Abstract
The ability to track moving objects is a crucial skill for performance in everyday spatial tasks. The tracking mechanism depends on representation of moving items as coherent entities, which follow the spatiotemporal constraints of objects in the world. In the present experiment, participants tracked 1 to 4 targets in a display of 8 identical objects. Objects moved randomly and independently (moving condition), passed behind an invisible bar (occluded condition), or momentarily disappeared by shrinking (implosion condition). Scholl and Pylyshyn (1999) found that adults can track entities under the moving and occluded conditions, but not under implosion. This finding suggests that the tracking mechanism is constrained by the spatiotemporal properties of physical objects as they move in the world. In the present study, we adapt these conditions to investigate whether this constraint holds for people with severe spatial impairments associated with Williams syndrome (WS). In Experiment 1, we compare the performance of individuals with WS and typically developing (TD) adults. TD adults replicated Scholl and Pylyshyn’s findings; performance was no different between the moving and occluded conditions but was worse under implosion. People with WS had reduced tracking capacity but demonstrated the same pattern across conditions. In Experiment 2, we tested TD 4-, 5-, and 7-year-olds. People with WS performed at a level that fell between TD 4- and 5-year-olds. These results suggest that the multiple object tracking system in WS operates under the same object-based constraints that hold in typical development.
The ability to track moving objects is a crucial skill for a wide range of visual functions, from simple tasks such as reaching to more complex tasks such as driving. In a seminal set of studies, Pylyshyn and colleagues demonstrated that adults are able to simultaneously track up to four or five objects as they move, change, or briefly disappear (Burkell & Pylyshyn, Citation1997; Pylyshyn, Citation1989, Citation1994; Pylyshyn et al., Citation1994; Pylyshyn & Storm, Citation1988; Scholl & Pylyshyn, Citation1999). They proposed that object tracking is accomplished by a limited number of “mental pointers” that act like “sticky fingers” and adhere to objects on the basis of their spatiotemporal continuity over paths of motion. This capacity allows adults to keep track of individual moving objects without explicitly representing their spatial location. These mental pointers have been called fingers of instantiation (FINSTs; Pylyshyn & Storm, Citation1988) or indexes (Leslie, Xu, Tremoulet, & Scholl, Citation1998; Pylyshyn, Citation2000) and are thought to underlie the ability to carry out multiple object tracking (MOT) from infancy onward. They may also play a role in other tasks that require that objects are represented as distinct individuated entities moving through space (Ballard, Hayhoe, Pook, & Rao, Citation1997; Carey & Xu, Citation2001; Leslie et al., Citation1998; Pylyshyn, Citation2000; Richardson & Kirkham, Citation2004; Scholl & Leslie, Citation1999; Ullman, Citation1984).
One clear signature of the tracking system is its dependence on representation of moving entities as objects. Several lines of evidence have shown that people track visual entities that behave according to the physical and spatiotemporal constraints of solid objects in the real world (Hollingworth & Franconeri, Citation2009; Scholl, Citation2001; Scholl & Pylyshyn, Citation1999; Scholl, Pylyshyn, & Feldman, Citation2001; Spelke, Citation1990). For example, performance breaks down when people attempt to track entities that move like substances (e.g., pouring; vanMarle & Scholl, Citation2003). In addition, tracking is successful when objects disappear for a short period, but only if their disappearance adheres to the properties of objects as they move behind and re-emerge from occluders (Scholl & Pylyshyn, Citation1999). In this kind of an event, an object’s visible boundaries undergo continuous smooth deletion from one edge as the object goes behind the occluder and continuous smooth accretion of that edge as it re-emerges from behind the occluder. The visual information specifying the object’s disappearance conforms to what occurs when objects in the real world come into and go out of view as they pass behind different surfaces (e.g., a person walking behind a car and appearing on the other side). Brief occludeds of this kind do not impair adults’ MOT performance—they can track as many objects under this kind of occluded as they can when the objects are fully visible throughout their trajectory (Scholl & Pylyshyn, Citation1999). In contrast, if an object undergoes disappearance by “implosion”—where it continuously shrinks to a point—people find it difficult to track the same object as it reappears in a different location by gradually growing in size from a point (“explosion”). Implosion/explosion events do not support the interpretation of an object going out of sight behind an occluding surface, but instead, they suggest that the object has gone out of and then come back into existence (Gibson, Kaplan, Reynolds, & Wheeler, Citation1969; Scholl & Pylyshyn, Citation1999). Scholl and Pylyshyn (Citation1999) found that in such cases of disappearance, adult MOT performance is significantly compromised. Presumably, in these cases, one can make “best guesses” about the trajectory linking an imploded object and a newly created object, but these guesses are not equivalent to the tracking mechanism that holds when a single object is tracked as it moves behind an occluder (Fencsik, Klieger, & Horowitz, Citation2007; Iordanescu, Grabowecky, & Suzuki, Citation2009). Sensitivity to the objecthood constraint on MOT exists even early in life; infants establish representations of constant number when objects disappear and reappear via occluded, but not when they do so via implosion (Cheries, Feigenson, Scholl, & Carey, Citation2005).
In the present article, we asked whether this signature pattern for visual tracking holds even when tracking capacity itself is impaired. We examined MOT in individuals with Williams syndrome (WS), a genetic disorder of approximately 25 deleted genes on chromosome 7q11.23 (Morris, Citation2006), occurring with an incidence of 1 in 7,500 (Strømme, Bjørnstad, & Ramstad, Citation2002). WS is characterized by a cognitive profile that includes mild-to-moderate intellectual disability (Mervis et al., Citation2000) and severe impairment on a range of spatial functions. Adults with WS show the capacity to track only approximately two moving objects at a time, comparable to typically developing (TD) 4-year-olds (O’Hearn, Landau, & Hoffman, Citation2005). We review these findings in more detail in the following sections, but for now, we note that this impairment suggests a substantial quantitative decrease in tracking capacity. We aim to discover whether this impaired tracking capacity nevertheless bears the same signature constraint on objecthood as has been shown for TD adults. If it does, this finding will suggest that the MOT system is deeply constrained by the requirement to track objects, even if it is significantly compromised. Alternatively, it is possible that the atypical visual-spatial capacities of people with WS, along with corresponding impairments at the neural level, will result in qualitative differences in tracking (cf. Karmiloff-Smith, Citation1998). For example, the WS tracking system may be blind to the spatiotemporal constraints of moving objects and operate instead on visual entities that are not analyzed as objects per se.
The latter hypothesis is in principle consistent with the broad range of spatial impairment shown by this population as well as the corresponding neurological abnormalities. The WS visual-spatial impairment has been documented across a variety of tasks, including block construction, figure copying, orientation discrimination, and visual-motor action (Brown et al., Citation2003; Dilks, Landau, & Hoffman, Citation2008; Georgopoulos, Georgopoulos, Kurz, & Landau, Citation2004; Hoffman, Landau, & Pagani, Citation2003; Meyer-Lindenberg et al., Citation2004; Nardini, Atkinson, Braddick, & Burgess, Citation2008; O’Hearn et al., Citation2005; Palomares, Landau, & Egeth, Citation2009; Vicari & Carlesimo, Citation2006; see Landau & Hoffman, Citation2012, for review). On these tasks, people with WS perform at levels below TD individuals who are of the same chronological age. They also often show levels of performance that are below those of TD children who are matched for mental age (MA), most often measured by the Differential Abilities Scale (Elliot, Citation1990) or the Kaufman Brief Intelligence Test (K-BIT II; Kaufman & Kaufman, Citation2004). The method of matching participants on MA attempts to factor out intellectual disability; however, it rests upon the assumption that one can identify with confidence the complete set of cognitive abilities that are measured by the standardized test. In the work presented here, we adopted the approach of comparing the performance of individuals with WS to those of TD children of different chronological ages. By examining the typical developmental trajectory for different spatial functions, we can a) gain an understanding of the shape of the growth curve and b) infer where people with WS fall relative to TD individuals who are at different points along the trajectory for the cognitive function of interest.
Research indicates that MOT is susceptible to impairment in WS. O’Hearn et al. (Citation2005) found that individuals with WS performed more poorly than MA-matched TD controls (Mage = 5;11) when asked to track three or four moving targets (moving condition) but were no different from these controls when asked to remember static locations (static condition). On average, people with WS performed at the level of TD 4-year-olds in the moving condition (tracking up to two moving targets) but were at the level of 5- to 6-year-olds in the static condition, as they remembered the locations of four non-moving targets. Because both the static and moving conditions required storing the locations of up to four objects, these findings indicate that the WS impairment does not result simply from a general limit in visual-spatial memory or attention. This suggests that MOT recruits one or more processes that are not required for static visual-spatial memory and that these processes are compromised in WS. In a separate study, O’Hearn, Hoffman, and Landau (Citation2011) tested individuals with WS on a subitizing task, thought to also engage visual-spatial pointers or “indexes” (Trick, Jaspers-Fayer, & Sethi, Citation2005). The findings revealed a similar pattern, with the WS group more likely to subitize three rather than four elements, comparable to TD 4-year-olds (O’Hearn et al., Citation2011). Finally, to better understand the limit on the number of objects people with WS are able to track, O’Hearn, Hoffman, and Landau (Citation2010) compared WS performance on an MOT task to that of TD children across a broader age range (3–7 years). They found that the typical developmental trajectory of MOT performance on this task shows growth in capacity during these years, from approximately one object at age 3 years to four objects at age 7 years. In contrast, the MOT system in adolescents and adults with WS was estimated to have a surprisingly small capacity of only approximately two objects, similar to the capacity of TD 4-year-olds.
The WS impairment in various visual-spatial tasks, including MOT, is consistent with findings of atypical brain structure and function in individuals with WS. The WS brain structurally differs from TD individuals in a number of ways, including reduced gray matter volume (Boddaert et al., Citation2005; Chiang et al., Citation2007; Eckert et al., Citation2005; Meyer-Lindenberg et al., Citation2004; Reiss et al., Citation2000), sulcal depth (Gaser et al., Citation2006; Jackowski & Schultz, Citation2005; Kippenhan et al., Citation2005; Van Essen et al., Citation2006), and atypical properties of white matter tracts (Faria et al., Citation2012; Hoeft et al., Citation2007; Marenco et al., Citation2007). Especially pertinent to MOT, the parietal lobe, which supports a variety of spatial functions, shows atypical functional properties (Boddeart et al., Citation2005; Meyer-Lindenburg et al., Citation2004, Citation2005; Mobbs et al., Citation2004). For example, during a visual-spatial construction task, individuals with WS show less activation in the intraparietal sulcus (IPS) compared with age-matched controls (Meyer-Lindenberg et al., Citation2004). These authors suggested that the WS brain has a structural bottleneck in this region, resulting in compromised ability to represent spatial information at higher levels. The IPS is engaged in MOT as well as related tasks (e.g., subitizing; Atmaca et al., Citation2013; Culham et al., Citation1998, Citation2001; Howe, Horowitz, Morocz, Wolfe, & Livingstone, Citation2009; Jovicich et al., Citation2001), consistent with the WS behavioral spatial impairment.
Despite the clear pattern of impairment, the existing data do not tell us whether MOT in people with WS is qualitatively different from that in TD children. Indeed, Landau and Hoffman (Citation2012) (see also Landau & Ferrara, Citation2013) have argued that the bulk of the data on visual-spatial functions in WS is consistent with the idea that these functions are quantitatively, but not qualitatively, atypical. Specifically, these authors proposed that people with WS possess the same architecture and constraints for visual-spatial function as is shown in TD systems; even if performance is quantitatively worse, it will be qualitatively similar to the TD system at some developmental point.
This hypothesis receives support from a broad range of studies on visual-spatial function in people with WS compared to TD children. In many spatial tasks, individuals with WS show impairment relative to TD children who are matched for MA. However, their profile often looks quite similar to that of TD children who are younger than these MA matches, suggesting that the WS spatial systems develop to the level of a young TD child but in some cases, may develop no further. For example, Landau, Hoffman, and Kurz (Citation2006) found that children and adults with WS and TD 4- and 6-year-highly accurate in recognizing briefly presented objects when presented in canonical orientations. However, when the objects were presented in unusual orientations, the accuracy of all groups suffered; individuals with WS and TD 4-year-olds fell to the same level of poor performance and were only able to identify the object on approximately half of the trials (Landau & Hoffman, Citation2012; Landau et al., Citation2006). The unusual orientation condition was quite difficult for all groups, and TD children did not reach adult levels of performance until 12 years of age. This finding suggests that recognition of objects under canonical versus unusual orientations develops along different timelines, with the former reaching maturity in typical development earlier than the latter. By adolescence, people with WS appear to reach only the level of a TD 4- or 6-year-old and perform well on canonical orientations but poorly on unusual orientations. It is unclear whether they progress further, but evidence has suggested that development of at least some spatial functions may become arrested during adolescence in WS, just when they have reached the level of a young TD child.
Other research has shown similar patterns. For example, in tasks of orientation discrimination, people with WS perform at the same level as TD 4-year-olds, far below the mature adult level, which emerges at 9 years of age (Palomares et al., Citation2009). In tasks requiring visual orientation integration (detection of an orientation-defined contour embedded among randomly oriented noise elements), people with WS also perform like TD 4-year-olds, but in this case, the performance of both groups is similar to that of TD adults, suggesting that orientation integration typically matures quite early in life (Palomares et al., Citation2009). Overall, the pattern of findings on visual-spatial cognition in people with WS suggests that they reach maturity for many spatial functions that typically mature early in life, but they do not do so for spatial functions that reach maturity in adolescence or adulthood (Landau & Ferrara, Citation2013; Landau & Hoffman, Citation2012). This pattern extends to related cognitive domains (e.g., numeric estimation; Libertus, Feigenson, Halberda, & Landau, Citation2014) and surprisingly can also account for many facts about language in WS (Landau & Hoffman, Citation2012; Musolino & Landau, Citation2010).
This view of the WS spatial impairment predicts that although there may be significant limits on the capacity to track multiple moving objects, the internal structure of the system may reflect the very same constraints that have been observed in TD infants and adults. Although people with WS may show a small capacity in the number of objects they are able to track, they may show the same qualitative signature pattern of tracking across different types of object disappearance. We hypothesized that WS tracking is relatively unaffected when targets disappear and reappear in a manner that reflects the spatiotemporal constraints of continuous objects in the world (e.g., those that move behind occluders and then re-emerge from the opposite side as unitary entities). In contrast, we predicted that people with WS will be selectively impaired in tracking the same entities when they disappear in a manner that does not follow principles of object persistence (e.g., when they disappear via implosion).
In Experiment 1, we examined MOT in TD adults and people with WS under three conditions, one in which the tracked entities are fully visible and two in which they undergo disappearance and reappearance (one following the principles of solid objects as they pass behind occluders and the other in which the objects disappear by “imploding” and reappear by “exploding”). Although individuals with WS may demonstrate smaller tracking capacity in comparison to TD adults, we predict that the two groups will show similar tracking patterns across the three conditions.
EXPERIMENT 1
Participants
We tested 12 individuals with WS and 12 TD adults. Individuals with WS ranged in age from 11;3 to 39;1 (Mage = 19;3, SD = 89 months, 5 male). The number of participants with WS within each age bracket was as follows: 4 participants aged 11 to 15 years, 4 participants aged 16 to 20 years, 2 participants aged 21 to 25 years, 1 participant aged 25 to 30 years, and 1 participant aged 30 years or older. All individuals with WS had been positively diagnosed by the FISH (fluorescence in situ hybridization) test (Ewart, Citation1993). They were recruited through the Williams Syndrome Association and received monetary compensation for their participation. The 12 TD adults ranged in age from 20;1 to 25;0 (Mage = 20;6, SD = 16 months, 6 male). All were undergraduates who received course credit for their participation. The WS and TD adult groups did not significantly differ from one another in terms of chronological age, t(25) = –0.61, p = .55, d = –0.22.
To assess the overall cognitive profile of participants with WS, the K-BIT II (Kaufman & Kaufman, Citation2004) was administered. The K-BIT II is a standardized measure normed for TD individuals aged 4 to 90 years. It consists of verbal measures that assess vocabulary and a nonverbal measure that assesses the ability to categorize items and complete matrices based on item similarity. These scores are combined to yield a composite standard score, the equivalent of IQ. On the verbal measure, participants with WS had an average standard score of 69.67 (SD = 15.26; range = 46–97). On the nonverbal measure, participants with WS had an average standard score of 69.83 (SD = 19.80; range = 43–103). The average composite standard score was 66.83 (SD = 16.18; range = 44–90). These standard scores are in the range typically found in empirical studies of people with WS (Landau & Hoffman, Citation2012; Mervis et al., Citation2000; Pitts & Mervis, Citation2016).
Design, Stimuli, and Procedure
Participants were seated at a table and viewed an LCD monitor from a distance of approximately 46 cm to 64 cm. The screen (resolution 1,024 × 768 × 32) subtended approximately 28 × 21 degrees of visual angle (dva). Each participant was tested in three conditions: moving, occluded, and implosion. The order of conditions was counterbalanced across participants. Each test condition was preceded by 2 practice trials, on which participants demonstrated understanding of the task, followed by 20 randomly ordered test trials, each with one, two, three, or four targets.
In each condition, eight black squares (described as “cards”) were presented in randomly assigned starting positions, with centers at least 5.7 dva apart and edges a minimum of 0.8 dva from the screen boundaries (). One to four of the items were identified as targets when the cards were flipped over to reveal a picture of a cat. Participants were instructed to keep track of the target cards throughout the trial. They were given as much time as they wanted to study the static display with the target(s) revealed. The experimenter then clicked the mouse and the target(s) were flipped back to their original state, resulting in eight identical black cards once again. All cards then began to move, following individual trajectories. The trajectories of the three conditions were matched so that each participant saw the same set of trajectories, which were the same across participant groups. Motion paths were computed by assigning each object an initial random starting direction, which changed as a function of the object’s distance from other objects or the sides of the display. If the center of one object was within 3.78 dva of the center of another object or one of the sides of the display, it was assigned a new random direction to avoid contact. Velocity was constant at 3.6 dva per second. Characteristics of the task and trajectories were similar to those in previous studies of MOT in people with WS and TD children (O’Hearn et al., Citation2005, Citation2010).
After 10 s, the cards stopped moving and the mouse pointer reappeared. Participants pointed to the card(s) that they thought corresponded to the target(s), and the experimenter clicked the mouse on each to record their choices. With each click, the selected item “turned over” to reveal whether it was a target (cat) or a nontarget (blank white card). Participants could choose as many cards as there were targets. A full report method was used, rather than the partial report of Pylyshyn and Storm (Citation1988), to maximize the amount of data extracted from each trial (see also Trick et al., Citation2005).
In the moving condition, the cards continuously moved around the screen before they stopped. The occluded and implosion conditions were the same, but the cards disappeared briefly, based on the Scholl and Pylyshyn (Citation1999) procedure for occluded and imploding objects. For the occluded and implosion conditions, the task was identical to that in the moving condition, except that the screen also included two invisible vertical bars (i.e., “occluders”) behind which the objects disappeared (even though the bars themselves were not visible). In the occluded condition, the cards moved behind the invisible bar such that the leading edge underwent smooth deletion as it moved behind the occluder and then underwent smooth accretion as it moved out from behind the occluder. In the implosion condition, when the object contacted the occluder, it imploded by shrinking continuously to a point and disappearing. Objects in the implosion condition reappeared by reversing this process (i.e., “explosion”), expanding in size from a point when they emerged from the other side of the bar. Both types of disappearance (occluded and implosion) were matched for the amount of material that was deleted over time and the timing of the disappearance event.
Results
illustrates the percentage of errors across conditions and groups. Overall, the participants with WS performed more poorly than TD adults. This impairment was most evident when more than one target was tracked. Importantly, for both adults and individuals with WS, within-group performance in the moving and occluded conditions was quite similar but showed decrements in implosion. A 2 (group) × 3 (condition) × 4 (target number) repeated-measures analysis of variance (ANOVA) on percentage error revealed main effects of group, F(1, 22) = 84.66, p < .001, ηp2 = .79, condition, F(2, 44) = 50.02, p < .001, ηp2 = .70, and target number, F(3, 66) = 28.42, p < .001, ηp2 = .56. Main effects showed that error rates differed across the groups and the conditions, with higher rates of error for larger target numbers. These effects were modulated by a two-way interaction between group and condition, F(2, 44) = 9.86, p = .001, ηp2 = .31. The interaction between target number and group was also significant, F(3, 66) = 14.36, p < .001, ηp2 = .40, as was the three-way interaction of group, target number, and condition, F(6, 132) = 5.63, p = .001, ηp2 = .20. The condition × target number interaction was not significant, F(6, 132) = 1.09, p = .38, ηp2 = .05. (All repeated-measures ANOVAs were computed using the Greenhouse Geisser correction to correct for possible violations of the sphericity assumption.)
Figure 2 Percentage of error as a function of number of targets in each of the three conditions (moving, occluded, and implosion), shown for both the typically developing (TD) adult and Williams syndrome (WS) groups.
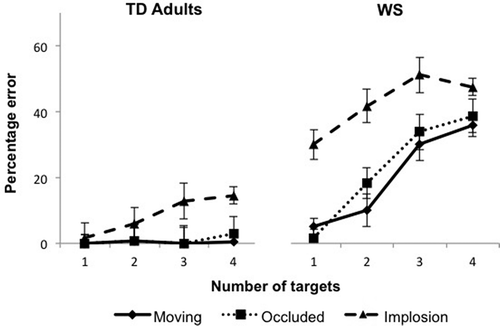
Planned comparisons showed that the WS group had higher rates of error than did TD adults on the three- and four-target trials in the moving condition: three targets, t(22) = 4.92, p < .001, d = 2.01; four targets, t(22) = 8.37, p < .001, d = 3.04 (Bonferroni-corrected alpha level of .004 for multiple comparisons). These findings replicate those of O’Hearn et al. (Citation2005), who also found that participants with WS had a greater percentage of errors than did TD adults in the moving condition for three- and four-target trials, but not one- and two-target trials. In the occluded condition of the present study, the WS and TD adult groups did not differ in the one-target condition, but the percentage error for participants with WS was greater than that of TD adults for the two-, three-, and four-target conditions: one target, t(22) = 1.00, p = .33, d = 0.41; two targets, t(22) = 3.16, p = .005, d = 1.28; three targets, t(22) = 7.25, p < .001, d = 2.96; four targets, t(22) = 8.82, p < .001, d = 3.60. In the implosion condition, the WS group performed reliably worse than TD adults for all target conditions: one target, t(22) = 5.17, p < .001, d = 2.11; two targets, t(22) = 5.47, p < .001, d = 2.23; three targets, t(22) = 7.72, p < .001, d = 3.15; four targets, t(22) = 9.32, p < .001, d = 3.80. This finding highlights the relatively greater decrement on WS performance that was caused by tracking through the unnatural event of implosion/explosion.
To further analyze these data, we used a high-threshold guessing model (Hulleman, Citation2005) to convert percent correct into a measure of capacity (k), which represents the number of objects tracked or remembered:
k = nc−t2
n + c−2t
Where n = the total number of objects, t = the number of targets to be tracked (from one to four), and c = the number of targets correctly identified. As the number of targets varied from one to four in this study, the upper limit of the k statistic also varies from 1 to 4.
shows the k values for both groups. TD adults performed at ceiling in both the moving (one target, 1.00; two targets, 1.98; three targets, 3.00; four targets, 4.00) and occluded conditions (one target, 1.00; two targets, 1.98; three targets, 2.98; four targets, 3.88) but performed worse in implosion (one target, 0.98; two targets, 1.77; three targets, 2.37; four targets, 3.37), especially as the number of targets increased. This adult pattern of performance echoes the pattern found in the original Scholl and Pylyshyn (Citation1999) study, which also showed decrements in performance when objects disappeared via implosion. Planned comparisons between the conditions showed that k values of TD adults in the moving and occluded conditions did not differ, regardless of the number of targets that were tracked, all ts(17) ≤ 2.34, ps > .006, ds < 0.16 (Bonferroni-corrected alpha level of .006). This finding replicates the original finding of Scholl and Pylyshyn, who found that the performance of adults does not differ when tracking moving objects and those that undergo occluded. Performance in the implosion condition suffered relative to the moving condition for two, three, and four targets, all ts(17) ≥ 3.04, ps < .006, ds > 0.41. The same was found for comparison of the implosion and occluded conditions, all ts(17) ≥ 2.92, ps < .006, ds > 0.64, again replicating the original Scholl and Pylyshyn findings.
Figure 3 Mean number of objects tracked (k) by the typically developing (TD) adult and Williams syndrome (WS) groups in Experiment 1. Error bars represent the standard error of the mean.
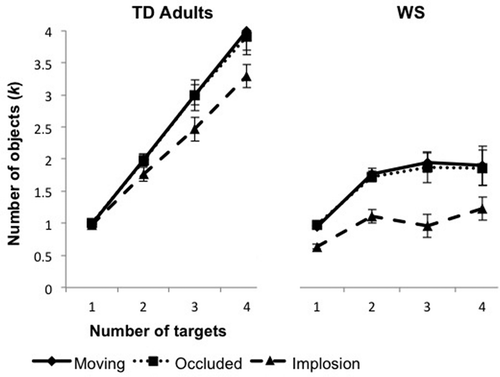
To estimate the capacity of each group within each condition, we compared k values for each increasing target number, where capacity is defined as the point at which there is no longer a significant increase in k value as the target set size increases. TD adults showed capacity limits of at least four targets (the highest target number tested) in the moving (three vs. four targets), t(11) = –1,201.00, p < .001, d = –714.18, occluded (three vs. four targets), t(11) = –14.23, p < .001, d = –6.15, and implosion conditions (three vs. four targets), t(11) = –5.32, p < .001, d = –2.06 (Bonferroni-corrected alpha level of .006). Overall, individuals with WS had lower capacity than TD adults. They showed a capacity limit of only two targets in the moving (two vs. three targets): t(11) = –0.74, p = .475, d = –0.23, occluded (two vs. three targets), t(11) = –1.07, p = .309, d = –0.33, and implosion conditions (two vs. three targets), t(11) = 1.05, p = .316, d = –0.34. This capacity of two targets is consistent with previous findings (O’Hearn et al., Citation2005, Citation2010).
Further comparison across the conditions revealed that for TD adults, k values for the moving and occluded conditions did not significantly differ from one another across all target conditions, all ts(11) < 2.18, ps > .08, ds < 0.40. The implosion condition, however, did show lower k values in comparison with the moving condition for the three-target, t(11) = 4.26, p = .001, d = 1.75, and four-target, t(11) = 4.81, p = .001, d = 1.96, conditions. The k values of participants with WS varied across conditions in ways that mirrored the TD adult pattern. The moving (one target, 0.95; two targets, 1.79; three targets, 1.94; four targets, 1.86) and occluded (one target, 0.98; two targets, 1.70; three targets, 1.84; four targets, 1.75) conditions did not differ from one another for any of the target conditions, all ts(11) < 0.72, ps > .435, ds < 0.14 (Bonferroni-corrected alpha level of .006). In contrast, in the implosion condition (one target, 0.63; two targets, 1.13; three targets, 0.93; four targets, 1.21), the WS group showed significantly smaller k values in comparison with the moving condition for the one-target, t(11) = 4.15, p = .002, d = 1.86, two-target, t(11) = 4.60, p = .001, d = 1.49, and three-target conditions, t(11) = 4.58, p = .001, d = 1.33.
These findings illustrate several points. First, as predicted, the capacity of individuals with WS was below that of TD adults. Individuals with WS showed a tracking capacity of approximately two objects, whereas the adult controls showed a ceiling capacity of at least four objects, consistent with other studies (e.g., Burkell & Pylyshyn,Citation1997; Intriligator & Cavanaugh, Citation2001; Pylyshyn & Annan, Citation2006; Pylyshyn & Storm, Citation1988; Yantis, Citation1992). Second, within-group comparisons revealed that both groups showed equivalent levels of performance across the moving and occluded conditions. Although capacity overall differed dramatically between groups, neither showed within-group differences between tracking fully visible objects (moving condition) versus objects that were periodically occluded (occluded condition). Third, both groups showed a decrement for objects that periodically disappeared through implosion. We should note that the original Scholl and Pylyshyn (Citation1999) findings revealed substantially more impairment for adults in the implosion condition than reported here (for tracking four objects, 64% correct vs. 80% correct in the current study). We suspect this finding is due to the fact that our task, with timing parameters designed for an impaired population as well as for TD children, was considerably easier than the original task.
As a whole, these findings are consistent with the idea that the WS MOT system is impaired in the sense that the capacity for tracking multiple objects is reduced in number. At the same time, however, it shows the same pattern of performance across different types of disappearance as that of TD adults—namely, equivalent tracking of objects that are visible or occluded while moving, but reduced tracking of objects that undergo disappearance by implosion, which does not provide the spatiotemporal cues to support the continuous representation of an object. The findings for the WS group raise the question of whether their performance resembles that of TD children of much younger ages. In previous research, we estimated that adults and adolescents with WS have an MOT capacity roughly similar to that of TD 4-year-olds. However, it is unknown whether this similarity will also hold for tracking through interruptions of occluded or implosion. In Experiment 2, we examined the performance of 4-, 5-, and 7-year-olds in these conditions.
EXPERIMENT 2
Participants
Thirty-six children aged 4 to 7 years old were tested. Children were divided into three age groups (n = 12 each): 4-year-olds (Mage = 4;6, SD = 4 months, range = 4;0–4;11, 6 boys), 5-year-olds (Mage = 5;6, SD = 4 months, range = 5;0–5;11, 5 boys), and 7-year-olds (Mage = 7;4, SD = 3 months, range = 6;11–7;11, 7 boys). All TD children were recruited through local preschools or had older siblings who had participated in previous studies. Design, stimuli, and procedures were the same as those in Experiment 1, except for the case of the 4-year-old group. Because children of this young age had difficulty completing the full experimental task due to its demands on behavior and sustained attention, data were collected only for conditions with one and two targets.
Results
illustrates the error rates across age and condition. Four-year-olds showed the highest rates of error overall. To first consider trends across development, we analyzed the percentage error for solely the one- and two-target trials so as to facilitate comparison to the 4-year-old group. A 3 (group) × 3 (condition) × 2 (target number) repeated-measures ANOVA revealed main effects of group, F(1, 33) = 17.08, p < .001, ηp2 = .51, condition, F(2, 66) = 14.96, p < .001, ηp2 = .31, and target number, F(1, 33) = 5.96, p = .02, ηp2 = .15. The two-way interaction between group and condition was significant, F(4, 66) = 2.58, p = .045, ηp2 = .14. The target number × group interaction was not significant, F(2, 33) = 0.99, p = .38, ηp2 = .06, nor was the condition × target number interaction, F(2, 66) = 0.04, p = .96, ηp2 = .001. The three-way interaction of group, target number, and condition was also nonsignificant, F(4, 66) = 1.68, p = .17, ηp2 = .09.
Figure 4 Percentage of errors as a function of number of targets in each of the three conditions (moving, occluded, and implosion), shown for the 4-year-old, 5-year-old, and 7-year-old groups.
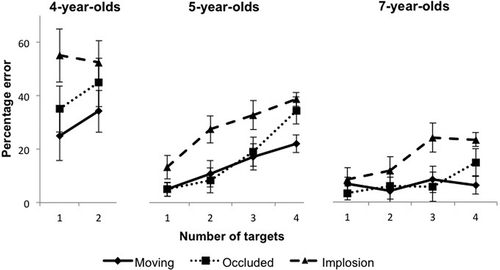
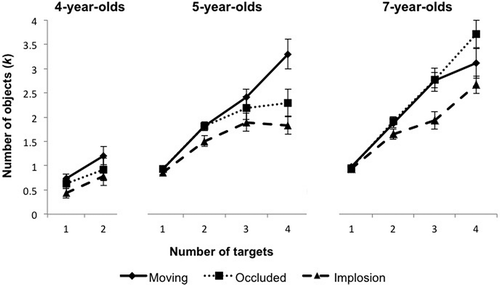
Figure 5 Mean number of objects tracked (k) by the 4-, 5-, and 7-year-olds in Experiment 2. Error bars represent the standard error of the mean.
To consider performance across all four target conditions, a 2 (group) × 3 (condition) × 4 (target number) repeated-measures ANOVA was conducted for percentage error data among the 5- and 7-year-olds. This ANOVA revealed a main effect of group, F(1, 22) = 8.82, p = .007, ηp2 = .29, reflecting a higher percentage of error among 5-year-olds compared with 7-year-olds. There were also main effects of condition, F(2, 44) = 35.29, p < .001, ηp2 = .62, and target number, F(3, 66) = 22.74, p < .001, ηp2 = .51. These effects were modulated by two-way interactions between target number and group, F(3, 66) = 4.53, p = .006, ηp2 = .17, and condition and target number, F(6, 132) = 3.54, p = .006, ηp2 = .14. The group × condition interaction was not significant, F(2, 44) = 0.78, p = .47, ηp2 = .03, nor was the three-way interaction of group, condition, and target number, F(6, 132) = 1.19, p = .315, ηp2 = .05. These improvements with age are consistent with the findings of previous studies, which have identified MOT growth in capacity from 5 to 7 years of age (O’Hearn et al., Citation2005) and shown that MOT reaches adult levels of performance at 11 to 13 years of age (Trick, Hollinsworth, & Brodeur, Citation2009).
We next analyzed k values to determine whether capacity limits varied across conditions and across the age groups (). For 4-year-olds, k values increased with increasing target number for all three conditions (moving, one target, 0.73, two targets, 1.21; occluded, one target, 0.63, two targets, 0.98; implosion, one target, 0.43, two targets, 0.79). These values showed significant increases for all conditions: moving, one versus two targets, t(11) = –3.30, p = .008, d = –0.90; occluded, one versus two targets, t(11) = –2.72, p = .05, d = –0.62; implosion, one versus two targets, t(11) = –2.09, p = .02, d = –0.67 (Bonferroni-corrected alpha level of .017). Similar to the pattern that was found for TD adults and WS participants, the moving and occluded conditions did not significantly differ from one another for either the one-target condition, t(11) = 1.87, p = .09, d = 0.29, or two-target condition, t(11) = 1.59, p = .139, d = 0.28. The implosion condition, however, did show lower k values in comparison with moving for the one-target, t(11) = 2.64, p = .023, d = 0.87, and two-target, t(11) = 3.54, p = .005, d = 0.63, conditions. This finding indicates that even for children of this younger age who track just one or two targets, the moving and occluded conditions lead to similar performance while implosion leads to decrements in performance.
For the 5-year-old group, k values in the moving condition steadily increased as the number of targets increased (one target, 0.92; two targets, 1.81; three targets, 2.42; four targets, 3.29), with a capacity limit of at least four targets: three versus four targets, t(11) = –1.83, p = .02, d = –0.22 (Bonferroni-corrected alpha level of .006). This finding illustrates that MOT in 5-year-olds is relatively mature when items remain in sight during motion. The k values of 5-year-olds similarly increased in the occluded condition (one target, 0.95; two targets, 1.89; three targets, 2.24; four targets, 2.51). In the occluded condition, 5-year-olds showed a capacity of three tracked targets—three versus four targets, t(11) = –1.18, p = .263, d = –0.40—which was true of the implosion condition as well—three versus four targets, t(11) = 1.90, p = .09, d = 0.45. In the implosion condition, 5-year-olds showed lower k values overall (one target, 0.86; two targets, 1.50; three targets, 1.89; four targets, 1.83). Comparison of k values across conditions revealed that moving and occluded did not differ for any of the target conditions, all ts(11) < 1.24, ps > .15, ds < 0.18. The implosion condition showed significantly smaller k values in comparison with the moving condition for the four-target condition, t(11) = 3.70, p = .004, d = 1.32. This finding again indicates that tracking through the event of implosion, which breaks the spatiotemporal cues necessary to maintain object representation, results in impairment of tracking ability in 5-year-olds.
The 7-year-olds also showed the familiar pattern of similar performance in the moving and occluded conditions but impairment in implosion. These children were close to ceiling for both the moving and occluded conditions, and like adults, they showed increasing k values as the number of targets increased (moving, one target, 0.97, two targets, 1.81, three targets, 2.76, four targets, 3.11; occluded, one target, 0.94, two targets, 1.92, three targets, 2.77, four targets, 3.71). The moving condition showed an estimated capacity of at least four targets—three versus four targets, t(11) = –6.98, p < .001, d = –2.81 (Bonferroni-corrected alpha level of .006)—and the occluded condition showed a capacity of four targets—three versus four targets, t(11) = –1.74, p = .11, d = –0.62. In the implosion condition, the k values of 7-year-olds were lower (one target, 0.93; two targets, 1.65; three targets, 1.93; four targets, 2.66), with a capacity of three targets—two versus three targets, t(11) = –1.93, p = .09, d = –0.59. The moving and occluded conditions did not differ from one another for one, two, or three targets, all ts(11) ≤ 2.85, all ps > .004, demonstrating the same pattern found for the other participant groups. Performance did significantly differ between moving versus occluded for four targets, t(11) = 3.71, p = .003, d = 1.17, where performance in the occluded condition was higher. We can only speculate about the source of this puzzling finding; it may be due to 7-year-olds maintaining a more sustained focus in the occluded condition, perhaps triggered by the momentary disappearance of the objects. Performance in the implosion condition did not differ from that in the moving condition for one, t(11) = 1.76, p = .105, d = 0.73, or two targets, t(11) = 2.31, p = .06, d = 1.06, but it was worse than the moving condition for three, t(11) = 5.10, p < .001, d = 1.68, and four, t(11) = 3.46, p = .005, d = 1.31, targets.
We next directly compared the WS group to TD children. We first considered the percentage error made by TD 4-year-olds in comparison to individuals with WS. A 2 (group) × 3 (condition) × 2 (target number) repeated-measures ANOVA revealed main effects of group, F(1, 22) = 9.07, p = .006, ηp2 = .29, condition, F(2, 44) = 21.63, p < .001, ηp2 = .50, and target number, F(1, 22) = 7.12, p = .014, ηp2 = .25. The two-way interactions of condition and group, F(2, 44) = 1.09, p = .346, ηp2 = .05, target number and group, F(1, 22) = 0.79, p = .383, ηp2 = .04, and condition and target number, F(2, 44) = 1.49, p = .24, ηp2 = .06, were not significant. The three-way interaction of condition, target number, and group was also not significant, F(2, 44) = 1.58, p = .222, ηp2 = .07. Planned comparisons revealed that individuals with WS had significantly greater k scores than TD 4-year-olds for the one-target, t(22) = 3.79, p = .001, d = 1.55, and two-target occluded conditions, t(22) = 3.25, p = .004, d = 1.32 (Bonferroni-corrected alpha level of .008). The groups did not differ in the moving or implosion conditions, all ts(22) < 1.73, ps > .10, ds < 0.70. Overall, this comparison indicates that participants with WS and 4-year-olds were largely similar in their tracking capacities across the conditions, with the WS group showing slightly larger capacities for object tracking under the event of occluded.
We next compared individuals with WS to TD 5-year-olds, who both completed all four target conditions. A 2 (group) × 3 (condition) × 4 (target number) repeated-measures ANOVA revealed main effects of group, F(1, 22) = 5.31, p = .031, ηp2 = .19, condition, F(2, 44) = 49.44, p < .001, ηp2 = .69, and target number, F(3, 66) = 37.56, p < .001, ηp2 = .63. The interaction between condition and target number was significant, F(6, 132) = 2.74, p = .027, ηp2 = .11. The condition × group interaction, F(2, 44) = 2.75, p = .085, ηp2 = .11, target number × group interaction, F(3, 66) = 1.42, p = .24, ηp2 = .06, and three-way interaction of condition, target number, and group were not significant, F(6, 132) = 2.21, p = .075, ηp2 = .09. Planned comparisons revealed that the WS and TD 5-year-old groups did not significantly differ for one, two, or three targets in the moving condition, all ts(22) ≤ 1.30, all ps > .21, ds ≤ 0.54, but 5-year-olds did attain significantly higher k scores for four targets, t(22) = 3.89, p < .001, d = 1.5 (Bonferroni-corrected alpha level of .004). This finding replicates the results of O’Hearn et al. (Citation2005, Citation2010), who found that the tracking capacity of people with WS is smaller than that of TD 5-year-olds. No group differences were found for the occluded condition, all ts(22) ≤ 1.42, all ps > .25, ds ≤ 0.83. However, in the implosion condition, 5-year-olds showed significantly higher levels of performance than those of individuals with WS when two or three targets were tracked, all ts(22) ≥ 3.15, all ps < .004, ds ≥ 1.6. The results for the implosion condition show that people with WS often had greater difficulty than TD 5-year-olds in tracking objects through spatiotemporal gaps that do not provide cues in support of continuous object representation.
Overall, comparison of WS performance to that of two age groups of TD children revealed that WS MOT performance falls between the performance of TD 4- and 5-year-olds. Participants with WS showed equivalent performance to 4-year-olds in the moving condition. They had higher capacities than TD 4-year-olds when tracking through occluded but lower capacities than 5-year-olds in this same condition. In the implosion condition, WS capacity was found to be roughly equivalent to that of TD 4-year-olds.
DISCUSSION
The goal of this study was to determine whether the signature tracking pattern found in studies of TD adults would also emerge in people with WS, who have severely compromised visual-spatial functions, including a small MOT capacity for their chronological age. Therefore, we examined the ability of participants in both groups to track moving objects that a) remained continuously visible, b) periodically disappeared behind an occluder, or c) “imploded” and “exploded” in a nearby location a short time later. We found that TD adults conformed to the pattern first shown by Scholl and Pylyshyn (Citation1999): comparable performance in the moving and occluded conditions, with decrements in the implosion condition. Based on the results of O’Hearn et al. (Citation2005; Citation2010), we hypothesized that TD adults would track more objects than would people with WS. This prediction was upheld, as we found individuals with WS to have a capacity of roughly two objects in the moving condition (compared with four objects for TD adults). Strikingly, WS performance in the occluded condition did not differ from their performance in the moving condition but dropped significantly in the implosion condition. Indeed, people with WS were able to track only one object in this condition. These results suggest that although there are striking differences in the numerical capacity of the tracking mechanism in people with WS and TD adults, both systems share similar constraints in terms of the type of spatial-temporal continuity that contributes to accurate tracking.
We also explored the developmental trajectory of MOT under conditions when the objects do not remain continuously in sight. In previous work, O’Hearn et al. (Citation2010) found that TD children grow from 4 to 7 years of age in the number of targets they are able to track (without the interruption of occluded): At age 4 years, children can track approximately two targets, and this number increases to approximately four targets by age 7 years. In the current study, we tested TD 4-, 5-, and 7-year-olds in three conditions (moving, occluded, and implosion). We reported results from the moving condition that are similar to findings from a previous study (O’Hearn et al., Citation2010), with a capacity of approximately four objects for 5-year-olds and 7-year-olds. Within-group comparisons across conditions revealed that all groups showed similar levels of performance across the moving and occluded conditions, with decrements in the implosion condition. This finding follows the pattern shown by both TD adults and individuals with WS.
These findings clarify the mechanisms underlying MOT, its development, and its compromise within a neurodevelopmental disorder. First, the data lend additional support to the theory of MOT proposed by Pylyshyn and colleagues, which focuses on the fact that the tracking mechanism engages representations of objects. The performance of TD adults and people with WS in the moving and occluded conditions offers strong support for the idea that the entities being tracked need not be constantly visible. Whether fully visible or momentarily invisible, the tracking system of both groups followed the trajectories of these entities and revealed the same within-group capacities. Similarly, both groups showed poorer tracking performance in the implosion condition. This finding is not surprising: If the entities appear to simply go out of existence, then there are no grounds for predicting their reappearance. Clearly, however, TD adults did not completely fail in this condition. It is likely that they used strategic “best guesses” about where the targets may appear next, based on predictions of proximity and trajectory (Fencsik et al., Citation2007; Franconeri, Pylyshyn, & Scholl, Citation2012; Horowitz, Birnkrant, Fencsik, Tran, & Wolfe, Citation2006; Howe & Holcombe, Citation2012; Keane & Pylyshyn, Citation2006). The performance of TD adults was compromised in the implosion condition, but their capacity (k values) did increase with the number of targets, indicating that they were reasonably successful in predicting the future locations of the imploding objects (but notably still at rates significantly lower than in the moving and occluded conditions). By contrast, the individuals with WS were only able to track, on average, a single object in the imploding condition. They may have found it more difficult to make spatial interpolations based on trajector,y or elected to follow just one target on each trial (rather than attempting to track all at once).
TD children also demonstrated similar tracking performance in the moving and occluded conditions and worse performance in the implosion condition. They showed a pattern for the moving and occluded conditions that was highly similar to that of TD adults and individuals with WS (nearly identical within-group profiles across the two conditions for one, two, or three targets). The lower k values of TD 5- and 7-year-olds for four targets likely reflect the increase in difficulty of the task as the number of targets increased. Overall, children’s tracking performance suffered in the implosion condition, presumably because the spatiotemporal cues of this condition do not adhere to the preferred criteria of the object-based MOT process.
The performance of the WS group is informative because it provides evidence that even in cases of developmental impairment where overall capacity is limited, the MOT mechanism retains its hallmark constraint on spatiotemporal continuity (Scholl & Pylyshyn, Citation1999). As MOT is a complex process, it is possible that the performance of participants with WS may be due to factors beyond the spatial impairment, such as working memory or executive function, as suggested by research with individuals with Down syndrome (Brodeur, Trick, Flores, Marr, & Burack, Citation2013). However, if these more global factors drive task performance, we predict that we would have observed more uniform levels of impairment across the different conditions of object disappearance. These findings support the idea that even though the WS genetic deletion results in severe spatial impairment, the qualitative nature of spatial representation in people with WS is similar to that of TD individuals. What is striking about these data is that the WS pattern of performance in the three conditions conforms to the qualitative signature shown by TD adults and children (i.e., similar performance in the moving and occluded conditions, with greater decrements in performance under the object-violating parameters of the implosion condition). This finding parallels other recent findings that also suggest that many of the visual-spatial impairments in WS can be well characterized as quantitative differences from TD performance, rather than qualitative. Measures of object recognition, visual-motor action, orientation discrimination, and orientation integration all follow a pattern among people with WS that suggests that the internal structure of their spatial representations is similar to that of TD children, albeit at a much younger age (Dilks et al., Citation2008; Landau et al., Citation2006; Palomares et al., Citation2009). Other researchers have reported evidence of qualitatively similar representations in the domain of face representation (Tager-Flusberg, Plesa-Skwerer, Faja, & Joseph, Citation2003). These findings are remarkable in the context of what is known about the many atypicalities of the WS brain (as reviewed earlier), yet at the level of cognition, many representational properties—across domains as different as language and visual-spatial reasoning—are preserved (see Musolino & Landau, Citation2010).
The use of MOT tasks to study visual-spatial processing in atypical development has been growing. A decrease in tracking capacity has been reported in other developmental disorders, including autism spectrum disorder (Koldewyn, Weigelt, Kanwisher, & Jiang, Citation2013; O’Hearn, Franconeri, Wright, Minshew, & Luna, Citation2013), Down syndrome (Brodeur et al., Citation2013), Turner syndrome (Beaton et al., Citation2010), 22q11.2 deletion syndrome (Cabaral, Beaton, Stoddard, & Simon, Citation2012), and amblyopia (Giaschi, Chapman, Meier, Narasimhan, & Regan, Citation2015). The MOT atypicalities across this range of developmental disorders, all with varying degrees of spatial impairment, suggests that the mechanism is particularly fragile and vulnerable to neurocognitive impairment (Atkinson & Braddick, Citation2012). It is possible that the multiple underlying neural regions are differentially impacted across these disorders, which may reveal different behavioral profiles across the experimental manipulations included in the present study. The collective work with these populations also highlights the need for additional research to better understand why these impairments may persist and to develop effective treatment approaches to spur continued development. Studies with TD adults demonstrate that mature object-tracking performance is affected by factors such as spacing between the objects to be tracked (i.e., crowding; Franconeri, Lin, Enns, Pylyshyn, & Fisher, Citation2008; Shim, Alvarez, & Jiang, Citation2008; Tombu & Seiffert, Citation2008) and speed at which the objects travel (Alvarez & Franconeri, Citation2007). A promising avenue for future research is to investigate whether these manipulations similarly affect tracking performance in cases of atypical development.
In conclusion, we have shown that the case of WS provides unique insight into the development of the fundamental mechanisms underlying MOT. The parallel study of typical and atypical development provides evidence that the MOT mechanism is highly constrained in terms of the type of entities that may be tracked, even in cases of broad visual-spatial impairment. Future studies using the MOT task and similar lines of research will continue to characterize the underlying mechanisms of visual-spatial cognition and attention across both typical and atypical development.
FUNDING
This research was supported in part by an Integrative Graduate Education and Research Traineeship through the National Science Foundation (DGE 0549379 to KF), a T32 Postdoctoral Research Fellowship through the National Institutes of Health (5T32 HD 046388 to KF), and grants from the National Institute of Health (NINDS RO1 050876 to BL and JEH), the National Institute of Mental Health (K01 MH081191 to KH), and the National Science Foundation (BCS 1059560 to JEH).
Additional information
Funding
References
- Alvarez, G. A., & Franconeri, S. L. (2007). How many objects can you track? Evidence for a resource-limited attentive tracking mechanism. Journal of Vision, 7(13), 14. doi:10.1167/7.13.14
- Atkinson, J., & Braddick, O. (2012). Visual attention in the first years: Typical development and developmental disorders. Developmental Medicine & Child Neurology, 54, 589–595. doi:10.1111/j.1469-8749.2012.04294.x
- Atmaca, S., Stadler, W., Keitel, A., Ott, D. V. M., Lepsein, J., & Prinz, W. (2013). Prediction processes during multiple object tracking (MOT): Involvement of dorsal and ventral premotor cortices. Brain and Behavior, 3, 683–700. doi:10.1002/brb3.180
- Ballard, D., Hayhoe, M., Pook, P., & Rao, R. (1997). Deictic codes for the embodiment of cognition. Behavioral and Brain Sciences, 20, 723–767. doi:10.1017/S0140525X97001611
- Beaton, E. A., Stoddard, J., Lai, S., Lackey, J., Shi, J., Ross, J. L., & Simon, T. J. (2010). Atypical functional brain activation during a multiple object tracking task in girls with Turner syndrome: Neurocorrelates of reduced spatiotemporal resolution. American Journal on Intellectual and Developmental Disabilities, 115, 140–156. doi:10.1352/1944-7558-115.2.140
- Boddaert, N., Mochel, F., Meresse, I., Seidenwurm, D., Cachia, A., Brunelle, F., … Zilbovicius, M. (2005). Parieto-occipital grey matter abnormalities in children with Williams syndrome. NeuroImage, 30, 721–725. doi:10.1016/j.neuroimage.2005.10.051
- Brodeur, D. A., Trick, L. M., Flores, H., Marr, C., & Burack, J. A. (2013). Multiple-object tracking among individuals with Down syndrome and typically developing children. Development and Psychopathology, 25, 545–553. doi:10.1017/S095457941200123X
- Brown, J. H., Johnson, M. H., Paterson, S. J., Gilmore, R., Longhi, E., & Karmiloff-Smith, A. (2003). Spatial representation and attention in toddlers with Williams syndrome and Down syndrome. Neuropsychologia, 41, 1037–1046. doi:10.1016/S0028-3932(02)00299-3
- Burkell, J., & Pylyshyn, Z. W. (1997). Searching through selected subsets of visual displays: A test of the FINST indexing hypothesis. Spatial Vision, 11, 225–258. doi:10.1163/156856897X00203
- Cabaral, M. H., Beaton, E. A., Stoddard, J., & Simon, T. J. (2012). Impaired multiple object tracking in children with 22q11.2 deletion syndrome. Journal of Neurodevelopmental Disorders, 4, 6. doi:10.1186/1866-1955-4-6
- Carey, S., & Xu, F. (2001). Infants’ knowledge of objects: Beyond object files and object tracking. Cognition, 80, 179–213. doi:10.1016/S0010-0277(00)00154-2
- Cheries, E. W., Feigenson, L., Scholl, B. J., & Carey, S. (2005). Cues to object persistence in infancy: Tracking objects through occlusion vs. implosion. Journal of Vision, 5, 352. doi:10.1167/5.8.352
- Chiang, M.-C., Reiss, A. L., Lee, A. D., Bellugi, U., Galaburda, A., Korenberg, J. R. E. A., … Thompson, P. M. (2007). 3D pattern of brain abnormalities in Williams syndrome visualized using tensor-based morphometry. NeuroImage, 36, 1096–1109. doi:10.1016/j.neuroimage.2007.04.024
- Culham, J., Brandt, S., Cavanagh, P., Kanwisher, N., Dale, A., & Tootell, R. (1998). Cortical fMRI activation produced by attentive tracking of moving targets. Journal of Neurophysiology, 80, 2657–2670.
- Culham, J. C., Cavanagh, P., & Kanwisher, N. G. (2001). Attention response functions: Characterizing brain areas using fMRI activation during parametric variations of attentional load. Neuron, 32, 737–745. doi:10.1016/S0896-6273(01)00499-8
- Dilks, D., Landau, B., & Hoffman, J. (2008). Vision for perception and vision for action: Normal and unusual development. Developmental Science, 11, 474–486. doi:10.1111/j.1467-7687.2008.00693.x
- Eckert, M. A., Hu, D., Eliez, S., Bellugi, U., Galaburda, A., Korenberg, J., … Reiss, A. L. (2005). Evidence for superior parietal impairment in Williams syndrome. Neurology, 64, 152–153. doi:10.1212/01.WNL.0000148598.63153.8A
- Elliott, C. D. (1990). Differential Abilities Scales. San Antonio, TX: Psychological Corporation.
- Ewart, A. K., Morris, C. A., Atkinson, D., Jin, W., Sternes, K., Spallone, P., … Keating, M. T. (1993). Hemizygosity at the elastin locus in a developmental disorder, Williams syndrome. Nature Genetics, 5, 11–16. doi:10.1038/ng0993-11
- Faria, A. F., Landau, B., O’Hearn, K., Li, X., Jiang, H., Zhang, J., … Susumu, M. (2012). Quantitative analysis of gray and white matter in Williams syndrome. NeuroReport, 23, 283–289. doi:10.1097/WNR.0b013e3283505b62
- Fencsik, D. E., Klieger, S. B., & Horowitz, T. S. (2007). The role of location and motion information in the tracking and recovery of moving objects. Perception & Psychophysics, 69, 567–577. doi:10.3758/BF03193914
- Franconeri, S. L., Lin, J. Y., Enns, J. T., Pylyshyn, Z. W., & Fisher, B. (2008). Evidence against a speed limit in multiple-object tracking. Psychonomic Bulletin & Review, 15, 802–808. doi:10.3758/PBR.15.4.802
- Franconeri, S. L., Pylyshyn, Z. W., & Scholl, B. J. (2012). A simple proximity heuristic allows tracking of multiple objects through occlusion. Attention, Perception, & Psychophysics, 74, 691–702. doi:10.3758/s13414-011-0265-9
- Gaser, C., Luders, E., Thompson, P. M., Lee, A. D., Dutton, R. A., Geaga, J. A., … Korenberg, J. R. (2006). Increased local gyrification mapped in Williams syndrome. NeuroImage, 33, 46–54. doi:10.1016/j.neuroimage.2006.06.018
- Georgopoulos, M. A., Georgopoulos, A. P., Kurz, N., & Landau, B. (2004). Figure copying in Williams syndrome and normal subjects. Experimental Brain Research, 157, 137–146. doi:10.1007/s00221-004-1834-0
- Giaschi, D., Chapman, C., Meier, K., Narasimhan, S., & Regan, D. (2015). The effect of occlusion therapy on motion perception deficits in amblyopia. Vision Research, 114, 122–134. doi:10.1016/j.visres.2015.05.015
- Gibson, J. J., Kaplan, G. A., Reynolds, H. N., & Wheeler, K. (1969). The change from visible to invisible: A study of optical transitions. Perception & Psychophysics, 5, 113–116. doi:10.3758/BF03210533
- Hoeft, F., Barnea-Goraly, N., Haas, B. W., Golari, G., Ng, D., Mills, D., … Reiss, A. L. (2007). More is not always better: Increased fractional anisotropy of superior longitudinal fasciculus associated with poor visuospatial abilities in Williams syndrome. Journal of Neuroscience, 27, 11960. doi:10.1523/JNEUROSCI.3591-07.2007
- Hoffman, J., Landau, B., & Pagani, B. (2003). Spatial breakdown in spatial construction: Evidence from eye fixations in children with Williams syndrome. Cognitive Psychology, 46, 260–301. doi:10.1016/S0010-0285(02)00518-2
- Hollingworth, A., & Franconeri, S. L. (2009). Object correspondence across brief occlusion is established on the basis of both spatiotemporal and surface feature cues. Cognition, 113, 150–166. doi:10.1016/j.cognition.2009.08.004
- Horowitz, T. S., Birnkrant, R. S., Fencsik, D. E., Tran, L., & Wolfe, J. M. (2006). How do we track invisible objects? Psychonomic Bulletin & Review, 13, 516–523. doi:10.3758/BF03193879
- Howe, P. D. L., & Holcombe, A. O. (2012). Motion information is sometimes used as an aid to the visual tracking of objects. Journal of Vision, 12(3), 1–10. doi:10.1167/12.13.10
- Howe, P. D., Horowitz, T. S., Morocz, I. A., Wolfe, J., & Livingstone, M. S. (2009). Using fMRI to distinguish components of the multiple object tracking task. Journal of Vision, 9(4), 1–11. doi:10.1167/9.4.10
- Hulleman, J. (2005). The mathematics of multiple object tracking: From proportions correct to number of objects tracked. Vision Research, 45, 2298–2309. doi:10.1016/j.visres.2005.02.016
- Intriligator, J., & Cavanagh, P. (2001). The spatial resolution of visual attention. Cognitive Psychology, 43, 171–216. doi:10.1006/cogp.2001.0755
- Iordanescu, L., Grabowecky, M., & Suzuki, S. (2009). Demand-based dynamic distribution of attention and monitoring of velocities during multiple-object tracking. Journal of Vision, 9(4), 1–12. doi:10.1167/9.4.1
- Jackowski, A. P., & Schultz, R. T. (2005). Foreshortened dorsal extension of the central sulcus in Williams syndrome. Cortex, 41, 282–290. doi:10.1016/S0010-9452(08)70266-1
- Jovicich, J., Peters, R. J., Koch, C., Braun, J., Chang, L., & Ernst, T. (2001). Brain areas specific for attentional load in a motion-tracking task. Journal of Cognitive Neuroscience, 13, 1048–1058. doi:10.1162/089892901753294347
- Karmiloff-Smith, A. (1998). Development itself is the key to understanding developmental disorders. Trends in Cognitive Sciences, 2, 389–398. doi:10.1016/S1364-6613(98)01230-3
- Kaufman, A. S., & Kaufman, N. L. (2004). Kaufman Brief Intelligence Test (2nd ed.). Circle Pines, MN: American Guidance Service.
- Keane, B. P., & Pylyshyn, Z. W. (2006). Is motion extrapolation employed in multiple object tracking? Tracking as a low-level, non-predictive function. Cognitive Psychology, 52, 346–368. doi:10.1016/j.cogpsych.2005.12.001
- Kippenhan, J. S., Olsen, R. K., Mervis, C. B., Morris, C. A., Kohn, P., Meyer-Lindenberg, A., & Berman, K. F. (2005). Genetic contributions to human gyrification: Sulcal morphometry in Williams syndrome. Journal of Neuroscience, 25, 7840–7846. doi:10.1523/JNEUROSCI.1722-05.2005
- Koldewyn, K., Weigelt, S., Kanwisher, N., & Jiang, Y. (2013). Multiple object tracking in autism spectrum disorders. Journal of Autism and Developmental Disorders, 43, 1394–1405. doi:10.1007/s10803-012-1694-6
- Landau, B., & Ferrara, K. (2013). Space and language in Williams syndrome: Insights from typical development. WIREs Cognitive Science, 4, 693–706.
- Landau, B., & Hoffman, J. E. (2012). Spatial representation: From gene to mind. New York, NY: Oxford University Press.
- Landau, B., Hoffman, J. E., & Kurz, N. (2006). Object recognition with severe spatial deficits in Williams syndrome: Sparing and breakdown. Cognition, 100, 483–510. doi:10.1016/j.cognition.2005.06.005
- Leslie, A., Xu, F., Tremoulet, P., & Scholl, B. (1998). Indexing and the object concept: Developing ‘what’ and ‘where’ systems. Trends in Cognitive Science, 2, 10–18. doi:10.1016/S1364-6613(97)01113-3
- Libertus, M. E., Feigenson, L., Halberda, J., & Landau, B. (2014). Understanding the mapping between numerical approximation and number words: Evidence from Williams syndrome and typical development. Developmental Science, 17, 905–919. doi:10.1111/desc.2014.17.issue-6
- Marenco, S., Siuta, M. A., Kippenhan, J. S., Grodofsky, S., Chang, W., Kohn, P., … Meyer-Lindenburg, A. (2007). Genetic contributions to white matter architecture revealed by diffusion tensor imaging in Williams syndrome. Proceedings of the National Academy of Sciences USA, 104, 15117. doi:10.1073/pnas.0704311104
- Mervis, C. B., Robinson, B. F., Bertrand, J., Morris, C. A., Klein-Tasman, B. P., & Armstrong, S. C. (2000). The Williams syndrome cognitive profile. Brain and Cognition, 44, 604–628. doi:10.1006/brcg.2000.1232
- Meyer-Lindenberg, A., Kohn, P., Mervis, C. B., Kippenhan, J. S., Olsen, R., Morris, C. A., & Berman, K. F. (2004). Neural basis of genetically determined visuospatial construction deficit in Williams syndrome. Neuron, 43, 623–631. doi:10.1016/j.neuron.2004.08.014
- Meyer-Lindenberg, A., Mervis, C. B., Sarpal, D., Koch, P., Steele, S., Kohn, P., … Kippenhan, S. (2005). Functional, structural, and metabolic abnormalities of the hippocampal formation in Williams syndrome. Journal of Clinical Investigation, 115, 1888–1895. doi:10.1172/JCI24892
- Mobbs, D., Garrett, A. S., Menon, V., Rose, F. E., Bellugi, U., & Reiss, A. L. (2004). Anomalous brain activation during face and gaze processing in Williams syndrome. Neurology, 62, 2070–2076. doi:10.1212/01.WNL.0000129536.95274.DC
- Morris, C. A. (2006). The dysmorphology, genetics, and natural history of Williams-Beuren syndrome. In C. A. Morris, H. Lenhoff, & P. Wang (Eds.), Williams-Beuren syndrome: Research, evaluation, and treatment (pp. 3–17). Baltimore, MD: Johns Hopkins University Press.
- Musolino, J., & Landau, B. (2010). When theories don’t compete: Response to Thomas, Karaminis, and Knowland’s commentary on Musolino, Chunyo, and Landau. Language Learning and Development, 6, 126–161. doi:10.1080/15475440903507772
- Nardini, M., Atkinson, J., Braddick, O., & Burgess, N. (2008). Developmental trajectories for spatial frames of reference in Williams syndrome. Developmental Science, 11, 583–595. doi:10.1111/desc.2008.11.issue-4
- O’Hearn, K., Franconeri, S., Wright, C., Minshew, N., & Luna, B. (2013). The development of individuation in autism. Journal of Experimental Psychology: Human Perception and Performance, 39, 494–509.
- O’Hearn, K., Hoffman, J. E., & Landau, B. (2010). Developmental profiles for multiple object tracking and spatial memory: Typically developing preschoolers and people with Williams syndrome. Developmental Science, 13, 430–440. doi:10.1111/desc.2010.13.issue-3
- O’Hearn, K., Hoffman, J. E., & Landau, B. (2011). Small subitizing range in people with Williams syndrome. Visual Cognition, 19, 289–312. doi:10.1080/13506285.2010.535994
- O’Hearn, K., Landau, B., & Hoffman, J. E. (2005). Multiple object tracking in people with Williams syndrome and in normally developing children. Psychological Science, 16, 905–912. doi:10.1111/j.1467-9280.2005.01635.x
- Palomares, M., Landau, B., & Egeth, H. (2009). Orientation perception in Williams syndrome: Discrimination and integration. Brain and Cognition, 70, 21–30. doi:10.1016/j.bandc.2008.11.007
- Pitts, H. C., & Mervis, C. B. (2016). Performance on the Kaufman Brief Intelligence Test-2 by children with Williams syndrome. American Journal on Intellectual and Developmental Disabilities, 121, 33–47. doi:10.1352/1944-7558-121.1.33
- Pylyshyn, Z. W., & Annan, A. (2006). Dynamics of selection in multiple object tracking (MOT). Spatial Vision, 19, 485–504. doi:10.1163/156856806779194017
- Pylyshyn, Z. W. (1989). The role of location indexes in spatial perception: A sketch of the FINST spatial index model. Cognition, 32, 65–97. doi:10.1016/0010-0277(89)90014-0
- Pylyshyn, Z. W. (1994). Some primitive mechanisms of spatial attention. Cognition, 50, 363–384. doi:10.1016/0010-0277(94)90036-1
- Pylyshyn, Z. W. (2000). Situating the world in vision. Trends in Cognitive Science, 4, 197–207. doi:10.1016/S1364-6613(00)01477-7
- Pylyshyn, Z. W., Burkell, J., Fisher, B., Sears, C., Schmidt, W., & Trick, L. (1994). Multiple parallel access in visual attention. Canadian Journal of Experimental Psychology, 48, 260–283. doi:10.1037/1196-1961.48.2.260
- Pylyshyn, Z. W., & Storm, R. (1988). Tracking multiple independent objects: Evidence for a parallel tracking mechanism. Spatial Vision, 3, 179–197. doi:10.1163/156856888X00122
- Reiss, A. L., Eliez, S., Schmitt, J. E., Straus, E., Lai, Z., Jones, W., & Bellugi, U. (2000). IV. Neuroanatomy of Williams syndrome: A high-resolution MRI study. Journal of Cognitive Neuroscience, 12(Suppl. 1), 65–73. doi:10.1162/089892900561986
- Richardson, D. C., & Kirkham, N. Z. (2004). Multi-modal events and moving locations: Eye movements of adults and 6-month-olds reveal dynamic spatial indexing. Journal of Experimental Psychology: General, 133, 46–62. doi:10.1037/0096-3445.133.1.46
- Scholl, B. J. (2001). Spatiotemporal priority and object identity. Cahiers De Psychologie Cognitive, 20, 359–371.
- Scholl, B., & Leslie, A. (1999). Explaining the infant’s object concept: Beyond the perception/cognition dichotomy. In E. Lepore & Z. W. Pylshyn (Eds.), What is cognitive science? (pp. 26–73). Oxford: Blackwell.
- Scholl, B., & Pylyshyn, Z. (1999). Tracking multiple objects through occlusion: Clues to visual objecthood. Cognitive Psychology, 38, 259–290. doi:10.1006/cogp.1998.0698
- Scholl, B., Pylyshyn, Z. W., & Feldman, J. (2001). What is a visual object? Evidence from target merging in multiple object tracking. Cognition, 80, 159–177. doi:10.1016/S0010-0277(00)00157-8
- Shim, W. M., Alvarez, G. A., & Jiang, Y. V. (2008). Spatial separation between targets constrains maintenance of attention on multiple objects. Psychonomic Bulletin & Review, 15, 390–397. doi:10.3758/PBR.15.2.390
- Spelke, E. (1990). Principles of object perception. Cognitive Science, 14, 29–56. doi:10.1207/s15516709cog1401_3
- Strømme, P., Bjørnstad, P. G., & Ramstad, K. (2002). Prevalence estimation of Williams syndrome. Journal of Child Neurology, 17, 269–271. doi:10.1177/088307380201700406
- Tager-Flusberg, H., Plesa-Skwerer, D., Faja, S., & Joseph, R. M. (2003). People with Williams syndrome process faces holistically. Cognition, 89, 11–24. doi:10.1016/S0010-0277(03)00049-0
- Tombu, M., & Seiffert, A. E. (2008). Attentional costs in multiple-object tracking. Cognition, 108, 1–25. doi:10.1016/j.cognition.2007.12.014
- Trick, L. M., Hollinsworth, H., & Brodeur, D. (2009). Multiple-object tracking across the lifespan: Do different factors contribute to diminished performance in different age groups? In D. Dendrick & L. Trick (Eds.), Computation, cognition, and Pylyshyn (pp. 79–99). Cambridge, MA: MIT Press.
- Trick, L. M., Jaspers-Fayer, F., & Sethi, N. (2005). Multiple-object tracking in children: The ‘catch the spies’ task. Cognitive Development, 20, 373–387. doi:10.1016/j.cogdev.2005.05.009
- Ullman, S. (1984). Visual routines. Cognition, 18, 97–159. doi:10.1016/0010-0277(84)90023-4
- Van Essen, D. C., Dierker, D., Snyder, A. Z., Raichle, M. E., Reiss, A. L., & Korenberg, J. (2006). Symmetry of cortical folding abnormalities in Williams syndrome revealed by surface-based analyses. Journal of Neuroscience, 26, 5470–5483. doi:10.1523/JNEUROSCI.4154-05.2006
- vanMarle, K., & Scholl, B. J. (2003). Attentive tracking of objects vs. substances. Psychological Science, 14, 498–504. doi:10.1111/1467-9280.03451
- Vicari, S., & Carlesimo, G. A. (2006). Short-term memory deficits are not uniform in Down and Williams syndromes. Neuropsychology Review, 16, 87–94. doi:10.1007/s11065-006-9008-4
- Yantis, S. (1992). Multielement visual tracking: Attention and perceptual organization. Cognitive Psychology, 24, 295–340. doi:10.1016/0010-0285(92)90010-Y

