evaluated before soil sample collection.
The soil in Group 1 was collected from three pots using the point-centred quarter sampling method. The rhizosphere soil in Group 2 was collected following the Nazih et al. [Citation22] method. In the present study, soil clods were shaken off and only the soil adhering to roots was considered as rhizosphere soil. The soil samples were sieved through 40-mesh sieves. The soil samples, collected from Group 1 and Group 2, were stored at 4 and −70 °C, respectively.
The response index (RI; Williamson and Richardson [Citation23]) was measured based on the average of three replications as follows:(1)
(1)
In the above formula, C represents the control data and T is the treatment data. The absolute value of RI represents the phytotoxic effect. An RI value > 0 indicates a stimulatory effect, whereas a value < 0 indicates an inhibitory effect.
Soil DNA extraction and polymerase chain reaction-denaturing gradient gel electrophoresis
Total DNA was extracted from 0.20 g soil using the Ezup Column Soil DNA Purification Kit (Sangon Biotech Co., Ltd., Shanghai) following the protocol provided by the manufacturer. The DNA of each sample was amplified using primers 968-GC-F and 1401R [Citation24] for the 16 S rRNA gene. Nested PCR protocols were used to amplify the fungal partial internal transcribed spacer (ITS) regions. The extracted DNA was amplified using the primer sets ITS1F/ITS4 [Citation25] and ITS1-GC-F/ITS2 [Citation26] in the first and the second rounds of PCR amplifications, respectively.
The PCR was performed in a total volume of 25 µL containing of 2 µL buffer (500 mmol/L KCl, 100 mmol/L Tris-HCl and 15 mmol/L MgCl2), 400 nmol/L of each primer, 200 μmol/L of dNTPs, 0.5 U Polymerase and 1 µL of template DNA. The PCR conditions for bacteria were 95 °C for 5 min, followed by 30 cycles of 30 s at 94 °C, 30 s at 59 °C, 30 s at 72 °C and a final extension at 72 °C for 5 min. The PCR conditions for fungi were 94 °C for 7 min, followed by 32 cycles of 60 s at 94 °C, 60 s at 58 °C (59 °C for the second round), 60 s at 72 °C and a final extension at 72 °C for 15 min.
Aliquots (5 µL) of each PCR mixture were examined using eletrophoresis in 1% (w/v) agarose gel, stained to check the fragment size and integrity. A 20 µL aliquot of each bacterial and fungal PCR product was used for DGGE analysis.
DGGE was performed on 8% (w/v) acrylamide gel using a 30%–60% denaturant gradient for bacterial PCR products and acrylamide gel with a 15%–40% denaturant gradient for fungal PCR products. A 100% denaturing acrylamide was defined as containing 7 mol/L urea and 40% (v/v) formamide. The gels were run in a 1 × TAE [Tris-acetate-ethylenediaminetetraacetic acid (EDTA)] buffer (50 × TAE was made by using ddH2O to dissolve 242 g Tris, 37.2 g Na2EDTA∙2H2O and 57.1 mL acetic acid to a final volume of 1 L.) for 6.5 h under conditions of 60 ºC and 160 V for bacteria, and 14 h under 70 V for fungi.
DGGE was performed using a DCode universal mutation detection system (Bio-Rad Lab, LA). The gels were stained for 30 min in GeneFinder™ (1:10,000 dilution) and the images were captured using GelDocXR+ (Bio-Rad Lab, LA). All gels were digitized using Quantity One software (Bio-Rad Inc., Carlsbad, CA). The Shannon index was calculated by [Citation27](2)
(2) where pi is the ratio between the specific band intensity and the total intensity of all bands in a lane sample.
The index here was to evaluate the soil microbial community structural diversity.
Biolog EcoPlates method
Biolog EcoPlates were used to study the metabolic profiles of the microbial communities following Garland and Mills.[Citation28]
Biolog EcoPlates (USA) was used to evaluate the community level physiological profile. Briefly, 5 g of fresh soil was added to a tube containing 45 mL of sterile 0.85% NaCl solution and a soil suspension of 10−1 dilution was obtained after shaking the tube for 2 min. After the solution was diluted to a 10−3 dilution, using 0.85% NaCl solution, 150 µL of the 10−3 dilution was added to each well of a microplate. The microplate was incubated at 25 °C for seven days and the absorbance of the plates was measured every 24 h at a wavelength of 590 nm using a microplate reader (Biolog MicrostationTM).
The Shannon index was calculated by(3)
(3) where pi is the proportion of metabolic activity of the microbial community of a particular substrate, relative to all substrates.
The index here was to evaluate the soil microbial community functional diversity.
Statistical analysis
A principal component analysis (PCA, using Canoco for Windows 4.5) based on the data collected from the five treatment times was performed to analyse the functional profile of the treatments in the two groups. There were six types of carbon substrates in the Biolog EcoPlates.[Citation29]
The digitization of the DGGE profiles was performed using Quantity One software 4.6.2 (Bio-Rad Lab, USA). All the data obtained from the DGGE and Biolog EcoPlates was subjected to Tukey's honestly significant difference (HSD) test using IBM SPSS Statistics 20.0 for Windows. All treatments were replicated three times.
Results and discussion
Plant growth
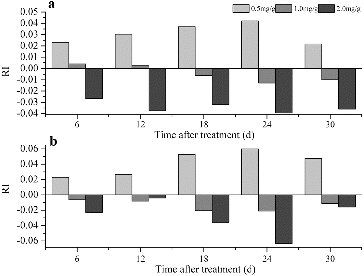
The different concentrations of 4-hydroxybenzoic acid exerted different effects. The RI values of the aboveground and belowground components of the grapevine cuttings increased in response to 0.5 mg/g 4-hydroxybenzoic acid, but were reduced with the application of 2.0 mg/g (). The growth of the belowground components was inhibited by treatment with 1.0 mg/g, whereas the growth of the aboveground components was promoted by treatment with 1.0 mg/g 6 and 12 d after the application ().
Soil microbial community structure diversity
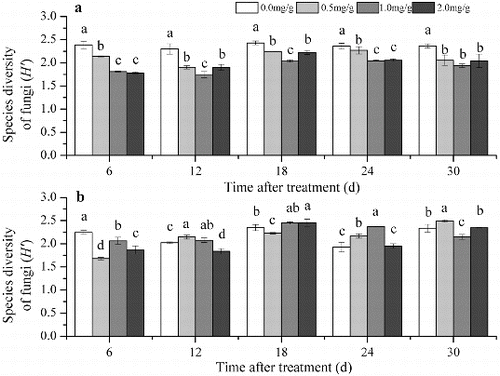
The Shannon index of the DGGE pattern reflects the soil microbial community composition.[Citation30,Citation31] In Group 1, the Shannon index of the bacterial community was significantly higher compared with the control 24 d after treatment with 0.5 mg/g 4-hydroxybenzoic acid, 6 and 18 d after treatment with 1.0 mg/g 4-hydroxybenzoic acid and 6, 18 and 24 d after treatment with the 2.0 mg/g 4-hydroxybenzoic acid. At the other time periods, the Shannon index was lower in the treated soil than in the control or the difference was not significant ((a)). In Group 1 the Shannon index of the fungal community in all 4-hydroxybenzoic acid-amended soils was lower than the control during all time periods ((a)). Numerous studies on replanting problems have focused on the variation in the soil microbial community.[Citation1,Citation9] In the present study, shifts in the soil microbial communities were observed after treatment with 4-hydroxybenzoic acid. After the treatment with 4-hydroxybenzoic acid, the community structural diversity of bacteria and fungi was altered in Group 1. Bacteria and fungi had different responses to 4-hydroxybenzoic acid in this group ((a) and (a)). Previous studies have shown that the bacteria and fungi of cucumber (C.sativus L) rhizosphere soil responded differently to vanillic acid. The increase in bacterial abundance was lower than that of fungi after the vanillic acid treatment.[Citation8] In the present study, the structural diversity of the bacterial community differed between the treated soil and control soil during the different stages. The average Shannon index of the bacteria was lower after treatment with 0.5 mg/g 4-hydroxybenzoic acid than that of the control, whereas the index was higher, when compared with the control, after treatment with the other concentrations of 4-hydroxybenzoic acid. The fungal community structural diversity decreased after treatment with 4-hydroxybenzoic acid.
Figure 2. The diversity based on the DGGE analysis of the bacterial community in Group 1 (a) and Group 2 (b). Note: Soil without grapevine cuttings (Group 1); soil with grapevine cuttings (Group 2); different letters indicate significant differences (P < 0.5, Tukey's HSD test).
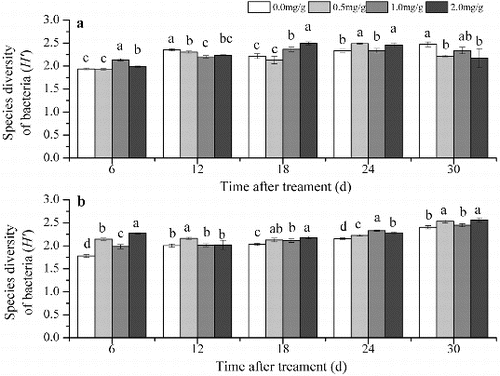
Figure 3. The diversity based on the DGGE analysis of the fungal community in Group 1 (a) and Group 2 (b). Note: Soil without grapevine cuttings (Group 1); soil with grapevine cuttings (Group 2); different letters indicate significant differences (P < 0.5, Tukey's HSD test).
In Group 2 after treatment with 0.5 mg/g 4-hydroxybenzoic acid, the Shannon index was higher than the index in the control soil during all time periods. The Shannon index was also higher after treatment with 1.0 mg/g and 2.0 mg/g 4-hydroxybenzoic acid compared with the control, or the difference between the treated soils and control ones was not significant at all time periods ((b)). In Group 2, 6 and 18 d after treatment with 0.5 mg/g 4-hydroxybenzoic acid, 6 and 30 d after treatment with 1.0 mg/g 4-hydroxybenzoic acid and 6 and 12 d after treatment with 2.0 mg/g 4-hydroxybenzoic acid, the Shannon index was significantly lower than that in the control soil. There was no difference or the index value of the treated soils was higher than the index value of the control soil at other times ((b)).
In contrast with Group 1, the average diversity of the bacterial community structure decreased in Group 2 except in the 0.5 mg/g treatment, whereas the average diversity of the fungal community structure increased, except for the fungal diversity in the control soil (). The difference between the treatments in these two groups might result from the role of root exudates. For example, the bacterial community structural diversity of the control in Group 2 was lower than that in Group 1. The diversity decreased in Group 1 when the soil was treated with 0.5 mg/g of 4-hydroxybenzoic acid. However, 0.5 mg/g of 4-hydroxybenzoic acid increased the bacterial community structural diversity in Group 2 (). These results show that 4-hydroxybenzoic acid can regulate the components of root exudates.
Table 1. Average Shannon index based on the DGGE of the soil microbial community at five sampling periods (6, 12, 18, 24 and 30 d after treatment).
Soil microbial functional diversity
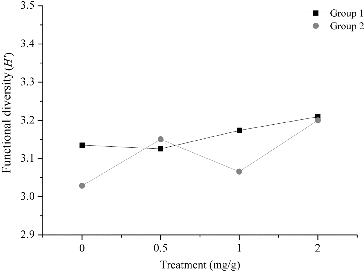
The Shannon index in this part represented the functional diversity of soil microbes. The Shannon index in Group 1 in soils amended with 4-hydroxybenzoic acid was higher than that in the control soil, or there were no significant differences ((a)). In Group 2 the Shannon index was higher compared with the control 18 d after treatment with 0.5 mg/g 4-hydroxybenzoic acid, 6, 18 and 30 d after treatment with 1.0 mg/g 4-hydroxybenzoic acid and 6, 18 and 24 d after treatment with 2.0 mg/g 4-hydroxybenzoic acid. At the other time periods, the Shannon index was lower in the treated soil compared with the control or there were no significant differences ((b)).
Figure 4. The functional diversity based on Biolog EcoPlates in Group 1 (a) and Group 2 (b). Note: Soil without grapevine cuttings (Group 1); soil with grapevine cuttings (Group 2); different letters indicate significant differences (P < 0.5, Tukey's HSD test).
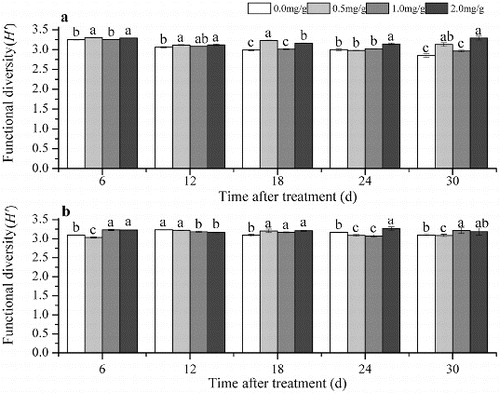
The results indicated that the involvement of grapevine cuttings increased the Shannon index in the control soil. After treatment with 4-hydroxybenzoic acid, the soil microbial function changed and the shifts differed between the two groups. Comparing the indexes during treatment with the same concentrations between Group 1 and Group 2, the average Shannon index was lower in the 0.5 mg/g treatment, but was higher in the 1.0 mg/g and 2.0 mg/g treatments in Group 1 than that in Group 2 ().
Figure 5. Average Shannon index based on Biolog EcoPlates in the soil microbial community at five sampling periods (6, 12, 18, 24 and 30 d after treatment). Note: Soil without grapevine cuttings (Group 1); soil with grapevine cuttings (Group 2).
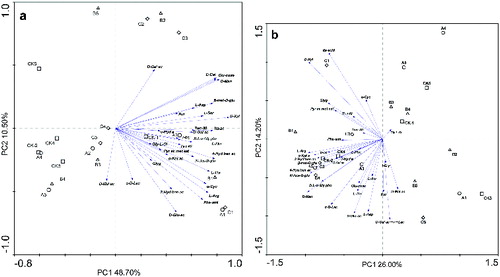
The observed shifts in the metabolic profiles of the microbial communities are caused by culturable bacteria that are able to grow rapidly under high-nutrient conditions in the substrate wells of the Biolog EcoPlates.[Citation32] Therefore, changes in the substrate use patterns indicate a shift in the bacterial species that use these substrates. The average well colour development data collected after five days for all treatments were used for the PCA. The first and second axes explained 59.20% of the variation of sole carbon utilization for soil microbial function in Group 1 (PC1 and PC2) ((a)) and explained 40.20% of the variance in Group 2 ((b)). Furthermore, there was a difference in the sole carbon used by bacteria between these two groups. All the treatments were highly correlated with the use of D-malic acid (0.899 in Group 1, −0.696 in Group 2), L-arginine (0.732 in Group 1, −0.690 in Group 2), 4-hydroxybenzoic acid (0.785 in Group 1, −0.623 in Group 2) and itaconic acid (0.771 in Group 1, 0.689 in Group 2) (). In addition, the treatments correlated with D-mannitol (0.821), glucose-1-phosphate (0.797), D-cellobiose (0.796), β-methyl-D-glucoside (0.770), L-threonine (0.743), α-cyclodextrin (0.714) and D-glucosaminic acid (0.664) in Group 1 ((a)), whereas, the treatments were mainly correlated with D-xylose (0.616), N-acetyl-D-glucosamine (−0.671), β-ketobutyric acid (−0.635) and D-galacturonic acid-γ-lactone (−0.640) in Group 2 ((b)). These results reiterate the fact that root exudates affect bacterial community diversity.
Figure 6. Principal component analysis of carbon substrate utilization patterns obtained using Biolog EcoPlates for Group 1 (a) and Group 2 (b). Note: CK, A, B and C represent 0.0, 0.5, 1.0 and 2.0 mg/g treatment, respectively; 1, 2, 3, 4 and 5 represent 6, 12, 18, 24 and 30 d after treatment, respectively.
Final remarks
Li et al. [Citation33] suggested that the application of autotoxins such as benzoic acid, diisobutyl phthalate, diisobutyl succinate, palmitic acid and 2,2-bis(4-hydroxyphenyl) propane would increase the content of phenol and phenolic acid in the root exudates of Panax ginseng. Our preliminary study showed that 4-hydroxybenzoic acid caused a shift in the components (i.e. amino acids and soluble carbohydrates) of grape root exudates (the data are yet to be published). The key effect of root exudates on the soil microbial community has been proven in many studies.[Citation34,Citation35] Peanut root exudates significantly promoted the growth of soil-borne pathogens and its stimulation was increased with increasing concentrations of the added peanut root exudates in vitro.[Citation36] Root exudates from grafted-root watermelon played a role in inhibiting Fusarium oxysporum f. sp. niveum.[Citation37] Root exudates can play stimulatory and repressive roles in certain microbes.[Citation38] However, Neumann et al. [Citation39] found that the soil type influenced the root exudates, which may be a result from plant interactions with soil-specific microbiomes. Therefore, it is not sure whether 4-hydroxybenzoic acid first affected the root exudates or the soil microbes, or whether the effect was simultaneous. Therefore, the relationship between root exudates, soil microbes and 4-hydroxybenzoic acid requires further study.
However, the diversity that we observed represented only a part of the soil microorganism community. In the studies of microbial communities, DGGE is a widely accepted method, but the technique might underestimate the community richness and diversity due to the similar migration behaviour caused by different sequences.[Citation40]
Moreover, the Biolog EcoPlates can only detect soil bacteria that can be cultivated. The method is not sufficient to clarify the expression of allelochemicals on target plants by focusing only on soil microbial diversity. Phenolic acids can act as specific substrates or signalling molecules for large groups of soil microbial species.[Citation38] Thus, the particular soil microbes that participated in the biotransformation process of 4-hydroxybenzoic acid should be studied in the future.
Conclusions
The present work showed that the 4-hydroxybenzoic acid changed the microbial functional diversity of the soil, as well as the structural diversity of the soil bacterial and fungal communities. Moreover, these shifts were different in soils with and without grapevine cuttings. The soil microbes and root exudates were also affected by treatment with 4-hydroxybenzoic acid. With the mediation of 4-hydroxybenzoic acid, the interaction between root exudates and soil microbes is a key factor for the expression of 4-hydroxybenzoic acid allelopathic potential on grape and this interaction may be the major reason for grapevine replant problem.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Qu XH, Wang JG. Effect of amendments with different phenolic acids on soil microbial biomass, activity, and community diversity. Appl Soil Ecol. 2008;39(2):172–179.
- Sampietro DA, Sgariglia MA, Soberón JR, Quiroga EN, Vattuone MA. Role of sugarcane straw allelochemicals in the growth suppression of arrowleaf sida. Environ Exp Bot. 2007;60(3):495–503.
- Zhao X, Zhen W, Qi Y, Liu X, Yin B. Coordinated effects of root autotoxic substances and Fusarium oxysporum Schl. f. sp. fragariae on the growth and replant disease of strawberry. Front Agric China. 2009;3(1):34–39.
- Singh HP, Batish RD, Kohli RK. Autotoxicity: concept, organisms, and ecological significance. Crit Rev Plant Sci. 1999;18(6):757–772.
- Teasdale JR, Rice CP, Cai G, Mangum RW. Expression of allelopathy in the soil environment: soil concentration and activity of benzoxazinoid compounds released by rye cover crop residue. Plant Ecol. 2012;213(12):1893–1905.
- Inderjit RK, Kaur R, Kaur S, Callaway RM. Impact of (±)-catechin on soil microbial communities. Commun Intgr Biol. 2009;2(2):127–129.
- Cantor A, Hale A, Aaron J, Traw MB, Kalisz S. Low allelochemical concentrations detected in garlic mustard-invaded forest soils inhibit fungal growth and AMF spore germination. Biol Invasions. 2011;13(12):3015–3025.
- Zhou X, Wu F. Artificially applied vanillic acid changed soil microbial communities in the rhizosphere of cucumber (Cucumis sativus L.). Can J Soil Sci. 2013;93(1):13–21.
- Zou L, Yuan XY, Li L, Wang XY. Effects continuous cropping on soil microbes on soybean roots. J Microbiol. 2005;2:27–30.
- Qi J, Yao H, Ma X, Zhou L, Li X. Soil microbial community composition and diversity in the rhizosphere of a Chinese medicinal plant. Commun Soil Sci Plant Anal. 2009;40(9–10):1462–1482.
- Wu F, Wang X, Xue C. Effect of cinnamic acid on soil microbial characteristics in the cucumber rhizosphere. Eur J Soil Biol. 2009;45(4):356–362.
- Huang LF, Song LX, Xia XJ, Mao WH, Shi K, Zhou YH, Yu JQ. Plant-soil feedbacks and soil sickness: from mechanisms to application in agriculture. J Chem Ecol. 2013;39(2):232–242.
- Deal DR, Mail WF, Boothyord CW. A survey of biotic relationships in grape replant situations. Phytopathology. 1972;62:503–507.
- Traquair JA. Etiology and control of orchard replant problems: a review. Can J Plant Pathol. 1984;6(1):54–62.
- Waschkies C, Schropp A, Marschner H. Relations between replant disease, growth parameters and mineral nutrition status of grapevines(Vitis sp.). Vitis. 1993;32(2):67–76.
- Schneider SM, Ajwa H, Trout TJ. Chemical alternatives to methyl bromide for nematode control under vineyard replant conditions. Am J Enol Vitic. 2006;57(2):183–193.
- Cabrera JA, Wang D, Schneider SM, Hanson BD. Subsurface drip application of alternative fumigants to methyl bromide for controlling nematodes in replanted grapevines. Pest Manag Sci. 2012;68(5):773–780.
- Westphal A, Browne GT, Schneider S. Evidence for biological nature of the grape replant problem in California. Plant Soil. 2002;242(2):197–203.
- Brinker A, Creasy L. Inhibitors as a possible basis for grape replant problem. J Am Soc Hortic Sci. 1988;113(3):304–309.
- Guo XW, Li K, Sun YN, Zhang LH, Hu XX, Xie HG. Allelopathic effects and identification of allelochemicals in grape root exudates. Acta Hortic Sinica. 2010;37(6):861–868.
- Li K, Han, X, Guo XW, Wang B, Jin GH. Dynamic changes of exogenous ρ-hydroxybenzoic acid in grape soil. J Shenyang Agric Univ. 2013;44(4):413–417.
- Nazih, N, Finlay-Moore O, Hartel, PG, Fuhrmann JJ. Whole soil fatty acid methyl ester (FAME) profiles of early soybean rhizosphere as affected by temperature and matric water potential. Soil Biol Biochem. 2001;33(4): 693–696.
- Williamson GB, Richardson D. Bioassays for allelopathy: measuring treatment responses with independent controls. J Chem Ecol. 1988;14(1):181–187.
- Heuer H, Krsek M, Baker P, Smalla K, Wellington E. Analysis of actinomycete communities by specific amplification of genes encoding 16S rRNA and gel-electrophoretic separation in denaturing gradients. Appl Environ Microb. 1997;63(8):3233–3241.
- White TJ, Bruns T, Lee S, Taylor J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ, editors. PCR protocols. New York, Taylor & Francis; 1990. p. 229–232.
- Gardes M, Bruns TD. ITS primers with enhanced specificity for basidiomycetes-application to the identification of mycorrhizae and rusts. Mol Ecol. 1993;2(2):113–118.
- Shannon CE, Weaver W. The mathematical theory of communication. Urbana: Taylor & Francis; 1949.
- Garland JL, Mills, AL. Classification and characterization of heterotrophic microbial communities on the basis of patterns of community-level sole-carbon-source utilization. Appl Environ Microb. 1991;57:2351–2359.
- Insam H. A new set of substrates proposed for community characterization in environmental samples. In: Insam H, Rangger A, editors. Functional versus structural approaches. Microbial Communities. Berlin: Taylor & Francis; 1997. p. 259–260.
- Kennedy AC, Gewin VL. Soil microbial diversity: present and future considerations. Soil Sci. 1997;162(9):607–617.
- Kennedy A, Smith K. Soil microbial diversity and the sustainability of agricultural soils. Plant Soil. 1995;170(1):75–86.
- Smalla K, Wachtendorf U, Heuer H, Liu WT, Forney L. Analysis of Biolog GN substrate utilization patterns by microbial communities. Appl Environ Microb. 1998;64(4)1220–1225.
- Li Y, Hu C, Ding W, Liu M. Effects of autotoxins stress on root exudates of Panax Ginseng. World Sci Technol Mod Tradit Chin Med. 2013;15(7):1499–1504.
- Broeckling CD, Broz AK, Bergelson J, Manter DK, Vivanco JM. Root exudates regulate soil fungal community composition and diversity. Appl Environ Microb. 2008;74(3):738–744.
- Garbeva P, van Veen JA, van Elsas JD. Microbial diversity in soil: selection of microbial populations by plant and soil type and implications for disease suppressiveness. Annu Rev Phytopathol. 2004;42:243–270.
- Li XG, Zhang TL, Wang XX, Hua K, Zhao L, Han ZM. The composition of root exudates from two different resistant peanut cultivars and their effects on the growth of soil-borne pathogen. Int J Biol Sci. 2013;9(2):164–173.
- Ling N, Zhang WW, Wang DS, Mao JG, Huang QW, Guo SW. Root exudates from grafted-root watermelon showed a certain contribution in inhibiting Fusarium oxysporumf.sp. niveum. PLoS One. 2013;8(5). doi:10.1371/journal.pone.0063383.g001.
- Badri DV, Chaparro JM, Zhang R, Shen Q, Vivanco JM. Application of natural blends of phytochemicals derived from the root exudates of Arabidopsis to the soil reveal that phenolic-related compounds predominantly modulate the soil microbiome. J Biol Chem. 2013;288(7):4502–4512.
- Neumann G, Bott S, Ohler M, Mock HP, Lippmann R, Grosch R, Smalla K. Root exudation and root development of lettuce (Lactuca sativa L. cv. Tizian) as affected by different soils. Front in Microb. 2014;5:2. doi:10.3389/fmicb.2014.00002. eCollection
- Kowalchuk GA, De Souza FA, Van Veen JA. Community analysis of arbuscular mycorrhizal fungi associated with Ammophila arenaria in Dutch coastal sand dunes. Mol Ecol. 2002;11(3):571–581.