ABSTRACT
Background: Transketolase-like protein 1 (TKTL1) is an isoform of tranketolase, a key protein in a cancer cell's glucose metabolism that causes rapid cell growth and controls the non-oxidative part of the pentose phosphate pathway (PPP). Its overexpression occurs in several human cancer types. Our purpose was to study whether TKTL1 expression in colorectal cancer tissue associates with these patients’ prognosis. Methods: We collected retrospectively patient data and tissue samples from 840 colorectal cancer patients treated at Helsinki University Hospital, then stained tumor tissue microarrays for TKTL1 by immunohistochemistry, and compared immunohistochemical tissue expression with clinico-pathological parameters and survival. Results: High expression of TKTL1 associated with high Dukes stage, non-mucinous adenocarcinoma, and left-sided disease. Patients with high TKTL1 expression had poorer prognosis than those with low expression, with a 5-year disease-specific survival of 55.7% vs. 62.7%. Conclusion: We show that high TKTL1 in tumor tissue can lead to poor survival in colorectal cancer. TKTL1 thus can serve as a candidate marker for identifying patients at risk of recurrent disease.
Abbreviation
| TKTL1 | = | transketolase-like protein 1 |
Introduction
Cancer cells show an increased glycolysis even in aerobic environments via the pentose phosphate pathway (PPP), a phenomenon known as the Warburg effect. Citation1 The non-oxidative part of the PPP is controlled by transketolase enzyme reactions.Citation2 Transketolase-like protein 1 (TKTL1) is an isoform of transketolase that is shown to cause rapid tumor-cell growth. Suppression of TKTL1, on the other hand, reduces glucose consumption, with reduced lactic acid production.Citation3
In human colon carcinoma, TKTL1-suppressed cells grow more slowly than do controls.Citation3 Overexpression of TKTL1 has been demonstrated in non-small-cell lung carcinoma, breast carcinoma, head and neck squamous cell carcinoma, follicular as well as papillary thyroid carcinoma, and in carcinomas of the prostate, pancreas, ovary, endometrium, cervix, rectum, and kidney.Citation4-10 In bladder cancer, its overexpression occurs only in invasive tumors, whereas non-muscle-invading tumors show no TKTL1 positivity. Moreover, noninvasive colon carcinomas (pTis tumors) are TKTL1 negative.Citation4 TKTL1 overexpression predicts, in locally advanced rectal cancer treated with preoperative chemoradiation, poor patient survival and tumor progression.Citation11 In many other cancer forms too, such as colon, ocular, non-small cell lung cancer, and oral squamous cell cancer, TKTL1 overexpression predicts poor outcome.Citation4,7,12-14
Colorectal cancer is the third most common cancer in men and the second most common in women globally. Some 1.4 million new cases are diagnosed annually, causing annually 694 000 deaths. Of the cases, 55% occur in the developed world.Citation15 Those with UICC stage I to II disease affecting only the mucosa and muscular layer of the colon or rectum with no lymph node metastasis are, after surgical removal of the bowel, usually offered follow-up only. However, among stage II patients, some 20% die of their cancer.Citation16 The question is: who are those patients with stage II disease with high risk of developing recurrent disease and to whom should we offer adjuvant therapy.
The aim of our study was to explore the prognostic impact of TKTL1 tumor expression on survival in colorectal cancer.
Results
Immunohistochemistry
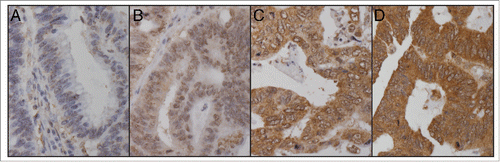
Of 840 patients, a total of 733 could be evaluated. Of these, 105 (12.5%) were scored as negative (score 0), 268 (31.9%) weakly positive (score 1), 235 (28.0%) moderately positive (score 2), and 125 (14.9%) strongly positive (score 3; ). For further analyses, those 373 with negative and weak TKTL1 expression were grouped as the low expression group, and those 360 with moderate and strong expression as high expression.
Association analysis
High expression of TKTL1 associated with Dukes stage B to D (P = 0.002), with adenocarcinoma (P ≤ 0.001), and with left-sided disease (P = 0.044, chi-square test). We found no association between TKTL1 and age, gender, or WHO grade, nor did any difference emerge in TKTL1 expression in the colon versus rectum ().
Table 1. Association of TKTL1 with clinicopathologic variables in 733 colorectal cancer patients.
Survival analysis
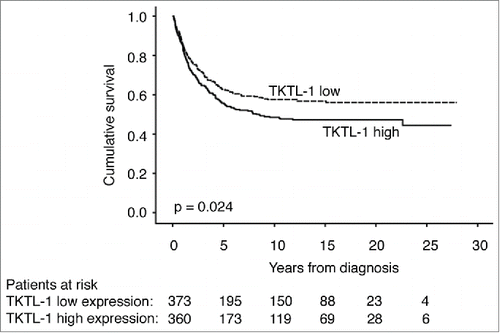
Patients with high TKTL1 expression had a poor prognosis. Disease-specific survival (DSS) at 5 y for all patients in this study was 58.9% (95% CI 55.4-62.4). For those with low TKTL1 expression 5-year DSS was 62.7% (95% CI 57.6–67.8), compared to those with high expression at 55.7% (95% CI 50.4–61.0; P = 0 .024, log-rank test; ). In subgroup analysis, TKTL1 served as a prognostic factor for patients under 65 (P = 0.015), patients with adenocarcinoma (P = 0.011), and patients with a high proliferation index (>10 % Ki-67 positivity) (P = 0.029, log-rank test; ).
Table 2. Kaplan-Meier analysis for disease-specific survival stratified for subgroups of colorectal cancer patients (P-value for logrank test).
Figure 2. Disease-specific survival according to TKTL1 expression in colorectal cancer patients. Prognosis was better in those with low expression of TKTL1, and worse in those with high expression of TKTL1 (p = 0 .024)
In multivariable survival analysis, patients’ age (hazard ratio (HR) 1.92, 95% CI 1.53-2.42, P < 0.001), Dukes classification, and WHO grade were independent risk factors, but TKTL1 showed no independent prognostic value (Cox regression analyses; ).
Table 3. Cox regression analysis for disease-specific survival of colorectal cancer patients.
Discussion
Transketolase is a key enzyme for the non-oxidative part of the pentose phosphate pathway in glucose metabolism in cancer cells.Citation2 We show here high expression of transketolase-like protein 1 (TKTL1), one of the members of the transketolase family, to predict worse survival in colorectal cancer patients, of being associated with Dukes stage B to D, non-mucinous adenocarcinoma, and left-sided disease.
To study glucose metabolism in cancer cells we used immunohistochemistry of the transketolase isoform TKTL1, upregulated in malignancies both at the mRNA and the protein level.Citation17-19 TKTL1 expression is not a direct measure of glucose metabolism, but mirrors the metabolic activity in cancer cells and works as a tool for estimating glucose metabolism in cancer cells at the time of surgery. TKTL1 tissue expression associates with poor patient survival in various types of cancer.
The few previous studies on tissue expression of TKTL1 in colorectal cancer have been based on rather small patient series. The results reported are, however, promising, and we decided to validate them in our large series of both colon and rectum carcinomas with long follow-up. Schwaab et al. used quantitative PCR to study 33 locally advanced rectal carcinomas receiving neoadjuvant chemotherapy.Citation11 Patients with high expression of TKTL1 had significantly shorter 3-year disease-free survival (DFS) than did those with low expression (34% vs. 87%, P = 0.017). In one series of 70 colon cancers, expression of TKTL1 correlated with tumor invasiveness and poor patient survival.Citation4 In another, with 63 patients with colorectal carcinoma, although TKTL1 expression correlated with local tumor progression and regional lymph node metastasis, TKTL1 expression in primary tumors decreased when distant metastasis occured.Citation20 Two alternative reasons for this development were presented, the first based on TKTL1 as enabling metastasis formation, but not being necessary after metastasis occurs. The other is based on a Darwinian assumption of only TKTL1-positive tumors as being able to form metastasis. Both these are hypotheses only. No one has been able to confirm why the decrease in primary tumor TKTL1 levels happens. In our study, high expression of TKTL1 correlated with Dukes stage B-D (P = 0.002), but we failed to see any decrease in primary tumor TKTL1 in Dukes D patients alone. These differences could be due to differing scoring methods. We used manual scoring by 2 researchers, whereas the Diaz-Moralli et al. study used computerized technology. In our study immunohistochemical expression of TKTL1 correlated with poor survival in both colon and rectal carcinomas.
TKTL1-protein expression in colorectal cancer cell lines, when studied with real-time PCR, Western blotting, and immunohistochemistry, was upregulated in about half of colorectal carcinomas but not in healthy tissue, and of 30 patients, 14 showed positive TKTL1 immunostaining.Citation18 The latter figure is much lower than in our study where 87.5% of tumors stained for TKTL1. Both studies had similar protocols for producing TMA. The difference in results may in part be due to different scoring protocols, as the other study used both staining intensity and percentage of positive tumor cells according to immunoreactive score. Differences were also possible in ischemic time from removal of the specimen to formalin fixation. In our hospital we have for many years paid attention to this part of the process. With the human colon cancer cell line LoVo, it appeared by PCR that mRNA expression of TKTL1 is upregulated.Citation17 Moreover, when these cells were treated with anti-TKTL1 siRNA, the cell-proliferation rate decreased. Unfortunately we lack data on the mRNA expression in our series.
One of the major differences between normal and cancer cells is this difference in glucose metabolism. The ability of cancer cells to enhance glycolysis is thought to associate with cell proliferation and survival. This specific property of cancer cells offers a possibility to interfere in order to find new treatment methods. As an enzyme, transketolase constitutes a potential target for future anti-cancer therapy. Inhibiting TKTL1 in order might hinder cancer-cells’ growth by limiting their energy sources. Thus far, clinical trials have focused on restricting the glucoseintake of cancer patients. In a study of 20 patients with recurrent glioblastoma, they were each allowed 60 g of carbohydrates per day, and had their urine ketones monitored with urine test sticks. Stable ketosis meant that 2 urine samples per week showed ketones. In patients with stable ketosis - although without any significant effect on progression-free survival (PFS) - a trend for longer PFS was evident, compared to the survival of those failing to reach stable ketosis.Citation21 Research on dietary intake of glucose in cancer patients is based on the knowledge of increased glucose metabolism in cancer cells. The theory is that when limiting dietary intake of glucose, less glucose would be available from which cancerous tissue could produce energy from.
The strength of our study is a large, well-characterized patient series of colorectal cancers with long follow-up time and reliable survival data. This material enables subgroup analyses. Although tissue microarray represents only a small part of the tumor, it has proven as good as, or even better than whole sections for analysis of a large patient series. Citation22-25
There is no absolute truth regarding scoring of immunohistochemical stainings of prognostic biomarkers. In this study we decided to use the highest score out of 3 spots from each patient for further analysis. We find this appropriate, since TKTL1 expression is known from other studies to correlate with outcome. Therefore, we assumed that the focus with the highest expression represents an aggressive tumor population, that most likely contribute to invasion and metastasis.
In conclusion, our results confirm previous smaller studies’ findings that colorectal cancer patients with high TKTL1 expression have a poor prognosis. Future studies should focus on stage II (Dukes B) colorectal cancer patients of whom 20% develop recurrent disease.Citation16 In any country these patients will receive adjuvant therapy, although 80% would be cured by surgery alone. It would be important to identify those patients at risk. TKTL1 is thus one candidate marker for risk identification.
Materials and methods
Patients and tissues
This study comprised 840 colorectal cancer patients surgically treated in the Department of Surgery at Helsinki University Hospital, 1989-2000. Of these, 466 (55.5%) were men, and 480 (57.1%) were over 65. The tumor was located in the colon in 429 patients (51.1%), and in the rectum in 411 (48.9%). According to the modified Dukes classification, 125 patients (14.9%) were classified as Dukes A, 294 (35.0%) as Dukes B, 231 (27.5%) as Dukes C, and 190 as Dukes D (22.6%). Disease-specific survival (DSS) at 5 y for all patients was 58.9% (95% CI 55.4-62.4). The median follow-up time was 5.1 y (0–28.0).
Surgical tumor samples were fixed in formalin for more than 24 hours, embedded in paraffin, and stored in the archives of the Department of Pathology. All specimens underwent re-evaluation by an experienced pathologist. Thereafter, the tumor tissue microarray (TMA) blocks constructed included all colorectal cancer tumor samples.Citation22-24 We collected 3 1.0-mm diameter punches of tissue from representative areas of each of these tumor blocks.
Immunohistochemistry
The TMA blocks were cut into 0.4-mm-thick sections, fixed on slides, and dried for 12 to 24 hours at 37°C. Sections were subsequently deparaffinized in xylene and rehydrated through graded ethanol and distilled water. For antigen retrieval, the slides were treated with Tris-HCl (pH 8.5) in a PreTreatment module (LabVision Corp.) for 20 minutes at 98°C. Staining of sections took place in an Autostainer 480 (LabVision) with an anti-human TKTL1 antibody (Rida Pentocheck IHC, Clone JFC12T10, R-Biopharm AG) diluted 1:200 with Dako REAL Antibody Diluent S2022 (Dako). The primary antibody was kept on glasses over night (O/N) followed by a 30-minute incubation with the secondary peroxide-conjugated rabbit/mouse ENV (K5007) Dako REAL Envision/HRP antibody (Dako). The slides were finally visualized with Dako REAL DAB+Chromogen kept on glasses for 10 minutes. Between each step of the staining procedure, slides were washed with PBS-0.04% Tween20. The slides were counterstained with Meyer's hematoxylin, washed in tap water for 10 minutes, and finally mounted in aqueous mounting medium (Aquamount, BHD). We used a gastric and a colon cancer specimen known to be positive for TKTL1 as positive controls in each staining. Ki67 in this material has been stained in an earlier study using a mouse monoclonal antibody (clone MIB-1, Dako) with a 1:100 dilution.Citation26
Scoring
The samples were scored independently by K.A. and J.H. Cytoplasmic positivity was scored on a 4-grade scale, where absence of staining was scored as 0, mild staining as 1, moderate staining as 2, and strong staining as 3. The highest score out of 3 was chosen to represent the tumor, as high TKTL1 has been shown to associate with poor survival. Samples with no tumor tissue or with too few cells for adequate judging were excluded. Samples receiving different scores from the 2 researchers were discussed to reach consensus.
Statistical analysis
Associations between TKTL1 and clinicopathologic variables were assessed by the chi-square test. Disease-specific overall survival calculation was from date of surgery to date of death from colorectal cancer or until end of follow-up. Survival curves were constructed according to the Kaplan-Meier method and compared with the logrank test. For univariable and multivariable survival analysis, the Cox proportional hazard model had the following covariates entered: age, gender, grade, Dukes classification, histologic type, side (right vs. left), tumor location (colon vs. rectum), and TKTL1 expression. Dukes’ classification, grade, histologic type, and expression of TKTL1 were entered as categorical covariates. A p-value of <0 .05 was considered statistically significant. All statistical analyses were performed with IBM SPSS Statistics version 20.0 for Mac (IBM Corporation).
The Surgical Ethics Committee of Helsinki University Hospital (Dnro HUS 226/E6/06, extension TMK02 §66 17.4.2013) and the National Supervisory Authority of Welfare and Health (Valvira Dnro 10041/06.01.03.01/2012) approved the study.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Päivi Peltokangas and Elina Aspiala for their excellent technical assistance. This work was financially supported by Finska Läkaresällskapet, the Sigrid Jusélius Foundation, and Medicinska Understödsföreningen Liv och Hälsa.
Funding
This work was financially supported by Finska Läkaresällskapet, the Sigrid Jusélius Foundation, and Medicinska Understödsföreningen Livoch Hälsa.
References
- Warburg O, Posener K, Negelein E. Über den Stoffwechsel der Carcinomzelle. Biochem Z 1924; 152:309-44; http://dx.doi.org/10.1007/BF01504608
- Wittig R, Coy JF. The role of glucose metabolism and glucose-associated signalling in cancer. Perspect Medicin Chem 2008; 1:64-82; PMID:19812737
- Xu X, Hausen Zur A, Coy JF, Löchelt M. Transketolase-like protein 1 (TKTL1) is required for rapid cell growth and full viability of human tumor cells. Int J Cancer 2009; 124:1330-7; PMID:19065656; http://dx.doi.org/10.1002/ijc.24078
- Langbein S, Zerilli M, Hausen Zur A, Staiger W, Rensch-Boschert K, Lukan N, Popa J, Ternullo MP, Steidler A, Weiss C, et al. Expression of transketolase TKTL1 predicts colon and urothelial cancer patient survival: Warburg effect reinterpreted. Br J Cancer 2006; 94:578-85; PMID:16465194; http://dx.doi.org/10.1038/sj.bjc.6602962
- Schultz H, Kähler D, Branscheid D, Vollmer E, Zabel P, Goldmann T. TKTL1 is overexpressed in a large portion of non-small cell lung cancer specimens. Diagn Pathol 2008; 3:35; PMID:18700018; http://dx.doi.org/10.1186/1746-1596-3-35
- Sun W, Liu Y, Glazer CA, Shao C, Bhan S, Demokan S, Zhao M, Rudek MA, Ha PK, Califano JA. TKTL1 is activated by promoter hypomethylation and contributes to head and neck squamous cell carcinoma carcinogenesis through increased aerobic glycolysis and HIF1alpha stabilization. Clin Cancer Res 2010; 16:857-66; PMID:20103683; http://dx.doi.org/10.1158/1078-0432.CCR-09-2604
- Kayser G, Sienel W, Kubitz B, Mattern D, Stickeler E, Passlick B, Werner M, Hausen Zur A. Poor outcome in primary non-small cell lung cancers is predicted by transketolase TKTL1 expression. Pathology 2011; 43:719-24; PMID:22027741; http://dx.doi.org/10.1097/PAT.0b013e32834c352b
- Krockenberger M, Engel JB, Schmidt M, Kohrenhagen N, Häusler SFM, Dombrowski Y, Kapp M, Dietl J, Honig A. Expression of transketolase-like 1 protein (TKTL1) in human endometrial cancer. Anticancer Res 2010; 30:1653-9; PMID:20592357
- Schmidt M, Voelker H-U, Kapp M, Krockenberger M, Dietl J, Kammerer U. Glycolytic phenotype in breast cancer: activation of Akt, up-regulation of GLUT1, TKTL1 and down-regulation of M2PK. J Cancer Res Clin Oncol 2010; 136:219-25; PMID:19655166; http://dx.doi.org/10.1007/s00432-009-0652-y
- Chen H, Yue J-X, Yang S-H, Ding H, Zhao R-W, Zhang S. Overexpression of transketolase-like gene 1 is associated with cell proliferation in uterine cervix cancer. J Exp Clin Cancer Res 2009; 28:43; PMID:19331662; http://dx.doi.org/10.1186/1756-9966-28-43
- Schwaab J, Horisberger K, Ströbel P, Bohn B, Gencer D, Kähler G, Kienle P, Post S, Wenz F, Hofmann W-K, et al. Expression of Transketolase like gene 1 (TKTL1) predicts disease-free survival in patients with locally advanced rectal cancer receiving neoadjuvant chemoradiotherapy. BMC Cancer 2011; 11:363; PMID:21854597; http://dx.doi.org/10.1186/1471-2407-11-363
- Lange CA, Tisch-Rottensteiner J, Böhringer D, Martin G, Schwartzkopff J, Auw-Haedrich C. Enhanced TKTL1 expression in malignant tumors of the ocular adnexa predicts clinical outcome. Ophthalmology 2012; 119:1924-9; PMID:22658715; http://dx.doi.org/10.1016/j.ophtha.2012.03.037
- Grimm M, Hoefert S, Luz O, Reinert S, Polligkeit J. Transketolase-like protein 1 expression in recurrent oral squamous cell carcinoma after curative resection: a case report. Oral Surg Oral Med Oral Pathol Oral Radiol 2013; 116:e173-8; PMID:22819458; http://dx.doi.org/10.1016/j.oooo.2011.12.022
- Grimm M, Munz A, Teriete P, Nadtotschi T, Reinert S. GLUT-1(+)/TKTL1(+) coexpression predicts poor outcome in oral squamous cell carcinoma. Oral Surg Oral Med Oral Pathol Oral Radiol 2014; 117:743-53; PMID:24703406; http://dx.doi.org/10.1016/j.oooo.2014.02.007
- Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2014; 136:359–386; PMID:25220842
- Böckelman C, Engelmann BE, Kaprio T, Hansen TF, Glimelius B. Risk of recurrence in patients with colon cancer stage II and III: A systematic review and meta-analysis of recent literature. Acta Oncol 2015; 54:5-16; PMID:25430983; http://dx.doi.org/10.3109/0284186X.2014.975839
- Hu L-H, Yang J-H, Zhang D-T, Zhang S, Wang L, Cai P-C, Zheng J-F, Huang J-S. The TKTL1 gene influences total transketolase activity and cell proliferation in human colon cancer LoVo cells. Anticancer Drugs 2007; 18:427-33; PMID:17351395; http://dx.doi.org/10.1097/CAD.0b013e328013d99e
- Bentz S, Cee A, Endlicher E, Wojtal KA, Naami A, Pesch T, Lang S, Schubert P, Fried M, Weber A, et al. Hypoxia induces the expression of transketolase-like 1 in human colorectal cancer. Digestion 2013; 88:182-92; PMID:24193262; http://dx.doi.org/10.1159/000355015
- Coy JF, Dressler D, Wilde J, Schubert P. Mutations in the transketolase-like gene TKTL1: clinical implications for neurodegenerative diseases, diabetes and cancer. Clin Lab 2005; 51:257-73; PMID:15991799
- Diaz-Moralli S, Tarrado-Castellarnau M, Alenda C, Castells A, Cascante M. Transketolase-like 1 expression is modulated during colorectal cancer progression and metastasis formation. PLoS ONE 2011; 6:e25323; PMID:21980427; http://dx.doi.org/10.1371/journal.pone.0025323
- Rieger J, Bähr O, Maurer GD, Hattingen E, Franz K, Brucker D, Walenta S, Kammerer U, Coy JF, Weller M, et al. ERGO: a pilot study of ketogenic diet in recurrent glioblastoma. Int J Oncol 2014; 44:1843-52; PMID:24728273; http://dx.doi.org/10.3892/ijo.2014.2382
- Kononen J, Bubendorf L, Kallioniemi A, Bärlund M, Schraml P, Leighton S, Torhorst J, Mihatsch MJ, Sauter G, Kallioniemi OP. Tissue microarrays for high-throughput molecular profiling of tumor specimens. Nat Med 1998; 4:844-7; PMID:9662379; http://dx.doi.org/10.1038/nm0798-844
- Nocito A, Kononen J, Kallioniemi OP, Sauter G. Tissue microarrays (TMAs) for high-throughput molecular pathology research. Int J Cancer 2001; 94:1-5; PMID:11668471; http://dx.doi.org/10.1002/ijc.1385
- Kallioniemi OP, Wagner U, Kononen J, Sauter G. Tissue microarray technology for high-throughput molecular profiling of cancer. Hum Mol Genet 2001; 10:657-62; PMID:11257096; http://dx.doi.org/10.1093/hmg/10.7.657
- Torhorst J, Bucher C, Kononen J, Haas P, Zuber M, Köchli OR, Mross F, Dieterich H, Moch H, Mihatsch M, et al. Tissue microarrays for rapid linking of molecular changes to clinical endpoints. Am J Pathol 2001; 159:2249-56; PMID:11733374; http://dx.doi.org/10.1016/S0002-9440(10)63075-1
- Böckelman C, Koskensalo S, Hagström J, Lundin M, Ristimäki A, Haglund C. CIP2A overexpression is associated with c-Myc expression in colorectal cancer. Cancer Biol Ther 2012; 13:289-95; PMID:22310977; http://dx.doi.org/10.4161/cbt.18922