Abstract
Honey adulteration has been on the rise with the increasing demand for high-quality honey, and the authentication of A. cerana honey is a major problem that the current bee industry needs to solve. The aim of this research was to establish the proofreading enzyme-mediated probe cleavage combined with ladder-shape melting temperature isothermal amplification (Proofman-LMTIA) method for the authentication of Apis cerana honey. The LMTIA primers were designed to target the specific 18S rRNA sequence of A. cerana, and the conditions for Proofman-LMTIA amplification were optimized. The specificity and sensitivity of the method were determined and compared with that of the droplet digital polymerase chain reaction (PCR). The optimal temperature of the established Proofman-LMTIA method was 62 °C. The method can specially to detect the A. cerana gene without cross-reactivity with the genomes from other animal and plant species, and the amplification reaction can be finished within 20 min with the sensitivity of 10 pg A. cerana genomic DNA, which was more sensitive than the droplet digital PCR method. The obtained results demonstrate that the established Proofman-LMTIA procedure was specific, sensitive, and rapid, and can be used for authentication of A. cerana honey.
Introduction
Honey adulteration has been on the rise with the increasing demand for high-quality honey, among which the mislabeling of bee species is the one form of honey fraud [Citation1]. Apis cerana and Apis mellifera are the two largest bee colonies for honey production in China, South Korea, and some southeast Asian countries [Citation2], because A. cerana is less suitable for modern breeding and less productive than A. mellifera, and the tense competition for habitats between the two species has resulted in the decline of A. cerana populations [Citation3]. Due to the production decline of A. cerana honey and the local consumer preferences, A. cerana honey is usually three to five times more expensive than A. mellifera honey [Citation2]. Thus, fraudilent mislabeling of A. mellifera honey as A. cerana honey or mixing A. mellifera honey into A. cerana honey are not uncommon [Citation1]. To protect consumers and promote fair competition among producers, it is increasingly necessary to assess the authenticity of honey, especially with regard to entomological origin.
At present, many techniques for the identification of honey adulteration have been reported, including sensory identification, pollen identification, physical and chemical identification, chromatography [Citation4], mass spectrometry [Citation5], near-infrared spectroscopy [Citation6], SDS-PAGE (Sodium Dodecyl Sulfate-PolyAcrylamide Gel Electrophoresis) electrophoresis [Citation7], and other modern analytical techniques. However, due to the long detection time and other reasons, the results are unspecific, and the more stable and accurate technology needs to be developed for identification. Because honey contains bee DNA, it can be used to authenticate the entomological origin of honey [Citation8–10].
The DNA molecules in honey are highly stable, so the DNA-based methods are used as a powerful tool to authenticate the authenticity of honey. At present, polymerase chain reaction (PCR) and loop-mediated isothermal amplification (LAMP) are the most advanced nucleic acid detection techniques [Citation11]. Their development is limited due to the need of thermal cycling apparatus for PCR or due to the nonspecific amplification problems of LAMP reaction [Citation12].
In 2021, Wang et al. [Citation13] developed a novel nucleic acid isothermal amplification technology, Ladder-shape melting temperature isothermal amplification of nucleic acids (LMTIA), which had not only realized the isothermal amplification of one pair of PCR primers [Citation13–15], but also clarified the single-template generation mechanism of LAMP reaction [Citation13, Citation16]. Compared with PCR technology and LAMP technology, the LMTIA technology has the advantages of simple mechanism, easy primer design, Quick turn-around time, high specificity, high sensitivity, low requirement for target sequence length and wide application range [Citation13], and the LMTIA technology has been successfully applied to the detection of African swine fever virus (ASFV) [Citation17], meat adulteration detection [Citation18], noddle adulteration detection [Citation19], Chinese herbal medicine adulteration detection [Citation20], and detection plant-derived components in food [Citation21]. It is proved that this technique has the advantages of high sensitivity and strong specificity. The purpose of this study was to establish a method that combines the LMTIA technology with the proofreading enzyme-mediated probe cleavage (Proof-man probe) for detection of a specific A. cerana gene, and to provide technical support for authentication of A. cerana honey.
Materials and methods
Materials, reagents, and instruments
Materials: A. cerana was purchased from Taobao Shopping Mall (Hangzhou City, Zhejiang Province, China); A. cerana honey was purchased from Xuchang Supermarket (Xuchang City, Henan Province, China).
Reagents: 2 × Mix Premix (Catalogue No: MB205-P01): Shandong Merit Biotechnology Co., LTD, Heze, Shandong Province, China; GPV8 Ultra-fidelity DNA polymerase (Catalogue No: PM06500): General Biological Systems Co., LTD, Chuzhou, Anhui Province, China; Animal Genome DNA Extraction Kit (Catalogue No: DP341): Beijing Tiangen Biochemical Technology Co., LTD, Beijing, China; Plant Genome DNA Extraction Kit (Catalogue No: DP350): Beijing Tiangen Biochemical Technology Co., LTD; NucleoSpin® Food (Catalogue No: 1911/001): MACHEREY-NAGEL GmbH & Co. KG, Duren, Germany; Primers and Probes: General Biological Systems Co., LTD.
Main instruments: Gentier 96E Automatic Medical PCR Analysis System: Xian Tianlong Technology Co., LTD, Xi’an, Shanxi Province; Nano Drop One Ultra Micro Nucleic Acid/Protein Analyzer: Thermo Fisher Scientific Inc., Waltham, MA; SCI-VS Adjustable Mixer: Thermo Fisher Scientific Inc.; TGL-16C Table Low speed Centrifuge: Shanghai Anting Scientific Instrument Factory, Shanghai, China; DH300 Dry Thermostat (Metal Bath): Hangzhou Ruicheng Instrument Co., LTD, Hangzhou, China-Preparation Instrument (Drop Maker M1): TargetingOne Corporation, Beijing, China; Biochip Analyzer: TargetingOne Corporation.
Primers and probe design of Proofman-LMTIA
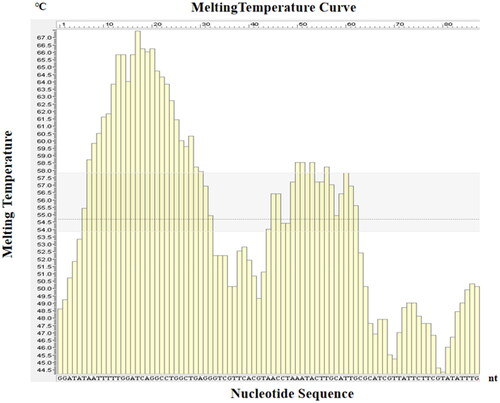
The principle for the target sequence selection of LMTIA included three points: first, the melting temperature curve of target sequence was ladder-type; second, the GC (Guanine and Cytosine) content of target sequence was usually 40–80%; lastly, the target sequence was of high specificity [Citation22]. The specific gene sequences of 18S rRNA sequence of A. cerana were selected as the target sequences with the Oligo 7 software (https://oligo.net/downloads.html). The fragments with a ladder curve of melting temperature were selected, and the selected sequences were verified to be specific with NCBI BLAST. The online software Primer 3 Plus (http://www.primer3plus.com) was used to design the LMTIA primers. The primers included the primer F, the primer B, and loop primer LB, which were similar to the forward inner primer, backward inner primer, and loop B primer (LB) [Citation22], and the Proofman probe was designed, as shown in and .
Table 1. Primers and probe sequences of the Proofman-LMTIA method.
Preparation of A. cerana genomic DNA
The Animal genome DNA Extraction Kit was used to extract the A. cerana genomic DNA. First, the honeybee tissue was first broken into cell suspension, centrifuged at 11200 g for 1 min, the supernatant was removed, the sediment was suspended in the buffer GA of the Animal genome DNA Extraction Kit; 20 µL Proteinase K of the Animal genome DNA Extraction Kit was added to above suspension liquid, and the tissue lysis was performed at 56 °C for 1 h. Then, 200 µL anhydrous ethanol was added to the lysis solution, the mixture was transferred to the adsorption column of the Animal genome DNA Extraction Kit and was centrifuged at 13400 g for 2 min. The supernatant was removed; the 500 µL buffer GD of the Animal genome DNA Extraction Kit was added to the adsorption column. Following centrifugation at 13400 g for 30 s, the supernatant was removed; the 600 µL rinsing solution PW of the Animal genome DNA Extraction Kit was added to the adsorption column. After centrifugation at 13400 g for 30 s, the supernatant was removed; the silicon film in the adsorption column was dried at room temperature for 10 min, and DNA was eluted with 100 µL TE buffer by centrifugation at 13400 g for 2 min. Finally, the A. cerana genomic DNA was obtained, and its concentration and purity were determined by Nano Drop One Ultra-micro Nucleic Acid/Protein Analyzer. The A. cerana genomic DNA samples were stored at −20 °C.
Proofman-LMTIA reaction temperature optimization
A 10 μL system was prepared in a PCR reaction tube, which included 5 μL 2 × Mix premix, 0.4 μL GPV8 ultra-fidelity DNA polymerase, and 0.16 μL100 μmol/L primers F and B each. 0.04 μL μmol/L Primer LB, 0.3 μL 10 μmol/L Proofman probe, 2 μL DNA template, supplemented with ddH2O to 10 μL. The reaction tubes were placed in the real-time fluorescence quantitative PCR instrument, and the optimal reaction temperature was selected according to the amplification efficiency. Triplicate samples were used.
Specificity determination of the Proofman-LMTIA method
The seeds of the linden tree, twigs of the chaste tree, jujube, acacia, rapeseed, rice, and beet were purchased from Taobao Shopping Mall, and the genomic DNAs from these seeds were extracted with the Plant Genome DNA Extraction Kit. The samples of chicken, duck, pork, beef, and mutton were purchased from Xuchang Supermarket, and the genomic DNAs from these samples were extracted with the Animal Genome DNA Extraction Kit. These genomic DNAs were used to determine the specificity of the established method with ddH2O as the negative control.
Sensitivity determination of the Proofman-LMTIA method
The extracted genomic DNA of A. cerana was diluted in a 10-fold series to five gradient concentrations of 10 ng, 1 ng, 100, 10, and 1 pg, respectively. The reaction tube was placed in the real-time fluorescence PCR instrument. The fluorescence signal was collected every 30 s, total of 40 times, and the sensitivity of the established method was determined.
Sensitivity comparison with droplet digital PCR method
A 30 μL system was prepared in a PCR reaction tube, which included: 7.5 μL 4 × Probe dPCR Unimix premix, 2.4 μL upstream primer (10 μmol/L), 2.4 μL downstream primer (10 μmol/L), 0.75 μL probe (10 μmol/L), 6 μL A. cerana genomic DNA, supplemented with ddH2O to 30 μL. The drops were prepared with the Preparation Instrument. Then PCR reaction was performed, and the program was set as follows: predenaturation at 95 °C for 15 s, 1 cycle; denatured at 94 °C for 30 s, annealed at 60 °C, extended for 60 s, 40 cycles; cooled at 12 °C for 5 min. Finally, the Biochip Analyzer was used to detect the signal of the reaction water-in-oil droplets.
Honey samples tested with the Proofman-LMTIA method
The DNAs were extracted from honey samples with the Food Genome Extraction Kit, the concentration and purity of the DNA extracted from the honey samples were detected by the Nano Drop One Ultra-micro Nucleic Acid/Protein Analyzer, and the DNAs were stored at −20 °C. The genomic DNA extracted from bees was used as positive control, and ddH2O was used as negative control. The reaction tube was placed in the real-time fluorescent quantitative PCR instrument, and the determination was performed at the optimal temperature of the established Proofman-LMTIA method.
Results
Proofman-LMTIA temperature optimization
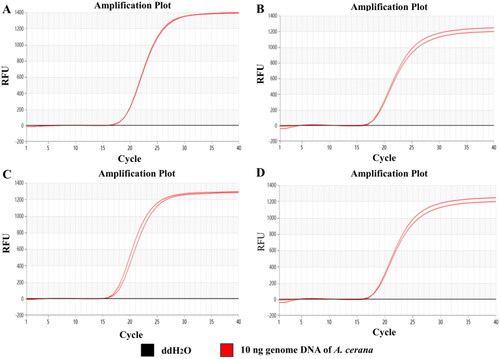
The Proofman-LMTIA reaction system was prepared in PCR reaction tube, and 20 μL paraffin oil was added to avoid aerosol pollution. The tube was covered, mixed well, and centrifuged briefly. The genomic DNA of A. cerana was used as the positive control and ddH2O as the negative control. The Proofman-LMTIA-Proofman reaction system was heated at 60 °C, 61 °C, 62 °C and 63 °C with the Real-time Fluorescence Quantitative PCR Instrument. The fluorescence signal was collected every 30 s, total 40 times. The results showed that the reaction system with ddH2O as the negative control had no amplification, and 62 °C was selected as the optimal reaction temperature, for it gave the highest amplification efficiency; the Ct was 16, which was equivalent to 8 min, as shown in .
Specificity determination of the Proofman-LMTIA method
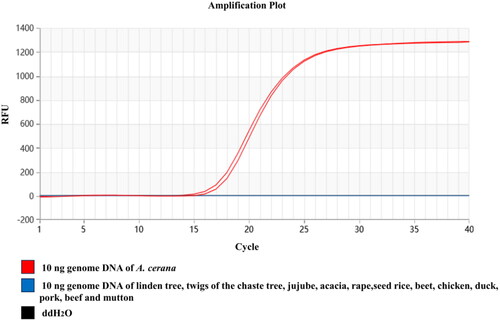
To verify the specificity of the designed LMTIA primers and Proofman probe, the genomic DNAs from the linden tree, twigs of the chaste tree, jujube, acacia, rapeseed, rice, beet, chicken, duck, pork, beef, and mutton were used for determination, with ddH2O used as the negative control. The fluorescence signal was collected every 30 s at 62 °C, total 40 times. The results showed that, at 62 °C, only the genomic DNA of A. cerana had the typical ‘S’ type amplification curve, while the genomic DNAs for other animals and plants had no amplification, indicating that the established Proofman-LMTIA procedure was of high specificity, as shown in .
Sensitivity determination of the Proofman-LMTIA method
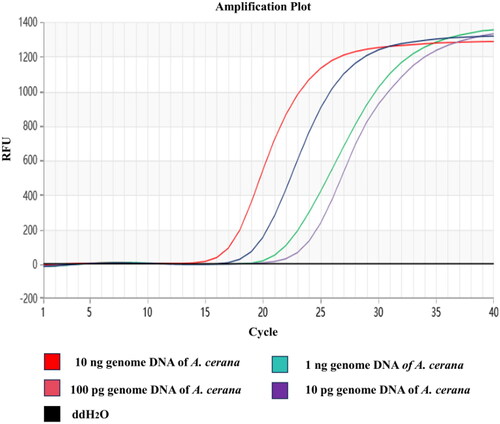
To verify the sensitivity of the developed Proofman-LMTIA method, the A. cerana genomic DNA was diluted to 10 ng/μL, 1 ng/μL, 100 pg/μL, 10 pg/μL, and 1 pg/μL, respectively, which were used to determine the sensitivity of the developed Proofman-LMTIA method with ddH2O as the negative control. The fluorescence signal was collected every 30 s at 62 °C, total 40 times. The results showed that the detection limit was 10 pg A. cerana genomic DNA, indicating that the established Proofman-LMTIA method was of high sensitivity, as shown in .
Sensitivity comparison with droplet digital PCR method
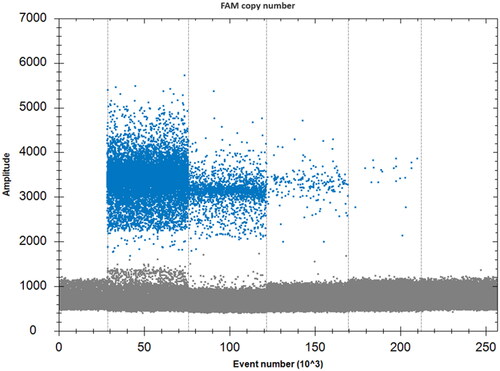
The genomic DNA of A. cerana was diluted to 50 ng/μL, 10 ng/μL, 1 ng/μL, 100 pg/μL, and 10 pg/μL, respectively. The fluorescence threshold was determined with the FAM (Fluorescein Amidite) scatter diagram analysis, and the linear equation was obtained from the detection results. The results showed that the detection limit of the by droplet digital PCR was 100 pg/μL A. cerana genomic DNA, as shown in and .
Table 2. Copy number and total drop number determined with the droplet digital PCR method.
Authenticity of the commercially available A. cerana honey samples
Twelve A. cerana honey samples from Chinese Aksu of Xinjiang Uygur Autonomous Region, Jincheng of Shanxi Province, Kunming of Yunnan Province, Luoyang of Henan Province, Xuchang of Henan Province, Jinhua of Zhejiang Province, which were labeled as the A. cerana honey, were collected. DNA samples were extracted from these honey samples and tested with the established Proofman-LMTIA-method at 62 °C. The results indicated that only three honey samples from Chinese Aksu of Xinjiang Uygur Autonomous Region, Jincheng of Shanxi Province were A. cerana genomic DNA positive, while the nine honey samples from Chinese Kunming of Yunnan Province, Luoyang of Henan Province, Xuchang of Henan Province, and Jinhua of Zhejiang Province were A. cerana genomic DNA negative, suggesting that such adulteration of honey products in Chinese market was very common, as shown in .
Table 3. Results of A. cerana honey samples determined with the Proofman-LMTIA.
Discussion
A. cerana is quick in flight, has a keen sense of smell, leaves the nest early, returns to the nest late, takes a long time to collect honey every day, and has excellent honey collecting performance. A. cerana honey is of high nutritional value, known as ‘honey treasures’. With the continuous improvement of living standards, local food has become more and more popular among the public. The price of A. cerana honey produced in China is usually 3–5 times that of A. mellifera honey [Citation1]. In the current market, some attempts to make profits by seizing the public’s preference for A. cerana honey and deliberately mislabelling A. mellifera honey as A. cerana honey have not been uncommon [Citation2]. Therefore, the established Proofman-LMTIA method can authenticate A. cerana honey and effectively prevent such food fraud.
At present, there are many techniques for the detection of A. cerana honey adulteration, but due to their various limitations, there needs to be developed more sensitive technology. Nucleic acid detection technology has become the hotspot in recent years because of its high specificity and sensitivity. In view of the problems such as the need for thermal cycling apparatus and the long reaction time of the PCR technique, scientists are committed to developing nucleic acid amplification technologies that do not require a thermal cycling apparatus, and more than 20 isothermal amplification methods have been developed since 1990. LAMP has become the most valuable biological analysis tool due to its high sensitivity, but its false positive rate limits the application of LAMP. Upon PCR and LAMP technologies, Wang et al. [Citation13] developed the LMTIA technology. The biggest advantages of this technology are that it can react at a constant temperature, does not require a thermal cycle instrument, has simple primer design, has low target sequence length requirement, is of rapidity and visualization, and can complete the amplification within 20 min, which has a wide application range.
For the LMTIA reaction did not have the false positive problems caused by aerosol contamination and nonspecific amplification as in LAMP reaction [Citation13, Citation23], the LMTIA had been widely applied for gene detection. The LMTIA had been used for detection p72 gene of ASFV with the same sensitivity as that of the commercial real-time PCR kit [Citation17], detection of chicken adulteration in beef with ≥0.1% (w/w) detection limit [Citation18], for identification of cassava component in sweet potato starch noodles with detection limit of 0.01% [Citation19], for detection of duck or pork adulteration in beef with the sensitivity of 1 ng genomic DNA of Sus scrofa [Citation24, Citation25] and for detection of soybean components in edible oil [Citation22]. Moreover, the LMTIA technique had been successfully combined with the Proofman probe for discrimination of Panax quinquefolium and Panax ginseng, detection of soybean-derived components in dairy products, detection of Listeria monocytogenes in food [Citation20, Citation26].
In this study, several sets of LMTIA primers and Proofman probe were designed for the specific 18S rRNA sequence of A. cerana, and an optimal set of LMTIA primers and Proofman probe was selected. The reaction temperature of the Proofman-LMTIA method was optimized to be 62 °C. It was verified by the experiments that the technology could specifically detect the genomic DNA of A. cerana at 62 °C, and there was no cross-reaction with the genomic DNAs of the linden tree, twigs of the chaste tree, jujube, acacia, rapeseed, rice, beet, chicken, duck, pork, beef, and mutton, and the sensitivity was 10 pg of genomic A. cerana DNA per reaction. Compared with the droplet digital PCR method, the detection limit of the Proofman-LMTIA method was ten times higher, and the Proofman-LMTIA method for A. cerana was successfully established. The method not only maintained the function of ring primers to accelerate amplification, but also realized the function of labeling amplification products in amplification reaction, with rapidity and high sensitivity. It had effectively avoided the ‘false positive’ caused by non-specific amplification and provided the technical support for honey authentication.
Conclusions
In this study, the Proofman-LMTIA assay was developed to authenticate A. cerana honey. The method was of high specificity, and the sensitivity was 10 pg genomic DNA of A. cerana. The experiment provided a reference for authentication of honey from other species, including botanical origin.
Authors’ contribution
Conceptualization, D.W. and Y.W.; methodology, D.X. and W.Y.; formal analysis, J.D., Z.Y., and J.T.; resources, X.Z. and J.L.; data curation, D.Z. and D.X.; writing-original draft preparation, D.X., Y.W., and D.W.; writing-review and editing, Y.W. and D.W.; supervision, D.W.; funding acquisition, D.W. and X.Z. All authors have read and agreed to the published version of the manuscript.
Acknowledgments
The authors wish to thank all members from Key Laboratory of Biomarker Based Rapid-Detection Technology for Food Safety of Henan Province, and Dr. Yanhong Liu from US Department of Agriculture for critical reading of the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
The data that support this study are available from the corresponding author upon reasonable request.
Additional information
Funding
References
- Zhang YZ, Wang S, Chen YF, et al. Authentication of Apis cerana honey and Apis mellifera honey based on major royal jelly protein 2 gene. Molecules. 2019;24(2):1. doi: 10.3390/molecules24020289.
- Soares S, Grazina L, Mafra I, et al. Novel diagnostic tools for asian (Apis cerana) and european (Apis mellifera) honey authentication. Food Res Int. 2018;105:686–9. doi: 10.1016/j.foodres.2017.11.081.
- He X, Wang W, Qin Q, et al. Assessment of flight activity and homing ability in asian and european honey bee species, Apis cerana and Apis mellifera, measured with radio frequency tags. Apidologie. 2013;44(1):38–51. doi: 10.1007/s13592-012-0156-7.
- Chen CT, Chen BY, Nai YS, et al. Novel inspection of sugar residue and origin in honey based on the 13C/12C isotopic ratio and protein content. J Food Drug Anal. 2019;27(1):175–183. doi: 10.1016/j.jfda.2018.08.004.
- Karabagias IK. Seeking of reliable markers related to greek nectar honey geographical and botanical origin identification based on sugar profile by HPLC-RI and electro-chemical parameters using multivariate statistics. Eur Food Res Technol. 2019;245(4):805–816. doi: 10.1007/s00217-018-3216-z.
- Soares S, Amaral JS, Oliveira MBPP, et al. A comprehensive review on the main honey authentication issues: production and origin. Compr Rev Food Sci Food Saf. 2017;16(5):1072–1100. doi: 10.1111/1541-4337.12278.
- Naila A, Flint SH, Sulaiman AZ, et al. Classical and novel approaches to the analysis of honey and detection of adulterants. Food Control. 2018;90:152–165. doi: 10.1016/j.foodcont.2018.02.027.
- Prosser SWJ, Hebert PDN. Rapid identification of the botanical and entomological sources of honey using DNA metabarcoding. Food Chem. 2017;214:183–191. doi: 10.1016/j.foodchem.2016.07.077.
- Kek SP, Chin NL, Tan SW, et al. Molecular identification of honey entomological origin based on bee mitochondrial 16S rRNA and COI gene sequences. Food Control. 2017;78:150–159. doi: 10.1016/j.foodcont.2017.02.025.
- Kek SP, Chin NL, Tan SW, et al. Comparison of DNA extraction methods for entomological origin identification of honey using simple additive weighting method. Int J Food Sci Tech. 2018;53(11):2490–2499. doi: 10.1111/ijfs.13840.
- Mohamadzade Namin S, Ghosh S, Jung C. Honey quality control: review of methodologies for determining entomological origin. Molecules. 2023;28(10):4232. doi: 10.3390/molecules28104232.
- Wang Z, Yu W, Xie R, et al. A strip of lateral flow gene assay using gold nanoparticles for point-of-care diagnosis of African swine fever virus in limited environment. Anal Bioanal Chem. 2021;413(18):4665–4672. doi: 10.1007/s00216-021-03408-2.
- Wang D, Wang Y, Zhang M, et al. Ladder-shape melting temperature isothermal amplification of nucleic acids. Biotechniques. 2021;71(1):358–369. doi: 10.2144/btn-2020-0173.
- Saiki RK, Scharf S, Faloona F, et al. Enzymatic amplification of Beta-Globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985;230(4732):1350–1354. doi: 10.1126/science.2999980.
- Saiki RK, Gelfand DH, Stoffel S, et al. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988;239(4839):487–491. doi: 10.1126/science.239.4839.487.
- Notomi T, Okayama H, Masubuchi H, et al. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 2000;28(12):e63-e63–63. doi: 10.1093/nar/28.12.e63.
- Wang Y, Wang B, Xu D, et al. Development of a ladder-shape melting temperature isothermal amplification (LMTIA) assay for detection of african swine fever virus (ASFV). J Vet Sci. 2022;23(4):e51. doi: 10.4142/jvs.22001.
- Wang Y, Wang B, Wang D. Detection of chicken adulteration in beef via ladder-shape melting temperature isothermal amplification (LMTIA) assay. Biotechnol Biotechnolo Equip. 2022;36(1):339–345. doi: 10.1080/13102818.2022.2081514.
- Zhang Y, Wang Y, Ouyang X, et al. Development of a ladder-shape melting temperature isothermal amplification (LMTIA) assay for the identification of cassava component in sweet potato starch noodles. Molecules. 2022;27(11):3414. doi: 10.3390/molecules27113414.
- Zhang X, Li Z, Zhang Y, et al. Rapid discrimination of Panax quinquefolium and Panax ginseng using the Proofman-Duplex-LMTIA technique. Molecules. 2023;28(19):6872. doi: 10.3390/molecules28196872.
- Xiao F, Gu M, Zhang Y, et al. Detection of soybean-derived components in dairy products using proofreading enzyme-mediated probe cleavage coupled with ladder-shape melting temperature isothermal amplification (Proofman-LMTIA). Molecules. 2023;28(4):1685. doi: 10.3390/molecules28041685.
- Gu M, Xiao F, Wang B, et al. Study on detection of soybean components in edible oil with ladder-shape melting temperature isothermal amplification (LMTIA) assay. Anal Methods. 2023;15(5):581–586. doi: 10.1039/d2ay01719a.
- Glökler J, Lim TS, Ida J, et al. Isothermal amplifications—A comprehensive review on current methods. Crit Rev Biochem Mol Biol. 2021;56(6):543–586. doi: 10.1080/10409238.2021.1937927.
- Wang Y, Wang B, Wang D. Development of a ladder-shape melting temperature isothermal amplification (LMTIA) assay for detection of duck adulteration in beef. J Food Prot. 2022;85(8):1203–1209. doi: 10.4315/JFP-22-015.
- Wang Y, Wang B, Wang D. Detection of pork adulteration in beef with ladder-shape melting temperature isothermal amplification (LMTIA) assay. CyTA J Food. 2022;20(1):244–250.
- Song C, Wang B, Wang Y, et al. Detection of Listeria monocytogenes in food using the Proofman-LMTIA assay. Molecules. 2023;28(14):5457. doi: 10.3390/molecules28145457.