Abstract
Objective
The Practitioner’s Toolkit for Managing the Menopause, developed in 2014, provided an accessible desk-top tool for health-care practitioners caring for women at midlife. To ensure the Toolkit algorithms and supporting information reflect current best practice, the Toolkit has been revised in accordance with the published literature.
Methods
A systematic search for guidelines, position and consensus statements pertaining to the menopause and published after 2014 was undertaken, and key recommendations extracted from the Clinical Practice Guidelines determined to be the most robust by formal evaluation. The peer-reviewed literature was further searched for identified information gaps.
Results
The revised Toolkit provides algorithms that guide the clinical assessment and care of women relevant to menopause. Included are the reasons why women present, information that should be ascertained, issues that may influence shared decision-making and algorithms that assist with determination of menopausal status, menopause hormone therapy (MHT) and non-hormonal treatment options for symptom relief. As clear guidelines regarding when MHT might be indicated to prevent bone loss and subsequent osteoporosis in asymptomatic women were found to be lacking, the Toolkit has been expanded to support shared decision-making regarding bone health.
Conclusions
The 2023 Toolkit and supporting document provide accessible desk-top information to support health-care providers caring for women at midlife.
The Toolkit has been endorsed by the International Menopause Society, Australasian Menopause Society, British Menopause Society, Endocrine Society of Australia and Jean hailes for Women’s Health.
摘要
目的:2014年开发的《从业人员绝经管理工具包》为从事中年女性保健人员提供了一个简便易用的桌面工具。为了确保工具包的算法和支持信息反映当前的最佳实践, 我们根据已发表的文献对该工具包进行了修订。
方法:系统检索2014年以后发表的与绝经相关的指南、立场和共识声明, 并从临床实践指南中提取关键建议, 经正式评估确定为最有力的建议。进一步检索同行评议的文献以确定信息缺口。
结果:修订后的工具包提供了算法指导女性绝经相关的临床评估和保健。其中包括女性出现症状的原因、应确定的信息、可能影响共同决策的问题、有助于确定绝经状态的算法、绝经激素治疗(MHT)和缓解症状的非激素治疗选择。由于缺乏明确指南关于何时可以采用MHT预防无症状女性的骨质流失和随后骨质疏松症, 我们扩展了该工具包以支持有关骨骼健康的共同决策。
结论:2023年工具包和支持文件提供了可访问的桌面信息, 为从事中年女性卫生保健从业者提供支持。
该工具包得到了国际绝经学会、澳大利亚绝经学会、英国绝经学会、澳大利亚内分泌学会和女性健康组织珍·海尔斯的认可。
Introduction
The Practitioner’s Toolkit for Managing Menopause was developed to provide health-care providers with a simple assessment and decision-making tool for use during a clinical consultation [Citation1]. The Toolkit alerted clinicians that women might present with symptoms or concerns, and included a pragmatic algorithm to assess a woman’s menopausal status, including assessment of women who had a prior hysterectomy or endometrial ablation and those using hormonal contraception [Citation2]. The assessment, treatment options and symptom management algorithms were derived from the published literature, and the Toolkit, endorsed by the International Menopause Society, has had global uptake and use. Informed by subsequent research and the availability of additional therapeutic options, the original Toolkit has been reviewed and revised. Again, the included therapies are comprehensive to support global application, with the caveat that not all menopause hormone therapy (MHT) and non-hormonal treatment options are universally available, and indications for their use by regulatory bodies varies between countries. Similarly, recommendations for investigations may vary between regions, depending on availability and cost. Our focus has been to recommend the minimum best care for all women.
The assessment and care algorithms are for use during a clinical consultation. This article is not intended as a comprehensive review of menopause and its management, but provides clinicians with a brief text to support the use of the algorithms. The article does not address the care of early menopause or premature ovarian insufficiency (POI). While the term ‘woman’ is used throughout, it should be read as including nonbinary, gender diverse and transgender people who experience menopause.
To our knowledge, this remains the only clinical practice tool for menopause-related care that has international application.
Methods
We conducted a systematic search of Ovid MEDLINE, EMBASE, PsycINFO and Web of Science for guidelines, recommendations, position statements and consensus statements on menopause published since 2015. The identified 25 menopause guidance papers were then evaluated by four independent reviewers using the Appraisal of Guidelines for Research and Evaluation II (AGREE II) tool for the assessment of the quality of guidelines [Citation3]. We extracted the recommendations regarding MHT and non-hormonal therapy from the guidance documents determined to be at least of moderate quality by AGREE II [Citation4–10]. As an updated version of the North American Menopause Society position statement on non-hormonal therapy became available after this process [Citation11], the updated recommendations were incorporated. Most guidelines recommend MHT to prevent osteoporosis and fragility fracture [Citation4, Citation5, Citation7, Citation9]. As dedicated guidance regarding when to recommend MHT for this purpose are scant, we further searched the literature for the best evidence to support recommendations.
The 2023 Practitioner’s Toolkit for Managing the Menopause supporting notes
The menopause is a physiological process experienced by all women who live beyond midlife. The perception and experience of the menopause transition and years post menopause is unique to each woman and will be influenced by the age at which menopause occurs, whether it is natural or iatrogenic, past and current physical and psychological health and well-being, and ethnicity, environment and culture. The use of hormonal or non-hormonal therapy will be determined by symptom severity, weighing of benefits and risks, and each woman’s expectations and wishes.
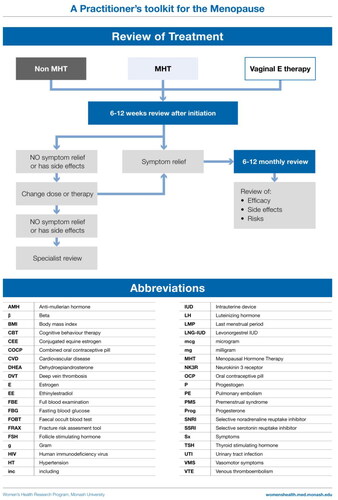
Definitions
Menopause is the permanent cessation of menstruation in a non-hysterectomized woman. As many women may not be naturally menstruating when their menopause transition begins, for example due to hormonal contraception, having had an endometrial ablation or hysterectomy or pre-existing oligo-amenorrhea, a pragmatic definition of menopause is the permanent cessation of ovarian function.
The average age of natural menopause has been reported as being at 51.5 years in high-income countries, with a range between the ages of 45 and 55 years [Citation12]. However, menopause has been reported as occurring earlier in countries such as India, where the average age of menopause has been reported as 46 years [Citation13]. Therefore, the age definitions of early menopause and POI provided in the following may not be appropriate in all populations.
Perimenopause is the time from the onset of cycle irregularity through until 12 months after the final menstrual period.
The menopause transition is defined as the time from the onset of cycle irregularity until the time of the final menstrual period.
Postmenopause starts 12 months after the final menstrual period.
Surgical menopause is the removal of both ovaries.
Early menopause is menopause before the age of 45 years (may not be appropriate in all populations).
POI is cessation of ovarian function before the age of 40 years (may not be appropriate in all populations).
Factors associated with earlier menopause include hysterectomy, smoking, lower level of education, living at an altitude above 2000 m and some medical conditions such as human immunodeficiency virus infection. Factors that have been associated with a later menopause include parity, higher body mass index (BMI) and oral contraceptive use [Citation14–16].
Basic physiology
Menopause occurs when ovulatory and related function of the ovary ends. The basic reproductive unit of the ovary is the follicle and each ovarian follicle contains a single oocyte. A female infant at birth has approximately 300,000 ovarian follicles. By approximately 37 years of age this number is depleted to about 25,000, and at menopause few/none remain [Citation17, Citation18].
Loss of ovarian follicles is associated with diminished estradiol and ovarian inhibin production, and increased production of pituitary follicle stimulating hormone (FSH). Loss of follicles also results in a fall in the production of anti-Müllerian hormone (AMH) which is produced by developing ovarian follicles. Hence, AMH concentrations decline with age and indicate ovarian aging. Measurement of AMH is useful in predicting a woman’s ovarian response to ovulation induction (low blood levels predict a poor response) but is not clinically helpful in predicting menopause in women aged <40 years [Citation19]. The predictive value of AMH for menopause increases with age but still lacks accuracy for women before the average age of 48 years [Citation19]. Changes in FSH, estradiol, inhibin B and AMH may precede or coincide with the development of menstrual irregularity or symptoms.
The stages of menopause were originally classified by the Stages of Reproductive Ageing Workshop (STRAW), further updated as STRAW + 10 which relies only on menstrual cycle characteristics to stage women [Citation20]. The stages are summarized in , modified to allow for inclusion of women without regular menses premenopause.
Table 1. Menopausal stages.
Androgens and the menopause
Testosterone and the pre-androgen androstenedione are produced by the ovaries, and the pre-androgens dehydroepiandrosterone (DHEA), DHEA sulfate (DHEAS) and androstenedione are produced by the adrenal cortex. Circulating blood levels of testosterone and the pre-androgens decline with age, with the decline commencing in the early reproductive years [Citation21].
There is no acute change in testosterone or the pre-androgens across the natural menopause transition or perimenopause [Citation22, Citation23].
Surgical menopause is associated with a significant reduction in testosterone [Citation22, Citation24], and lower testosterone concentrations have been reported in women with POI [Citation25].
Perimenopausal symptoms
Due to fluctuating ovarian hormone production during the menopause transition, women might present with symptoms of relative estrogen excess, estrogen insufficiency or a random mix of both. Typical estrogen excess symptoms include breast tenderness, menorrhagia, migraine, nausea, shorter cycle length and a shorter follicular phase [Citation26]. Estrogen insufficiency symptoms are described in the following.
Symptoms of the menopause
There is substantial variability between women in symptom occurrence due to the hormonal changes of menopause. Many symptoms are non-specific and may be due to other conditions. The cardinal symptoms listed in the following are recognized as occurring as a result of systemic estrogen insufficiency, and most are alleviated by estrogen therapy [Citation4–9].
Menopausal symptoms that are considered indications for MHT [Citation4–9] include the following:
Vasomotor symptoms (VMS)
Hot flushes (flashes).
Sweats and/or night sweats.
Urogenital symptoms
Vaginal irritation, burning, dryness and dyspareunia.
Urinary frequency, urgency, recurrent urinary infections.
Symptoms that may be menopause-associated include the following:
Psychological symptoms
Low mood, but not clinical depression.
Anxiety/irritability.
Disturbed sleep with frequent awakenings
Lessened sexual desire
Despite Asian women being more likely to experience new onset musculoskeletal symptoms at menopause than VMS [Citation27], to date these have not been included in recent guidelines as a primary indication for MHT [Citation4–9]. However, many women with new onset musculoskeletal symptoms will gain relief with MHT.
Other commonly reported symptoms that are considered less menopause-specific include fatigue, headaches and impaired memory and concentration [Citation4–9]. Overall, cognitive complaints alone are not considered an indication for MHT [Citation4–9]. This is because clinical trials to date have not demonstrated objective improvement in cognitive performance with MHT over placebo post menopause [Citation28]. Research regarding cognitive effects of MHT during the perimenopause is lacking and urgently required. Women frequently experience an array of other symptoms not listed that may or may not improve with MHT.
Symptom prevalence
Most women will experience symptoms associated with menopause. Findings from large Australian epidemiology studies have revealed that 74% of postmenopausal women aged <55 years have VMS [Citation29], 28% of postmenopausal women aged <55 years have moderate to severely bothersome VMS [Citation29], and 42% and 33% of women aged 60–64 years and 65–79 years, respectively, still have VMS [Citation30]. VMS severely impact well-being, the effect being similar to having insecure housing [Citation31].
Women with moderate to severe VMS are up to three-fold more likely to have moderate–severe depressive symptoms than other women [Citation32, Citation33]. Other common menopause-associated symptoms include anxiety, disturbed sleep, joint pain and vaginal dryness [Citation29]. Similar prevalences of VMS have been reported across the world, for example in Japan [Citation34], Bangladesh [Citation27] and Iran [Citation32], dispelling the belief that menopausal symptoms are phenomena of westernized countries. However, in many countries menopause-associated musculoskeletal pain is more problematic than VMS [Citation27, Citation35–37]. Urogenital atrophy symptoms include vaginal irritation, itch, dryness, dyspareunia, bladder irritability, urinary frequency and urge, and urinary tract infections. These symptoms, when due to estrogen deficiency, persist unless treated, and potentially all untreated postmenopausal women are affected.
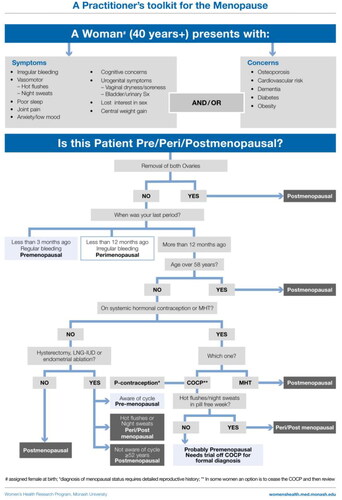
Diagnosing menopause
The diagnosis of menopause is mostly straightforward for women over the age of 45 years who report cessation of menstruation for more than 12 months, with or without symptoms. If a woman has had a hysterectomy and has classic menopausal symptoms, treatment can be instituted without a firm diagnosis, as menstrual bleeding is not an issue if estrogen is prescribed. Challenging situations, in terms of diagnosis, include women who have had an endometrial ablation, have a progestin-releasing intrauterine device (IUD), are using systemic hormonal contraception, have pre-existing oligo-amenorrhea or have atypical symptoms and are <45 years old. In the 2014 Toolkit the diagnostic algorithm classified women as postmenopausal if they were aged >56 years [Citation1]. However, there is evidence that the age of menopause is increasing [Citation38, Citation39]. Therefore, to be conservative, the updated algorithm now classifies women as postmenopausal if they are aged >58 years irrespective of symptoms.
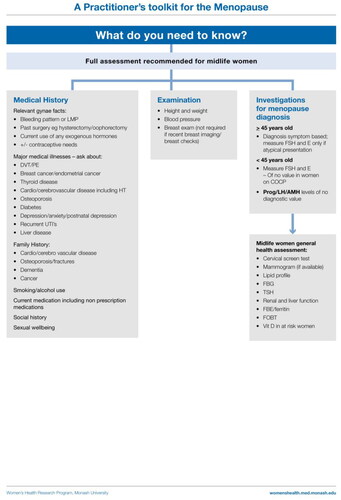
Measurement of hormones, when and why
Hormonal testing should not be performed to diagnose menopause for the majority of women aged 45 years and above [Citation4–9].
Progestin IUD in situ: the patient can usually be treated with estrogen if symptomatic without any blood tests for diagnosis.
Endometrial ablation: still need to prescribe progestogen as protection for the endometrium. It is appropriate to institute treatment if the patient is symptomatic without any hormonal tests.
Using systemic hormonal contraception that suppresses ovulation: hormone tests are uninformative. The only way to ascertain menopausal status is to cease the hormonal contraception.
Testosterone should not be measured to diagnose insufficiency as there is no blood level below which a woman can be considered to have insufficient testosterone [Citation40]. The only diagnostic indication for measurement of testosterone is the investigation of androgen excess.
Hormone measurement may be useful for the following:
Amenorrheic women with subtle/fluctuating symptoms, for example predominant mood change and no/few VMS. A single observation of normal FSH and estradiol does not exclude perimenopause as hormone levels fluctuate at this time.
Women aged 40–45 years.
Hormone measurements are required for the following:
POI diagnosis: requires FSH to be elevated and estradiol to be low on at least two occasions at least 4–6 weeks apart. Other investigations are usually indicated once POI is diagnosed.
Other biochemical investigations based on clinical assessment:
Exclude other causes of amenorrhea if the diagnosis is uncertain (pregnancy, hyperprolactinemia, thyroid disease, hypothalamic amenorrhea [Citation41]).
Exclude other common causes of fatigue, mood change, hotness (thyroid disease – measure thyroid stimulating hormone; iron deficiency – measure hemoglobin/iron stores; type 2 diabetes – measure fasting blood glucose).
Consider whether fasting lipids or vitamin D measurement is required.
Other health consequences of the change in hormones at menopause
The fall in estradiol at menopause has a number of adverse metabolic effects and health effects [Citation12, Citation28, Citation42]:
Metabolic
Increased central abdominal fat deposition (even in slim women).
Insulin resistance and increased risk of type 2 diabetes.
Cardiovascular
Impaired endothelial function (impaired vascular integrity).
A more adverse lipid profile.
Skeletal
Accelerated bone loss commencing prior to the final menstrual period.
Direct/indirect contribution to sarcopenia.
Increased fracture risk.
Neurological
Some women may experience trouble with verbal memory during the perimenopause that may improve post menopause.
Management
Considerations for all women at menopause
The importance of improving lifestyle factors such as good nutrition, being physically active, cessation of smoking, limiting alcohol and stress management should be highlighted, as healthy behaviors confer benefits to all women.
All women should be reviewed in terms of the following:
Cardiovascular disease risk (blood pressure and lipids).
Diabetes (fasting blood glucose).
Urogenital health (consider local hormonal/non-hormonal therapy).
Cancer screening – breast check, cervical cancer screening, mammogram (availability and recommended frequency varies between countries).
General advice for symptom management
Overweight and obesity, and smoking are risk factors for VMS [Citation43]. Weight reduction may result in reduced VMS in overweight women [Citation11, Citation44], and smoking cessation should be encouraged.
Exercise, yoga and relaxation methods have not been found to be effective for VMS [Citation11], but these activities may improve sleep and general well-being.
Menopausal hormone therapy
The most robust menopause Clinical Practice Guidelines (CPGs) support MHT as the most effective treatment to alleviate VMS [Citation4–9].
There is general consensus [Citation4–9] on the following:
Endometrial protection with a progestogen is essential in non-hysterectomized women.
Hysterectomized women should be prescribed estrogen-only therapy unless they have a history of moderate to severe endometriosis or subtotal hysterectomy with residual endometrium in the cervical stump.
Oral estrogen is associated with an increased risk of venous thromboembolic disease (VTE), although the absolute risk is small for women aged <60 years. The risk is lower/not at all with transdermal estradiol, which is preferred for women at increased risk of VTE (i.e. smokers, obese women and diabetic women).
Breast cancer is a contraindication to the use of MHT.
The prescription of individually formulated and compounded hormone preparations is not recommended.
MHT prevents bone loss and fractures in postmenopausal women.
With respect to androgen therapy, the Global Consensus Position Statement [Citation40] advises the following:
Transdermal testosterone therapy for postmenopausal women with sexual desire dysfunction, given in a female-appropriate dose, may improve sexual desire, arousal, orgasm and pleasure.
There is presently no other evidence-based indication for testosterone therapy for women.
Oral DHEA is not effective for the treatment of postmenopausal sexual dysfunction. In addition, systemic DHEA has not been found to be clinically beneficial for the treatment or prevention of any other symptoms or conditions [Citation45].
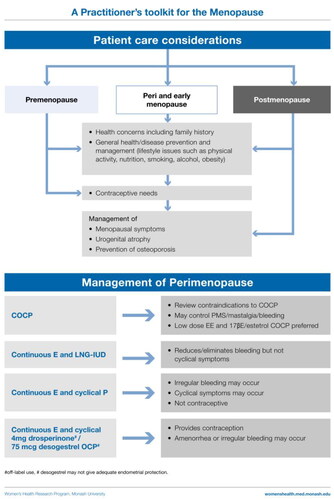
Perimenopausal women
Treatment goals are cycle control, contraception and symptom relief
Combined oral contraceptive pill
For the perimenopausal woman needing contraception, the combined oral contraceptive pill (COCP) provides contraception, menstrual cycle control and relief from VMS and other symptoms. It also prevents bone loss and treats acne that can occur at this time. Each woman’s risks must be assessed including smoking status, blood pressure, lipid profile, migraine with aura history, thrombosis and cardiovascular disease risk, and family history.
Low-dose ethinylestradiol COCP (20 μg) can be used and a 30 μg COCP can probably be used with equal safety (UK Medical Eligibility Criteria for Contraceptive Use: https://www.fsrh.org/documents/ukmec-2016/). However, some women have VMS when using ethinylestradiol that may be alleviated when they are switched to an estradiol-containing COCP.
Estradiol-containing COCPs are available as well as an estetrol-containing COCP. Estradiol appears to have more neutral blood pressure effects [Citation46], and estetrol more favorable lipid effects [Citation47]. Therefore, these may be preferred over ethinylestradiol COCPs.
VMS in the pill-free week can be managed by eliminating the placebo tablets or adding a low dose of supplemental estrogen.
In some countries, weekly transdermal, monthly injectable or monthly intravaginal contraceptive options will provide the same benefits with cyclical bleeding.
Women can transition from the contraceptive hormone therapy to MHT when contraception is no longer required.
Progestogen-only regimens
The levonorgestrel-releasing intrauterine device (LNG-IUD) provides contraception and suppresses the endometrium, and is an excellent option for the management of heavy bleeding. Although initial breakthrough bleeding may occur, 80% of women are amenorrheic at 1 year [Citation48]. The 52 mg LNG-IUD can be combined with oral/transdermal estrogen and can be left in situ for up to 8 years for contraception and provides endometrial protection for up to 5 years [Citation48].
Progestin-only oral contraceptives that suppress ovulation (e.g. drospirenone 4 mg or desogestrel 75 μg pills) are available, and off-label can potentially be used with transdermal estradiol for symptom relief when oral estrogen is contraindicated (e.g. migraine with aura).
High-dose oral progestogen-only regimens (medroxyprogesterone acetate or micronized progesterone) may alleviate VMS and treat endometrial hyperplasia. However, side effects (weight gain, mastalgia, fluid retention, vaginal discharge and dry mouth) can occur at these doses. Lower doses can be tried but may be less effective. Short-term use may be applicable in women who do not want to take estrogen. It can be used cyclically for the first 12–14 days of the cycle and produce predictable bleeding in most women.
Cyclical MHT
This can be initiated during perimenopause, with the progestogen dose timed to commence 14 days after bleeding day 1 of a woman’s own cycle. However, these regimens do not suppress ovulation and are not contraceptive. Women frequently experience symptoms of estrogen excess including mastalgia and erratic bleeding due to their underlying ovarian function.
Menopausal hormone therapy after the perimenopause
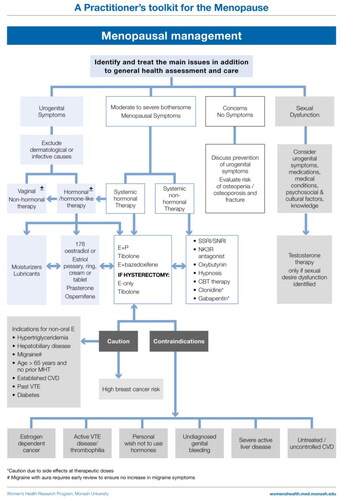
Estrogen ± progestogen therapy
MHT is indicated to alleviate symptoms of the menopause, namely VMS, menopause-associated sleep disturbance or mood change and vaginal dryness [Citation4–9]. As already suggested, alleviation of menopause-associated musculoskeletal pain merits a trial of therapy.
For women with an intact uterus, progestogen therapy is required with estrogen to protect the lining of the uterus from overstimulation (thickening) by estrogen. This can be continuous estrogen with cyclic progestogen for 12–14 days out of a monthly cycle (which can be a calendar month for simplicity), or continuous-combined MHT where both the estrogen and the progestogen are taken every day/ a LNG-IUD is in situ. Cyclic MHT results in scheduled menstrual bleeding after the progestogen is ceased. Continuous-combined MHT results in no bleeding in 90% of women by 12 months. Breakthrough bleeding is common in the first few months of this type of regimen. Persistent, prolonged breakthrough bleeding or new onset bleeding after several months of therapy requires investigation. If an unexpected bleed is preceded by classic premenstrual symptoms in the early postmenopausal years this may be due to a random ovulation.
For women who have undergone a hysterectomy, estrogen-only therapy is appropriate, with no progestogen required unless recent severe endometriosis or a subtotal hysterectomy with possible endometrial tissue in the retained cervix.
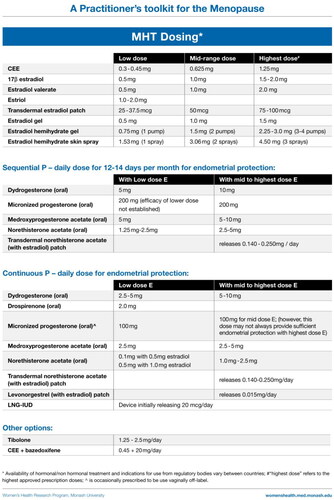
MHT formulations and options
Estrogen therapy
Estrogen can be used systemically as oral conjugated equine estrogen, estradiol valerate, estrone sulfate or micronized estradiol; transdermal estradiol (patches, gels, spray); a vaginal estradiol ring; and implanted estradiol pellets (mostly not regulator-approved). Vaginal pessaries and creams are used to treat urogenital symptoms.
Oral estrogen preparations
Advantages:
Convenience and reliable absorption for most users.
Disadvantages:
Increased risk of VTE events, and cholelithiasis.
Increased thyroid binding globulin (TBG) – may need to adjust thyroxine dose.
May significantly increase triglyceride blood levels.
Transdermal estradiol preparations
Advantages:
Avoidance of gut and first-pass hepatic metabolism: no change in TBG, null effect on hepatic coagulation proteins at standard doses.
Little/no increase in VTE disease at standard doses.
Convenience (e.g. once or twice a week patch).
Neutral effect of blood lipids.
Disadvantages:
Topical preparations may cause skin irritation and rarely general allergic reaction. Skin irritation is less likely with topical gels.
Occasional poor absorption.
Women may forget to change patch twice weekly.
Progestogen therapy
Progestogen therapy is required for all women taking estrogen unless they have had a hysterectomy [Citation4–9].
Progestogens include micronized progesterone and synthetic progestins which may be combined with estradiol in a tablet or patch, or taken in addition to the estrogen therapy. The 52 mg LNG-IUD is an alternative to systemic progestogen therapy. Compounded progesterone preparations are not recommended as evidence for endometrial protection is lacking [Citation4–9].
Estrogen ± bazedoxifene, a selective estrogen receptor modulator
Conjugated estrogens 0.45 mg/day combined with 20 mg bazedoxifene (BZE) provides an alternative to estrogen–progestogen therapy in a fixed-dose combination [Citation49, Citation50]. The estrogen dose cannot be modified if symptom relief is not achieved.
Tibolone
Tibolone provides an alternative to estrogen–progestogen therapy. Tibolone should not be commenced until 12 months after the last natural menstrual period. For hysterectomized women, treatment can be commenced with the onset of bothersome symptoms. It should not be prescribed with other MHT and, like estrogen, is contraindicated in women with breast cancer. Tibolone is metabolized in the gastrointestinal tract and target tissues to metabolites that have estrogenic, progestogenic and androgenic effects. As tibolone does not stimulate the endometrium it does not require concurrent progestogen therapy [Citation51]. It may improve sexual interest and responsiveness [Citation51]. Breast tenderness is uncommon and tibolone does not increase mammographic density.
Ospemifene for urogenital atrophy
Ospemifene is an oral selective estrogen receptor modulator indicated for vulvo-vaginal atrophy symptoms in postmenopausal women (60 mg/day). Ospemifene has an estrogen-like effect in the vagina (increases superficial cells and decreases parabasal cells, and lowers vaginal pH) and alleviates dyspareunia [Citation52]. The most common adverse effect is VMS, which occur in 10% of treated women [Citation53].
Testosterone
Testosterone is not a standard component of MHT [Citation40]. The only evidence-based indication for testosterone therapy is for the treatment of postmenopausal women who experience loss of sexual desire that causes them to experience concern/distress [Citation54]. When prescribed for this indication, treatment should be transdermal and ideally with a preparation formulated for women [Citation40]. Alternatively, a modified dose of a regulatory-approved male testosterone therapy could be used [Citation40]. With both options, women should be monitored for evidence of clinical or biochemical androgen excess. Compounded testosterone formulations are not recommended due to lack of evidence of safety or efficacy [Citation40]. Detailed guidance regarding testosterone use in women can be found in the Global Consensus Position Statement for Testosterone use in Women [Citation40].
Effectiveness
Estrogen therapy or tibolone alleviates VMS in most women [Citation55–57]. Low-dose estrogen therapy can be highly effective.
There is evidence for improved sleep quality with MHT, including tibolone [Citation56, Citation57].
Regarding mood, although menopause-associated anxiety and depressive symptoms (but not clinical depression) are generally considered indications for MHT, systematic reviews and meta-analyses of randomized controlled trials found no benefit of estrogen therapy on depressive symptoms, alone or with a progestogen, over placebo in postmenopausal women [Citation58, Citation59]. While benefits for perimenopausal women have been suggested [Citation59], the data to support this are too scant to draw conclusions [Citation58, Citation59].
Caution with systemic MHT
Oral estrogens increase the risk of VTE, but there is general agreement that non-oral estrogen is associated with little/no increase in VTE risk [Citation4–9]. However, as the absolute risk of VTE is low in healthy women at the average age of menopause, the route of administration is best determined by the patient’s preference. Clinical trials have demonstrated an increased risk of breast cancer with oral estrogen plus progestogen therapy [Citation60] which was not seen with oral conjugate equine estrogen-only therapy [Citation61]. Whether breast cancer risk differs between oral and non-oral MHT regimens has not been established in randomized clinical trials. In addition, observational studies implicate progesterone as safer than synthetic progestogens (progestins) with regards to breast cancer risk [Citation62, Citation63]. There are few long-term observational data (>5 years) and no evidence from randomized controlled trials to inform whether progesterone is safer than progestins. However, expert opinion is that progesterone may confer less breast cancer risk [Citation4, Citation6].
Tibolone did not increase breast cancer risk or VTE risk in randomized controlled trials of women mostly aged <65 years [Citation57]. Tibolone has not been associated with an increased risk of endometrial cancer or cardiovascular disease in women aged <60 years [Citation57] (and unpublished data [Citation64]). In women aged >60 years, 1.25 mg/day tibolone was associated with a small increase in risk of ischemic stroke while invasive breast cancer and colon cancer were significantly reduced by 68% and 69%, respectively [Citation65].
Circumstances that require prescribing caution, and ideally specialist review, include prescribing for women with high risk of VTE or breast cancer, untreated cardiovascular disease, undiagnosed vaginal bleeding, active liver disease and migraine, notably migraine with aura.
Managing clinical side effects of MHT therapy
Initiating treatment with low-dose MHT will minimize the likelihood of adverse effects. Common adverse effects of estrogen include nausea (mostly limited to oral therapy) and breast tenderness. Breakthrough bleeding is not unexpected in the first 3 months of continuous-combined estrogen–progestogen therapy, and occasionally heavy bleeding can occur. Progestogen therapy may cause low mood or irritability. When this occurs either the dose needs to be reduced or another progestogen tried. Micronized progesterone may improve sleep and sometimes causes somnolence, and so should be taken at bedtime [Citation66]. Changing from one regimen to another in many cases will alleviate adverse effects.
Potential adverse effects of tibolone include fluid retention, mild weight gain and initial vaginal bleeding or spotting.
Optimally, women using systemic MHT should have a medical review at 3 months to assess symptom relief and side effects. This is also an opportunity to discuss any patient concerns and ensure correct use of the prescribed therapy. Further follow-up will be determined by whether any treatment adjustments are made or investigations initiated. Long-term follow-up should be at least yearly. Review should include updating medical history and a general medical examination and breast assessment as indicated. Investigations should be individually determined, with mammography frequency according to local recommendations. Unscheduled or prolonged bleeding 3–6 months after commencing MHT needs investigation (ultrasound and/or biopsy), and where indicated, specialist referral. The need for ongoing MHT, the formulation and dose requirement should be reviewed.
Treatment tips
If symptoms persist on high-dose oral therapy, there is little point increasing beyond the recommended upper dose. Switch to non-oral. If symptoms persist on high-dose non-oral therapy, check serum estradiol as evidence that the patient is absorbing any of the administered dose. Note that different assays have different reference ranges. When a patient does not respond to adequate dose therapy another cause for the symptoms should be sought.
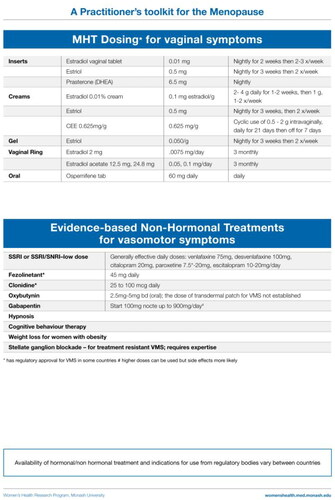
Local treatment of urogenital atrophy
Urogenital symptoms due to estrogen insufficiency are under-recognized and under-treated. These symptoms can be effectively treated with an array of local therapies including intravaginal estrogen preparations or DHEA (prasterone) and intravaginal moisturizers. Concurrent progestogen therapy is not required. Many women require local therapy, in addition to systemic MHT, to relieve urogenital symptoms. It is important patients understand that treatment needs to be ongoing and is not simply a short course of therapy.
Non-hormonal options with evidence to support efficacy
While women are commonly told to avoid VMS triggers and dress in layers, there is no evidence that such advice is of value with respect to the severity or frequency of VMS. Activities such as yoga, mindfulness, relaxation and exercise have health benefits but have not been shown to be meaningful treatments for VMS [Citation4–9, Citation11]. Similarly, nutritional supplements and botanicals have not been found to be more effective than placebo for moderately to severely bothersome VMS in robust clinical trials [Citation4–9, Citation11].
The following non-hormonal therapies have evidence to support their use to alleviate VMS [Citation4–9, Citation11].
The selective serotonin reuptake inhibitors and serotonin-norepinephrine reuptake inhibitors are effective in some, but not all, women with VMS. Paroxetine, 7.5 mg/day, has regulatory approval for VMS in the USA [Citation11].
Fezolinetant is a neurokinin 3B receptor antagonist that acts centrally in the brain to reduce VMS [Citation67]. It may improve sleep quality by reducing nocturnal VMS. Fezolinetant has been approved for the treatment of VMS at a dose of 45 mg/day in some countries [Citation11].
Low-dose oxybutynin has been found to be effective for treatment of VMS either as a standard low-dose or extended-release formulation [Citation11, Citation68].
Other potential, but probably less effective, options include clonidine and gabapentin/pregabalin. Clonidine may be prescribed for VMS for women who cannot take estrogen at a dose of 100–150 μg/day, although the effect is modest [Citation4–9, Citation69, Citation70] and it is not consistently recommended [Citation11]. The most common side effect, dry mouth, is dose related. Gabapentin (300–900 mg/day [Citation71, Citation72] may alleviate VMS, but side effects such as headache, dizziness and somnolence, which are dose related, are not uncommon and withdrawal may occur [Citation4–8].
Hypnosis may diminish VMS frequency and severity in postmenopausal women and can be considered a treatment option for women who are unable to take MHT [Citation11, Citation73].
Cognitive behavior therapy employs psychotherapeutic behavior modification to help women deal with VMS. Cognitive behavior therapy has been shown to significantly reduce VMS [Citation4–9, Citation11].
Stellate ganglion blockade at the anterolateral aspect of the C6 vertebra on the right side under fluoroscopy can alleviate severe VMS for up to 12 weeks [Citation74]. This procedure requires a highly skilled practitioner and availability is scant.
General guidance to prevent fragility fracture
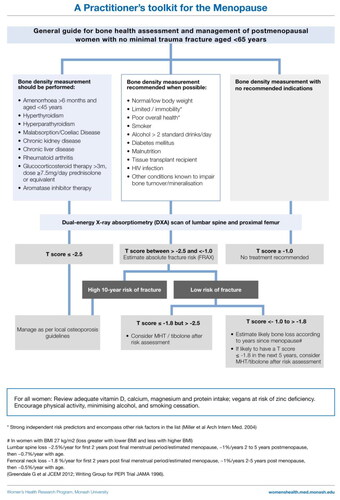
The majority of postmenopausal women who sustain a fragility fracture have a bone mineral density (BMD) T-score that categorizes them as having ‘osteopenia’ (T-score between −2.5 and −1.0) or what is considered normal BMD (T-score > −1.0) [Citation75]. An Australian study has shown that fractures occurring in people with osteopenia accounted for 50% of direct fracture treatment costs [Citation76]. Hence, a BMD diagnosis of osteoporosis (T-score ≤ –2.5) will miss most postmenopausal women who would benefit from fracture prevention.
The UK NICE Guideline [Citation9], and other high-quality guidelines [Citation4, Citation5], recommend MHT for the prevention of osteoporosis and fragility fracture, and MHT is licensed in many countries for this purpose [Citation77, Citation78]. However, guidance as to when postmenopausal women not requiring MHT for symptoms, but who have osteopenia, merit MHT to protect against fragility fracture is starkly absent in osteoporosis guidelines. We have assembled an algorithm to provide general guidance for the assessment and care of asymptomatic postmenopausal women with no prior fragility fracture. This guidance should be used with the understanding that overall fracture risk is influenced by other factors that determine bone strength, including factors intrinsic to bone (geometry and degree of mineralization), as well as age, BMI, mobility and balance, and an array of iatrogenic factors.
Which asymptomatic women should have a BMD study?
While various tools have been developed to identify the women likely to have osteoporosis on BMD study [Citation79–82], those that do not take into account menopause status perform poorly in women aged 50–64 years [Citation79, Citation80]. The bone health assessment and management algorithm summarizes the clinical conditions widely accepted as indicating that a BMD study should be performed, and the additional characteristics that identify women at increased likelihood of having low bone density [Citation83].
When to institute fracture prevention therapy
While the initiation of fracture prevention therapy in some guidelines hinges on the probability of a major fracture estimated by the Fracture Risk Assessment tool (FRAX) [Citation84], unfortunately this approach lacks validity in many healthy postmenopausal women aged <65 years [Citation79, Citation85]. Bone loss accelerates from the onset of the menopause transition in naturally menopausal women and peaks at about 2 years after the final menstrual period [Citation86]. Overall vertebral bone loss from 1 year before to 2 years after the final menstrual period is in the order of 7.4% for the lumbar spine and 5.8% for the femoral neck [Citation86]. The utility of FRAX is limited in women aged <65 years as it does not take into account menopausal status or the use of MHT.
The 2023 recommendation of the American College of Physicians is for bone-specific fracture prevention therapy to be indicated for postmenopausal women with a BMD T-score ≤ −2.5 and women aged >65 years with low bone mass [Citation87]. There are few data to guide intervention for postmenopausal women aged <65 years with osteopenia who are not identified as being at high fracture risk by FRAX.
The analysis of data from the National Osteoporosis Risk Assessment (NORA) Study showed that a peripheral BMD T-score of −1.8 or less was associated with an increased likelihood of short-term fracture in postmenopausal women aged 50 years and older. Women aged 50–64 years experienced one-third of all fractures and one-fifth of hip fractures [Citation83]. Irrespective of the T-score, a prior fracture was associated with a 4.1% risk over 12 months and a T-score of −1.8 or less with a 2.2% risk over 12 months [Citation88]. Other independent risk factors for fracture were poor mobility (1.9% risk) and poor health status (2.2% risk) [Citation88]. Interestingly, the 1-year risk of fracture was similar for women aged 50–59 years and 60–69 years [Citation88]. A limitation of the NORA study data is that BMD was assessed at peripheral sites by single X-ray absorptiometry and ultrasound such that absolute T-scores by these methods may not equate to T-scores for hip and spine dual-energy X-ray absorptiometry scan. However, to our knowledge this paper remains the only one to provide a T-score cut-off point that best differentiates women at increased risk of fracture among those with no prior fracture.
A consistent finding comes from the placebo arm of a clinical trial that included 309 postmenopausal women, mean age 64 years, not treated with estrogen or estrogen-like drugs within 3 months of study entry or for more than 1 month within 6 months, with a baseline femoral neck T-score of −1.84, and who were calcium and vitamin D replete, in which the 3-year cumulative fragility fracture incidence was 6.9% [Citation89]. The cumulative vertebral and non-vertebral fracture incidences were 4.2% and 5.4%, respectively [Citation89]. While these data are imperfect, together these studies support a peripheral or hip T-score of −1.8 to be a useful indicator of increased fracture risk in postmenopausal women aged <65 years.
The Women’s Health Initiative (WHI) study clearly demonstrated that MHT reduced vertebral and non-vertebral fracture incidence by 34% in 16,608 women aged 50 years and older unselected for BMD. MHT reduced total fractures in a subset of women who had BMD measured at baseline (osteoporosis present in 4% in MHT arm and 6% in placebo arm) [Citation90]. Importantly, fracture risk reduction was unaffected by age, BMI, smoking, history of falls, personal and family history of fracture, total calcium intake, past MHT use, baseline BMD or summary fracture risk score [Citation90].
The accelerated bone loss associated with menopause in white US women is in the order of 2.46% and 1.8% per year for the first 2 years postmenopause from the lumbar spine and neck of femur, respectively, and then about 1% per year from both sites after that, with less loss in women with a BMI above 27 kg/m2 and more loss in women with a BMI below this [Citation86]. Loss is likely to differ in women of other ethnicities [Citation86]. These data allow for crude prediction of percentage loss in postmenopausal women with a BMD T-score < –1.8 according to years postmenopause that might guide MHT use for bone loss prevention.
In summary, for postmenopausal women not identified as at high fracture risk by FRAX, no study to date has provided a clear T-score cut-off for the initiation of MHT solely for fracture prevention. On the one hand, MHT should be considered in all patients with osteopenia before age 65 years, taking into account other fracture risk factors. Alternatively, considering the available data, a peripheral or femoral neck T-score of −1.8 or lower offers a pragmatic, conservative, cut-off point after which fracture risk increases in postmenopausal women aged <65 years. When applying this cut-off, both the individual’s BMI and time since menopause need to be taken into consideration. MHT has been shown to prevent bone loss and fragility fractures in all postmenopausal women irrespective of BMD and other risk factors [Citation90]. In asymptomatic postmenopausal women aged <65 years with a T-score of −1.8 or less, MHT use is therefore likely to reduce future fractures, and for many the benefit will outweigh any potential risk.
Conclusion
The aim of the Toolkit is to provide evidence-based, general guidance to optimize menopause-related care. It provides fundamental information that will enable appropriate care of women across the globe and is intentionally not prescriptive. Consequently, the recommendations need to be applied in the context of local availability and cost of investigations and pharmacotherapies. Most importantly, the Toolkit provides the full spectrum of available options and therefore can be used to support shared decision-making and patient-informed care.
Potential conflict of interest
S.R.D. reports honoraria from Besins Healthcare, Mayne Pharma, Abbott, Health Ed, BioSyent, Lawley Pharmaceuticals and Que Oncology, has served on Advisory Boards for Mayne Pharma, Astellas Pharmaceuticals, Theramex and Gedeon Richter, and has been an institutional investigator for Que Oncology and Ovoca Bio. P.R.E. reports research grants from Amgen, Alexion and Sanofi, and honoraria from Amgen, Pfizer and Alexion.
Source of funding
This work was funded by Australian National Health and Medical Research Council (NHMRC) [Grant 2015514]. S.R.D. holds an NHMRC Leadership [Grant 2016627].
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
References
- Jane FM, Davis SR. A practitioner’s toolkit for managing the menopause. Climacteric. 2014;17(5):564–579. doi: 10.3109/13697137.2014.929651.
- Bell RJ, Lijovic M, Fradkin P, et al. A pragmatic approach to the classification of menopausal status for community-based research. Menopause. 2008;15(5):978–983. doi: 10.1097/gme.0b013e318162c487.
- Brouwers MC, Kho ME, Browman GP, et al. AGREE II: advancing guideline development, reporting and evaluation in health care. CMAJ. 2010;182(18):E839–42. doi: 10.1503/cmaj.090449.
- Meeta DL, Agarwal N, Vaze N, et al. Clinical practice guideline on menopause, 2nd ed. India: Indian Menopause Society; 2020.
- Obstetrical and Gynaecological Society of Malaysia MMS. Clinical practice guidelines management of menopause in Malaysia. Kuala Lumpur: Obstetrical and Gynaecological Society of Malaysia, Malaysian Menopause Society; 2022.
- Stuenkel CA, Davis SR, Gompel A, et al. Treatment of symptoms of the menopause: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2015;100(11):3975–4011. doi: 10.1210/jc.2015-2236.
- Ortmann O, Beckermann MJ, Inwald EC, et al. Peri- and postmenopause-diagnosis and interventions interdisciplinary S3 guideline of the association of the scientific medical societies in Germany (AWMF 015/062): short version. Arch Gynecol Obstet. 2020;302(3):763–777. doi: 10.1007/s00404-020-05682-4.
- Inwald EC, Albring C, Baum E, et al. Perimenopause and postmenopause – diagnosis and interventions. Guideline of the DGGG and OEGGG (S3-Level, AWMF registry number 015-062, september 2020). Geburtshilfe Frauenheilkd. 2021;81(6):612–636. doi: 10.1055/a-1361-1948.
- National Institute for Health and Care Excellence. Clinical guidelines. Menopause: full guideline. London (UK): National Institute for Health and Care Excellence; 2015.
- Carpenter J, Gass MLS, Maki PM, et al. Nonhormonal management of menopause-Associated vasomotor symptoms: 2015 position statement of the North American menopause society. Menopause. 2015;22(11):1155–1174.
- The 2023 nonhormone therapy position statement of the North American menopause society. Menopause. 2023;30(6):573–590.
- Davis SR, Lambrinoudaki I, Lumsden MA, et al. Menopause. Nat Rev Dis Primers. 2015;1(1):15004. doi: 10.1038/nrdp.2015.4.
- Singh M. Early age of natural menopause in India, a biological marker for early preventive health programs. Climacteric. 2012;15(6):581–586. doi: 10.3109/13697137.2011.643514.
- Qiu C, Chen H, Wen J, et al. Associations between age at menarche and menopause with cardiovascular disease, diabetes, and osteoporosis in Chinese women. J Clin Endocrinol Metab. 2013;98(4):1612–1621. doi: 10.1210/jc.2012-2919.
- Gold EB, Crawford SL, Avis NE, et al. Factors related to age at natural menopause: longitudinal analyses from SWAN. Am J Epidemiol. 2013;178(1):70–83. doi: 10.1093/aje/kws421.
- Castelo-Branco C, Blumel JE, Chedraui P, et al. Age at menopause in Latin America. Menopause. 2006;13(4):706–712. doi: 10.1097/01.gme.0000227338.73738.2d.
- Tesarik J, Galan-Lazaro M, Mendoza-Tesarik R. Ovarian aging: molecular mechanisms and medical management. Int J Mol Sci. 2021;22(3):1371.
- Richardson SJ, Senikas V, Nelson JF. Follicular depletion during the menopausal transition: evidence for accelerated loss and ultimate exhaustion. J Clin Endocrinol Metab. 1987;65(6):1231–1237. doi: 10.1210/jcem-65-6-1231.
- Nelson SM, Davis SR, Kalantaridou S, et al. Anti-Müllerian hormone for the diagnosis and prediction of menopause: a systematic review. Hum Reprod Update. 2023;29(3):327–346. doi: 10.1093/humupd/dmac045.
- Harlow SD, Gass M, Hall JE, et al. Executive summary of the stages of reproductive aging workshop + 10: addressing the unfinished agenda of staging reproductive aging. J Clin Endocrinol Metab. 2012;97(4):1159–1168. doi: 10.1210/jc.2011-3362.
- Skiba MA, Bell RJ, Islam RM, et al. Androgens during the reproductive years, What’s normal for women? J Clin Endocrinol Metab. 2019;104(11):5382–5392. doi: 10.1210/jc.2019-01357.
- Davison SL, Bell R, Donath S, et al. Androgen levels in adult females: changes with age, menopause, and oophorectomy. J Clin Endocrinol Metab. 2005;90(7):3847–3853. doi: 10.1210/jc.2005-0212.
- Burger HG, Dudley EC, Cui J, et al. A prospective longitudinal study of serum testosterone, dehydroepiandrosterone sulfate, and sex hormone-binding globulin levels through the menopause transition. J Clin Endocrinol Metab. 2000;85(8):2832–2838. doi: 10.1210/jc.85.8.2832.
- Judd HL, Lucas WE, Yen SSC. Effect of oopherectomy on circulating testosterone and androstenedione levels in patients with endometrial cancer. Am J Obstet Gynecol. 1974;118(6):793–798. doi: 10.1016/0002-9378(74)90490-6.
- Soman M, Huang LC, Cai WH, et al. Serum androgen profiles in women with premature ovarian insufficiency: a systematic review and meta-analysis. Menopause. 2019;26(1):78–93. doi: 10.1097/GME.0000000000001161.
- Van Look PF, Lothian H, Hunter WM, et al. Hypothalamic-pituitary-ovarian function in perimenopausal women. Clin Endocrinol. 1977;7(1):13–31. doi: 10.1111/j.1365-2265.1977.tb02936.x.
- Islam RM, Bell RJ, Billah B, et al. Prevalence and severity of vasomotor symptoms and joint pain in women at midlife in Bangladesh: a population-based survey. Menopause. 2016;23(7):731–739. doi: 10.1097/GME.0000000000000615.
- Maki PM, Jaff NG. Brain fog in menopause: a health-care professional’s guide for decision-making and counseling on cognition. Climacteric. 2022;25(6):570–578. doi: 10.1080/13697137.2022.2122792.
- Gartoulla P, Worsley R, Bell RJ, et al. Moderate-severe vasomotor and sexual symptoms remain problematic for 60-65 year old women. Menopause. 2015;22(7):694–701. doi: 10.1097/GME.0000000000000383.
- Zeleke BM, Bell RJ, Billah B, et al. Vasomotor and sexual symptoms in older Australian women: a cross-sectional study. Fertil Steril. 2016;105(1):149–155 e1. doi: 10.1016/j.fertnstert.2015.09.017.
- Gartoulla P, Bell RJ, Worsley R, et al. Moderate-severely bothersome vasomotor symptoms are associated with lowered psychological general wellbeing in women at midlife. Maturitas. 2015;81(4):487–492. doi: 10.1016/j.maturitas.2015.06.004.
- Fooladi E, Bell RJ, Masoumi M, et al. Bothersome menopausal symptoms amongst postmenopausal iranian women. Climacteric. 2018;21(6):586–593. doi: 10.1080/13697137.2018.1493452.
- Worsley R, Bell RJ, Gartoulla P, et al. Moderate-severe vasomotor symptoms are associated with moderate-severe depressive symptoms. J Womens Health. 2017;26(7):712–718. doi: 10.1089/jwh.2016.6142.
- Akiko S, Kawaharada M. Associations between hot flashes, obesity, sense of coherence and qol among japanese farmers and part-time workers. Climacteric. 2011;14:187.
- Chuni N, Sreeramareddy CT. Frequency of symptoms, determinants of severe symptoms, validity of and cut-off score for Menopause Rating Scale (MRS) as a screening tool: a cross-sectional survey among midlife Nepalese women. BMC Womens Health. 2011;11(1):30. doi: 10.1186/1472-6874-11-30.
- Olaolorun FM, Lawoyin TO. Experience of menopausal symptoms by women in an urban community in Ibadan, Nigeria. Menopause. 2009;16(4):822–830. doi: 10.1097/gme.0b013e318198d6e7.
- Waidyasekera H, Wijewardena K, Lindmark G, et al. Menopausal symptoms and quality of life during the menopausal transition in Sri Lankan women. Menopause. 2009;16(1):164–170. doi: 10.1097/gme.0b013e31817a8abd.
- Appiah D, Nwabuo CC, Ebong IA, et al. Trends in age at natural menopause and reproductive life span among US women, 1959–2018. JAMA. 2021;325(13):1328–1330. doi: 10.1001/jama.2021.0278.
- Gottschalk MS, Eskild A, Hofvind S, et al. Temporal trends in age at menarche and age at menopause: a population study of 312 656 women in Norway. Hum Reprod. 2020;35(2):464–471. doi: 10.1093/humrep/dez288.
- Davis SR, Baber R, Panay N, et al. Global consensus position statement on the use of testosterone therapy for women. Climacteric. 2019;22(5):429–434. doi: 10.1080/13697137.2019.1637079.
- Ng E, Sztal-Mazer S, Davis SR. Functional hypothalamic amenorrhoea: a diagnosis of exclusion. Med J Aust. 2022;216(2):73–76. doi: 10.5694/mja2.51376.
- Yoh K, Ikeda K, Horie K, et al. Roles of estrogen, estrogen receptors, and Estrogen-Related receptors in skeletal muscle: regulation of mitochondrial function. Int J Mol Sci. 2023;24(3):1853.
- Gartoulla P, Worsley R, Bell RJ, et al. Moderate to severe vasomotor and sexual symptoms remain problematic for women aged 60 to 65 years. Menopause. 2018;25(11):1331–1338. doi: 10.1097/GME.0000000000001237.
- Kroenke CH, Caan BJ, Stefanick ML, et al. Effects of a dietary intervention and weight change on vasomotor symptoms in the women’s health initiative. Menopause. 2012;19(9):980–988. doi: 10.1097/gme.0b013e31824f606e.
- Elraiyah T, Sonbol MB, Wang Z, et al. Clinical review: the benefits and harms of systemic dehydroepiandrosterone (DHEA) in postmenopausal women with normal adrenal function: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2014;99(10):3536–3542. doi: 10.1210/jc.2014-2261.
- Langrish JP, Mills NL, Bath LE, et al. Cardiovascular effects of physiological and standard sex steroid replacement regimens in premature ovarian failure. Hypertension. 2009;53(5):805–811. doi: 10.1161/HYPERTENSIONAHA.108.126516.
- Klipping C, Duijkers I, Parke S, et al. Hemostatic effects of a novel estradiol-based oral contraceptive: an open-label, randomized, crossover study of estradiol valerate/dienogest versus ethinylestradiol/levonorgestrel. Drugs R D. 2011;11(2):159–170. doi: 10.2165/11591200-000000000-00000.
- Chrisman C, Ribeiro P, Dalton VK. The levonorgestrel-releasing intrauterine system: an updated review of the contraceptive and noncontraceptive uses. Clin Obstet Gynecol. 2007;50(4):886–897. doi: 10.1097/GRF.0b013e318159c0d9.
- Pinkerton JV, Abraham L, Bushmakin AG, et al. Evaluation of the efficacy and safety of bazedoxifene/conjugated estrogens for secondary outcomes including vasomotor symptoms in postmenopausal women by years since menopause in the selective estrogens, menopause and response to therapy (SMART) trials. J Womens Health. 2014;23(1):18–28. doi: 10.1089/jwh.2013.4392.
- Mirkin S, Komm BS, Pan K, et al. Effects of bazedoxifene/conjugated estrogens on endometrial safety and bone in postmenopausal women. Climacteric. 2013;16(3):338–346. doi: 10.3109/13697137.2012.717994.
- Nijland EA, Weijmar Schultz WC, Nathorst BJ, et al. Tibolone and transdermal E2/NETA for the treatment of female sexual dysfunction in naturally menopausal women: results of a randomized active-controlled trial. J Sex Med. 2008;5(3):646–656. doi: 10.1111/j.1743-6109.2007.00726.x.
- Portman DJ, Bachmann GA, Simon JA. Ospemifene, a novel selective estrogen receptor modulator for treating dyspareunia associated with postmenopausal vulvar and vaginal atrophy. Menopause. 2013;20(6):623–630. doi: 10.1097/gme.0b013e318279ba64.
- Simon J, Portman D, Mabey RG.Jr. Long-term safety of ospemifene (52-week extension) in the treatment of vulvar and vaginal atrophy in hysterectomized postmenopausal women. Maturitas. 2014;77(3):274–281. doi: 10.1016/j.maturitas.2013.12.005.
- Islam RM, Bell RJ, Green S, et al. Safety and efficacy of testosterone for women: a systematic review and meta-analysis of randomised controlled trial data. Lancet Diabetes Endocrinol. 2019;7(10):754–766. doi: 10.1016/S2213-8587(19)30189-5.
- NIH-Pannel. NIH state-of-the-Science conference statement on management of menopause-related symptoms. Annals Internal Med. 2005;142(12):1003–1–13.
- Welton AJ, Vickers MR, Kim J, et al. Health related quality of life after combined hormone replacement therapy: randomised controlled trial. BMJ. 2008;337(2):a1190–a1190. doi: 10.1136/bmj.a1190.
- Formoso G, Perrone E, Maltoni S, et al. Short-term and long-term effects of tibolone in postmenopausal women. Cochrane Database Syst Rev. 2016;10: CD008536.
- Whedon JM, KizhakkeVeettil A, Rugo NA, et al. Bioidentical estrogen for menopausal depressive symptoms: a systematic review and Meta-Analysis. J Womens Health. 2017;26(1):18–28. doi: 10.1089/jwh.2015.5628.
- Zhang J, Yin J, Song X, et al. The effect of exogenous estrogen on depressive mood in women: a systematic review and meta-analysis of randomized controlled trials. J Psychiatr Res. 2023;162:21–29. doi: 10.1016/j.jpsychires.2023.04.002.
- Rossouw J, Anderson G, Prentice R, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the women’s health initiative randomised controlled trial. JAMA. 2002;288(3):321–333.
- Anderson GL, Limacher M, Assaf AR, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the women’s health initiative randomized controlled trial. JAMA. 2004;291(14):1701–1712.
- Vinogradova Y, Coupland C, Hippisley-Cox J. Use of hormone replacement therapy and risk of breast cancer: nested case-control studies using the QResearch and CPRD databases. BMJ. 2020;371:m3873. doi: 10.1136/bmj.m3873.
- Fournier A, Fabre A, Mesrine S, et al. Use of different postmenopausal hormone therapies and risk of histology- and hormone receptor-defined invasive breast cancer. J Clin Oncol. 2008;26(8):1260–1268. doi: 10.1200/JCO.2007.13.4338.
- Archer DF, Hendrix S, Ferenczy A, et al. Tibolone histology of the endometrium and breast endpoints study: design of the trial and endometrial histology at baseline in postmenopausal women. Fertil Steril. 2007;88(4):866–878. doi: 10.1016/j.fertnstert.2006.12.052.
- Cummings SR, Ettinger B, Delmas PD, et al. The effects of tibolone in older postmenopausal women. N Engl J Med. 2008;359(7):697–708. doi: 10.1056/NEJMoa0800743.
- Gambacciani M, Ciaponi M, Cappagli B, et al. Effects of low-dose, continuous combined hormone replacement therapy on sleep in symptomatic postmenopausal women. Maturitas. 2005;50(2):91–97. doi: 10.1016/j.maturitas.2004.04.006.
- Johnson KA, Martin N, Nappi RE, et al. Efficacy and safety of fezolinetant in moderate-to-Severe vasomotor symptoms associated with menopause: a phase 3 RCT. J Clin Endocrinol Metab. 2023;108(8):1981–1997. doi: 10.1210/clinem/dgad058.
- Simon JA, Gaines T, LaGuardia KD. Extended-release oxybutynin therapy for vasomotor symptoms in women: a randomized clinical trial. Menopause. 2016;23(11):1214–1221. doi: 10.1097/GME.0000000000000773.
- Bolli P, Simpson FO. Clonidine in menopausal flushing: a double-blind trial. N Z Med J. 1975;82(548):196–197.
- Pandya KJ, Raubertas RF, Flynn PJ, et al. Oral clonidine in postmenopausal patients with breast cancer experiencing tamoxifen-induced hot flashes: a University of Rochester Cancer Center Community Clinical Oncology Program study. Ann Intern Med. 2000;132(10):788–793. doi: 10.7326/0003-4819-132-10-200005160-00004.
- Lavigne JE, Heckler C, Mathews JL, et al. A randomized, controlled, double-blinded clinical trial of gabapentin 300 versus 900 mg versus placebo for anxiety symptoms in breast cancer survivors. Breast Cancer Res Treat. 2012;136(2):479–486. doi: 10.1007/s10549-012-2251-x.
- Pandya KJ, Morrow GR, Roscoe JA, et al. Gabapentin for hot flashes in 420 women with breast cancer: a randomised double-blind placebo-controlled trial. Lancet. 2005;366(9488):818–824. doi: 10.1016/S0140-6736(05)67215-7.
- Elkins GR, Fisher WI, Johnson AK, et al. Clinical hypnosis in the treatment of postmenopausal hot flashes: a randomized controlled trial. Menopause. 2013;20(3):291–298. doi: 10.1097/gme.0b013e31826ce3ed.
- Lipov EG, Joshi JR, Sanders S, et al. Effects of stellate-ganglion block on hot flushes and night awakenings in survivors of breast cancer: a pilot study. Lancet Oncol. 2008;9(6):523–532. doi: 10.1016/S1470-2045(08)70131-1.
- Pasco JA, Seeman E, Henry MJ, et al. The population burden of fractures originates in women with osteopenia, not osteoporosis. Osteoporos Int. 2006;17(9):1404–1409. doi: 10.1007/s00198-006-0135-9.
- Tatangelo G, Watts J, Lim K, et al. The cost of osteoporosis, osteopenia, and associated fractures in Australia in 2017. J Bone Miner Res. 2019;34(4):616–625. doi: 10.1002/jbmr.3640.
- National Collaborating Centre for Women’s and Children’s Health. Menopause. London: National Institute for Health and Care Excellence (UK); 2015.
- The North American menopause S. Management of osteoporosis in postmenopausal women: the 2021 position statement of the North American menopause society. Menopause. 2021;28(9):973–997.
- Crandall CJ, Larson J, Gourlay ML, et al. Osteoporosis screening in postmenopausal women 50 to 64 years old: comparison of US preventive services task force strategy and two traditional strategies in the women’s health initiative. J Bone Miner Res. 2014;29(7):1661–1666. doi: 10.1002/jbmr.2174.
- Gourlay ML, Miller WC, Richy F, et al. Performance of osteoporosis risk assessment tools in postmenopausal women aged 45-64 years. Osteoporos Int. 2005;16(8):921–927. doi: 10.1007/s00198-004-1775-2.
- Cadarette SM, Jaglal SB, Kreiger N, et al. Development and validation of the osteoporosis risk assessment instrument to facilitate selection of women for bone densitometry. CMAJ. 2000;162(9):1289–1294.
- Davis SR, Tan A, Bell RJ. Targeted assessment of fracture risk in women at midlife. Osteoporos Int. 2015;26(6):1705–1712. doi: 10.1007/s00198-015-3046-9.
- Siris ES, Brenneman SK, Miller PD, et al. Predictive value of low BMD for 1-year fracture outcomes is similar for postmenopausal women ages 50-64 and 65 and older: results from the National Osteoporosis Risk Assessment (NORA). J Bone Miner Res. 2004;19(8):1215–1220. doi: 10.1359/JBMR.040508.
- Kanis JA, Cooper C, Rizzoli R, et al. European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int. 2019;30(1):3–44. doi: 10.1007/s00198-018-4704-5.
- Ghannam S, Blaney H, Gelfond J, et al. The use of FRAX in identifying women less than 65 years needing bone mineral density testing. J Clin Densitom. 2021;24(1):36–43. doi: 10.1016/j.jocd.2020.05.002.
- Greendale GA, Sowers M, Han W, et al. Bone mineral density loss in relation to the final menstrual period in a multiethnic cohort: results from the Study of Women’s Health across the Nation (SWAN). J Bone Miner Res. 2012;27(1):111–118. doi: 10.1002/jbmr.534.
- Qaseem A, Hicks LA, Etxeandia-Ikobaltzeta I, et al. Pharmacologic treatment of primary osteoporosis or low bone mass to prevent fractures in adults: a living clinical guideline from the American college of physicians. Ann Intern Med. 2023;176(2):224–238. doi: 10.7326/M22-1034.
- Miller PD, Barlas S, Brenneman SK, et al. An approach to identifying osteopenic women at increased short-term risk of fracture. Arch Intern Med. 2004;164(10):1113–1120. doi: 10.1001/archinte.164.10.1113.
- Siris ES, Simon JA, Barton IP, et al. Effects of risedronate on fracture risk in postmenopausal women with osteopenia. Osteoporos Int. 2008;19(5):681–686. doi: 10.1007/s00198-007-0493-y.
- Cauley JA, Robbins J, Chen Z, et al. Effects of estrogen plus progestin on risk of fracture and bone mineral density: the women’s health initiative randomized trial. Jama. 2003;290(13):1729–1738. doi: 10.1001/jama.290.13.1729.